Abstract
Objectives
Diabetic neuropathy (DNP) is a widespread and debilitating complication with complex pathophysiology that is caused by neuronal dysfunction in diabetic patients. Conventional therapeutics for DNP are quite challenging due to their serious adverse effects. Hence, there is a need to investigate novel effective and safe options. The novelty of the present study was to provide available therapeutic approaches, emerging molecular mechanisms, signaling pathways and future directions of DNP as well as polyphenols’ effect, which accordingly, give new insights for paving the way for novel treatments in DNP.
Evidence acquisition
A comprehensive review was done in electronic databases including Medline, PubMed, Web of Science, Scopus, national database (Irandoc and SID), and related articles regarding metabolic pathways on the pathogenesis of DNP as well as the polyphenols’ effect. The keywords “diabetic neuropathy” and “diabetes mellitus” in the title/abstract and “polyphenol” in the whole text were used. Data were collected from inception until May 2019.
Results
DNP complications is mostly related to a poor glycemic control and metabolic imbalances mainly inflammation and oxidative stress. Several signaling and molecular pathways play key roles in the pathogenesis and progression of DNP. Among natural entities, polyphenols are suggested as multi-target alternatives affecting most of these pathogenesis mechanisms in DNP.
Conclusion
The findings revealed novel pathogenicity signaling pathways of DNP and affirmed the auspicious role of polyphenols to tackle these destructive pathways in order to prevent, manage, and treat various diseases.
Graphical Abstract.
.
Keywords: Diabetic neuropathy, Polyphenols, Therapeutic targets, Signaling pathways
Introduction
As one of the most chronic and common endocrine-metabolic disorders, diabetes mellitus (DM) is predicted to afflict up to 300 million people or more by the year 2025 [1, 2]. The elevated blood glucose concentration, in addition to macrovascular incidents (heart attack, stroke and peripheral vascular disease) also leads to microvascular complications in body organs specially kidney (diabetic nephropathy), eyes (retinopathy), and nerves (neuropathy) [1–3]. Diabetic neuropathy (DNP) has been considered the most common complication of diabetes resulting from prolonged periods of hyperglycemia, damaging fragile nerve fibers and the walls of their blood vessels [4]. The prevalence of DNP in diabetic patients ranges from 7% within 1 year of diagnosis, to 50% for those suffering from diabetes for more than 25 years [3]. DNP is divided into several subcategories, including; hyperglycaemia, generalized (autonomic neuropathy, sensorimotor polyneuropathy, and acute painful sensory neuropathy), focal and multifocal (thoracolumbar radiculoneuropathy, cranial neuropathy, proximal and focal limb DNP) neuropathies, and superimposed chronic inflammatory demyelinating polyneuropathy [5]. Diabetic sensory polyneuropathy or peripheral neuropathy is the most common form of DNP can present as sensory symptoms include hypesthesia, neuropathic pain, allodynia, paresthesias (tingling or pricking), dysesthesia and paresis [6]. Neurovascular insufficiency, autoimmune damage, neuro-hormonal growth factor deficiency and hyperglycemia-induced formation of advanced glycation end products (AGEs) are also among the multiple etiologies of DNP [7–9].
Despite clinical developments in the treatment of diabetes complications specially DNP, it still remained a clinical challenge with no effective solution. Thus, it rises the needs to develop novel multi-target therapeutics to regulate more involving destructive signaling pathways of individuals with DNP. As a heterogeneous and large group of phytochemicals containing phenol rings, polyphenols [10] are multi-target agents with the most antioxidants and anti-inflammatory effects have the potentials to combat several diseases like diabetes and its complications [11, 12]. Recently, polyphenols have also been introduced as potential neuroprotective agents in diabetes [13]. Understanding the signaling pathways underlying the progress of DNP and the way polyphenols prevent the progress of these destructive pathways, may lead to introduce polyphenols as new therapeutic agents in DNP.
The aim of the current review was to address available therapeutic targets, signaling pathways, molecular mechanisms, and future directions of DNP as well as polyphenols’ effect, in order to pave the way for novel treatments.
Metabolic pathways involved in the pathogenesis of DNP
Different pathways have been reported as being involved in the development of DNP pathogenesis e.g., imbalances in peripheral nerves blood supply, the vascular system of the thalamic gland, gene expression of sodium and calcium channels, and autoimmune diseases characterized by activation of glial cells [14].
In terms of mechanism, polyol pathway, oxidative stress and advanced glycation, inflammation, trophic factors, channels, and glutamate pathway are the main mediators and signaling pathways associated with DNP [15–19].
Polyol pathway
Polyol pathway is present in several tissues like peripheral nerve and blood vessels and certainly contributes to the development of DNP [20]. Two main enzymes are involved in the polyol pathway; aldose reductase (AR, a rate-limiting enzyme) and sorbitol dehydrogenase which the first is found in various tissues, including nerve, lens, retina, glomerulus, and vascular cells [21]. High blood glucose level leads to the activation of AR that produces sorbitol from glucose. This reaction consumes nicotinic acid adenine dinucleotide phosphate (NADPH) and produces NADP+. High consumption of NADPH reduces the level of reduced glutathione (GSH) and increases the level of oxidized glutathione (GSSG). Nevertheless, due to the inability of sorbitol to cross the cell membrane, the accumulated sorbitol elevates the blood osmolality resulting in the loss of electrolytes [17, 22]. High osmosis damages the cells surrounding peripheral neurons (Schwann cells) and causes the schwannopathy-related phenotype of DNP [22, 23].
The other key enzyme sorbitol dehydrogenase converts the accumulated sorbitol to fructose via oxidation and producing nicotinic acid adenine dinucleotide (NADH). However, the increase in both sorbitol and fructose leads to some deleterious effects on nerve cells due to several reasons, including the decrease in concentration of osmolality regulator (taurine or 2-aminoethanesulfonic acid, a bile component), and insulin sensitivity regulator (myoinositol), inhibition of Na+/K+ ATPase pump, accumulation of intracellular Na+, ionic homoeostasis via diminution of Protein kinase C (PKC) activity which cause swelling of axon and axon-glia dysfunction, and reduction of nerve conduction velocity (NCV) [15].
The accumulated glucose enters the hexosamine pathway and produces fructose-6-phosphate which, in turn, is converted to uridine diphosphate-N-acetylglucosamine (UDP- GlcNac). GlcNac is one of the sugar moieties used in N- or O-glycosylation of such translated proteins (posttranslational modification) as the SP-1 transcription factor which leads to overexpression of plasminogen activator inhibitor-1 (PAI-1) and transformation of growth factor-β1 (TGF-β1). As a result, these factors cause the injury of nerves by producing mitochondrial superoxides [24]. Given that, AR inhibitors were highly effective in attenuating DNP in animal models [25]. In contrast, they were not as effective in human clinical studies [26], which was partly due to the administration of much lower doses than in animal in-vivo studies. Therefore, not adequate concentrations were reached to prevent the flux via the polyol pathway [25].
Oxidative stress and advanced glycation
The term oxidative stress is used in order to describe the state in which oxidation exceeds the antioxidant capacity in cells due to an imbalance of the enzymatic antioxidant catalase (CAT) and superoxide dismutase (SOD) or non-enzymatic factor glutathione (GSH). As mentioned above, the consumption of NADPH in the polyol pathway has a negative effect on the level of a reduced antioxidant key player, GSH. High level of reactive oxygen species (superoxide O2-·, the hydroxyl radical OH·, and hydrogen peroxide H2O2) damages the cells’ lipid and proteins. It has been reported that ROS leads to damage of lipids in the myelin sheath [27]. Edwards et al. showed that high level of nitrosative products (peroxynitrite ONOO−and nitrotyrosine NT) in diabetic patients are positively correlated with diabetic peripheral neuropathic pain [28]. Consequently, they would increase the lipid peroxidation, DNA and protein damage rate. Advanced glycation end products (AGEs) are made by non-enzymatic reactions between the damaged DNA, lipids or proteins and aldehyde groups of reducing sugars, which induce ROS production both during their interaction and formation with AGE receptor (RAGE) [7, 29]. Moreover, advanced lipoxidation end products (ALEs) are formed by increased oxidative stress-induced lipid per-oxidation coupled with altered lipid metabolism [30]. NADPH-oxidase, Na+/H+ exchanger, 12/15-lipoxygenase, and PKC-β are also among the enzymes involved in ROS production in DNP patients [31–33]. PKC-β plays a central role in nerve function and pathogenesis of DNP [34]. Streptozotocin (STZ)-induced diabetic rats showed the positive effects of PKC-β inhibitor on DNP in reducing free radicals [35].
Mitochondria is the also house for electron transport chain and normally produces a low level of free radicals which are neutralized by antioxidants. In hyperglycemia, the potential of the mitochondrial membrane is disrupted and it releases cytochrome c which activates procaspase 9 together with apoptotic protease activating factor-1 (Apaf-1) cause the activation of apoptotic executioner caspase 3 in neurons [36, 37]. In a study by Zherebitskaya et al. on STZ-induced diabetes rat model, high glucose decreased manganese-containing superoxide dismutase (MnSOD) and increased ROS level in axons which mainly caused the impairment in the axons outgrowth and dystrophic structures [38]. The aforementioned data on oxidative stress indicates that controlling ROS level in diabetic patients could be a plausible approach to prevent DNP.
Inflammation
Inflammation is a cellular process activated by an injury in the nerve, skin, spinal cord or dorsal root ganglion (DRG) leading to painful sensation. It is correlated with diabetes and high levels of inflammatory cytokines like C-reactive protein (CRP) and tumor necrosis factor α (TNF-α) in neuropathic patients [39]. Conti et al. found that the induction of diabetes with STZ led to the infiltration of immune cells (macrophages and monocytes), the neuronal overexpression of pro-inflammatory cytokines interleukin-1 beta (IL-1β) and expression of neurotrophin receptor p75 (p75 NTR) [40]. The involvement of inflammation in DNP was also confirmed in an animal model with STZ-induced diabetes. It was found that thiazolidinedione pioglitazone altered the expression level of protein kinase C-alpha, decreased phosphorylated extracellular signal-regulated kinases (ERK), and decreased the number of accumulated macrophages in Schwann cells [41].
As a transcriptional factor composed of two subunits of p65 and p50, nuclear factor kappa B (NF-κB) is localized in the cytoplasm in an inhibitory state bound to its inhibitor iκB. Upon simulation, iκB is tagged by ubiquitin for proteasomal degradation leaving NF-κB in an active state. The activated NF-κB is translocated to the nucleus where it promotes the expression of different inflammatory and survival genes. Andorfer et al. showed that the p65 subunit of NF-κB is upregulated in the myelin sheath of neurons in demyelinating polyneuropathies [42]. Ha et al showed that hyperglycemia in glial cells activated NF-κB leading to an increase in the level of inflammatory genes (p38MAPK, TNF-α, IL-1β, IL-6, COX-2, iNOS) and cell adhesion genes (Endothelin-1, ICAM-1, and VCAM-1) [43]. Bierhaus et al., localized NF-κBp65 subunit, advanced glycation end-product receptor and IL-6 in sural nerve biopsies collected from diabetic patients [16, 44]. All these findings prove that inflammatory signaling cascades play important roles in the pathogenesis of DNP and makes them interesting pharmacological targets by natural entities.
Trophic factors
Neurotrophic factors promote the normal physiological functions of surviving neurons, increase their resistance to injury, and stimulate nerve regeneration as well, which all improve the clinical condition of DNP patients [45]. Neurotrophin deficiency contributes to the pathogenesis of DNP. Brain-derived neurotrophic factor (BDNF), neurotrophin-3/4/5 (NT-3/4/5), ciliary neurotrophic factor (CNTF) and maybe insulin-like growth factors (IGFs) were all reduced in the muscle of DNP patients [46]. Nerve growth factor (NGF) could attenuate these neurotrophic imbalances [47]. In fact, preclinical studies support the notion that affecting neurotrophic factors by natural products might be effective treatments for many different types of peripheral nerve disease.
