Abstract
Rationale. Methamphetamine (METH) abuse is generally attributed to the d-isomer. Self-administration of l-METH has been examined only in rhesus monkeys with a history of cocaine self-administration or drug-naïve rats using high toxic doses. Objectives. In this study, the ability of l-METH and, for comparison, d-METH to engender self-administration in experimentally-naïve rats, as well as to decrease d-METH self-administration and food-maintained responding were examined. Methods. Male Sprague-Dawley rats were used in 3 separate experiments. In Experiment 1, the acquisition of l- or d-METH self-administration followed by dose-response determinations was studied. In Experiment 2, rats were trained to self-administer d-METH (0.05 mg/kg/infusion) and, then, various doses of l- or d-METH were given acutely prior to the session; the effect of repeated l-METH (30 mg/kg) also was examined. In Experiment 3, rats were trained to respond for food reinforcement and, then, various doses of l- or d-METH were given acutely prior to the session; the effect of repeated l-METH (3 mg/kg) also was examined. Results. Reliable acquisition of l- and d-METH self-administration was obtained at unit doses of 0.5 and 0.05 mg/kg/infusion respectively. The dose-response function for l-METH self-administration was flattened and shifted rightward compared to d-METH self-administration, with peak responding for l- and d-METH occurring at unit doses of 0.17 and 0.025 respectively. l-METH also was approximately 10-fold less potent than d-METH in decreasing d-METH self-administration and 2-fold lower in decreasing food-maintained responding. Tolerance did not occur to repeated l-METH pretreatments on either measure. Conclusions. As a potential pharmacotherapeutic, l-METH has less abuse liability than d-METH and its efficacy in decreasing d-METH self-administration and food-maintained responding is sustained with repeated treatment.
Keywords: l-Methamphetamine, d-Methamphetamine, Self-administration, Stimulant use disorders, Food reinforcement, Dose-response, Rat
Methamphetamine (METH) use disorder is primarily associated with the dextro (d) isomer, rather than the levo (l) isomer, likely due to the greater potency of d-METH to evoke dopamine release (Kuczenski et al. 1995; Rothman and Baumann 2003). Illicit distribution of METH occurs as both the racemate and the pure d-isomer, depending on the synthetic methods utilized. As for licit use, d-METH is prescribed for attention deficit hyperactivity disorder and narcolepsy, while l-METH is available in over-the-counter nasal decongestant inhalers. In METH users, both isomers produce a similar peak plasma concentration following i.v. administration, although the elimination half-life is longer for l-METH compared to d-METH; ~14 hr vs. ~10 hr (Mendelson et al. 2006). More important, that same study showed that while l-METH produced subjective drug “liking” using a visual analog rating scale, d-METH was “liked” more than l-METH at equivalent doses, and peak ratings for “arousal”, “positive mood” and “vigor” using the Profile of Mood States (POMS) were higher with d-METH compared to equivalent doses of l-METH.
There are currently no FDA-approved medications for the treatment of METH use disorder. Since METH abuse is associated primarily with the d isomer, the reduced abuse liability of the l isomer suggests that it may serve as a potential substitution therapy for METH use disorder. Consistent with this view, both l and d-METH function as substrates at the vesicular monoamine transporter (VMAT2), with l-METH being approximately 2-fold less potent than d-METH in inhibiting vesicular uptake (IC50 values of 19.3 and 9.1 μM, respectively) and being approximately 3-fold less potent than d-METH in evoking vesicular release (EC50 values of 34 and 11 μM, respectively) (Partilla et al. 2006). This is an important finding because VMAT2 is a target for medication development for METH use disorder (Zheng et al. 2006).
In addition to similar mechanisms of action at VMAT2, l- and d-METH can produce similar stimulus effects. For example, in drug discrimination studies, l and d-METH show cross-substitution in rats (Bondareva et al. 2002) and generalization in cocaine-trained rhesus monkeys (Kohut et al. 2016; Kohut et al. 2017b), with l-METH being 5 times less potent than d-METH. On the other hand, preclinical research also has shown that the behavioral profiles of the METH isomers can differ. For example, l-METH, but not d-METH, has antinociceptive activity in rats (Siemian et al. 2017).
Notwithstanding the potential value of l-METH as a therapeutic for stimulant use disorders, there is only limited information comparing the reinforcing effects of l- and d-METH in self-administration studies. In rhesus monkeys, l-METH substituted for cocaine and decreased cocaine self-administration (Kohut et al. 2016; Kohut et al. 2017a); however, it is unknown whether l-METH can engender reliable self-administration in drug naïve monkeys. In rats, an early study showed that while both l- and d- methamphetamine engendered self-administration responding, doses needed to maintain responding were higher for l-METH than for d-METH (Yokel and Pickens 1973). However, in that study, rats were first trained to lever press for food reinforcement before the introduction of l- or d-METH, and the i.v. doses d-METH (0.5–2.5 mg/kg/infusion) were 20- to 100-fold higher than those sufficient to maintain d-METH self-administration (Harrod et al. 2001). In addition, the extended access period (6 or 24 hr) of self-administration resulted in lethality for a majority of rats tested.
In the current study, we sought to determine the acquisition and dose effect relation for l- and d-METH, using low doses on a limited access schedule (60 min) in rats with no history of lever-press training. In addition, to examine the ability of l-METH to selectively attenuate the abuse-related reinforcing effects d-METH, we assessed the effect of l-METH pretreatment on d-METH self-administration and, separately, on food-maintained responding.
