Abstract
Background
Protocatechuic acid is an antioxidant which is shown to have analgesic activity in limited studies. However, the mechanisms of action remain unclear.
Objectives
It is aimed to investigate the possible contribution of cannabinoid system that supresses the nociceptive process by the activation of CB1 and CB2 receptors in central and peripheral levels of pain pathways, to the analgesic activity of protocatechuic acid.
Methods
The analgesic activity of protocatechuic acid was determined at the doses of 75, 150 and 300 mg/kg (i.p.) by acetic acid-induced writhing and tail-immersion tests in mice. The results were compared to the analgesic effect of 300 mg/kg (i.p.) dipyrone and non-specific CB receptor agonist 5 mg/kg (i.p.) WIN 55,212–2. For investigating the contribution of cannabinoid system to protocatechuic acid analgesia; pre-treatment with 8 mg/kg (i.p.) CB1 antagonist AM251 and 8 mg/kg (i.p.) CB2 antagonist AM630 were performed separately before 300 mg/kg protocatechuic acid administration.
Results
It was determined that protocatechuic acid has dose-dependent analgesic effect independently from locomotor activity and is comparable with effects of dipyrone and WIN 55,212–2. Pre-treatment with CB1 receptor antagonist AM251 significantly antagonized the protocatechuic acid-induced analgesia in the tail-immersion and writhing tests, whereas pre-treatment of CB2 receptor antagonist AM630 was found to be effective only in the tail-immersion test.
Conclusion
It is concluded that cannabinoid modulation contributes to the analgesic effect of protocatechuic acid in spinal level rather than peripheral. CB1 receptor stimulation rather than CB2 receptor stimulation mediates the analgesic effect of protocatechuic acid in both levels, especially peripheral.
Graphical abstract.
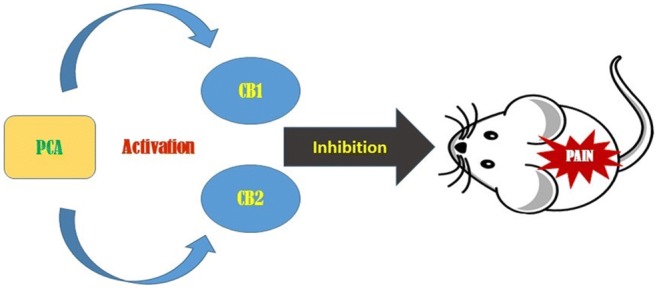
Protocatechuic acid inhibits pain response via cannabinoidergic system
Keywords: Protocatechuic acid, Pain, CB1 receptor, CB2 receptor, Cannabinoid system
Introduction
Pain is described as a sensory and emotional unpleasant experience associated with existing or possible tissue damage [1]. In the relief of pain, various drug groups such as opioids, non-steroidal anti-inflammatory drugs (NSAIDs), tricyclic antidepressants and anticonvulsants are used according to the type and intensity of pain. However, there is a need to discover new agents due to drug interactions, side effects and tolerability problems encountered with existing drugs. Thus, the interest in treatment with herbal medicines, which have fewer side effects and stronger therapeutic results, has considerably increased [2–4]. Protocatechuic acid (3,4-dihydroxybenzoic acid) is a phenolic compound that has been recently drawing attention for its biologic effects [5, 6]. Protocatechuic acid can easily crosses the blood brain barrier. Thereby, it gains attention in the inhibition of neurodegenerative progress based on existing data [7]. Protocatechuic acid also shows antioxidant, antihyperglycemic, anti-inflammatory and analgesic effects in different animal models [8, 9]. However, the mechanisms of action still remain unclear. For rational drug use, it is of great importance to investigate and elaborate the mechanisms of action of drugs.
Pain modulation is an extremely complex process that is managed with the compatible function of many endogenous inhibitory or excitatory mediators and different receptor groups ranging from the periphery to the central [10–12]. One of these endogenous systems is the endocannabinoid system. Studies of animal pain models using both endocannabinoids and synthetic receptor agonists have shown that cannabinoids are effective against acute pain induced by chemical, mechanical and thermal stimuli [13, 14]. Besides, there is increasing evidence that cannabinoids are also effective in chronic pain, such as inflammatory and neuropathic pain [15, 16]. Cannabinoids demonstrate their analgesic effects directly by activation of CB1 and CB2 receptors at central and peripheral levels of pain pathways [17] and also indirectly by interacting with other systems involved in pain modulation, such as endogenous opioids, glutamate and serotonin [15]. Recently, studies are focused on discovering new agents that activate the endocannabinoid system to provide effective pain control.