Peroxisome proliferator-activated receptors
Peroxisome proliferator-activated receptors (PPAR) belong to nuclear hormone receptor proteins. Bound to the lipophilic stimulant, PPAR, induces the expression of proximal genes involved in β-oxidation of fatty acids, proximal proliferation, lipid hemostasis and hepatocarcinogenesis [48, 49]. α, β/δ, and γ are the main three subtypes of PPARs which play a critical role in controlling the metabolism, storage, and mobilization of lipids, glucose, morphogenesis and inflammatory processes [50][49, 51]. PPARs cooperate with other cellular transcription factors such as NF-κB, signal transducer/activator of transcription-1 (STAT-1) and activated protein-1 (AP-1) [51]. PPARs repress the expressions of pro-inflammatory genes (IL-1β and TNFα), and chemokines (MCP-1 and CCR2) and reduce the pain sensation [52]. As insulin-sensitizing drugs, PPAR gamma (PPARγ) agonists (e.g. pioglitazone and rosiglitazone), are widely recommended for the treatment of insulin resistance-hyperglycemia [53] and to attenuate spinal nociceptive neuron activation in type II diabetic rats [54]. The recent clinical application of PPARs agonist provides a promising future to evaluate their potential as novel analgesics in the treatment of different chronic pain conditions such as DNP. However, it is important to note their potential adverse effects while targeting the PPAR signaling system as analgesics [55]. These results make necessary the research for natural PPAR agonists in order to reduce the DNP.
Channels activation
The transient receptor potential vanilloid 1 (TRPV1) channel is involved in various modalities of nociceptive stimuli. In a STZ-induced painful DNP, TRPV1 expression was significantly increased in hyperalgesic compared to hypoalgesic and normoalgesic skin [56, 57]. Studies showed that early stages of DNP are due to up-regulation of TRPV1 by PKC and PKA [58]. It indicated the key role of TRPV1 channels in the expression of hyperalgesia [58]. Other TRPV receptors have not been yet investigated enough in DNP [59]. In general, TRPV might be considered as a promising therapeutic target to develop new treatments for DNP. Activation of TRPV1 evoked [Ca2+] transients and also reciprocally alters in hyperalgesia [58]. Thus, calcium channels (Cav) are thought to be involved in painful DNP [60]. The alpha(2)delta subunits increase the expression and trafficking of these channels, but may also play a role in synaptogenesis within the central and peripheral nervous system [61]. In addition to Cav, voltage-gated sodium channels (Nav) have also been shown to increase at the site of neuronal damage in DNP [62]. Methylglyoxal, as an AGE, increased in the serum of painful DNP patients, and causes the thermal and mechanical hyperalgesia when injected into diabetic mice (not in sodium channel Nav1.8 knockout mice) [63]. These all show the importance of TRPV1, Nav and Cav channels by multi-target natural products on the development of DNP. Besides, targeting TRPV1 is co-expressed with glutamate receptors [64].
Glutamate pathway
Glutamate plays a crucial role such processes as the synapse plasticity, cell differentiation, cell migration, death [65] and peripheral transduction of sensory inputs in the central nervous system (CNS) [66]. Several studies have shown the involvement of glutamate-mediated toxicity in both acute and chronic neurodegenerative diseases of CNS and PNS [67]. Glutamatergic ligands cause nociceptive behaviors, which suggests that glutamate is involved in nociceptive pathways and peripheral sensory transduction. On the other hand, hyperglycemia significantly increased the expression of N-methyl-D-aspartate (NMDA) receptors in a mice model of type I diabetes [68]. The role of spinal NMDARs has also been demonstrated in nerve injury-induced pain [69]. Spinal NMDAR subunit 2B (NR2B) was upregulated in both protein and mRNA levels of STZ-induced DNP resulting in the hyperactivity of spinal cord dorsal horn neurons [70]. Glutamate, especially NR2B, trigger cascades of oxidative stress, inflammation, and apoptosis [71] which are among the important pathways involved in DNP. For the same reason, targeting the glutamate pathway and NR2B as upstream factors of oxidative, inflammatory and apoptotic pathways by natural entities is very promising to combat DNP.
Conventional pharmacotherapy of painful DNP
There are different drugs proposed for treating or controlling DNP complications. Conventional treatments for neuropathic pain include tricyclic antidepressants (TCA, e.g., nortriptyline or amitriptyline), serotonin-norepinephrine reuptake inhibitor (SNRI, e.g., duloxetine), anticonvulsants therapies (e.g., gabapentin, pregabalin), and the dual-effect drug tapentadol (an opioid receptor agonist and norepinephrine reuptake inhibitor) which are used to treat neuropathy induced disorders [72]. Other medications like opioids (e.g., Tramadol) are also prescribed but not recommended [19]. A recent systematic review and network meta-analysis of the randomized controlled trial confirmed the effectiveness of SNRIs, TCAs, anticonvulsants and topical capsaicin in painful DNP. Moreover, SNRIs had a greater effect on attenuating the pain than the opioids and anticonvulsants [73].
Nevertheless, there is no definitive treatment for DNP with most potency and less side effects. Thus controlling the blood sugar can significantly alter the course of neuropathy. Of another point of view, many of these agents have a number of serious adverse effects. This necessitates the search for effective and safe medications among which herbal medicine is a potential regimen for diabetes and diabetes-related complications such as neuropathy [74, 75].
Polyphenols as alternative therapies for DNP
The ethnobotanical data indicates that there are about 800 plants that may possess anti-diabetic properties. Several herbal treatments including curcumin, kaempferol, quercetin, naringenin, resveratrol, kolaviron, etc have been so far administered for DNP patients. But, first it is necessary to identify DNP syndromes some of which are potentially treatable by traditional herbal medicine.
Therefore, the current review has attempted to provide a helpful classification of herbal medicine used in the management of DNP. Most relevant pharmacological targets and cellular signaling involved in the therapeutic effect of polyphenols in diabetic DNP are as follows:
Enzymatic and non-enzymatic antioxidant performance
Hyperglycemia reduces the activity of antioxidant enzymes in diabetic animals with non-enzymatic glycosylation and causes oxidative stress [76]. Due to the induction of some damaging effects like free radical generation via ischemia, hyperglycemia, increased mitochondrial leak, catecholamine and leukocytes oxidation, decrease of glutathione peroxidase (GPx), glutathione-S-transferase (GST), Cu/Zn SOD and lower levels of GSH, oxidative stress plays a major destructive role in the development of DNP [76–79]. Several antioxidants specially polyphenols have shown some activities promising for the treatment of experimental DNP. Treatment with α-lipoic acid prevents neurovascular abnormalities in experimental DNP. It also attenuates reduced digital NCV, nerve blood flow and GSH levels in diabetic rats by enhancing oxygen free radical scavenging activity [80, 81]. On the other hand, probucol, as an LDL-oxidation inhibitor, and a powerful free radical scavenger, normalizes both nerve blood flow and electrophysiology [82].
Al-Rejaie et al. reported that naringenin possesses antioxidant activity. It also suppressed the levels of thiobarbituric acid reactive substances (TBARS) and nitric oxide (NO), and attenuated the reduced level of CAT, and GPx in STZ-induced diabetic rats [83, 84]. As another polyphenol, resveratrol protected neural tissues against diabetes-induced oxidative stress either by reducing NO, XO, MDA levels of the cerebellum, hippocampus, cortex, spinal cord and brain stem by enhancing GSH level in diabetic rats [85]. Curcumin and apocynin also attenuated the increased level of spinal H2O2 and MDA level and enhanced SOD level in STZ-induced diabetic rats, as indicated by Zhao et al. It has also been demonstrated that curcumin inhibited the activation of spinal NADPH oxidases, the main enzymes that produce ROS by reversing the upregulation of both phagocyte NADPH oxidase subunits (p47phox and gp91phox) [86].
More relevant pharmacological targets and cellular signaling involved in antioxidant effect of polyphenols in DNP are shown in Table 1.
Table 1.
Polyphenols as alternative therapies for DNP
| Polyphenol name | Animal models and Tests | Dosage/day | Outcome | References |
|---|---|---|---|---|
| Astragaloside IV | STZ-induced diabetes rats | 3, 6 & 12 mg/kg BID for 12 weeks, p.o. | ↑Myelinated fiber area, myelinated fiber density & segmental demyelination | [120] |
| ↓HbA1C levels | ||||
| ↑MNCV & GPx | ||||
| ↓AR in erythrocyte | ||||
| ↑ Na+/K+ ATPase activity in both the nerves and erythrocytes | ||||
| Bacosine | Mechanical & thermal hyperalgesia, tactile allodynia & hot plate test in STZ-induced diabetes rats | 5 & 10 mg/kg for 30 days, p.o. | diabetes-associated cognitive deficit | [190] |
| ↓Hyperalgesia | ||||
| ↑MNCV | ||||
| ↓ AGEs & ROS | ||||
| Carvacrol | Morris water maze and biochemical evaluations in STZ-induced diabetic rats | 25, 50 & 100 mg/kg for 7 weeks, i.p. | ↓MDA | [100] |
| ↑SOD | ||||
| ↓IL-1β &TNF-α | ||||
| Chlorogenic acid | Formalin-induced pain model in STZ-induced diabetic rats | 100 mg/kg, i.p. | ↑Mechanical hyperalgesia threshold | [108] |
| ↓Formalin-induced nociceptive behavior | ||||
| Chromane | Tail-immersion test and hot-plate test in STZ-induced diabetic rats | 5 & 10 mg/kg for 30 days, p.o. | ↓Thermal hyperalgesia & mechanical allodynia, | [107] |
| ↑paw withdrawal threshold | ||||
| ↑MNCV | ||||
| ↓AGEs & ROS | ||||
| Curcumin | Mechanical allodynia, Hot plate & tail flick test in STZ-induced diabetic rats | 100 mg/kg, p.o. for 6 weeks | ↓Thermal nociception | [111] |
| ↑Paw withdrawal threshold & tail flick latency | ||||
| ↓TNF-α & IL-10 | ||||
| Hot plate test, allodynia and Rota-rod test in STZ-induced diabetic rats | 200 mg/kg, p.o. for 3 weeks | ↓Thermal hyperalgesia & mechanical allodynia | [112] | |
| ↑Paw withdrawal threshold | ||||
| ↓COX & prostaglandin peroxidase | ||||
| ↓AR | ||||
| Mechanical allodynia in STZ-induced diabetic rats | 200 mg/kg, p.o. for 14 days | ↑Paw withdrawal threshold | [86] | |
