Abstract
Infections of herpes simplex virus type 1 (HSV-1), cytomegalovirus (CMV), Helicobacter pylori, and Chlamydia pneumoniae may play a role in cognitive decline via systemic inflammation. We hypothesized that Atherosclerosis Risk in Communities study participants who were seropositive in midlife for antibodies to HSV-1, CMV, H pylori, or C pneumoniae would have an accelerated rate of cognitive decline over 20 years. Atherosclerosis Risk in Communities performed a case-cohort study involving a stratified random sample of participants tested for serum immunoglobulin G antibodies to the pathogens of interest. We conducted a longitudinal study using this cohort. Cognitive change was measured using Z scores from the Delayed Word Recall (DWR), Digit Symbol Substitution (DSS), and Word Fluency (WF) Tests administered at visits 2 (1990-1992), 4 (1996-1998), and 5 (2011-2013). Linear regression models with generalized estimating equations and inverse probability of attrition weights were used to evaluate associations between infection and cognitive performance. Four hundred twenty-six participants were analyzed, of which 3% were seronegative for all 4 infections, 14% seropositive for one, 33% and 34% seropositive for 2 and 3, respectively, and 16% seropositive for all infections. At baseline, test scores were significantly lower for participants seropositive for H pylori and C pneumoniae. After baseline covariate adjustment, the rate of decline in DWR, DSS, WF, and global Z scores did not differ significantly by infection status for any of the 4 infections. There was also no significant association between the number of infections for which participants were seropositive and cognitive decline. Our study provides no evidence supporting a longitudinal relationship between seropositivity and cognitive decline.
Keywords: cognitive decline, cohort study, infection
Introduction
As people age, changes in cognition occur across a continuum that can range from normal aging to pathologic decline. Cognitive decline is a change in cognition that exceeds the decline expected due to aging alone.1, 2 Cognitive changes among older adults are important because pathologic decline indicates increased risk of cognitive impairment and dementia.2 Past or chronic infections with herpes simplex virus type 1 (HSV-1), cytomegalovirus (CMV), Helicobacter pylori, and Chlamydia pneumoniae have been proposed as possible risk factors for cognitive decline.
Herpes simplex virus type 1 is a virus typically transmitted via oral-to-oral contact and while often asymptomatic, it can cause painful lesions at the site of infection, including cold sores.3, 4 The infection is highly contagious with the majority of transmissions occurring in childhood and adolescence and a seroprevalence of 65% among those ages 70 and older. Cytomegalovirus is in the family of herpes viruses, is spread via bodily fluids, and can cause mild illness including fever, sore throat, and fatigue, but is usually asymptomatic.5 The seroprevalence of CMV is estimated to be 83% by age 70, but varies considerably by race and socioeconomic factors.6 Helicobacter pylori is a gram-negative bacillus that typically inhabits the mucous layer over the gastric epithelial cells.7 The seroprevalence of H pylori is 57% among adults in the United States aged ≥70 with infection often occurring in childhood and usually remaining asymptomatic.8 Chlamydia pneumoniae is an intracellular bacterium that typically infects the lungs causing mild upper respiratory infection, and in more severe cases, bronchitis and pneumonia.9 However, 90% of people infected with C pneumoniae are asymptomatic.10 The seroprevalence is estimated to be 50% among adults.11
These common infections have long been hypothesized to have a role in the pathogenesis of atherosclerosis and coronary heart disease (CHD) by way of systemic inflammation; however, their association with cardiovascular disease has been inconsistent.12 There is, however, renewed interest in the role past infection may play in cognitive decline. Polymerase chain reaction procedures have detected elementary bodies of HSV-1, CMV, and C pneumoniae in brain tissues of dementia patients, and while H pylori likely does not infiltrate the brain directly, the pathogen is associated with persistent infection and can lead to a systemic inflammatory response implicated in dementia pathogenesis.13-16
The current literature is inconsistent as to whether HSV-1, CMV, H pylori, or C pneumoniae are associated with cognitive decline. Major limitations of previous studies include small sample sizes (n < 100) and reliance on case–control or cross-sectional study designs.17 The association between past infections and cognitive decline is biologically plausible, and the Atherosclerosis Risk in Communities (ARIC) Study offers an opportunity to examine this relationship longitudinally in a moderately sized subset of the cohort. We therefore hypothesized that ARIC participants whose baseline serum tested positive for antibodies to HSV-1, CMV, H pylori, or C pneumoniae would have an accelerated rate of cognitive decline over 20 years compared to participants whose serum tested negative.
