Abstract
Abuse of alcohol is a major clinical problem with far-reaching health consequences. Understanding the environmental and genetic factors that contribute to alcohol-related behaviors is a potential gateway for developing novel therapeutic approaches for patients that abuse the drug. To this end, we have used Drosophila melanogaster as a model to investigate the effect of diet, an environmental factor, on ethanol sedation. Providing flies with diets high in yeast, a routinely used component of fly media, increased their resistance to ethanol sedation. The yeast-induced resistance to ethanol sedation occurred in several different genetic backgrounds, was observed in males and females, was elicited by yeast from different sources, was readily reversible, and was associated with increased nutrient intake as well as decreased internal ethanol levels. Inhibition of serotonergic neuron function using multiple independent genetic manipulations blocked the effect of yeast supplementation on ethanol sedation, nutrient intake, and internal ethanol levels. Our results demonstrate that yeast is a critical dietary component that influences ethanol sedation in flies and that serotonergic signaling is required for the effect of dietary yeast on nutrient intake, ethanol uptake/elimination and ethanol sedation. Our studies establish the fly as a model for diet-induced changes in ethanol sedation and raise the possibility that serotonin might mediate the effect of diet on alcohol-related behavior in other species.
Keywords: ethanol, alcohol, Drosophila, behavior, sedation, diet
Introduction
Consumption of alcohol (ethanol) has a wide range of pleasurable effects including psychomotor stimulation1, 2, general improvement in mood and relief of stress3. Additionally, however, abuse of alcohol has far-reaching, negative health consequences4, 5. Alcohol abuse contributes to 3–4% of all preventable deaths worldwide, increases the risk for specific forms of cancer, and is responsible for hundreds of billions of dollars in costs annually within the United States alone4. Both environmental and heritable genetic factors contribute to the risk for abusing alcohol6–9. A better understanding of these environmental and genetic risk factors could ultimately facilitate prevention and treatment of alcohol abuse.
Drosophila melanogaster (fruit fly or fly) is a leading invertebrate model for investigating molecular-genetic mechanisms that influence alcohol-related behaviors10–13. Behavioral responses of flies to alcohol include locomotor stimulation at low doses14, 15, sedation at higher doses16–22, development of seizures upon withdrawal of alcohol23, and development of tolerance after prior exposure to the drug24–26. Additionally, flies will voluntarily consume alcohol27 and they develop exposure-dependent alcohol preference28–31. All of the behavioral responses to ethanol in flies are also found in other species including humans11, strongly suggesting that alcohol likely has conserved effects on nervous system function. Consistent with this possibility, many genes or genetic pathways that influence behavioral responses to alcohol in flies have also been implicated in various aspects of alcohol-related behaviors in other model organisms (e.g. Clic 20, GABA signaling32, 33, slo potassium channels34 and NPF/NPY signaling35, 36) as well as various aspects of alcohol consumption and abuse in humans (e.g. Adh31, 37–39, Rsu140, AUTS241, Ryr16). Thus, at least some of the mechanisms underlying alcohol-related behavior in model organisms might also impact alcohol abuse in humans.
In addition to genetic factors, ~50% of the risk for abusing alcohol is influenced by environment6–9. Diet is possibly one of the key—but largely underappreciated—environmental factors that influences alcohol phenotypes in humans. Supplementation of the diet with tryptophan decreases alcohol craving in human binge drinkers exposed to stress42. Additionally, patients with higher body mass indexes (BMI) are at an increased risk for heavy alcohol intake43, development of alcohol dependence44 and alcohol abuse45. Diet also influences multiple alcohol-related behaviors in rodents46–49 and C. elegans 50. Furthermore, variants in the genes FTO and CPNE5 are associated with both obesity and multiple alcohol phenotypes in humans51–53 and several genes in flies might regulate both food intake and behavioral responses to alcohol54. These studies collectively suggest that diet and diet × genotype interactions might play important roles in multiple aspects of alcohol-related behavior in animals and impact risk for alcohol-related phenotypes in humans.
Several studies suggest that the serotonin (5-hydroxytryphtophan, 5-HT) system might modulate or mediate the effects of diet on behavioral responses to alcohol. In flies, for example, dietary yeast influences brain 5-HT levels55, serotonergic neurons regulate feeding56–58, the 5-HT2A receptor impacts dietary protein consumption55, and 5-HT is implicated in ethanol sedation59. Additionally, there is a large literature linking 5-HT to alcohol problems in humans (e.g.60–64). Despite the insights of the studies summarized here, the possibility that 5-HT signaling underlies diet-induced changes in behavioral responses has not been formally addressed.
In this report, we describe results from our studies on the role of diet in alcohol sedation in Drosophila. We chose flies for these studies because of their conserved alcohol-related behaviors11, 14–31, the powerful tools available for performing genetic analyses in this model65, the ability to measure both ethanol sedation (see above) and food intake66, the ability to control food composition55, 66, 67, and the known genetic connections between fly alcohol behavior and human alcohol abuse16, 31, 37–41. We report that dietary yeast significantly impacts ethanol sedation in flies, possibly by influencing ethanol uptake/elimination. We also report that the effect of dietary yeast on ethanol sedation and uptake/elimination depends on serotonergic neuron function. Our studies establish flies as a model for exploring diet-induced changes in alcohol sedation and suggest that the serotonergic system might be a conserved regulator of the underlying processes.
Materials and methods
Materials
Drosophila agar type II and cotton plugs for vials were from Apex BioResearch Products (Genesee Scientific, San Diego, CA); saf-instant yeast, Lesaffre Yeast Corp. (Milwaukee, WI); yellow corn meal, The Quaker Oats Co. (Chicago, IL); MP Bakers (101400) and MP Brewers (903312) yeast, MP Biomedicals (Solon, OH); table sugar (sucrose), Richmond Restaurant Service (Richmond, VA); methyl 4-hydroxybenzoate (Tegosept), chloramphenicol, tetracycline and ampicillin, Sigma-Aldrich (St. Louis, MO); FD&C Blue No. 1, Spectrum Chemical Manufacturing Corp. (Gardena, CA); polypropylene culture bottles (AS-355) and cotton plugs, Fisher Scientific; polystyrene narrow vials (89092–722), VWR International; gas drying tube caps (199610000), Bel-Art Products (Wanye, NJ); feeder caps for Con-Ex studies (FCS13/16NA1), MOCAP (Park Hills, MO); 200 (41–6304) and 400 (41–6140) μm mesh, Ted Pella, Inc. (Redding, CA); Alcohol Reagent Set (A7504), Pointe Scientific, Inc. (Canton, MI).
Fly stocks and husbandry
The w[A], Lausanne-S (LS), Oregon-R (OR) and Samarkand (Sam) strains (stock numbers 5905, 4268, 25211 and 4270, respectively), UAS-Tetanus Toxin Light Chain68 (stock number 28837), and two Trh-Gal469 drivers (stock numbers 38388 and 38389) were obtained from the Bloomington Drosophila stock center (Bloomington, IN). The r[A] strain was generated by backcrossing the w+ allele in Canton-S (supplied by Ron Davis, Scripts, Florida) into w[A] for 7 generations. Flies containing the UAS-Kir2.170 transgene were generated in a w1118 genetic background (supplied by Scott Pletcher, University of Michigan).
Flies were grown to adulthood at 25°C/65% relative humidity with a 12-hour light/dark cycle on standard food medium (2Y10S3C: 2% saf-instant yeast, 10% sugar, 3.3% cornmeal, 1% agar, 2 g/L Tegosept, 0.125 g/L chloramphenicol, 0.02 g/L tetracycline and 0.1 g/L ampicillin) supplemented with live yeast. Flies (3 to 5 d-old) were collected under light CO2 anesthesia, sex separated, and placed in fresh food vials containing the media indicated in the main text prior to the described studies.
In studies using yeast paste (live or heat-killed (autoclaved at 122˚C for 1 h using the dry cycle of a Hirayama HV-50 autoclave) saf-instant yeast (35% w/v) in water), flies were collected and placed into fresh food vials (containing 2Y10S3C as described above) and provided yeast paste (1 g/vial) via inverted caps from 50 mL conical tubes placed in the open ends of the vials. For studies using nylon mesh barriers, caps from gas drying tubes were bored out, circular pieces of nylon mesh were melted into the caps, and the cap-nylon mesh inserts were placed in the vials to provide an ~2 cm gap between the flies and the yeast paste.
The media in vials were 2Y10S3C (described above); 2Y10S3C missing antimicrobials, missing one or two nutrient components, or with all components diluted as described in the main text; 2Y10S3C supplemented with additional yeast, sugar or cornmeal as described in the main text; or 2Y, 10Y, 20Y or 30Y (2, 10, 20 or 30% yeast w/v in 1% agar). Unless otherwise noted, yeast indicates saf-instant bakers yeast.
Ethanol sedation, ethanol rapid tolerance and internal ethanol.
Ethanol sedation (determined as sedation time 50 (ST50), the time required for 50% of flies to become sedated) and rapid tolerance (the ratio of a second ST50 to a first ST50) were measured as previously described17, 19 using vapor from 85% ethanol. For analysis of internal ethanol, flies were exposed to ethanol vapor for the times indicated in the figure legends and then homogenized in 200 μl of distilled water. Homogenates were centrifuged to pellet debris and ethanol content in the supernatants was determined as previously described via a spectrophotometric method17, 19.
Media and nutrient consumption
Intake of food medium was measured using consumption-excretion of 1% FD&C Blue 1 in the indicated media using the sum of the dye excreted in the vial (ExVial) and the internal dye (INT) to reflect the volume of media consumption as described66. Flies were reared and collected as described above, placed on the indicated food medium containing 1% FD&C Blue 1 for 24 h, and then ExVial and INT were determined. Nutrient consumption (fold of 2Y) was estimated as ([ExVial+INT] x [yeast concentration]) ÷ ([mean 2Y ExVial+INT] x [yeast concentration]).
Total, dry and water weight
Adult flies were reared and collected as above and weighed to determine total weight in groups of 11 while anesthetized in tared 1.5 ml snap-cap tubes with perforated lids. Tubes of flies were incubated at 50˚C (ambient humidity) for 24 or more h to volatilize water content and weighed to obtain dry weight. Water weight was determined as the difference between total and dry weight. Total, dry and water weights for each tube were expressed as mg/fly. Each tube of 11 flies generated a single datum.
Brain 5-HT levels
r[A] females, reared and collected as described above, were fed 2Y or 30Y media for 2 days. Brains were dissected from flies and 5-HT was measured essentially as previously described71–73. In brief, single fly brains were dissected, homogenized, diluted with 10 µL 20 µM perchloric acid (to prevent transmitter degradation) and then tissue content determined with capillary electrophoresis with fast scan cyclic voltammetry detection.
Statistical analyses
All data were normally distributed (Prism 6.07, GraphPad Software Inc., San Diego, CA) and were therefore analyzed with standard parametric tests (two-tailed t tests, one- and two-way ANOVAs, Bonferroni’s multiple comparisons (BMC)) using Prism 6.07 (GraphPad Software Inc., San Diego, CA). P values < 0.05 were considered to represent statistically discernable differences. All P values are reported in the figure legends and all data are reported as mean ± S.E.M.
Results
Drugs, enzyme inhibitors and other molecules can be administered (i.e. fed) to flies via a simple paste made of yeast (Saccharomyces cerevisiae) and water (e.g.74–76). While establishing this drug treatment regimen for investigating alcohol behavior in flies, we found that flies exposed to a standard food medium supplemented with a paste made from live yeast and water were substantially resistant to ethanol sedation (Fig. 1A, time-courses; Fig. 1B, sedation-time 50 (ST50) values) compared to flies provided only a standard medium containing 2% yeast, 10% sucrose and 3.3% cornmeal (hereafter 2Y10S3C). The resistance to ethanol sedation was evident by 2 d of exposure to yeast paste and persisted during at least 4 d of continuous exposure (Fig. 1C).
Figure 1. Exposure to dietary yeast paste alters ethanol sedation sensitivity.