| ↓MDA, H2O2 in the spinal cord | ||||
| ↑SOD | ||||
| Curcumin & gliclazide | Hot-plate & tail-flick test in STZ-induced diabetic rats | 100 mg/kg, p.o. for 5 weeks | ↑Mechanical hyperalgesia threshold | [110] |
| ↑Hot-plate & tail-flick latencies | ||||
| ↓peroxynitrite, LPO & TNF-α | ||||
| Curcumin & resveratrol | Tail-immersion test & hot- plate test in STZ-induced diabetes | Resveratrol 20 mg/kg & curcumin 60 mg/kg, p.o. for 4 weeks | ↑Nociceptive threshold | [191] |
| ↓TNF-α | ||||
| ↓brain nitrite level | ||||
| Diosmin | Tail immersion test & walking function test in STZ-induced diabetes | 50 & 100 mg/kg p.o. for 4 weeks | ↑Tail flick latency, ↓duration for traveling | [114] |
| ↑SOD & GSH | ||||
| ↓MDA & NO | ||||
| Epigallocatechin-gallate | Tactile allodynia & mechanical hyperalgesia in STZ-induced diabetes | 2 g/L, p.o. | ↓Thermal hyperalgesia & mechanical allodynia | [192] |
| ↑Paw withdrawal pressure | ||||
| ↓8-OHdG immunoreaction, numbers of Fos-immunoreacted neurons & co-localization of 8-OHdG and Fos in laminae I–III | ||||
| Epigallocatechin-gallate | Tail immersion test & formalin-induced pain in STZ-induced diabetic rats | 20 & 40 mg/kg, p.o. for 7 weeks | ↑Tail flick latency & nociceptive threshold | [106] |
| ↓Formalin-induced nociceptive behavior | ||||
| ↓Nitrite | ||||
| ↓TBARS, MDA & ↑SOD | ||||
| Grape seed proanthocyanidins | Hot plate test in STZ-induced diabetic rats | 125, 250 & 500 mg/kg, p.o. | ↑Hot-plate latency | [121] |
| ↑Nerve conduction velocity | ||||
| ↓Concentration of free Ca2+, ↑ATPase activities in sciatic nerves | ||||
| Hydroxytyrosol | Hot plate paw withdrawal and Randall−Selitto paw withdrawal tests in STZ-induced diabetes | 10 & 100 mg/kg, p.o. for 6 weeks | ↓Thermal nociception | [193] |
| ↑Paw withdrawal threshold | ||||
| ↑MNCV | ||||
| ↑ Na+/K+ ATPase activity | ||||
| Kaempferol | Formalin-induced and tail immersion test in STZ-induced diabetic mice | 25, 50 & 100 mg/kg, p.o. | ↓Formalin-induced nociceptive behavior in phase 1, phase 2 & edema size | [102] |
| ↓Hyperalgesia, ↑thermal pain threshold, ↓IL-1β, TNF-α, LPO & nitrite | ||||
| Kolaviron | STZ-induced diabetic rats | 100 & 200 mg/kg, p.o. for 6 weeks | ↓Oxidative stress | [194] |
| ↓IL-1β & TNF-α | ||||
| ↑GSH, CAT & GPx | ||||
| ↓MDA, TBARS | ||||
| Morin | Tail flick test in STZ-induced diabetic rats | 15 & 30 mg/kg, p.o. for 3 weeks | ↑Paw-withdrawal & tail flick latency | [118] |
| ↑NGF & IGF-1 in sciatic nerves | ||||
| ↓IL-1β, TNF-α & LPO | ||||
| Mulberry flavonoids | ALX- induced diabetic rats | 0.3 & 0.1 g/kg, p.o. for 8 weeks | ↓Peripheral nerve injury & numbers of extramedullary fiber of sciatic nerves | [195] |
| ↓myelin breakdown & myelinated fiber cross-sectional area | ||||
| Naringenin | Tail-flick, Randall & Selitto tests in STZ-induced diabetic rats | 25 & 50 mg/kg, p.o. for 5 weeks | ↑Paw-withdrawal & tail-flick latency | [84] |
| ↑NGF & IGF-1 in sciatic nerves | ||||
| ↓IL-1β & TNF-α | ||||
| ↑CAT, GSH & GPx | ||||
| Naringin | Mechanical hyperalgesia, tactile allodynia & tail-immersion test in STZ-induced diabetic rats | 40 & 80 mg/kg, p.o. for 4 weeks | ↓Mechano-tactile allodynia | [83] |
| ↑Nociceptive threshold & tail flick latency | ||||
| ↑MNCV | ||||
| ↑SOD | ||||
| ↓oxidative-nitrosative stress & TNF-α | ||||
| Oryzanol | Hot-plate test & Tail-immersion test in STZ-induced diabetic rats | 50 & 100 mg/kg, p.o. | ↑Pain threshold & hot plate latency | [113] |
| ↓Flinching in diabetic rats during both quiescent phase & phase 2 but not in phase 1 | ||||
| ↓Nitrite, MDA | ||||
| ↑GSH | ||||
| Attenuating Na+-K+ ATPase | ||||
| Pepino polyphenolic extract | STZ-induced diabetic mice | 0.5 or 1% for 12 weeks | ↓TNF-α & IL-6 | [196] |
| ↑GSH & GPx | ||||
| ↓AGEs &ROS | ||||
| ↑fascicle with numerous small myelinated fibers | ||||
| Quercetin | Tail-flick test, Hot-plate method & the walking function test in STZ-induced diabetic rats | 40 mg/kg, p.o. for 2 weeks | ↑Tail-withdrawal latency & hot plate and cold allodynia latency | [75] |
| ↓Number of foot slips | ||||
| Mechanical hyperalgesia, tactile allodynia & tail immersion test in STZ-induced diabetic rats | 10, 20 & 40 mg/kg, p.o. for 8 weeks | ↓Thermal hyperalgesia & mechanical allodynia, | [103] | |
| ↑MNCV | ||||
| ↑SOD & GPx, | ||||
| ↓TNF-α &IL-1β | ||||
| Tail-immersion assay in STZ-induced diabetic rats | 10 mg/kg, p.o. for 4 weeks | ↑Tail-flick latencies & nociceptive threshold in both diabetic and non-diabetic mice | [104] | |
| Tail-immersion assay & hot-plate test in STZ-induced diabetic rats | 10 mg/kg, p.o. for 4 weeks | ↓Thermal nociception | [197] | |
| ↑tail-withdrawal latencies & nociceptive threshold | ||||
| Resveratrol | Cold immersion & hot immersion tests in STZ-induced diabetes rats | 10 & 20 mg/kg, i.p. for 2 weeks | ↑Tail flick latency & paw withdrawal pressure | [98] |
| ↑MNCV | ||||
| ↓MDA | ||||
| ↑CAT | ||||
| STZ-induced diabetic rat | 10 & 20 mg/kg, i.p. for 2 weeks | ↑MNCV | [198] | |
| ↓MDA | ||||
| ↓p65, IκB-α & NF-κB | ||||
| ↓TNF-α, IL-6 & COX-2 | ||||
| Cerebral ischemia-reperfusion in STZ-induced diabetic rats | 20 mg/kg, p.o. for 6 weeks | ↓Cerebral MDA & COX-2 | [99] | |
| ↑cerebral IL-4 | ||||
| ↑cerebral GSH contents | ||||
| STZ-induced diabetic rats | 10 mg/kg, i.p. over 6 weeks | ↓MDA, XO & NO | [85] | |
| ↑GSH in hippocampus, cortex, cerebellum, brain stem & spinal cord | ||||
| Tail-immersion test in STZ-induced diabetes | 20 mg/kg, p.o. for 2 weeks | ↑Tail withdrawal threshold & tail withdrawal latencies | [199] | |
| Silymarin | Tail-immersion test & formalin-induced pain test in STZ-induced diabetic rats | 100 & 200 mg/kg, i.p. for 8 weeks | ↑Tail flick latency | [200] |
| ↓Nociceptive scores in both phases of the formalin test | ||||
| 6-Methoxyflavanone | Hot plate test in STZ-induced diabetic rats | 10 & 30 mg/kg, i.p. | ↓Thermal nociception, | [109] |
| ↑paw withdrawal threshold & latency | ||||
| ↑flinching response threshold & latency by a preference for the δ- & ĸ-opioid receptors, involvement of GABA receptors | ||||
| 7-Hydroxy-3,4-dihydrocadalin | Formalin-induced pain & tactile allodynia in STZ-induced diabetic rat and mice | 0.3–30 and 30-300 mg/kg, p.o. | ↓Mechanical hyperalgesia and allodynia & formalin-evoked hyperalgesia | [116] |
| ↑withdrawal threshold | ||||
| ↓MDA |
DNP Diabetic neuropathy; STZ Streptozotocin; ALX alloxan; TNF-α Tumor necrosis factor-α; IL-10 Interleukin-10; MDA Malondialdehyde; GABA Gamma-Amino Butyric Acid; GPx Glutathione Peroxidase; CAT Catalase; GSH Glutathione; NGF Nerve growth factor; IGF-1 Insulin-Like Growth Factor; XO Xanthine oxidase; NO Nitric oxide; NOS neuronal Nitric oxide synthase; SOD Superoxide dismutase; MNCV Motor nerve conduction velocity; AGEs Advanced glycation end products; AR aldose reductase; ROS Reactive oxygen species; 8-OHdG 8-hydroxy-2′-deoxyguanosine; LPO Lipid peroxidation; TBARS thiobarbituric acid reactive substances; COX Cyclooxygenase; p.o. per-oral; i.p. intraperitoneal
Prevention of pro-inflammatory cytokines and inflammatory reaction
Pro-inflammatory changes observed in diabetes play a major role in the pathogenesis of atherosclerosis, neuropathy, nephropathy, and retinopathy [87]. Generation of hyperglycemia-induced ROS is directly involved in the pathogenesis of DNP. These radical oxygen species may cause the production of TNF-α and IL-1β. Hyperglycemia and insulin resistance are associated with TNF-α system in the CNS which may induce pain and hyperalgesia in DNP [88–90]. Moreover, studies showed that inhibiting TNF-α reduced hyperalgesia in models of painful DNP [91]. Meanwhile, it has been demonstrated that intraplantar injection of TNF-α is associated with mechanical allodynia and thermal hyperalgesia in rats [17, 88, 92]. IL-1β is derived from many cell types, like fibroblasts, synoviocytes, mononuclear cells, schwann and endothelial cells, and plays a central role in the generation of mechanical hyperalgesia. It also neutralized IL-1 receptors, which in return, reduced the pain-associated behavior in mice model of experimental neuropathy [93–95].
Hyperglycemia and higher lipid level lead to NF-κB activation that plays a central role in TNF-α and ROS production inducing inflammatory demyelination. It has been reported that NF-κB may elevate in metabolic diseases like diabetes and initiates inflammation [96]. P65 and IκB-α are among the subunits of NF-κB which are overexpressed in sural nerve macrophages in acute and chronic inflammatory demyelinating polyneuropathies [42, 97].
Kumar and Sharma demonstrated that resveratrol possessed anti-inflammatory activity by decreasing the expression of p65 and IκB-α and ameliorating the elevated levels of TNF-α, COX-2, IL-6, and NF-κB in STZ-induced DNP in rats [98]. In addition, resveratrol significantly decreased the serum glucose level, atherogenic index, and the expression of cerebral MDA and COX-2 [99].
As revealed by Deng et al., carvacrol (25, 50, and 100 mg/kg) diminished STZ-induced DNP via decreasing the level of NF-κB p65 subunit, IL-1β, caspase-3, and TNF-α [100]. As another polyphenol, kaempferol attenuated STZ-induced DNP by decreasing the levels of IL-1β and TNF-α and suppressing the formalin-induced nociceptive behavior. It also ameliorated LPS-induced inflammatory mediators (NO, prostaglandins, TNF-α, IL-1β, ROS and phagocytosis) in the microglial cells [101, 102]. More relevant pharmacological targets and cellular signaling involved in the anti-inflammatory effects of polyphenols in DNP have been shown in Table 1.
Anti-nociceptive effects
Hyperglycemia-triggered lipid peroxidation and ROS generation in sciatic nerves, accelerated the dysfunction of sciatic nerves and reduced endoneurial blood flow in diabetes-induced neuropathy. Painful DNP can occur as spontaneously, hyperalgesia or allodynia. Neuropathic pain is one of the most common diabetic complications induced by abnormal function of the peripheral or central nervous system and results in sensory abnormalities, changes of primary afferent nerves, and central sensitization. Studies have demonstrated the effectiveness of γ-aminobutyric acid (GABA) and opioids, TCAs, gabapentin, pregabalin, phenytoin, lamotrigine, dextromethorphan, and tramadol on painful sensory neuropathy. Although these therapies would relieve the pain 30–50%, they are often restricted due to significant side effects [87]. So, it raises the needs to polyphenols as alternative therapies.
