Abstract
Rationale
Although the influence of gestational cocaine exposure on offspring has been the focus of a sustained research effort, the effect of preconception cocaine self-administration by dams on progeny has received far less attention.
Method
In the current study, adult female rats were allowed to self-administer cocaine 2-hours a day for 60 days and then, after a ten-day wash out period, bred to naïve males. Maternal behavior was measured in dams until weaning. When male and female progeny reached adulthood, anxiety-like behavior, memory and cocaine self-administration were assessed in separate cohorts of rats.
Results
Despite a total of at least 30 days of cocaine abstinence, the quality of maternal behaviors was negatively affected by previous cocaine exposure as reflected by less time spent with pups as well as an excess of other maladaptive maternal behaviors. Measures of anxiety-like behavior and memory were not affected by maternal cocaine intake in either male or female offspring. In contrast, male, but not female, progeny of dams exposed to cocaine showed increased reinforcing efficacy of cocaine as measured by cocaine self-administration under a progressive ratio schedule. The fact that cocaine self-administration was influenced only in the male offspring of cocaine exposed dams argues against this phenotype being linked to altered maternal behavior, although this possibility cannot be ruled out completely.
Conclusions
Collectively, these results indicate that preconception cocaine self-administration by dams results in the relatively selective enhancement of cocaine addiction-like behavior in male offspring.
Introduction
The initial crack cocaine abuse epidemic in the 1980s focused attention on the potential deleterious effects on children exposed to cocaine prenatally. Fortunately, the initial fears that so-called crack babies would suffer from severe and persistent neurological and psychiatric disorders ultimately proved to be greatly exaggerated (Betancourt et al. 2011; Hurt et al. 2009). That said, children exposed to cocaine prenatally tend to be small at birth and subsets of these individuals display developmental and cognitive issues (Dos Santos et al. 2018; McCarthy et al. 2014). Consistent with these results, rodent studies in which offspring were exposed to cocaine in utero show impaired executive function and memory as well as disrupted cortical and hippocampal development (Dow-Edwards 2011; Lidow 2003).
Less is known about the influence of maternal drug use prior to, but not during, pregnancy on offspring behavior and physiology. Although there are some relevant clinical findings, such as decreased birth weight in children of alcoholic women that abstain from use during pregnancy (Livy et al. 2004; Ramsay 2010), most work of this sort is preclinical. For example, adolescent female rats were exposed to cannabinoids during adolescence and then were maintained drug-free for over three weeks and bred during adulthood. Using this paradigm, it was shown that male offspring of adolescent cannabinoid-exposed dams demonstrated enhanced expression of morphine-induced behavioral sensitization and conditioned place preference (Byrnes et al. 2012; Vassoler et al. 2013a). A similar model focusing on preconception morphine indicated that adult offspring show sex-specific changes in measures of anxiety as well as morphine-induced behavioral sensitization, morphine reinforcement and morphine conditioned reward as well as cocaine reward (Byrnes 2005; Byrnes et al. 2011; Vassoler et al. 2017; Vassoler et al. 2018; Vassoler et al. 2016). In terms of cocaine, the offspring of rat dams that received experimenter-delivered cocaine prior to pregnancy showed increased cocaine-induced behavioral hyperactivity but no changes in the stress-induced corticosterone response (Sasaki et al. 2014). Here, we allowed rat dams to self-administer cocaine prior to breeding and examined cocaine self-administration, anxiety-related behaviors and measures of learning and memory in the adult female and male offspring.
Materials and Methods
F0 dam cocaine self-administration and breeding
Adult female Sprague Dawley rats were acquired from Taconic Laboratories. Surgery was performed to implant animals with jugular catheters as described previously (Schmidt et al. 2009; Vassoler et al. 2008). Following a 7-day recovery period, females were allowed to self-administer cocaine (0.25 mg cocaine/56 μl saline/infusion over 5 sec; the cocaine dose was not adjusted for animal weight) on a fixed ratio 1 (FR1) schedule of reinforcement 2 hours daily for 60 days. This time frame more than encompasses the 45-day oocyte maturation time in rats. After a wash-out period of 10 days during which cocaine self-administration was discontinued and animals remained individually housed, females were placed into a cage with naïve males at a ratio of 1 to 1. Animals remained pair-housed for a maximum of 10 days; vaginal plugs were checked every day and the breeding pair was immediately separated if a plug was detected. Offspring were weaned and group-housed by sex at P21. When the offspring reached P60, one to three male and female rats per litter were selected and randomly assigned to one behavioral experiment. This experimental design prevented over-representation of any litter in each of our assessments. All experiments were conducted between PND 60–90.