Methods
Atherosclerosis Risk in Communities is a prospective cohort study that enrolled 15 792 participants, after institutional review board approval and written informed consent, ages 45 to 64 from 4 communities: Forsyth County, North Carolina; Jackson, Mississippi; the northwestern suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Atherosclerosis Risk in Communities has performed a number of case-cohort studies, for one of which the cohort component included a stratified random sample of 556 participants free from CHD who were tested for serum antibodies to HSV-1, CMV, H pylori, and C pneumoniae at visit 1 (1987-1989). These CHD-free participants were selected within strata based on age, race, and sex with oversampling of African American men ages 55 years and older. For the present analysis, a longitudinal analysis was conducted using this cohort random sample to assess the association between these 4 infections and cognitive decline over 20 years of follow-up, between ARIC clinic visits 2 (1990-1992) and 5 (2011-2013). When the cohort random sample was selected, cognitive decline had not yet been ascertained allowing us to assess the association longitudinally. After exclusions for participants who were non-white or African American as well as African Americans from Minnesota and Maryland (due to small numbers), those with missing serum antibody measures, those missing baseline covariates, and those who had a history of stroke, 426 participants were included in the analysis.
In the mid-1990s, visit 1 serum samples were thawed and tested for immunoglobulin (IgG) antibodies to several different infectious pathogens. VP123 for HSV-1 and AD169 for CMV were tested using solid-phase radio-immunoassay performed at the Division of Molecular Virology, Baylor College of Medicine, Houston, Texas with positive cutoff indicated at ≥2.25 for HSV and >2.05 for CMV.18 Immunoglobulin G antibodies to the high-molecular-weight cell-associated proteins of H pylori were measured using enzyme-linked immunosorbent assay (HM-CAP, EPI, Westbury, New York) with positive cutoff indicated at >2.2.19 Immunoglobulin G antibodies to C pneumoniae strain Taiwan acute respiratory (TWAR) were measured using a micro immunofluorescence specific to TWAR at the University of Washington, Seattle, WA with positive cutoff indicated at a titer of 1:64.20
Cognitive change was measured using scores from the Delayed Word Recall (DWR) test, Digit Symbol Substitution (DSS) test, and Word Fluency (WF) test. These generally reflect cognitive domains of memory, executive function and processing speed, and language, respectively. The DWR, DSS, and WF tests were administered at visits 2 (1990-1992), 4 (1996-1998), and 5 (2011-2013). Test scores were converted to Z scores and standardized to visit 2. The cognitive variables of interest were therefore difference in change, from 1990 to 1992 to 2011 to 2013, in cognitive test Z score by each infection status.
Other covariates included age, sex, race, apolipoprotein E genotype (APOE ϵ4-yes/no), income, and education measured at visit 1. Covariates measured at visit 2 included body mass index, smoking status, alcohol drinking status, hypertension (defined as a systolic blood pressure of ≥140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or report of taking hypertensive medication), diabetes (defined as nonfasting glucose ≥ 200 mg/dL, fasting blood glucose ≥ 126 mg/dL, self-report of physician diagnosis of diabetes, or reporting taking medication for diabetes or high blood sugar), prevalent CHD, and measured plasma high density lipoprotein and total cholesterol.
To assess the association between past infection status (positive/negative) and change in cognitive scores (measured at visits 2, 4, and 5) we calculated Z scores ([test score — mean score]/standard deviation) for the 3 cognitive tests as well as a combined global test. Linear regression models were used to estimate the difference in baseline cognitive levels by infection status for HSV-1, CMV, H pylori, and C pneumoniae. Linear regression models with generalized estimating equations were used to assess the association between the 4 infections and 20-year cognitive decline as well as the number of seropositives for infection and 20-year cognitive decline using robust variance and an unstructured correlation matrix. There was an approximate 6-year difference between measures from visit 2 to visit 4, and a 14-year difference between measures from visit 4 to visit 5. To account for the different slopes of decline during the 20 years of total follow-up, models included a linear spline for time with a knot at 6 years (visit 4) and an interaction term between time and infection status. Interactions between follow-up time and covariates were explored as appropriate.
