Abstract
Rationale
The α7 nicotinic acetylcholine receptor (nAChR) has been implicated as a target in modulating nicotine reward. However, the effect of pharmacological agents that have been shown to alter the channel properties of the α7 nAChR is not well understood in nicotine reward.
Objectives
This study aimed to investigate the impact of α7 nAChR pharmacological modulation on nicotine conditioned place preference (CPP) in mice by using positive allosteric modulators (PAMs) and a silent agonist.
Methods
The effect of the orthosteric α7 nAChR full agonist PNU282987 (1.3 and 9 mg/kg, s.c.), Type I α7 PAM NS1738 (1 and 10 mg/kg; i.p.), the Type II α7 PAM PNU120596 (0.3, 1, and 3 mg/kg, i.p.), and the α7 silent agonist NS6740 (1 and 3 mg/kg, i.p) on nicotine CPP was measured in mice. Mice were conditioned with either saline or nicotine (0.5 mg/kg) for 3 days in the CPP paradigm.
Results
The α7 full orthosteric agonist PNU282987 and the Type II α7 nAChR PAM PNU120596 reduced nicotine CPP, while the silent agonist NS6740 and Type I PAM NS1738 had no effect. The effects of PNU282987 and PNU120596 did not have an effect on morphine CPP.
Conclusions
Taken together, our results suggest that modulation of the α7 nAChR can play important roles in nicotine CPP in mice. In addition, the Type II α7 nAChR PAM PNU120596 attenuated nicotine reward suggesting that endogenous acetylcholine/choline tone is sufficient to reduce nicotine CPP. These findings highlight a beneficial effect of using α7 nAChR PAMs in nicotine reward.
Keywords: Nicotine, Mice, Conditioned place preference, Reward
Introduction
Activation of the homomeric α7 nicotinic acetylcholine receptors (nAChR) has been shown to induce dopamine release in the mesolimbic pathway (Livingstone et al. 2009; Schilström et al. 1998); however, early behavioral studies suggested little involvement of the α7 nAChR in nicotine reward (Brioni et al. 1996; Grottick et al. 2000). Recently, it has been shown that ArIB, a selective α7 nAChR antagonist, infused in the NAc shell increased nicotine intake in nicotine i.v. self-administration procedure (Brunzell and McIntosh 2012). Similarly, the genetic deletion of α7 nAChR in mice enhanced nicotine reward as measured in the CPP test (Harenza et al. 2014). In contrast, α7 knock-in mice (mice heterozygous for a Leu250-to-Thr substitution in the channel domain of α7 subunit, which creates a gain-of-function mutation) had abolished nicotine preference (Harenza et al. 2014). Furthermore, PNU282987, an α7 nAChR agonist administered systemically reduced nicotine conditioned place preference in mice (Jackson et al. 2017) and PNU282987 infused locally into the NAc shell was found to reduce nicotine intake in i.v. self-administration in rats (Brunzell and McIntosh 2012). Galantamine, a compound which acts as both an acetylcholinesterase inhibitor and a positive allosteric modulator (PAM) at α7 and α4β2-containing nAChRs (Harvey 1995; Maelicke and Albuquerque 2000; Samochocki 2003) [however, see also Kowal et al. 2017, where galantamine was shown not to be a PAM of human α4β2 or α7 nicotinic acetylcholine receptors], has been shown to attenuate nicotine i.v. self-administration in rats and reduce nicotine-seeking behaviors (Hopkins et al. 2012). Collectively, these studies suggest that stimulation of α7 nAChR subtypes supports a pathway that counters nicotine reward and reinforcement. Therefore, the need for further investigation of the role of α7 nAChRs in nicotine reward is evident.
The homomeric α7 nAChR has unique pharmacological and physiological features that includes high calcium permeability, rapid and reversible desensitization, and low probability of channel opening (Séguéla et al. 1993; Williams et al. 2011a). In addition, the subtype can be stimulated by an array of pharmacological ligands, including a variety of selective agonists and partial agonists and by highly selective positive allosteric modulators (PAMs), which, to varying degrees, allow orthosteric agonists to overcome the channel’s limited open probability. Type I PAMs, such as NS1738, enhance the channel opening probability of α7 nAChRs, while Type II PAMs, such as PNU120596, both increase the opening probability of α7 nAChRs and destabilize the desensitized states of the receptor to support more prolonged bursts of channel opening (Gronlien et al. 2007; Williams et al. 2011b). Adding to the rich pharmacology of the α7 nAChR subtype-selective ligands, recent studies have identified a class of compounds termed silent agonists. These agonists bind to α7 nAChRs selectively with low or absent partial agonist activity and induce a desensitized state in the α7 nAChR which renders it inactive in response to ACh. For example, the silent agonist NS6740 is a high affinity orthosteric ligand that desensitizes the receptor by inducing conformational changes that favor the desensitization state over the active state (Papke et al. 2015). Interestingly, although silent agonist compounds can reduce ACh stimulation of α7 nAChRs, they differ from antagonists in their ability to induce PAM-sensitive non-conducing (desensitized) states of the α7 nAChR receptors. So that, although ineffective at activating the channel when applied alone, they produce significant amounts of channel activation when co-applied with a7 PAMs (Papke et al. 2015). To date, the impact of these various α7 nAChR modulators in nicotine withdrawal has been examined (Jackson et al. 2018), but their effect on nicotine reward has not been reported.
