Abstract
Background
Myocardial pathologies are significant causes of morbidity and mortality in patients worldwide. Ischemic and non-ischemic cardiomyopathies have become a worldwide epidemic of the 21st century with an increasing impact on health care systems. The 2012 European Society of Cardiology and 2013 American College of Cardiology Foundation/American Heart Association guidelines provide current therapy guidance to reduce mortality and morbidity.
Methods
This was a systematic review involving cardiac magnetic resonance (CMR) studies for the diagnosis of cardiomyopathy from January 2013 to April 2017. Out of 62 reviewed studies, only 12 were included in our study.
Results
The average sensitivity and specificity of CMR in the diagnosis of cardiomyopathy was 86.75% (95% confidence interval [CI], 70.30% to 92.58%) and 81.75% (95% CI, 73.0% to 87.6%), respectively, and the positive predictive and negative predictive values were 80.17% and 86.75%, respectively.
Conclusion
Despite some limitations, our study shows that CMR has high sensitivity, specificity, and positive predictive value in diagnosing different types of cardiomyopathy. CMR may be used to differentiate types of cardiomyopathy, accurately quantify the chamber dimensions, volumes, and cardiac function, which make it useful for prognosis as well.
Keywords: Cardiac magnetic resonance, dilated cardiomyopathy, hypertrophic cardiomyopathy, ischemic cardiomyopathy, late gadolinium enhancement
Introduction
Myocardial pathologies are significant causes of morbidity and mortality in patients worldwide. Ischemic and non-ischemic cardiomyopathies have become a worldwide epidemic of the 21st century with an increasing impact on health care systems. The 2012 European Society of Cardiology and 2013 American College of Cardiology Foundation/American Heart Association guidelines provide current therapy guidance to reduce mortality and morbidity1,2,3,4. The revascularization of coronary arteries in acute myocardial infarction has become the treatment of choice, and revascularization procedures have evolved significantly. Coronary angiography is invasive and provides information only on the anatomical status of obstructive coronary lesions. Several non-invasive methods have been developed to aid in the assessment of the functional state of the myocardium, namely contraction and perfusion, as well as microvascular and cellular integrity, including positron emission tomography and contrast, enhanced echocardiography5,6,7,8. More recently, the development of delayed contrast-enhanced magnetic resonance imaging (DE-MRI) has served a purpose as another imaging tool. In ischemic cardiomyopathy, the sub-endocardium is always enhanced on DE-MRI, while a patchy mid-myocardial enhancement is observed in dilated cardiomyopathy (DCM). Furthermore, patients with restrictive cardiomyopathy showed delayed myocardial improvement over the entire sub-endocardial circumference9,10,11,12.
Heart failure represents the final stage in the continuum of cardiovascular diseases. Cardiac remodelling is a key component of heart failure that progresses from adaptive to maladaptive as the disorder worsens. Increased myocardial wall stress during diastole contributes to the development and progression of adverse cardiac remodeling13,14,15.
Cardiac magnetic resonance is superior to other cardiac imaging modalities such as echocardiography, computed tomography angiography and coronary angiography in determining the type of cardiomyopathy, and cardiac function16,17.
Methods
Patient characteristics
The age of participants in the included 12 cardiomyopathy studies ranged from 18 to 87 years, with dilated cardiomyopathy being a more seen pathology in the studies followed by ischemic cardiomyopathy. The patient characteristics are summarized in Table 1.
Table 1.