Separate models were run for each cognitive test (DWRT, DSST, and WFT) and the global cognitive score. Models were adjusted for the baseline (visit 1 or 2) covariates. There were 426 participants included at baseline and of those, 358 participants attended visit 4 and 186 attended visit 5. To account for this attrition during follow-up, inverse probability of attrition weights (IPAW) were used in regression models. Weights for each participant were calculated at visits 4 and 5 by estimating the probability of (1) being alive at the time of the follow-up visit and (2) attending the visit, conditional on being alive at the time of the examination. These weights were stabilized by the baseline covariates of age, sex, race, and education. Due to the sampling design for the stratified cohort random sample, we also included weights to correct for the selection criteria used when creating the cohort random sample. With 80% power and an α of .05, the minimum detectable difference in cognitive test Z scores ranged from 0.2 to 0.3 for the 4 infections.
We conducted a sensitivity analysis using latent variable methods to incorporate all cognitive tests administered in ARIC at visits 2, 4, and 5 (including cognitive tests administered only at visit 5 that did not have repeated measures). However, results did not differ and are not presented here. All statistical analyses were performed with SAS version 9.4 (SAS Inc, Cary, North Carolina).
Results
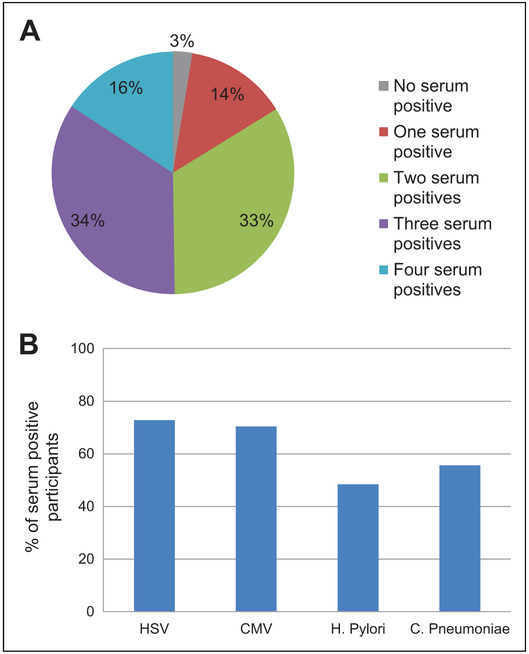
At the time of the first cognitive assessment (visit 2) the 426 participants with serologic infectious pathogen measurements was 59 years old. Those who were seropositive for HSV-1, CMV, or H pylori were more likely to be African American, have less than a high school education, have an income less than US$16 000 (measured at visit 1), be a current smoker, have diabetes, and have higher total cholesterol compared to those who were seronegative (Table 1). Chlamydia pneumoniae status followed similar baseline prevalence patterns as the other 3 pathogens, but did not differ by socioeconomic measures (race, education, or income). Only 3% of participants were seronegative for all 4 infections, 14% were seropositive for 1 infection, 33% were seropositive for 2 infections, 34% were seropositive for 3 infections, and 16% were seropositive for all 4 infections (Figure 1).
Table 1.
Baseline Characteristics of Cohort Random Sample by Serology Status, Visit 2 ARIC 1990 to 1992.