Maternal behavior
Maternal behavior of F0 cocaine- and saline-exposed dams (n=4 for each condition) was monitored for two hours daily, for five to six days between P3 and P9. During each observation period, the time dams spent in each of five different postures was quantified: mother handling or grooming any pup, mother nursing in an arched position, blanket posture, passive posture (in contact or close proximity with pups without active interaction), and time spent away from pups, as described previously (Vassoler et al. 2013b; Weaver et al. 2004).
F1 cocaine self-administration
Adult offspring (PND 60–90) were randomly selected from litters and assigned for single housing and catheter implantation. Following a 7-day recovery from surgery, offspring born from cocaine- or saline-exposed dams were allowed to lever press for cocaine (0.75 mg/kg/infusion) in standard operant chambers (Med Associates; Fairfax, VT). Animals were allowed to lever press for cocaine during daily 2-hour self-administration sessions under an FR1 schedule of reinforcement for a total of 11 days. The following day, rats were switched to an FR5 schedule (for a total of 2 days) and then to a progressive ratio (PR) schedule for one day. Under the PR schedule, the response requirement for each subsequent drug delivery increased until the subject took >30 minutes to meet a requirement, at which point the session ended. The response requirement for the “ith” reinforcement was R(i) = [5e0.2i-5] (Richardson and Roberts 1996). The breakpoint was operationally defined as the total number of responses per session.
Object location memory task
As described previously (Wimmer et al. 2017), prior to the onset of training, animals were habituated to the training context (30” × 30” × 17”) during two 5-min sessions on two separate days. On the day of training, rats were placed in the training arena with two identical objects for a total of three 5-min sessions with an intersession interval of 10 min, during which animals were returned to their home cage. The objects used were two glass Erlenmeyer flasks. The objects were fixed to the floor using double sided tape. Thirty min after training, animals were returned to the training context for 5 min with one object displaced to a new location, while the other object was not moved. All sessions were videotaped and time spent exploring each object was scored by researchers blind to the experimental groups. Exploration of the objects was defined as the amount of time rats were oriented toward an object with their nose within ~1 cm of the object. Grooming near the object was not considered exploration. Percent preference was calculated as time spent exploring the displaced object relative to the total time spent exploring both objects.
Novel object recognition task
Object recognition memory experiments were performed in the same training arenas and using the same objects as described above. Animals were pre-exposed to the training context for 10 min per day on 5 days prior to the onset of training. Twenty-four hours after the last context exposure, rats were placed in the training arena with 2 identical objects (Erlenmeyer flasks) for 10 min. Thirty minutes after training, animals were placed back in the training arena for 10 min with one familiar object and one novel object (an L-shaped aluminum piece) in the same locations used during training. Exploration time was measured as described above. Percent preference was calculated as time spent exploring the novel object relative to the total time exploring both objects.
Novelty-induced hypophagia
For all steps of novelty-induced hypophagia assessment, food and water were removed from the home cage 90 min prior. As in our previous work (White et al. 2016), rats were habituated to the testing room in their home cages for 30 minutes. During 6 days of training, animals had access to peanut butter chips (Reese’s Peanut Butter Chips, 2.257 g) for 15 minutes in their home cages. On day 7, animals were given access to the peanut butter chips in a novel environment located in the same room. The novel environment was a brightly lit (1380 lux) polycarbonate cage of the same dimensions as the home cage (48 cm L × 26 cm W × 20 cm H) with a wire mesh floor and white walls. The novel arena was cleaned with 70% ethanol between trials. On day 8, animals were again given access to peanut butter chips in the testing room in their home cage to assess persistence of chip appetence. Across training and testing, the latency to consume the peanut butter chips was recorded. Latency was determined as the time to start eating peanut butter chips by an observer blinded to mothering.
Statistical Analysis
Data were analyzed in GraphPad Prism 7 and p<0.05 was the criteria for significance. Maternal behavior in cocaine-exposed vs. saline-exposed dams was analyzed by Chi-square test.
Unpaired t-test was used to compare litter size and sex distribution from cocaine-exposed vs. saline-exposed dams. Male and female offspring cocaine self-administration on FR1 or FR5 schedules were analyzed with separate two-way mixed model analyses of variance (ANOVAs) with a between-subject factor of dam exposure (saline- vs. cocaine-exposed) and repeated measures over time (days). Bonferroni correction was used for post-hoc comparisons. Unpaired t-test was used to analyze progressive ratio responding in male or female offspring from cocaine-exposed vs. saline-exposed dams. Male and female novel object recognition and object location memory data were analyzed with separate two-way mixed model ANOVAs with dam exposure as the between subject factor (saline- vs. cocaine-exposed) and session (train vs test) as the within subject factor. Bonferroni correction was used for post-hoc comparisons. Novelty induced hypophagia in male and female offspring was analyzed by separate two-way mixed model ANOVAs with dam exposure as the between subject factor (saline- vs. cocaine-exposed) and environment as the within-subject factor. Bonferroni correction was used for post-hoc comparisons.