In this study, two different methods were used as tail-immersion and acetic acid-induced writhing test to evaluate the analgesia. Tail-immersion test measures the activity of the spinal reflex induced by nociceptive stimulation, not the pain-related functions managed by the upper centers [18, 19]. Namely; tail-withdrawal response induced by acute thermal stimulation is a simple spinal reflex and occurs after spinal cord transection [20]. Acetic acid-induced writhing model is one of the oldest and most sensitive methods used for acute visceral pain with peripheral origin in mice [2, 21]. Acetic acid provokes a very stereotypical behavior in mice characterized by abdominal contractions, bending and twisting of the trunk, elongation of the hind legs, reduced motor coordination and motor activity [22].
Based on this information, the contribution of cannabinoid CB1 and CB2 receptor activation to the analgesic activity of protocatechuic acid was investigated in mice using acetic acid-induced writhing and tail-immersion tests.
Methods
Chemicals
Protocatechuic acid and dipyrone (NSAID) were purchased from Sigma (St. Louis, MO, USA). Cannabinoid CB1 receptor antagonist AM251, cannabinoid CB2 receptor antagonist AM630 and non-specific cannabinoid agonist WIN 55,212-2 were purchased from Cayman (Michigan, USA).
Animals
Experimental studies were performed using 120 adult CD-1 male mice. All animals were housed in a well-ventilated room with 12h light/dark cycle at 22 ± 1 °C and allowed free access to food and water ad libitum. Experimental procedures were performed in accordance with ethical principles of Helsinki Declaration and ethic approval was supplied from by the Local Ethics Committee of Anadolu University (Approval Number: 2018-05).
Experimental groups
Protocatechuic acid was dissolved in mixture of % 50 polyethylene glycol (PEG) and % 8 dimethyl sulfoxide (DMSO) (1:1, v/v). The tested doses of protocatechuic acid, WIN 55,212-2 and cannabinoid receptor antagonists are determined based on previous studies [23–26]. Two experimental group were formed for comparing the analgesic effect of protocatechuic acid to the effects of positive control drugs, dipyrone and WIN 55,212-2. A group was formed as combination group to evaluate the possible interaction between protocatechuic acid and WIN 55,212-2. Two separate groups were formed that are administered with protocatechuic acid (14th group) and solvent (15th group) for investigating the effect on locomotor activity. All drug administrations were performed intraperitoneally (i.p.). Mice were randomly divided into 15 groups (n=8 per group). Treatment schedule is as follows;
Group 1: Equal volume of solvent vehicle, control group
Group 2: 75 mg/kg protocatechuic acid
Group 3: 150 mg/kg protocatechuic acid
Group 4: 300 mg/kg protocatechuic acid
Group 5: 300 mg/kg dipyrone
Group 6: 5 mg/kg WIN 55,212–2
Group 7: 150 mg/kg protocatechuic acid +2.5 mg/kg WIN 55,212–2, combination group
Group 8: Pre-treatment with 8 mg/kg AM251, 30 min after vehicle
Group 9: Pre-treatment with 8 mg/kg AM251, 30 min after 300 mg/kg protocatechuic acid
Group 10: Pre-treatment with 8 mg/kg AM251, 30 min after 5 mg/kg WIN 55,212–2
Group 11: Pre-treatment with 8 mg/kg AM630, 30 min after vehicle
Group 12: Pre-treatment with 8 mg/kg AM630, 30 min after 300 mg/kg protocatechuic acid
Group 13: Pre-treatment with 8 mg/kg AM630, 30 min after 5 mg/kg WIN 55,212–2
Group 14: Equal volume of solvent vehicle
Group 15: 300 mg/kg protocatechuic acid
Pain thresholds were measured in the tail-immersion test before drug administration and the base-line values were recorded as pre-drug latency. Analgesia test procedures were performed 30 minutes after the last drug injection
Tail-immersion test
3 cm from the end of the tail of the animal was immersed in water at a temperature of 52.5 ± 0.2 °C [27]. The nociceptive threshold of the animal was determined measuring tail withdrawal latency. In order to prevent tail tissue damage, the cut-off time was determined as 15 s. The analgesic effect was presented as the percentage of the maximum possible analgesic effect (MPE %). Enhancement in MPE % values were interpreted as antinociception. MPE % formula as below [28]:
Acetic acid-induced writhing test
Writhing formed in animals following acetic-acid (i.p.) administration is characterized by the contraction of abdominal muscles, stretching of the back legs backward and friction of abdomen. 40 min after the last drug injection, 0.6% solution of acetic acid was administered (i.p.) to animals. Following a 5-min waiting period, writhing behaviour of every animal were monitored for 10 min and the number of writhes were recorded [29].