| Herpes Simplex Virus 1 |
Cytomegalovirus |
Helicobacter pylori |
Chlamydia pneumoniae |
|||||
|---|---|---|---|---|---|---|---|---|
| Positive (n = 310) |
Negative (n = 116) |
Positive (n = 300) |
Negative (n = 126) |
Positive (n = 206) |
Negative (n = 220) |
Positive (n = 237) |
Negative (n = 189) |
|
| Age,a years | 59.4 (4.8) | 58.5 (5.3) | 59.3 (4.9) | 58.9 (5.2) | 59.2 (4.8) | 59.1 (5.1) | 59.3 (5.1) | 59.0 (4.9) |
| Male, % | 51.9 | 68.1 | 54.0 | 61.9 | 60.2 | 52.7 | 59.5 | 52.4 |
| African American, % | 26.8 | 6.0 | 27.7 | 5.6 | 34.0 | 9.1 | 19.0 | 23.8 |
| < High school education,b % | 27.7 | 14.8 | 27.3 | 16.8 | 31.6 | 17.4 | 24.1 | 24.5 |
| < US$16 000 Income,b % | 24.5 | 11.2 | 25.0 | 11.1 | 29.1 | 13.2 | 16.5 | 26.5 |
| Current drinking, % | 53.6 | 67.2 | 56.3 | 59.5 | 53.4 | 60.9 | 59.9 | 54.0 |
| Current Smokers, % | 20.0 | 14.7 | 19.0 | 17.5 | 22.8 | 14.6 | 20.3 | 16.4 |
| Diabetes,c % | 9.7 | 3.5 | 8.7 | 6.4 | 9.2 | 6.8 | 10.1 | 5.3 |
| Hypertension,d % | 30.0 | 20.7 | 29.3 | 23.0 | 29.6 | 25.5 | 30.0 | 24.3 |
| Body mass index,a kg/m2 | 27.6 (4.7) | 26.3 (4.2) | 27.2 (4.7) | 27.2 (4.5) | 27.3 (4.7) | 27.2 (4.5) | 27.4 (4.9) | 27.1 (4.2) |
| HDL Cholesterol,a mg/dL | 50.0 (16.4) | 48.6 (15.0) | 50.1 (15.7) | 48.6 (16.8) | 48.9 (15.4) | 50.2 (16.5) | 48.9 (16.6) | 50.6 (15.2) |
| Total Cholesterol,a mg/dL | 207.5 (39.5) | 202.0 (36.9) | 207.5 (40.2) | 202.5 (35.2) | 205.8 (37.0) | 206.2 (40.6) | 207.6 (40.0) | 204.0 (37.3) |
| APOE ϵ4, % | 29.7 | 29.3 | 30.3 | 27.8 | 31.1 | 28.2 | 32.5 | 25.9 |
| CHD,e% | 1.0 | 1.7 | 1.0 | 1.6 | 0.5 | 1.8 | 2.11 | 0 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease; HDL, high density lipoprotein.
Presented as mean (standard deviation).
Determined from self-report at visit 1,ARIC 1987-1989.
Defined as nonfasting blood glucose ≥200 mg/dL, fasting blood glucose ≥126 mg/dL, self-report of diabetes, or reporting taking medication for diabetes or high blood sugar at visit 2.
Based on diastolic blood pressure ≥90 mm Hg, systolic blood pressure of ≥140 mm Hg, or use of hypertensive medication at visit 2.
CHD: prevalent coronary heart disease defined as myocardial infarction or coronary revascularization.
Figure 1.
(A) Distribution of baseline serum positives and (B) proportion of serum positives for each pathogen.
At baseline, there were no statistically significant differences in any of the cognitive test scores by infection status for HSV-1 or CMV after full adjustment for baseline covariates (Table 2). Cognitive test scores were statistically significantly lower in those seropositive for H pylori for the DWR test and global score, and in those seropositive for C pneumoniae for the WF test after full adjustment.
Table 2.