Results
Maternal preconception cocaine self-administration negatively impacts maternal behaviors
In order to assess the impact of maternal preconception cocaine intake on offspring behavior, female Sprague-Dawley rats to self-administer i.v. cocaine (6.12±0.66 mg/day on average) for 60 days. This time frame largely encompasses the duration of oocyte maturation in rats (45 days), and ensures that a significant amount of the available pool of oocytes will have been exposed to cocaine throughout maturation. Control rats received yoked i.v. saline injections. After the last self-administration session, females underwent a wash-out period where they were individually housed in their home cage. In addition to ensuring the elimination of all cocaine metabolites (Mets et al. 1999), this increased the likelihood that ovulating oocytes had been exposed to cocaine throughout their maturation period. After 10 days, F0 females were mated with naïve males to produce first generation (F1) offspring (see Figure 1A). A total of 10 litters from cocaine-exposed females and 13 litters from saline-exposed females were generated. Litter size (an average of 7.7 for cocaine-exposed females vs 6.9 for saline-exposed animals) and litter sex-ratio (0.48 for cocaine-exposed females vs 0.50 for saline-exposed animals) were unaffected by cocaine self-administration [t(28)=−0.56247, p=0.54 and t(28)=−0.4354, p=0.67, respectively].
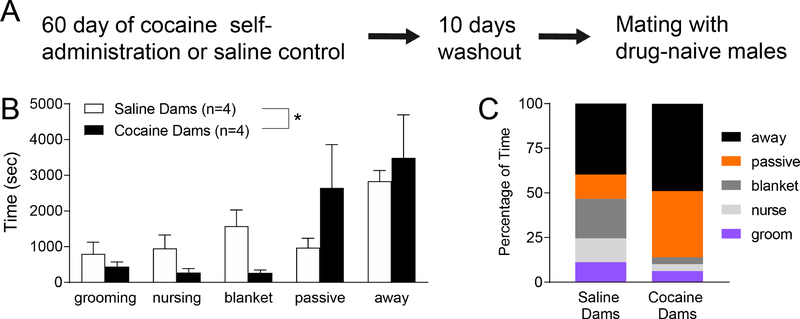
Figure 1: Maternal cocaine exposure prior to conception alters maternal behavior.
A. Dams self-administered cocaine for 60 consecutive days followed by a 10 day drug-free washout period. They were then paired with drug naïve males to produce F1 offspring. B. Cocaine-exposed dams showed significant changes in a range of maternal and passive behaviors. * p<0.0001. C. Average time spent on each behavior in saline vs. cocaine dams.
As cocaine can affect pup care in dams (Febo and Ferris 2007; Nephew and Febo 2010), maternal behavior of cocaine-exposed and saline-exposed dams was monitored after offspring birth. As compared to saline-exposed females, cocaine-exposed dams spent more time away or in passive contact with their pups (Fig.1B and 1C, p<0.0001) and less time providing active care. These results suggest that preconception cocaine self-administration negatively affects maternal behavior.