Characteristics of patients
| Category | |
| Sample size information | |
| Total number of patients included | 999 |
| Number of studies reporting on sample size | 12 |
| Range of sample size reported | 23–150 |
| Age of participants | |
| Number of studies reporting on age | 10 |
| Age range, yrs | 18–87 |
| Type of cardiomyopathy | |
| Ischemic cardiomyopathy | 193 |
| Dilated cardiomyopathy | 337 |
| Dual pathologies (NICM and DCM) | 16 |
| Hypertrophic cardiomyopathy | 70 |
| Tachycardia-induced cardiomyopathy | 55 |
| Myocarditis | 138 |
| Cardiac amyloidosis | 119 |
| Cardiac sarcoidosis | 14 |
| Left ventricle non-compaction | 57 |
| Other tests | |
| Histopathology | 1 |
| Echocardiography | 2 |
| Coronary angiography | 1 |
Abbreviations: NICM= nonischemic cardiomyopathy, DCM= dilated cardiomyopathy
Identification of studies and journals
We identified published studies using CMR in the diagnosis of different types of cardiomyopathy in original and review articles by systematic searches of PubMed, MedLine, Cochrane database and Embase, and by manual searches of listed references in the papers, we found. We limited our search to studies published from January 2013 to April 2017 because, during this period, we noted an increase in the application of CMR in diagnosing different types of cardiomyopathy. The keywords used were: “dilated cardiomyopathy”, “ischemic cardiomyopathy”, “hypertrophic cardiomyopathy”, “myocarditis”, “cardiac amyloidosis”, “cardiac sarcoidosis”, and “cardiac MR”, or “LGE CMR” (late gadolinium enhancement cardiovascular magnetic resonance), “Cine CMR”, and “sensitivity” or “specificity”. We identified 62 studies through this search strategy. We further screened the reference list of the retrieved studies for any additional publications. There were no restrictions on studies based on their sample size.
Eligibility criteria
We considered all eligible studies that evaluated the role of CMR in the diagnosis of cardiomyopathy. We also included studies with sufficient information to allow the calculation of sensitivity and specificity. We excluded meeting reports, abstracts, and reviews whose final stories were unavailable.
Data extraction
For each eligible study, we extracted the following information: author names, journal, year of publication, number of enrolled patients, the age of study patients, study design, and CMR protocol.
Statistical analysis
Statistical analysis was done using MedCalc for Windows version 64 bits; we calculated the sensitivity, specificity, positive predictive value, negative predictive value, positive likely-hood ratio, and negative likelihood ratio.
Results
Eligible studies
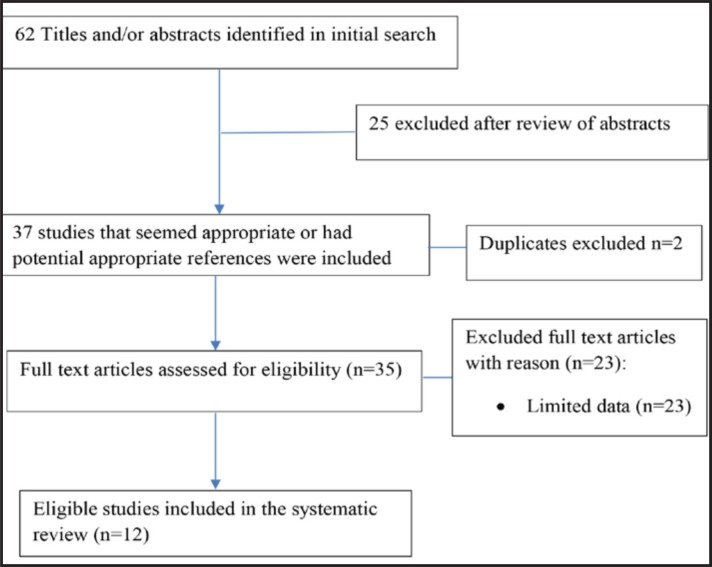
The search yielded 35 relevant studies. Of these, due to limited data, 23 studies were excluded, and 12 studies were available for systematic review. Figure 1 summarizes the flow diagram of how eligible studies were obtained. These studies were selected because they related to our analysis, they were recent, and they had likely extractable data as shown in figure 1.
Figure 1.
The flow diagram of study selection process for systematic review
Study description and patient characteristics
The 12 studies had a total of 999 patients. The sample size of the studies ranged from 23 to 150. The age range of the study subjects, 18 to 87 years, was reported in 10 reviews. Dilated cardiomyopathy was more prevalent in the studies, followed by ischemic cardiomyopathy and the least pervasive was cardiac sarcoidosis. The average sensitivity and specificity of CMR in the diagnosis of cardiomyopathy was 86.75% (95% confidence interval [CI], 70.30% to 92.58%) and 81.75% (95% CI, 73.0% to 87.6%), respectively. The positive predictive and negative predictive values were 80.17% and 86.75%. Tables 1, 2, 3, and 4 summarize the results.
Table 2.