Therefore, the current study investigated the impact of pharmacological modulation of the α7 nAChR in the nicotine conditioned place preference (CPP) test in mice. The Type I PAM NS1738 and Type II PAM PNU120596 were used to evaluate the effect of channel opening probability and modulation of endogenous acetylcholine or choline tone. The Type II PAM PNU120596 and silent agonist NS6740 were used to evaluate the role of enhanced channel opening and receptor desensitization, respectively, in nicotine reward. The orthosteric full α7 agonist PNU282987 was used as a reference compound. The findings of this study will advance a better understanding of the role of the α7 nAChR in nicotine reward.
Materials and methods
Animals
Drug-naive, ICR male mice (8 weeks old upon arrival; Harlan Laboratories, Indianapolis, IN) served as subjects. Mice were housed four per cage with ad libitum access to food and water on a 12-h light cycle (lights on at 0600) in a humidity and temperature-controlled vivarium that was approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Mice received corn cob bedding and were fed Envigo Teklad mouse/rat diet 7102 (LM-485). Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs
(−)-Nicotine hydrogen tartrate [(−)-1-methyl-2-(3- pyridyl) pyrrolidine (+)-bitartrate] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). PNU120596 [1-(5-chloro-2, 4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)] and PNU282987 [N-(3R)-1 Azabicyclo [2.2.2] oct-3-yl-4-chlorobenzamide] were obtained from the National Institute on Drug Abuse (NIDA) supply program (Bethesda, MD). NS6740 (1, 4-diazabicyclo [3.2.2] nonan-4-yl (5-(3-(trifluoromethyl) phenyl) furan-2-yl) methanone). NS1738 was purchased from Tocris Biosciences (Minneapolis, MN). Morphine sulfate was obtained from the National Institute on Drug Abuse (Baltimore, MD, USA). Nicotine, morphine, NS6740, and PNU282987 were dissolved in physiological saline. NS1738 and PNU120596 were dissolved in a mixture of 1:1:18 [1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ) and 18 volumes distilled water]. Nicotine, morphine, and PNU282987 were injected subcutaneously (s.c.), while all other drugs were administered intraperitoneally (i.p.). The nicotine solution pH was neutralized to pH 7.0 with sodium bicarbonate as needed. Freshly prepared solutions were given to mice at 10 ml/kg, s.c. Doses are expressed as the free base of the drug.
Nicotine and morphine conditioned place preference studies
An unbiased CPP paradigm was performed, as previously described (Kota et al. 2007). Briefly, the CPP apparatus consisted of three chambers in a linear arrangement (Med Associates, St Albans, VT). The CPP apparatus (MedAssociates, St. Albans, VT, ENV3013) consisted of white and black chambers (20 × 20 × 20 cm each), which differed in overall color and floor texture (white mesh or black rod), separated by a smaller gray chamber with a smooth PVC floor. Removeable partitions could isolate mice to chambers or could be removed to allow access between the gray, black, and white chambers. On day 1, animals were confined to the middle gray chamber for a 5-min habituation and then allowed to freely move between all three chambers for 15 min. Time spent in each chamber was recorded, and these data were used to populate groups of approximately equal bias in baseline chamber preference. Twenty-minute conditioning sessions occurred twice a day (days 2–4) for nicotine conditioning, while 30-min conditioning was used for morphine CPP. During conditioning sessions, mice were confined to one of the larger black and white chambers. The saline groups received saline in one large chamber in the morning and saline in the other large chamber in the afternoon. The nicotine group received nicotine (0.5 mg/kg, s.c.) in one large chamber and saline in the other large chamber. Treatments were counterbalanced equally to ensure that some mice received the unconditioned stimulus in the morning, while others received it in the afternoon. The nicotine-paired chamber was randomized among all groups. Sessions were 4 h apart and were conducted by the same investigator. On each of the conditioning days, mice were pretreated with PNU282987 (1, 3, and 9 mg/kg, s.c.), NS1738 (1 and 10 mg/kg, i.p.), PNU120596 (0.3, 1, and 3 mg/kg, i.p.), NS6740 (1 and 3 mg/kg, i.p.) or their respective vehicle 15 min prior to morphine (10 mg/kg, s.c.), nicotine (0.5 mg/kg, s.c.), or saline injection. On test day (day 5), mice were allowed access to all chambers for 15 min in a drug-free state. The preference score was calculated by determining the difference between the time spent in the drug paired side during test day versus the time in drug paired side during the baseline day.