As concluded by Kandhare et al., quercetin inhibited a significant increase of paw withdrawal threshold (PWT) in STZ-induced diabetic rats in a plantar heat hyperalgesia test that was evaluated by Hargreaves’ test. In addition, it significantly increased the mechanical PWT, compared to STZ-diabetic control rats on a Randall-Selitto paw pressure device [103]. It has also been shown that quercetin (100 mg/kg, p.o) increased the tail withdrawal latency in both diabetic and nondiabetic mice [104]. Besides, it has significantly increased the tail and paw withdrawal latency and decreased the number of foot slips of STZ-induced diabetic rats in a dose-dependent manner, compared to normal control [75, 105, 106].
Studying the anti-nociceptive effect of other polyphenols, Kaur et al., showed that chromane significantly corrected the decreased PWT of STZ-induced diabetic rats in tail-immersion and hot-plate tests, compared to the control group [107]. Chlorogenic acid and 6-methoxy flavanones also increased the thermal and mechanical PWT in diabetic rats, respectively [108, 109].
As Attia et al. articulated, the combined administration of curcumin and gabapentin caused a significant increase in the mechanical PWT as well as hot-plate and tail-flick latencies in STZ-induced diabetic rats [110]. Curcumin showed significant pain threshold elevations, increased reaction times and tail-flick response latencies [111]. Treatment with curcumin also enhanced the anti-nociceptive activity in hot-plate and allodynia- tests in STZ-induced DNP via increasing pain threshold level compared to untreated diabetic rats [112]. Oryzanol and diosmin both significantly increased the tail-flick latency in tail-immersion test and reduced the thermal hyperalgesia in STZ-induced diabetes [113]. In addition, diosmin treatment significantly improved shortening of time on walking function test in diabetic rats [114].
Kumar et al., demonstrated that the decrease of PWT and tail flick latency in cold and hot immersion performance was significantly corrected by resveratrol treatment at 10 and 20 mg/kg [98]. As two other polyphenols, silymarin and 7-hydroxy-3,4-dihydrocadalin caused a significant decrease in pain scores of the formalin-test [115, 116].
More relevant pharmacological targets and cellular signaling involved in anti-nociceptive effects of polyphenols in DNP are shown in Table 1.
Improvement of nerve growth factors
There are complex mechanisms behind the DNP involving several molecule alterations and sensory modalities. Several neurotrophic factors have been found to effects the population of specific neurons in the nervous system, among the most promising ones is NGF [45]. Evidence suggests that DNP could be modulated by neurotrophins like transient receptor potential ion channels, including vanilloid receptor 1 (VR-1) in one hand and nerve growth factor (NGF), such as its receptors p75 and tyrosine kinase A (TrkA) as well as their down streams on the other hand. It has been well-documented that NGF has an important neuroprotective function and causes axonal growth. Pathological conditions that alter the levels of NGF can cause neurons to lose their function and die. After inflammation and nerve injury, NGF increases in the nervous system and facilitates hyperalgesia and pain that can be reduced by anti-NGF therapy. The complex of TrkA and NGF sensitizes VR1 thereby elevates pain. Following the binding of NGF to TrkA as related receptor, nerve regeneration, cell survival, and neurite-growth pathways will begin [117].
As a structurally similar hormone to insulin, IGF-1 plays an auspicious anabolic role in cellular proliferation and growth. It has also been considered as a strong inhibitor of apoptosis. It also controls the growth and development of nerve cells and DNA synthesis, as well [118]. It has been demonstrated that sciatic nerve levels of NGF and IGF-1 in morin treated diabetic animals significantly elevated in comparison with the negative control group [118].
As indicated by Song-Tao et al., mulberry flavonoids and methycobal alleviated the suppression of the average optical density of myelin sheath and myelinated extramedullary fiber cross-sectional area in diabetic rats. Furthermore, animals pretreated with 0.3 g/kg mulberry flavonoids showed ultrastructural features of myelin, remarkable axonal improvement and considerable decrement of myelin breakdown [119]. Astragaloside IV, as another polyphenol, suppressed a decrease in myelinated fiber area and density and segmental demyelination in diabetic rats by decreasing the hemoglobin A1c (HbA1C) and AR levels in erythrocytes, increasing the plasma insulin levels, and GPx activity in nerves. Moreover, astragaloside IV elevated Na+/K+-ATPase activity in both nerves and erythrocytes in STZ-induced diabetic rats [120].
Ding et al., showed that grape seed proanthocyanidins treatment (500 mg/kg dose) improved the abnormal function of peripheral nerve and impaired nerve tissues. They also showed that it reduced the NCV level and concentration of free Ca2+, elevating Ca2+-ATPase activity in sciatic nerves [121]. Diabetic rats treated with curcumin attenuated the reduction of sciatic nerve Na+/K+ ATPase activity compared to normal control. In addition, curcumin treatment of diabetic rats has shown a gradual recovery of cyclooxygenase activity in sciatic nerve [122].
Glutamate pathway and NMDA receptors
As previously mentioned, glutamate receptors and ligands are thought to be involved in nociceptive behaviors in experimental models of DNP. It seems that glutamate and the NMDA receptor are involved in nociceptive pathways and peripheral sensory transduction [123]. NMDA receptors are coupled to mitogen-activated protein kinase (MAPK) and ERK phosphorylation and activation in the superficial laminae of the spinal cord which can be suppressed by NMDA receptor antagonist treatment [124–126]. In painful DNP, NMDA receptor ion channel-mediated calcium entry play a key role for the activation of MAPK and ERK pathways [123].
Resveratrol prevents glutamate damages by blocking the NMDA receptor and suppressing glutamatergic neurotransmission [127, 128]. To prevent diabetic retinopathy, it significantly decreased glutamine synthetase, transportation, and expression, [129]. Resveratrol inhibits microglia activation, mitochondrial dysfunction, intracellular ROS production and impairments in Na+/K+-ATPase [130, 131]. It also down-regulates a glutamate-induced tissue plasminogen activator via ERK and AMPK/mammalian target of rapamycin (m-TOR) pathways and decreases MAPK activation, which subsequently suppresses the voltage-dependent Ca2+ channel activity and inhibits evoked glutamate release [132, 133]. Similar to resveratrol, piceatannol induces the expression of nuclear factor erythroid 2–related factor 2 (Nrf2)-dependent and heme oxygenase-1 (HO-1) and thereby, protects HT22 neuronal cells against glutamate-induced cell death [134].
As another polyphenol, chlorogenic acid in coffee protects neurons from glutamate neurotoxicity through its caffeoyl acid group, and its hydrolysate, the caffeic acid by regulating Ca2+ entry into neurons [135, 136].
Protection of motor neuron by epigallocatechin-3-gallate is associated with regulating glutamate level [137]. It inhibits glutamate dehydrogenase in pancreatic ß-cells and activates AMPK to positively affect diabetes [138]. It also reduces the glutamate-induced Ca2+ increase by attenuating PKC and ionotropic Ca2+ influx [139, 140], as quercetin does [141].
Curcumin attenuated NMDA receptor-mediated excitotoxicity in diabetic rats [142], ameliorated both glutamate level and gene expression of NR2B [143, 144], and prevented intracellular Ca2+ elevation [145]. In addition, it affected PI3K/AKT pathway and downstream signaling through TrKβ and BDNF, possibly by decreasing MAPK/ERK activation [146, 147].
Since, Apigenin 8-C-glucoside, chlorogenic acid, and naringin regulate glutamate pathway [148, 149] and astragaloside IV and kaempferol attenuate glutamate-induced toxicity and oxidative stress [150, 151], they could be good options for preventing DNP complications (Table 1).
More relevant pharmacological targets and cellular signaling involved in the therapeutic effect of polyphenols in DNP have been shown in Table 1, Figs 1 and 2.
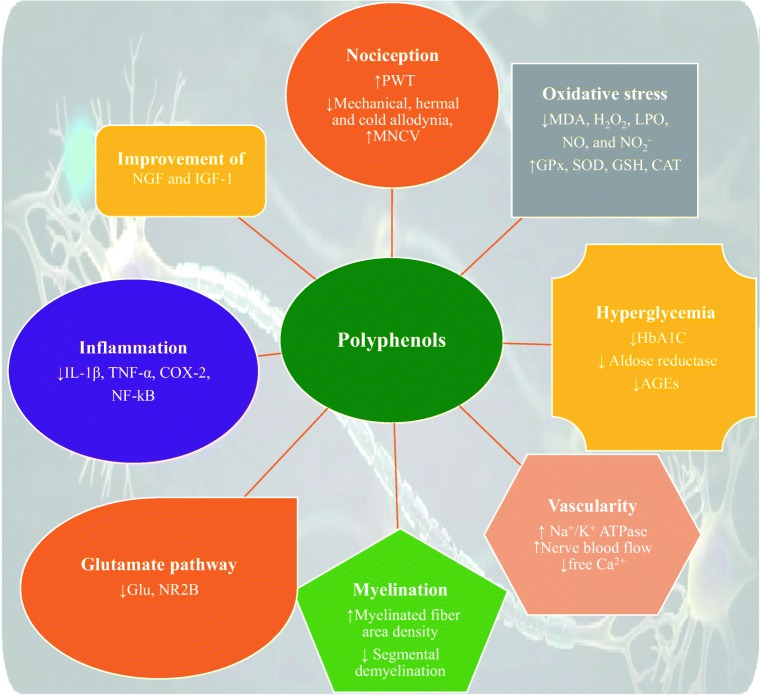
Fig. 1.
General effects of polyphenols on DNP. PWT: Paw withdrawal threshold, NGF: Nerve growth factor, IGF-1: Insulin-like growth factor-1, MDA: Malondialdehyde, H2O2: Hydrogen peroxide, LPO: Lipid peroxidation, NO: Nitric oxide, XO: Xanthine oxidase, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, GSH: Glutathione, CAT: Catalase, IL: Interleukin, TNF-α: Tumor necrosis factor-α, COX: Cyclooxygenase, HbA1C: Hemoglobin A1C, Glu:Glutamate, NMDA: n-methyl-D-aspartate, NR2B: NMDA type 2B, MNCV: Motor nerve conduction velocity, AGEs: Advanced glycation end products
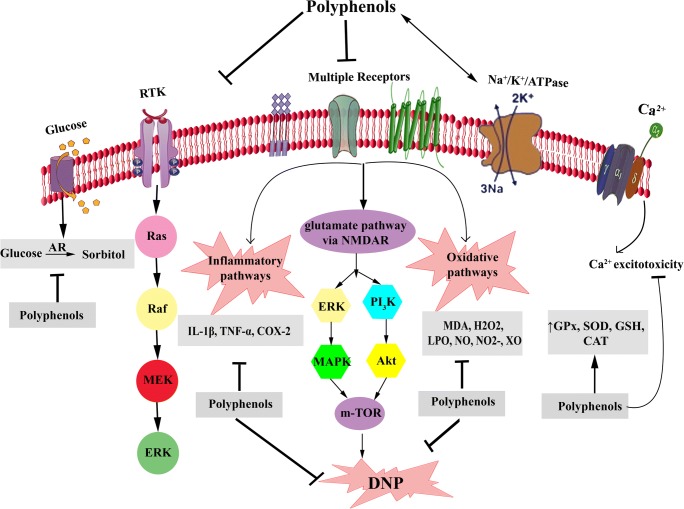
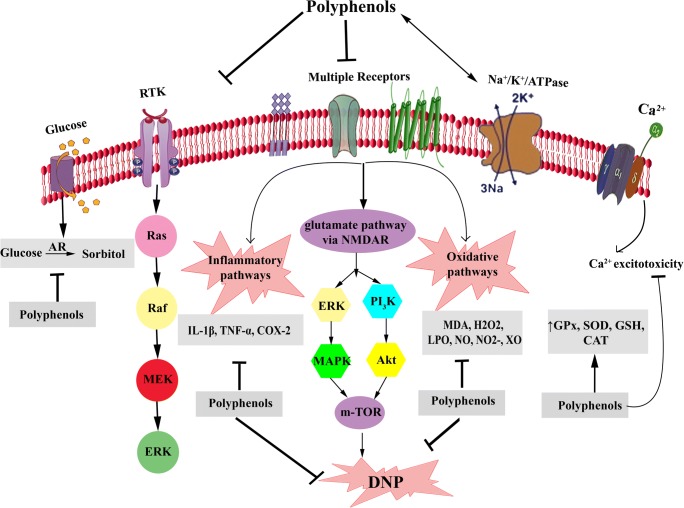
Fig. 2.