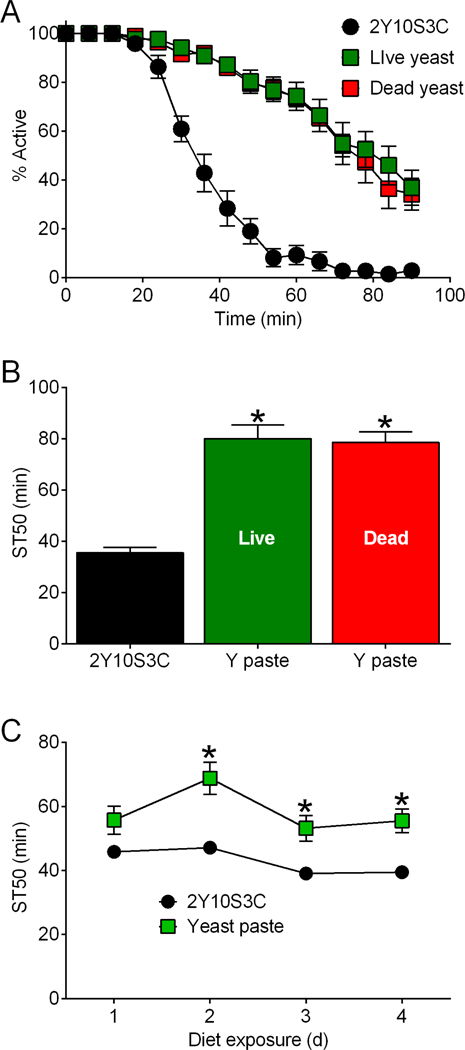
(A) w[A] females fed a paste of live or dead yeast (35% w/v) for 2 d took longer to become sedated compared to flies fed 2Y10S3C (standard) medium (two-way ANOVA; time, p<0.0001; yeast, p<0.0001; interaction, p<0.0001; *Bonferroni’s multiple comparisons (BMC) versus 2Y10S3C; p<0.0001; n=7–8 per data point). (B) ST50 values derived from panel A. Yeast (Y) paste had a significant overall effect on ST50s (one-way ANOVA, p<0.0001, n=7–8). ST50s were greater in flies fed live or dead yeast versus 2Y10S3C medium (*BMC, p<0.0001). ST50s were not distinguishable between flies fed live or dead yeast paste (BMC, p=0.9682). (C) Dietary yeast paste increased ST50 values in r[A] females (two-way ANOVA; yeast, p<0.0001; time, p=0.0029; interaction, p=0.3486; *BMC, p=0.0136 to <0.0001; n=8).
Yeast produce ethanol via fermentation77–80, including under conditions used to rear Drosophila17. To address the possibility that the ethanol resistance in flies fed yeast paste reflected tolerance in response to ethanol produced by the supplemented yeast, we fed flies paste made of heat-killed yeast (which would be incapable of fermentation) and then assessed ethanol sedation. Flies fed heat-killed yeast paste were resistant to ethanol sedation compared to flies fed standard food, and ethanol sedation in flies fed heat-killed and live yeast paste were indistinguishable (Fig. 1A, time-courses; Fig. 1B, ST50 values). Therefore fermentation and ethanol production by supplemented yeast is not required for the yeast-induced change in resistance to ethanol sedation.
Flies were provided with supplemental yeast paste in the studies reported in Fig. 1. To address the possibility that increasing the concentration of yeast incorporated into agar-based fly media (versus supplementation with yeast paste) was capable of altering ethanol sedation, we assessed ST50 values in flies fed our standard fly medium (2% yeast, 2Y10S3C) and in media containing 10% (10Y10S3C), 20% (20Y10S3C) and 30% (30Y10S3C) yeast. Increasing the yeast concentration increased ST50 values in males (Fig. 2A) and females (Fig. 2B). Flies fed 20% yeast medium had increased ST50s after exposure to the diet for 2 or more d, whereas flies fed medium with 30% yeast had greater ST50 values after 1 or more d on the diet (Fig. 2). Increasing the yeast concentration in agar-based medium, like supplemental yeast paste, is therefore capable of eliciting resistance to ethanol sedation. Rearing flies on 2Y10S3C and 30Y10S3C promoted comparable patterns of adult emergence over time (Fig. S1A) and comparable total numbers of progeny (Fig. S1B), suggesting that our standard 2Y01S3C medium is not nutrient deficient and therefore the yeast-driven changes in ST50 (Fig. 2) are likely to be related to nutrient supplementation versus restoration of sufficient nutrients. The data in Fig. 2 also suggest that dietary yeast did not need to be alive to elicit resistance to ethanol sedation since the agar-based media were generated by boiling.
Figure 2. Supplementation of dietary media with yeast alters ethanol sedation.
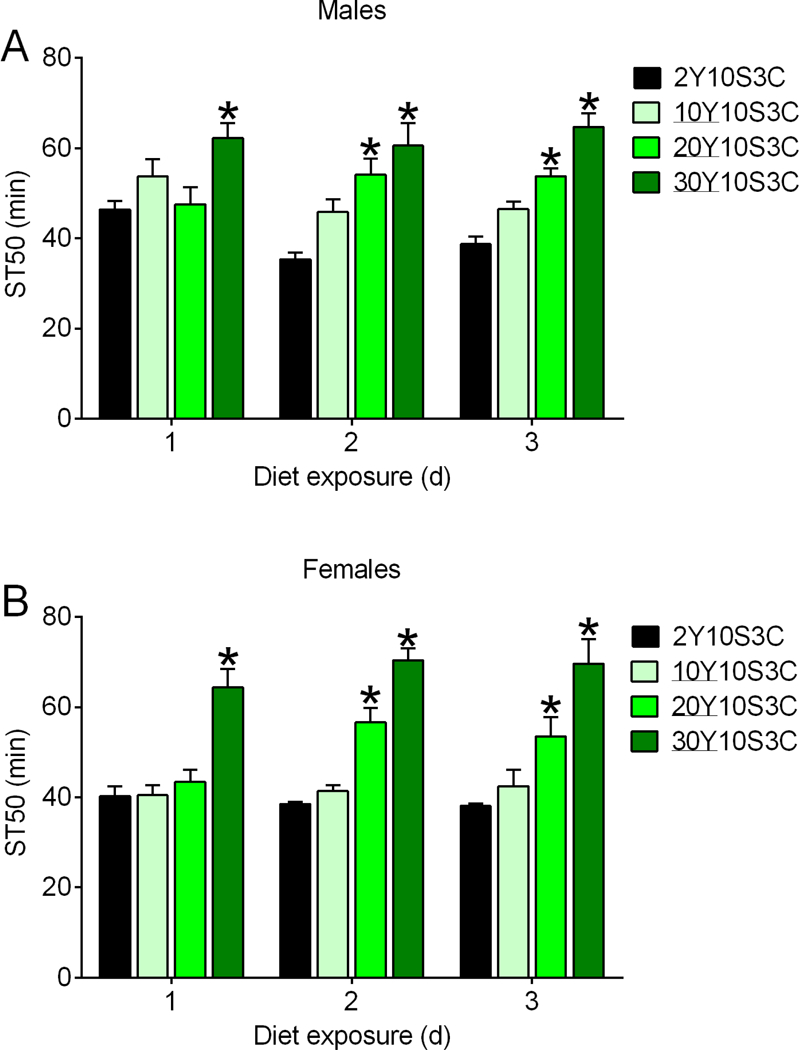
Flies were fed the indicated media for 1–3 d. Concentrations (w/v) of yeast (Y) used are underlined. ST50s in r[A] males (A) and females (B) were influenced by supplementing the diet with yeast (two-way ANOVA; yeast, p<0.0001; diet exposure time in males, p=0.2665; diet exposure time in females, p=0.0852; interaction in males, p=0.0681; interaction in females, p=0.2749; n=6). Compared to flies fed 2Y10S3C medium, ST50s were increased in flies fed media supplemented with yeast (*BMCs, p=0.068 to <0.0001).
It seemed possible that increasing nutrient components other than yeast in dietary media might also influence ethanol sedation. We therefore fed flies standard agar-based media supplemented with sucrose or cornmeal and then measured their ST50s. We found that increasing these other nutrient components of dietary media for 1–3 d of feeding did not systematically or substantially alter ST50 values in males (Figs. S2A and S2C) or females (Figs. S2B and S2D). Although these experiments do not formally rule out a potential role for dietary sucrose or cornmeal in fly ethanol sedation resistance, they do indicate that altering these two components of the diet likely has a much more modest (if any) effect on ethanol sedation compared to yeast.
It also seemed possible that omitting other components of the fly media could affect ethanol sedation. We therefore measured ST50 values in male and female flies fed 2Y10S3C media with (+ATC) or without (−ATC) the antibiotics ampicillin, chloramphenicol, and tetracycline (Fig. S3A), and with (+TEG) and without (−TEG) the antifungal Tegosept (Fig. S3B). Additionally, to test whether omission of one or more of the nutrient components of 2Y10S3C medium could alter ethanol sedation, we assessed ST50 values in flies fed media that did not contain yeast, sucrose, or cornmeal individually (Fig. S4A), lacked combinations of yeast and sucrose, sucrose and cornmeal, or yeast and cornmeal (Fig. S4B), contained diluted media components (0.5X and 0.25X, Fig. S4C), or contained no yeast, sucrose or cornmeal (0X, Fig. S4C). Ethanol sedation was not significantly affected by the omission of antibiotics from the media (Fig. S3A), consistent with a previous report from our group17, nor by omitting or reducing Tegosept (Fig. S3B), yeast, sugar, or cornmeal (Figs. S4A and S4B), or all nutrient components (Fig. S4C).
The results in Figs. 1-2 and S2-S4 collectively indicate that increasing dietary yeast is capable of increasing resistance to ethanol sedation. To more directly test this possibility, we assessed ST50 values in males and females fed our standard 2Y10S3C medium, a medium with 2% yeast as the only nutrient (2Y), or a medium with 30% yeast as the only nutrient (30Y). ST50 values were indistinguishable in flies fed 2Y10S3C and 2Y media (Fig. 3A; left, males; right, females), consistent with our previous studies using media lacking sucrose or cornmeal (Fig. S4). As expected, ST50 values were significantly greater in male and female flies fed a 30Y diet compared to both 2Y10S3C and 2Y (Fig. 3A). These results confirm that manipulating the concentration of dietary yeast in the absence of other nutrients is sufficient for altering ethanol sedation.
Figure 3. Effects of multiple types of dietary yeast influences ethanol sedation.
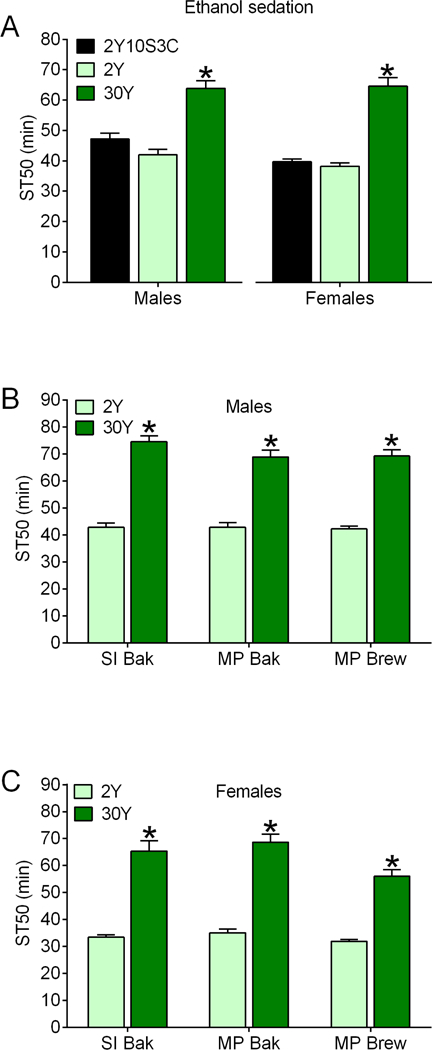
(A) Flies were fed the indicated media for 2 d. ST50s were greater in r[A] males and females fed 30% yeast (30Y) compared to 2Y10S3C or 2% yeast (2Y) media (one-way ANOVAs; males, p<0.0001; females, p<0.0001; *BMC versus other groups, p<0.0001; n=8). ST50s were increased in male (B) and female (C) flies fed 30Y versus 2Y media for 2 d (individual two-way ANOVAs: males—yeast concentration, p<0.0001; yeast source, p=0.2509; interaction, p=0.3232; females—yeast concentration, p<0.0001; yeast source, p=0.0048; interaction, p=0.1087; *BMC versus 2Y, p<0.0001; n=8 for all groups). ST50s in females were lower on 30Y MP Brew than in 30Y SI Bak and MP Bak (BMC, p=0.0202 and 0.0012, respectively).