Maternal cocaine increases cocaine self-administration in male offspring
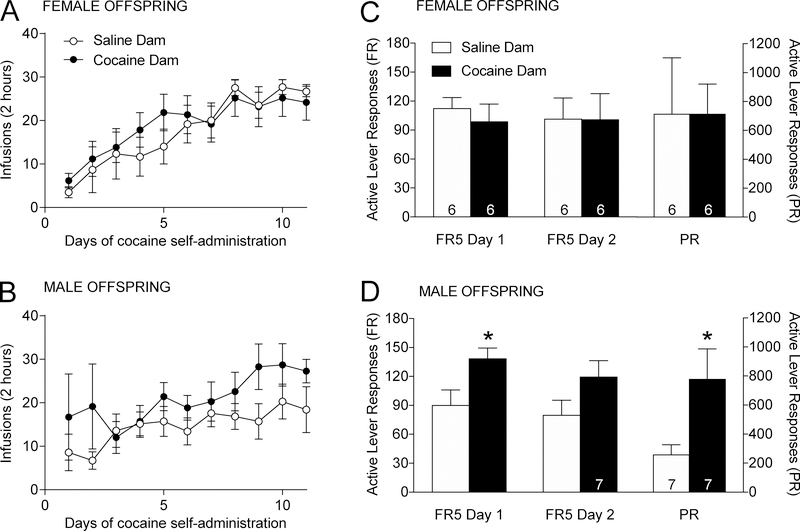
We assessed the acquisition of cocaine self-administration under an FR1 schedule in adult male and female progeny. There was no statistical difference in the rate of acquisition or the level of cocaine intake among female or male offspring of cocaine-exposed females relative to saline-exposed animals (Figs. 2A and 2B). There was a significant main effect of time [F(10,120)=3.184, p=0.0012 for male offspring; F(10,100)=14.89, p<0.0001 for female offspring], but no effect of dam drug exposure [F(1,12)=1.667, p=0.221 for male offspring; F(1,10)=0.097, p=0.762 for female offspring]. After 11 daily sessions of cocaine self-administration, offspring were switched to an FR5 schedule for two days (Figs. 2C and 2D). For male offspring, the ANOVAs revealed a significant main effect of time [F(1,12)=12.162, p=0.004], and a nonsignificant trend towards a significant main effect of dam exposure [F(1,12)=4.661, p=0.0518]; Bonferroni post-hoc comparisons revealed that dam exposure had a significant effect on the first day of FR5 schedule (p=0.046). For female offspring, no significant main effect of time [F(1,10)=0.0989, p=0.760] nor dam exposure [F(1,10)=0.004, p=0.951] were observed.
Figure 2. Pre-conception maternal cocaine exposure increases the reinforcing efficacy of cocaine in male but not female progeny.
A. Adult F1 females from cocaine- or salineexposed dams identically self-administered cocaine (0.75 mg/kg/infusion) on a fixed ratio 1 schedule for 11 daily two-hour sessions. B. Similarly, adult F1 males did not differ in cocaine self-administration (0.75 mg/kg/infusion) on an FR1 for 11 days. C. Number of active responses during two days of FR5 schedule are shown on the left, total number of active responses during the PR session are shown on the right. Female offspring similarly responded for cocaine on an FR5 and on a PR schedule (right). D. Number of active responses during two days of FR5 schedule are shown on the left, total number of active responses during the PR session are shown on the right. Male offspring from cocaine-exposed dams displayed significantly higher lever presses for cocaine on an FR5 schedule as well as on a PR schedule. (*p<0.05). Group size (n) is indicated by the number located in the bar. Number of litters represented: female offspring; n=4 for saline, n=3 for cocaine; male offspring n=3 for saline; n=4 for cocaine.
Following the acquisition of cocaine self-administration, all subjects were switched to a progressive ratio (PR) schedule, under which the response requirement for each subsequent drug delivery increases until the subject fails to meet a requirement. Dam cocaine exposure did not influence cocaine self-administration under a PR schedule among female offspring (Fig 2C, t(12)=0.00114, p=0.999). However, male offspring from cocaine-exposed dams showed increased breakpoints compared to males descended from saline-exposed dams (Fig 2D, t(14)=2.389, p=0.034). These findings indicate that pre-conception maternal cocaine exposure increases cocaine reinforcing efficacy in male, but not female progeny.
Litter effects are an important consideration in all multigenerational studies. We chose to address this potential confound by ensuring that no single litter was over-represented in any of our experiments. Thus, for each behavioral measurement, we used no more than 3 individuals from a single litter. Importantly, it is difficult to assess within-litter variability in our experiments because some litters are only represented by one animal. Therefore, we analyzed the data for each group, using litter as a between subject factor and day as the within subject factor. These ANOVAs revealed an overall effect of day for each group, but no overall effect of litter for any of the cohorts (male saline effect of litter: [F(2,4)=0.2305; p=0.8030] effect of day [F(10,40)=2.668; p=0.0134]; male cocaine effect of litter: [F(3,3)=0.9369; p=0.5207]; effect of day [F(10,30)=2.357, p=0.0340]; female saline effect of litter: [F(3,2)=2.483; p=0.3000]; effect of day [F(10,20)=12.7; p<0.0001]; female cocaine effect of litter: [F(2,3)=1.852; p=0.2993]; effect of day [F(10,30)=4.125; p=0.0012]). These data suggest all groups increased cocaine taking over days of cocaine self-administration but litter did not affect drug taking within each group.