MATERIALS AND METHODS
Animals
A total of 38 adult male Sprague-Dawley rats (Envigo-Harlan, Indianapolis, IN, USA) weighing 250–350 g at the outset of Experiments 1, 2, and 3 were used (n=18, 12, and 8, respectively, each rat participated in only one experiment). Males were selected for this initial study to permit comparison to data from relevant previous studies that were conducted in males (Siemian et al. 2017; Yokel and Pickens 1973). In Experiment 1, rats were allowed free access to food and water in the home cage throughout the experiment, whereas in Experiments 2 and 3, food was restricted in the home cage to maintain a body weight of approximately 85% free feed weight; Experiments 2 and 3 involved lever-press training for food reinforcement as described below. Rats were housed individually in temperature- and humidity-controlled rooms that were maintained on a light-dark cycle (lights on at 07:00). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical Procedures
Rats underwent jugular catheter implantation surgery. Briefly, rats were anesthetized with a combination of ketamine (Butler Schein, Dublin, OH, USA), xylazine (Akorn, Inc., Decatur, IL, USA) and acepromazine (Boehringer Ingelheim, St. Joseph, MO, USA), which were mixed together to yield a single cocktail (75/7.5/0.75 mg/kg; 0.15ml/100g body weight; i.p.). A silastic catheter was inserted into the right jugular vein, threaded under the skin, and exited the body via an incision on the scalp. A cannula was connected to the end of the catheter and secured to the skull with dental acrylic and four jeweler’s screws.
Apparatus
All self-administration sessions were conducted in operant conditioning chambers (28 × 24 × 25 cm; ENV-001; MED Associates, St. Albans, VT, USA) housed in sound-attenuation chambers containing a fan to dampen extraneous noise (ENV-018M; MED Associates). Each chamber was equipped with two retractable levers with a white cue light located above each lever, a 45 mg recessed food tray between the levers for delivery of food pellets (ENV-203–45; MED Associates), a 28-V houselight centered on the opposite wall and a syringe pump for drug delivery (PHM-100; MED Associates). For the self-administration sessions, rats were connected to the syringe pump via tubing passed through a metal leash (C313CS; Plastics One) that was attached to a swivel (375/22PS; Instech) above the chamber.
Procedures
Experiment 1: Acquisition and dose-response for l- and d-METH self-administration.
In Experiment 1, seven days after catheter implant surgery, separate groups of rats began acquisition training for self-administration of l- or d-METH using a 7-day autoshaping procedure (Carroll and Lac 1993). On each of 7 consecutive days, rats underwent a 60-min autoshaping session, followed 60 min later by a 60-min contingent l- or d-METH self-administration session. On each autoshaping session, the house light was illuminated and an inactive lever (no programmed consequence, counterbalanced for position across rats) was extended continuously. For the first 15 min of each autoshaping session, an active lever was also extended 10 times at random intervals and remained extended for 10 sec. If the active lever was pressed, l- or d-METH (0.05 mg/kg/infusion, 5.9 sec duration, 0.1 ml volume) was delivered immediately; if the active lever was not pressed, the lever was retracted and a non-contingent infusion of l- or d-METH was delivered. For the remaining 45 min of the autoshaping session, only the inactive lever remained extended. On each contingent self-administration session, both levers were extended and an infusion of l- or d-METH (0.05 mg/kg/infusion, 5.9 sec duration, 0.1 ml volume) was administered following pressing on the active lever (same lever as during autoshaping session) under a continuous reinforcement schedule (fixed ratio 1; FR1). Each contingent drug infusion was signaled by illuminating both cue lights above the levers for 20 sec; responses during cue light illumination were recorded but not reinforced.
Following the 7-day autoshaping procedure, rats continued in daily 60-min response-contingent self-administration sessions to assess acquisition. An FR1 schedule was maintained for both groups (l- and d-METH) for 15 additional sessions (Sessions 8–22) and until evidence of drug-reinforced responding was obtained for each isomer. Across these sessions, the dose of l-METH increased incrementally as follows: Sessions 8–10 at 0.05 mg/kg/infusion; Sessions 11–13 at 0.17 mg/kg/infusion; Sessions 14–16 at 0.5 mg/kg/infusion; Sessions 17–22 at 1.7 mg/kg/infusion. Across the next 9 sessions (Sessions 23–31), with the unit dose at 1.7 mg/kg/infusion l-METH, the FR value was then increased to FR2 (Sessions 23–25), to FR3 (Sessions 26–28) and then to a terminal FR5 (Sessions 29–31). Rats in the d-METH group were treated identically, except the unit dose of d-METH was held at 0.05 mg/kg/infusion.
Beginning on Session 32, dose-response functions for self-administration under the FR5 schedule were determined in both l- and d-METH groups. Rats were allowed to self-administer different doses of l-METH (0, 0.05, 0.17, 0.5, 1, 1.7, 3, and 5 mg/kg/infusion) or d-METH (0, 0.001, 0.01, 0.025, 0.05, 0.1, 0.25 mg/kg/infusion) in pseudo-random order. Each dose was presented for 3 consecutive sessions; for each dose, mean values in the last 2 sessions were averaged across subjects to determine the dose-response curve.
Experiment 2: Effect of l- and d-METH on d-METH self-administration.
In Experiment 2, to facilitate acquisition of d-METH self-administration, rats were initially trained to lever-press for food reinforcement (45 mg; F0021 dustless precision pellet, Bio-Serve, Frenchtown, NJ). As described previously in our laboratory (Beckmann et al. 2012; Harrod et al. 2001), rats were trained briefly (12–15 sessions) to respond on one lever for food reinforcement (active lever) under an FR 5 schedule, while responding on the other lever had no consequence (inactive lever; counterbalanced). Rats were then implanted with a jugular catheter and allowed to self-administer d-METH (0.05 mg/kg/infusion) for 60 min daily using the same active lever that was used in food pretraining. Each infusion was followed by a 20-sec timeout signaled by illumination of both cue lights. The response requirement was gradually increased from FR1 to a terminal FR 5 schedule of reinforcement until responding stabilized. Stability was defined as 20% or less variability in the number of infusions earned across 3 successive sessions, at least a 2:1 ratio of active to inactive lever responses, and at least 10 infusions earned per session.
