Abstract
Celiac disease (CeD) is a common form of enteropathy with frequent extraintestinal manifestations (EIM). Misrecognition of these presentations may lead to significant delays in diagnosis. Any organ may be involved, either through an immune / inflammatory phenomenon, or nutritional deficiencies. Some EIM, such as gluten ataxia, may be irreversible if left untreated, but most will improve with gluten-free diet (GFD). Knowledge of the various EIM as well as the associated conditions which do not improve on a GFD will avoid delays in the diagnosis and management of CeD and associated manifestations.
Keywords: Celiac disease, extraintestinal manifestations, gluten-free diet
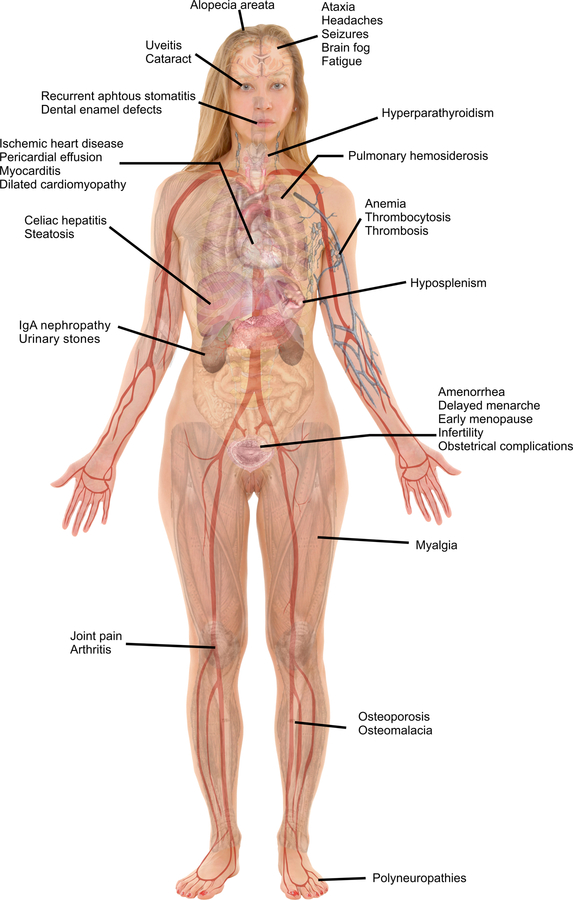
Celiac disease (CeD) is common with an estimated global prevalence of 0.7 to 1.4% 1. It is a T-cell enteropathy induced by gluten exposure among genetically predisposed individuals. Classically, CeD was diagnosed among children presenting with gastrointestinal manifestations and failure to thrive. The discovery of tissue transglutaminase as an immunological target offered a convenient first line diagnostic test2, and liberalization of screening led to recognition of many other presentations of CeD that are unrelated to the digestive tract3. A recent retrospective cohort study showed that 62% of adults had extraintestinal manifestations (EIM) of CeD at diagnosis, which were unaccompanied by intestinal manifestations in 9% of cases4. Antigenic mimicry, circulating auto-antibodies, inflammatory cytokines, and nutritional deficiencies are all factors contributing to EIM. Lack of knowledge of the various atypical manifestations of CeD may lead to delays in diagnosis. In an adult cohort study, the mean time to diagnosis was 2.3 months for those with GI symptoms, compared to 42 months for those without GI symptoms 5. Children presenting with primarily EIM had more severe small intestinal histologic injury at the time of diagnosis6. This review will first summarize common vitamin and mineral deficiencies associated with CeD and their clinical manifestations, then provide a system-oriented overview of the various EIM of CeD. The latter will detail extra-intestinal manifestations responding to GFD, as well as complications from long term untreated disease, including those that are generally associated with systemic inflammation (Figure 1). Finally, associated diseases without a causative link with CeD will be mentioned.
Figure 1:
Main Extraintestinal Manifestations Associated with Celiac Disease
Overview of GI symptoms and associated vitamin deficiencies
Classic presentation of CeD includes diarrhea, steatorrhea, abdominal pain, bloating and weight loss, a symptomatology mainly attributed to the maldigestion and malabsorption associated with intestinal villous atrophy. However, some individuals present with constipation, recurrent vomiting or heartburn, which may be mistaken for a functional disorder or irritable bowel syndrome 7.
Due to insufficient nutritional intake, malabsorption and maldigestion, deficiencies in electrolytes, vitamins and minerals are very frequent among untreated CeD. Indeed, a recent prospective found deficiencies in almost 90%, notably zinc deficiency in 66.7%8. These deficiencies may have some systemic consequences (Table 1). Although rare nowadays, untreated CeD may result in severe malabsorption and malnutrition.
Table 1:
Nutritional deficiencies associated with CeD and their related clinical manifestations
| Nutritional deficiency | Most frequent signs and symptoms |
|---|---|
| Irona | Hypochromic, microcytic anemia, glossitis, koilonychia, fatigue, pallor, cognitive impairment9 |
| Folate | Megaloblastic anemia, glossitis, diarrhea, cognitive impairment10 |
| Vitamin B12 | Megaloblastic anemia, posterior columns syndrome, dementia, depression, psychosis |
| Vitamin D | Osteomalacia (deformity of bone, pathologic fractures), osteoporosis, cognitive impairment11, secondary hyperparathyroidism 12, 13 |
| Zinc | Growth retardation, hypogonadism, infertility, dysgueusia, poor wound healing, diarrhea, dermatitis on the extremities and periorificial, glossitis, alopecia, corneal clouding |
| Less frequently occurring | |
| Protein | Edema, muscular atrophy |
| Vitamin B1 (thiamine) | Irritability, fatigue, headaches, peripheral neuropathy, wet Beriberi: congestive heart failure; Wernicke: nystagmus, ophtalmoplegia, ataxia; Korsakoff: hallucinations, impaired short-term memory and confabulation |
| Vitamin B3 (niacin) | Pellagra: diarrhea, dementia, pigmented dermatitis; Glossitis, stomatitis, vaginitis, vertigo, burning dysesthesias |
| Vitamin B6 (pyridoxine) | Stomatitis, angular cheilosis, glossitis, irritability, depression, confusion, normochromic normocytic anemia |
| Vitamin A | Follicular hyperkeratosis, night blindness, conjunctival xerosis, keratomalacia |
| Vitamin E | Hemolytic anemia, peripheral neuropathies, ophtalmoplegia, posterior columns syndrome |
| Vitamin K | Easy bleeding |
Iron deficiency is very common among individuals with CeD and may be the leading manifestation in up to 46% of subclinical or asymptomatic cases 14.
As mentioned in Table 1, vitamin D deficiency has several consequences, notably secondary hyperparathyroidism which is found in 19 to 28% of individuals with CeD 12, 13 and may progress to primary hyperparathyroidism if untreated 15; some with initial normocalcemic hyperparathyroidism subsequently develop hypercalcemic hyperparathyroidism despite vitamin D repletion and gluten avoidance 16.
In addition to oligosaccharides and oligopeptides maldigestion due to loss of brush border enzymes, fat maldigestion and malabsorption may also occur and is a well known cause of hyperoxaluria and oxalate kidney stones 17. Some have proposed that urinary stones may be the first manifestations of subclinical CeD, which could account for 1–1.5% of all cases of urinary stone disease 18. Studies have shown an increased risk of urinary stones among untreated CeD 18, 19 but that risk was comparable to the general population among treated CeD18. Hyperoxaluria was not associated with overt fat malabsorption in the presence of CeD 18.
Refractory celiac disease (RCD) is defined as persistent immune activation, villous atrophy and signs and symptoms of malabsorption despite adhering strictly to a GFD for at least one year20. There are two types of RCD; type 1 is indistinguishable from active CeD, whereas type 2 is characterized by the presence of aberrant T cells and clonal T-cell receptor gene rearrangement 21. Individuals with RCD are at high risk of malnutrition and related complications, and those with RCD type 2 may suffer from gastrointestinal bleeds, obstruction or perforation as a result of ulcerative jejunitis. RCD is defined clinically based on intestinal symptoms (diarrhea, weight loss, abdominal pain, bloating) and EIM have not been well described. A recent series of RCD cases treated with oral budesonide reported that 72% had a classical presentation (i.e diarrhea) and 18% had dermatitis herpetiformis22. Only 21% reported fatigue22. In a similar retrospective cohort including 21 well-defined RCD type 1 cases from two US Celiac Centers, 29% reported fatigue and 19% had other EIM, including brain fog, joint pain, headaches, dermatitis herpetiformis and neuropathy (Therrien et al., submitted manuscript).
Extraintestinal manifestations and complications of CeD
Neurological manifestations
The prevalence of neurological symptoms among individuals with CeD is estimated to be between 6 and 10%23, but may be as high as 42% in untreated CeD 24. Many mechanisms have been implicated, notably cross-reacting antibodies, immune-complex deposition, direct neurotoxicity and vitamin or nutrient deficiencies 24, 25.