The studies reported in all figures discussed thus far used saf-instant bakers (SI Bak) yeast. To address whether SI Bak yeast was unique in its ability to elicit resistance to ethanol sedation, we tested whether yeast from other sources could alter ST50 values (Figs. 3B, 3C). Males (Fig. 3B) and females (Fig. 3C) fed media containing 30% (30Y) SI Bak, MP bakers (MP Bak) or MP brewers (MP Brew) yeast were resistant to ethanol sedation compared to their sex-matched counterparts fed media with 2% yeast (2Y) from each source. Media with 30% of all three yeast sources had comparable effects on resistance to ethanol sedation in males (Fig. 3B), whereas 30% MP Brew yeast had a smaller effect than the other 2 yeast sources in females (Fig. 3C). The ability to induce resistance to ethanol sedation appears to be a common property of yeast. Additionally, our studies suggest that there could be subtle yeast x sex effects on ethanol sedation.
Like mammals, flies develop rapid ethanol tolerance, quantified as the change in resistance to ethanol during a second ethanol challenge after recovery from an initial exposure to the drug24. To determine whether a high yeast diet altered rapid tolerance in flies, we fed flies 2Y or 30Y media, measured their ethanol-naive ST50 values, allowed them to recover for 4 h, and then measured their ST50 values during a second ethanol exposure. Males (Fig. S5A) and females (Fig. S5B) fed 2Y and 30Y media developed robust rapid tolerance, but the development of rapid tolerance to ethanol was not altered by diet in either sex (Figs. S5A and S5B). This suggests that high concentrations of dietary yeast influence initial ethanol sedation, but not the development of rapid tolerance.
Flies from different genetic backgrounds can vary substantially in their feeding66, 81, alcohol22, 82, and other behaviors83, 84. To determine whether the effect of dietary yeast supplementation on resistance to ethanol sedation was a common property of flies, we measured ST50 values in four additional control strains (w[A], Lausanne-S (LS), Oregon-R (OR) and Samarkand (SAM)) after feeding them 2Y or 30Y media for two days (Fig. 4) or one day (Fig. S6). Males and females fed 30Y medium had elevated ST50 values compared to flies fed 2Y medium in all cases. The magnitude of the supplemental yeast effect on ST50 values varied across the additional control strains tested (e.g. compare w[A] and SAM in Fig. 4A and 4D), consistent with widely appreciated genetic background effects on behavior. Although the effect of dietary yeast on ST50 values varied across the control strains tested, these data indicate that the increased resistance to ethanol sedation in response to supplemental dietary yeast is a common feature of flies. Additionally, these data confirm that providing flies with an elevated yeast diet for 1 or more days is sufficient to increase their resistance to ethanol sedation.
Figure 4. Dietary yeast impacts ethanol sedation in flies from several different genetic backgrounds.
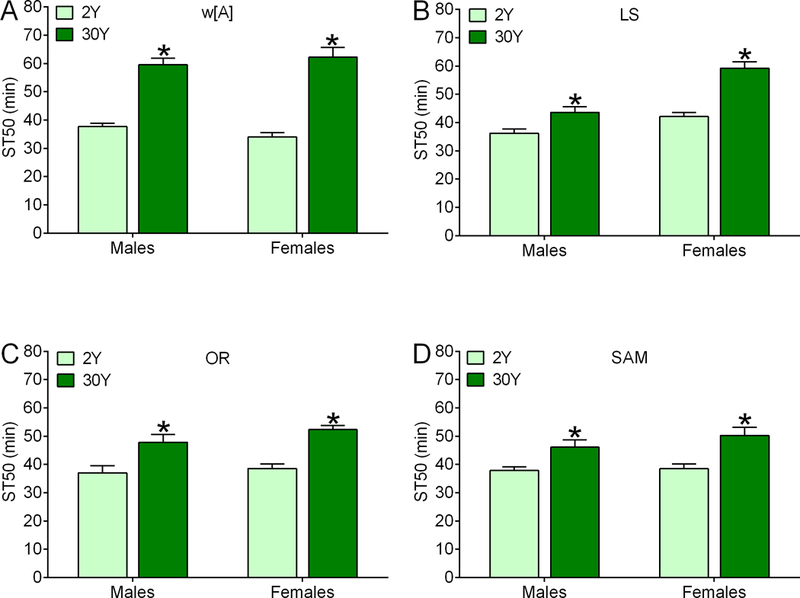
Compared to flies fed 2Y medium, ST50s were increased in male and female w[A] (A), LS (B), OR (C) and Sam (D) after 2 d of feeding on 30Y medium (individual two-way ANOVAs; w[A]—yeast concentration, p<0.0001; sex, p=0.8266; interaction, p=0.1857; LS— yeast concentration, p<0.0001; sex, p<0.0001; interaction, p=0.0137; OR— yeast concentration, p<0.0001; sex, p=0.1756; interaction, p=0.4990; Sam— yeast concentration, p=0.0002; sex, p=0.2905; interaction, p=0.4390; *BMC versus 2Y, p=0.0299 to <0.0001; n=6 for all groups in all panels).
Altering the diet can lead to changes in the body mass of flies85, 86. To determine if yeast supplementation altered body mass in our experiments, we measured total, dry and water weight in several different control flies fed 2Y and 30Y media for 1 d. Compared to flies fed 2Y medium, flies on 30Y had increased total body mass in 9 of 10 cases, increased dry mass in 7 of 10 cases, and increased water weight in 8 of 10 cases (Supplementary Table 1). To address if body mass might impact ethanol sedation, we explored whether total, dry, or water weight correlated with ST50 values in flies on 30Y vs 2Y media. Total, dry, and water weight did not correlate with ST50s in males or females (Supplementary Table 2). Additionally, feeding 30Y medium for 1 d increased ST50 values in males and females of all genotypes tested (Figs. 2 and S6), even though some groups of animals did not have changes in total, dry, or water weight (Supplementary Table 1). Thus, flies fed 30Y medium had increased total, dry, and water weight in most cases, but these changes were not required for altered ethanol sedation and body mass did not predict ST50 values.
To determine whether the effect of a high yeast diet on resistance to ethanol sedation was reversible, we fed flies 30Y medium for two days, switched them to 2Y for two days, then assessed their ST50 values. Flies fed 30Y food for two days were resistant to ethanol sedation compared to flies fed 2Y for two days as expected (Fig. 5A, males; Fig. 5B, females). In contrast, flies fed 30Y medium for two days and then switched to 2Y food for two days had ST50 values that were indistinguishable from flies fed 2Y medium only (Fig. 5A, males; Fig. 5B, females). The resistance to ethanol sedation driven by supplemental dietary yeast is therefore readily reversible in both males and females.
Figure 5. Reversible effects of dietary yeast on ethanol sedation.
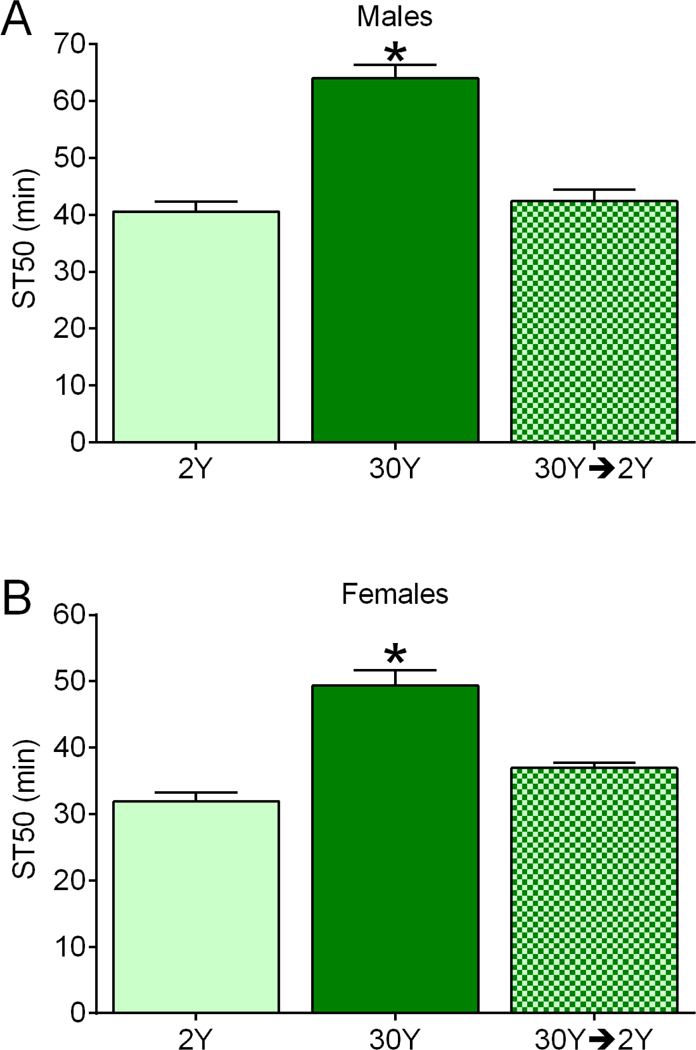
Dietary regimen impacted ST50 values in males (A) and females (B) (individual one-way ANOVAs for effect of diet; males, p<0.0001; females, p<0.0001; n=8). Compared to flies fed only 2Y medium, ST50 values were increased in males and females fed 30Y medium for 2 d (*BMC, p<0.0001; n=8), but not in flies fed 30Y for 2d and then switched to 2Y for an additional 2d (BMC; males, p>0.9999; females, p=0.1097).
Flies are well known to adjust the volume of media they consume in response to changes in nutrient concentration in their diet66, 81, 87. This compensatory feeding is thought to help maintain steady total nutrient intake81, 87, although this phenomenon does not always occur66. To address whether flies provided with 30Y medium consumed more nutrients than flies fed 2Y medium, we performed consumption-excretion (Con-Ex) studies using FD&C Blue 1 as a food tracer66. Males and females both consumed decreased volumes of 30Y versus 2Y media as anticipated (Fig. 6A). Given that 30Y medium has 15-fold the yeast concentration of 2Y medium, the level of consumption represented in Fig. 6A results in 30Y-fed flies ingesting at least 2-fold the total nutrients as flies fed 2Y (Fig. 6B; males, left; females; right). Importantly, consumption of media from the feeder caps in Con-Ex experiments and the presence of FD&C Blue 1 in the media did not have discernable effects on yeast-induced resistance to ethanol sedation (Fig. 6C). These data show that increased yeast nutrient consumption accompanies the increase in resistance to ethanol sedation, suggesting that they are causally linked.
Figure 6. Control r[A] flies consume more nutrients from 30Y versus 2Y media.