Polyphenols mechanisms of action in DNP. DNP: Diabetic neuropathy, AR: Aldose reductase, RTK: Receptor tyrosine kinase, NGF: Nerve growth factor, IGF-1: Insulin-like growth factor-1, MDA: Malondialdehyde, H2O2: Hydrogen peroxide, LPO: Lipid peroxidation, NO: Nitric oxide, XO: Xanthine oxidase, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, GSH: Glutathione, CAT: Catalase, IL: Interleukin, TNF-α: Tumor necrosis factor-α, COX: Cyclooxygenase, NMDAR: n-methyl-D-aspartate receptor, MAPK: mitogen-activated protein kinase, ERK: extracellular signal-regulated kinases, m-TOR: Mammalian target of rapamycin, Activation  , Modulation:
, Modulation:  Inhibition
Inhibition 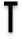
Therapeutic applications and health benefits of polyphenols: Diabetes, DNP and beyond
Polyphenols benefit human health status and play promising roles in the management, prevention and treatment of several chronic diseases, including cardiovascular disease, cancer, obesity, pancreatitis, gastrointestinal problems, osteoporosis, lung damage, neurodegenerative diseases, and gut microbiota based on their antioxidant and anti-inflammatory effects [152–157]. Polyphenols have an auspicious role against several inflammation-mediated diseases through inhibiting inflammatory mediators and signaling pathways [158], as we described previously. Polyphenols also induce cell apoptosis and arrest cellular growth in order to be tumor suppressors [159].
Several studies reported that resveratrol prevents platelet aggregation and decreased blood pressure and low-density lipoprotein (LDL) cholesterol as well [11, 160–163]. Of the compounds contain the polyphenol curcumin, it was hypothesized to contribute to the low incidence of cognitive impairment and Alzheimer’s disease in individuals with a high rate of consumption [164]. Randomized-controlled trial studies reported that polyphenolic compounds reduce the differentiation, proliferation and genesis of adipocytes [165]. Catechin polyphenols, have been linked to antioxidant, anti-inflammatory, and anti-mutagenic properties and thought to prevent weight gain by promoting greater fat oxidation and energy expenditure [166–169]. Resveratrol has also shown anti-obesogenic properties in both animal and human studies [170]. Curcumin has been shown to reduce adiposity through suppressed angiogenesis, reducing inflammation, and increasing energy metabolism [171]. Of the gastrointestinal benefits, polyphenol may inhibit invasive species while promoting beneficial gut bacteria, may promote beneficial actions of probiotics with unknown mechanisms [172–174]. Evidence suggests modulatory potential impacts of polyphenols on chronic disease risk, such as the hepatoprotective and atheroprotective effects and improving insulin sensitivity [175–177].
Although, there are yet limited human studies to tackle diabetic chronic complications by polyphenols, purified polyphenols, as well as diets rich in polyphenols, also prevent the progress of human chronic complications in diabetes like DNP [178, 179]. As inflammation, oxidative stress, trophic factors, channels, and glutamate pathway are the main signaling mediators and pathways associated with DNP, so polyphenols with anti-inflammatory and antioxidant effects could carry out their significant health benefits through tackling them in diabetes. In the clinical trial testing, curcumin attenuated inflammatory mediators like TGF-β and IL-8 [180] as well as oxidative stress markers including lipid peroxidation and MDA [181], thereby could be a promising polyphenol to combat DNP. In type II diabetic patients, resveratrol [182], berberine [183, 184] and catechin [185] resulted in a decrease in insulin resistant and an increase in insulin level. A meta-analysis of clinical studies in diabetic patients showed that cinnamon or cinnamon extracts decrease fasting blood glucose levels [186]. As high blood glucose level leads to the activation of AR enzyme of the polyol pathway in DNP [21], so resveratrol, catechin and cinnamon could be effective compounds to combat DNP. As another polyphenol, ginger in addition to blood glucose lowering effects it also decreased inflammation and oxidative stress in patients with type II diabetes [187, 188]. Growing evidence have revealed positive effects of various dietary polyphenols on blood glucose and polyol pathway, oxidative stress, and inflammatory mediators may also help prevent and control diabetes complication as DNP, however, more clinical trials are needed [189].
Conclusion and future perspective
As the most distressing complication of diabetes, DNP affects more than 30% of diabetic people worldwide. There are increasing types of diabetes-induced peripheral nerve injury, including autonomic and small fiber predominant neuropathy, diabetic amyotrophy, radiculopathy, mononeuropathy and mononeuritis multiplex. The pathogenesis of DNP is multifactorial with the main categories being metabolic and ischemic. Although opioid therapy and neuromodulating medications, including TCA and anticonvulsants have been found to be effective in the treatment of neuropathic pain, the high costs associated with social and personal healthcare of DNP in one hand, and lack of consistently effective and safe treatment for DNP on the other hand rise the needs to develop novel herbal therapies to improve the life quality of individuals with DNP [201]. Among natural entities, several evidence indicated that polyphenols possess protective effects via antioxidant and anti-inflammatory pathways. Polyphenols are multi-target agents [10] with different structures, signaling pathways and physiological roles possessing the rich antioxidants and anti-inflammatory effects. They have the potentials to combat several chronic diseases like diabetes and its complications with less toxicity in-vitro and in animal models, so play a vital role in human health [11, 12]. These features have made polyphenols an emerging area of interest in nutrition [202, 203]. The current review, introduced polyphenols as strong multi-target compounds to tackle DNP through affecting different signaling pathways with fewer side effects. The findings affirm that herbal treatments such as curcumin, kaempferol, quercetin, naringenin, resveratrol, kolaviron, and etc may positively effect in the management of DNP.
Further area of research on novel pathogenicity signaling pathways of DNP as well as the safety and efficacy of polyphenols in human, will expand the more potential applications of polyphenols in the management, prevention, and treatment of several diseases. However, more studies are still needed to introduce more effective treatments for DNP.
Compliance with ethical standards
Conflicts of interest
The authors stated no conflicts of interest
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Said G, Goulon-Goeau C, Slama G, Tchobroutsky G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus: a clinical and pathological study. New England Journal of Medicine. 1992;326(19):1257–1263. doi: 10.1056/NEJM199205073261905. [DOI] [PubMed] [Google Scholar]
- 2.Patel D, Kumar R, Prasad S, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin–induced diabetic rats. Asian Pacific journal of tropical biomedicine. 2011;1(4):316–322. doi: 10.1016/S2221-1691(11)60051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes care. 1978;1(3):168–188. [Google Scholar]
- 4.Johnson PC, Doll SC, Cromey DW. Pathogenesis of diabetic neuropathy. Annals of neurology. 1986;19(5):450–457. doi: 10.1002/ana.410190505. [DOI] [PubMed] [Google Scholar]
- 5.Watkins P, Thomas P. Diabetes mellitus and the nervous system. Journal of Neurology, Neurosurgery & Psychiatry. 1998;65(5):620–632. doi: 10.1136/jnnp.65.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes care. 2006;29(7):1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14(10):953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 8.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocrine reviews. 2004;25(4):612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 9.Nemni R, Sanvito L, Quattrini A, Santuccio G, Camerlingo M, Canal N. Peripheral neuropathy in hepatitis C virus infection with and without cryoglobulinaemia. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74(9):1267–1271. doi: 10.1136/jnnp.74.9.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, et al. Impact of dietary polyphenols on carbohydrate metabolism. International journal of molecular sciences. 2010;11(4):1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative medicine and cellular longevity. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Critical reviews in food science and nutrition. 2005;45(4):287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 13.Cásedas G, Les F, González-Burgos E, Gómez-Serranillos MP, Smith C, López V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. South African Journal of Botany. 2019;120:241–246. [Google Scholar]
- 14.Kern TS, Kowluru RA, Engerman RL. Abnormalities of retinal metabolism in diabetes or galactosemia: ATPases and glutathione. Investigative ophthalmology & visual science. 1994;35(7):2962–2967. [PubMed] [Google Scholar]
- 15.Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends in neurosciences. 2013;36(8):439–449. doi: 10.1016/j.tins.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. European Journal of Pharmacology. 2018;833:472–523. doi: 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. European journal of pharmacology. 2016;791:8–24. doi: 10.1016/j.ejphar.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Bayram EH, Sezer AD. Elçioğlu HKb. InTech: Diabetic Neuropathy and Treatment Strategy–New Challenges and Applications. Smart Drug Delivery System; 2016. [Google Scholar]
- 20.Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? Journal of Diabetes Investigation. 2011;2(1):18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res. 2010;106(9):1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nature Reviews Neuroscience. 2008;9(1):36. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 23.Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2007;36(2):144–166. doi: 10.1002/mus.20785. [DOI] [PubMed] [Google Scholar]
- 24.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proceedings of the National Academy of Sciences. 2000;97(22):12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008;9(1):14–36. doi: 10.2174/138945008783431781. [DOI] [PubMed] [Google Scholar]
- 26.Hotta N, Kawamori R, Fukuda M, Shigeta Y. Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29(12):1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nature clinical practice Endocrinology & metabolism. 2006;2(5):269–281. doi: 10.1038/ncpendmet0142. [DOI] [PubMed] [Google Scholar]
- 28.Edwards JF, Casellini CM, Parson HK, Obrosova IG, Yorek M, Vinik AI. Role of Peroxynitrite in the Development of Diabetic Peripheral Neuropathy. Diabetes care. 2015;38(7):e100–e1e1. doi: 10.2337/dc14-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron NE, Gibson TM, Nangle MR, Cotter MA. Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Ann N Y Acad Sci. 2005;1043(1):784–792. doi: 10.1196/annals.1333.091. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam PS, Cotter MA, Bravenboer B, Cameron NE. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur J Pharmacol. 2013;719(1-3):180–186. doi: 10.1016/j.ejphar.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Obrosova IG, Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Nadler JL, et al. Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. The American journal of pathology. 2010;177(3):1436–1447. doi: 10.2353/ajpath.2010.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupachyk S, Watcho P, Maksmchyk Y, Shevalye H, Vareniuk I, Oltman C et al., editors. Na+/H+-exchanger-1 inhibition reverses functional and structural manifestations of peripheral diabetic neuropathy. Diabetes; 2010: AMER DIABETES ASSOC 1701 N BEAUREGARD ST, ALEXANDRIA, VA 22311-1717 USA.
