Abstract
Rationale
Approximately 20 million adults in the United States have an alcohol use disorder (AUD). There are clinical and preclinical data suggesting that psychedelics may have benefits for AUD.
Objective
To investigate the effects of the synthetic psychedelic 2,5-dimethoxy-4-iodoamphetamine (DOI) on the behavioral effects of ethanol.
Methods
The effects of DOI were examined using ethanol-induced place conditioning (1.8 g/kg ethanol) and 2-bottle choice ethanol drinking (20% v/v), using a dose of DOI (3 mg/kg) that produced the peak response in the serotonin 2A (5-HT2A) receptor dependent head-twitch assay. Interactions between DOI and ethanol (3 g/kg) were examined using the ethanol-induced loss of righting reflex procedure and blood-ethanol analysis. To examine additional mechanisms by which psychedelics may interact with ethanol, we determined whether DOI reverses ethanol-induced nitric oxide release in macrophages, a marker of inflammation.
Results
DOI significantly attenuated ethanol-induced place conditioning and ethanol drinking. DOI-induced suppression of alcohol drinking depended upon 5-HT2A receptors, was selective for alcohol over water, and was selective for high alcohol preferring subjects. DOI had no apparent pharmacokinetic interactions with ethanol, and DOI reduced ethanol-induced nitric oxide release.
Conclusions
Our findings demonstrate that DOI blocks ethanol place conditioning and selectively reduces voluntary ethanol consumption. This may be related to modulation of the effects of ethanol in the reward circuitry of the brain, ethanol-induced neuroinflammation, or a combination of both. Additional studies to elucidate the mechanisms through which psychedelics attenuate the effects of ethanol would inform the pathophysiology of AUD and potentially provide new treatment options.
Introduction
There has been a recent renaissance in research on the potential benefits of carefully controlled pharmacotherapy with psychedelics for anxiety, depression, and substance-use disorders, especially in combination with psychotherapy. This has culminated in the Food and Drug Administration recently granting breakthrough therapy status for psilocybin for treatment-resistant depression. From the 1950–1970s, a literature was published showing psychedelics have a positive effect on substance dependence (Bogenschutz and Johnson 2016; Dyck 2005). Early studies focused on using lysergic acid diethylamide (LSD) for the treatment of alcohol-use disorder (AUD) (Abuzzahab and Anderson 1971; Grinspoon and Bakalar 1986; Mangini 1998), and reported reduced drinking and/or improved social and professional function or complete sobriety in approximately 50% of the subjects. A recent study reported significant decreases in alcohol consumption after 1–2 sessions of psilocybin in 10 alcohol-dependent subjects using an open-label design (Bogenschutz et al. 2015). Similar to AUD, psilocybin treatment in heavily tobacco-dependent smokers resulted in approximately 60–80% abstinence rates at 6 (Johnson et al. 2014) and 12 (Johnson et al. 2017) months. Such effects have led to a renewed interest in the therapeutic benefits of psychedelics. However, many of these studies suffered from small samples sizes, a lack of randomization or other controls, and possible selection bias. Therefore, more research is necessary to evaluate and optimize psychedelic therapy for AUD.
To the best of our knowledge, there have been few previous animal studies of the effects of psychedelics in models of alcohol abuse, with only a few recent exceptions (Alper et al. 2018; Cata-Preta et al. 2018; Oliveira-Lima et al. 2015). Animal models are an important component of drug development. Among other things they offer the capacity to define neural mechanisms, determine pharmacological interactions, generate objective biomarkers of pharmacotherapeutic efficacy, and determine the long-term behavioral and neurochemical effects of drug exposure under tightly controlled conditions (Murnane 2018). For example, as it relates to psychedelics, animal studies have been critical to establishing the serotonin 2A (5-HT2A) receptor as their primary molecular target. Yet, despite the excitement generated by the recent revival of psychedelic research, there has been limited preclinical investigation of the pharmacotherapeutic effects of psychedelics in laboratory animals (Murnane 2018). As such studies would benefit the evaluation and optimization of psychedelic therapy for AUD, here, we examined the effects of the psychedelic 5-HT2A receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) in several preclinical models of the behavioral and pharmacokinetic effects of ethanol.
In this study, we investigated the effects of DOI on ethanol induced place conditioning and voluntary alcohol consumption in mice. We then investigated whether suppression of alcohol drinking by DOI is mediated by 5-HT2A receptors using the selective antagonist M100907 (Hall et al. 2000) in the two-bottle choice assay. We also examined whether the effects of DOI are attributable to pharmacokinetic or pharmacodynamic interactions with ethanol. To examine additional mechanisms by which psychedelics may interact with ethanol, we lastly determined whether DOI reverses ethanol-induced nitric oxide release in macrophages, a marker of inflammation. Alcohol affects both the innate and adaptive immune system, leading to increased production of proinflammatory cytokines and neuroinflammation (Lippai et al. 2013; Szabo and Saha 2015), which may contribute to subsequent ethanol seeking and taking behavior. Consistent with this, we have recently reported that anti-inflammatory cannabinoids (Tung et al. 2008) attenuate ethanol place conditioning and 2-bottle choice drinking (Oppong-Damoah et al. In Press). Likewise, psychedelics have anti-inflammatory effects (Nau et al. 2013; Yu et al. 2008b) and this may contribute to their therapeutic properties, but whether psychedelics can reverse ethanol-related inflammation has not been studied.
Methods
Drugs
OmniPur®, pure 200 proof, ethanol was commercially purchased (VWR, Radnor, PA) and was diluted with saline for intraperitoneal injection. For oral consumption, OmniPur®, pure 200 proof ethanol was diluted with tap water to the desired final concentration (3% v/v, 6% v/v, 10% v/v and 20% v/v). (+/−)-2,5-Dimethoxy-4-iodoamphetamine (DOI) was synthesized at the Research Triangle Institute (Research Triangle Park, NC) and dissolved in normal saline. M100907 is a selective serotonin 2A receptor antagonist (Hall et al. 2000) that was synthesized at the Chemical Biology Research Branch (Ullrich and Rice 2000) and diluted in sterile water with minor addition of hydrochloric acid (HCl) at 100ul per 10ml of sterile water for the stock solution, as we have done previously (Murnane et al. 2013a; Murnane et al. 2019; Murnane et al. 2012a; Murnane et al. 2013b). A final concentration of 0.01 mg/ml was made from the stock solution by dilution with sterile water only.