Headaches and migraine
Headaches, especially migraines, may be the first manifestation of CeD and may be of higher intensity compared to the general population, leading those affected to seek medical care more frequently26, 27, 28. A pooled estimated prevalence of headache in adults with CeD is 26%, slightly higher than a pooled prevalence of 18.3% in children 28, 29. Idiopathic headache, migraine and tension headache are more frequent among individuals with CeD, while there is no association with cluster headache, hemicrania continua or trigeminal neuralgia26, 27,28. Furthermore, an increased rate of medical visits for headaches and migraines in the setting of potential CeD has been reported 26, and these symptoms may also occur with gluten exposure in treated CeD30. Fortunately, both frequency and intensity of headaches decrease with the GFD, especially in children 4, 24, 27, 28.
Peripheral neuropathy
There is a 2.5 to 3.4 -fold increased risk of peripheral neuropathy in the CeD population compared to the general population31, 32. However, an even more common entity, called “gluten-induced neuropathy”, is a peripheral idiopathic neuropathy associated with positive celiac serology but without enteropathy33. When associated with CeD, peripheral neuropathy may present either before or after the diagnosis or may be the only manifestation of the disease 32, 34. A population-based study recently showed an association between polyneuropathy and being subsequently diagnosed with CeD (OR 5.4 95%CI 3.6–8.2)31. Various type of neuropathies have been described; small fiber sensory neuropathy, symmetric predominantly sensory neuropathy, mononeuritis multiplex and multifocal motor or sensorimotor polyneuropathy34. According to the type of neuropathy, clinical signs and symptoms may include painful paresthesia (may be facial or oral), mild gait instability, mild lower limbs or focal weakness 34. It is associated with a significant reduction of quality of life 33.
Molecular mimicry between ganglioside molecules of peripheral nerves and gluten is a proposed mechanism34 and antiganglioside antibodies were present in serum of 65% of a small cohort of CeD patients with neuropathy 35. Improvement on GFD is variable, and may depend on dietary compliance, the duration of the inflammatory process, and the etiology of the neuropathy; the presence of anti-neuronal antibodies is an indicator of non response to GFD 25, 36.
Gluten Ataxia
Although gluten ataxia is the most extensively studied neurological manifestation in CeD, most of the available literature is from a single group from the United Kingdom. Gluten ataxia is also considered as a “gluten related disorder”, implying that it can be triggered by gluten in the absence of CeD. Gluten ataxia typically develops among middle-age adults37 and is characterized by gait ataxia and mostly lower limb ataxia, dysarthria, nystagmus and other oculomotor disorders25, 37. Less than 10% have GI symptoms but about 40% have duodenal histology compatible with CeD37, 38. Elevated celiac serology and HLADQ2/DQ8 may help discriminate between CeD and gluten ataxia in the absence of enteropathy. Although anti-gliadin antibodies may be found in gluten ataxia without CeD, tTG antibodies are rarely elevated 38, 39.
Gluten ataxia is believed to be related to antibody cross-reactivity between Purkinje and other cerebellar cells and gluten peptides, and IgA tissue transglutaminase 6 (TTG6) deposits can be observed in the cerebellum37. These antibodies were found in circulation in 73% of individuals with gluten ataxia (of which 51% had an enteropathy), and the titers significantly decreased with a GFD 40.
A GFD seems to be an effective treatment for gluten ataxia, but it may depend on the duration of symptoms prior to the diagnosis, since there may be irreversible loss of Purkinje cells in the cerebellum 37, 41. Nevertheless, improvement after one year of GFD was independent of the presence of enteropathy41. Screening for CeD with anti-tTG-2 IgA and IgG deamidated gliadin peptide as well as other antibodies associated with gluten ataxia (native anti-gliadin antibodies and, if available, anti-tTG-6), and subsequent initiation of GFD should be performed promptly in this population. MR spectroscopy of the cerebellum may be a useful tool for follow-up39.
Interestingly, a recent prospective cohort of 100 consecutive newly diagnosed CeD patients reported gait instability in 24%, gait ataxia in 29%, nystagmus in 11% and the presence of circulating tTG6 among 40%24. Moreover, 47% had abnormal MR spectroscopy of the cerebellum and 25% had white matter lesions on brain MRI. There was an overall improvement after one year of GFD. Although a referral bias cannot be excluded, and some manifestations may be attributed to vitamin deficiencies, these data provide compelling evidence about a potentially frequent neurological immune related manifestation of CeD and how GFD may prevent progression to the more severe condition that is gluten ataxia24.
Gluten-induced cognitive impairment (Brain fog)
Although “brain fog” is not a medically or psychologically recognized condition, this term is often used colloquially by patients to refer to difficulty concentrating, lack of attentiveness, short-term memory deficits, word-finding difficulties, reduced creativity, confusion and disorientation after gluten exposure42, 43.
Interestingly, this type of cognitive impairment is not specific to gluten ingestion; this phenomenon has also been observed in many other conditions, notably multiple sclerosis, fibromyalgia, and those undergoing chemotherapy42. Few studies are available on gluten-induced cognitive impairment in CeD, but a small cohort study showed objective minor deficits to the short-term memory, processing speed and visual-spatial memory at diagnosis that improved after one year on a GFD43. Cognitive improvement also significantly correlated with the extent of mucosal healing43. Proposed mechanisms for gluten-induced cognitive impairment involve circulating pro-inflammatory cytokines that may act directly upon neurons43 or affect blood-brain barrier permeability enhancing inflammation by increasing leukocytes migration42. Other hypotheses include reduced brain tryptophan concentration leading to a deficit in serotonin, effects of exorphins (which are opioid peptides derived from partially digested gluten) and microbiome changes43. Individuals who experience gluten-induced cognitive impairment upon accidental gluten exposure often report that this symptom may be more prolonged (one to four weeks) than gastrointestinal symptoms44.
Epilepsy
The risk of epilepsy is increased among individuals with CeD45, with complex partial seizures being the most frequent type 23. There is a well-known triad of CeD, bilateral parieto-occipital calcifications, and seizures46, 47. Interestingly, in this presentation, the seizures do not arise specifically from the sites of the calcifications46, 47, which may also be associated with headaches rather than epilepsy39. Many hypotheses have been proposed for these lesions, notably folic acid deficiency or an autoimmune phenomenon, being often associated with white matter abnormalities 23, 46, 47. Interestingly, this syndrome appears to be restricted to HLADQ2 47. Although incidence and seizure activity may be reduced by strict adherence to GFD 48, 49, cerebral calcifications are possibly irreversible 48.
Other rare presentations
Many other neurological presentations of CeD have been described, notably acute generalized chorea 50, pseudotumor cerebri 51 and cerebral vasculitis 52, all of which appear responsive to GFD.
Psychiatric conditions
Although anxiety, depression, attention-deficit/hyperactivity and eating disorders have been associated with CeD 53, distinguishing between specific manifestations of CeD, and the consequences of suffering from a chronic GI disorder and following a strict diet is challenging53. For instance, a recent study showed an association between the development of anxiety after a CeD diagnosis and achieving mucosal recovery, illustrating the impact of strict adherence to the GFD on psychological well-being 54. Nevertheless, psychiatric disorders were found among 24% of adults at diagnosis and tended to improve after 24 months of GFD4. More specifically, some studies still showed improvement in anxiety symptoms on a GFD, but there is controversy whether depressive symptoms improve 55, 56. One possible explanation is that treating CeD may increase circulating L-tryptophan levels and production of serotonin and dopamine 57.
Although there is scant literature on the subject, it is the authors experience that intermittent gluten exposure may trigger feelings of depression, anxiety and personality changes in some individuals with CeD 30.
Fatigue
Fatigue is common in CeD, with an incidence as high as 37% at diagnosis 4. There is no standard definition for fatigue and studies have used different instruments to measure it 58. Chronic fatigue may be an indication to screen for CeD, since it was found more often among undiagnosed cases of CeD 59. Acute fatigue may also be a sign of gluten exposure 30. There is controversy about the effects of the GFD on fatigue, as only 50% of adults may improve after two years on a GFD 4, 60. The etiology of fatigue in the non-responders is multifactorial, and screening for depression, sleep disorders, and thyroid disease is advisable, particularly in the absence of anemia, 60, 61.
Ocular manifestations
There are few ocular complications of untreated CeD. In addition to retinopathy related to vitamin A deficiency, cataracts (HR 1.28 95CI 1.19–1.36)62 and uveitis (HR 1.32 95CI 1.10 – 1.58)63 have been reported, with subsequent resolution of the uveitis on a GFD 64. Oxidative stress, vitamin deficiencies and hypocalcemia have been hypothesized as an explanation for the association between cataract formation and CeD65. The risk was the highest among individuals diagnosed with CeD at 40–49 years (HR 1.40 95CI 1.11–1.77) and 50–59 years (HR 1.23 95CI 1.04–1.43)62. Interestingly, individuals with previous cataract were also at increased risk to be later diagnosed with CeD (OR 1.14 95CI 1.02–1.30) 62.
Dermatologic manifestations
Many skin conditions occur more frequently among individuals with CeD, such as eczema, psoriasis, atopic dermatitis and urticaria (Table 2). Non-specific rashes have also been associated with gluten exposure 30. The following section will focus on specific skin and hair conditions which typically improve on a GFD; however, only dermatitis herpetiformis has been demonstrated to have a clear gluten-related immune mechanism.