Statistical analysis
Data were analyzed using the GraphPad software version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. A one-way analysis of variance (ANOVA) in conjunction with Holm-Šídák comparison tests was conducted to determine significant effects of drug treatments vs controls. Comparisons were considered statistically significant when p < 0.05.
Results
Nicotine CPP attenuated by α7 nAChR full orthosteric agonist PNU282987
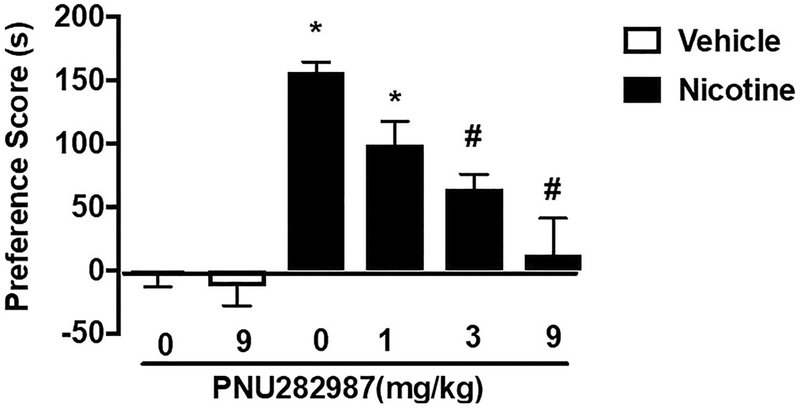
Mice were conditioned with either saline or nicotine (0.5 mg/kg) for 3 days in the CPP paradigm. A robust CPP was observed in nicotine-conditioned mice pre-treated with vehicle [F (5, 42) = 16.08, p < 0.0001]. PNU282987 reduced nicotine reward in the CPP test. Post hoc analysis revealed that pretreatment with a lower dose of PNU282987 (1 mg/kg) did not significantly alter nicotine CPP (p > 0.05), but higher doses of the agonist (3 and 9 mg/kg) did (p < 0.05) (Fig. 1). PNU282987 at the dose of 9 mg/kg did not produce a preference or aversion in saline-treated mice. PNU282987 was administered within the range of doses and pretreatment time used for other behavior studies (de Moura and McMahon 2017; Vicens et al. 2013).
Fig. 1.
α7 nAChR full orthosteric agonist PNU282987 blocks nicotine CPP. Mice were conditioned with either saline (s.c.) or nicotine(0.5 mg/kg) for 3 days. A robust CPP was observed in nicotine-conditioned mice pretreated with vehicle. PNU282987 (1, 3, and 9 mg/kg; s.c.) attenuated nicotine reward as measured by the CPP. Asterisk denotes p < 0.05 from vehicle-vehicle. Number sign denotes p < 0.05 from nicotine control. Each point represents the mean ± SEM of n = 8 mice per group
α7 nAChR Type I PAM NS1738 had no effect on nicotine CPP
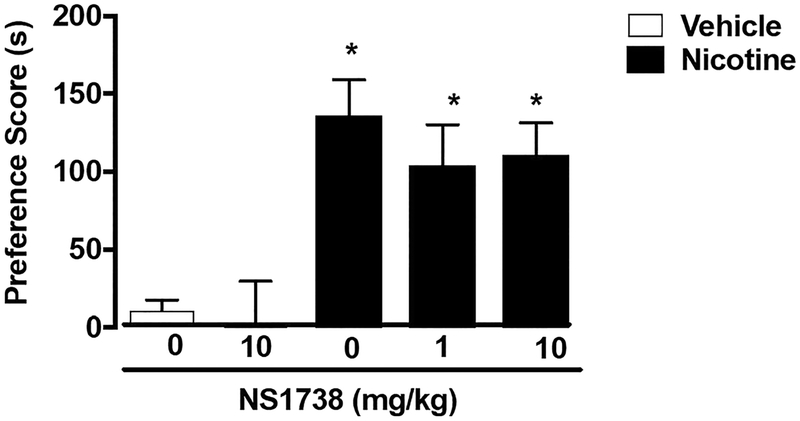
CPP conditioning with either saline or nicotine (0.5 mg/kg) was performed for 3 days. CPP was observed in nicotine-conditioned mice pre-treated with vehicle [F (4, 36) = 7.365, p = 0.0002]. NS1738 did not reduce nicotine reward at either dose tested (1 and 10 mg/kg) (Fig. 2). NS1738 at the dose of 10 mg/kg did not produce a preference or aversion in saline-treated mice. NS1738 was used at doses and a pretreatment time previously described (Freitas et al. 2013a, c).