- 33.Eichberg J. Protein kinase C changes in diabetes: is the concept relevant to neuropathy? Int Rev Neurobiol. 2002;50:61–82. doi: 10.1016/s0074-7742(02)50073-8. [DOI] [PubMed] [Google Scholar]
- 34.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasase T, Yamada H, Sakoda K, Imagawa N, Abe T, Ito M, et al. Novel protein kinase C-β isoform selective inhibitor JTT-010 ameliorates both hyper-and hypoalgesia in streptozotocin-induced diabetic rats. Diabetes Obes Metab. 2005;7(5):586–594. doi: 10.1111/j.1463-1326.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 36.Edwards J, Quattrini A, Lentz S, Figueroa-Romero C, Cerri F, Backus C, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53(1):160. doi: 10.1007/s00125-009-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent AM, Mclean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. The FASEB Journal. 2005;19(6):638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 38.Zherebitskaya E, Akude E, Smith DR, Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58(6):1356–1364. doi: 10.2337/db09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular Reactivity and Inflammatory Cytokines in Painful and Painless Peripheral Diabetic Neuropathy. The Journal of Clinical Endocrinology & Metabolism. 2009;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conti G, Scarpini E, Baron P, Livraghi S, Tiriticco M, Bianchi R, et al. Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: a process concomitant with endoneurial induction of IL-1beta and p75NTR. Journal of the neurological sciences. 2002;195(1):35–40. doi: 10.1016/s0022-510x(01)00684-0. [DOI] [PubMed] [Google Scholar]
- 41.Yamagishi S, Ogasawara S, Mizukami H, Yajima N, Wada R, Sugawara A, et al. Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J Neurochem. 2008;104(2):491–499. doi: 10.1111/j.1471-4159.2007.05050.x. [DOI] [PubMed] [Google Scholar]
- 42.Andorfer B, Kieseier BC, Mathey E, Armati P, Pollard J, Oka N, et al. Expression and distribution of transcription factor NF-κB and inhibitor IκB in the inflamed peripheral nervous system. Journal of neuroimmunology. 2001;116(2):226–232. doi: 10.1016/s0165-5728(01)00306-x. [DOI] [PubMed] [Google Scholar]
- 43.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99(5):3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bierhaus A, Haslbeck K-M, Humpert PM, Liliensiek B, Dehmer T, Morcos M, et al. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. The Journal of clinical investigation. 2004;114(12):1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apfel S. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl. 1):27–34. doi: 10.1159/000052077. [DOI] [PubMed] [Google Scholar]
- 46.Jeong J-O, Kim M-O, Kim H, Lee M-Y, Kim S-W, Ii M, et al. Dual angiogenic and neurotrophic effects of bone marrow–derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009;119(5):699–708. doi: 10.1161/CIRCULATIONAHA.108.789297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apfel SC. Neurotrophic factors in the therapy of diabetic neuropathy. The American journal of medicine. 1999;107(2):34–42. doi: 10.1016/s0002-9343(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 48.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. Journal of advanced pharmaceutical technology & research. 2011;2(4):236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116(3):598–606. doi: 10.1172/jci27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janani C, Kumari BR. PPAR gamma gene–a review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(1):46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira AC, Bertollo CM, Rocha LT, Nascimento EB, Jr, Costa KA, Coelho MM. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR alpha, and pioglitazone, an agonist of PPAR gamma. Eur J Pharmacol. 2007;561(1-3):194–201. doi: 10.1016/j.ejphar.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 52.Freitag CM, Miller RJ. Peroxisome proliferator-activated receptor agonists modulate neuropathic pain: a link to chemokines? Frontiers in cellular neuroscience. 2014;8:238. doi: 10.3389/fncel.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mooradian AD, Chehade J, Thurman JE. The role of thiazolidinediones in the treatment of patients with type 2 diabetes mellitus. Treatments in endocrinology. 2002;1(1):13–20. doi: 10.2165/00024677-200201010-00002. [DOI] [PubMed] [Google Scholar]
- 54.Griggs RB, Donahue RR, Adkins BG, Anderson KL, Thibault O, Taylor BK. Pioglitazone inhibits the development of hyperalgesia and sensitization of spinal nociresponsive neurons in type 2 diabetes. The Journal of Pain. 2016;17(3):359–373. doi: 10.1016/j.jpain.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okine BN, Gaspar JC, Finn DP. PPARs and pain. British journal of pharmacology. 2019;176(10):1421–1442. doi: 10.1111/bph.14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pabbidi RM, Yu S-Q, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4(1):9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khomula EV, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan PV, Voitenko NV. Specific functioning of Cav3. 2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2013;1832(5):636-49. [DOI] [PubMed]
- 58.Pabbidi MR, Premkumar LS. Role of Transient Receptor Potential Channels Trpv1 and Trpm8 in Diabetic Peripheral Neuropathy. Journal of diabetes and treatment. 2017;2017(4). [PMC free article] [PubMed]
- 59.Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci. 2013;92(8-9):415–424. doi: 10.1016/j.lfs.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernyhough P, Calcutt NA. Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium. 2010;47(2):130–139. doi: 10.1016/j.ceca.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolphin AC. Calcium channel auxiliary α 2 δ and β subunits: trafficking and one step beyond. Nature Reviews Neuroscience. 2012;13(8):542. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 62.Craner MJ, Klein JP, Renganathan M, Black JA, Waxman SG. Changes of sodium channel expression in experimental painful diabetic neuropathy. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 2002;52(6):786–792. doi: 10.1002/ana.10364. [DOI] [PubMed] [Google Scholar]
- 63.Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, et al. Methylglyoxal modification of Na v 1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18(6):926. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 64.Carlton SM. Peripheral excitatory amino acids. Curr Opin Pharmacol. 2001;1(1):52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 65.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 66.Rustioni A. Modulation of sensory input to the spinal cord by presynaptic ionotropic glutamate receptors. Arch Ital Biol. 2005;143(2):103–112. [PubMed] [Google Scholar]
- 67.Chizh B. Novel approaches to targeting glutamate receptors for the treatment of chronic pain. Amino Acids. 2002;23(1-3):169–176. doi: 10.1007/s00726-001-0124-4. [DOI] [PubMed] [Google Scholar]
- 68.Roshanravan H, Kim EY, Dryer SE. NMDA receptors as potential therapeutic targets in diabetic nephropathy: increased renal NMDA receptor subunit expression in Akita mice and reduced nephropathy following sustained treatment with memantine or MK-801. Diabetes. 2016:db160209. [DOI] [PMC free article] [PubMed]
- 69.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, et al. Depletion of capsaicin sensitive afferents prevents lamina-dependent increases in spinal N-methyl-d-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008;12(5):552–563. doi: 10.1016/j.ejpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Bai H-P, Liu P, Wu Y-M, Guo W-Y, Guo Y-X, Wang X-L. Activation of spinal GABAB receptors normalizes N-methyl-D-aspartate receptor in diabetic neuropathy. J Neurol Sci. 2014;341(1-2):68–72. doi: 10.1016/j.jns.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Fakhri S, Abbaszadeh F, Dargahi L. Jorjani M. A mechanistic review on its biological activities and health benefits. Pharmacological research: Astaxanthin; 2018. [DOI] [PubMed] [Google Scholar]
- 72.Mendell JR, Sahenk Z. Painful sensory neuropathy. New England Journal of Medicine. 2003;348(13):1243–1255. doi: 10.1056/NEJMcp022282. [DOI] [PubMed] [Google Scholar]
- 73.Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Leon BGC, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161(9):639–649. doi: 10.7326/M14-0511. [DOI] [PubMed] [Google Scholar]
- 74.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. Jama. 1998;280(21):1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 75.Dureshahwar K, Mubashir M, Une HD. Quantification of quercetin obtained from Allium cepa Lam. leaves and its effects on streptozotocin-induced diabetic neuropathy. Pharmacognosy research. 2017;9(3):287. doi: 10.4103/pr.pr_147_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Low P. A., Nickander K. K., Tritschler H. J. The Roles of Oxidative Stress and Antioxidant Treatment in Experimental Diabetic Neuropathy. Diabetes. 1997;46(Supplement_2):S38–S42. doi: 10.2337/diab.46.2.s38. [DOI] [PubMed] [Google Scholar]
- 77.Romero FJ. Antioxidants in peripheral nerve. Free Radical Biology and Medicine. 1996;20(7):925–932. doi: 10.1016/0891-5849(95)02183-3. [DOI] [PubMed] [Google Scholar]
- 78.Sahoo DK, Roy A, Chainy GB. Protective effects of vitamin E and curcumin on L-thyroxine-induced rat testicular oxidative stress. Chemico-biological interactions. 2008;176(2-3):121–128. doi: 10.1016/j.cbi.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free radical biology and medicine. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 80.Ziegler D, Hanefeld M, Ruhnau K-J, Hasche H, Lobisch M, Schütte K, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes care. 1999;22(8):1296–1301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
- 81.Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, et al. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes care. 1995;18(8):1160–1167. doi: 10.2337/diacare.18.8.1160. [DOI] [PubMed] [Google Scholar]
- 82.Cameron N, Cotter M, Archibald V, Dines K, Maxfield E. Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia. 1994;37(5):449–459. doi: 10.1007/s001250050131. [DOI] [PubMed] [Google Scholar]
- 83.Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83(4):650–659. doi: 10.1016/j.fitote.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, et al. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 2015;37(10):924–933. doi: 10.1179/1743132815Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 85.Ates O, Cayli SR, Yucel N, Altinoz E, Kocak A, Durak MA, et al. Central nervous system protection by resveratrol in streptozotocin-induced diabetic rats. Journal of Clinical Neuroscience. 2007;14(3):256–260. doi: 10.1016/j.jocn.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, et al. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014;560:81–85. doi: 10.1016/j.neulet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience letters. 2004;361(1-3):184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 88.Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. Journal of Neuroscience. 2003;23(7):2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.González-Clemente J, Mauricio D, Richart C, Broch M, Caixas A, Megia A, et al. Diabetic neuropathy is associated with activation of the TNF-α system in subjects with type 1 diabetes mellitus. Clinical endocrinology. 2005;63(5):525–529. doi: 10.1111/j.1365-2265.2005.02376.x. [DOI] [PubMed] [Google Scholar]
- 90.Shafer DM, Assael L, White LB, Rossomando EF. Tumor necrosis factor-α as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. Journal of oral and maxillofacial surgery. 1994;52(8):786–791. doi: 10.1016/0278-2391(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 91.Satoh J, Yagihashi S, Toyota T. The possible role of tumor necrosis factor-α in diabetic polyneuropathy. Journal of Diabetes Research. 2003;4(2):65–71. doi: 10.1155/EDR.2003.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Current drug targets. 2008;9(1):60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- 93.Hansson P, Lacerenza M, Marchettini P. Aspects of clinical and experimental neuropathic pain: the clinical perspective. Progress in Pain Research and Management. 2001;21:1–18. [Google Scholar]