Table 2:
Association between CeD and other conditions
| System | Disease | Odds ratio or Hazard ratio |
|---|---|---|
| Endocrine | Hypothyroidism | OR 3.38(95% CI 2.88–6.56) 210 HR 4.4 (95% CI 3.4–5.6) 211 |
| Hyperthyroidism | OR 1.28 (95% CI 0.37–4.46)210
HR 2.9 (95% CI 2.0–4.2)211 |
|
| Type 1 diabetes | HR 2.4 a (95% CI 1.9–3.0)212 | |
| Addison’s disease | HR 11.4 (95% CI 4.4–29.6)213 | |
| Renal | Diabetic nephropathy among type 1 diabetes | HR 1.36 (95% CI 1.23–1.50)128 |
| Skin | Psoriasis | OR 1.64 (95%CI 1.40–1.92) 59 OR 3.09 (95% CI 1.92 – 4.97)79 |
| Urticaria | HR 1.51 (95%CI 1.36 – 1.68)81 | |
| Chronic urticariab | HR 1.92 (95% CI 1.48 – 2.48)81 | |
| Atopic dermatitis | OR 3.17 (95% CI 1.02–9.82)214 | |
| Rosacea | HR 1.46 (95%CI 1.11–1.93)215 | |
| Liver | Primary biliary cholangitis | HR 10.16 (95% CI 2.61–39.49) 216 |
| Primary sclerosing cholangitis | HR 4.46 (95%CI 2.50–7.98) 216 | |
| Immunology | Selective IgA deficiency | 10–20 fold increased riskc217 |
| Lung | Chronic obstructive pulmonary disease | HR 1.24 (95%CI 1.10–1.38) 121 |
| Connective tissue disorders | Systemic lupus erythematous | HR 3.49 (95% CI 2.48 – 4.90)218 |
| Ehlers-Danlos syndrome | HR 2.43 (95%CI 1.20–4.91) 219 | |
| Genetic syndromes | Turner syndrome | OR 3.29 (95%CI1.94–5.56) 220 |
| Down’s syndrome | OR 6.15 (95%CI 5.09–7.43) 221 |
Risk of subsequent type I diabetes among children and adolescent diagnosed with CeD
Duration ≥ 6 weeks
13% of a cohort with CeD and selective IgA deficiency had subclinical CeD
Dermatitis herpetiformis
Dermatitis herpetiformis (DH) is the most frequent dermatologic manifestation of CeD. The rash is characterized by extremely pruritic small vesicles and papules on the elbows, knees and buttocks that may be obscured by excoriations. Oral involvement is very rare 66. In contrast to CeD, the incidence of DH seems to be decreasing over time, probably since it is mostly diagnosed among untreated CeD and the disease’s global awareness is increasing 68, 69. Indeed, compared to previous cohort studies showing a 17–20% prevalence of DH among untreated CeD14, 70, the rate in a more recent ten-year cohort in Finland was only 4%71. Diagnosis before 16 years of age is rare and the highest incidence is among men between 60 and 69 years old and women between 50 and 59 years old 68. Although most with DH have enteropathy, they rarely report gastrointestinal symptoms 72, 73. DH is characterized by deposits in the superficial papillary dermis of anti-tissue transglutaminase 3, an epidermal transglutaminase 74. Thus, in addition to the clinical characteristics, DH diagnosis is confirmed by a skin biopsy showing pathognomonic granular IgA deposits on the papillary dermis by immunofluorescence 75. Typically, DH lesions respond to GFD 76, therefore, when DH develops or persists in a patient with treated CeD, ongoing gluten exposure must be suspected and adherence to the GFD reassessed. Fortunately, the rash tends to heal without scaring, but remission may take months or years to achieve77. Dapsone is often used initially because the GFD prevents further inflammation, but does not affect the ongoing skin process77.
Psoriasis
Psoriasis can be associated with CeD, with a risk 1.44 to 3.09 times greater than the general population 78, 79. Some studies have reported that the GFD improves psoriasis in CeD 80. It may be due to a better absorption of vitamin D, or improved intestinal barrier function 77. Some authors have suggested that psoriasis may be the only clinical manifestation of CeD 77.
Chronic urticaria
Chronic urticaria (hives lasting more than six weeks) is associated with HLADQ8 and is more common among individuals with CeD (HR 1.92, 95%CI 1.48 – 2.48)77, 81. A GFD may lead to complete remission of urticaria, allowing some to conclude that urticaria may be a manifestation of CeD 82.
Leucocytoclastic vasculitis
Leucocytoclastic vasculitis is a small vessel vasculitis presenting with palpable purpura, hemorrhagic vesicles and livedo reticularis 73. There are few reports of cutaneous vasculitis associated with CeD, including most interestingly, a 38 year-old woman with untreated CeD and leucocytoclastic vasculitis who had complete resolution of her skin lesions after adoption of a strict GFD 83.
Alopecia areata
Alopecia areata is an autoimmune disease presenting with well circumscribed patches of hair loss without scarring 73. The prevalence of CeD in alopecia areata is the same as general population73; however, some cases, especially children, may improve with a GFD 84.
Oral manifestations
There is no pathognomonic oral manifestation of CeD. As mentioned in Table 1, many vitamin deficiencies may induce glossitis or stomatitis. In addition, recurrent aphtous stomatitis (RAS) and dental enamel defects have been observed more frequently among those with CeD and may be partly immune-mediated and gluten responsive.
Recurrent aphthous stomatitis
RAS is characterized by painful small well circumscribed ulcers in the oral cavity 73. It is more frequent among individuals with CeD than the general population (OR 3.79 95%CI 2.67 – 5.39) 85. It is unclear whether this condition is related to direct immune effects from gluten exposure or nutritional deficiencies, such as iron, folic acid and vitamin B12 73, 86. In addition to nutritional deficiencies, some drugs such as NSAIDs, captopril, and phenobarbital have been associated with RAS87. Differential diagnoses include Behcet’s syndrome, inflammatory bowel disease, Sweet syndrome, cyclic neutropenia and HIV infection 88. Nevertheless, in favor of its being gluten-related, GFD may lead to complete remission of the stomatitis, and gluten exposure induces recurrence 30, 89.
Dental enamel defects
Dental enamel defects include defects in color, pitting, grooving and complete loss of enamel and are most commonly described involving the secondary incisors in children 67, 86. Severity seems to be correlated with the duration of gluten exposure 90. Disease pathways may be either immune-mediated or nutrition-related 86. Interestingly, dental enamel defects have also been found among healthy first-degree relatives of CeD patients carrying HLA DQ2 with normal duodenal histology91.
Musculoskeletal manifestations
Osteoporosis
Reduced bone density at diagnosis in CeD is found at a variable rate ranging from 26 to 72%, and may be independent of the presence of GI symptoms92, 93. Although many meta-analyses found an increased risk of osteoporosis in CeD 94, the inverse has not been shown as CeD is not more prevalent among those with osteoporosis 95. A recent meta-analysis of prospective studies showed that CeD was associated with a 30% increased risk of any fracture and a 69% increased risk of hip fracture96. Factors associated with osteoporosis at the time of CeD diagnosis, justifying an earlier DEXA scan, include male sex, age >45 years old, being underweight and severe histological damage (Marsh 3c) 97. Many mechanisms link decrease in bone density and CeD. Calcium and vitamin D malabsorption may lead to hyperparathyroidism, and overall malnutrition leads to low body mass index and hypogonadism 98. Furthermore, circulating cytokines and autoantibodies against osteoprotegerin may affect bone turnover99, 100. Some studies showed improvement or stabilization of bone density on a GFD, but persistence of fracture risk 101, 102, while a systematic review concluded that bone density improves after one year on a GFD and may even return to normal after five or more years on a GFD103. Persistent villous atrophy may be an important risk modifier 104, 105. A DEXA scan is recommended, either at diagnosis or one year after the initiation of GFD 20, 101.
Osteomalacia
Osteomalacia is a decreased bone mineralization at sites of bone turnover caused by vitamin D deficiency. A recent meta-analysis showed a mean incidence of osteomalacia of 18.3% (4.7–38.1%) in CeD 94. Osteomalacia may be asymptomatic, or cause bone pain, muscle spasm or tenderness, and fractures 106. Common laboratory findings include elevated alkaline phosphatase, reduced serum calcium and phosphorus, reduced urinary calcium, markedly reduced ergocalciferol and elevated PTH 106. GFD and vitamin D supplementation are the current treatments for osteomalacia in CeD.
Myalgia
Myalgia are common initial symptoms of CeD 4 which may be related to either nutritional deficiencies or systemic inflammation. In contrast to children, only half of adults with CeD had decreased muscle pain after 24 months of GFD 4. However, other causes of muscle pain, such as idiopathic inflammatory myopathies and fibromyalgia, are also frequently associated with CeD and may not respond to the GFD98.