Once d-METH self-administration was stable, rats were tested across 3 separate experimental phases: (1) dose-effect for acute l-METH pretreatment; (2) effect of repeated l-METH pretreatment; and (3) dose-effect for acute d-METH pretreatment. In the first phase, rats were treated in a pseudo-random order with varying unit doses of l-METH (0, 0.3, 1, 3, 10 or 30 mg/kg, s.c., 15 min prior to the session). At least two maintenance sessions (i.e., no pretreatment) separated test sessions to ensure stable d-METH self-administration. In the second phase, rats were pretreated with l-METH (30 mg/kg, s.c., 15 min prior to session) on 7 consecutive sessions, followed by 6 consecutive sessions with saline pretreatment. In the third phase, rats were pretreated in a pseudo-random order with d-METH (0, 0.1, 0.3, 1, 3 mg/kg, s.c., 15 min prior to the session) with 2 maintenance sessions between each test session.
Experiment 3: Effect of l- and d-METH on food-maintained responding.
Experiment 3 was similar to Experiment 2, except that rats responded for food reinforcement throughout the experiment. After reaching stability under the FR5 schedule, rats were tested across 3 separate experimental phases: (1) dose-effect for acute l-METH pretreatment; (2) effect of repeated l-METH pretreatment; and (3) dose-effect for acute d-METH pretreatment. In the first phase, rats were treated in a pseudo-random order with varying unit doses of l-METH (0, 0.3, 1, 3, 10 or 30 mg/kg, s.c., 15 min prior to the session). At least two maintenance sessions (i.e., no pretreatment) separated test sessions to ensure stable food-maintained responding. In the second phase, rats were pretreated with l-METH (30 mg/kg, s.c., 15 min prior to session) on 7 consecutive sessions, followed by 6 consecutive sessions with saline pretreatment. In the third phase, rats were pretreated in a pseudo-random order with d-METH (0, 0.1, 0.3, 1, 3 mg/kg, s.c., 15 min prior to the session) with 2 maintenance sessions between each dose.
Data Analysis
Active and inactive lever presses during autoshaping were analyzed using a 2 (isomer) × 2 (lever) × 7 (session) mixed ANOVA. Responding during the period of incremental l-METH increase was broken into 3 session blocks, corresponding to each dose of l-METH, and analyzed with a 2 (isomer) × 4 (block / dose) × 2 (lever) × 3 (session within block) mi×ed ANOVA. Similarly, lever-press responding during the period of incremental FR increase was analyzed with a 2 (isomer) × 4 (FR) × 2 (lever) × 3 (session within block) mixed ANOVA; the number of infusions was also analyzed using a 2 (isomer) × 4 (FR) × 3 (session within block) mixed ANOVA. Dose-response data were analyzed using a trend analysis in Prism (v7.04, GraphPad Software, Inc.) to determine if the curves were linear, quadratic, or cubic.
The acute effects of pretreatment on responding for d-METH or food was analyzed using ID50 analysis in Prism; ID50 was defined as the pretreatment dose that produced 50% inhibition of the average number of baseline responses. The effects of repeated pretreatment were analyzed using a 2 (isomer) × 3 (treatment period) mixed ANOVA, with session within treatment period included as a continuous covariate. All ANOVAs were conducted using the GLIMMIX package in SAS (v9.4). Tukey’s HSD post hoc analyses were used to investigate significant interactions; p values of less than 0.05 were deemed significant.
Drugs
d-METH was obtained through the NIDA Drug Supply Program. l-METH was synthesized by the laboratory of Dr. Bruce Blough following the procedure described in Supplementary Material. The final product was > 95% pure and in > 95% enantiomeric excess with an optical rotation of [α] = −18.2 (c = 1.15 mg/mL H2O). Since purity was not 100%, this should be considered when comparing potency differences across experiments.
RESULTS
Experiment 1: Acquisition and dose-response for l- and d-METH self-administration.
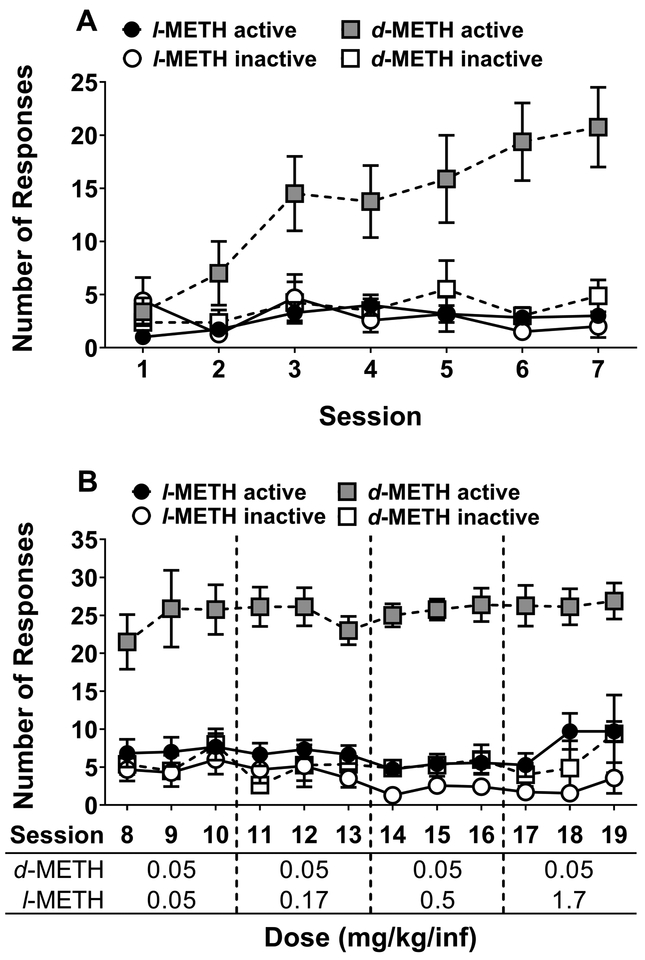
Figure 1 shows the acquisition of self-administration from Experiment 1. During initial acquisition using an autoshaping procedure with a unit dose of 0.05 mg/kg/infusion for both l- and d-METH (Fig 1A), analyses revealed isomer × lever (F1,13 = 17.4, p < 0.001) and isomer × session (F6,76 = 2.83, p < 0.05) interactions. Tukey’s post-hoc analyses revealed that rats receiving d-METH responded more on the active lever than inactive lever beginning on session 3. In contrast, rats receiving l-METH failed to show a significant difference in responding between the active and inactive levers on any session.