- 94.Copray J, Mantingh I, Brouwer N, Biber K, Küst B, Liem R, et al. Expression of interleukin-1 beta in rat dorsal root ganglia. Journal of neuroimmunology. 2001;118(2):203–211. doi: 10.1016/s0165-5728(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 95.Cunha J, Cunha F, Poole S, Ferreira S. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. British journal of pharmacology. 2000;130(6):1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bujalska M, Tatarkiewicz J, De Cordé A, Gumułka SW. Effect of cyclooxygenase and nitric oxide synthase inhibitors on streptozotocin-induced hyperalgesia in rats. Pharmacology. 2008;81(2):151–157. doi: 10.1159/000110787. [DOI] [PubMed] [Google Scholar]
- 97.Kellogg AP, Wiggin TD, Larkin DD, Hayes JM, Stevens MJ, Pop-Busui R. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes. 2007;56(12):2997–3005. doi: 10.2337/db07-0740. [DOI] [PubMed] [Google Scholar]
- 98.Kumar A, Kaundal RK, Iyer S, Sharma SS. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007;80(13):1236–1244. doi: 10.1016/j.lfs.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 99.Mohamed HE, El-Swefy SE, Hasan RA, Hasan AA. Neuroprotective effect of resveratrol in diabetic cerebral ischemic-reperfused rats through regulation of inflammatory and apoptotic events. Diabetology & metabolic syndrome. 2014;6(1):88. doi: 10.1186/1758-5996-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deng W, Lu H, Teng J. Carvacrol attenuates diabetes-associated cognitive deficits in rats. Journal of Molecular Neuroscience. 2013;51(3):813–819. doi: 10.1007/s12031-013-0069-6. [DOI] [PubMed] [Google Scholar]
- 101.Park S, Sapkota K, Kim S, Kim H, Kim S. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. British journal of pharmacology. 2011;164(3):1008–1025. doi: 10.1111/j.1476-5381.2011.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abo-Salem OM. Kaempferol attenuates the development of diabetic neuropathic pain in mice: Possible anti-inflammatory and anti-oxidant mechanisms. Macedonian Journal of Medical Sciences. 2014;7(3):424–430. [Google Scholar]
- 103.Kandhare AD, Raygude KS, Kumar VS, Rajmane AR, Visnagri A, Ghule AE, et al. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomedicine & Aging Pathology. 2012;2(4):173–186. [Google Scholar]
- 104.Anjaneyulu M, Chopra K. Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Progress in neuro-psychopharmacology and biological psychiatry. 2003;27(6):1001–1005. doi: 10.1016/S0278-5846(03)00160-X. [DOI] [PubMed] [Google Scholar]
- 105.Baluchnejadmojarad T, Roghani M, Khastehkhodaie Z. Chronic treatment of silymarin improves hyperalgesia and motor nerve conduction velocity in diabetic neuropathic rat. Phytotherapy research. 2010;24(8):1120–1125. doi: 10.1002/ptr.3078. [DOI] [PubMed] [Google Scholar]
- 106.Baluchnejadmojarad T, Roghani M. Chronic oral epigallocatechin-gallate alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: involvement of oxidative stress. Iranian journal of pharmaceutical research: IJPR. 2012;11(4):1243. [PMC free article] [PubMed] [Google Scholar]
- 107.Kaur N, Kishore L, Singh R. Chromane isolated from leaves of Dillenia indica improves the neuronal dysfunction in STZ-induced diabetic neuropathy. J Ethnopharmacol. 2017;206:19–30. doi: 10.1016/j.jep.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 108.Bagdas D, Ozboluk HY, Cinkilic N, Gurun MS. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J Med Food. 2014;17(6):730–732. doi: 10.1089/jmf.2013.2966. [DOI] [PubMed] [Google Scholar]
- 109.Akbar S, Subhan F, Karim N, Shahid M, Ahmad N, Ali G, et al. 6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic pain. Biomedicine & Pharmacotherapy. 2016;84:962–971. doi: 10.1016/j.biopha.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 110.Attia HN, Al-rasheed NM, Al-rasheed NM, Maklad YA, Ahmed AA, Kenawy SAB. Protective effects of combined therapy of gliclazide with curcumin in experimental diabetic neuropathy in rats. Behav Pharmacol. 2012;23(2):153–161. doi: 10.1097/FBP.0b013e3283512c00. [DOI] [PubMed] [Google Scholar]
- 111.Abd Allah ES, Gomaa AM. Effects of curcumin and captopril on the functions of kidney and nerve in streptozotocin-induced diabetic rats: role of angiotensin converting enzyme 1. Applied Physiology, Nutrition, and Metabolism. 2015;40(10):1061–1067. doi: 10.1139/apnm-2015-0145. [DOI] [PubMed] [Google Scholar]
- 112.Nagilla B, Pratap RK. Neuroprotective and antinociceptive effect of curcumin in diabetic neuropathy in rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(5):131–138. [Google Scholar]
- 113.Ghatak SB, Panchal SS. Protective effect of oryzanol isolated from crude rice bran oil in experimental model of diabetic neuropathy. Revista Brasileira de Farmacognosia. 2012;22(5):1092–1103. [Google Scholar]
- 114.Jain D, Bansal MK, Dalvi R, Upganlawar A, Somani R. Protective effect of diosmin against diabetic neuropathy in experimental rats. Journal of integrative medicine. 2014;12(1):35–41. doi: 10.1016/s2095-4964(14)60001-7. [DOI] [PubMed] [Google Scholar]
- 115.Baluchnejadmojarad T, Roghani M, Mafakheri M. Neuroprotective effect of silymarin in 6-hydroxydopamine hemi-parkinsonian rat: involvement of estrogen receptors and oxidative stress. Neurosci Lett. 2010;480(3):206–210. doi: 10.1016/j.neulet.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 116.Rocha-González HI, Ramírez-Aguilar M, Granados-Soto V, Reyes-García JG, Torres-López JE, Huerta-Cruz JC, et al. Antineuropathic effect of 7-hydroxy-3, 4-dihydrocadalin in streptozotocin-induced diabetic rodents. BMC complementary and alternative medicine. 2014;14(1):129. doi: 10.1186/1472-6882-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shieh K, Yi C, Liu T, Tseng H, Ho H, Hsieh H, et al. Evidence for neurotrophic factors associating with TRPV1 gene expression in the inflamed human esophagus. Neurogastroenterology & Motility. 2010;22(9):971–e252. doi: 10.1111/j.1365-2982.2010.01530.x. [DOI] [PubMed] [Google Scholar]
- 118.AlSharari SD, Al-Rejaie SS, Abuohashish HM, Aleisa AM, Parmar MY, Ahmed MM. Ameliorative potential of morin in streptozotocin-induced neuropathic pain in rats. Tropical Journal of Pharmaceutical Research. 2014;13(9):1429–1436. [Google Scholar]
- 119.Song-Tao M, Dong-lian L, Jing-jing D, Yan-juan P. Protective effect of mulberry flavonoids on sciatic nerve in alloxan-induced diabetic rats. Brazilian Journal of Pharmaceutical Sciences. 2014;50(4):765–771. [Google Scholar]
- 120.Yu J, Zhang Y, Sun S, Shen J, Qiu J, Yin X, et al. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Canadian journal of physiology and pharmacology. 2006;84(6):579–587. doi: 10.1139/y06-015. [DOI] [PubMed] [Google Scholar]
- 121.Ding Y, Dai X, Jiang Y, Zhang Z, Li Y. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus. Phytother Res. 2014;28(7):1082–1087. doi: 10.1002/ptr.5104. [DOI] [PubMed] [Google Scholar]
- 122.Nagilla B, Reddy P. Neuroprotective and antinociceptive effect of curcumin in diabetic neuropathy in rats. Int J Pharm Sci. 2014;5:131–138. [Google Scholar]
- 123.A Carozzi V, Ceresa C. The role of glutamate in diabetic and in chemotherapy induced peripheral neuropathies and its regulation by glutamate carboxypeptidase II. Curr Med Chem. 2012;19(9):1261-1268. [DOI] [PubMed]
- 124.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E. Privat A-M et al. Mol Pharmacol: Diabetes-induced mechanical hyperalgesia involves spinal MAPKs activation in neurons and microglia via NMDA-dependent mechanisms; 2006. [DOI] [PubMed] [Google Scholar]
- 125.Fakhri S, Dargahi L, Abbaszadeh F. Jorjani M. Brain Res Bull: Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury; 2018. [DOI] [PubMed] [Google Scholar]
- 126.Fakhri Sajad, Dargahi Leila, Abbaszadeh Fatemeh, Jorjani Masoumeh. Effects of astaxanthin on sensory‐motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. European Journal of Pain. 2018;23(4):750–764. doi: 10.1002/ejp.1342. [DOI] [PubMed] [Google Scholar]
- 127.Takehana S, Kubota Y, Uotsu N, Yui K, Iwata K, Shimazu Y, et al. The dietary constituent resveratrol suppresses nociceptive neurotransmission via the NMDA receptor. Mol Pain. 2017;13:1744806917697010. doi: 10.1177/1744806917697010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moldzio R, Radad K, Krewenka C, Kranner B, Duvigneau JC, Rausch W-D. Protective effects of resveratrol on glutamate-induced damages in murine brain cultures. J Neural Transm. 2013;120(9):1271–1280. doi: 10.1007/s00702-013-1000-6. [DOI] [PubMed] [Google Scholar]
- 129.Zeng K, Yang N, Wang D, Li S, Ming J, Wang J, et al. Resveratrol prevents retinal dysfunction by regulating glutamate transporters, glutamine synthetase expression and activity in diabetic retina. Neurochem Res. 2016;41(5):1050–1064. doi: 10.1007/s11064-015-1793-9. [DOI] [PubMed] [Google Scholar]
- 130.Girbovan C, Plamondon H. Resveratrol downregulates type-1 glutamate transporter expression and microglia activation in the hippocampus following cerebral ischemia reperfusion in rats. Brain Res. 1608;2015:203–214. doi: 10.1016/j.brainres.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 131.Quincozes-Santos A, Bobermin LD, Tramontina AC, Wartchow KM, Tagliari B, Souza DO, et al. Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: neuroprotective effect of resveratrol. Toxicol In Vitro. 2014;28(4):544–551. doi: 10.1016/j.tiv.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 132.Chang Y, Wang S-J. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by resveratrol. Neurochem Int. 2009;54(2):135–141. doi: 10.1016/j.neuint.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Cho KS, Lee EJ, Kwon KJ, Gonzales ELT, Kim YB, Cheong JH, et al. Resveratrol down-regulates a glutamate-induced tissue plasminogen activator via Erk and AMPK/mTOR pathways in rat primary cortical neurons. Food & function. 2014;5(5):951–960. doi: 10.1039/c3fo60397k. [DOI] [PubMed] [Google Scholar]
- 134.Son Y, Byun SJ, Pae H-O. Involvement of heme oxygenase-1 expression in neuroprotection by piceatannol, a natural analog and a metabolite of resveratrol, against glutamate-mediated oxidative injury in HT22 neuronal cells. Amino Acids. 2013;45(2):393–401. doi: 10.1007/s00726-013-1518-9. [DOI] [PubMed] [Google Scholar]
- 135.Mikami Y, Yamazawa T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015;139:69–74. doi: 10.1016/j.lfs.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Rebai O, Belkhir M, Sanchez-Gomez MV, Matute C, Fattouch S, Amri M. Differential Molecular Targets for Neuroprotective Effect of Chlorogenic Acid and its Related Compounds Against Glutamate Induced Excitotoxicity and Oxidative Stress in Rat Cortical Neurons. Neurochem Res. 2017;42(12):3559–3572. doi: 10.1007/s11064-017-2403-9. [DOI] [PubMed] [Google Scholar]
- 137.Yu J, Jia Y, Guo Y, Chang G, Duan W, Sun M, et al. Epigallocatechin-3-gallate protects motor neurons and regulates glutamate level. FEBS Lett. 2010;584(13):2921–2925. doi: 10.1016/j.febslet.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 138.Pournourmohammadi S, Grimaldi M, Stridh MH, Lavallard V, Waagepetersen HS, Wollheim CB, et al. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ß-cells: A potential beneficial effect in the pre-diabetic state? The international journal of biochemistry & cell biology. 2017;88:220–225. doi: 10.1016/j.biocel.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 139.Chou C-W, Huang W-J, Tien L-T, Wang S-J. (−)-Epigallocatechin gallate, the most active polyphenolic catechin in green tea, presynaptically facilitates Ca2+-dependent glutamate release via activation of protein kinase C in rat cerebral cortex. Synapse. 2007;61(11):889–902. doi: 10.1002/syn.20444. [DOI] [PubMed] [Google Scholar]
- 140.Lee JH, Song DK, Jung CH, Shin DH, Park J, Kwon TK, et al. (–)-Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca2+ modulation in PC12 cells. Clin Exp Pharmacol Physiol. 2004;31(8):530–536. doi: 10.1111/j.1440-1681.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 141.Lu C-W, Lin T-Y, Wang S-J. Quercetin inhibits depolarization-evoked glutamate release in nerve terminals from rat cerebral cortex. Neurotoxicology. 2013;39:1–9. doi: 10.1016/j.neuro.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 142.Jayanarayanan S, Smijin S, Peeyush K, Anju T, Paulose C. NMDA and AMPA receptor mediated excitotoxicity in cerebral cortex of streptozotocin induced diabetic rat: ameliorating effects of curcumin. Chem Biol Interact. 2013;201(1-3):39–48. doi: 10.1016/j.cbi.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 143.Khalil RM, Khedr NF. Curcumin protects against monosodium glutamate neurotoxicity and decreasing NMDA2B and mGluR5 expression in rat hippocampus. Neurosignals. 2016;24(1):81–87. doi: 10.1159/000442614. [DOI] [PubMed] [Google Scholar]