Arthralgia and arthritis
Another frequent musculoskeletal manifestation is joint pain, occurring in 20–30% at CeD diagnosis94. Some have described a presentation similar to seronegative spondyloarthropathies with non-erosive, non-deforming oligoarthritis and axial or sacroiliac involvement 107. However, a recent meta-analysis did not show an increased risk of arthritis in CeD 94. Studies are contradictory regarding whether joint pain improves on a GFD 4, 108, and response to GFD may help distinguish whether joint pain is directly related to CeD and gluten exposure, as opposed to synovial effusion, sacroiliitis and joint stiffness, which may be signs of associated autoimmune rheumatologic disease and do not improve on a GFD 109, 110, 111. Although the evidence is limited, some individuals on a longstanding GFD may experience joint pain upon accidental gluten exposure 44. Finally, a higher prevalence of lower limb enthesopathy has been found among untreated CeD 107, 112.
Cardiovascular system
Pericardial effusion, myocarditis and cardiomyopathy
CeD may present with asymptomatic pericardial effusion, which resolves on a GFD 113, 114. Untreated silent CeD is frequent among individuals with auto-immune myocarditis, with an incidence of biopsy-confirmed CeD of 4.4% 115. The myocarditis is mostly lymphocytic infiltrate and is usually not associated with any chronic GI symptoms 115. While some studies showed an increased prevalence of untreated CeD among individuals with dilated or idiopathic cardiomyopathies116, other population studies showed that cases with CeD are not at increased risk for these conditions 117, 118. Although the precise immune mechanism has not been elucidated yet, idiopathic dilated cardiomyopathy with coexisting CeD improves on a GFD, compared to nonadherent individuals 119, 120.
Pulmonary manifestations
Lungs are rarely involved in CeD. An increased risk of developing chronic obstructive lung disease before and after CeD diagnosis has been reported, although no strong causative relationship was established121 (Table 2). To our knowledge, the only proven lung involvement of CeD is pulmonary hemosiderosis.
Pulmonary hemosiderosis
Pulmonary hemosiderosis is characterized by recurrent alveolar hemorrhages. Lane-Hamilton syndrome is the association of CeD and pulmonary hemosiderosis caused by an increased permeability of alveolar capillaries due to deposition of immune complexes 122. This syndrome may occur in both children and adults, and is not always associated with GI symptoms 123,124. GFD allows some improvement, possibly in part because of a better absorption of the immunosuppressive treatment 124. Some recommend that pulmonary hemosiderosis is an indication to screen for CeD 122.
Renal disease
The prevalence of CeD is increased among individuals with chronic kidney diseases125, and although controversial, an increased risk of end-stage renal disease has been reported in CeD 126. The strongest associations remain IgA nephropathy and diabetic nephropathy127; interestingly, CeD may be a specific risk factor for the development of diabetic nephropathy in type I diabetes 128. In addition, it has been hypothesized that nutritional deficiencies may exacerbate renal insults17.
IgA Nephropathy
IgA nephropathy (Maladie de Berger) is an immune-complex mediated glomerulonephritis caused by glomerular IgA deposits and is characterized by macroscopic hematuria and impaired renal function. Although the overall prevalence is low (0.026%) 129, a metanalysis showed an increased risk among in CeD, especially among males 127. Recently, shared disease pathways between these two disorders were elucidated, including overexpression of the IgA1 mesangial receptor (TfR1)130, 131. Antigliadin IgA complexes trigger the activity of transglutaminase 2 present in the kidney and upregulate the expression of TfR1 and enhance glomerular injury 132. Some individuals without CeD experienced clinical and histological improvement of the IgA nephropathy with GFD, but the diet did not prevent renal failure 133, 134.
Other nephropathies
A few case series described other glomerulopathies in the context of CeD, such as membranous nephropathy and membranoproliferative nephropathy, the later being improved with GFD in four case reports 17.
Endocrine disorders
Autoimmune endocrine disorders (thyroid diseases, Addison disease, type 1 diabetes) are conditions associated with CeD, because of common genetic predisposition and shared TH1 immunity pathways (Table 2); they are not manifestations of CeD per se 135, 136. Nevertheless, an elegant study showed anti-tissue transglutaminase 2 deposits in primate thyroid tissue as well as a correlation between tTG IgA and TPO antibodies titers in individuals with CeD, suggesting that tTG may play a role in auto-immune thyroiditis among CeD patients 137.
Reproductive system
CeD is associated with unexplained infertility and increases the risk of several obstetrical complications 138. Initial presentation of CeD has been described during the third trimester of pregnancy or the postpartum period 139.
Amenorrhea, delayed menarche and early menopause
CeD has been associated with delayed menarche of two years or more among untreated girls 140. Amenorrhea is a frequent complication of untreated CeD 140, 141. Menopause may also start earlier among untreated CeD women 140, 142. Interestingly, although functional hypopituitarism associated with malnutrition may reduce gonadotrophins and sexual hormones, it has been proposed that circulating gluten peptides may also have direct immunologic effect on the hypothalamo-pituitary-gonadal axis 138. GFD may correct these effects; those with treated CeD have menarche and menopause at typical ages 141.
Infertility
There is a well-known association between unexplained infertility and CeD 143. Initial studies suggested that the prevalence of asymptomatic CeD among unexplained infertility may be from 4 to 10.3% 67, 144, 145, 146. More recently, this concept has been challenged by two studies from fertility centers 147, 148. However, these recent studies included women who had been referred for fertility treatment in countries where screening for CeD may be more intensive. Infertility affects mostly untreated women. In men, studies more than 30 years ago described hormonal imbalances and alterations in spermatozoid morphology and motility, but recent epidemiologic studies have failed to show this association. 149, 150, 151. In addition to hormonal imbalances, nutritional deficiencies, such as iron, folate, zinc and selenium deficiencies may be linked to infertility 152, which may resolve with a GFD 138, 153. However, infertility is also frequent among women without any severe malabsorption or trace elements deficiency, suggesting that immune mechanisms may also prevent trophoblast implantation 154, 155.
Obstetrical complications
Untreated CeD is associated with recurrent miscarriage and intra-uterine growth restriction 154, 156. The mechanisms may be similar to infertility; malnutrition, zinc and selenium deficiencies having a deleterious effect on gonadotropin secretion leading to spontaneous abortion 152. Furthermore, because tissue transglutaminases are active in the placenta and endometrium tissues, anti-tissue transglutaminase 2 antibodies can impair placental implantation and development, leading to intrauterine growth restriction 154. Finally, CeD is more frequently found among women with a history of pre-term deliveries and low birth weight infants, who may be otherwise asymptomatic from their CeD 156, 157. Fortunately, GFD appears to reduce these risks to the same as general population157.
Hematologic manifestations
Anemia
Anemia is the most frequent EIM of CeD 4. Although iron deficiency is the most common cause, B12, folate deficiency and systemic inflammation account for more than 15% of anemia in CeD 158. In a recent systematic review and meta-analysis, CeD was present in 3.2–5.5% of individuals with iron deficiency anemia 159. Furthermore, in a large cohort from Italy of subclinical CeD, iron deficiency anemia was the most frequent manifestation in both adults (46.3%) and children (34.8%) 14. Iron deficiency anemia improves with iron supplementation on a GFD 4; lack of improvement requires further investigation.
Hyposplenism
The spleen plays a key role in mounting the immune response to encapsulated micro-organisms160. The extent of the association between these two conditions is not well known, since the reported incidence of hyposplenism in CeD is highly variable according to studies and diagnostic modalities 162, 163. It does not appear to occur among children 164 and may be associated with other auto-immune comorbidities 161, 165. Clinical clues of hyposplenism include thrombocytosis and Howell-Jolly bodies on the blood smear. Other diagnostic modalities are scintigraphy and detection of pitted red cells by phase-interference microscopy 160. An association between splenic atrophy and mesenteric lymph node cavitation has been described in over 30 cases 166. Hyposplenism causes a susceptibility to infections, and studies conducted over the past fifty years showed that those with CeD were at increased risk for pneumococcal infections 167. Some societies now recommend pneumococcal vaccination for those with a diagnosis of CeD 101, 168. The World Gastroenterology Organization also recommends vaccination against Haemophilus influenzae and meningococci 168. Hyposplenism may be reversible with the GFD, but that remains controversial 169, 170.
Response to hepatitis B virus vaccine
An attenuated response to the hepatitis B virus (HBV) vaccine has been observed among individuals with CeD 171, 172. A predisposition to poor immune response to the vaccine may be due to the specific haplotypes, especially DQ2, which may fail to induce a Th2 response to the HBV surface antigen 171, 173. However, this non-response is mostly seen in the context of ongoing gluten ingestion, and may be reversible with a GFD174. Some have proposed to screen for CeD among non-responders to the HBV vaccine, and to revaccinate once adherence to the GFD is optimal 172, 174. Confirming HBV vaccine response among individuals with CeD is advisable.
Liver
Autoimmune liver diseases have been associated with CeD (Table 2). Active intestinal inflammation and immune reaction towards gluten and tissue transglutaminase may also have direct effects on the liver.