Difference (95% Confidence Interval) in Baseline Cognitive Test Z Score by Baseline Antibody Status (Positive vs Negative), 1990 to 1992.a
| Baseline Cognitive Test |
Delayed Word Recall |
Digit Symbol Substitution |
Word Frequency |
Global Scoreb |
||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model l | Model 2 | Model l | Model 2 | Model l | Model 2 | |
| HSV-1 | 0.1 [−0.2 to 0.3] |
0.2 [−0.03 to 0.4] |
−0.2 [−0.3 to 0.01] |
−0.04 [−0.2 to 0.1] |
−0.02 [−0.2 to 0.2] |
0.2 [−0.01 to 0.4] |
−0.1 [−0.3 to 0.1] |
0.1 [−0.03 to 0.3] |
| CMV | −0.1 [−0.3 to 0.1] |
−0.04 [−0.2 to 0.2] |
−0.2c [−0.3 to −0.004] |
−0.1 [−0.2 to 0.1] |
−0.3c [−0.5 to −0.1] |
−0.1 [−0.3 to 0.04] |
−0.2c [−0.4 to −0.05] |
−0.1 [−0.3 to 0.1] |
| Helicobacter pylori | −0.3c [−−0.5 to −0.2] |
−0.2c [−0.4 to −0.03] |
−0.2c [−0.3 to −0.05] |
−0.04 [−0.2 to 0.1] |
−0.4c [−0.6 to −0.2] |
−0.1 [−0.3 to 0.04] |
−0.4c [−0.6 to −0.2] |
−0.2c [−0.3 to −0.02] |
| Chlamydia pneumoniae | 0.1 [−0.1 to 0.3] |
0.05 [−0.1 to 0.2] |
0.05 [−0.1 to 0.2] |
−0.01 [−0.1 to 0.1] |
−0.2c [−0.4 to −0.01] |
−0.3c [−0.4 to −0.1] |
−0.03 [−0.2 to 0.1] |
−0.1 [−0.2 to 0.04] |
Abbreviations: APOE ϵ4, apolipoprotein E genotype; BMI, body mass index; CHD, coronary heart disease; CMV, cytomegalovirus; HDL, high density lipoprotein; HSV-1, Herpes simplex virus type 1.
n = 426.
A composite (global) Z score was calculated from the average Z scores for the 3 cognitive test Z scores. All Z scores were standardized to visit 2. Model 1: adjusted for age, sex, race, and APOE ϵ4. Model 2: adjusted for model 1 plus income, education, BMI, hypertension status, diabetes status, drinking status, smoking status, HDL cholesterol, total cholesterol, and prevalent CHD. BMI, hypertension status, diabetes status, total cholesterol, and prevalent CHD interaction terms with time were also included in the model.
Statistically significant P < .05.
Over 20 years of follow-up (with aging), cognitive performance declined across all groups. However, after model 1 adjustments for baseline age, sex, race, and APOE ϵ4, the rate of decline in DWR, DSS, WF, or global test Z scores did not differ significantly by infection status for any of the 4 infections. After adjustment for the remaining covariates in model 2, the associations remained statistically insignificant (Table 3). For example, in the fully adjusted model after adjustment for IPAW, those with HSV at baseline had 0.1 (95% confidence interval: −0.1 to 0.4) less decline in global Z score over time compared to those without HSV-1, though this difference was not statistically significant (Table 3, final column). Further, there was no statistically significant association between the number of infections and cognitive decline over the follow-up period for any of the cognitive tests (results not shown).
Table 3.
Difference (95% Confidence Interval) in 20 Year Change in Cognitive Test Z Scores by Baseline Antibody Status (Positive vs Negative), 1990 to 2013.a
| Cognitive Test |
Delayed Word Recall |
Digit Symbol Substitution |
Word Frequency |
Global Scoreb |
||||
|---|---|---|---|---|---|---|---|---|
| Model l | Model 2 | Model l | Model 2 | Model l | Model 2 | Model l | Model 2 | |
| HSV-1 | 0.05 [−0.3 to 0.4] | 0.08 [−0.3 to 0.4] | 0.01 [−0.2 to 0.2] | 0.01 [−0.1 to 0.2] | 0.1 [−0.06 to 0.3] | 0.2 [−0.04 to 0.4] | 0.1 [−0.1 to 0.3] | 0.1 [−0.1 to 0.4] |
| CMV | 0.1 [−0.2 to 0.5] | 0.1 [−0.2 to 0.5] | 0.1 [−0.1 to 0.2] | 0.1 [−0.1 to 0.2] | −0.1 [−0.2 to 0.1] | −0.01 [−0.3 to 0.3] | 0.04 [−0.2 to 0.3] | 0.1 [−0.2 to 0.4] |
| Helicobacter pylori | 0.2 [−0.1 to 0.5] | 0.2 [−0.1 to 0.5] | 0.03 [−0.1 to 0.2] | 0.05 [−0.1 to 0.2] | 0.09 [−0.08 to 0.3] | 0.1 [−0.07 to 0.3] | 0.1 [−0.05 to 0.3] | 0.2 [−0.03 to 0.4] |
| Chlamydia pneumoniae | −0.07 [−0.4 to 0.3] | −0.05 [−0.4 to 0.3] | −0.04 [−0.2 to 0.1] | −0.05 [−0.2 to 0.1] | −0.04 [−0.2 to 0.1] | −0.06 [−0.2 to 0.1] | −0.06 [−0.3 to 0.1] | −0.05 [−0.3 to 0.2] |
Abbreviations: APOE ϵ4, apolipoprotein E genotype; BMI, body mass index; CHD, coronary heart disease; CMV, cytomegalovirus; HDL, high density lipoprotein; HSV-1, Herpes simplex virus type 1.
n = 426.