Self-administration by dams has no influence on spatial or object recognition in male or female offspring
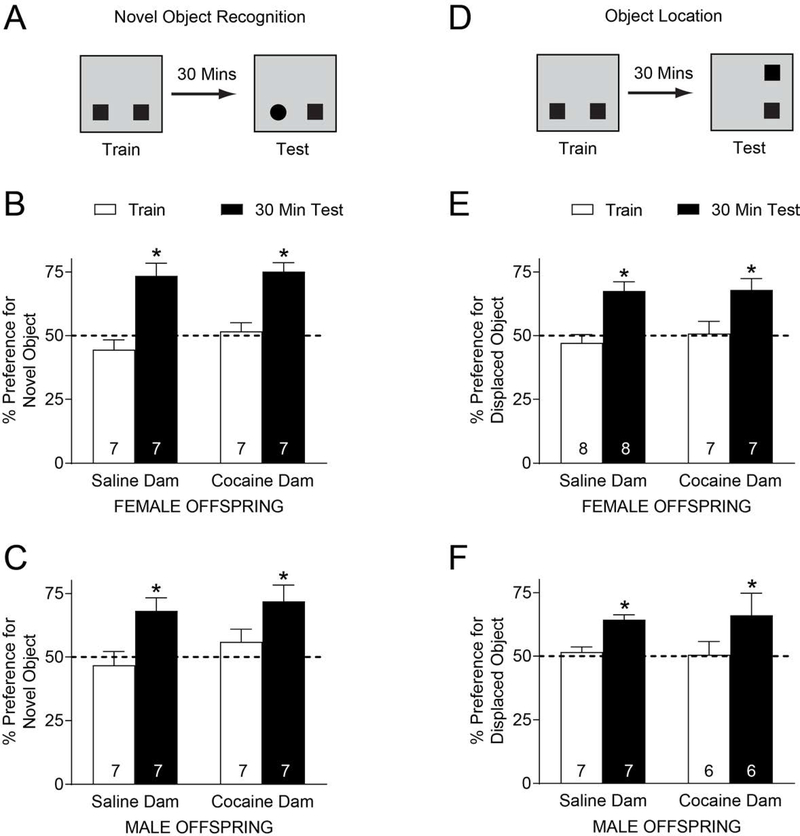
Sire cocaine exposure has been shown to affect short-term memory and anxiety in offspring (Fischer et al. 2017; He et al. 2006; Killinger et al. 2012; White et al. 2016; Wimmer et al. 2017). As such, we investigated the effects of F0 dam cocaine exposure on these behaviors in F1 offspring. First, we used a hippocampus-independent object recognition memory test (Forwood et al. 2005; Mumby 2001; Oliveira et al. 2010). Adult F1 animals were exposed to two identical objects in a familiar arena lacking spatial cues. Thirty minutes after training, a point at which animals born from cocaine-exposed sires exhibit memory defects (Wimmer et al. 2017), F1 offspring were returned to the training area where one of the objects was replaced by a novel one at the same location (Fig. 3A). Percent preference for the novel object (measured as the amount of time spent exploring the novel object over the total amount of time spent exploring both objects) was measured. For female offspring, although a significant main effect of session was detected [F(1,12)=28.98, p=0.0002], indicating a preference for the novel object, there was no significant effect of dam exposure [F(1,12)=2.439, p=0.1443] (Fig. 3B). Results were identical for male offspring, with a significant main effect of session [F(1,12)=10.65, p=0.007] and no effect of dam exposure [F(1,12)=1.461, p=0.250] (Fig. 3C). Taken together, these results demonstrate that object recognition is not affected by preconception maternal cocaine exposure.
Figure 3. Pre-conception maternal cocaine exposure has no effect on offspring memory.
A. In novel object recognition tests, the ability of offspring to recognize object novelty 30 min after training was assessed. B. Female F1 offspring exhibited a preference for the novel object regardless of dam exposure. C. Male F1 offspring exhibited a preference for the novel object regardless of dam exposure. D. In object location tests, the ability of offspring to recognize object displacement 30 min after training was assessed. E. Female F1 offspring exhibited a preference for the displaced object regardless of dam exposure. F. Male F1 offspring exhibited a preference for the displaced object regardless of dam exposure. * p<0.05 relative to saline. Group size (n) is indicated by the number located in the bar. Number of litters represented: female offspring NOR; n=6 for saline, n=5 for cocaine; male offspring NOR; n=3 for saline; n=4 for cocaine; female offspring SOR; n=5 for saline, n=3 for cocaine; male offspring SOR; n=3 for saline; n=4 for cocaine.