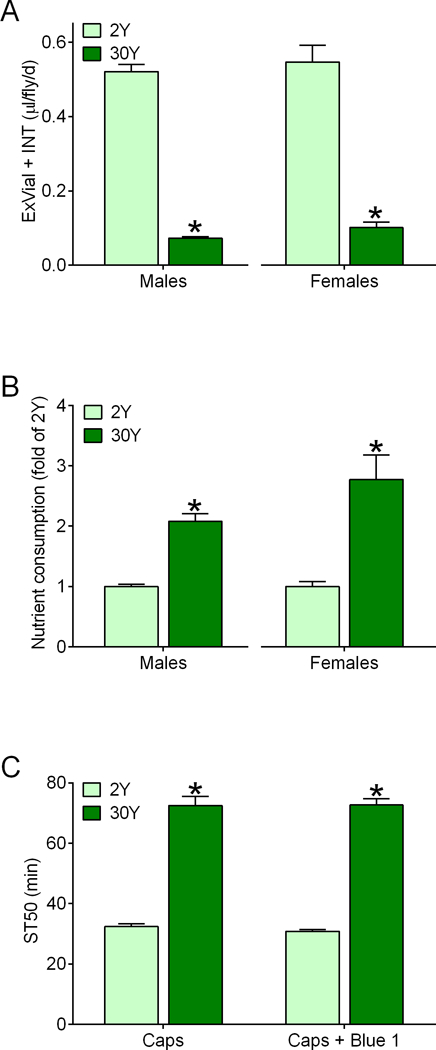
(A) Flies consumed-excreted lower volumes (ExVial+INT) of 30Y medium compared to 2Y medium during 24 h Con-Ex studies (*two-tailed t tests, p<0.0001; males, n=8; females, n=6). (B) Flies consumed more nutrients (relative to 2Y, calculated from panel A) from 30Y medium compared to 2Y (*two-tailed t tests; males, p<0.0001, n=8; females, p=0.0016; n=6). (C) Flies fed 30Y medium had increased ST50 values compared to flies fed 2Y medium when all media were provided in feeder caps (Caps) for 2 d. Including Blue 1 in the media had no effect on ST50 values (two-way ANOVA; yeast concentration, p<0.0001; Blue 1, p=0.7200; interaction, p=0.6652; *BMCs versus 2Y, p<0.0001; n=12).
Olfactory cues from yeast influence life span in flies88. To determine if olfactory cues from supplemental yeast are sufficient to elicit resistance to ethanol sedation, we assessed whether mesh barriers that prevented flies from directly contacting the yeast paste blocked the change in ST50 values. We used barriers with two different mesh sizes to test this possibility because (i) we reasoned that barriers of both sizes would eliminate the ability of flies to contact the food surface and (ii) the lager mesh size would be more porous to olfactory cues from the yeast paste. Compared to flies fed standard medium, flies that physically contacted supplemental yeast paste were resistant to ethanol sedation (Fig. 7A and 7B) as expected (Fig. 1). In contrast, ST50 values in flies that could not contact the supplemental yeast due to mesh barriers were indistinguishable from flies fed a standard diet only (Fig. 7A and 7B). The yeast-induced resistance to ethanol sedation therefore requires physical contact with, and presumably consumption of, the supplemental yeast to produce resistance to ethanol sedation.
Figure 7. Effect of dietary yeast paste on ST50 values requires physical contact.
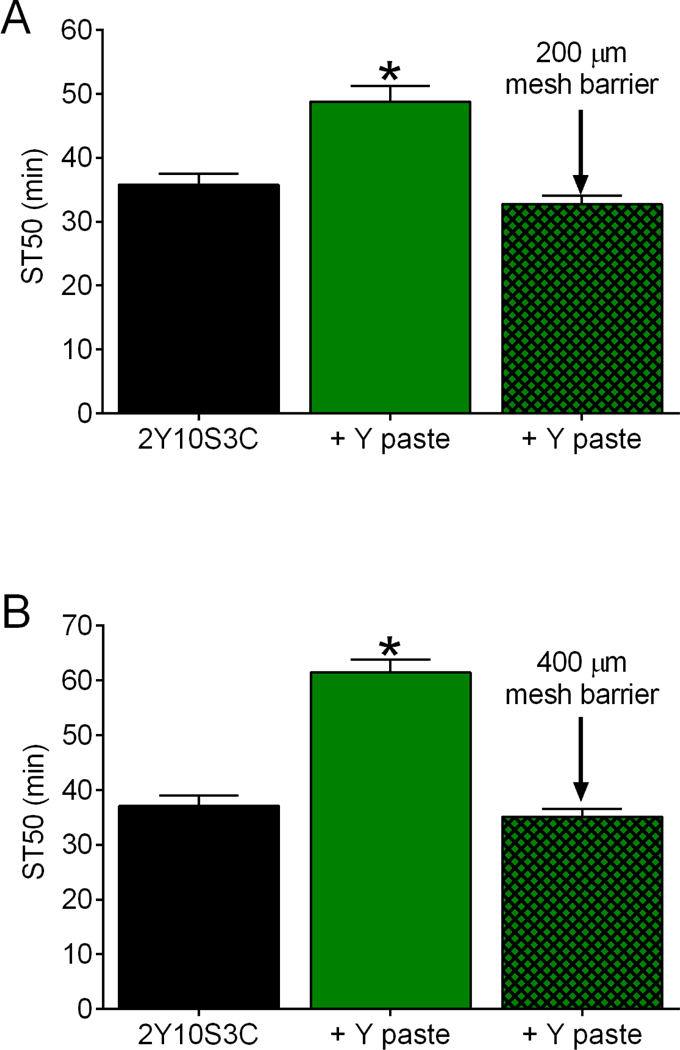
Flies had access to the indicated media for 2 d. (A, B) Compared to flies fed 2Y10S3C medium, ST50 values were increased in flies that had access to yeast paste (green bars), but not in flies that were prevented from physically contacting the yeast paste by a mesh barrier (green hatched bars). There was an overall effect of treatment group in w[A] (panel A) and r[A] (panel B) females (one-way ANOVAs, p<0.0001, n=8 in A and B). ST50s were greater in flies with access to yeast paste compared to the other groups (*BMC, p=0.0003 to <0.0001). ST50s were indistinguishable in flies fed 2Y10S3C and in flies prevented from physically contacting the yeast paste (BMC; panel A, p=0.8415; panel B, p>0.9999).
The mechanism by which dietary yeast influences ethanol sedation in flies is of obvious interest. Intriguingly, a high yeast diet increases brain 5-HT levels in flies [55, confirmed here (30Y: 439.8 ± 89.0 fmol/brain, n=11; 2Y: 231.7 ± 36.9 fmol/brain, n=14; t test, p=0.0282)]. Additionally, serotonergic neuron function is important for regulating food consumption in larval and adult flies56–58, the 5-HT2A receptor plays a role in preference for dietary protein consumption in flies55, and 5-HT has been implicated in fly ethanol sedation59. Furthermore, there is a large literature linking 5-HT to alcohol problems in humans (e.g. 60–64). These findings collectively suggested that there could be mechanistic connections between serotonergic neurons and the effect of dietary yeast on ethanol sedation. To address this possibility, we determined if suppression of serotonergic neurons influenced the effect of dietary yeast on ST50 or the consumption of high yeast medium.
Compared to 2Y medium, ST50 values were increased by 30Y diet in control flies with the Trh-Gal4.long3 or the Trh-Gal4.long2 driver alone, a UAS-Kir2.1 transgene alone, or a UAS-TeTxLC(E2) transgene alone (first four bars, Figs. 8A, 9A, S7A, S8A). These control flies also consumed more nutrients when fed 30Y medium (first four bars, Figs. 8B, 9B, S7B, S8B). Inhibition of serotonergic neurons by expression of UAS-Kir2.1 (which hyperpolarizes neurons69) via Trh-Gal4.long3 (Fig. 8A, hatched bars) or via Trh-Gal4.long2 (Fig. S7A, hatched bars) blocked the effect of 30Y medium on ST50 values. Similarly, Trh-Gal4-driven expression of tetanus toxin light chain (UAS-TeTxLC(E2), which inhibits vesicle release68) in serotonergic neurons blocked the effect of yeast supplementation on ST50 values (Figs. 9A, S8A, hatched bars). The effect of a high yeast diet on ethanol sedation therefore requires functional serotonergic neurons.
Figure 8. Inhibition of serotonergic neurons with Kir2.1 blunts the effect of a high yeast diet on ethanol sedation, nutrient consumption and internal ethanol levels.
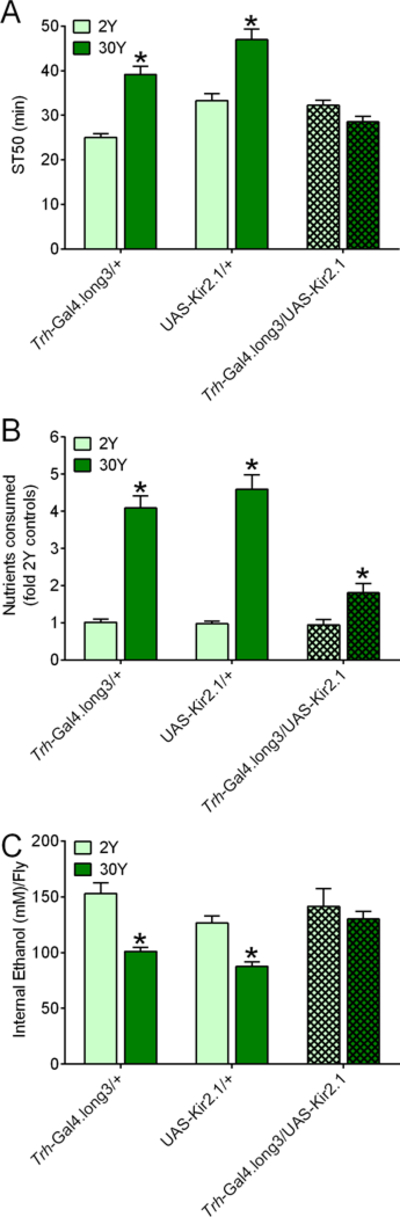
Male flies of the indicated genotypes consumed 2Y or 30Y media for 1 d prior to determination of ST50s, nutrient consumption, and internal ethanol. (A) There were overall effects of yeast concentration and genotype on ST50s, and an interaction between the two factors (two-way ANOVA; yeast, p<0.0001; genotype, p<0.0001; interaction, p<0.0001; n=8). Compared to flies fed 2Y medium, ST50s were greater in control flies (Trh-Gal4.long3/+ and UAS-Kir2.1/+) on 30Y (*BMC, p<0.0001), but not in flies with inhibition of serotonergic neurons (Trh-Gal4.long3/+; UAS-Kir2.1/+; hatched bars; BMC, p=0.3174). (B) Overall, yeast concentration and genotype influenced nutrient consumption and there was an interaction between yeast and genotype (two-way ANOVA; yeast, p<0.0001; genotype, p<0.0001; interaction, p<0.0001; n=8). All genotypes consumed more nutrients from 30Y than 2Y (*BMC, p≤<0.001). (C) Overall, the concentration of dietary yeast and genotype influenced internal ethanol levels after exposure to vapor from 85% ethanol for 36 minutes (two-way ANOVA; yeast, p<0.0001; genotype, p=0.0072; interaction, p=0.0733; n=8). Internal ethanol was decreased in control flies (Trh-Gal4.long3/+ and UAS-Kir2.1/+) fed 30Y versus 2Y media (*BMC, p≤0.0094), but yeast concentration had no effect on internal ethanol in Trh-Gal4.long3/+; UAS-Kir2.1/+ flies (hatched bars; BMC, p>0.9999).
Figure 9. Expression of tetanus toxin in serotonergic neurons dampens the effect of dietary yeast on ethanol sedation, nutrient intake and internal ethanol levels.