Individual studies with sample sizes and types of cardiomyopathy
| Study | Sample size |
CMR | Findings |
| Goebel et al.18 2016 |
150 | T1 mapping CMR |
Does not differentiate healthy and diffusely diseased myocardium |
| Makoto et al.19 2016 |
44 | LGE CMR | LE in CS predominantly basal, mid septum and throughout the LV, while in DCM, LE was localized in the basal and mid septum |
| Mikami et al.20 2016 |
118 | LGE CMR | Septal fibrosis |
| Okada et al.21 2016 |
102 | LGE CMR | TIC had a significant lower RVEF, and a larger RVEDV and RVEDS |
| Kwong et al.22 2015 |
81 | Cine SSFP and LGE |
CA mean proportion of atrial enhancement was significantly greater compared to SH and NIDCM |
| Maurizio et al.23 2015 |
77 | LGE CMR | Hypertrophied septum indicating regional fibrosis in HCM |
| Schwab et al.24 2015 |
43 | CMR | Wall motion abnormalities |
| Nguyen et al.25 2015 |
23 | CMR/LGE CMR |
Diffuse myocardial fibrosis |
| Dungu et al.26 2013 |
97 | LGE CMR | Distinguished ATTR from AL cardiac amyloidosis |
| Ferreira et al.27 2013 |
50 | T1 mapping CMR |
T1 mapping is a criterion for detection of acute myocarditis with a higher sensitivity |
| Choi et al.28 2016 |
114 | Cine CMR/ LGE CMR |
Classification of distribution of trabeculation, 43.9% global type, 56.2% apical type |
| Gulsin et al.29 2017 |
100 | Cine CMR/ LGE CMR |
Global LV hypokinesis |
Abbreviations: CMR= cardiac magnetic resonance, LGE= late gadolinium enhancement, LE= late enhancement, CS= cardiac sarcoidosis, DCM= dilated cardiomyopathy, TIC= tachycardia induced cardiomyopathy, RVEDV= right ventricle end diastolic volume, RVEDS= right ventricle end systolic volume, CA= cardiac amyloidosis, SH= systemic hypertension, NIDCM= Nonischemic dilated cardiomyopathy, HCM= hypertrophic cardiomyopathy, AL= amyloid light chain, ATTR= transthyretin related Amyloidosis, SSFP= steady state free precision
Table 3.
Showing individual studies with sensitivity, specificity and confidence intervals
| Study | Sensitivity | 95% CI | Specificity | 95% CI | FPR(1-TPR) |
| Goebel et al.18 2016 |
0.85 | 0.75–0.91 | 0.75 | 0.66–0.83 | 0.15 |
| Makoto et al.19 2016 |
0.68 | 0.57–0.78 | 0.63 | 0.53–0.72 | 0.32 |
| Mikami et al.20 2016 |
0.70 | 0.60–0.79 | 0.71 | 0.61–0.80 | 0.30 |
| Okada et al.21 2016 |
0.88 | 0.79–0.95 | 0.75 | 0.66–0.82 | 0.12 |
| Kwong et al.22 2015 |
0.93 | 0.85–0.97 | 0.80 | 0.71–0.87 | 0.07 |
| Maurizio et al.23 2015 |
0.99 | 0.93–1.00 | 0.83 | 0.75–0.89 | 0.01 |
| Schwab et al.24 2015 |
0.86 | 0.72–0.95 | 1.0 | 0.90–1.0 | 0.14 |
| Nguyen et al.25 2015 |
0.84 | 0.75–0.95 | 0.80 | 0.72–0.88 | 0.16 |
| Dungu et al.26 2013 |
0.96 | 0.89–0.99 | 0.88 | 0.80–0.93 | 0.04 |
| Ferreira et al.27 2013 |
0.91 | 0.83–0.96 | 0.90 | 0.82–0.95 | 0.09 |
| Choi et al.28 2016 |
0.85 | 0.76–0.92 | 0.76 | 0.64–0.83 | 0.15 |
| Gulsin et al.29 2017 |
0.96 | 0.90–0.99 | 1.00 | 0.96–1.0 | 0.04 |
Abbreviations: CI= confidence interval, FPR= false positive rate, TPR= true positive rate
Table 4.