Animals
Male Swiss-Webster mice (CFW; Charles River Laboratories, Inc.; Wilmington, MA) served as the subjects of all in vivo experiments. All mice were housed in a temperature and humidity-controlled room. With the exception of the two-bottle choice study, all mice were housed in groups of 4 and weighed between 25 and 40 grams. For the two-bottle choice experiment, mice weighed between 25 and 55 grams and were housed individually to collect individual drinking data. Swiss-Webster mice were utilized for these studies because they are a general purpose strain that has been used extensively to study behavior, physiology, and neurochemistry (Goeders et al. 2009; Lindsey et al. 2019; Murnane et al. 2019; Murnane et al. 2012b; Oppong-Damoah et al. 2019; Ray et al. 2019; Ray et al. 2018). Moreover, we have consistently observed that this strain of mice exhibits two distinct and stable phenotypes of ethanol consumption. Approximately 50% of mice drink more than 10 g/kg of ethanol per day and the other 50% drink less than 10 g/kg of ethanol per day (Oppong-Damoah et al. In Press). This allows for the study of drugs treatments and brain mechanisms in two discrete phenotypes. Animals had access to food (Laboratory Rodent Diet) and water ad libitum. Mice were housed in rooms maintained in a 12-hour light/dark cycle. The lights were turned off at 7 pm every evening and turned back on at 7 am every morning, and experiments were conducted during the light phase, with the exception of the 2-bottle choice experiments, as outlined below. All animals employed in this study were treated according to protocols evaluated and approved by Institutional Animal Care and Use Committee of Mercer University.
Head-twitch response (HTR)
The drug-elicited HTR is a selective behavioral model of 5-HT2A receptor stimulation. Head-twitch behavior occurs in rodents spontaneously, but increases in frequency following the administration of various psychedelic drugs (Fantegrossi et al. 2008; Murnane 2018; 2019). The HTR methods have been described previously (Murnane et al. 2019). In brief, HTR behavior was video recorded in each subject from 5–15 minutes after the injection of (+/−)-DOI (N=8 per group).
Conditioned Place Preference (CPP)
CPP is a commonly used procedure to assess the positive appetitive conditioned effects of a drug (Prus et al. 2009); however, the results from this assay can be difficult to interpret when an animal has a strong innate preference for one compartment of the conditioning apparatus (Bardo et al 2000). CPP experiments were performed in plastic 46 cm × 30 cm boxes with each separated by a removable partition into two compartments of equal size. The boxes were placed individually in soundproof boxes (Med Associates, St. Albans, VT). Each compartment had a different pattern on its walls (vertical versus horizontal stripes) and a different floor texture (smooth versus hole board). We used an expedited CPP process (Calcagnetti and Schechter 1992) where mice (N=8 per group) undergo two conditioning sessions each day for four days. Prior to conditioning, mice underwent 2 days of habituation with a 30-minute session each day, where they had unrestricted access to both sides of the chamber. On the third day, mice were placed in the chamber for a 15-minute pretest with unrestricted access to establish which environment was preferred. A biased CPP protocol was used where the less preferred compartment was paired with the drug. A dose-response curve of ethanol conditioning was performed to determine the peak dose of ethanol, which was used to assess the effects of DOI on the ethanol CPP. Vehicle or DOI (3 mg/kg) was administered 30 minutes prior to introduction of the mouse to the CPP chamber during the final post-test session, which we refer to as the acute treatment. The 3 mg/kg dose of DOI was used because it elicited the strongest effect in the HTR assay. To control for state-dependent effects, and assess the effects of DOI on the consolidation of the ethanol CPP, in separate groups, vehicle or DOI (3 mg/kg) was also administered immediately following removal of the mouse from the CPP apparatus for each of the four afternoon ethanol conditioning sessions, which we refer to as the subchronic treatment. The proportion of time spent in the less preferred compartment served as the dependent measure of conditioning (N=8 per group).
Two-Bottle Choice
Two-bottle choice is a widely used animal model that captures aspects of voluntary alcohol consumption in humans (Voikar et al. 2005), but requires individual housing, which can affect behavior such as aggression and responsivity to novelty and may also affect ethanol consumption. To induce high rates of ethanol consumption, intermittent access to 20% ethanol was conducted using procedures previously described (Hwa et al. 2011) with slight modifications. Briefly, mice were presented with two 25 ml pipettes fitted with stainless steel metal sippers, with one containing water and the other containing ethanol. During acquisition, a 3%, 6%, and 10% (v/v) ethanol solution was presented on the first Monday, Wednesday, and Friday, respectively. Subsequently, mice received 20% ethanol in one pipette and water in the other pipette each Monday, Wednesday, and Friday. The bottles were always provisioned as close to the beginning of the dark period as possible (at 1 to 3 hours before the beginning of the dark cycle) and animal allowed to drink overnight (Thiele and Navarro 2014). The pipette placement was alternated for each session to prevent the development of a side preference. Acquisition was complete when a stable drinking pattern was observed, which was operationally defined as consumption (in g/kg) that varied by less than 20% across 4 consecutive sessions. Stable drinking typically emerged within 9 sessions, after which mice were divided into the high and low drinking groups. On Mondays, mice were injected with DOI (3 mg/kg) or saline intraperitoneally 30 minutes prior to the provision of ethanol using a counter balanced administration schedule. M100907 (0.1 mg/kg) injections were administered intraperitoneally 15 minutes prior to administration of DOI. Ethanol consumption and preference was assessed 1 hour and 24 hours after the provision of ethanol.
Loss of Righting Reflex (LORR)
We used the LORR assay to test whether there are direct pharmacodynamic interactions between DOI and ethanol. The LORR procedure was conducted using procedures previously described (Al Mansouri et al. 2014). Mice (N=8 per group) were administered saline or DOI (3 mg/kg) 15 minutes prior to the injection of ethanol, at a 3 g/kg dose that was determined to have sedative effects in previous studies (Murnane et al. 2019). Ten minutes after the injection, each mouse was placed in the supine position in a plastic trough and tested to ensure presence of the righting reflex. Recovery from the ethanol-induced LORR was defined as the time from ten minutes after the injection of ethanol until three righting responses were observed within 30 seconds of each other, as has been done previously (Al Mansouri et al. 2014; Murnane et al. 2019).
Blood Ethanol Concentration (BEC)
Mice (N=6 for each group) were injected with saline or DOI (3 mg/kg) intraperitoneally 15 minutes prior to the injection of ethanol (3 g/kg). The sampling time points were 1, 20, 60, 120 and 240 minutes following ethanol injection. The experiment was conducted using a randomized order and repeated measures design over 5 weeks. Blood samples were collected via the facial vein (Golde et al. 2005). Each blood sample was collected into prechilled BD microtainer K2EDTA tubes (Franklin Lakes, NJ) and immediately placed on ice. Blood samples were spun at 1659×g for 10 minutes at 4°C, after which 10ul of plasma from each sample was analyzed using Nicotinamide Adenine Dinucleotide-Alcohol Dehydrogenase reagent (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s protocol. The absorbance of the test solutions was read at 339 nm against the blank using a 2000C NanoDrop spectrophotometer (ThermoFisher, Waltham, MA).