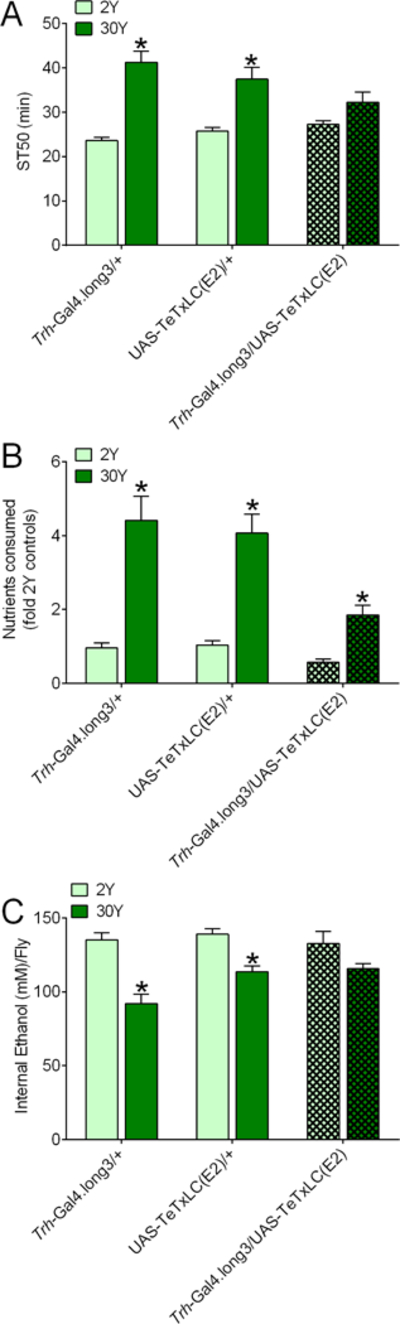
Male flies were fed 2Y or 30Y media for 1 d. (A) Overall, yeast concentration, but not genotype, influenced ST50s, and there was an interaction between yeast and genotype (two-way ANOVA; yeast, p<0.0001; genotype, p=0.3451; interaction, p=0.0058; n=8). Compared to flies fed 2Y medium, control Trh-Gal4.long3/+ and UAS-TeTxLC(E2)/+ flies fed 30Y had greater ST50s (*BMCs, p≤0.0002), but dietary yeast had no discernable effect on ST50s in flies expressing Tetanus Toxin Light Chain in serotonergic neurons (Trh-Gal4.long3/+; UAS-TeTxLC(E2)/+; hatched bars; BMC, p=0.1996). (B) Yeast and genotype had significant overall effects on nutrient consumption and there was an interaction between the factors (two-way ANOVA; yeast, p<0.0001; genotype, p<0.0001; interaction, p=0.0053; n=6–8). All genotypes consumed more nutrients on 30Y versus 2Y (*BMCs, p≤0.0257). (C) Overall, internal ethanol was affected by yeast concentration and genotype, but there was no interaction between the factors (two-way ANOVA; yeast, p<0.0001; genotype, p=0.0472; interaction, p=0.0524; n=8). Compared to flies fed 2Y, internal ethanol was decreased in control Trh-Gal4.long3/+ and UAS-TeTxLC(E2)/+ flies fed 30Y (*BMCs, p≤0.0045), but not in Trh-Gal4.long3/+; UAS-TeTxLC(E2)/+ flies (BMC, p=0.0807).
Regarding media consumption, flies expressing Kir2.1 via Trh-Gal4.long3 had greater intake of nutrients when fed 30Y vs 2Y media (Fig. 8B), but not when Kir2.1 was expressed by Trh-Gal4.long2 (Fig. S7B). Flies expressing tetanus toxin via both Trh-Gal4 drivers consumed significantly more nutrients from 30Y versus 2Y media (Figs. 9B, S8B). Thus, inhibition of serotonergic neurons did not consistently block the increase in nutrient intake on 30Y medium, but these flies appeared to consume fewer nutrients than control genotypes when on 30Y.
We postulated that a high yeast diet might impact net uptake/elimination of ethanol and, if true, that suppression of serotonergic neurons might influence internal ethanol levels in flies on a high yeast diet. We therefore measured internal ethanol in control flies and in flies expressing either UAS-Kir2.1 or UAS-TeTxLC(E2) in serotonergic neurons when fed 2Y or 30Y media. Internal ethanol concentrations during sedation from exogenous ethanol were decreased in control flies on 30Y vs 2Y media (Figs. 8C, 9C, first four bars), indicating that a high yeast diet influences ethanol uptake and/or elimination. Interestingly, the effect of 30Y diet on internal ethanol levels was blocked by inhibition of serotonergic neurons via expression of UAS-Kir2.1 (Fig. 8C, hatched bars) or UAS-TeTxLC(E2) (Fig. 9C, hatched bars).
The data in Figs. 8, 9, S7, and S8 raised the possibility that serotonergic neurons drive yeast consumption which in turn drives internal ethanol levels and ethanol sedation. To further explore this possibility, we determined whether there were correlations between nutrient consumption, internal ethanol levels, and ST50 values using data from Figs. 8, 9, S7, and S8. We found strong, significant correlations between all pairs of measures (Fig. 10). ST50 and nutrient intake exhibited a positive correlation (Fig. 10A), while ST50 and internal ethanol (Fig. 10B) as well as nutrient intake and internal ethanol (Fig. 10C) exhibited negative correlations. These results support a model in which a high yeast diet leads to serotonergic neuron-dependent increases in nutrient intake, and that increased nutrient intake subsequently alters the uptake or elimination of ethanol resulting in lower internal ethanol levels, ultimately leading to increased ST50 values (Fig. 11).
Figure 10. Correlations between ST50, nutrient intake, and internal ethanol levels.
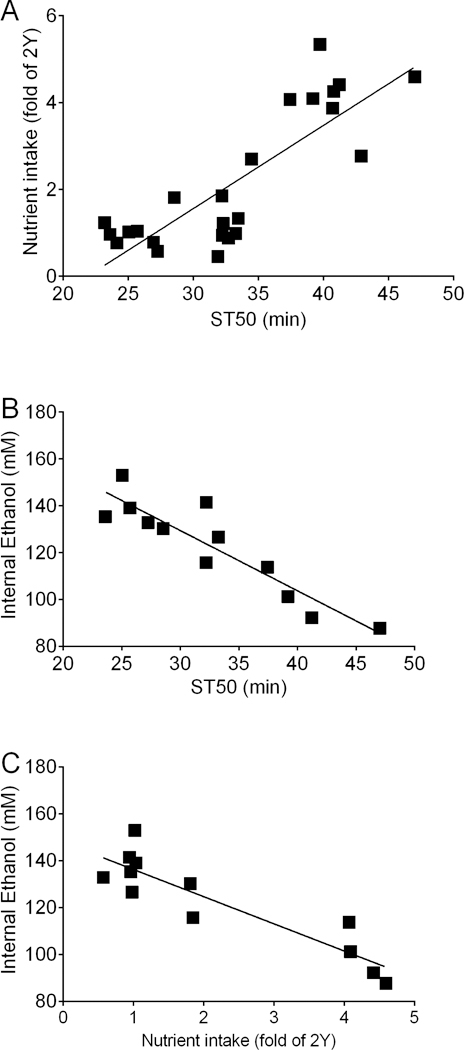
Data from figures 8, 9, S7, and S8 were combined to assess correlations between ST50, nutrient intake, and internal ethanol levels. (A) There was a positive correlation between ST50 and nutrient intake (Pearson r=0.827, p<0.0001, n=24). (B) ST50 values inversely correlated with internal ethanol levels (Pearson r=−0.913, p<0.0001, n=12). (C) Nutrient intake negatively correlated with internal ethanol levels (Pearson r=−0.903, p<0.0001, n=12). Lines are best fit linear regressions.
Figure 11. Model for effect of dietary yeast on ethanol sedation.
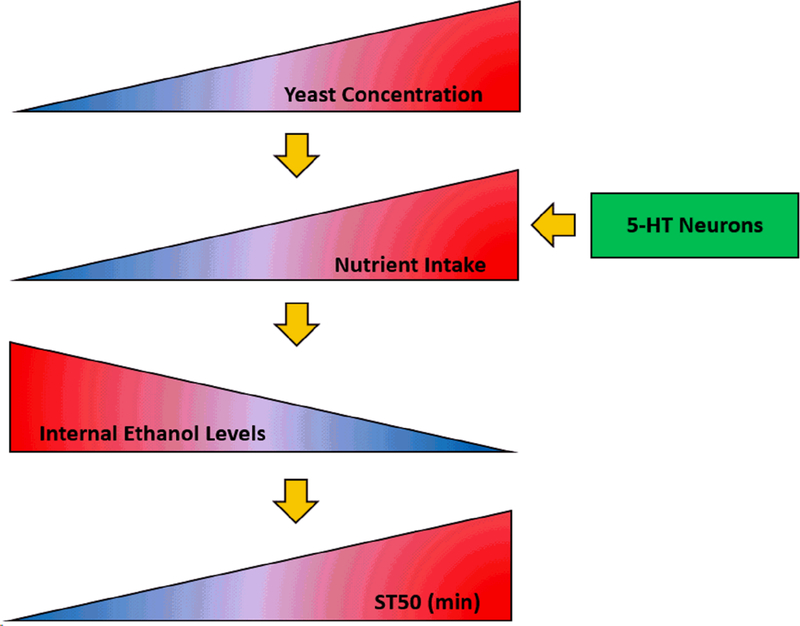
As the yeast concentration in the diet increases, nutrient intake increases, internal ethanol levels decrease, and the time to sedation (ST50) is extended. 5-HT neurons positively regulate nutrient intake and thereby influence the effect of dietary yeast on internal ethanol and ST50.
Discussion
Fruit flies are an important genetic model organism for investigating the molecular basis of a plethora of physiological outputs including alcohol-related behaviors11, 14–31, food consumption66, 81, and responses to diet67, 85, 89–97. To the best of our knowledge, our studies are the first to integrate these three areas of biology in the fly. We find that increasing the concentration of yeast in the diet, but not increasing other dietary components or decreasing all components of our standard medium, makes flies resistant to ethanol sedation. The resistance to ethanol sedation requires physical access to dietary yeast, is a common property of yeast, is seen in both males and females of multiple control strains, is reversible, appears to be caused by a mechanism independent of rapid tolerance, and is associated with increased yeast nutrient consumption as well as decreased internal ethanol levels. Importantly, the effect of a high yeast diet on ethanol sedation and internal ethanol levels is blunted by inhibition of serotonergic neurons.
In principle, our data on yeast supplementation and ethanol sedation could be interpreted in two ways. One interpretation is that yeast supplementation of a diet otherwise capable of supporting growth and normal behavior causes resistance to ethanol sedation. A second, alternative interpretation is that decreasing the concentration of dietary yeast below that required for normal growth and behavior leads to ethanol sedation sensitivity. We favor the former interpretation for several reasons. In previous studies, adult flies reared on our standard medium weigh approximately the same (e.g. ~1 mg for females19, 20, 22) as flies grown under routine conditions used in other labs (e.g. 98). In the studies reported here, flies reared on our standard 2Y10S3C and supplemented 30Y10S3C media emerged with similar time-courses and in the same numbers. These results suggest that flies grown on 2Y10S3C are not nutrient-deprived. Additionally, the increased resistance to alcohol sedation in our studies requires yeast concentrations in excess of 10%, which is higher than yeast concentrations used in routine fly culture. Our interpretation of these observations is that yeast supplementation of a diet otherwise sufficient in nutrients is capable of increasing resistance to ethanol sedation. It is extremely challenging, however, to formally rule out the possibility that flies fed our standard medium are not at least somewhat nutrient-deprived. Thus, it is a matter of perspective whether our data are interpreted to mean that yeast-supplementation increases resistance to ethanol sedation or that yeast-restriction decreases resistance to ethanol sedation. Importantly, either interpretation wholly supports the hypothesis that the concentration of yeast in the fly diet influences ethanol sedation.
Each Drosophila laboratory can and often does use a unique recipe for fly media. Differences in fly media composition could lead to variability in baseline ethanol sedation or potentially a lack of reproducibility of results across laboratories. We suggest that it become standard practice in the field to report all components and the concentrations used for fly media for studies on alcohol sedation as has been suggested previously for studies in other areas67.
The ability to manipulate ethanol sedation by changing the yeast concentration in the fly diet expands the utility of the Drosophila model for investigating genes and genetic pathways that underlie alcohol-related behaviors. With our data as a backdrop, the fly model should be suitable for pursuing at least three major areas of research: molecular and cellular mechanisms like serotonergic signaling that drive nutrient consumption as it relates to ethanol sedation, nutrient-driven changes in ethanol uptake and/or elimination, and pathways downstream of nutrient intake that change behavioral responses to alcohol. It is interesting to speculate that at least some genetic manipulations known to influence resistance to ethanol sedation in flies or other species might relate to one or more of these three areas.