Showing individual studies with positive predictive value and negative predictive value
| Study | PPV | NPV |
| Goebel et al.18 2016 | 0.71 | 0.87 |
| Makoto et al.19 2016 | 0.57 | 0.73 |
| Mikami et al.20 2016 | 0.72 | 0.69 |
| Okada et al.21 2016 | 0.69 | 0.91 |
| Kwong et al.22 2015 | 0.76 | 0.94 |
| Maurizio et al.23 2015 | 0.80 | 0.99 |
| Schwab et al.24 2015 | 1.00 | 0.85 |
| Nguyen et al.25 2015 | 0.81 | 0.85 |
| Dungu et al.26 2013 | 0.87 | 0.96 |
| Ferreira et al.27 2013 | 0.97 | 0.90 |
| Choi et al.28 2016 | 0.72 | 0.88 |
| Gulsin et al.29 2017 | 1.00 | 0.96 |
Abbreviations: PPV= positive predictive value, NPV= negative predictive value
Discussion
Cardiomyopathy has been diagnosed and assessed by echocardiogram or cardiac computed tomography for many years. With technological advancements and further research, several studies have addressed the role of cardiac magnetic resonance (CMR) as a functional modality in the diagnosis and quantification of cardiac function in different types of cardiomyopathy. CMR can measure and quantify chamber sizes and left ventricle (LV) systolic function accurately. Therefore, Cardiac Magnetic Resonance has potential as a tool to assess patient prognosis.
In our systematic review of 12 studies, we found moderately high sensitivity and specificity values for CMR, which implies that CMR is a standard valuable imaging modality for diagnosing different types of cardiomyopathy. Goebel et al. could differentiate between dilated cardiomyopathy, hypertrophic cardiomyopathy (HCM), and healthy heart function through left ventricle quantification; patients with dilated cardiomyopathy had higher left ventricle end-diastolic volume, end-systolic volume, and systolic volume and significantly lower ejection fractions compared to patients with healthy hearts18. Hypertrophic cardiomyopathy patients had substantially higher end-diastolic septum thickness compared to healthy subjects18. This reinforces the importance of cardiac magnetic resonance in diagnosing cardiomyopathy. CMR can also be useful in differentiating ischemic and non-ischemic cardiomyopathy, which might facilitate optimal management of patients.
According to this study, we appreciate the ability of CMR to diagnose and differentiate types of cardiomyopathies, through its high spatial resolution and tomographic image capabilities. Late gadolinium magnetic resonance was able to distinguish cardiac sarcoidosis and dilated cardiomyopathy19. Furthermore, Late Gadolinium Enhancement cardiac magnetic resonance was also used to diagnose non-ischemic Dilated cardiomyopathy by revealing septal fibrosis and other studies identified a mid-wall septal striae pattern of Late gadolinium enhancement to be the most reliable predictor of future events20. Moreover, Cardiac magnetic resonance was also used to assess the diagnostic value of early right ventricular dysfunction to predict tachycardia-induced cardiomyopathy, in which the studies revealed that CMR imaging assessing right ventricular function might be valuable compared with echocardiography21.
Ischemic cardiomyopathy can easily be missed in routine screening of suspected coronary heart disease patients particularly in microvascular coronary artery disease whereby coronary angiogram may be the standard investigation expected to be done. But with the current new technology of Positron emission magnetic resonance imaging (PETMRI), it will be easy to know which areas are poorly perfused and confirmed by measuring coronary flow reserve30. Among other non-invasive imaging modalities, CMR is emerging as a highly sensitive and specific test for myocardial ischemia and infarction. Resting perfusion in CMR is used to evaluate microvascular obstruction, which is shown to predict adverse left ventricular remodelling31. Thus, as previous studies have indicated, the significance of magnetic resonance in diagnosing different types of cardiomyopathies especially in differentiating ischemic versus nonischemic cardiomyopathy, we concur with our results of MRI having high sensitivity and specificity.
Clinical implications
CMR is superior in evaluating cardiac function, LV dimensions, and capable of differentiating types of cardiomyopathies with a specificity of 81.75% and sensitivity of 86.75%. In the clinical setting, the ability to diagnose the form of cardiomyopathy helps in choosing the specific treatment. In a study of Choi et al.,28 they used cardiac magnetic resonance imaging to establish refined diagnostic criteria for left ventricle non-compaction. As a quantitative approach, we have shown that a trabeculated left ventricle volume of >35% of the LV myocardial volume is diagnostic for left ventricle non-compaction with high specificity. Our study has also shown the diagnostic accuracy of late gadolinium-enhanced cardiac magnetic resonance for establishing the etiology of heart failure. Late gadolinium enhancement-cardiac magnetic resonance (LGE-CMR) was able to differentiate between ischemic cardiomyopathy and non-ischemic cardiomyopathy. Furthermore, the addition of adenosine stress perfusion-cardiac magnetic resonance (SP-CMR) to cine and LGE-CMR provided minimal incremental diagnostic yield for determining the etiology of heart failure in patients with severe left ventricle systolic dysfunction29.