We also assessed the effect of dam cocaine exposure on a hippocampus-dependent memory task in a separate cohort of F1 offspring. We used an object location memory test, where adult F1 animals were exposed to two identical objects. Thirty minutes after training, offspring were returned to the training arena in which one of the objects was displaced to a novel location (Fig. 3D). Percent preference for the novel location (measured as the amount of time spent exploring the novel object over the total amount of time spent exploring both objects) was measured. Although there was a significant main effect of session [F(1,13)=15.88, p=0.002], indicating a preference for the displaced object no effect of dam exposure was detected for female offspring [F(1,13)=0.4292, p=0.524] (Fig. 3E). Similarly, male offspring results showed a significant main effect of session [F(1,11)=18.06, p=0.0014] and no effect of dam exposure [F(1,11)=0.0028, p=0.958]. Therefore, preconception cocaine dam exposure has no effect on offspring hippocampus-dependent object location memory.
No changes in offspring anxiety-like behavior when dams self-administered cocaine
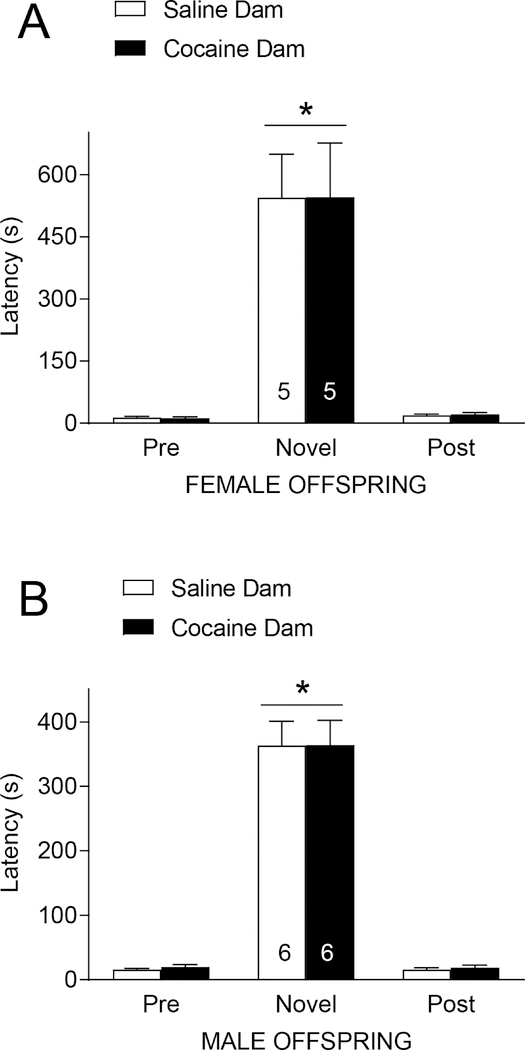
Finally, we measured anxiety-like behavior in male and female progeny using the novelty-induced hypophagia paradigm using a paradigm similar to our previous work (White et al. 2016). Latency to start eating peanut butter chips in the home cage for pre- and post-tests or in a novel environment on test day was measured. Analysis of female latencies revealed a significant main effect of environment [F(2,14)=41.81, p<0.0001], demonstrating an increase in latency to feed in a novel environment, but no effect of dam exposure [F(1,7)=0.0001394, p=0.991] (Fig. 4A). In a similar fashion, a significant main effect of environment [F(2,20)=166.6, p<0.0001] was detected in male offspring (Fig. 4B), but no effect of dam exposure [F(1,10)=0.01945, p=0.892]. These results indicate that preconception cocaine dam exposure has no effect on offspring anxiety-like behavior.
Figure 4. Pre-conception maternal cocaine exposure has no effect on offspring anxiety.
A. Female F1 offspring exhibited an increased latency to feed in a novel environment. Dam exposure had no effect on latency. B. Male F1 offspring exhibited an increased latency to feed in a novel environment. Dam exposure had no effect on latency. *p<0.05 comparing “novel” to “pre” and “novel” to “post”. Group size (n) is indicated by the number located in the bar. Number of litters represented: female offspring; n=5 for saline, n=5 for cocaine; male offspring n=6 for saline; n=6 for cocaine.
Discussion
The present findings indicate that preconception dam cocaine exposure resulted in increased cocaine reinforcing efficacy in male but not female adult offspring, which is consistent with previous results showing increased acute cocaine-induced behavioral hyperactivity in male offspring of dams exposed to cocaine before conception (Sasaki et al. 2014). Enhancement of the behavioral effects of cocaine is the opposite of what was observed when cocaine was self-administered by the sire, which produced reduced cocaine self-administration in male progeny (Le et al., 2017; Vassoler et al., 2013). The current results also showed no effect of dam cocaine self-administration on measures of anxiety and memory in offspring, both of which were previously shown to be impacted by paternal cocaine self-administration in mice and rats (Fischer et al. 2017; White et al. 2016; Wimmer et al. 2017).