Nitric Oxide Assay
RAW 264.7 cells were obtained from ATCC (Rockville, MD) and cultured in 100 mm plates containing Dulbecco’s Modified Eagle’s Medium, supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Life Technologies, Grand Island, NY). Cells were maintained at 5% CO2, 37°C, and 80% humidity in a cell-culture incubator. The media was replaced every two days. At 80% confluence, cells were washed with pre-warmed Dulbecco’s phosphate buffered saline, scraped, and centrifuged at 130×g for 5 minutes at 25°C. The cell pellet was resuspended in complete media, plated at 50,000 cells per well in a 96 well plate and incubated for 24 hours. Cells were treated with ethanol (0.56, 3.2, 17.8, and 100 mmol/L) alone or in combination with 0.3 nM DOI, which was determined to be an effective concentration in preliminary experiments. After 24 hours, 50 ul of supernatant was removed from all wells and transferred into another 96 well plate. 50 ul of 1% sulfanilamide solution was added to each well and incubated for 10 minutes at 25°C in the dark. After that 50 ul of N-(1-Napthyl)ethylenediamine (NED) solution was added to each well and incubated for 10 minutes at 25°C in the dark. The optical density of each well was then measured at 540 nm using a BioTek S1AFR HT synergy spectrophotometer (Winooski, VT)
Data Analysis
Graphical presentation of all data depicts mean ± standard error of the mean (SEM). All graphical data presentations and statistical tests were generated using GraphPad Prism (La Jolla, CA), and significance was arbitrated at a p < 0.05. Head-twitch behavior was analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc was used for group comparisons. Ethanol-induced place conditioning was analyzed two-way ANOVA followed by paired t-test. Ethanol drinking was analyzed by one-way repeated measures ANOVA followed by Tukey’s post-hoc test. The LORR data were analyzed by paired t-test. The areas under the curve of blood ethanol levels were assessed by Student’s t-test. The nitric oxide results were assessed by two-way ANOVA with Tukey’s post-hoc analysis.
Results
Head-twitch behavior
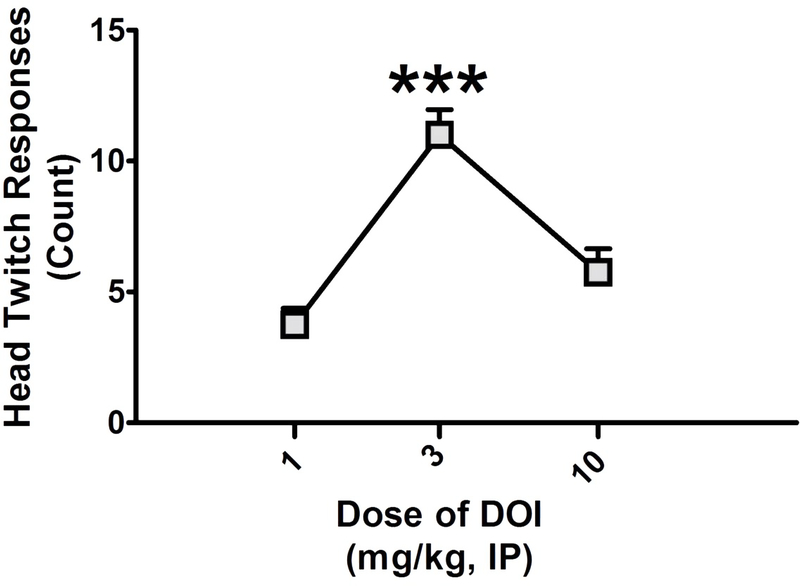
5-HT2A receptor antagonists selectively block the HTR, the potency with which they do so is correlated with their affinity for 5-HT2A receptors (Fantegrossi et al. 2008; Gonzalez-Maeso et al. 2007), and activation of the 5-HT2C receptor acts in opposition to the 5-HT2A receptor and suppresses the HTR (Fantegrossi et al. 2010). As DOI has agonist activity at 5-HT2C receptors, the dose at which DOI elicits the strongest HTR is the dose when it induces the most selective agonism of 5-HT2A receptors. Consistent with our previous studies (Murnane et al. 2019), and those of others (Fantegrossi et al. 2010), we found a main effect of (+/−)-DOI on the HTR (F2,23 = 19.79; p < 0.001), with a dose of 3 mg/kg inducing significantly more head twitches than either 1 (q = 8.614) or 10 (q = 6.238) mg/kg (p < 0.001) mg/kg (Figure 1), with no significant difference between 1 and 10 mg/kg. Therefore, the 3 mg/kg dose of DOI was used for all subsequent studies.
Figure 1.
The head-twitch response dose-response curve for racemic DOI. The head-twitch response is expressed as a raw count and DOI was tested at 1, 3, and 10 mg/kg. All values represent the mean ± SEM. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 as assessed by one-way analysis of variance followed by Tukey’s test.
Ethanol-Induced Place Conditioning
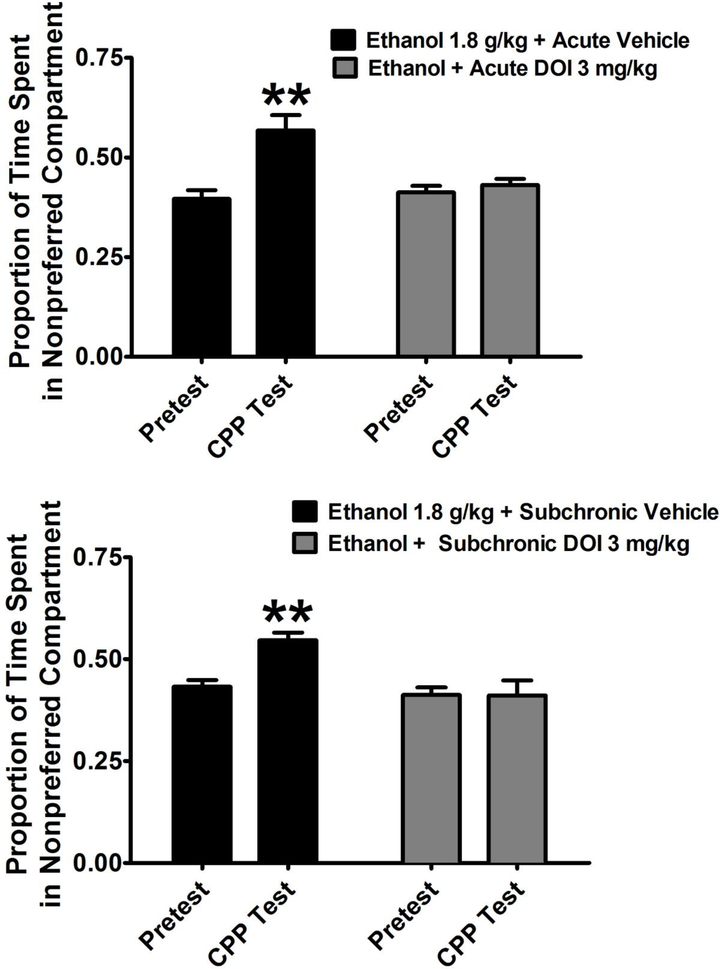
In preliminary CPP experiments, an initial dose response function was established with ethanol at 1, 1.8, and 3 g/kg. 1.8 g/kg was chosen for further drug interaction experiments as it induced the peak ethanol-induced CPP. For the acute experiment, two-way ANOVA revealed a significant main effect of trial (F1,28 = 18.80; p < 0.001), significant main effect of treatment (F1,28 = 14.55; p < 0.001), significant interaction (F1,28 = 12.97; p < 0.01). For the subchronic experiment, two-way ANOVA revealed a significant main effect of trial (F1,28 = 5.42; p < 0.05), significant main effect of treatment (F1,28 = 10.30; p < 0.01), and significant interaction (F1,28 = 5.75; p < 0.05). Post-hoc analysis by paired t-test showed that ethanol induced a significant CPP following either acute pretreatment with saline (T7 = 4.33; p < 0.01) or subchronic treatment with saline (T7 = 3.99; p < 0.01) as assessed by paired t-test. In contrast, both acute or subchronic treatment with DOI prevented the expression of ethanol CPP (Figure 2).