Limitations
The inclusion of studies with small sample sizes may influence the statistical power of the individual research and lead to imprecise and inconclusive results. Other limitations include bias through selection, publication, and verification of the studies.
Conclusion
Despite some limitations, our study shows that cardiac magnetic resonance (CMR) has high sensitivity, specificity, and positive predictive value in diagnosing different types of cardiomyopathy. CMR may be used to differentiate types of cardiomyopathy, accurately quantify the chamber dimensions, volumes, and cardiac function which make it useful for prognosis as well.
Disclosure
The authors declare no conflict of interest.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeed M, Hetts SW, ablonowski R, Wilson MW. Magnetic resonance imaging and multi-detector computed tomography assessment of extracellular compartment in ischemic and non-ischemic myocardial pathologies. World J Cardiol. 2014;6(11):1192–1208. doi: 10.4330/wjc.v6.i11.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziaeian B, Fonarow GC. Epidemiology and etiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayala HA, Mayala M, Mutta R, Bakari KH, Hui WZ. The Effect of interleukin-6, microRNA-21 and the role of atorvastatin in dilated cardiomyopathy- A review of literature. EJBPS. 2017;4(9):103–106. [Google Scholar]
- 5.Foussas SG, Tsiaousis GZ. Revascularization treatment in patients with coronary artery disease. Hippokratia. 2008;12(1):3–10. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H-Y, Choi J-H. How to utilize coronary computed tomography angiography in the treatment of coronary artery disease. J Cardiovasc Ultrasound. 2015;23(4):204–208. doi: 10.4250/jcu.2015.23.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah T, Geleris JD, Zhong M, Swaminathan RV, Kim LK, Feldman DN. Fractional flow reserve to guide surgical coronary revascularization. J Thorac Dis. 2017;9(Suppl 4):S317–S326. doi: 10.21037/jtd.2017.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbab-Zadeh A. Stress testing and non-invasive coronary angiography in patients with suspected coronary artery disease: time for a new paradigm. Int Heart J. 2012;7(1):e2. doi: 10.4081/hi.2012.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermes E, Carbone I, Friedricha MG, Merchant N. Patterns of myocardial late enhancement: Typical and atypical features. Arch Cardiovasc Dis. 105:300–308. doi: 10.1016/j.acvd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Doltra A, Amundsen BH, Gebker R, Fleck E, Kelle S. Emerging concepts for myocardial late gadolinium enhancement MRI. Curr Cardiol Rev. 2013;9(3):185–190. doi: 10.2174/1573403X113099990030. 185-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh H, Sano M, Suwa K, Saitoh T, Nobuhara M, Saotome M, et al. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in the differential diagnosis, clinical features, and prognosis. World J Cardiol. 2014;6(7):585–601. doi: 10.4330/wjc.v6.i7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco A, Javidi S, Ruehm SG. Delayed myocardial enhancement in cardiac magnetic resonance imaging. J Radiol Case Rep. 2015;9(6):6–18. doi: 10.3941/jrcr.v9i6.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip GW, Fung JWH, Tan Y-T, Sanderson JE. Hypertension and heart failure: a dysfunction of systole, diastole or both? J Hum Hypertens. 2009;23:295–306. doi: 10.1038/jhh.2008.141. [DOI] [PubMed] [Google Scholar]
- 14.Burchfield JS, Xie M, Hill JA. Pathological Ventricular Remodeling: Mechanisms: Part 1 of 2. Circ. 2013;128(4):388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson J, Bolger AF, Ebbers T, Carlhäll CJ. Assessment of left ventricular hemodynamic forces in healthy subjects and patients with dilated cardiomyopathy using 4D flow MRI. Physiol Rep. 2016;4(3) doi: 10.14814/phy2.12685. pii: e12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assunção FB, de Oliveira DCL, Souza VF, M Nacif MS. Cardiac magnetic resonance and computed tomography in ischemic cardiomyopathy: An update. Radiol Bras. 2016;49(1):26–34. doi: 10.1590/0100-3984.2014.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman DS, Hachamovitch R, Shaw LJ, Friedman JD, Hayes SW, Thomson LE, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Assessment of patients with suspected coronary artery disease. J Nucl Med. 2006;47:74–82. [PubMed] [Google Scholar]