Figure 1. Acquisition of l- and d-METH self-administration on FR1 schedule in Experiment 1.
Panel A: Mean (±SEM) number of responses on active and inactive levers for l- and d-METH infusions (0.05 mg/kg/infusion) across the 7 operant sessions during the autoshaping phase. Panel B: Mean (±SEM) number of responses on active and inactive levers for l- and d-METH infusions using incrementing doses of l-METH (0.05, 0.17, 0.5 and 1.7 mg/kg/infusion) across sessions and a constant dose of d-METH (0.05 mg/kg/infusion). For the l-METH group (n=7), responses were significantly higher on the active lever compared to inactive only on sessions 14–16 (0.5 mg/kg/infusion) and 17–19 (1.7 mg/kg/infusion), each p<0.05. For the d-METH group (n=8), responses were significantly higher on the active lever compared to the inactive lever across sessions 3–19, each p<0.05.
Since the initial training dose of l-METH (0.05 mg/kg/infusion) failed to engender reliable self-administration, the dose of l-METH was progressively increased from 0.05 to 1.7 mg/kg/infusion in the next phase of acquisition; the dose of d-METH was held at 0.05 mg/kg/infusion in this phase (Fig 1B). Analysis of responding across the 3-session blocks revealed an isomer × lever interaction (F1,13 = 25.3, p < 0.001). In this overall ANOVA, there was no main effect of block and no interactions with block, indicating that the increasing doses of l-METH did not lead to greater responding. However, exploratory analysis of the isomer × lever interaction using a Tukey-Kramer test adjusted for multiple comparisons revealed that rats receiving l-METH showed greater responding on the active lever than the inactive lever on the last two blocks, i.e., unit doses of 0.5 and 1.7 mg/kg/infusion (t13 ≥ 2.42, p < 0.05 in both cases).
Figure 2 shows effect of incrementing the FR schedule up to the terminal FR5 using unit doses of either 1.7 mg/kg/infusion for l-METH or 0.05 mg/kg/infusion for d-METH. When plotted as number of lever presses (Fig 2A), analysis across the 3-session FR blocks revealed an isomer × lever × FR (F3,39 = 13.6, p < 0.001) interaction. Post-hoc analysis of the 3-way interaction revealed that active lever presses increased above FR1 levels, starting at FR3 for l-METH and starting at FR2 for d-METH. In addition, whereas responding on the active lever was greater than responding on the inactive lever for all FR requirements with d-METH, this difference did not reach significance until FR2 with l-METH. Finally, responding on the active lever was lower for l-METH than d-METH across all FR requirements.
Figure 2. Maintenance of l- and d-METH self-administration across incrementing FR requirements in Experiment 1.
Panel A: Mean (±SEM) number of responses on active and inactive levers for l- and d-METH infusions (1.7 and 0.05 mg/kg/infusion, respectively) across the incrementing FR requirements (FR1-FR5). Active responses were higher than inactive responses at FR2-FR5 for the l-METH group (n=7) and across all FR requirements for the d-METH group (n=8), each p<0.05. Panel B: Mean (±SEM) number of infusions earned for l- and d-METH infusions (1.7 and 0.05 mg/kg/infusion, respectively) across the incrementing FR requirements (FR1-FR5). Infusions earned were lower in the l-METH group (n=7) than the d-METH group (n=8) across all FR requirements, each p<0.05.
When the data from the incremental FR requirement phase were plotted as number of infusions earned (Fig 2B), there were main effects of isomer (F1,13 = 85.7, p < 0.001) and FR requirement (F3,39 = 4.06, p < 0.05). Tukey’s post-hoc analysis revealed that, overall, rats earned fewer infusions of l-METH than d-METH and that the number of infusions earned with each isomer was lower at FR5 than at FR1.
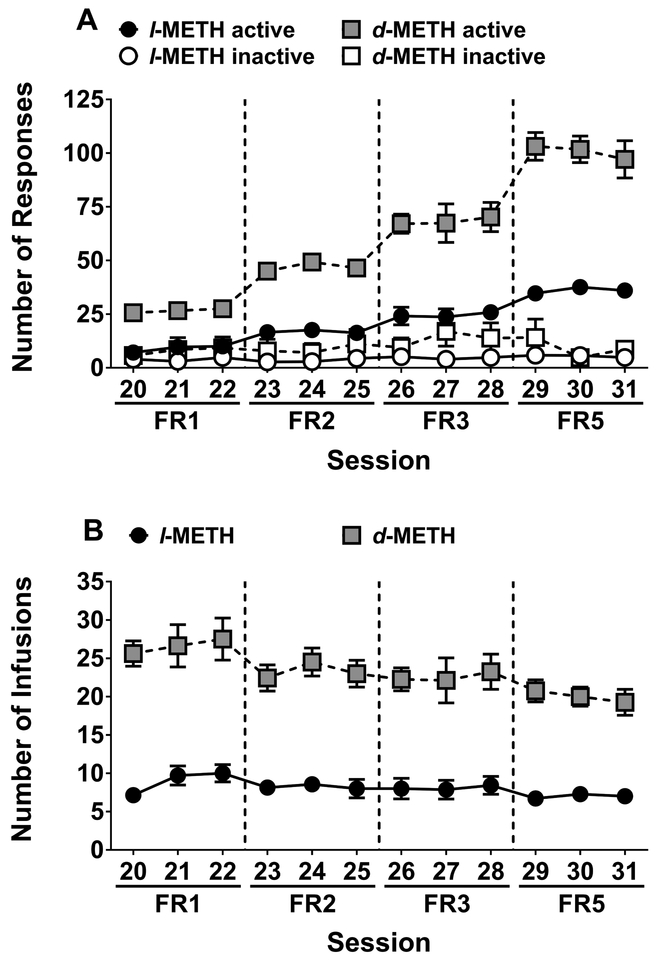
Figure 3 shows the dose-response curves for l- and d-METH, each of which reveals an inverted U-shaped function (Fig 3A). This was supported by trend analyses revealing that both l-METH (F1,46 = 28.4, p < 0.001) and d-METH (F1,45 = 96.8, p < 0.001) fit a quadratic model. Compared to d-METH, the peak of the dose-response curve for l-METH was flattened and shifted to the right, with the peak for l- and d-METH being obtained at unit doses of 0.17 and 0.025 mg/kg/infusion respectively.