Figure 2.
Effects of DOI (3 mg/kg) administered acutely (TOP) or sub-chronically (BOTTOM) on the ethanol-induced conditioned place preference (CPP). Ethanol was administered at a dose of 1.8 g/kg because this dose produced the strongest place conditioning in preliminary experiments. Acute injections occurred 30 minutes prior to the CPP test session. Sub-chronic injections were administered immediately after the four ethanol conditioning sessions. Data are expressed as a proportion of the session time of 900 seconds. All values represent the mean + SEM. ** = p < 0.01 compared to the pretest as assessed by paired t-test.
Ethanol Drinking
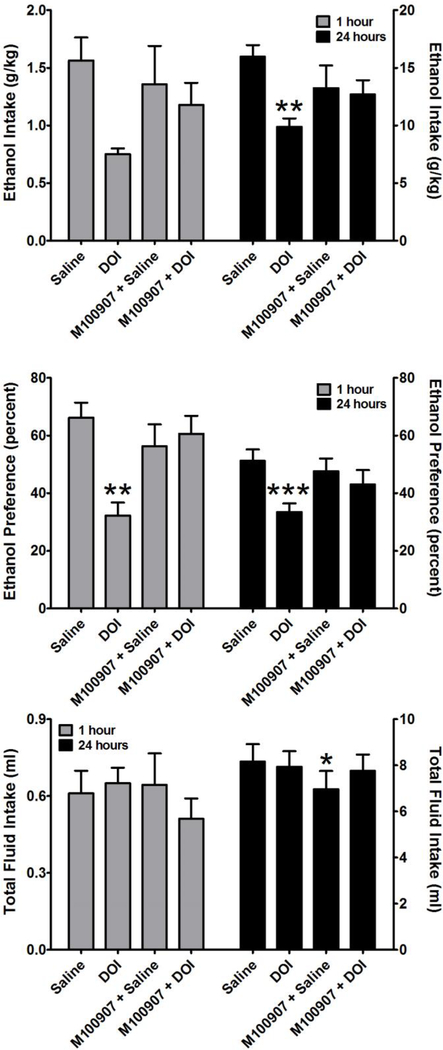
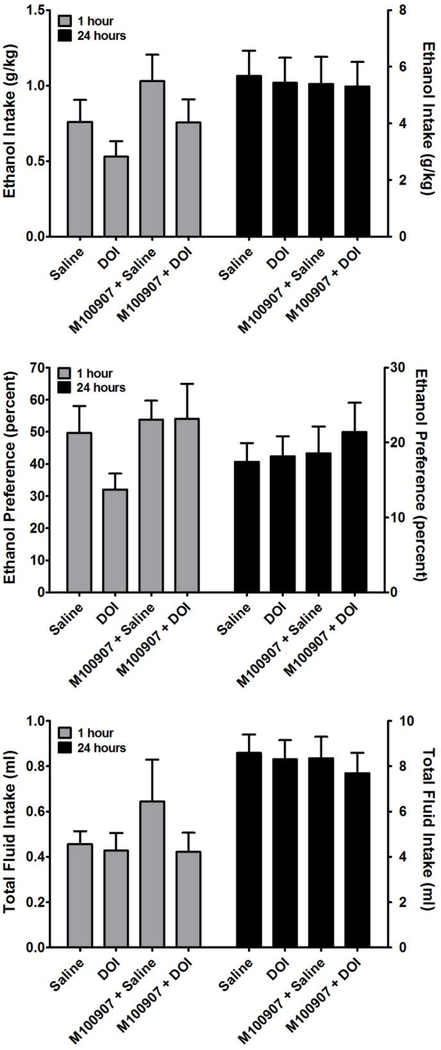
Following acquisition of ethanol drinking, the mice were split across the median into a high drinking group (N = 10) and a low drinking group (N=9). This was done to model aspects of excessive drinking behavior, and the effects of DOI were examined in both groups. One mouse in the low drinking group was removed from the analyses of the treatment effects as it was found to be an outlier by the Grubb’s test. In the high drinking group (Figure 3), one-way repeated measures ANOVA revealed a significant main effect of treatment on ethanol intake at 24 hours (F3,39 = 5.94; p < 0.01). Post-hoc analysis by Tukey’s test revealed that DOI significantly suppressed ethanol intake relative to the saline injection (q = 5.946, p < 0.01), which was reversed (p < 0.05) by pretreatment with the selective 5-HT2A receptor antagonist M100907. One-way repeated measures ANOVA did not reveal a significant main effect of treatment on ethanol intake at 1 hour. In contrast, one-way repeated measures ANOVA revealed a significant main effect of treatment on the preference for ethanol at 1 hour (F3,35 = 6.33; p < 0.01) and at 24 hours (F3,35 = 8.54; p < 0.001). Post-hoc analysis by Tukey’s test of the 1 hour data revealed that DOI significantly decreased the preference for ethanol relative to the saline injection (q = 5.716, p < 0.01), which was significantly attenuated (q = 4.041, p < 0.05) by pretreatment with M100907. Post-hoc analysis by Tukey’s test of the 24 hour data revealed that DOI significantly decreased the preference for ethanol relative to the saline injection (q = 6.754, p < 0.001), which was significantly attenuated (q = 5.389 p < 0.01) by pretreatment with M100907. In contrast, DOI had no significant effect on total fluid intake in the high drinking group. DOI also had no significant effect on ethanol intake, preference for ethanol, or total fluid intake in the low drinking group (Figure 4).
Figure 3.
Effects of DOI (3 mg/kg) on ethanol intake (TOP), preference for ethanol consumption over water consumption (MIDDLE), and total fluid intake (BOTTOM). M100907, a selective serotonin 2A receptor antagonist, reversed the effects of DOI. Data are subdivided to show the effects of ethanol 1 hour after treatment (Left Y-Axis) and 24 hours after treatment (Right Y-Axis). Data are presented from the top 50% of ethanol consuming mice. All values represent the mean ± SEM. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 as assessed by repeated measures one-way analysis of variance followed by Tukey’s test. No post-hoc analysis was performed for fluid intake because repeated measures one-way analysis of variance revealed no main effect of treatment on this measure.
Figure 4.