Activity cage
Plexiglass, cage-shaped device called activity cage was used to evaluate the spontaneous locomotor activity. Parts located in two vertical sides of the device produce infrared (IR) rays. Vertical and horizontal movements of animals cause the interruption of these rays and thus, recorded by photocell [30]. Animals separately administered with vehicle and 300 mg/kg protocatechuic acid were put into the activity cage 30 min after the injection and recorded for 15 min.
Data analyses
The statistical analyses of analgesia test procedures were carried out using GraphPad Prism version 5.0 and one-way analysis of variance (ANOVA) followed by Tukey HSD multiple comparison test. Data obtained from activity cage was evaluated with student-t test. The results were expressed as the mean ± standard error of the mean to show variation in groups. Differences were considered significant when P < 0.05. All graphs were created by using GraphPad Prism version 5.0.
Results
The analgesic activity in tail-immersion test
Analgesic effect of protocatechuic acid at the doses of 75, 150 and 300 mg/kg (i.p.) in the tail-immersion test is shown on Fig. 1 and Table 1. 75 mg/kg protocatechuic acid relatively increased MPE % whereas 150 and 300 mg/kg protocatechuic acid, 300 mg/kg dipyrone and 5 mg/kg WIN 55,212–2 caused a significant increase (P < 0.05, P < 0.001, P < 0.05, P < 0.001, respectively) compared to the control group. MPE % of combination group was found significantly higher than the control group (P < 0.001).
Fig. 1.

The antinociception induced by 75, 150, 300 mg/kg (i.p.) protocatechuic acid, 300 mg/kg (i.p.) dipyrone, 5 mg/kg (i.p.) WIN 55,212–2 and the combination of 150 mg/kg (i.p.) protocatechuic acid and 2.5 mg/kg (i.p.) WIN 55,212–2 in the tail-immersion test. PCA: Protocatechuic acid. MPE: Maximum possible effect. WIN: WIN 55,212–2. *P < 0.05, ***P < 0.001: significant difference based on the control group. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. (n = 8)
Table 1.
Analgesic activity of protocatechuic acid and reversal effect of AM251 and AM630 in the tail-immersion and writhing test
| Tail-immersion (MPE %) | Writhing (number) | |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Control | 3.094 ± 1.233 | 34.860 ± 3.003 |
| 75PCA | 9.043 ± 1.718 | 19.860 ± 5.031* |
| 150PCA | 11.42 ± 1.675* | 17.250 ± 3.584** |
| 300PCA | 16.11 ± 1.910*** | 13.500 ± 2.659*** |
| Dipyron | 13.68 ± 1.538* | 7.000 ± 2.266*** |
| WIN | 16.60 ± 1.054*** | 3.500 ± 1.195*** |
| WIN+PCA | 20.46 ± 2.600*** | 2.375 ± 1.426*** |
| AM251 | 3.507 ± 0.7985 | 34.630 ± 2.449 |
| AM251 + PCA | 8.049 ± 0.9089+++ | 25.250 ± 3.889+ |
| AM251 + WIN | 7.947 ± 0.7782&&& | 24.500 ± 2.766&&& |
| AM630 | 3.029 ± 0.5698 | 34.130 ± 1.586 |
| AM630 + PCA | 9.056 ± 1.7990*,++ | 16.750 ± 2.763*** |
| AM630 + WIN | 8.516 ± 1.0740&& | 14.800 ± 3.105***;& |
MPE: Maximum possible effect. PCA: Protocatechuic acid. WIN: WIN 55,212–2. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001: significant difference based on the control group. +P < 0.05, ++P < 0.01, +++P < 0.001: significant difference based on the 300PCA group. &P < 0.05, &&P < 0.01, &&&P < 0.001: significant difference based on the WIN group
Figure 2 and Table 1 show how pre-treatment with CB1 receptor antagonist AM251 (8 mg/kg, i.p.) affected the analgesic effect of 300 mg/kg protocatechuic acid in tail-immersion test. Although AM251 pre-treatment significantly reversed (P < 0.001) the MPE % of protocatechuic acid, analgesic effect was still significant (P < 0.05) in AM251 pre-treated group. Likewise, the analgesic effect of 5 mg/kg WIN 55,212–2 was prevented significantly (P < 0.001) by AM251 pre-treatment.