A composite (global) Z score was calculated from the average Z scores for the 3 cognitive test Z scores. All Z scores were standardized to visit 2. Model 1: adjusted for age, sex, race, and APOE ϵ4. Model 2: adjusted for model 1 plus income, education, BMI, hypertension status, diabetes status, drinking status, smoking status, HDL cholesterol, total cholesterol, and prevalent CHD. BMI, hypertension status, diabetes status, total cholesterol, and prevalent CHD interaction terms with time were also included in the model.
Discussion
In this longitudinal study, participants with baseline serologic evidence of infection with HSV-1, CMV, H pylori, and C pneumoniae had no significant difference in cognitive decline over 20 years compared with those who were seronegative at baseline. While rate of decline did not differ, those who were seropositive for H pylori or C pneumoniae had significantly lower Z scores at baseline for some tests compared to those who were seronegative. There are very few prospective studies examining infection and cognitive decline.
Key strengths of our study include the longitudinal design and measurement of antibody titers in middle age, which ensured that infection exposure information preceded change in cognitive status. Most prior assessments of infection and cognition have been cross-sectional. However, limitations of this analysis need to be considered. With only a small subset of the ARIC cohort tested for serum antibodies and a high prevalence of prior infection in the ARIC sample, we had limited statistical power to detect small differences in cognitive decline by infection status. Further, participants’ serum antibody levels were measured only once and antibody levels were dichotomized (positive/negative for past infection). This prevented us from determining severity of past infection as well as whether participants’ infections were reactivated during the follow-up period. In addition, we could not retest serum samples to ensure antibody levels remained stable over time. Infection status would have been misclassified for participants who seroconverted over the study period. Nonetheless, the infections studied are generally transmitted in childhood and adolescence, so seroconversion was likely uncommon.
The battery of cognitive tests available over the follow-up period was also limited. Visits 2 and 4 of ARIC included 3 cognitive tests, DWR, DSS, and WF. At visit 5, these 3 tests as well as Logical Memory, Incidental Learning, Trail Making Parts A and B, Digit Span Backwards, Boston Naming, and Semantic Fluency were also administered as part of the ARIC Neurocognitive Study. In our analysis, we did not incorporate all the available visit 5 cognition data in order to conduct a longitudinal analysis of DWR, DSS, and WF at visits 2, 4, and 5. A recent paper developed a latent variable model to assess domains of memory, executive function, and language using the full battery of testing available across all ARIC visits with cognitive testing.21 We conducted a sensitivity analysis using these latent variables, but as mentioned earlier, results did not differ and were not presented. Finally, misclassification may have resulted from error in serologic measurements, namely if participants had a weak immune response to infection. For that reason, we used a dichotomous infection status variable (positive or negative) with consideration of serum IgG levels.