Dietary yeast influences brain 5-HT content in flies55, 5-HT likely plays a role in fly ethanol sedation59, and 5-HT is connected to problematic alcohol consumption in humans (e.g. 60–64). Additionally, serotonergic neurons and serotonin signaling are important for hunger/satiety and feeding behavior in both larval and adults flies56–58, 99. Our studies in flies suggest that serotonergic neurons might influence ethanol sedation via effects on nutrient consumption and ethanol uptake/elimination, raising the possibility that there could be a link between 5-HT, diet, and alcohol-related behavior in other species.
The effect of diet on alcohol-related behavior is not unique to flies. In C. elegans, mutations that disrupt synthesis of eicosapentaenoic acid (EPA, an omega-3 polyunsaturated fatty acid) blunt the development of acute functional tolerance to alcohol and dietary supplementation with this fatty acid facilitates acute functional tolerance50. Reduced caloric intake in rats enhances the alcohol-deprivation effect and reinstatement of ethanol-seeking behavior46 and food deprivation decreases alcohol drinking in mice47. Furthermore, providing mice with different, but otherwise routinely used, laboratory diets influences ethanol drinking, ethanol consumption, and ethanol-induced locomotion48, and altering EPA in the diet of mice influences both ethanol sensitivity and consumption49. These results indicate that diet-induced changes in alcohol-related behavior are a common feature of metazoans. Therefore, identification of the underlying mechanisms via studies like those described here has the potential to be valuable for both prevention and treatment of AUD.
Supplementary Material
Supplementary Table 1. Effect of dietary yeast on fly weight. Data are total, dry, and water weight (mg/fly) for the indicated groups. P values are from two-tailed t testes comparing weight measures in 2Y and 30Y flies (significant differences are shaded).
Supplementary Table 2. Correlations between percent change in ST50s and weights. Male or female control fly strains (r[A], w[A], Lausanne, Oregon-R, and Samarkand) were exposed to 2Y or 30Ymedia for 1 d. Males have a significant correlation between the percent change in ST50 and the percent change in total weight (Pearson correlation, p=0.0420) and dry weight (Pearson correlation, p=0.0344), but not the percent change in water weight (Pearson correlation, p=0.2160). The percent change in total, dry, and water weights (Pearson correlation, p=0.3442, 0.9163, 0.2318, respectively) are not significantly correlated to the percent change of ST50s in females. Data is a combination of 6 individual, n=7–8/experiment, experiments to obtain the percent change in weight values for each males and females.
Supplementary Figure 1. Time to emergence of adult progeny on standard food medium. (A, B) Mated adult females were introduced into bottles containing 2Y10S3C or 30Y01S3C media and newly emerged adult flies were collected and counted daily. (A) Time course of emerging adult flies starting on day 9 and peaking on day 12. (B) Total number of adult flies eclosed from day 9 to day 15 (two-tailed t test; p=0.4607; n=4 bottles/media).
Supplementary Figure 2. Increasing sugar or cornmeal in dietary media does not substantially alter ST50 values. Flies were fed the indicated media for 1–3 days. Supplementation of dietary media with sugar (A and B) or cornmeal (C and D) did not robustly alter ST50s. Sugar supplementation influenced ST50s in r[A] males (panel A; two-way ANOVA; sugar, p<0.0001; diet exposure time, 0.5328; interaction, p=0.5471; *BMC versus 2Y10S3C, p=0.0047) and females (panel B; two-way ANOVA; sugar, p=0.0103; diet exposure time, p=0.3757; interaction, p=0.2862). Overall, there was a significant effect of cornmeal supplementation on ST50s in males (panel C; two-way ANOVA; cornmeal, p=0.0418; diet exposure time, p=0.0354; interaction, p=0.4242), but not in females (panel D; two-way ANOVA; cornmeal, p=0.0670; diet exposure time, p=0.2063; interaction, p=0.0833). N=6 in all panels.
Supplementary Figure 3. Dietary antimicrobials do not alter ST50 values. Flies were fed the indicated media for 2 d. (A) ST50s were indistinguishable in r[A] males and females fed media with (+ATC) or without (− ATC) ampicillin, tetracycline and chloramphenicol (two-way ANOVA; ATC, p=0.2452; sex, p=0.9481; interaction, p=0.6529; n=8). (B) Dietary media with (+TEG) or without (−TEG) Tegosept had no effect on ST50s in r[A] males and females (two-way ANOVA; TEG, p=0.1523; sex, p=0.4214; interaction, p=0.6527; n=8).
Supplementary Figure 4. Removal or dilution of media nutrients does not impact ST50 values. Flies were fed the indicated media for 2 d. (A) Omitting yeast (0Y), sugar (0S), or cornmeal (0C) from dietary media did not alter ST50s (one-way ANOVA, p=0.1989, n=6). (B) Removing 2 nutrient components from dietary media did not alter ST50s (one-way ANOVA, p=0.3001, n=6). (C) Dilution of 2Y10S3C medium (0.5X, 0.25X) and removal of yeast, sugar and cornmeal from the medium (0X) did not influence ST50s (one-way ANOVA, p=0.3364; n=8).
Supplementary Figure 5. Yeast supplementation does not impact rapid tolerance to ethanol. Rapid tolerance was not significantly different in r[A] males (A) or females (B) fed 2Y or 30Y media for 2 d (individual two-tailed t tests; males, p=0.9773, n=8; females, p=0.0970; n=8). The ST50s during the second ethanol exposure were greater than during the first exposure (paired two-tailed t tests; 2Y males, p=0.0218; 30Y males, p=0.0059; 2Y females, p<0.0001; 30Y females, p=0.0003).
Supplementary Figure 6. Dietary yeast impacts ethanol sedation in flies from several different genetic backgrounds. Compared to flies fed 2Y medium, ST50s were increased in male and female w[A] (A), LS (B), OR (C) and Sam (D) after 1 d of feeding on 30Y medium (individual two-way ANOVAs; w[A]—yeast concentration, p<0.0001; sex, p=0.0012; interaction, p=0.7528; LS— yeast concentration, p=0.0002; sex, p<0.1779; interaction, p=0.7468; OR— yeast concentration, p<0.0001; sex, p=0.9658; interaction, p=0.8976; Sam— yeast concentration, p<0.0001; sex, p=0.7948; interaction, p=0.4659; *BMC versus 2Y, p=0.0188 to 0.0003; n=8 for all groups in all panels).
Supplementary Figure 7. Inhibition of serotonergic neurons with Kir2.1 blunts the effect of a high yeast diet on ethanol sedation and nutrient consumption: replication with a second Trh-Gal4 driver. Male flies of the indicated genotypes consumed 2Y or 30Y media for 1 d prior to determination of ST50s and nutrient consumption. (A) Overall, yeast concentration, but not genotype, impacted ST50s and there was an interaction between the two factors (two-way ANOVA; yeast, p<0.0001; genotype, p=0.0724; interaction, p<0.0001; n=8). ST50s were greater in control flies (Trh-Gal4.long2/+ and UAS-Kir2.1/+) on 30Y versus 2Y media (*BMC, p≤0.0005), but yeast concentration did not alter ST50s in flies with inhibition of serotonergic neuron function (Trh-Gal4. long2/+; UAS-Kir2.1/+; hatched bars; BMC, p>0.9999). (B) Overall, yeast concentration and genotype influenced nutrient consumption and there was an interaction between yeast and genotype (two-way ANOVA; yeast, p<0.0001; genotype, p<0.0001; interaction, p<0.0001; n=8). Control (Trh-Gal4. long2/+ and UAS-Kir2.1/+) flies consumed more nutrients from 30Y than 2Y (*BMC p<0.0001), but nutrient consumption from 2Y and 30Y was indistinguishable in Trh-Gal4. long2/+; UAS-Kir2.1/+ flies (hatched bars; BMC, p=0.3767).
Supplementary Figure 8. Expression of tetanus toxin in serotonergic neurons dampens the effect of dietary yeast on ethanol sedation and nutrient intake: replication with a second Trh-Gal4 driver. Male flies of the indicated genotypes consumed 2Y or 30Y media for 1 d prior to determination of ST50s and nutrient consumption. (A) Overall, yeast concentration, but not genotype, impacted ST50s and there was an interaction between the two factors (two-way ANOVA; diet, p<0.0001; genotype, p=0.3555; interaction, p<0.0001; n=8). ST50s were greater in control flies (Trh-Gal4.long2/+ and UAS-TeTxLC(E2)/+) on 30Y versus 2Y media (*BMC, p<0.0001), but yeast concentration did not alter ST50s in flies with inhibition of serotonergic neuron function (Trh-Gal4. long2/+; UAS-TeTxLC(E2)/+; hatched bars; BMC, p=0.3990). (B) There were main effects of yeast concentration and genotype on nutrient consumption, but there was not an interaction between yeast and genotype (two-way ANOVA; yeast, p<0.0001; genotype, p=0.0004; interaction, p=0.0621; n=8). All genotypes consumed more nutrients on 30Y versus 2Y media (*BMC; p≤0.0006).
Acknowledgements
The authors thank the Bloomington Drosophila Stock Center (NIH P40 0018537) for stocks used in this study and Ron Davis (Scripts, Florida) for fly stocks, Ian Hines for technical assistance, Robin Chan for generating the r[A] strain, Kristen M. Lee and investigators in the VCU Alcohol Research Center (Kenneth Kendler, Michael Miles, Jill Bettinger, Andrew Davies, Laura Mathies, and Jennifer Wolstenholme) for helpful discussions and/or critical reading of the manuscript. The studies were supported by grants from the US National Institutes of Health, National Institute of Alcohol Abuse and Alcoholism (AA022357, AA020634) and the VCU Presidential Research Quest Fund to M.G., the US National Institutes of Health, National Institute on Drug Abuse (DA042181) to K.L.S., and the US National Institutes of Health, National Institute on Aging (AG030593 and AG023166) and the Glenn Medical Foundation to S.D.P.