Dams that self-administered cocaine prior to conception exhibited altered maternal behavior. Most notably, they spent disproportionate amounts of time on average not actively engaged with the pups. Previous work showed that females exposed to cocaine preconception either showed no changes in maternal behavior (Sasaki et al. 2014) or retrieved pups more quickly and spent more time caring for their pups (Nephew and Febo 2010). There were major differences in the manner and extent of cocaine administration in these studies compared to our own. In the previous experiments, a moderate dose of cocaine was administered systemically daily for 10 days (Nephew and Febo 2010; Sasaki et al. 2014). Here, a similar amount of cocaine was self-administered daily but the route was i.v. and the duration was 60 days. The total amount of cocaine administered and the duration of the exposure may have contributed to the differences in subsequent maternal behavior observed in the present relative to prior studies (Sasaki et al. 2014).
The present results indicate that a prolonged period of cocaine self-administration interferes with the subsequent architecture of typical maternal behavior, which can have significant effects on offspring physiology and behavior (Szyf et al. 2007; Vassoler et al. 2014; Weaver et al. 2004). Considerable alterations in maternal behaviors have been shown to affect offspring performance in memory- or anxiety-related tasks (Dalle Molle et al. 2012; Liu et al. 2000). Such effects were not detected in offspring from cocaine-exposed F0 dams, indicating that cocaine-induced alterations in maternal behavior were not sufficient to elicit well-described behavioral consequences in male or female offspring in our paradigm. Interestingly, enhanced cocaine self-administration in offspring of cocaine-experienced dams was observed only in males, whereas the prominent features of cocaine-induced alterations in maternal behavior, increased time away and passive interaction, were by definition evenly applied to all pups across sex. However, we cannot eliminate the possibility that males were differentially impacted by deficits in maternal care. Whether the sex of the pup can directly influence maternal behavior in rats remains somewhat debated. There is some evidence to suggest that maternal care can be affected by the sex of the pup (Hao et al. 2011). Other studies suggest that maternal care varies across dams but is not affected by litter sex ratio (Champagne et al. 2003). We did not assess maternal behavior toward male versus female pups in our analyses and therefore cannot determine whether maternal care toward female pups was different than that directed to male pups in cocaine-treated dams.
Perhaps more likely are direct or indirect effects of cocaine on oocytes, which may be epigenetic in nature, resulting in changes in the developmental trajectory. Cocaine exposure in sires results in epigenetic modifications in sperm, including DNA hypomethylation (Le et al., 2017) as well as increased acetylated histone H3 at the BNDF promoter (Vassoler et al., 2013); male cocaine-sired offspring express elevated BDNF protein in the prefrontal cortex, and cocaine self-administration is normalized by administration of an antagonist for the BDNF receptor TrkB (Vassoler et al., 2013). Given the bidirectional influence of cocaine sires and cocaine dams on cocaine intake in male offspring, it would be interesting to explore whether the same mechanism(s) are oppositely regulated by cocaine in sperm and oocytes or if independent epigenetic mechanisms produce opposing phenotypes. It also is possible that cocaine may result in persistent changes that impact the prenatal environment, such as altering hormone levels, that may produce sex-specific modifications in the developmental trajectory of the male offspring (Vassoler et al. 2014). It has been shown that preconception cocaine influences gene transcription in the mesolimbic system in that D1 dopamine receptor (D1DR) mRNA expression was selectively increased in the medial prefrontal cortex (mPFC) of male offspring (Sasaki et al. 2014); altered D1DR transmission in the mPFC is known to modulate both cocaine intake and seeking (Olsen and Duvauchelle 2006; Sanchez et al. 2003; Sun and Rebec 2005).
In summary, the male offspring of female rats that self-administered cocaine for 60 days prior to conception displayed enhanced intake of cocaine due to increases in the reinforcing efficacy of this stimulant. No changes in memory or anxiety-like behavior were observed in either male of female offspring of cocaine experienced dams. We also observed significant alterations in maternal behaviors in dams exposed to cocaine. Although changes in maternal behavior do not appear to have influenced cocaine reinforcement, memory or anxiety in offspring, it is possible that other physiological and behavioral responses not assessed may have been impacted.
Acknowledgements
This work was supported by the following grants from the National Institutes of Health: R01 DA33641 (RCP), T32 DA28874 (SES-J) and K01 DA39308 (MEW).