Fig. 2.
α7 nAChR Type I PAM NS1738 did not block nicotine CPP. Mice underwent 3 days of conditioning with either saline (s.c.) or nicotine (0.5 mg/kg). Nicotine produced a robust CPP in mice pre-treated with vehicle. The α7 Type I PAM NS1738 (1 and 10 mg/kg; i.p.) did not alter nicotine reward as measured by the CPP test at both doses tested. Asterisk denotes p < 0.05 from vehicle-vehicle. Each point represents the mean ± SEM of n = 7–10 mice per group
α7 nAChR Type II PAM PNU120596 reduced nicotine CPP
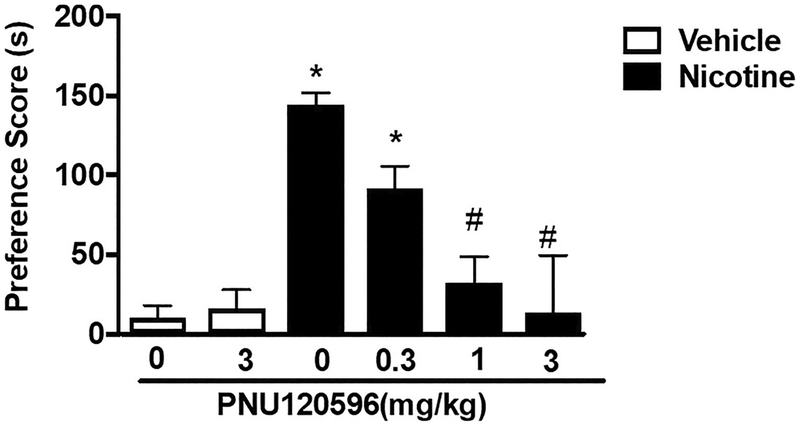
CPP conditioning with either saline or nicotine (0.5 mg/kg) was performed for 3 days. CPP was observed in nicotine-conditioned mice pre-treated with vehicle reward [F (5,60) = 7.825, p < 0.0001]. PNU120596 significantly reduced nicotine reward. Post hoc analysis revealed that pre-treatment with a lower dose of PNU120596 (0.3 mg/kg) did not significantly alter nicotine CPP (p > 0.05), but higher doses of the PAM (1 and 3 mg/kg) did (p < 0.05) (Fig. 3). PNU120596 at the dose of 3 mg/kg did not produce a preference or aversion in saline-treated mice. PNU120596 was used at similar doses and a pretreatment time previously described (Freitas et al. 2013a, b).
Fig. 3.
Attenuation of the development of nicotine CPP by α7 nAChR Type II PAM PNU120596. Mice were administered saline (s.c.) or nicotine (0.5 mg/kg; s.c.) for 3 days. Nicotine administration produced a significant CPP in mice pre-treated with vehicle. The α7 Type II PAM PNU120596 (0.3, 1, and 3 mg/kg; i.p.) reduced nicotine reward as measured by the CPP test at both doses tested. Asterisk denotes p < 0.05 from vehicle-vehicle. Number sign denotes p < 0.05 from nicotine control. Each point represents the mean ± SEM of n = 9–15 mice per group
α7 nAChR silent agonist NS6740 did not attenuate nicotine CPP
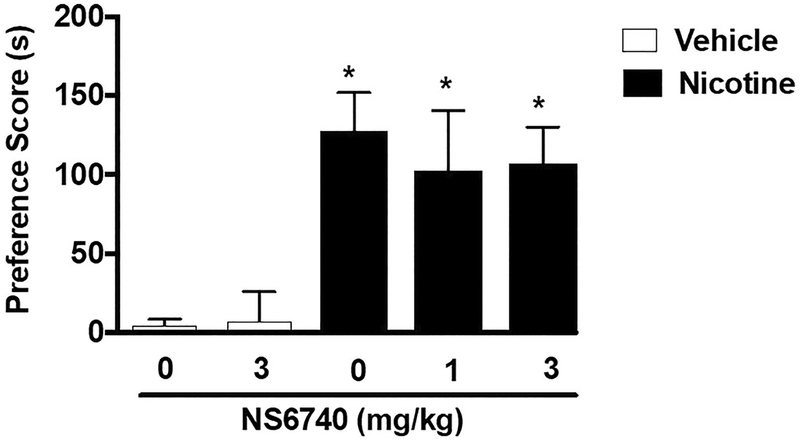
CPP conditioning with either saline or nicotine (0.5 mg/kg) was performed for 3 days. CPP was observed in nicotine-conditioned mice pre-treated with vehicle [F (4, 39) = 6.674, p = 0.0003]. NS6740 had no effect on nicotine reward at both doses tested (1 and 3 mg/kg) (Fig. 4). NS6740 at the dose of 3 mg/kg did not produce a preference or aversion in saline-treated mice. NS6740 was used at a range of doses and a pretreatment time previously described (Papke et al. 2015; Briggs et al. 2009).