Celiac hepatitis
Mild elevation of liver enzymes is frequent at the time of CeD diagnosis175, 176. As such, CeD may be the etiology of unexplained transaminitis in 2–12% 176, 177. Celiac hepatitis is characterized by an asymptomatic elevation of less than five times the upper limit of normal and AST/ALT <1, in the setting of a normal physical exam and liver ultrasound 178. Histologic features of celiac hepatitis include mild reactive hepatitis, focal microvesicular fatty changes and chronic hepatitis, without features suggestive of autoimmune hepatitis 177. Serum transaminase levels usually normalize after 6–12 months of GFD 175, so persistent elevation justifies additional investigations 178. Rarely, CeD presents with acute liver failure, chronic hepatitis or cryptogenic cirrhosis, with some improvement on the GFD 179, 180.
Steatosis
Liver steatosis is common in CeD, with the prevalence being higher in the first five years after the diagnosis 181. Liver steatosis in the absence of metabolic syndrome may justify testing for CeD 178, 182. Altered intestinal permeability leading to increased LPS, bacterial antigens and cytokines in the portal circulation is a putative mechanism 183. Interestingly, a recent animal model study showed that inactivation of tissue transglutaminase in the liver lead to worsening of fatty liver disease184; alteration of the liver tissue transglutaminase by CeD antibodies could thus be hypothesized as another mechanism for the steatosis in CeD. Finally, the GFD is richer in saturated fatty acids and caloric density than a gluten-containing diet, which may predispose to weight gain and obesity185, 186. It is thus difficult to assess if untreated CeD associated steatosis improves on a GFD.
Other complications related to systemic inflammation
Similarly to other inflammatory bowel diseases, active CeD may be a risk factor for atherosclerosis, arterial thrombosis and venous thromboembolism. Potential mechanisms are linked to hyperhomocysteinemia, thrombophilia, circulating cytokines and deficiencies in protein C and S 122, 187.
Stroke
Individuals with untreated asymptomatic CeD, as well as CeD in the first year following diagnosis may be at increased risk for stroke 188. Some have suggested to screen for CeD among those with cryptogenic strokes, especially children 189. The risk of atrial fibrillation is increased in CeD, both before and after diagnosis, which is an additional risk factor for cerebrovascular accident 190.
Ischemic heart disease
Untreated CeD is associated with an increased risk of atherosclerosis, even in childhood 191, 192. Altered cholesterol metabolism and inflammation (increased erythrocyte sedimentation rate, hyperhomocysteinemia) are cardiovascular risk factors that tend to improve on a GFD 191; however, there is limited evidence that an increased risk of incident ischemic heart disease persists for more than a year after CeD diagnosis 193, 194. This may be explained by the GFD leading to a higher intake of saturated fatty acids and caloric load, being a risk factor for obesity 185, 186.
Thrombocytosis
Thrombocytosis may be common at the time of CeD diagnosis, particularly in association with iron-deficiency anemia; however, this phenomenon is mostly reported in older studies, such as a pediatric study from India fifteen years ago showing a prevalence of 60% 195. It is unclear nowadays how frequent thrombocytosis is at the time of CeD diagnosis 196, but it may still be a marker of ongoing inflammation with untreated CeD.
Thrombosis
In addition to atherosclerosis, both arterial and venous thrombosis may occur in undiagnosed CeD, without any other identifiable risk factors 187. Individuals with CeD are at increased risk of venous thromboembolism 197 and silent CeD may be unmasked by pulmonary embolism 187. Other examples of venous thrombosis may involve the hepatic, mesenteric 198, portal, splenic, retinal199 and cerebral veins 200. CeD has been identified as the cause of Budd-Chiari in 11.4% of the cases in a study from North Africa 201. As for active inflammatory bowel disease or cancer, RCD lead to a higher risk of thromboembolism, with deep vein thrombosis involving the superior mesenteric vein, the superior vena cava, pulmonary veins as well as central vein catheters 202. Moreover, increased levels of thrombin-activatable fibrinolysis inhibitor (TAFI) have been reported among newly diagnosed CeD, which could be an additional risk factor for venous thromboembolism 203. There is no existing data about the duration of anticoagulation in the context of venous thrombosis with no risks factors other than CeD or among well-established treated CeD 187.
Common coexisting conditions
Many other autoimmune diseases are associated with CeD without being gluten-responsive (Table 2). Putative mechanisms include common genetic predisposition, shared immune pathways and potential triggers (virus, microbiota, environment), increased intestinal permeability and molecular mimicry (especially with primary biliary cholangitis) 204. The risk of auto-immune disorders increases with age and possibly also the duration of gluten exposure, especially when signs and symptoms are present more than ten years before diagnosis 205, 206. Other risk factors for coexisting auto-immune disorders include a family history of auto-immune disorders and being overweight at the time of CeD diagnosis 206.
Sjogren syndrome, systemic sclerosis, rheumatoid arthritis and idiopathic inflammatory myopathies may also be associated with CeD, but well-designed population or case-control studies are lacking 98, 111, 207. This also applies to autoimmune hepatitis (AIH) as although single center studies have shown an increased prevalence of CeD among individuals with AIH 208, 209, population studies with well-characterized AIH and CeD are lacking.
Conclusion
The discovery of tissue transglutaminase as a target of autoantibodies associated with CeD and the development of various assays for TTG antibodies have liberalized screening for this condition. However, even in Europe and North America, diagnostic delays of more than three years persist, especially among individuals without GI complaints 5, 222, 223, 224. Contrary to previous belief, CeD is a multisystem disease, and physicians from all fields should be aware of the various presentations of CeD and consider it in the diagnostic algorithm of many conditions. Furthermore, most of the EIM of CeD improve with the GFD; other specific diagnoses should be considered among non-responders. EIM should be taken into account in the drug development for CeD.
Take-home messages:
Headaches are frequent in the CeD population and may be associated with gluten exposure
Gluten ataxia must be recognized and GFD initiated early may avoid permanent sequelae.
Peripheral neuropathy is also frequently reported in the CeD population but may not always improve with the GFD.
Gluten-induced cognitive impairment is increasingly reported by individuals with CeD and may be associated with gluten exposure.
Take-home messages:
Fatigue is fairly common among individuals with CeD and is multifactorial; screening for depression, sleep disorders, and thyroid disease is advised, especially if there is no improvement on the GFD.
Take-home messages:
Dermatitis herpetiform is the most frequent dermatologic manifestation of CeD
Diagnosis of DH requires skin biopsy for histology and direct IgA immunofluorescence.
Skin rashes are frequent among individuals with CeD (Table 2). Some may respond to the GFD, but a dermatology evaluation is advised.
Take-home messages:
Oral manifestations of CeD are mostly related to nutritional deficiencies (Table 1).
Recurrent aphthous stomatitis occurs more frequently among children, and recurrence may be a sign of gluten exposure.
Dental enamel defects should prompt to testing for CeD.
Take-home messages:
Low bone density is frequent among individuals with CeD and a DEXA scan is recommended after one year of GFD, or sooner among males, those older than 45 years, underweight or with severe histological damage (Marsh 3c)
Myalgia and arthralgia are common at the time of CeD diagnosis. Failure to improve with a GFD should prompt rheumatology evaluation to rule out other associated conditions.
Take-home messages:
Cardiac manifestations are rare, but screening for CeD is suggested among individuals with idiopathic or dilated cardiomyopathy.
Take-home messages:
CeD may be a risk factor for diabetic nephropathy in type I diabetes.
- Testing for CeD should be considered in those with:
- IgA nephropathy, especially males
- Oxalate kidney stones in the absence of inflammatory bowel disease
Take-home messages:
Evaluation for CeD is suggested in cases of unexplained infertility or frequent miscarriages
The risk of obstetrical complications or fetal bad outcomes is similar to the general population among treated CeD women
Take-home messages:
Evaluation for CeD must be part of the work-up of iron deficiency anemia
Lack of improvement of iron deficiency anemia on a GFD should lead to investigations for other causes of anemia
CeD may be an indication for pneumococcal vaccination
Untreated CeD may be associated with poor response to HBV vaccine; the booster should be administered during treatment with a GFD.
Take-home messages:
Mild elevation of serum transaminases is present in about 40% at the time of CeD diagnosis and usually resolves on a GFD 175. Persistent elevated liver enzymes should be further investigated.
Evaluation for CeD should be performed among individuals with unexplained elevated liver enzymes or steatosis.
Take-home messages:
Untreated CeD may be a risk factor for early atherosclerosis, but dietary guidance should also be given to avoid excessive weight gain on a GFD.
Venous thrombosis at many locations have been associated with CeD, and investigation for CeD should be part of the work-up of a venous thrombosis of unknown etiology.
Acknowledgments
Funding: This research was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Numbers K23DK119584 and T32DK07760 awarded to JAS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. AT was supported by the DG Kinnear Award from the Association Quebecoise des Gastroenterologues du Quebec and Phase 2 Award from the Fonds de Recherche Sante Quebec (Programme FRQS/MSSS de formation pour médecins résidents en médecine spécialisée visant une carrière en recherche).
Footnotes
Disclosures: JAS has received consulting fees from Takeda Pharmaceuticals International Co, and research support from Cour Pharmaceuticals, Biomedal SL and Glutenostics LLC. CPK has acted as a scientific advisor to companies attempting to develop new diagnostic and management approaches for Celiac disease including Cour Pharmaceuticals, Glutenostics, Innovate, ImmunogenX and Takeda Pharmaceuticals. He also acts as Principal Investigator on research grants on Celiac disease supported by Aptalis and Takeda Pharmaceuticals International Co. AT has no conflict to disclose.