Footnotes
Conflict of Interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Betancourt LM, Yang W, Brodsky NL, Gallagher PR, Malmud EK, Giannetta JM, Farah MJ, Hurt H (2011) Adolescents with and without gestational cocaine exposure: Longitudinal analysis of inhibitory control, memory and receptive language. Neurotoxicol Teratol 33: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM (2005) Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 182: 537–44. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM (2011) Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res 218: 200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, Byrnes EM (2012) Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. J Psychopharmacol 26: 1348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ (2003) Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79: 359–71. [DOI] [PubMed] [Google Scholar]
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, Manfro GG, Silveira PP (2012) Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry 2: e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos JF, de Melo Bastos Cavalcante C, Barbosa FT, Gitai DLG, Duzzioni M, Tilelli CQ, Shetty AK, de Castro OW (2018) Maternal, fetal and neonatal consequences associated with the use of crack cocaine during the gestational period: a systematic review and meta-analysis. Arch Gynecol Obstet 298: 487–503. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D (2011) Translational issues for prenatal cocaine studies and the role of environment. Neurotoxicol Teratol 33: 9–16. [DOI] [PubMed] [Google Scholar]
- Febo M, Ferris CF (2007) Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience 148: 400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DK, Rice RC, Martinez Rivera A, Donohoe M, Rajadhyaksha AM (2017) Altered reward sensitivity in female offspring of cocaine-exposed fathers. Behav Brain Res 332: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ (2005) Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 15: 347–55. [DOI] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA, Kosten TA (2011) Litter gender composition and sex affect maternal behavior and DNA methylation levels of the oprm1 gene in rat offspring. Front Psychiatry 2: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS (2006) Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol 28: 198–209. [DOI] [PubMed] [Google Scholar]
- Hurt H, Betancourt LM, Malmud EK, Shera DM, Giannetta JM, Brodsky NL, Farah MJ (2009) Children with and without gestational cocaine exposure: a neurocognitive systems analysis. Neurotoxicol Teratol 31: 334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger CE, Robinson S, Stanwood GD (2012) Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse 66: 902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS (2003) Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res 147: 23–36. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ (2000) Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci 3: 799–806. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Maier SE, West JR (2004) Long-term alcohol exposure prior to conception results in lower fetal body weights. Birth Defects Res B Dev Reprod Toxicol 71: 135–41. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Kabir ZD, Bhide PG, Kosofsky BE (2014) Effects of prenatal exposure to cocaine on brain structure and function. Prog Brain Res 211: 277–89. [DOI] [PubMed] [Google Scholar]
- Mets B, Diaz J, Soo E, Jamdar S (1999) Cocaine, norcocaine, ecgonine methylester and benzoylecgonine pharmacokinetics in the rat. Life Sci 65: 1317–28. [DOI] [PubMed] [Google Scholar]
- Mumby DG (2001) Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res 127: 159–81. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Febo M (2010) Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharmacology (Berl) 209: 127–35. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learning & memory 17: 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Duvauchelle CL (2006) Prefrontal cortex D1 modulation of the reinforcing properties of cocaine. Brain Res 1075: 229–35. [DOI] [PubMed] [Google Scholar]
- Ramsay M (2010) Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Med 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA (2003) Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience 119: 497–505. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Constantinof A, Pan P, Kupferschmidt DA, McGowan PO, Erb S (2014) Cocaine exposure prior to pregnancy alters the psychomotor response to cocaine and transcriptional regulation of the dopamine D1 receptor in adult male offspring. Behav Brain Res 265: 163–70. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC (2009) The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci 30: 1358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV (2005) The role of prefrontal cortex D1-like and D2-like receptors in cocaineseeking behavior in rats. Psychopharmacology (Berl) 177: 315–23. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M (2007) Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol 24: 9–19. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Byrnes EM, Pierce RC (2014) The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology 76 Pt B: 269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Johnson NL, Byrnes EM (2013a) Female adolescent exposure to cannabinoids causes transgenerational effects on morphine sensitization in female offspring in the absence of in utero exposure. J Psychopharmacol 27: 1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, Byrnes EM (2017) Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology 113: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, Knapp CM, Pierce RC (2008) Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci 28: 8735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Toorie AM, Byrnes EM (2018) Increased cocaine reward in offspring of females exposed to morphine during adolescence. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013b) Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16: 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Wright SJ, Byrnes EM (2016) Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology 103: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7: 847–54. [DOI] [PubMed] [Google Scholar]
- White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME (2016) Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol 21: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Briand LA, Fant B, Guercio LA, Arreola AC, Schmidt HD, Sidoli S, Han Y, Garcia BA, Pierce RC (2017) Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]