Effects of DOI on ethanol intake (TOP), preference for ethanol consumption over water consumption (MIDDLE), and total fluid intake (BOTTOM). Data are subdivided to show the effects of ethanol 1 hour after treatment (Left Y-Axis) and 24 hours after treatment (Right Y-Axis). Data are presented from the bottom 50% of ethanol consuming mice. All values represent the mean ± SEM. There was no main effect of DOI treatment on any of these three parameters as assessed by repeated measures one-way analysis of variance.
Interactions Between DOI and Ethanol
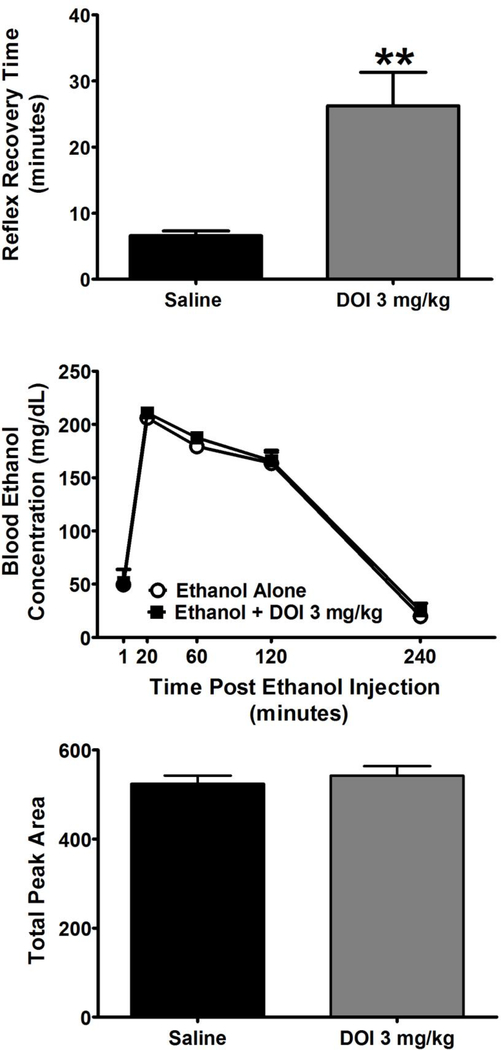
We then assessed whether DOI can alter other behavioral effects of ethanol using the LORR assay, and found that DOI significantly (T6 = 4.08; p < 0.01) increased sensitivity to the sedating effects of ethanol as assessed by paired t-test (Figure 5, TOP). We next determined whether the effects of DOI on the behavioral effects of ethanol may be mediated by altering the pharmacokinetics of ethanol. We observed no difference in the time course of blood ethanol levels following injection of a 3 g/kg bolus injection of ethanol that was administered after either vehicle or 3 mg/kg DOI (Figure 5, MIDDLE). Area under the curve analysis of these time courses revealed no significant effects of DOI pretreatment as assessed by Student’s t-test (Figure 5, BOTTOM).
Figure 5.
TOP: DOI (3 mg/kg) increases the duration of the loss of the righting reflex induced by ethanol (3 g/kg) as represented by the total time taken to recover the righting response following ethanol administration expressed in minutes. MIDDLE: Time course of blood ethanol concentrations (mg/dl) following a bolus injection of a sedating dose of ethanol (3 g/kg) after pretreatment with saline or DOI (3 mg/kg). BOTTOM: Area under the curve analysis of the blood ethanol time course determined across each individual mouse. All values represent the mean + SEM. ** = p < 0.01 as assessed by paired t test.
Nitric Oxide
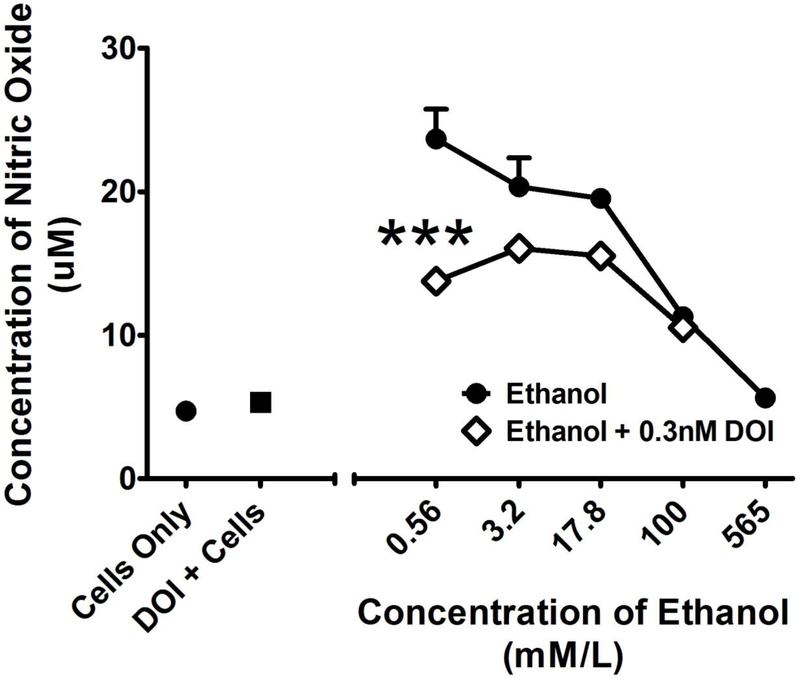
Finally, we determined whether DOI attenuated ethanol induced nitric oxide release from macrophages, which is an establish marker of an innate inflammatory response. This experiment was based on the rationale that psychedelics may have anti-inflammatory and neuroprotective effects that contribute to their pharmacotherapeutic effects (Nau et al. 2013), yet there has been little study of the anti-inflammatory effects of psychedelics in the context of alcohol dependence. For ethanol-induced nitric oxide release from macrophages, two-way ANOVA revealed a significant main effect of treatment (F1,16 = 35.94; p < 0.001), a significant main effect of concentration (F3,16 = 21.35; p < 0.001), and a significant interaction (F3,16 = 5.77; p < 0.01). Post-hoc analysis by Tukey’s test revealed that DOI significantly (P < 0.001) decreased ethanol-induced nitric oxide release at a concentration of 0.56 mmol/L (Figure 6).
Figure 6.
Effects of DOI (0.3 nM) on nitric oxide (NO) release from RAW 264.7 macrophages after 24 hours treatment with a range of concentrations of ethanol. The X-axis represents the concentration of ethanol in mmol/L whereas the Y-axis represents the micromolar concentration of released NO. All values represent the mean + SEM. *** = p < 0.001 as assessed by two-way analysis of variance followed by Tukey’s multiple comparison test.