References
- 1.Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed]
- 2.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797–801. [DOI] [PubMed] [Google Scholar]
- 3.Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med 2006;119:355 e9–14. [DOI] [PubMed] [Google Scholar]
- 4.Jericho H, Sansotta N, Guandalini S. Extraintestinal Manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. J Pediatr Gastroenterol Nutr 2017;65:75–9. [DOI] [PubMed] [Google Scholar]
- 5.Paez MA, Gramelspacher AM, Sinacore J, et al. Delay in Diagnosis of Celiac Disease in Patients Without Gastrointestinal Complaints. Am J Med 2017;130:1318–23. [DOI] [PubMed] [Google Scholar]
- 6.Nurminen S, Kivela L, Huhtala H, et al. Extraintestinal manifestations were common in children with coeliac disease and were more prevalent in patients with more severe clinical and histological presentation. Acta Paediatr 2018. [DOI] [PubMed]
- 7.Irvine AJ, Chey WD, Ford AC. Screening for Celiac Disease in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-analysis. Am J Gastroenterol 2017;112:65–76. [DOI] [PubMed] [Google Scholar]
- 8.Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, et al. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013;5:3975–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007;85:778–87. [DOI] [PubMed] [Google Scholar]
- 10.Ramos MI, Allen LH, Mungas DM, et al. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2005;82:1346–52. [DOI] [PubMed] [Google Scholar]
- 11.Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology 2012;79:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keaveny AP, Freaney R, McKenna MJ, et al. Bone remodeling indices and secondary hyperparathyroidism in celiac disease. Am J Gastroenterol 1996;91:1226–31. [PubMed] [Google Scholar]
- 13.Selby PL, Davies M, Adams JE, et al. Bone loss in celiac disease is related to secondary hyperparathyroidism. J Bone Miner Res 1999;14:652–7. [DOI] [PubMed] [Google Scholar]
- 14.Bottaro G, Cataldo F, Rotolo N, et al. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol 1999;94:691–6. [DOI] [PubMed] [Google Scholar]
- 15.Walker MD, Zylberberg HM, Green PHR, et al. Endocrine complications of celiac disease: a case report and review of the literature. Endocr Res 2018:1–19. [DOI] [PubMed]
- 16.Wu SC, Caravita S, Secchi MB. Hyperparathyroidism in celiac disease: always secondary? Intern Emerg Med 2012;7 Suppl 1:S11–3. [DOI] [PubMed] [Google Scholar]
- 17.Boonpheng B, Cheungpasitporn W, Wijarnpreecha K. Renal disease in patients with celiac disease. Minerva Med 2018;109:126–40. [DOI] [PubMed] [Google Scholar]
- 18.Ciacci C, Spagnuolo G, Tortora R, et al. Urinary stone disease in adults with celiac disease: prevalence, incidence and urinary determinants. J Urol 2008;180:974–9. [DOI] [PubMed] [Google Scholar]
- 19.Ludvigsson JF, Zingone F, Fored M, et al. Moderately increased risk of urinary stone disease in patients with biopsy-verified coeliac disease. Aliment Pharmacol Ther 2012;35:477–84. [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–76; quiz 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gils T, Nijeboer P, van Wanrooij RL, et al. Mechanisms and management of refractory coeliac disease. Nat Rev Gastroenterol Hepatol 2015;12:572–9. [DOI] [PubMed] [Google Scholar]
- 22.Mukewar SS, Sharma A, Rubio-Tapia A, et al. Open-Capsule Budesonide for Refractory Celiac Disease. Am J Gastroenterol 2017;112:959–67. [DOI] [PubMed] [Google Scholar]
- 23.Chin RL, Latov N, Green PH, et al. Neurologic complications of celiac disease. J Clin Neuromuscul Dis 2004;5:129–37. [DOI] [PubMed] [Google Scholar]
- 24.Hadjivassiliou M, Croall ID, Zis P, et al. Neurologic Deficits in Patients With Newly Diagnosed Celiac Disease Are Frequent and Linked With Autobodies to Transglutaminase 6. Clin Gastroenterol Hepatol 2019. [DOI] [PubMed]
- 25.Pennisi M, Bramanti A, Cantone M, et al. Neurophysiology of the “Celiac Brain”: Disentangling Gut-Brain Connections. Front Neurosci 2017;11:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebwohl B, Roy A, Alaedini A, et al. Risk of Headache-Related Healthcare Visits in Patients With Celiac Disease: A Population-Based Observational Study. Headache 2016;56:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimitrova AK, Ungaro RC, Lebwohl B, et al. Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache 2013;53:344–55. [DOI] [PubMed] [Google Scholar]
- 28.Zis P, Julian T, Hadjivassiliou M. Headache Associated with Coeliac Disease: A Systematic Review and Meta-Analysis. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sansotta N, Amirikian K, Guandalini S, et al. Celiac Disease Symptom Resolution: Effectiveness of the Gluten-free Diet. J Pediatr Gastroenterol Nutr 2018;66:48–52. [DOI] [PubMed] [Google Scholar]
- 30.Silvester JA, Graff LA, Rigaux L, et al. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther 2016;44:612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludvigsson JF, Olsson T, Ekbom A, et al. A population-based study of coeliac disease, neurodegenerative and neuroinflammatory diseases. Aliment Pharmacol Ther 2007;25:1317–27. [DOI] [PubMed] [Google Scholar]
- 32.Thawani SP, Brannagan TH 3rd, Lebwohl B, et al. Risk of Neuropathy Among 28,232 Patients With Biopsy-Verified Celiac Disease. JAMA Neurol 2015;72:806–11. [DOI] [PubMed] [Google Scholar]
- 33.Zis P, Sarrigiannis PG, Rao DG, et al. Quality of Life in Patients with Gluten Neuropathy: A Case-Controlled Study. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin RL, Latov N. Peripheral Neuropathy and Celiac Disease. Curr Treat Options Neurol 2005;7:43–8. [DOI] [PubMed] [Google Scholar]
- 35.Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003;60:1581–5. [DOI] [PubMed] [Google Scholar]
- 36.Tursi A, Giorgetti GM, Iani C, et al. Peripheral neurological disturbances, autonomic dysfunction, and antineuronal antibodies in adult celiac disease before and after a gluten-free diet. Dig Dis Sci 2006;51:1869–74. [DOI] [PubMed] [Google Scholar]
- 37.Hadjivassiliou M, Sanders DD, Aeschlimann DP. Gluten-related disorders: gluten ataxia. Dig Dis 2015;33:264–8. [DOI] [PubMed] [Google Scholar]
- 38.Hadjivassiliou M, Grunewald RA, Chattopadhyay AK, et al. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet 1998;352:1582–5. [DOI] [PubMed] [Google Scholar]
- 39.Hadjivassiliou M, Grunewald RA, Sanders DS, et al. The Significance of Low Titre Antigliadin Antibodies in the Diagnosis of Gluten Ataxia. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadjivassiliou M, Aeschlimann P, Sanders DS, et al. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 2013;80:1740–5. [DOI] [PubMed] [Google Scholar]
- 41.Hadjivassiliou M, Davies-Jones GA, Sanders DS, et al. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry 2003;74:1221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yelland GW. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J Gastroenterol Hepatol 2017;32 Suppl 1:90–3. [DOI] [PubMed] [Google Scholar]
- 43.Lichtwark IT, Newnham ED, Robinson SR, et al. Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment Pharmacol Ther 2014;40:160–70. [DOI] [PubMed] [Google Scholar]
- 44.Therrien A, Silvester JA, Leffler DA, et al. Efficacy of Enteric-Release Oral Budesonide in Treatment of Acute Reactions to Gluten in Patients With Celiac Disease. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- 45.Ludvigsson JF, Zingone F, Tomson T, et al. Increased risk of epilepsy in biopsy-verified celiac disease: a population-based cohort study. Neurology 2012;78:1401–7. [DOI] [PubMed] [Google Scholar]
- 46.Canales P, Mery VP, Larrondo FJ, et al. Epilepsy and celiac disease: favorable outcome with a gluten-free diet in a patient refractory to antiepileptic drugs. Neurologist 2006;12:318–21. [DOI] [PubMed] [Google Scholar]
- 47.Gobbi G, Bouquet F, Greco L, et al. Coeliac disease, epilepsy, and cerebral calcifications. The Italian Working Group on Coeliac Disease and Epilepsy. Lancet 1992;340:439–43. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez MA, Colina G, Ortigosa L. Epilepsy, cerebral calcifications and clinical or subclinical coeliac disease. Course and follow up with gluten-free diet. Seizure 1998;7:49–54. [DOI] [PubMed] [Google Scholar]
- 49.Cernibori A, Gobbi G. Partial seizures, cerebral calcifications and celiac disease. Ital J Neurol Sci 1995;16:187–91. [DOI] [PubMed] [Google Scholar]