Figure 3. Dose-response functions for l- and d-METH self-administration in Experiment 1.
Mean (±SEM) number of infusions earned for l- and d-METH across varying unit doses self-administered on an FR5 schedule; l-METH group n=7 and d-METH group n=8.
Experiment 2: Effect of l- and d-METH on d-METH self-administration.
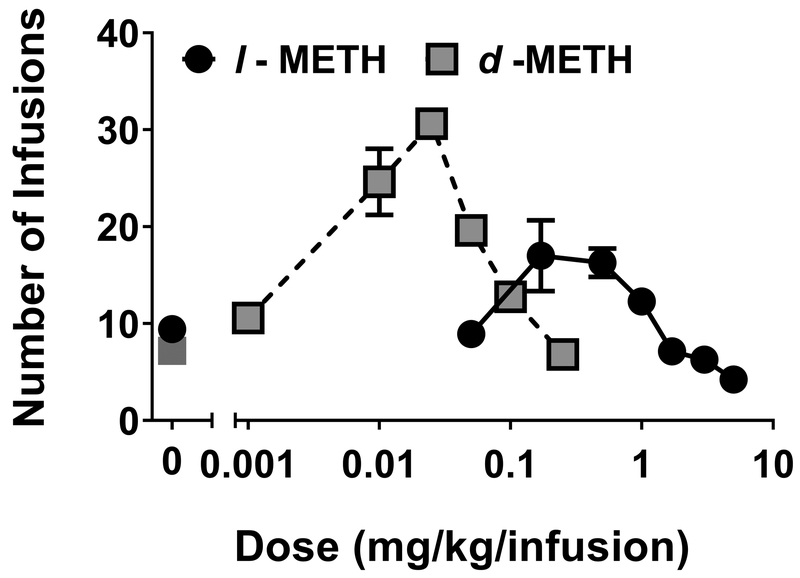
Figure 4 shows the effects of l- and d-METH pretreatment on self-administration of d-METH (0.05 mg/kg/infusion). Acute pretreatment with either l- or d-METH dose-dependently decreased self-administration of d-METH (Fig 4A), with l-METH being approximately 10-fold less potent than d-METH. The ID50 for was 6.52 mg/kg for l-METH and was 0.64 mg/kg for d-METH; this difference was statistically significant (F1,67 = 16.1, p < 0.001).
Figure 4. Effect of l- and d-METH on d-METH self-administration in Experiment 2.
Panel A: Mean (±SEM) number of d-METH infusions (0.05 mg/kg/infusion) earned on an FR5 schedule following varying doses of either l- or d-METH; n=10 for l-METH dose-response curve and n=7 for d-METH dose-response curve. Panel B: Mean (±SEM) number of d-METH infusions (0.05 mg/kg/infusion) earned on an FR5 schedule across repeated pretreatments. All rats (n=8) were first tested at maintenance baseline (BL) and following an injection of saline (SAL), and then were split into groups (n=4 per group) receiving 7 pretreatments with either l-METH (30 mg/kg) or SAL, followed by 6 more sessions in which all pretreatments were SAL. The number of d-METH infusions earned was lower in the l-METH pretreatment group compared to the SAL pretreatment group across all 7 pretreatment sessions, each p<0.05.
Repeated pretreatment with 30 mg/kg l-METH consistently reduced self-administration of d-METH (0.05 mg/kg/infusion; Fig 4B). Analysis of these data revealed an isomer × block interaction (F2,12 = 15.9, p < 0.001). Post-hoc analysis of the interaction confirmed the reduced responding for d-METH during blocks in which rats were pretreated with l-METH compared to rats pretreated with saline. There were no significant differences between groups during either the baseline/saline or the post-treatment blocks.
Experiment 3: Effect of l- and d-METH on food-maintained responding.