While the relationship between infection and cognitive decline seems to be biologically plausible, our study does not support a meaningful longitudinal relation for the 4 infections studied. However, due to the limitations in how past infection and cognitive impairment were measured, generalizability of these results is somewhat limited. This study suggests the association between these 4 past infections and cognitive decline may be weak and not explain differential rates of cognitive decline with aging, unlike other variables such as education, APOE ϵ4, diabetes, hypertension, smoking, drinking, obesity, and hypercholesteremia.22-27 Further study using a larger, representative cohort with multiple serum measures is warranted to better characterize the relationship between past infection and cognitive impairment.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ms George was supported by National Heart, Lung, and Blood Institute (NHLBI) Training Grant T32HL007779. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN26820110000 8C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01-HL70825.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Salthouse T Consequences of age-related cognitive declines. Annu Rev Psychol. 2012;63:201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systemic review of factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):183–193. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population based study. Lancet Neurol. 2006;5(9):735–741. [DOI] [PubMed] [Google Scholar]
- 4.Herpes Simplex Virus. World Health Organization; 2017; http://www.who.int/mediacentre/factsheets/fs400/en/. Accessed February 5, 2017. [Google Scholar]
- 5.Harris SA, Harris EA. Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer’s disease. J Alzheimers Dis. 2015;48(2):319–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cytomegalovirus (CMV) and Congenital CMV Infection. Centers for Disease Control and Prevention; 2016; https://www.cdc.gov/cmv/overview.html. Accessed February 5, 2017. [Google Scholar]
- 7.Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet. 1997;349(9047):241–244. [DOI] [PubMed] [Google Scholar]
- 8.Helicobacter pylori. World Health Organization; 2008; http://www.who.int/immunization/topics/helicobacter_pylori/en/. Accessed April 21, 2017. [Google Scholar]
- 9.Neurological Consequences of Cytomegalovirus Infection. National Institute of Neurobiological Disorders and Stroke; 2017; http://www.brainfacts.org/diseases-disorders/diseases-a-to-z-from-ninds/neurological-consequences-of-cytomegalovrius-infection/. Accessed February 5, 2017. [Google Scholar]
- 10.Chlamydia Pneumoniae infection. Centers for Disease Control and Prevention; 2016; https://www.cdc.gov/pneumonia/atypical/cpneumoniae/about/fast-facts.html. Accessed February 6, 2017. [Google Scholar]
- 11.Hyman CL, Roblin PM, Gaydos CA, Quinn TC, Schachter J, Hammerschlag MR. Prevalence of asymptomatic nasopharyngeal carriage of Chlamydia pneumoniae in subjectively healthy adults: assessment by polymerase chain reaction-enzyme immunoassay and culture. Clin Infect Dis. 1995;20(5):1174–1178. [DOI] [PubMed] [Google Scholar]
- 12.Roivainen M, Viik-Kajander M, Palosuo T, et al. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101(3):252–257. [DOI] [PubMed] [Google Scholar]
- 13.Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type DNA is located within Alzheimer’s disease amyloid plaques. J Pathol. 2009;217(1):131–138. [DOI] [PubMed] [Google Scholar]
- 14.Lin WR, Wozniak MA, Wilcock GK, Itzhaki RF. Cytomegalo-virus is present in a very high proportion of brains from vascular dementia patients. Neurobiol Dis. 2002;9(1):82–87. [DOI] [PubMed] [Google Scholar]
- 15.Balin BJ, Gerard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, Whittum-Hudson Hudson AP. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol. 1998;187(1):23–42. [DOI] [PubMed] [Google Scholar]
- 16.Sanders MK, Peura DA. Helicobacter pylori-associated diseases. Curr Gastroenterol Rep. 2002;4(6):448–454. [DOI] [PubMed] [Google Scholar]
- 17.Mawanda F, Wallace R. Can infections cause Alzheimer’s disease? Epidemiol Rev. 2013;35(1):161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorlie PD, Adam E, Melnick SL, et al. Cytomegalovirus/herpesvirus and carotid atherosclerosis: the ARIC study. J Med Virol. 1994;42(1):33–37. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Nieto FJ, Sorlie P, Chambless LE, Graham DY. Helicobacter pylori seropositivity and coronary heart disease incidence. Circulation. 1998;98(9):845–850. [DOI] [PubMed] [Google Scholar]
- 20.Melnick SL, Shahar E, Folsom AR, et al. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95(5):499–504. [DOI] [PubMed] [Google Scholar]
- 21.Gross AL, Power MC, Albert MS, et al. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26(6):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brookmeyer R, Evans DA, Hebert L, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang EY, Harrison SL, Errington L, et al. Current developments in dementia risk prediction modelling: an updated systematic review. PLoS One. 2015;10(9):e0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelick PB, Scuteri A, Black SE, et al. ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol. 2017;4(10):1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;1(10):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso A, Mosley TH, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalization associated with cardiovascular risk factors in midlife and older age: the atherosclerosis risk in communities (ARIC) study. J Neurol Nuerosurg Psychiatr. 2009;80(11):1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]