Discussion
The major finding of the present study is that the synthetic psychedelic DOI attenuates ethanol consumption. This is consistent with a literature documenting that psychedelics may treat alcohol dependence, as outlined in the introduction. The human literature suggests that psychedelic assisted therapy in alcohol-dependent subjects may reduce drinking, improve social and professional function, or yield complete sobriety in approximately 50% of treated subjects when conducted without selection (Abuzzahab and Anderson 1971; Bogenschutz et al. 2015; Grinspoon and Bakalar 1986; Mangini 1998). A single dose of LSD in 24 patients with AUD who agreed to a 2–4 week hospital stay documented that 12 patients remained “unchanged,” whereas 12 patients were either “improved” or “much improved” (Smith 1958). It is interesting that our animal data appear to parallel these findings as we report that DOI selectively reduces ethanol intake and preference in the top 50% of ethanol consuming mice. This suggests that there may be a discernable biological basis for these individual differences in the therapeutic response to psychedelics that could undergo study to elucidate its pharmacological and neurobiological mechanisms. Another area of individual differences that should be explored in future studies is sex differences in the response to psychedelics. The present study was limited to male subjects, and females should be included in subsequent research. Importantly, DOI selectively attenuated ethanol intake in the absence of any significant effect on fluid consumption. This later finding indicates that this dose of DOI does not induce general suppression of drinking behavior. However, it will be important in the future to examine whether the effects of DOI are specific to ethanol, or whether DOI will suppress drinking of other palatable solutions such as saccharin, as has been done previously (Maurel et al. 2000).
The present study has begun to elucidate the mechanisms underlying the psychedelic-mediated reductions in ethanol consumption. Our CPP data suggest that DOI can modify the reward-related effects of ethanol. It is well established that CPP procedures are sensitive to the positive appetitive conditioned effects of drugs, including ethanol (Prus et al. 2009), and we herein report that DOI attenuated the expression of the ethanol-induced CPP. It is unlikely that this is related to state-dependent disruption of the expression of conditioning during the CPP post-test, as DOI attenuated the expression of the ethanol-induced CPP when given after ethanol. This is an important control as it is well known that DOI can enhance learning and memory under a variety of conditions (Murnane 2019) and may have pronounced psychoactive effects, suggesting the possibility of state dependent effects on learning and memory. Nevertheless, given the known effects of DOI on learning and memory, future research should continue to delineate effects on cognition versus effects on reward systems, especially when it comes to assays such as CPP that have a learning component.
To examine the role of 5-HT2A receptors in DOI-induced suppression of the behavioral effects of ethanol, the dose of DOI that was tested was selected because it was the most effective dose in the HTR assay, which suggests that it has maximum selectivity for 5-HT2A receptors. Moreover, the effects of DOI on ethanol drinking were largely reversed by pretreatment with the selective 5-HT2A receptor antagonist M100907, at a dose that had no significant effect on ethanol intake by itself. Likewise, DOI has no effect on fluid intake and was only effective in high alcohol drinking mice. These data indicate that the DOI produces a selective suppression of alcohol drinking that depends on the 5-HT2A receptor, which is consistent with previous findings in alcohol preferring rats (Maurel et al. 1999). It should be noted, however, that administration of DOI can impact several neurotransmitter, neuromodulator, and hormonal systems, especially as it relates to the mesocortical systems that have been related to psychosis. Systemic administration of DOI increases the firing rates of dopamine neurons in the ventral tegmental area (VTA) and induces cortical dopamine release, and these effects are attenuated by selective antagonism of the 5-HT2A receptor (Bortolozzi et al. 2005; Pehek et al. 2006) or genetic deletion of the 5-HT2A receptor (Di Matteo et al. 2000; Huang et al. 2011). Direct administration of DOI into the nucleus accumbens (NAcc) significantly increases local dopamine levels (Bowers et al. 2000; Yan 2000). Furthermore, these receptors are capable of modulating cortical glutamate neurotransmission as DOI increases glutamate release (Scruggs et al. 2003) through a 5-HT2A receptor-dependent mechanism (Gewirtz and Marek 2000; Zhang and Marek 2008). Whether DOI has similar effects in the mesolimbic pathways that have been tied to substance abuse remains more speculative, and should be explored. Such complex neurophysiological changes could result in general suppression of consumatory behavior. However, we have found that DOI selectively suppresses alcohol intake in absence of an effect on fluid intake. This is consistent with previous reports that DOI selectively suppresses alcohol intake in alcohol preferring rats (Maurel et al. 1999), its anti-alcohol effects are more selective than those of meta-chlorophenylpiperazine (mCPP) or fluoxetine (Maurel et al. 1999; Maurel et al. 2000), and the availability of sucrose or saccharin (Maurel et al. 2000) does not affect the selectivity of DOI.
The data reported in this study are consistent with a preclinical literature documenting a role for psychedelics in the behavioral effects of ethanol. For example, systemic administration psychedelic cocktail ayahuasca blocks ethanol-induced behavioral sensitization (Oliveira-Lima et al. 2015) and the expression of an ethanol-induced conditioned place preference (Cata-Preta et al. 2018). Similarly, systemic administration of LSD attenuates ethanol drinking in C57 mice in a two-bottle choice paradigm (Alper et al. 2018), and DOI selectively suppresses alcohol intake in alcohol preferring rats (Maurel et al. 1999). Importantly, however, the present study extends that research by directly demonstrating that the effects of DOI are specific for alcohol preferring animals, which as outlined above, appears to be consistent with the human literature. This suggests that the subjects with AUD chosen for psychedelic therapy should be carefully selected and suggests that an interrogation of the neurophysiological basis for differential responses to psychedelic therapy could be informative.
It is generally regarded that 5-HT2A receptors are the common molecular target of psychedelics. The present study builds upon this previous research by directly documenting that 5-HT2A receptors are critical for suppression of ethanol drinking by DOI in mice, as has been shown in rats (Maurel et al. 1999). Future studies should examine the receptor pharmacology underlying the effects of LSD, ayahuasca, psilocybin, and other psychedelics. It is important to note that other studies have reported that decreased activity at 5-HT2A receptors also decreases ethanol-mediated behavior, as self-infusion of alcohol directly into brain reward regions is attenuated by co-infusion of the non-selective 5-HT2 receptor antagonist R-96544 (Ding et al. 2009). Moreover, 5-HT2A receptor antagonism attenuates the dopamine releasing and behavioral effects of amphetamine, MDMA, and cocaine (Murnane et al. 2013b), as well as drug- and cue-induced reinstatement of extinguished cocaine self-administration in rats (Fletcher et al. 2002; Nic Dhonnchadha et al. 2009) and primates (Murnane et al. 2013b). These conflicting results may be because acute agonism of 5-HT2A receptors by psychedelics causes rapid 5-HT2A receptor desensitization followed by sustained receptor downregulation (Buckholtz et al. 1985; Leysen et al. 1989; McKenna et al. 1989; Smith et al. 1999). Likewise, 5-HT2C receptor agonist activity could be an important part of the reduced ethanol drinking by psychedelics as DOI and many other psychedelics have relevant agonist affinity for 5-HT2C receptors and 5-HT2C receptor agonists are known to reduce self-administration of ethanol (Kasper et al. 2013; Tomkins et al. 2002), and it has been recently argued that 5-HT2C receptor agonism by psychedelics limits their own potential for addiciton (Canal and Murnane 2017). We selected the dose of DOI tested to maximize selectivity for 5-HT2A receptors and found that the effects of DOI were attenuated by selective antagonism of 5-HT2A receptors at a dose of M100907 that had no effect by itself. These data are not supportive of the involvement of 5-HT2C receptors in selective DOI-induced suppression of ethanol drinking in alcohol preferring subjects, consistent with previous studies in alcohol preferring rats (Maurel et al. 1999). However, it should be noted that higher doses of DOI that combine agonism 5-HT2A and 5-HT2C receptors could be more effective than doses of DOI selective for 5-HT2A receptors, especially in the low drinking group, as the mixed 5-HT2C receptor agonist 5-HT releaser mCPP also suppressed drinking in alcohol preferring rats (Maurel et al. 1999). Unlike the selective effects of DOI, however, mCPP significantly suppressed fluid intake in addition to alcohol intake, and so more selective 5-HT2C receptor agonists, such as WAY-163909 (Murphy and Murnane 2019), should be examined in future studies. The present study sets the stage for these new mechanistic studies to clarify the pharmacological and neurobiological mechanisms mediating the therapeutic effects of the psychedelics.