- 50.Andrade C, Rocha H, Albuquerque A, et al. Gluten chorea. Clin Neurol Neurosurg 2015;138:8–9. [DOI] [PubMed] [Google Scholar]
- 51.Rani U, Imdad A, Beg M. Rare Neurological Manifestation of Celiac Disease. Case Rep Gastroenterol 2015;9:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittstock M, Grossmann A, Kunesch E, et al. Symptomatic vascular dystonia in Celiac disease. Mov Disord 2006;21:427–9. [DOI] [PubMed] [Google Scholar]
- 53.Slim M, Rico-Villademoros F, Calandre EP. Psychiatric Comorbidity in Children and Adults with Gluten-Related Disorders: A Narrative Review. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludvigsson JF, Lebwohl B, Chen Q, et al. Anxiety after coeliac disease diagnosis predicts mucosal healing: a population-based study. Aliment Pharmacol Ther 2018;48:1091–8. [DOI] [PubMed] [Google Scholar]
- 55.Addolorato G, Capristo E, Ghittoni G, et al. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand J Gastroenterol 2001;36:502–6. [DOI] [PubMed] [Google Scholar]
- 56.Corvaglia L, Catamo R, Pepe G, et al. Depression in adult untreated celiac subjects: diagnosis by the pediatrician. Am J Gastroenterol 1999;94:839–43. [DOI] [PubMed] [Google Scholar]
- 57.Hallert C, Sedvall G. Improvement in central monoamine metabolism in adult coeliac patients starting a gluten-free diet. Psychol Med 1983;13:267–71. [DOI] [PubMed] [Google Scholar]
- 58.Jelsness-Jorgensen LP, Bernklev T, Lundin KEA. Fatigue as an Extra-Intestinal Manifestation of Celiac Disease: A Systematic Review. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hujoel IA, Van Dyke CT, Brantner T, et al. Natural history and clinical detection of undiagnosed coeliac disease in a North American community. Aliment Pharmacol Ther 2018;47:1358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siniscalchi M, Iovino P, Tortora R, et al. Fatigue in adult coeliac disease. Aliment Pharmacol Ther 2005;22:489–94. [DOI] [PubMed] [Google Scholar]
- 61.Jorda FC, Lopez Vivancos J. Fatigue as a determinant of health in patients with celiac disease. J Clin Gastroenterol 2010;44:423–7. [DOI] [PubMed] [Google Scholar]
- 62.Mollazadegan K, Kugelberg M, Lindblad BE, et al. Increased risk of cataract among 28,000 patients with celiac disease. Am J Epidemiol 2011;174:195–202. [DOI] [PubMed] [Google Scholar]
- 63.Mollazadegan K, Kugelberg M, Tallstedt L, et al. Increased risk of uveitis in coeliac disease: a nationwide cohort study. Br J Ophthalmol 2012;96:857–61. [DOI] [PubMed] [Google Scholar]
- 64.Klack K, Pereira RM, de Carvalho JF. Uveitis in celiac disease with an excellent response to gluten-free diet: third case described. Rheumatol Int 2011;31:399–402. [DOI] [PubMed] [Google Scholar]
- 65.Martins TG, Costa AL, Oyamada MK, et al. Ophthalmologic manifestations of celiac disease. Int J Ophthalmol 2016;9:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fraser NG, Kerr NW, Donald D. Oral lesions in dermatitis herpetiformis. Br J Dermatol 1973;89:439–50. [DOI] [PubMed] [Google Scholar]
- 67.Laurikka P, Nurminen S, Kivela L, et al. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salmi TT, Hervonen K, Kautiainen H, et al. Prevalence and incidence of dermatitis herpetiformis: a 40-year prospective study from Finland. Br J Dermatol 2011;165:354–9. [DOI] [PubMed] [Google Scholar]
- 69.Reunala T, Salmi TT, Hervonen K, et al. Dermatitis Herpetiformis: A Common Extraintestinal Manifestation of Coeliac Disease. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collin P, Huhtala H, Virta L, et al. Diagnosis of celiac disease in clinical practice: physician’s alertness to the condition essential. J Clin Gastroenterol 2007;41:152–6. [DOI] [PubMed] [Google Scholar]
- 71.Virta LJ, Saarinen MM, Kolho KL. Declining trend in the incidence of biopsy-verified coeliac disease in the adult population of Finland, 2005–2014. Aliment Pharmacol Ther 2017;46:1085–93. [DOI] [PubMed] [Google Scholar]
- 72.Mansikka E, Hervonen K, Kaukinen K, et al. Prognosis of Dermatitis Herpetiformis Patients with and without Villous Atrophy at Diagnosis. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sardy M, Karpati S, Merkl B, et al. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 2002;195:747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Meer JB. Granular deposits of immunoglobulins in the skin of patients with dermatitis herpetiformis. An immunofluorescent study. Br J Dermatol 1969;81:493–503. [DOI] [PubMed] [Google Scholar]
- 76.Fry L, Seah PP, Riches DJ, et al. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet 1973;1:288–91. [DOI] [PubMed] [Google Scholar]
- 77.Graziano M, Rossi M. An update on the cutaneous manifestations of coeliac disease and non-coeliac gluten sensitivity. Int Rev Immunol 2018:1–10. [DOI] [PubMed]
- 78.Egeberg A, Griffiths CEM, Mallbris L, et al. The association between psoriasis and coeliac disease. Br J Dermatol 2017;177:e329–e30. [DOI] [PubMed] [Google Scholar]
- 79.Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and Risk of Celiac Disease: A Systematic Review and Meta-analysis. Indian J Dermatol 2017;62:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol 2014;71:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ludvigsson JF, Lindelof B, Rashtak S, et al. Does urticaria risk increase in patients with celiac disease? A large population-based cohort study. Eur J Dermatol 2013;23:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caminiti L, Passalacqua G, Magazzu G, et al. Chronic urticaria and associated coeliac disease in children: a case-control study. Pediatr Allergy Immunol 2005;16:428–32. [DOI] [PubMed] [Google Scholar]
- 83.Meyers S, Dikman S, Spiera H, et al. Cutaneous vasculitis complicating coeliac disease. Gut 1981;22:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naveh Y, Rosenthal E, Ben-Arieh Y, et al. Celiac disease-associated alopecia in childhood. J Pediatr 1999;134:362–4. [DOI] [PubMed] [Google Scholar]
- 85.Nieri M, Tofani E, Defraia E, et al. Enamel defects and aphthous stomatitis in celiac and healthy subjects: Systematic review and meta-analysis of controlled studies. J Dent 2017;65:1–10. [DOI] [PubMed] [Google Scholar]
- 86.Rashid M, Zarkadas M, Anca A, et al. Oral manifestations of celiac disease: a clinical guide for dentists. J Can Dent Assoc 2011;77:b39. [PubMed] [Google Scholar]
- 87.Preeti L, Magesh K, Rajkumar K, et al. Recurrent aphthous stomatitis. J Oral Maxillofac Pathol 2011;15:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis 2006;12:1–21. [DOI] [PubMed] [Google Scholar]
- 89.Pastore L, Carroccio A, Compilato D, et al. Oral manifestations of celiac disease. J Clin Gastroenterol 2008;42:224–32. [DOI] [PubMed] [Google Scholar]
- 90.Ciacci C, Iovino P, Amoruso D, et al. Grown-up coeliac children: the effects of only a few years on a gluten-free diet in childhood. Aliment Pharmacol Ther 2005;21:421–9. [DOI] [PubMed] [Google Scholar]
- 91.Maki M, Aine L, Lipsanen V, et al. Dental enamel defects in first-degree relatives of coeliac disease patients. Lancet 1991;337:763–4. [DOI] [PubMed] [Google Scholar]
- 92.Lucendo AJ, Garcia-Manzanares A. Bone mineral density in adult coeliac disease: an updated review. Rev Esp Enferm Dig 2013;105:154–62. [DOI] [PubMed] [Google Scholar]
- 93.Larussa T, Suraci E, Nazionale I, et al. Bone mineralization in celiac disease. Gastroenterol Res Pract 2012;2012:198025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daron C, Soubrier M, Mathieu S. Occurrence of rheumatic symptoms in celiac disease: A meta-analysis: Comment on the article “Osteoarticular manifestations of celiac disease and non-celiac gluten hypersensitivity” by Dos Santos and Liote. Joint Bone Spine 2016, doi: 10.1016/j.jbspin.2016.09.007. [DOI] [PubMed] [Google Scholar]; Joint Bone Spine 2017;84:645–6. [DOI] [PubMed] [Google Scholar]
- 95.Laszkowska M, Mahadev S, Sundstrom J, et al. Systematic review with meta-analysis: the prevalence of coeliac disease in patients with osteoporosis. Aliment Pharmacol Ther 2018;48:590–7. [DOI] [PubMed] [Google Scholar]
- 96.Heikkila K, Pearce J, Maki M, et al. Celiac disease and bone fractures: a systematic review and meta-analysis. J Clin Endocrinol Metab 2015;100:25–34. [DOI] [PubMed] [Google Scholar]