Fig. 2.

The reversal effect of 8 mg/kg (i.p.) AM251 on 300 mg/kg (i.p.) protocatechuic acid and 5 mg/kg (i.p.) WIN 55,212–2 induced antinociception in the tail-immersion test. PCA: Protocatechuic acid. MPE: Maximum possible effect. WIN: WIN 55,212–2. *P < 0.05, ***P < 0.001: significant difference based on the control group. +++P < 0.001: significant difference based on the 300PCA group. &&&P < 0.001: significant difference based on the WIN group. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. (n = 8)
Figure 3 and Table 1 show how pre-treatment with CB2 receptor antagonist AM630 (8 mg/kg, i.p.) affected the analgesic effect of 300 mg/kg protocatechuic acid in tail-immersion test. Although AM630 pre-treatment significantly reversed (P < 0.01) the MPE % of protocatechuic acid, analgesic effect was still significant (P < 0.05) in AM630 pre-treated group. Similarly, the analgesic effect of 5 mg/kg WIN 55,212–2 was prevented significantly (P < 0.01) by AM630 pre-treatment.
Fig. 3.

The reversal effect of 8 mg/kg (i.p.) AM630 on 300 mg/kg (i.p.) protocatechuic acid and 5 mg/kg (i.p.) WIN 55,212–2 induced antinociception in the tail-immersion test. PCA: Protocatechuic acid. MPE: Maximum possible effect. WIN: WIN 55,212–2. *P < 0.05, ***P < 0.001: significant difference based on the control group. ++P < 0.01: significant difference based on the 300PCA group. &&P < 0.01: significant difference based on the WIN group. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. (n = 8)
The analgesic activity in acetic acid-induced writhing test
Analgesic effect of protocatechuic acid at the doses of 75, 150 and 300 mg/kg in acetic acid-induced writhing test is shown in Fig. 4 and Table 1. 75, 150, 300 mg/kg protocatechuic acid, 300 mg/kg dipyrone and 5 mg/kg WIN 55,212–2 administrations significantly decreased the number of writhes compared to the control group (P < 0.05, P < 0.01, P < 0.001, P < 0.001, P < 0.001, P < 0.001, respectively). A significant (P < 0.001) decrease in the number of writhes was observed in combination group in comparison with control group.
Fig. 4.

The antinociception induced by 75, 150, 300 mg/kg (i.p.) protocatechuic acid, 300 mg/kg (i.p.) dipyrone, 5 mg/kg (i.p.) WIN 55,212–2 and the combination of 150 mg/kg (i.p.) protocatechuic acid and 2.5 mg/kg (i.p.) WIN 55,212–2 in the acetic acid-induced writhing test. PCA: Protocatechuic acid. WIN: WIN 55,212–2. *P < 0.05, **P < 0.01, ***P < 0.001: significant difference based on the control group. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. (n = 8)
Figure 5 and Table 1 show how pre-treatment with CB1 receptor antagonist AM251 (8 mg/kg, i.p.) changed the peripheral analgesic effect of 300 mg/kg protocatechuic acid in acetic acid-induced writhing test. AM251 pre-treatment significantly (P < 0.05) reversed the effect of protocatechuic acid. Similarly, the analgesic effect of 5 mg/kg WIN 55,212–2 was prevented significantly (P < 0.001) with AM251 pre-treatment.
Fig. 5.

The reversal effect of 8 mg/kg (i.p.) AM251 on 300 mg/kg (i.p.) protocatechuic acid and 5 mg/kg (i.p.) WIN 55,212–2 induced antinociception in the acetic acid-induced writhing test. PCA: Protocatechuic acid. WIN: WIN 55,212–2. ***P < 0.001: significant difference based on the control group. +P < 0.05: significant difference based on the 300PCA group. &&&P < 0.001: significant difference based on the WIN group. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. (n = 8)
Figure 6 and Table 1 show how pre-treatment with CB2 receptor antagonist AM630 (8 mg/kg, i.p.) changed the peripheral analgesic effect of 300 mg/kg protocatechuic acid in acetic acid-induced writhing test. AM630 pre-treatment was not successful in reversing the effect of protocatechuic acid, in fact the effect of AM630 pre-treated group was significant (P < 0.001) compared to control group. Although the analgesic effect of 5 mg/kg WIN 55,212–2 was reversed significantly (P < 0.05) with AM630 pre-treatment, analgesic effect of WIN 55,212–2 was still significant (P < 0.001) in AM630 pre-treated group.