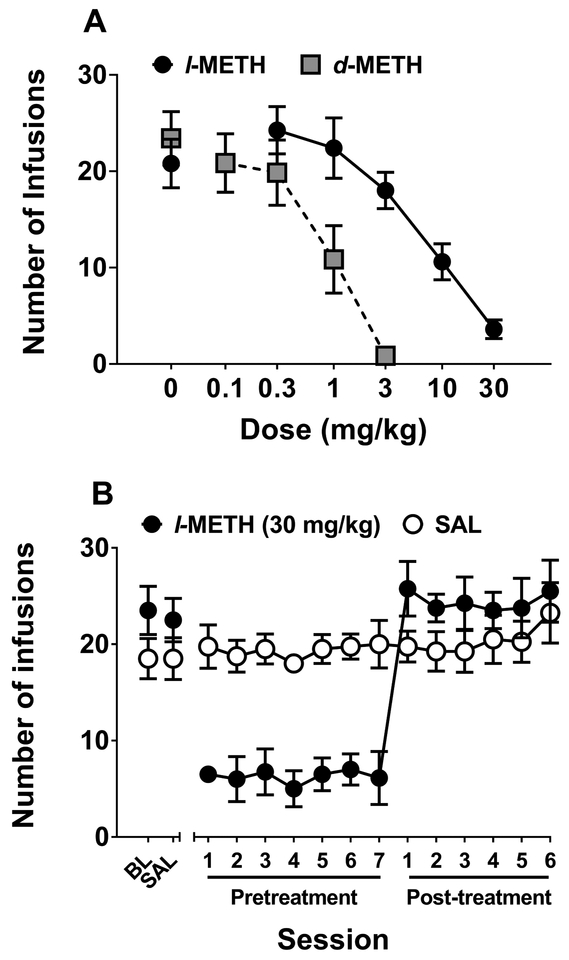
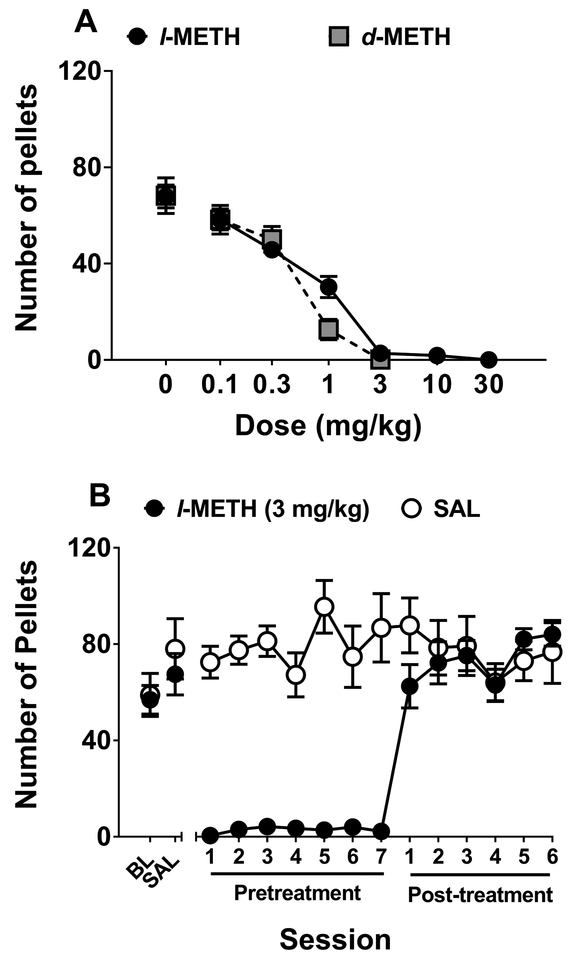
Figure 5 shows the effects of l- and d-METH pretreatment on food-maintained responding. Acute pretreatment with either l- or d-METH dose-dependently decreased food-maintained responding (Fig 5A), with l- and d-METH being equipotent. The ID50 was 0.56 mg/kg for l-METH and 0.29 mg/kg for d-METH; this difference was not statistically significant (p = 0.107).
Figure 5. Effect of l- and d-METH on food-maintained responding in Experiment 3.
Panel A: Mean (±SEM) number of pellets earned on an FR5 schedule following varying doses of either l- or d-METH; n=8 for l-METH dose-response curve and n=8 for d-METH dose-response curve. Panel B: Mean (±SEM) number of pellets earned on an FR5 schedule across repeated pretreatments. All rats (n=8) were first tested at maintenance baseline (BL) and following an injection of saline (SAL), and then were split into groups (n=4 per group) receiving 7 pretreatments with either l-METH (3 mg/kg) or SAL, followed by 6 more sessions in which all pretreatments were SAL. The number of pellets earned was lower in the l-METH pretreatment group compared to the SAL pretreatment group across all 7 pretreatment sessions, each p<0.05.
Repeated pretreatment with 3 mg/kg l-METH consistently reduced food-maintained responding (Fig 5B). Analysis of these data revealed l-METH × block (F2,12 = 6.56, p < 0.05) and session × block (F2,102 = 3.65, p < 0.05) interactions. Post-hoc analysis of the l-METH × block interaction confirmed that repeated l-METH pretreatment reduced food-maintained responding compared to repeated saline pretreatment. There were no significant differences between groups during either the baseline/saline or the post-treatment blocks.
DISCUSSION
Previous studies of the self-administration of l- and d-METH were conducted in rhesus monkeys following a history of cocaine self-administration (Kohut et al. 2017a) or in rats tested with high unit doses that induced significant lethality (Yokel and Pickens 1973). There has been no research comparing the acquisition of self-administration behavior or, once acquired, the dose-response effects of the two isomers of METH. In the present work, acquisition studies were conducted in experimentally-naïve rats to determine threshold doses needed to engender l-METH self-administration; acquisition using a standard unit dose of d-METH (0.5 mg/kg/infusion) was studied in a separate group of rats for comparison. Results indicated that reliable l-METH self-administration could be obtained using escalating doses and FR values (0.05 to 1.7 mg/kg/infusion; FR1 to FR5). On an FR1, a significant difference between responses on the active and inactive levers was not observed until the unit dose of l-METH reached 0.5 mg/kg/infusion. Some caution is needed in describing 0.5 mg/kg/infusion as the “threshold reinforcing dose” of l-METH, however, because a reinforcing effect may be revealed at a lower unit dose if active lever pressing was compared to a saline control or if more than 3 sessions were implemented at each unit dose. In addition, the initial exposure to low inactive doses of l-METH may have hindered subsequent acquisition of self-administration at higher doses. With regard to the incrementing FR requirements, direct comparison between l- and d-METH self-administration is also limited because of the potency difference at the unit doses used (1.7 vs. 0.05 mg/kg/infusion).