- 18.Goebel J, Seifert I, Nensa F, Schemuth HP, Maderwald S, Quick HH, et al. Can native T1 mapping differentiate between healthy and diffuse diseased myocardium in clinical routine cardiac MR imaging? PLoS ONE. 2016;11(5):e0155591. doi: 10.1371/journal.pone.0155591. https://doi.org/10.1371/journal. pone.0155591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sano M, Satoh H, Suwa K, Saotome M, Urushida T, Katoh H, et al. Intra-cardiac distribution of late gadolinium enhancement in cardiac sarcoidosis and dilated cardiomyopathy. World J Cardiol. 2016;8(9):496–503. doi: 10.4330/wjc.v8.i9.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikami Y, Cornhill A, Heydari B, Joncas SX, Almehmadi F, Zahrani M, et al. Objective criteria for septal fibrosis in non-ischemic dilated cardiomyopathy: validated for the prediction of future cardiovascular events. J Cardiovasc Magn Reson. 2016;18:82. doi: 10.1186/s12968-016-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada A, Nakajima I, Morita Y, Inoue YY, Kamakura T, Wada M, et al. Diagnostic value of right ventricular dysfunction in tachycardia-induced cardiomyopathy using cardiac magnetic resonance imaging. Circ J. 2016;80:2141–2148. doi: 10.1253/circj.CJ-16-0532. [DOI] [PubMed] [Google Scholar]
- 22.Kwong R, Sara P, Sharmila D. Multimodality imaging in the assessment of myocardial viability. Heart Fail Rev. 2011;16(4):381–395. doi: 10.1007/s10741-010-9201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurizio G, Nuno C, Antonello D'A, Oliver B, Bernard C, Laurent D, et al. The multi-modality cardiac imaging approach to the Athlete's heart: an expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 24.Schwab J, Rogg HJ, Pauschinger M, Fessele K, Bareiter T, Bär I, et al. Functional and Morphological Parameters with Tissue Characterization of Cardiovascular Magnetic Imaging in Clinically Verified “Infarct-like Myocarditis”. Rofo. 2016;188(4):365–373. doi: 10.1055/s-0041-108200. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen C, Minjie L, Zhaoyang F, Xiaoming B, Peter K, Shihua Z, et al. Contrast-free detection of myocardial fibrosis in hypertrophic cardiomyopathy patients with diffusion-weighted cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance. 2015;17:107. doi: 10.1186/s12968-015-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dungu JN, Oswaldo V, Jennifer H, Simon D J G, Dorota R, Janet A G, et al. CMR-Based Differentiation of AL and ATTR Cardiac Amyloidosis. Jacc: cardiovascular imaging. 2014;7(2) doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6(10):1048–1058. doi: 10.1016/j.jcmg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Choi Y, Kim SM, Lee S-C, Chang S, Jang SY, Choe YH. Quantification of left ventricular trabeculae using cardiovascular magnetic resonance for the diagnosis of left ventricular non-compaction: evaluation of trabecular volume and refined semi-quantitative criteria. J Cardiovasc Magn Reson. 2016;18:24. doi: 10.1186/s12968-016-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulsin GS, Shetye A, Khoo J, Swarbrick DJ, Levelt E, Lai FY, et al. Does stress perfusion imaging improve the diagnostic accuracy of late gadolinium-enhanced cardiac magnetic resonance for establishing the etiology of heart failure? BMC Cardiovasc Dis. 2017;17:98. doi: 10.1186/s12872-017-0529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heo R, Nakazato R, Kalra D, Min JK. Noninvasive imaging in coronary artery disease. Semin Nucl Med. 2014;44(5):398–409. doi: 10.1053/semnuclmed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamirani YS, Kramer CM. Cardiac MRI assessment of myocardial perfusion. Future Cardiol. 2014;10(3):349–358. doi: 10.2217/fca.14.18. [DOI] [PMC free article] [PubMed] [Google Scholar]