Fig. 6.

The reversal effect of 8 mg/kg (i.p.) AM630 on 300 mg/kg (i.p.) protocatechuic acid and 5 mg/kg (i.p.) WIN 55,212–2 induced antinociception in the acetic acid-induced writhing test. PCA: Protocatechuic acid. WIN: WIN 55,212–2. ***P < 0.001: significant difference based on the control group. &P < 0.001: significant difference based on the WIN group. One-way ANOVA followed by Tukey’s HSD multiple comparison post-hoc test was performed. Values expressed as mean ± S.E.M. (n = 8)
The effect on locomotor activity in activity cage
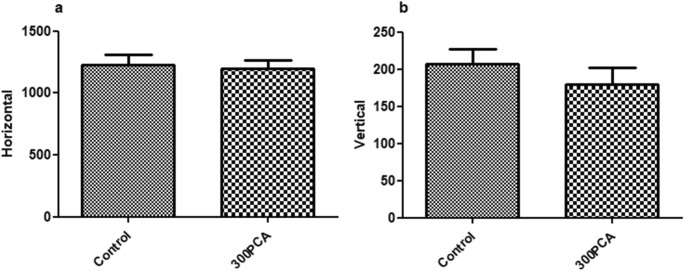
The effect of 300 mg/kg protocatechuic acid on locomotor activity was determined through horizontal (a) and vertical (b) movements in activity cage. As seen in Fig. 7, protocatechuic acid at the dose of 300 mg/kg did not cause any changes in horizontal and vertical movements.
Fig. 7.
The effect of 300 mg/kg protocatechuic acid on locomotor activity through horizontal (a) and vertical (b) movements in the activity cage. PCA: Protocatechuic acid. Student-t test was performed. Values expressed as mean ± S.E.M. (n = 8)
Discussion
In this study, the role of cannabinoid CB1 and CB2 receptor involvement in the analgesic effect of protocatechuic acid was elucidated. It was determined in the tail-immersion test that protocatechuic acid at the doses of 75, 150 and 300 mg/kg demonstrate dose-dependent analgesic effect by increasing the threshold of mice against thermal stimulus. Protocatechuic acid also demonstrated peripheral analgesic activity by reducing acetic acid-induced writhing behavior. Dipyrone was used as reference drug in this study due to be an acknowledged analgesic. Its mechanism of action involves both central and peripheral pathways [31]. In both methods, the analgesic effect of protocatechuic acid at 300 mg/kg dose was very similar to the analgesic effect of 300 mg/kg dipyrone and CB agonist 5 mg/kg WIN 55,212–2. These results correspond to the limited number of studies and those performed in our laboratory [9, 32]. In summary, it can be said that protocatechuic acid shows analgesic effect associated with both peripheral and spinal mechanisms. CB agonist WIN 55,212–2, which was shown to be an effective analgesic in experimental studies [33], was used as a positive control and also half-dose of effective dose of WIN 55,212–2 and protocatechuic acid were combined. The analgesic effect of this combination was found to be similar or even higher than the analgesic effect provided by the administration of 300 mg/kg of protocatechuic acid and 5 mg/kg WIN 55,212–2 alone. It is concluded that the combined therapy may be advantageous in terms of achieving the cannabinoid system-targeted analgesic effect with fewer side effects such as nausea-vomiting, appetite changes, cardiovascular disorders, respiratory depression and confusion limiting the use of CB agonist [34]. Because protocatechuic acid has analgesic effect associated with endocannabinoid system, as mentioned below, and it also enhances the analgesia induced by direct cannabinoid system-targeted WIN 55,212–2. It was also determined that the protocatechuic acid at 300 mg/kg does not change the locomotor activity. The compounds which are centrally effective are preferred to show their efficacy independent from the locomotor activity [35]. Therefore, it may be considered an advantage to provide analgesia without altering locomotor activity.