Determination of dose-response functions revealed that, in contrast to the relatively sharp function for d-METH, the function for l-METH was flattened and shifted rightward. Maximal responding for l-METH occurred at 0.17 mg/kg/infusion, whereas maximal responding with d-METH occurred at 0.025 mg/kg/infusion. These results are consistent with previous results obtained using an FR10 procedure in rhesus monkeys with a history of cocaine self-administration (Kohut et al. 2017a), indicating that the differences in potency of reinforcing effects between l- and d-METH is not species-specific and is not readily altered by prior exposure to stimulant drugs. However, since the dose-response curves for both isomers were inverted U-shaped functions, it is also possible that isomeric differences exist in disruptive behavior (at high doses), rather than in reinforcing effect.
Another important finding from this study is that pretreatment with l-METH, like d-METH, produced a dose-dependent decrease in d-METH self-administration. Thus, the dose-response function for the l-METH-induced decrease in d-METH self-administration (0.05 mg/kg/infusion) was parallel to and approximately 10-fold to the right of the d-METH dose-response function. While there are no previous reports regarding l-METH pretreatment on d-METH self-administration, continuous infusion of l-METH across 5 consecutive days was shown previously to dose-dependently decrease cocaine self-administration in rhesus monkeys (Kohut et al. 2016). As in that previous report, no significant tolerance across 7 consecutive sessions of pretreatment with l-METH (30 mg/kg, s.c.) was observed. These results, combined with findings of weak reinforcing efficacy, provide preclinical evidence that l-METH may have utility as a pharmacotherapy for stimulant use disorders.
From a clinical perspective, there is controversy in the field regarding substitution therapies for stimulant use disorders (e.g., cocaine, METH). Preclinical and clinical evidence suggests that drugs that increase extracellular dopamine levels such as d-amphetamine and lisdexamfetamine attenuate the reinforcing effects of cocaine and methamphetamine (Banks et al. 2015; Greenwald et al. 2010; Rush et al. 2010). However, these drugs have not advanced as pharmacotherapies for stimulant abuse, perhaps due to mixed findings regarding their clinical efficacy (Castells et al. 2010; Mooney et al. 2015; Shearer et al. 2003), as well as their own abuse liability, which is a key barrier for the general use of agonist drug replacement strategies. The current results suggest that l-METH would have reduced abuse liability compared to d-METH. Unfortunately, since l-METH was approximately 10-fold less potent on self-administration than food-reinforced behavior suggests it may not be a good candidate for development as a treatment for methamphetamine abuse. We are not aware of any studies that have evaluated the efficacy of l-METH to reduce stimulant use in humans.
Another key finding from this study is that l-METH produced a dose-dependent decrease in food-maintained responding in drug-naïve rats. l-METH had only an approximately 2-fold lower potency than d-METH in decreasing food-maintained responding (ID50 0.56 mg/kg vs 0.29 mg/kg), which was not statistically significant. These findings agree reasonably well with a previous report showing a 2.6-fold difference in the potency with which the isomers decreased food-maintained responding in rats (Siemian et al. 2017). In addition, the current report shows that l-METH decreased food-maintained responding at doses that were substantially lower than those that decreased d-METH self-administration (ID50 of 0.56 and 6.5 mg/kg, respectively). The greater potency of l-METH to decrease food-maintained responding than d-METH self-administration may relate, at least in part, to the higher rate of responding in the food experiment compared to the self-administration experiment, i.e., rate-dependency effect (Sanger and Blackman 1976). In any case, this finding contrasts with work in rhesus monkeys showing that l-METH failed to decrease food-maintained responding at doses that produced a robust decrease in cocaine self-administration under a second-order schedule (Kohut et al. 2016). While this discrepancy between studies may reflect a species difference, there are also many procedural differences that prevent a direct comparison across studies, including the self-administered drug (d-METH vs. cocaine), the operant schedule (FR5 vs. second-order FR2:VR16:S) and the treatment delivery (acute s.c. vs. continuous i.v. infusion).
The current finding that l-METH is approximately equipotent to d-METH in decreasing food-maintained responding but approximately 10-fold less potent than d-METH in decreasing d-METH self-administration, suggests that the isomers employ different mechanisms of action in decreasing food and d-METH reinforcement. Regarding d-METH reinforcement, among its many complex effects, d-METH reverses the dopamine transporter (DAT) at the plasmalemma and the vesicular monoamine transporter (VMAT2) within the intracellular compartment, while also inhibiting monoamine oxidase activity, yielding a rise in extracellular dopamine levels in reward-relevant limbic structures (Fleckenstein et al. 2007; Sulzer et al. 2005). The approximately 10-fold difference in isomeric potency to decrease d-METH self-administration in the current report does not likely reflect differential inhibition of either MAOA or MAOB, as these enzymes which show negligible stereoselectivity in rat liver (Robinson 1985). In addition, the 10-fold isomeric potency difference does not likely reflect differential inhibition of dopamine uptake or release at VMAT2, as these neurochemical actions show only a 2- to 3-fold difference in stereoselectivity in vesicles isolated from rat brain (Partilla et al. 2006). Instead, the 10-fold isomeric potency difference aligns most closely with in vitro data showing a 17-fold lower potency of l- than d-METH in evoking dopamine release in synaptosomes isolated from rat striatum (Rothman and Baumann 2003; Rothman et al. 2001), suggesting that the isomeric difference may relate to actions at the plasmalemma DAT. Unfortunately, this conclusion is complicated because additional studies assessing [3H]-dopamine uptake in rat striatal synaptosomes show an approximately 42-fold lower potency of l-METH compared to d-METH (Rothman and Baumann 2003; Rothman et al. 2001), thus suggesting that multiple cellular processes are likely involved in the 10-fold potency difference between l- and d-METH to inhibit d-METH self-administration. Moreover, in vivo microdialysis studies have demonstrated that changes in extracellular dopamine levels in striatum do not necessarily parallel the profiles of dopamine-related behaviors in rats (Kuczenski et al. 1995).