Fig. 4.
No effect of α7 nAChR silent agonist NS6740 on the development of nicotine CPP. Mice underwent 3 days of conditioning with either saline (s.c.) or nicotine (0.5 mg/kg). Nicotine produced a robust CPP in mice pre-treated with vehicle. The α7 silent agonist NS6740 (1 and 3 mg/kg;i.p.) did not reduce nicotine reward as measured by the CPP test at both doses tested. Asterisk denotes p < 0.05 from vehicle-vehicle. Each point represents the mean ± SEM of n = 7–10 mice per group
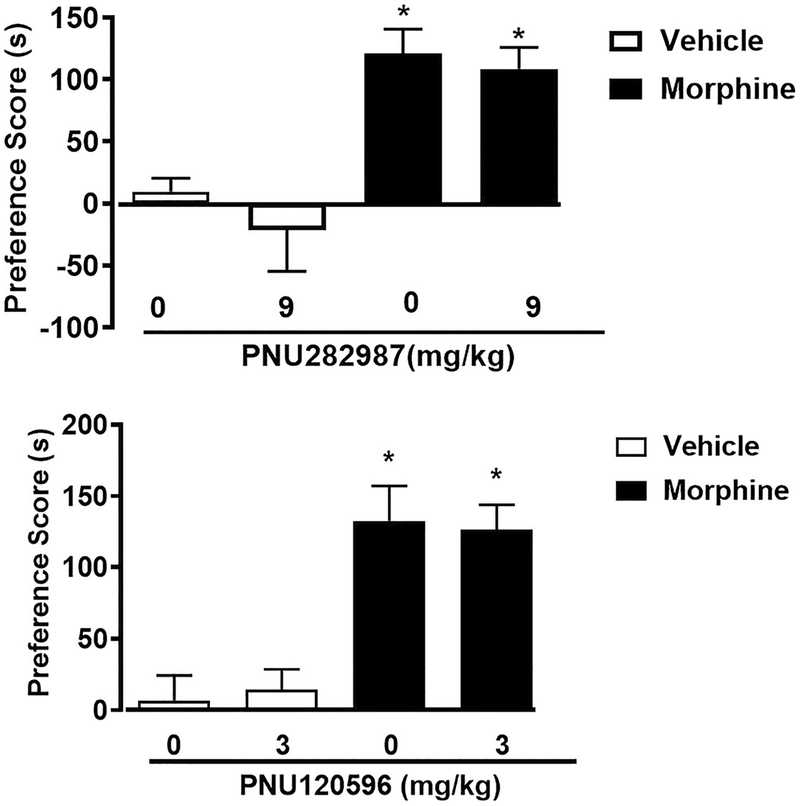
α7 nAChR full orthosteric agonist and PAM Type II did not attenuate morphine CPP
To assess the generalizability of these effects for other CPP learning, we tested the effects of α7 nAChR full orthosteric agonist PNU282987 and α7 Type II PAM PNU120596 on morphine CPP. CPP conditioning with either saline or morphine (10 mg/kg) was performed for 3 days. CPP was observed in morphine-conditioned mice pre-treated with vehicle [F (3, 28) = 10.51, p < 0.0001]. PNU282987 had no effect on morphine reward at the 9 mg/kg dose (p > 0.05) (Fig. 5a). PNU282987 at the dose of 9 mg/kg did not produce a preference or aversion in saline-treated mice.
Fig. 5.
PNU282987 and PNU120596 did not block morphine CPP. Mice were conditioned with saline (s.c.) or morphine (10 mg/kg) for 3 days. Morphine produced a robust CPP in mice pre-treated with vehicle. The α7 full orthosteric agonist PNU282987 (9 mg/kg; s.c.) did not attenuate morphine reward as measured by the CPP. Similarly, the α7 Type II PAM PNU120596 (3 mg/kg; i.p.) did not reduce morphine reward as measured by the CPP. Asterisk denotes p < 0.05 from vehicle-vehicle. Each point represents the mean ± SEM of n = 8 mice per group
Similarly, in another cohort of mice, mice conditioned with morphine (10 mg/kg for 3 days) demonstrated CPP compared to vehicle treated mice [F (3, 28) = 13.41, p = 0.0001]. PNU120596 (3 mg/kg) did not attenuate morphine CPP (Fig. 5b). PNU120596 (3 mg/kg) did not produce a preference or aversion in saline-treated mice (p > 0.05).