As mentioned in the introduction, picomolar concentrations of DOI inhibit the expression of a number proinflammatory signaling molecules that can be induced by tumor necrosis factor alpha (TNF-α), such as intracellular adhesion molecule, interleukin 6, and nitric oxide synthase (Yu et al. 2008a). These data suggest that DOI may have benefits for ethanol-induced neuroinflammation that may synergize with its effects in the reward circuitry to elicit favorable pharmacotherapeutic outcomes, but this has largely been unexplored in the context of AUD. Our cell culture studies represent an initial foray into this area, and are generally supportive of the idea that psychedelics may reduce neuroinflammation associated with ethanol. However, it is important to clearly recognize that much research remains to be conducted to evaluate this hypothesis. Likewise, we used pure 200 proof ethanol, with a nonvolatile residue of <0.0003% by the certificate of analysis, as has been done previously (Crabbe et al. 2012; Silva et al. 2018). It is difficult to purify ethanol to 100%, and so if any proinflammatory residue remained within the ethanol, it could have increased the contribution of inflammation to ethanol drinking in these animals, and perhaps augmented the efficacy of DOI. Future research could examine the efficacy of DOI using 190 proof ethanol, as is done in other studies.
In conclusion, we report that the synthetic psychedelic DOI significantly attenuates both ethanol-induced place conditioning and ethanol drinking. As has been reported previously in rats, the effects of DOI were selective for alcohol intake over fluid intake, and for the first time, we directly show that the effects of DOI were selective for high alcohol drinking subjects. DOI has apparent pharmacodynamic interactions with ethanol, in the absence of pharmacokinetic interactions, as there was no difference in clearance of ethanol following administration of DOI. Moreover, DOI elicits significant reductions in nitric oxide release from macrophages, a marker of activation of acute inflammation. The effects of DOI appear to depend on the 5-HT2A as they are reversed by M100907, but it should be noted that the 5-HT2A receptor is the major excitatory serotonin receptor in the brain and has very complex responses, and DOI produces a complex pattern of effects on neurotransmitters, and neuromodulators, and hormones. Elucidating the neurophysiological systems, neuropharmacological interactions, and neural circuitry through which psychedelics reverse alcohol dependence and reduce alcohol drinking holds tremendous promise for elucidating the pathophysiology of AUD and the development of new treatment options for this devastating disorder.
Acknowledgments
These studies were supported by the National Institutes of Health [DA040907 and NS100512)] and by funding from the Mercer University College of Pharmacy to KSM. The work of the Drug Design and Synthesis Section was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA). These studies represent partial fulfillment of AOs PhD dissertation research project at Mercer University
Footnotes
Disclosure:
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Abuzzahab FS Sr, Anderson BJ (1971) A review of LSD treatment in alcoholism. Int Pharmacopsychiatry 6: 223–35. [DOI] [PubMed] [Google Scholar]
- Al Mansouri S, Ojha S, Al Maamari E, Al Ameri M, Nurulain SM, Bahi A (2014) The cannabinoid receptor 2 agonist, beta-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacol Biochem Behav 124: 260–8. [DOI] [PubMed] [Google Scholar]
- Alper K, Dong B, Shah R, Sershen H, Vinod KY (2018) LSD Administered as a Single Dose Reduces Alcohol Consumption in C57BL/6J Mice. Frontiers in pharmacology 9: 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. Journal of psychopharmacology (Oxford, England) 29: 289–99. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Johnson MW (2016) Classic hallucinogens in the treatment of addictions. Prog Neuropsychopharmacol Biol Psychiatry 64: 250–8. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F (2005) The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem 95: 1597–607. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Henry MB, Thielen RJ, McBride WJ (2000) Serotonin 5-HT(2) receptor stimulation of dopamine release in the posterior but not anterior nucleus accumbens of the rat. J Neurochem 75: 1625–33. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Freedman DX, Middaugh LD (1985) Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur J Pharmacol 109: 421–5. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD (1992) Reducing the time needed to conduct conditioned place preference testing. Prog Neuropsychopharmacol Biol Psychiatry 16: 969–76. [DOI] [PubMed] [Google Scholar]
- Canal CE, Murnane KS (2017) The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens. Journal of psychopharmacology (Oxford, England) 31: 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata-Preta EG, Serra YA, Moreira-Junior EDC, Reis HS, Kisaki ND, Libarino-Santos M, Silva RRR, Barros-Santos T, Santos LC, Barbosa PCR, Costa JL, Oliveira-Lima AJ, Berro LF, Marinho EAV (2018) Ayahuasca and Its DMT- and beta-carbolines - Containing Ingredients Block the Expression of Ethanol-Induced Conditioned Place Preference in Mice: Role of the Treatment Environment. Frontiers in pharmacology 9: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P (2012) Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol and alcoholism (Oxford, Oxfordshire) 47: 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E (2000) Biochemical and electrophysiological evidence that RO 60–0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res 865: 85–90. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Toalston JE, Oster SM, McBride WJ, Rodd ZA (2009) Involvement of local serotonin-2A but not serotonin-1B receptors in the reinforcing effects of ethanol within the posterior ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 204: 381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck E (2005) Flashback: psychiatric experimentation with LSD in historical perspective. Canadian journal of psychiatry Revue canadienne de psychiatrie 50: 381–8. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ (2008) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335: 728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA (2002) Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27: 576–86. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23: 569–76. [DOI] [PubMed] [Google Scholar]
- Goeders JE, Murnane KS, Banks ML, Fantegrossi WE (2009) Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav 93: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL (2005) A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab animal 34: 39–43. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–52. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB (1986) Can drugs be used to enhance the psychotherapeutic process? American journal of psychotherapy 40: 393–404. [DOI] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G (2000) Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse (New York, NY 38: 421–31. [DOI] [PubMed] [Google Scholar]
- Huang M, Dai J, Meltzer HY (2011) 5-HT(2A) and 5-HT(2C) receptor stimulation are differentially involved in the cortical dopamine efflux-Studied in 5-HT(2A) and 5-HT(2C) genetic mutant mice. Eur J Pharmacol 652: 40–5. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35: 1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR (2014) Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of psychopharmacology (Oxford, England) 28: 983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR (2017) Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 43: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper J, Tikamdas R, Kim MS, Macfadyen K, Aramini R, Ladd J, Bisceglia S, Booth R, Peris J (2013) The serotonin-2 receptor modulator, (−)-trans-PAT, decreases voluntary ethanol consumption in rats. Eur J Pharmacol 718: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen JE, Janssen PF, Niemegeers CJ (1989) Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol 163: 145–9. [DOI] [PubMed] [Google Scholar]