In contrast to d-METH reinforcement, considerable evidence implicates extracellular norepinephrine levels in the control of food intake (Wellman 2005). Research indicates that l- and d-METH display only approximately a 2-fold difference in potency to increase [3H]-norepinephrine release in whole rat brain synaptosomes (Rothman et al. 2001). Moreover, in vivo evidence suggests that l-METH has a similar potency to d-METH in increasing extracellular norepinephrine from rat hippocampus using in vivo microdialysis (Kuczenski et al. 1995). While the hippocampus is not thought to be involved directly in food reinforcement, noradrenergic input from hypothalamus to reward-relevant limbic dopamine systems plays an important role in food reinforcement processes (Wellman 2005). Thus, the negligible stereoselectivity of METH isomers in decreasing food-maintained responding suggests that these isomers decrease food reinforcement by altering noradrenergic input that modulates dopamine reward circuitry, rather than by altering dopamine reward circuitry directly.
Finally, regardless of cellular mechanisms, the current behavioral findings suggest the possibility of using l-METH as a pharmacotherapy for obesity. The clinical use of stimulant medications for the treatment of obesity is generally avoided due to their abuse liability and lack of long-term efficacy. However, as shown here, l-METH, which is similarly potent to d-METH in decreasing food-maintained responding acutely and produces no tolerance across repeated pretreatments, has reduced abuse potential across species. While there are no reports on the effects of l-METH on food intake or body weight measurements, limited data provide some evidence that l-amphetamine indeed may be more potent than d-amphetamine as an anorexigenic agent in rats (Lawlor et al. 1969). Thus, in addition to its potential pharmacotherapeutic value for METH abuse, l-METH may have utility as a medication for obesity and its associated metabolic syndrome.
Supplementary Material
Acknowledgments
Supported by NIH grants K01 DA039306 (SJK), P50 DA05312 (MTB), U01 DA13519 (LPD), U01 DA043908 (LPD) and T32 DA016176 (LPD).
Footnotes
No conflicts to report.
REFERENCES
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS (2015) Use of Preclinical Drug vs. Food Choice Procedures to Evaluate Candidate Medications for Cocaine Addiction. Current treatment options in psychiatry 2: 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Denehy ED, Zheng G, Crooks PA, Dwoskin LP, Bardo MT (2012) The effect of a novel VMAT2 inhibitor, GZ-793A, on methamphetamine reward in rats. Psychopharmacology 220: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareva TS, Young R, Glennon RA (2002) Central stimulants as discriminative stimuli. Asymmetric generalization between (−)ephedrine and S(+)methamphetamine. Pharmacology, biochemistry, and behavior 74: 157–62. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST (1993) Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology 110: 5–12. [DOI] [PubMed] [Google Scholar]
- Castells X, Casas M, Perez-Mana C, Roncero C, Vidal X, Capella D (2010) Efficacy of psychostimulant drugs for cocaine dependence. The Cochrane database of systematic reviews: Cd007380. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR (2007) New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology 47: 681–98. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL (2010) Sustained release d-amphetamine reduces cocaine but not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35: 2624–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT (2001) Lobeline attenuates d-methamphetamine self-administration in rats. The Journal of pharmacology and experimental therapeutics 298: 172–9. [PubMed] [Google Scholar]
- Kohut SJ, Bergman J, Blough BE (2016) Effects of L-methamphetamine treatment on cocaine- and food-maintained behavior in rhesus monkeys. Psychopharmacology 233: 1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Blough BE, Bergman J (2017a) Reinforcing effects of l-methamphetamine in non-human primates Annual Meeting of the College of Problems of Drug Dependence San Diego CA. [Google Scholar]
- Kohut SJ, Jacobs DS, Rothman RB, Partilla JS, Bergman J, Blough BE (2017b) Cocaine-like discriminative stimulus effects of “norepinephrine-preferring” monoamine releasers: time course and interaction studies in rhesus monkeys. Psychopharmacology 234: 3455–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W (1995) Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience 15: 1308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor RB, Trivedi MC, Yelnosky J (1969) A determination of the anorexigenic potential of dl-amphetamine, d-amphetamine, l-amphetamine and phentermine. Archives internationales de pharmacodynamie et de therapie 179: 401–7. [PubMed] [Google Scholar]
- Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P 3rd, Everhart ET, Jones RT (2006) Human pharmacology of the methamphetamine stereoisomers. Clinical pharmacology and therapeutics 80: 403–20. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J (2015) Pilot study of the effects of lisdexamfetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence 153: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (2006) Interaction of amphetamines and related compounds at the vesicular monoamine transporter. The Journal of pharmacology and experimental therapeutics 319: 237–46. [DOI] [PubMed] [Google Scholar]
- Robinson JB (1985) Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine, N-methylamphetamine and deprenyl. Biochemical pharmacology 34: 4105–8. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH (2003) Monoamine transporters and psychostimulant drugs. European journal of pharmacology 479: 23–40. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse (New York, NY) 39: 32–41. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR (2010) Cocaine choice in humans during D-amphetamine maintenance. Journal of clinical psychopharmacology 30: 152–9. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE (1976) Rate-dependent effects of drugs: a review of the literature. Pharmacology, biochemistry, and behavior 4: 73–83. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J (2003) Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction (Abingdon, England) 98: 1137–41. [DOI] [PubMed] [Google Scholar]
- Siemian JN, Xue Z, Blough BE, Li JX (2017) Comparison of some behavioral effects of d- and l-methamphetamine in adult male rats. Psychopharmacology 234: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Progress in neurobiology 75: 406–33. [DOI] [PubMed] [Google Scholar]
- Wellman PJ (2005) Modulation of eating by central catecholamine systems. Current drug targets 6: 191–9. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R (1973) Self-administration of optical isomers of amphetamine and methylamphetamine by rats. The Journal of pharmacology and experimental therapeutics 187: 27–33. [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA (2006) Vesicular monoamine transporter 2: role as a novel target for drug development. The AAPS journal 8: E682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.