Discussion
The results of this study produced interesting findings about the impact of α7 nAChR modulation and conformations on nicotine reward in mice. The α7 full orthosteric agonist PNU282987 and the Type II α7 nAChR PAM PNU120596 reduced nicotine CPP (Figs. 1 and 3), while the silent agonist NS6740 and Type I PAM NS1738 had no effect (Figs. 2 and 4). In addition, the PNU282987 and PNU120596 had no effect on morphine CPP (Fig. 5a, b) at doses that reduced nicotine CPP. This is one of the first reports of α7 nAChR PAMs and a silent agonist used in a preclinical test of nicotine reward.
Using an optimal dose of 0.5 mg/kg s.c. nicotine to establish CPP, co-administration of an α7 nAChR-selective agonist or a Type II PAM effectively attenuated nicotine CPP. This is perhaps in part due to the ability of the Type II PAM to decrease the equilibrium desensitization of the α7 nAChRs (Williams et al. 2011b). PNU120596 not only increases the chance of ion conductance but also allows the channels to enter open states for a longer duration, which also results in an increase of possible ion conductance (Williams et al. 2011b). Similarly, this may explain the divergent effects of the Type I and Type II PAMs used in this study. NS1738 had no effect on nicotine CPP at both doses tested (1 and 10 mg/kg i.p.). In a mouse model of tonic pain, the Type II PAM PNU120596, but not the Type I PAM NS1738, also reduced pain-related behaviors in the early and late phase of the formalin test (Freitas et al. 2013b). This is not due to lack of absorption, however, as systemic administration of NS1738 at similar doses produced brain concentrations (Freitas et al. 2013b) that were shown to enhance the channel opening of acetylcholine in vitro (Timmermann et al. 2007). Although these preclinical data suggest that stimulation of α7 nAChRs ought to reduce nicotine reward in smokers, it should be noted that JNJ-39393406, an α7 PAM, was shown to have no effect on smoking behaviors in humans (Perkins et al. 2018). It is unclear from previous studies if JNJ-39393406 acts as a Type I α7 PAM, Type II α7 PAM, or both (Winterer et al. 2013; Perkins et al. 2018). However, given the small sample size for healthy smokers, duration and the use of only one dose, this within subjects, cross-over design clinical study may not have sufficiently evaluated the effect of an α7 PAM on smoking behaviors.
In contrast to the other compounds used, the silent agonist NS6740 does not activate α7 nAChRs but rather induces a desensitized state with the absence of an open state. In these studies, NS6740 did not alter nicotine CPP at either dose tested (1 and 3 mg/kg i.p.). Higher doses of NS6740 were not used due to aversion it caused on its own that would confound interpretation of these data. NS6740 at similar doses used in this study was also effective at reducing chronic pain and inflammation in mice (Papke et al. 2015). NS6740 has been shown to antagonize the cognitive effects of an α7 nAChR full agonists (Briggs et al. 2009; Pieschl et al. 2017). An α7 nAChR antagonist has been shown to increase nicotine i.v. self-administration (Brunzell and McIntosh 2012), and genetic deletion of the receptor has been shown to increase nicotine CPP (Harenza et al. 2014); thus, it would be expected that NS6740 would enhance nicotine CPP. However, while typical silent agonists may be characterized as simply very weak partial agonists (Papke et al. 2014), NS6740 has numerous and complex effects on α7 conformational dynamics. NS6740 causes protracted suppression of α7 channel activation, and it effectively activates α7-mediated signal transduction (Thomsen and Mikkelsen 2012) and cholinergic anti-inflammatory pathways (Papke et al. 2015). On its own, it can reduce synaptic function (Papke et al. 2018), but it can also synergize with a Type II PAM to produce persistent α7 currents (Papke et al. 2017). Therefore, due to the complexities of NS6740’s interaction with the α7 nAChR, there is a need for more studies to fully understand the impact of this compound on nicotine reward-related behaviors. Furthermore, it is possible that different pharmacokinetic profiles of NS1738 and NS6740 explain their lack of effects in the nicotine CPP model. However, previous studies in mice showed that these two alpha7 ligands were active in various mouse models of pain (Freitas et al. 2013b; Papke et al. 2015).