There is limited information about the mechanisms that mediate the detected analgesic effect of protocatechuic acid [6, 26]. However, clarifying the action mechanisms of a chemical is important in terms of determining the effect profile of the drug candidate compound and using in the correct indication. The fact that the cannabinoid system reveals an effective analgesia the studies to discover new agents that activate this system has accelerated. In this study, the connection between protocatechuic acid and endocannabinoid system was investigated. The pain-relieving effects of cannabinoids occur at different levels of the central nervous system, spinal cord and peripheral nervous system that contain CB receptors at different concentrations [36, 37]. It is also known that endocannabinoids interact with other systems that are involved in pain modulation, such as endogenous opioids, glutamate and serotonin [15]. In this study, the pre-administration of CB1 antagonist AM251 in the tail-immersion and writhing tests significantly inhibited the analgesia induced by protocatechuic acid. Thus, CB1 receptor stimulation was thought to contribute to both spinal and peripheral analgesia. In addition, the fact that the antagonism provided by AM251 in tail-immersion test was more remarkable than the antagonism in writhing test, suggests that spinal CB1-mediated cannabinoid modulation may have a more important role in protocatechuic acid analgesia. Additionally, in the presence of AM251, the partial disappearance of protocatechuic acid effect suggests that other mechanisms as well as cannabinoid system are involved in the activity. It is known that CB1 receptors are concentrated in supraspinal and spinal pain regions and provide pain control by interacting with different systems [15, 38, 39]. Moreover, it has been shown in our laboratory that the antinociceptive effect of protocatechuic acid in hot-plate (supraspinal response) and tail-immersion (spinal reflex) tests in mice is modulated by the cholinergic and especially opioid systems at the spinal level [26]. The cannabinoid system may therefore undertake the spinal organization of protocatechuic acid analgesia along with other systems associated with pain. Although the antagonism observed in the presence of AM251 in the writhing test was less pronounced than the antagonism observed in the tail-immersion test, the pre-administration of AM251 prevented the protocatechuic acid to show significant effect. This result demonstrates that CB1 receptor stimulation is also effective in protocatechuic acid analgesia in the periphery. Furthermore, the deprivation of the analgesic effect of WIN 55,212–2 by pre-treatment with AM251 in the tail-immersion and writhing tests is important in order to indicate the reliability and functionality of our test setup. Pre-administration of the CB2 receptor antagonist AM630 in tail-immersion test antagonized the protocatechuic acid effect, although the effect was still present. On the contrary, significant antagonism was not observed in the writhing test and ongoing effect of protocatechuic acid was similar to the effect when administered alone. The analgesic effect of WIN 55,212–2 was also prevented and disappeared by AM630 pre-treatment in the tail-immersion test whereas the significance in the effect was ongoing besides the significant antagonism in writhing test. At this point, it can be thought that cannabinoid CB2 stimulation contributes to the spinal organization of protocatechuic acid analgesia but does not play an effective role at the peripheral level. In view of the fact that the analgesic effect of WIN 55,212–2 was not completely lost in the presence of AM630 in writhing test and also the reversal effect was relatively less than the tail-immersion test; it could be thought that CB1 stimulation has a predominant role in pain than CB2 stimulation and the results may be affected by this physiology. It is known that in contrast to CB1 receptors, CB2 receptors are more abundant in the periphery than in the central nervous system, and are negligible in neural tissues, but are commonly present in glia cells, lymphoid tissues, and immune system cells [15, 16, 39, 40]. Thus, antinociception via CB2 receptors is thought to be indirect. For instance; it has been shown that endorphin released by stimulation of CB2-expressing cells indirectly provides antinociception by the opioid receptors on the terminals of primary afferent neurons [41]. The recent data also demonstrates the presence of CB2 receptor expression on primary afferent nociceptive neurons in the periphery [42, 43]. Thereby, it can be implicated that peripheral CB2 receptor stimulation can provide antinociception as well as CB1 stimulation. At this point, it may be suggested that the CB2 receptor antagonist AM630 may have been insufficient at dose to antagonize the antinociceptive effect via CB2 receptor stimulation in the experimental conditions of this study. However, in order to confirm this idea, the study should be elaborated with varying doses of antagonists.
Conclusion
It has been determined that cannabinoid modulation contributes to the analgesic effect of protocatechuic acid in spinal level rather than peripheral. Also, it has been concluded that CB1 receptor stimulation rather than CB2 receptor stimulation mediates the analgesic effect of protocatechuic acid in both levels, especially peripheral. Protocatechuic acid may serve safely as a potential analgesic drug targeting the cannabinoid system.