References
- 1.Quinn PD, Fromme K. Individual differences in subjective alcohol responses and alcohol-related disinhibition. Experimental and clinical psychopharmacology 2016;24(2):90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caneto F, Pautassi RM, Pilatti A. Ethanol-induced autonomic responses and risk taking increase in young adults with a positive family history of alcohol problems. Addictive behaviors 2018;76:174–81. [DOI] [PubMed] [Google Scholar]
- 3.Spanagel R Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiological reviews 2009;89(2):649–705. [DOI] [PubMed] [Google Scholar]
- 4.DHHS. 10th Special Report to the U.S. Congress on Alcohol and Health: Highlights from Current Research 2000. [Google Scholar]
- 5.WHO. Global status report on alcohol and health 2014 2014. [Google Scholar]
- 6.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological medicine 1997;27(6):1381–96. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Archives of general psychiatry 1997;54(2):178–84. [DOI] [PubMed] [Google Scholar]
- 8.True WR, Heath AC, Bucholz K, Slutske W, Romeis JC, Scherrer JF, et al. Models of treatment seeking for alcoholism: the role of genes and environment. Alcoholism, clinical and experimental research 1996;20(9):1577–81. [DOI] [PubMed] [Google Scholar]
- 9.Kalu N, Ramchandani VA, Marshall V, Scott D, Ferguson C, Cain G, et al. Heritability of Level of Response and Association with Recent Drinking History in Nonalcohol-Dependent Drinkers. Alcoholism, clinical and experimental research 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Human genetics 2012;131(6):959–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devineni AV, Heberlein U. The evolution of Drosophila melanogaster as a model for alcohol research. Annual review of neuroscience 2013;36:121–38. [DOI] [PubMed] [Google Scholar]
- 12.Rothenfluh A, Troutwine BR, Ghezzi A, Atkinson NS. The Genetics of Alcohol Responses of Invertebrate Model Systems. In: Noronha A, Cui C, Harris RA, Crabbe JC, editors. Neurobiology of Alcohol Dependence London: Academic Press; 2014. p. 467–95. [Google Scholar]
- 13.Grotewiel M, Bettinger JC. Drosophila and Caenorhabditis elegans as Discovery Platforms for Genes Involved in Human Alcohol Use Disorder. Alcoholism, clinical and experimental research 2015;39(8):1292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapfhamer D, King I, Zou ME, Lim JP, Heberlein U, Wolf FW. JNK pathway activation is controlled by Tao/TAOK3 to modulate ethanol sensitivity. PloS one 2012;7(12):e50594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci 2002;22(24):11035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adkins AE, Hack LM, Bigdeli TB, Williamson VS, McMichael GO, Mamdani M, et al. Genomewide Association Study of Alcohol Dependence Identifies Risk Loci Altering Ethanol-Response Behaviors in Model Organisms. Alcoholism, clinical and experimental research 2017;41(5):911–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandhu S, Kollah AP, Lewellyn L, Chan RF, Grotewiel M. An inexpensive, scalable behavioral assay for measuring ethanol sedation sensitivity and rapid tolerance in Drosophila. Journal of visualized experiments : JoVE 2015(98). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acevedo SF, Peru YCdPRL, Gonzalez DA, Rodan AR, Rothenfluh A S6 Kinase Reflects and Regulates Ethanol-Induced Sedation. J Neurosci 2015;35(46):15396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan RF, Lewellyn L, DeLoyht JM, Sennett K, Coffman S, Hewitt M, et al. Contrasting Influences of Drosophila white/mini-white on Ethanol Sensitivity in Two Different Behavioral Assays. Alcoholism, clinical and experimental research 2014;38(6):1582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandari P, Hill JS, Farris SP, Costin B, Martin I, Chan CL, et al. Chloride intracellular channels modulate acute ethanol behaviors in Drosophila, Caenorhabditis elegans and mice. Genes, brain, and behavior 2012;11(4):387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maples T, Rothenfluh A. A simple way to measure ethanol sensitivity in flies. Journal of visualized experiments : JoVE 2011(48):e2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhandari P, Kendler KS, Bettinger JC, Davies AG, Grotewiel M. An assay for evoked locomotor behavior in Drosophila reveals a role for integrins in ethanol sensitivity and rapid ethanol tolerance. Alcoholism, clinical and experimental research 2009;33(10):1794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghezzi A, Krishnan HR, Atkinson NS. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addiction biology 2012;19(3):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron 2000;28(1):261–71. [DOI] [PubMed] [Google Scholar]
- 25.Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcoholism, clinical and experimental research 2004;28(10):1469–80. [DOI] [PubMed] [Google Scholar]
- 26.Ghezzi A, Al-Hasan YM, Larios LE, Bohm RA, Atkinson NS. slo K(+) channel gene regulation mediates rapid drug tolerance. Proc Natl Acad Sci U S A 2004;101(49):17276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A 2007;104(20):8253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel GL, Marella S, Kaun KR, Wu J, Adhikari P, Kong EC, et al. Sir2/Sirt1 Links Acute Inebriation to Presynaptic Changes and the Development of Alcohol Tolerance, Preference, and Reward. J Neurosci 2016;36(19):5241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peru YCdPRL, Ojelade SA, Penninti PS, Dove RJ, Nye MJ, Acevedo SF, et al. Long-lasting, experience-dependent alcohol preference in Drosophila. Addiction biology 2014;19(3):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohl JB, Baldwin BA, Dinh BL, Rahman P, Smerek D, Prado FJ 3rd, et al. Ethanol Preference in Drosophila melanogaster is Driven by Its Caloric Value. Alcoholism, clinical and experimental research 2012;36(11):1903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogueta M, Cibik O, Eltrop R, Schneider A, Scholz H. The influence of Adh function on ethanol preference and tolerance in adult Drosophila melanogaster. Chemical senses 2010;35(9):813–22. [DOI] [PubMed] [Google Scholar]
- 32.Dzitoyeva S, Dimitrijevic N, Manev H. Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci U S A 2003;100(9):5485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology 2009;205(4):529–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettinger JC, Davies AG. The role of the BK channel in ethanol response behaviors: evidence from model organism and human studies. Frontiers in physiology 2014;5:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A 2005;102(6):2141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron 2004;42(5):731–43. [DOI] [PubMed] [Google Scholar]
- 37.Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, et al. Genomewide Association Study for Maximum Number of Alcoholic Drinks in European Americans and African Americans. Alcoholism, clinical and experimental research 2015;39(7):1137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Zhao H, Gelernter J. Further clarification of the contribution of the ADH1C gene to vulnerability of alcoholism and selected liver diseases. Human genetics 2012;131(8):1361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biological psychiatry 2011;70(6):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci U S A 2015;112(30):E4085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci U S A 2011;108(17):7119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nesic J, Duka T. Effects of stress and dietary tryptophan enhancement on craving for alcohol in binge and non-binge heavy drinkers. Behavioural pharmacology 2014;25(5–6):503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahti-Koski M, Pietinen P, Heliovaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982–1997 FINRISK Studies. The American journal of clinical nutrition 2002;75(5):809–17. [DOI] [PubMed] [Google Scholar]
- 44.Lichenstein SD, Jones BL, O’Brien JW, Zezza N, Stiffler S, Holmes B, et al. Familial risk for alcohol dependence and developmental changes in BMI: the moderating influence of addiction and obesity genes. Pharmacogenomics 2014;15(10):1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barry D, Petry NM. Associations between body mass index and substance use disorders differ by gender: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addictive behaviors 2009;34(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guccione L, Paolini AG, Penman J, Djouma E. The effects of calorie restriction on operant-responding for alcohol in the alcohol preferring (iP) rat. Behavioural brain research 2012;230(1):281–7. [DOI] [PubMed] [Google Scholar]
- 47.Anji A, Kumari M. Supplementing the liquid alcohol diet with chow enhances alcohol intake in C57 BL/6 mice. Drug and alcohol dependence 2008;97(1–2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, Thiele TE. Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcoholism, clinical and experimental research 2015;39(8):1406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolstenholme JT, Bowers MS, Pais AB, Pais AC, Poland RS, Poklis JL, et al. Dietary Omega-3 Fatty Acids Differentially Impact Acute Ethanol-Responsive Behaviors and Ethanol Consumption in DBA/2J Versus C57BL/6J Mice. Alcoholism, clinical and experimental research 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raabe RC, Mathies LD, Davies AG, Bettinger JC. The omega-3 fatty acid eicosapentaenoic acid is required for normal alcohol response behaviors in C. elegans. PloS one 2014;9(8):e105999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Liu X, Luo X, Zeng M, Zuo L, Wang KS. Genetic variants in the fat mass- and obesity-associated (FTO) gene are associated with alcohol dependence. Journal of molecular neuroscience : MN 2013;51(2):416–24. [DOI] [PubMed] [Google Scholar]
- 52.Wang KS, Zuo L, Pan Y, Xie C, Luo X. Genetic variants in the CPNE5 gene are associated with alcohol dependence and obesity in Caucasian populations. Journal of psychiatric research 2015;71:1–7. [DOI] [PubMed] [Google Scholar]
- 53.Mattoo SK, Chakraborty K, Basu D, Ghosh A, Vijaya Kumar KG, Kulhara P. Prevalence & correlates of metabolic syndrome in alcohol & opioid dependent inpatients. The Indian journal of medical research 2011;134:341–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Sekhon ML, Lamina O, Hogan KE, Kliethermes CL. Common genes regulate food and ethanol intake in Drosophila. Alcohol 2016;53:27–34. [DOI] [PubMed] [Google Scholar]
- 55.Ro J, Pak G, Malec PA, Lyu Y, Allison DB, Kennedy RT, et al. Serotonin signaling mediates protein valuation and aging. eLife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gasque G, Conway S, Huang J, Rao Y, Vosshall LB. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci Rep 2013;3:srep02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsao CH, Chen CC, Lin CH, Yang HY, Lin S. Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior. eLife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albin SD, Kaun KR, Knapp JM, Chung P, Heberlein U, Simpson JH. A Subset of Serotonergic Neurons Evokes Hunger in Adult Drosophila. Curr Biol 2015;25(18):2435–40. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Zhang Y, Shen P. Protein kinase C deficiency-induced alcohol insensitivity and underlying cellular targets in Drosophila. Neuroscience 2010;166(1):34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plemenitas A, Kastelic M, o Porcelli S, Serretti A, Dolzan V, Kores Plesnicar B. Alcohol Dependence and Genetic Variability in the Serotonin Pathway among Currently and Formerly Alcohol-Dependent Males. Neuropsychobiology 2015;72(1):57–64. [DOI] [PubMed] [Google Scholar]
- 61.Tikkanen R, Tiihonen J, Rautiainen MR, Paunio T, Bevilacqua L, Panarsky R, et al. Impulsive alcohol-related risk-behavior and emotional dysregulation among individuals with a serotonin 2B receptor stop codon. Translational psychiatry 2015;5:e681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cope LM, Munier EC, Trucco EM, Hardee JE, Burmeister M, Zucker RA, et al. Effects of the serotonin transporter gene, sensitivity of response to alcohol, and parental monitoring on risk for problem alcohol use. Alcohol 2017;59:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang FL, Chassin L. Negative Urgency Mediates the Relation between Genetically-Influenced Serotonin Functioning and Alcohol Problems. Clinical psychological science : a journal of the Association for Psychological Science 2018;6(1):106–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang FL, Chassin L, Bates JE, Dick D, Lansford JE, Pettit GS, et al. Serotonin functioning and adolescents’ alcohol use: A genetically informed study examining mechanisms of risk. Development and psychopathology 2018;30(1):213–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hales KG, Korey CA, Larracuente AM, Roberts DM. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 2015;201(3):815–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shell B, Schmitt R, Lee KM, Johnson JC, Pletcher S, Grotewiel M. Measurement of solid food intake in Drosohpila via consumption-excretion of a dye tracer. submitted 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piper MD, Blanc E, Leitao-Goncalves R, Yang M, He X, Linford NJ, et al. A holidic medium for Drosophila melanogaster. Nature methods 2014;11(1):100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 1995;14(2):341–51. [DOI] [PubMed] [Google Scholar]