Taken together, our results suggest that ion conductance and channel opening of the α7 nAChR play important roles in the modulation of nicotine reward behaviors in mice, such that stimulation of α7 nAChR appears to counter nicotine reward. In addition, it has been shown that α7 nAChR modulation with PAMs reduces nicotine withdrawal symptoms in mice (Jackson et al. 2018). α7 nAChR PAMs may provide less perturbation of the endogenous cholinergic system in comparison to orthosteric α7 nAChR agonists’ activation. Electrophysiological studies demonstrate that PAMs on their own have no apparent effect, rather they are only able to amplify the effects of ACh or other agonists (Timmermann et al. 2007; Wang et al. 2012). In addition, PAMs also may provide better selectivity than putatively selective agonists for α7 nAChRs compared to other nAChR subtypes associated with nicotine reward, since all the brain nAChR have a high homology of their ligand binding domain (Gurley and Lanthorn 1998) and the PAMs bind to an allosteric site that is unique to α7 receptor and distinct from the orthosteric agonist binding site. The silent agonist NS6740 used in this study provided understanding by contrasting the role of α7 nAChR desensitization and ion conductance in nicotine reward. Of importance, these compounds have been shown to interact in a similar fashion with human α7 receptors (Papke et al. 2015; Quadri et al. 2016; Timmermann et al. 2007; Wang et al. 2012), supporting the possible clinical benefits of these compounds. To our knowledge, there are only three published reports on PAMs in humans and these studies did not report any serious side effects (Gee et al. 2017; Perkins et al. 2018; Winterer et al. 2013). Thus, PAMs and silent agonists may serve as useful complementary tools to understand the effect of α7 nAChR modulation in nicotine reward.
Funding
This study was supported by NIH grant [DA 005274 and DA032246] to MID. AJ was supported by T32 (DA007027) from NIH. RLP was supported by [GM57481].
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Briggs CA, Grønlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M (2009) Role of channel activation in cognitive enhancement mediated by Alpha7 nicotinic acetylcholine receptors. Br J Pharmacol 158:1486–1494. 10.1111/j.1476-5381.2009.00426.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioni JD, Kim DJ, O’Neill AB (1996) Nicotine cue: lack of effect of the alpha 7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol 301:1–5. 10.1016/0014-2999(96)00010-6 [DOI] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM (2012) Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology 37: 1134–1143. 10.1038/npp.2011.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura FB, McMahon LR (2017) The contribution of α4β2 and non-α4β2 nicotinic acetylcholine receptors to the discriminative stimulus effects of nicotine and varenicline in mice. Psychopharmacology 234:781–792. 10.1007/s00213-016-4514-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI (2013a) The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther 344:264–275. 10.1124/jpet.112.197871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Carroll FI, Lichtman AH, Damaj MI (2013b) Effects of alpha 7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65:156–164. 10.1016/j.neuropharm.2012.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Negus S, Carroll FI, Damaj MI (2013c) In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol 169:567–579. 10.1111/j.1476-5381.2012.02226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Olincy A, Kanner R, Johnson L, Hogenkamp D, Harris J, Tran M, Edmonds SA, Sauer W, Yoshimura R, Johnstone T, Freedman R, 2017. First in human trial of a type I positive allosteric modulator of alpha7-nicotinic acetylcholine receptors: pharmacokinetics, safety, and evidence for neurocognitive effect of AVL-3288 1–8. doi: 10.1177/0269881117691590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlien JH, Hakerud M, Ween H, Thorin-Hagene K, Briggs CA,Gopalakrishnan M, Malysz J (2007) Distinct profiles of 7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724. 10.1124/mol.107.035410 [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA (2000) Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine.J. Pharmacol. Exp. Ther 294, 1112–1119. [PubMed] [Google Scholar]
- Gurley DA, Lanthorn TH (1998) Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci Lett 247:107–110. 10.1016/S0304-3940(98)00306-1 [DOI] [PubMed] [Google Scholar]
- Harenza JL, Muldoon PP, De Biasi M, Damaj MI, Miles MF (2014) Genetic variation within the Chrna7 gene modulates nicotine reward-like phenotypes in mice. Genes Brain Behav 13:213–225. 10.1111/gbb.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL (1995) The pharmacology of galanthamine and its analogues. Pharmacol Ther 68:113–128. 10.1016/0163-7258(95)02002-0 [DOI] [PubMed] [Google Scholar]
- Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD (2012) Galantamine, an acetylcholinesterase inhibitor and positive allosteric modulator of nicotinic acetylcholine receptors, attenuates nicotine taking and seeking in rats. Neuropsychopharmacology 37: 2310–2321. 10.1038/npp.2012.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Bagdas D, Muldoon PP, Lichtman AH, Carroll FI, Greenwald M, Miles MF, Damaj MI, 2017. In vivo interactions between α7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-α: implication for nicotine dependence. Neuropharmacology 118. doi: 10.1016/j.neuropharm.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Papke RL, Damaj MI (2018) Pharmacological modulation of the α7 nicotinic acetylcholine receptor in a mouse model of mecamylamine - precipitated nicotine withdrawal. Psychopharmacology 235:1897–1905. 10.1007/s00213-018-4879-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI (2007) Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther 322:399–407. 10.1124/jpet.107.121616 [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JNC, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S (2009) alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci 29:539–550. 10.1111/j.1460-9568.2009.06613.x [DOI] [PubMed] [Google Scholar]
- Maelicke A, Albuquerque EX (2000) Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 393:165–170. 10.1177/0095327X0403100111 [DOI] [PubMed] [Google Scholar]
- Kowal NM, Ahring PK, Liao VWY, Indurti DC, Harvey BS, O’Connor SM, Chebib M, Olafsdottir ES, Balle T, (2017) Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors. British Journal of Pharmacology 175 (14):2911–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Chojnacka K, Horenstein NA (2014) The minimal pharmacophore for silent agonism of the α7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther 350:665–680. 10.1124/jpet.114.215236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bagdas D, Kulkarni AR, Gould T, Alsharari SD, Thakur GA, Damaj MI (2015) The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology 0:34–42. 10.1016/j.neuropharm.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R, Stokes C, Damaj M, Thakur G, Manther K, Treinin M, Bagdas D, Kulkarni AR, Horenstein NA (2017) Persistent activation of α 7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br J Pharmacol 1–17:1838–1854. 10.1111/bph.13851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Peng C, Kumar A, Stokes C (2018) NS6740, an α7 nicotinic acetylcholine receptor silent agonist, disrupts hippocampal synaptic plasticity. Neurosci Lett 677:6–13. 10.1586/14737175.2015.1028369.Focused [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Roy Chengappa KN, Karelitz JL, Boldry MC, Michael V, Herb T, Gannon J, Brar J, Ford L, Rassnick S, Brunzell DH (2018) Initial cross-over test of a positive allosteric modulator of Alpha-7 nicotinic receptors to aid cessation in smokers with or without schizophrenia. Neuropsychopharmacology 43:1334–1342. 10.1038/npp.2017.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieschl RL, Miller R, Jones KM, Post-Munson DJ, Chen P, Newberry K, Benitex Y, Molski T, Morgan D, McDonald IM, Macor JE, Olson RE, Asaka Y, Digavalli S, Easton A, Herrington J, Westphal RS, Lodge NJ, Zaczek R, Bristow LJ, Li YW (2017) Effects of BMS-902483, an α7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents. Eur J Pharmacol 807:1–11. 10.1016/j.ejphar.2017.04.024 [DOI] [PubMed] [Google Scholar]
- Quadri M, Papke RL, Horenstein NA (2016) Dissection of N,N-diethyl-N′-phenylpiperazines as α7 nicotinic receptor silent agonists. Bioorg Med Chem 24:286–293. 10.1016/j.bmc.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochocki M (2003) Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 305:1024–1036. 10.1124/jpet.102.045773 [DOI] [PubMed] [Google Scholar]
- Schilström B, Nomikos GG, Nisell M, Hertel P, Svensson TH (1998) N-methyl-D-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience 82:781–789. 10.1016/S0306-4522(97)00243-1 [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD (2012) Type I and II positive allosteric modulators differentially modulate agonist-induced up-regulation of α7 nicotinic acetylcholine receptors. J Neurochem 123:73–83. 10.1111/j.1471-4159.2012.07876.x [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, Ahring PK, Peters D, Holst D, Chrsitensen JK, Malysz J, Briggs CA, Gopalakrishnan M, Olsen GM (2007) An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther 323:294–307. 10.1124/jpet.107.120436.vidual [DOI] [PubMed] [Google Scholar]
- Vicens P, Ribes D, Heredia L, Torrente M, Domingo JL (2013) Motor and anxiety effects of PNU-282987, an alpha7 nicotinic receptor agonist, and stress in an animal model of Alzheimer’s disease. Curr Alzheimer Res 10:516–523. 10.2174/15672050113109990130 [DOI] [PubMed] [Google Scholar]
- Wang J, Papke RL, Stokes C, Horenstein NA (2012) Potential state-selective hydrogen bond formation can modulate activation and desensitization of the ␣ 7 nicotinic acetylcholine receptor * □. J Biol Chem 287:21957–21969. 10.1074/jbc.M112.339796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL (2011a) The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol 137:369–384. 10.1085/jgp.201010587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL (2011b) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930. 10.1016/j.bcp.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, Rujescu D, Favis R, Sun Y, Franc MA, Ouwerkerk-Mahadevan S, Janssens L, Timmers M, Streffer JR (2013) Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study. Neuropharmacology 64:197–204. 10.1016/j.neuropharm.2012.06.040 [DOI] [PubMed] [Google Scholar]