Future prospective
It can be said that protocatechuic acid is a natural agent that may be safely used alone or as an auxiliary drug in pain management. In order to support this speculation, further in vivo and in vitro pharmacological studies should be completed and, the mechanism of action and results of chronic use should be elaborated.
Acknowledgements
This article is based on the M.Sc. degree thesis of Duygu YESIM DIKMEN and supported financially the Anadolu University Research Foundation (Eskisehir, Turkey), Project no: 1802S035.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2. Seattle: IASP Press; 1994. [Google Scholar]
- 2.Mehrotra A, Shanbhag R, Chamallamudi MR, Singh VP, Mudgal J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur J Pharmacol. 2011;666:80–86. doi: 10.1016/j.ejphar.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Zamani SS, Hossieni M, Etebari M, Salehian P, Ebrahimi SA. Pharmacokinetics of calycopterin and xanthmicrol, two polymethoxylated hydroxyflavones with anti-angiogenic activities from Dracocephalum kotschyi Bioss. Daru. 2016;24:22. doi: 10.1186/s40199-016-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zareba G. Phytotherapy for pain relief. Drugs Today (Barc) 2009;45:445–467. doi: 10.1358/dot.2009.45.6.1354120. [DOI] [PubMed] [Google Scholar]
- 5.Cazacu I, Mogosan C, Loghin F. Safety issues of current analgesics: an update. Clujul Med. 2015;88:128–136. doi: 10.15386/cjmed-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semaming Y, Pannengpetch P, Chattipakorn S, Chattipakorn N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid Based Complement Alternat Med. 2015;2015:1–11. doi: 10.1155/2015/593902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krzysztoforska K, Mirowska-Guzel D, Widy-Tyszkiewicz E. Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: review on the basis of in vitro and in vivo studies in rodents and humans. Nutr Neurosci. 2017;26:1–11. doi: 10.1080/1028415X.2017.1354543. [DOI] [PubMed] [Google Scholar]
- 8.Masella R, Santangelo C, D'Archivio M, Li Volti G, Giovannini C, Galvano F. Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr Med Chem. 2012;19:2901–2917. doi: 10.2174/092986712800672102. [DOI] [PubMed] [Google Scholar]
- 9.Lende A, Kshirsagar A, Deshpande A, Muley M, Patil R, Bafna P. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology. 2011;19:255–263. doi: 10.1007/s10787-011-0086-4. [DOI] [PubMed] [Google Scholar]
- 10.Jinsmaa Y, Fujita Y, Shiotani K, Miyazaki A, Li T, Tsuda Y, Okada Y, Ambo A, Sasaki Y, Bryant SD, Lazarus LH. Differentiation of opioid receptor preference by [Dmt1]endomorphin-2-mediated antinociception in the mouse. Eur J Pharmacol. 2005;509:37–42. doi: 10.1016/j.ejphar.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Mizoguchi H, Takagi H, Watanabe C, Yonezawa A, Sato T, Sakurada T, Sakurada S. Involvement of multiple μ-opioid receptor subtypes on the presynaptic or postsynaptic inhibition of spinal pain transmission. Peptides. 2014;51:15–25. doi: 10.1016/j.peptides.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Schaible H. Peripheral and central mechanisms of pain generation. Handb Exp Pharmacol. 2007;177:3–28. doi: 10.1007/978-3-540-33823-9_1. [DOI] [PubMed] [Google Scholar]
- 13.Guindon J, De Lean A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Ulugol A, Ozyigit F, Yesilyurt O, Dogrul A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth Analg. 2006;102:443–447. doi: 10.1213/01.ane.0000194587.94260.1d. [DOI] [PubMed] [Google Scholar]
- 15.Fine P, Rosenfeld M. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med J. 2013;4:e0022. doi: 10.5041/RMMJ.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manzanares J, Julian M, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4:239–257. doi: 10.2174/157015906778019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie W. Assessment of pain in animals. In: Ma C, Zhang JM, editors. Animal models of pain. New York: Humana Press; 2011. pp. 1–22. [Google Scholar]
- 19.Uludag MO. Deneysel agri, inflamasyon ve sepsis modelleri. Ankara: Gazi Üniversitesi Laboratuvar Hayvanlari Yetistirme ve Deneysel Arastirmalar Merkezi; 2017. [Google Scholar]
- 20.Flores JA, El Banoua F, Galan-Rodrıguez B, Fernandez-Espejo E. Opiate antinociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain. 2004;110:205–214. doi: 10.1016/j.pain.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Bennett G. Animal models of pain. In: Kruger L, editor. Methods in pain research. New York: CRC Press; 2001. pp. 67–93. [Google Scholar]