- Lindsey LP, Daphney CM, Oppong-Damoah A, Uchakin PN, Abney SE, Uchakina ON, Khusial RD, Akil A, Murnane KS (2019) The cannabinoid receptor 2 agonist, beta-caryophyllene, improves working memory and reduces circulating levels of specific proinflammatory cytokines in aged male mice. Behav Brain Res 372: 112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G (2013) Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. Journal of leukocyte biology 94: 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangini M (1998) Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoactive Drugs 30: 381–418. [DOI] [PubMed] [Google Scholar]
- Maurel S, De Vry J, De Beun R, Schreiber R (1999) 5-HT2A and 5-HT2C/5-HT1B receptors are differentially involved in alcohol preference and consummatory behavior in cAA rats. Pharmacol Biochem Behav 62: 89–96. [DOI] [PubMed] [Google Scholar]
- Maurel S, Schreiber R, De Vry J (2000) Palatable fluids do not affect alcohol intake and its reduction by serotonergic compounds in alcohol-preferring cAA rats. Eur Neuropsychopharmacol 10: 351–3. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Nazarali AJ, Himeno A, Saavedra JM (1989) Chronic treatment with (+/−)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain. Neuropsychopharmacology 2: 81–7. [DOI] [PubMed] [Google Scholar]
- Murnane KS (2018) The renaissance in psychedelic research: What do preclinical models have to offer. Prog Brain Res 242: 25–67. [DOI] [PubMed] [Google Scholar]
- Murnane KS (2019) Serotonin 2A receptors are a stress response system: implications for post-traumatic stress disorder. Behavioural pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Andersen ML, Rice KC, Howell LL (2013a) Selective serotonin 2A receptor antagonism attenuates the effects of amphetamine on arousal and dopamine overflow in non-human primates. J Sleep Res 22: 581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Guner OF, Bowen JP, Rambacher KM, Moniri NH, Murphy TJ, Daphney CM, Oppong-Damoah A, Rice KC (2019) The adrenergic receptor antagonist carvedilol interacts with serotonin 2A receptors both in vitro and in vivo. Pharmacol Biochem Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Kimmel HL, Rice KC, Howell LL (2012a) The neuropharmacology of prolactin secretion elicited by 3,4-methylenedioxymethamphetamine (“ecstasy”): a concurrent microdialysis and plasma analysis study. Horm Behav 61: 181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Perrine SA, Finton BJ, Galloway MP, Howell LL, Fantegrossi WE (2012b) Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: correlations between behavior and neurochemistry. Psychopharmacology (Berl) 220: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, Rice KC, Howell LL (2013b) Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci 33: 13367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TJ, Murnane KS (2019) The serotonin 2C receptor agonist WAY-163909 attenuates ketamine-induced hypothermia in mice. Eur J Pharmacol 842: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau F Jr., Yu B, Martin D, Nichols CD (2013) Serotonin 5-HT2A receptor activation blocks TNF-alpha mediated inflammation in vivo. PLoS One 8: e75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA (2009) Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci 123: 382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Lima AJ, Santos R, Hollais AW, Gerardi-Junior CA, Baldaia MA, Wuo-Silva R, Yokoyama TS, Costa JL, Malpezzi-Marinho EL, Ribeiro-Barbosa PC, Berro LF, Frussa-Filho R, Marinho EA (2015) Effects of ayahuasca on the development of ethanol-induced behavioral sensitization and on a post-sensitization treatment in mice. Physiol Behav 142: 28–36. [DOI] [PubMed] [Google Scholar]
- Oppong-Damoah A, Makriyannis A, Blough BE, Murnane KS (In Press) The Sesquiterpene Βeta-Caryophyllene Oxide Attenuates Ethanol Drinking and Place Conditioning in Mice. Heliyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppong-Damoah A, Zaman RU, D’Souza MJ, Murnane KS (2019) Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Horm Behav 108: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS (2006) Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology 31: 265–77. [DOI] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA (2009) Frontiers in Neuroscience Conditioned Place Preference In: Buccafusc o JJ (eds) Methods of Behavior Analysis in Neuroscience. CRC Press/Taylor & Francis Taylor & Francis Group, LLC, Boca Raton (FL: ) [PubMed] [Google Scholar]
- Ray A, Canal CE, Ehlen JC, Rice KC, Murnane KS (2019) M100907 and BD 1047 attenuate the acute toxic effects of methamphetamine. Neurotoxicology 74: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Chitre NM, Daphney CM, Blough BE, Canal CE, Murnane KS (2018) Effects of the second-generation “bath salt” cathinone alpha-pyrrolidinopropiophenone (alpha-PPP) on behavior and monoamine neurochemistry in male mice. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY (2003) The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett 346: 137–40. [DOI] [PubMed] [Google Scholar]
- Silva CP, Horton WJ, Caruso MJ, Sebastian A, Klein LC, Albert I, Kamens HM (2018) The influence of adolescent nicotine exposure on ethanol intake and brain gene expression. 13: e0198935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM (1958) A new adjunct to the treatment of alcoholism: the hallucinogenic drugs. Quarterly journal of studies on alcohol 19: 406–17. [PubMed] [Google Scholar]
- Smith RL, Barrett RJ, Sanders-Bush E (1999) Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology (Berl) 144: 248–54. [DOI] [PubMed] [Google Scholar]
- Szabo G, Saha B (2015) Alcohol’s Effect on Host Defense. Alcohol research : current reviews 37: 159–70. [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M (2014) “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol (Fayetteville, NY) 48: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA (2002) An investigation of the role of 5-HT(2C) receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav 71: 735–44. [DOI] [PubMed] [Google Scholar]
- Tung YT, Chua MT, Wang SY, Chang ST (2008) Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol 99: 3908–13. [DOI] [PubMed] [Google Scholar]
- Ullrich T, Rice KC (2000) A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorganic & medicinal chemistry 8: 2427–32. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H (2005) Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav 4: 240–52. [DOI] [PubMed] [Google Scholar]
- Yan QS (2000) Activation of 5-HT2A/2C receptors within the nucleus accumbens increases local dopaminergic transmission. Brain research bulletin 51: 75–81. [DOI] [PubMed] [Google Scholar]
- Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD (2008a) Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. Journal of Pharmacology and Experimental Therapeutics 327: 316–323. [DOI] [PubMed] [Google Scholar]
- Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD (2008b) Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 327: 316–23. [DOI] [PubMed] [Google Scholar]
- Zhang C, Marek GJ (2008) AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuropsychopharmacol Biol Psychiatry 32: 62–71. [DOI] [PubMed] [Google Scholar]