- 144.Zhang L, Xu T, Wang S, Yu L, Liu D, Zhan R, et al. NMDA GluN2B receptors involved in the antidepressant effects of curcumin in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:12–17. doi: 10.1016/j.pnpbp.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 145.Huang H-C, Chang P, Lu S-Y, Zheng B-W, Jiang Z-F. Protection of curcumin against amyloid-β-induced cell damage and death involves the prevention from NMDA receptor-mediated intracellular Ca2+ elevation. Journal of Receptors and Signal Transduction. 2015;35(5):450–457. doi: 10.3109/10799893.2015.1006331. [DOI] [PubMed] [Google Scholar]
- 146.Lin TY, Lu CW, Huang SK, Wang SJ. Curcumin inhibits glutamate release from rat prefrontal nerve endings by affecting vesicle mobilization. International journal of molecular sciences. 2012;13(7):9097–9109. doi: 10.3390/ijms13079097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Srivastava P, Dhuriya YK, Kumar V, Srivastava A, Gupta R, Shukla RK, et al. PI3K/Akt/GSK3β induced CREB activation ameliorates arsenic mediated alterations in NMDA receptors and associated signaling in rat hippocampus: Neuroprotective role of curcumin. Neurotoxicology. 2018. [DOI] [PubMed]
- 148.Aseervatham GSB, Suryakala U, Sundaram S, Bose PC, Sivasudha T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomedicine & Pharmacotherapy. 2016;82:54–64. doi: 10.1016/j.biopha.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 149.Ramakrishnan A, Vijayakumar N, Renuka M. Naringin regulates glutamate-nitric oxide cGMP pathway in ammonium chloride induced neurotoxicity. Biomedicine & Pharmacotherapy. 2016;84:1717–1726. doi: 10.1016/j.biopha.2016.10.080. [DOI] [PubMed] [Google Scholar]
- 150.Yue R, Li X, Chen B, Zhao J, He W, Yuan H, et al. Astragaloside IV attenuates glutamate-induced neurotoxicity in PC12 cells through Raf-MEK-ERK pathway. PLoS ONE. 2015;10(5):e0126603. doi: 10.1371/journal.pone.0126603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang E-J, Kim G-S, Jun M, Song K-S. Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food & function. 2014;5(7):1395–1402. doi: 10.1039/c4fo00068d. [DOI] [PubMed] [Google Scholar]
- 152.Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. European journal of clinical nutrition. 2010;64(S3):S112. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- 153.Singh A, Holvoet S, Mercenier A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clinical & experimental allergy. 2011;41(10):1346–1359. doi: 10.1111/j.1365-2222.2011.03773.x. [DOI] [PubMed] [Google Scholar]
- 154.Orgogozo J-M, Dartigues J-F, Lafont S, Letenneur L, Commenges D, Salamon R, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. 1997. [PubMed] [Google Scholar]
- 155.Fujiki H, Sueoka E, Watanabe T, Suganuma M. Primary cancer prevention by green tea, and tertiary cancer prevention by the combination of green tea catechins and anticancer compounds. Journal of cancer prevention. 2015;20(1):1. doi: 10.15430/JCP.2015.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martín-Peláez S, Covas MI, Fitó M, Kušar A, Pravst I. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Molecular nutrition & food research. 2013;57(5):760–771. doi: 10.1002/mnfr.201200421. [DOI] [PubMed] [Google Scholar]
- 157.Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular aspects of medicine. 2010;31(6):435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 158.Additives EPoF. Food NSat Risk assessment for peri-and post-menopausal women taking food supplements containing isolated isoflavones. EFSA Journal. 2015;13(10):4246. [Google Scholar]
- 159.Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science. 2016;8:33–42. [Google Scholar]
- 160.Guo R, Li W, Liu B, Li S, Zhang B, Xu Y. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Medical science monitor basic research. 2014;20:82. doi: 10.12659/MSMBR.890858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang H, Liu Q, Lin J, Wang Y, Chen S, Hou J. GW27-e0657 Resveratrol Protects against oxidized LDL-induced foam cells formation and apoptosis through inhibition of ER stress and downregulation of CD36. Journal of the American College of Cardiology. 2016;68(16 Supplement):C26. [Google Scholar]
- 162.Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ precision oncology. 2017;1(1):35. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guo H, Chen Y, Liao L, Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovascular drugs and therapy. 2013;27(3):189–198. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- 164.Ng T-P, Chiam P-C, Lee T, Chua H-C, Lim L, Kua E-H. Curry consumption and cognitive function in the elderly. American journal of epidemiology. 2006;164(9):898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- 165.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, et al. Novel insights of dietary polyphenols and obesity. The Journal of nutritional biochemistry. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2(7):737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee K-S, Lee J-K, Kim H-G, Kim HR. Differential effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine on motor behavior and dopamine levels at brain regions in three different mouse strains. The Korean Journal of Physiology & Pharmacology. 2013;17(1):89–97. doi: 10.4196/kjpp.2013.17.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miyatake N, Sakano N, Numata T. Comparison of coffee, tea and green tea consumption between Japanese with and without metabolic syndrome in a cross-sectional study. Open Journal of Epidemiology. 2012;2(02):44. [Google Scholar]
- 169.Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L, Rumpler W, et al. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obesity reviews. 2011;12(7):e573–ee81. doi: 10.1111/j.1467-789X.2011.00862.x. [DOI] [PubMed] [Google Scholar]
- 170.Fernández-Quintela A, Carpéné C, Fernández M, Aguirre L, Milton-Laskibar I, Contreras J, et al. Anti-obesity effects of resveratrol: Comparison between animal models and humans. Journal of physiology and biochemistry. 2016;73(3):417–429. doi: 10.1007/s13105-016-0544-y. [DOI] [PubMed] [Google Scholar]
- 171.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. The Journal of nutrition. 2009;139(5):919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 172.Laparra JM, Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacological research. 2010;61(3):219–225. doi: 10.1016/j.phrs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 173.Marín Laura, Miguélez Elisa M., Villar Claudio J., Lombó Felipe. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Research International. 2015;2015:1–18. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.de Souza EL, de Albuquerque TMR, dos Santos AS, Massa NML, de Brito Alves JL. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities–a review. Critical reviews in food science and nutrition. 2018:1–15. [DOI] [PubMed]
- 175.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly) phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants & redox signaling. 2013;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zanotti I, Dall'Asta M, Mena P, Mele L, Bruni R, Ray S, et al. Atheroprotective effects of (poly) phenols: a focus on cell cholesterol metabolism. Food & function. 2015;6(1):13–31. doi: 10.1039/c4fo00670d. [DOI] [PubMed] [Google Scholar]
- 177.Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radical Biology and Medicine. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 178.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. Journal of diabetes & metabolic disorders. 2013;12(1):43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, Delmas D, et al. Dietary polyphenols and type 2 diabetes: human study and clinical trial. Critical reviews in food science and nutrition. 2018:1–9. [DOI] [PubMed]
- 180.Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scandinavian journal of urology and nephrology. 2011;45(5):365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- 181.Selvi NMK, Sridhar M, Swaminathan R, Sripradha R. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian Journal of Clinical Biochemistry. 2015;30(2):180–186. doi: 10.1007/s12291-014-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 183.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57(5):712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moazezi Z, Qujeq D. Berberis fruit extract and biochemical parameters in patients with type II diabetes. Jundishapur journal of natural pharmaceutical products. 2014;9(2). [DOI] [PMC free article] [PubMed]
- 185.Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2009;17(2):310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 186.Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. Journal of medicinal food. 2011;14(9):884–889. doi: 10.1089/jmf.2010.0180. [DOI] [PubMed] [Google Scholar]
- 187.Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. International journal of food sciences and nutrition. 2014;65(4):515–520. doi: 10.3109/09637486.2014.880671. [DOI] [PubMed] [Google Scholar]
- 188.Khandouzi N, Shidfar F, Rajab A, Rahideh T, Hosseini P, Taheri MM. The effects of ginger on fasting blood sugar, hemoglobin A1c, apolipoprotein B, apolipoprotein AI and malondialdehyde in type 2 diabetic patients. Iranian journal of pharmaceutical research: IJPR. 2015;14(1):131. [PMC free article] [PubMed] [Google Scholar]
- 189.Aryaeian N, Sedehi SK, Arablou T. Polyphenols and their effects on diabetes management: A review. Medical journal of the Islamic Republic of Iran. 2017;31:134. doi: 10.14196/mjiri.31.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kishore L, Kaur N, Singh R. Bacosine isolated from aerial parts of Bacopa monnieri improves the neuronal dysfunction in Streptozotocin-induced diabetic neuropathy. Journal of Functional Foods. 2017;34:237–247. doi: 10.1016/j.jff.2017.04.044. [DOI] [Google Scholar]
- 191.Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: Participation of nitric oxide and TNF-alpha. Phytotherapy Research. 2007;21(3):278–283. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- 192.Raposo D, Morgado C, Pereira-Terra P, Tavares I. Nociceptive spinal cord neurons of laminae I-III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin-gallate (EGCG) Brain research bulletin. 2015;110:68–75. doi: 10.1016/j.brainresbull.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 193.Ristagno G, Fumagalli F, Porretta-Serapiglia C, Orru A, Cassina C, Pesaresi M, et al. Hydroxytyrosol attenuates peripheral neuropathy in streptozotocin-induced diabetes in rats. Journal of agricultural and food chemistry. 2012;60(23):5859–5865. doi: 10.1021/jf2049323. [DOI] [PubMed] [Google Scholar]
- 194.Ayepola OR, Cerf ME, Brooks NL, Oguntibeju OO. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine. 2014;21(14):1785–1793. doi: 10.1016/j.phymed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 195.Ma ST, Liu DL, Deng JJ, Peng YJ. Protective effect of mulberry flavonoids on sciatic nerve in alloxan-induced diabetic rats. Brazilian Journal of Pharmaceutical Sciences. 2014;50(4):765–772. doi: 10.1590/S1984-82502014000400012. [DOI] [Google Scholar]
- 196.Ma CT, Chyau CC, Hsu CC, Kuo SM, Chuang CW, Lin HH, et al. Pepino polyphenolic extract improved oxidative, inflammatory and glycative stress in the sciatic nerves of diabetic mice. Food & function. 2016;7(2):1111–1121. doi: 10.1039/c5fo01358e. [DOI] [PubMed] [Google Scholar]
- 197.Anjaneyulu M, Chopra K. Quercetin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian journal of experimental biology. 2004;42(8):766–769. [PubMed] [Google Scholar]
- 198.Kumar A, Sharma SS. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochemical and biophysical research communications. 2010;394(2):360–365. doi: 10.1016/j.bbrc.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 199.Sharma S, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian journal of experimental biology. 2006;44(7):566–569. [PubMed] [Google Scholar]
- 200.Baluchnejadmojarad T, Roghani M, Khaste Khodaie Z. Evaluation of the effect of chronic administration of silymarin on thermal and chemical hyperalgesia in an experimental model of diabetic neuropathy in male rats. Iranian Journal of Endocrinology and Metabolism. 2010;11(5):583–608. [Google Scholar]
- 201.Eisenberg E, McNicol ED, Carr DB. Opioids for neuropathic pain. Cochrane database of systematic reviews. 2006;3. [DOI] [PubMed]
- 202.Lecour S. T Lamont K. Natural polyphenols and cardioprotection. Mini reviews in medicinal chemistry. 2011;11(14):1191–1199. doi: 10.2174/13895575111091191. [DOI] [PubMed] [Google Scholar]
- 203.Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: A Mini-Review. Frontiers in nutrition. 2018;5. [DOI] [PMC free article] [PubMed]