- 22.Le Bars D, Gozariu M, Cadden S. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 23.Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, Simone DA. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 24.Godin AM, Araújo DP, César IC, Menezes RR, Brito AS, Melo IS, Coura GM, Bastos LF, Almeida MO, Byrro RM, Matsui TC, Batista CR, Pianetti GA, de Fátima Â, Machado RR, Coelho MM. Activities of 2-phthalimidethyl nitrate and 2-phthalimidethanol in the models of nociceptive response and edema induced by formaldehyde in mice and preliminary investigation of the underlying mechanisms. Eur J Pharmacol. 2015;756:59–66. doi: 10.1016/j.ejphar.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, Verri WA., Jr Naringenin reduces inflammatory pain in mice. Neuropharmacology. 2016;105:508–519. doi: 10.1016/j.neuropharm.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Arslan R, Aydin S, Samur D, Bektas N. The possible mechanisms of protocatechuic acid-induced central analgesia. Saudi Pharm J. 2018;26:541–545. doi: 10.1016/j.jsps.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmauss C, Yaksh TL. In vivo studies on spinal receptor systems mediating antinociception II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984;228:1–12. [PubMed] [Google Scholar]
- 28.Kondo D, Saegusa H, Yabe R, Takasaki I, Kurihara T, Zong S, Tanabe T. Peripheral-type benzodiazepine receptor antagonist is effective in relieving neuropathic pain in mice. J Pharmacol Sci. 2009;110:55–63. doi: 10.1254/jphs.09028fp. [DOI] [PubMed] [Google Scholar]
- 29.De Oliveira ED, Schallenberger C, Böhmer AE, Hansel G, Fagundes AC, Milman M, Silva MD, Oses JP, Porciúncula LO, Portela LV, Elisabetsky E, Souza DO, Schmidt AP. Mechanisms involved in the antinociception induced by spinal administration of inosine or guanine in mice. Eur J Pharmacol. 2016;772:71–82. doi: 10.1016/j.ejphar.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Marazioti A, Spyraki C, Thermos K. GABA antagonists reverse the somatostatin dependent attenuation of rat locomotor activity. Neuropeptides. 2009;43:207–212. doi: 10.1016/j.npep.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Jasiecka A, Maślanka T, Jaroszewski JJ. Pharmacological characteristics of metamizole. Pol J Vet Sci. 2014;17:207–214. doi: 10.2478/pjvs-2014-0030. [DOI] [PubMed] [Google Scholar]
- 32.Bektas N, Arslan R. The involvement of NO–cGMP–ATP sensitive K+ channels pathway in protocatechuic acid peripheral analgesia. Ind J Pharm Edu Res. 2017;51:355–358. [Google Scholar]
- 33.Pascual D, Goicoechea C, Suardiaz M, Martin MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005;118:23–34. doi: 10.1016/j.pain.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Ibiloglu AO, Atlı A, Gunes M. Sentetik kannabinoidler. Psikiyatride Guncel Yaklasimlar. 2017;9:317–328. [Google Scholar]
- 35.Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- 37.Brusberg M, Arvidsson S, Kang D, Larsson H, Lindström E, Martinez V. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents. J Neurosci. 2009;29:1554–1564. doi: 10.1523/JNEUROSCI.5166-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrie N, Manolios N. The endocannabinoid system in pain and inflammation: its relevance to rheumatic disease. Eur J Pharmacol. 2017;4:210–218. doi: 10.5152/eurjrheum.2017.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo ER. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4:245–259. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pascual D, Sanchez-Robles EM, Garcia MM, Goicoechea C. Chronic pain and cannabinoids: great expectations or a christmas carol. Biochem Pharmacol. 2018;157:33–42. doi: 10.1016/j.bcp.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan P. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beltramo M. Cannabinoid type 2 receptor as a target for chronic pain. Mini Rev Med Chem. 2009;9:11–25. doi: 10.2174/138955709787001785. [DOI] [PubMed] [Google Scholar]
- 43.Valenzanoa KJ, Tafesseb L, Leeb G, Harrisona JE, Bouleta JM, Gottshalla SL, Marka L, Pearsona MS, Millera W, Shana S, Rabadic L, Rotshteync Y, Chaffera SM, Turchina PI, Elsemorea DA, Totha M, Koetznera L, Whitesidea GT. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]