- 69.Alekseyenko OV, Lee C, Kravitz EA. Targeted Manipulation of Serotonergic Neurotransmission Affects the Escalation of Aggression in Adult Male Drosophila melanogaster. PloS one 2010;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci 2001;21(5):1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang H, Vickrey TL, Venton BJ. Analysis of biogenic amines in a single Drosophila larva brain by capillary electrophoresis with fast-scan cyclic voltammetry detection. Analytical chemistry 2011;83(6):2258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denno ME, Privman E, Borman RP, Wolin DC, Venton BJ. Quantification of Histamine and Carcinine in Drosophila melanogaster Tissues. ACS chemical neuroscience 2016;7(3):407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denno ME, Privman E, Venton BJ. Analysis of neurotransmitter tissue content of Drosophila melanogaster in different life stages. ACS chemical neuroscience 2015;6(1):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poirier L, Shane A, Zheng J, Seroude L. Characterization of the Drosophila gene-switch system in aging studies: a cautionary tale. Aging cell 2008;7(5):758–70. [DOI] [PubMed] [Google Scholar]
- 75.Shaposhnikov M, Proshkina E, Shilova L, Zhavoronkov A, Moskalev A. Lifespan and Stress Resistance in Drosophila with Overexpressed DNA Repair Genes. Sci Rep 2015;5:15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 2004;429(6991):562–6. [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Alvarez A, Diaz-Perez AL, Sosa-Aguirre C, Macias-Rodriguez L, Campos-Garcia J. Ethanol yield and volatile compound content in fermentation of agave must by Kluyveromyces marxianus UMPe-1 comparing with Saccharomyces cerevisiae baker’s yeast used in tequila production. Journal of bioscience and bioengineering 2012;113(5):614–8. [DOI] [PubMed] [Google Scholar]
- 78.Shiroma S, Jayakody LN, Horie K, Okamoto K, Kitagaki H. Enhancement of ethanol fermentation in Saccharomyces cerevisiae sake yeast by disrupting mitophagy function. Applied and environmental microbiology 2014;80(3):1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snoek T, Verstrepen KJ, Voordeckers K. How do yeast cells become tolerant to high ethanol concentrations? Current genetics 2016;62(3):475–80. [DOI] [PubMed] [Google Scholar]
- 80.Aldrete-Tapia JA, Miranda-Castilleja DE, Arvizu-Medrano SM, Hernandez-Iturriaga M. Selection of Yeast Strains for Tequila Fermentation Based on Growth Dynamics in Combined Fructose and Ethanol Media. Journal of food science 2018;83(2):419–23. [DOI] [PubMed] [Google Scholar]
- 81.Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, et al. Quantifying Drosophila food intake: comparative analysis of current methodology. Nature methods 2014;11(5):535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Devineni AV, Heberlein U. Acute ethanol responses in Drosophila are sexually dimorphic. Proc Natl Acad Sci U S A 2012;109(51):21087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Experimental gerontology 2008;43(8):739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Experimental gerontology 2005;40(5):386–95. [DOI] [PubMed] [Google Scholar]
- 85.Rovenko BM, Kubrak OI, Gospodaryov DV, Perkhulyn NV, Yurkevych IS, Sanz A, et al. High sucrose consumption promotes obesity whereas its low consumption induces oxidative stress in Drosophila melanogaster. Journal of insect physiology 2015;79:42–54. [DOI] [PubMed] [Google Scholar]
- 86.Rovenko BM, Perkhulyn NV, Gospodaryov DV, Sanz A, Lushchak OV, Lushchak VI. High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comp Biochem Physiol A Mol Integr Physiol 2015;180:75–85. [DOI] [PubMed] [Google Scholar]
- 87.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nature methods 2005;2(11):813–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science 2007;315(5815):1133–7. [DOI] [PubMed] [Google Scholar]
- 89.Regalado JM, Cortez MB, Grubbs J, Link JA, van der Linden A, Zhang Y. Increased food intake after starvation enhances sleep in Drosophila melanogaster. Journal of genetics and genomics = Yi chuan xue bao 2017;44(6):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trindade de Paula M, Poetini Silva MR, Machado Araujo S, Cardoso Bortolotto V, Barreto Meichtry L, Zemolin AP, et al. High-Fat Diet Induces Oxidative Stress and MPK2 and HSP83 Gene Expression in Drosophila melanogaster. Oxidative medicine and cellular longevity 2016;2016:4018157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schriner SE, Coskun V, Hogan SP, Nguyen CT, Lopez TE, Jafari M. Extension of Drosophila Lifespan by Rhodiola rosea Depends on Dietary Carbohydrate and Caloric Content in a Simplified Diet. Journal of medicinal food 2016;19(3):318–23. [DOI] [PubMed] [Google Scholar]
- 92.Reis T Effects of Synthetic Diets Enriched in Specific Nutrients on Drosophila Development, Body Fat, and Lifespan. PloS one 2016;11(1):e0146758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navrotskaya V, Oxenkrug G, Vorobyova L, Summergrad P. Attenuation of high sucrose diet-induced insulin resistance in ABC transporter deficient white mutant of Drosophila melanogaster. Integrative obesity and diabetes 2016;2(2):187–90. [PMC free article] [PubMed] [Google Scholar]
- 94.Dew-Budd K, Jarnigan J, Reed LK. Genetic and Sex-Specific Transgenerational Effects of a High Fat Diet in Drosophila melanogaster. PloS one 2016;11(8):e0160857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laye MJ, Tran V, Jones DP, Kapahi P, Promislow DE. The effects of age and dietary restriction on the tissue-specific metabolome of Drosophila. Aging cell 2015;14(5):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holmbeck MA, Rand DM. Dietary Fatty Acids and Temperature Modulate Mitochondrial Function and Longevity in Drosophila. The journals of gerontology Series A, Biological sciences and medical sciences 2015;70(11):1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell metabolism 2010;12(5):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris SN, Coogan C, Chamseddin K, Fernandez-Kim SO, Kolli S, Keller JN, et al. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochimica et biophysica acta 2012;1822(8):1230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoofs A, Huckesfeld S, Pankratz MJ. Serotonergic network in the subesophageal zone modulates the motor pattern for food intake in Drosophila. Journal of insect physiology 2018;106(Pt 1):36–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Effect of dietary yeast on fly weight. Data are total, dry, and water weight (mg/fly) for the indicated groups. P values are from two-tailed t testes comparing weight measures in 2Y and 30Y flies (significant differences are shaded).
Supplementary Table 2. Correlations between percent change in ST50s and weights. Male or female control fly strains (r[A], w[A], Lausanne, Oregon-R, and Samarkand) were exposed to 2Y or 30Ymedia for 1 d. Males have a significant correlation between the percent change in ST50 and the percent change in total weight (Pearson correlation, p=0.0420) and dry weight (Pearson correlation, p=0.0344), but not the percent change in water weight (Pearson correlation, p=0.2160). The percent change in total, dry, and water weights (Pearson correlation, p=0.3442, 0.9163, 0.2318, respectively) are not significantly correlated to the percent change of ST50s in females. Data is a combination of 6 individual, n=7–8/experiment, experiments to obtain the percent change in weight values for each males and females.
Supplementary Figure 1. Time to emergence of adult progeny on standard food medium. (A, B) Mated adult females were introduced into bottles containing 2Y10S3C or 30Y01S3C media and newly emerged adult flies were collected and counted daily. (A) Time course of emerging adult flies starting on day 9 and peaking on day 12. (B) Total number of adult flies eclosed from day 9 to day 15 (two-tailed t test; p=0.4607; n=4 bottles/media).
Supplementary Figure 2. Increasing sugar or cornmeal in dietary media does not substantially alter ST50 values. Flies were fed the indicated media for 1–3 days. Supplementation of dietary media with sugar (A and B) or cornmeal (C and D) did not robustly alter ST50s. Sugar supplementation influenced ST50s in r[A] males (panel A; two-way ANOVA; sugar, p<0.0001; diet exposure time, 0.5328; interaction, p=0.5471; *BMC versus 2Y10S3C, p=0.0047) and females (panel B; two-way ANOVA; sugar, p=0.0103; diet exposure time, p=0.3757; interaction, p=0.2862). Overall, there was a significant effect of cornmeal supplementation on ST50s in males (panel C; two-way ANOVA; cornmeal, p=0.0418; diet exposure time, p=0.0354; interaction, p=0.4242), but not in females (panel D; two-way ANOVA; cornmeal, p=0.0670; diet exposure time, p=0.2063; interaction, p=0.0833). N=6 in all panels.
Supplementary Figure 3. Dietary antimicrobials do not alter ST50 values. Flies were fed the indicated media for 2 d. (A) ST50s were indistinguishable in r[A] males and females fed media with (+ATC) or without (− ATC) ampicillin, tetracycline and chloramphenicol (two-way ANOVA; ATC, p=0.2452; sex, p=0.9481; interaction, p=0.6529; n=8). (B) Dietary media with (+TEG) or without (−TEG) Tegosept had no effect on ST50s in r[A] males and females (two-way ANOVA; TEG, p=0.1523; sex, p=0.4214; interaction, p=0.6527; n=8).
Supplementary Figure 4. Removal or dilution of media nutrients does not impact ST50 values. Flies were fed the indicated media for 2 d. (A) Omitting yeast (0Y), sugar (0S), or cornmeal (0C) from dietary media did not alter ST50s (one-way ANOVA, p=0.1989, n=6). (B) Removing 2 nutrient components from dietary media did not alter ST50s (one-way ANOVA, p=0.3001, n=6). (C) Dilution of 2Y10S3C medium (0.5X, 0.25X) and removal of yeast, sugar and cornmeal from the medium (0X) did not influence ST50s (one-way ANOVA, p=0.3364; n=8).
Supplementary Figure 5. Yeast supplementation does not impact rapid tolerance to ethanol. Rapid tolerance was not significantly different in r[A] males (A) or females (B) fed 2Y or 30Y media for 2 d (individual two-tailed t tests; males, p=0.9773, n=8; females, p=0.0970; n=8). The ST50s during the second ethanol exposure were greater than during the first exposure (paired two-tailed t tests; 2Y males, p=0.0218; 30Y males, p=0.0059; 2Y females, p<0.0001; 30Y females, p=0.0003).
Supplementary Figure 6. Dietary yeast impacts ethanol sedation in flies from several different genetic backgrounds. Compared to flies fed 2Y medium, ST50s were increased in male and female w[A] (A), LS (B), OR (C) and Sam (D) after 1 d of feeding on 30Y medium (individual two-way ANOVAs; w[A]—yeast concentration, p<0.0001; sex, p=0.0012; interaction, p=0.7528; LS— yeast concentration, p=0.0002; sex, p<0.1779; interaction, p=0.7468; OR— yeast concentration, p<0.0001; sex, p=0.9658; interaction, p=0.8976; Sam— yeast concentration, p<0.0001; sex, p=0.7948; interaction, p=0.4659; *BMC versus 2Y, p=0.0188 to 0.0003; n=8 for all groups in all panels).
Supplementary Figure 7. Inhibition of serotonergic neurons with Kir2.1 blunts the effect of a high yeast diet on ethanol sedation and nutrient consumption: replication with a second Trh-Gal4 driver. Male flies of the indicated genotypes consumed 2Y or 30Y media for 1 d prior to determination of ST50s and nutrient consumption. (A) Overall, yeast concentration, but not genotype, impacted ST50s and there was an interaction between the two factors (two-way ANOVA; yeast, p<0.0001; genotype, p=0.0724; interaction, p<0.0001; n=8). ST50s were greater in control flies (Trh-Gal4.long2/+ and UAS-Kir2.1/+) on 30Y versus 2Y media (*BMC, p≤0.0005), but yeast concentration did not alter ST50s in flies with inhibition of serotonergic neuron function (Trh-Gal4. long2/+; UAS-Kir2.1/+; hatched bars; BMC, p>0.9999). (B) Overall, yeast concentration and genotype influenced nutrient consumption and there was an interaction between yeast and genotype (two-way ANOVA; yeast, p<0.0001; genotype, p<0.0001; interaction, p<0.0001; n=8). Control (Trh-Gal4. long2/+ and UAS-Kir2.1/+) flies consumed more nutrients from 30Y than 2Y (*BMC p<0.0001), but nutrient consumption from 2Y and 30Y was indistinguishable in Trh-Gal4. long2/+; UAS-Kir2.1/+ flies (hatched bars; BMC, p=0.3767).
Supplementary Figure 8. Expression of tetanus toxin in serotonergic neurons dampens the effect of dietary yeast on ethanol sedation and nutrient intake: replication with a second Trh-Gal4 driver. Male flies of the indicated genotypes consumed 2Y or 30Y media for 1 d prior to determination of ST50s and nutrient consumption. (A) Overall, yeast concentration, but not genotype, impacted ST50s and there was an interaction between the two factors (two-way ANOVA; diet, p<0.0001; genotype, p=0.3555; interaction, p<0.0001; n=8). ST50s were greater in control flies (Trh-Gal4.long2/+ and UAS-TeTxLC(E2)/+) on 30Y versus 2Y media (*BMC, p<0.0001), but yeast concentration did not alter ST50s in flies with inhibition of serotonergic neuron function (Trh-Gal4. long2/+; UAS-TeTxLC(E2)/+; hatched bars; BMC, p=0.3990). (B) There were main effects of yeast concentration and genotype on nutrient consumption, but there was not an interaction between yeast and genotype (two-way ANOVA; yeast, p<0.0001; genotype, p=0.0004; interaction, p=0.0621; n=8). All genotypes consumed more nutrients on 30Y versus 2Y media (*BMC; p≤0.0006).