- 97.Galli G, Lahner E, Conti L, et al. Risk factors associated with osteoporosis in a cohort of prospectively diagnosed adult coeliac patients. United European Gastroenterol J 2018;6:1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zylberberg HM, Lebwohl B, Green PHR. Celiac Disease-Musculoskeletal Manifestations and Mechanisms in Children to Adults. Curr Osteoporos Rep 2018;16:754–62. [DOI] [PubMed] [Google Scholar]
- 99.Fornari MC, Pedreira S, Niveloni S, et al. Pre- and post-treatment serum levels of cytokines IL-1beta, IL-6, and IL-1 receptor antagonist in celiac disease. Are they related to the associated osteopenia? Am J Gastroenterol 1998;93:413–8. [DOI] [PubMed] [Google Scholar]
- 100.Riches PL, McRorie E, Fraser WD, et al. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med 2009;361:1459–65. [DOI] [PubMed] [Google Scholar]
- 101.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 2014;63:1210–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer D, Stavropolous S, Diamond B, et al. Osteoporosis in a north american adult population with celiac disease. Am J Gastroenterol 2001;96:112–9. [DOI] [PubMed] [Google Scholar]
- 103.Grace-Farfaglia P Bones of contention: bone mineral density recovery in celiac disease--a systematic review. Nutrients 2015;7:3347–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lebwohl B, Michaelsson K, Green PH, et al. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metab 2014;99:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Posthumus L, Al-Toma A. Duodenal histopathology and laboratory deficiencies related to bone metabolism in coeliac disease. Eur J Gastroenterol Hepatol 2017;29:897–903. [DOI] [PubMed] [Google Scholar]
- 106.Basha B, Rao DS, Han ZH, et al. Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am J Med 2000;108:296–300. [DOI] [PubMed] [Google Scholar]
- 107.Atteno M, Costa L, Cozzolino A, et al. The enthesopathy of celiac patients: effects of gluten-free diet. Clin Rheumatol 2014;33:537–41. [DOI] [PubMed] [Google Scholar]
- 108.Norstrom F, Sandstrom O, Lindholm L, et al. A gluten-free diet effectively reduces symptoms and health care consumption in a Swedish celiac disease population. BMC Gastroenterol 2012;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lubrano E, Ciacci C, Ames PR, et al. The arthritis of coeliac disease: prevalence and pattern in 200 adult patients. Br J Rheumatol 1996;35:1314–8. [DOI] [PubMed] [Google Scholar]
- 110.Usai P, Boi MF, Piga M, et al. Adult celiac disease is frequently associated with sacroiliitis. Dig Dis Sci 1995;40:1906–8. [DOI] [PubMed] [Google Scholar]
- 111.Iqbal T, Zaidi MA, Wells GA, et al. Celiac disease arthropathy and autoimmunity study. J Gastroenterol Hepatol 2013;28:99–105. [DOI] [PubMed] [Google Scholar]
- 112.Atteno M, Costa L, Tortora R, et al. The occurrence of lower limb enthesopathy in coeliac disease patients without clinical signs of articular involvement. Rheumatology (Oxford) 2013;52:893–7. [DOI] [PubMed] [Google Scholar]
- 113.Riccabona M, Rossipal E. [Pericardial effusion in celiac disease--an incidental finding?]. Wien Klin Wochenschr 2000;112:27–31. [PubMed] [Google Scholar]
- 114.Riches PL, McRorie E, Fraser WD, et al. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med. 2009;361:1459–1465. [DOI] [PubMed] [Google Scholar]
- 115.Frustaci A, Cuoco L, Chimenti C, et al. Celiac disease associated with autoimmune myocarditis. Circulation 2002;105:2611–8. [DOI] [PubMed] [Google Scholar]
- 116.Curione M, Barbato M, De Biase L, et al. Prevalence of coeliac disease in idiopathic dilated cardiomyopathy. Lancet 1999;354:222–3. [DOI] [PubMed] [Google Scholar]
- 117.Elfstrom P, Hamsten A, Montgomery SM, et al. Cardiomyopathy, pericarditis and myocarditis in a population-based cohort of inpatients with coeliac disease. J Intern Med 2007;262:545–54. [DOI] [PubMed] [Google Scholar]
- 118.Emilsson L, Andersson B, Elfstrom P, et al. Risk of idiopathic dilated cardiomyopathy in 29 000 patients with celiac disease. J Am Heart Assoc 2012;1:e001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Curione M, Barbato M, Viola F, et al. Idiopathic dilated cardiomyopathy associated with coeliac disease: the effect of a gluten-free diet on cardiac performance. Dig Liver Dis 2002;34:866–9. [DOI] [PubMed] [Google Scholar]
- 120.McGrath S, Thomas A, Gorard DA. Cardiomyopathy responsive to gluten withdrawal in a patient with coeliac disease. BMJ Case Rep 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ludvigsson JF, Inghammar M, Ekberg M, et al. A nationwide cohort study of the risk of chronic obstructive pulmonary disease in coeliac disease. J Intern Med 2012;271:481–9. [DOI] [PubMed] [Google Scholar]
- 122.Ciaccio EJ, Lewis SK, Biviano AB, et al. Cardiovascular involvement in celiac disease. World J Cardiol 2017;9:652–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hendrickx GF, Somers K, Vandenplas Y. Lane-Hamilton syndrome: case report and review of the literature. Eur J Pediatr 2011;170:1597–602. [DOI] [PubMed] [Google Scholar]
- 124.Testa ME, Maffey A, Colom A, et al. [Pulmonary hemorrhage associated with celiac disease]. Arch Argent Pediatr 2012;110:e72–6. [DOI] [PubMed] [Google Scholar]
- 125.Sahin I, Eminbeyli L, Andic S, et al. Screening for celiac disease among patients with chronic kidney disease. Ren Fail 2012;34:545–9. [DOI] [PubMed] [Google Scholar]
- 126.Welander A, Prutz KG, Fored M, et al. Increased risk of end-stage renal disease in individuals with coeliac disease. Gut 2012;61:64–8. [DOI] [PubMed] [Google Scholar]
- 127.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, et al. Celiac disease and the risk of kidney diseases: A systematic review and meta-analysis. Dig Liver Dis 2016;48:1418–24. [DOI] [PubMed] [Google Scholar]
- 128.Rohrer TR, Wolf J, Liptay S, et al. Microvascular Complications in Childhood-Onset Type 1 Diabetes and Celiac Disease: A Multicenter Longitudinal Analysis of 56,514 Patients From the German-Austrian DPV Database. Diabetes Care 2015;38:801–7. [DOI] [PubMed] [Google Scholar]
- 129.Welander A, Sundelin B, Fored M, et al. Increased risk of IgA nephropathy among individuals with celiac disease. J Clin Gastroenterol 2013;47:678–83. [DOI] [PubMed] [Google Scholar]
- 130.Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 2008;205:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tamouza H, Vende F, Tiwari M, et al. Transferrin receptor engagement by polymeric IgA1 induces receptor expression and mesangial cell proliferation: role in IgA nephropathy. Contrib Nephrol 2007;157:144–7. [DOI] [PubMed] [Google Scholar]
- 132.Berthelot L, Papista C, Maciel TT, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 2012;209:793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coppo R, Roccatello D, Amore A, et al. Effects of a gluten-free diet in primary IgA nephropathy. Clin Nephrol 1990;33:72–86. [PubMed] [Google Scholar]
- 134.Ferri C, Puccini R, Longombardo G, et al. Low-antigen-content diet in the treatment of patients with IgA nephropathy. Nephrol Dial Transplant 1993;8:1193–8. [PubMed] [Google Scholar]
- 135.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008;57:2555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kahaly GJ, Frommer L, Schuppan D. Celiac disease and endocrine autoimmunity - the genetic link. Autoimmun Rev 2018;17:1169–75. [DOI] [PubMed] [Google Scholar]
- 137.Naiyer AJ, Shah J, Hernandez L, et al. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid 2008;18:1171–8. [DOI] [PubMed] [Google Scholar]
- 138.Casella G, Orfanotti G, Giacomantonio L, et al. Celiac disease and obstetrical-gynecological contribution. Gastroenterol Hepatol Bed Bench 2016;9:241–9. [PMC free article] [PubMed] [Google Scholar]
- 139.Smecuol E, Maurino E, Vazquez H, et al. Gynaecological and obstetric disorders in coeliac disease: frequent clinical onset during pregnancy or the puerperium. Eur J Gastroenterol Hepatol 1996;8:63–89. [DOI] [PubMed] [Google Scholar]
- 140.Molteni N, Bardella MT, Bianchi PA. Obstetric and gynecological problems in women with untreated celiac sprue. J Clin Gastroenterol 1990;12:37–9. [DOI] [PubMed] [Google Scholar]
- 141.Ferguson R, Holmes GK, Cooke WT. Coeliac disease, fertility, and pregnancy. Scand J Gastroenterol 1982;17:65–8. [DOI] [PubMed] [Google Scholar]
- 142.Sher KS, Mayberry JF. Female fertility, obstetric and gynaecological history in coeliac disease. A case control study. Digestion 1994;55:243–6. [DOI] [PubMed] [Google Scholar]
- 143.Singh P, Arora S, Lal S, et al. Celiac Disease in Women With Infertility: A Meta-Analysis. J Clin Gastroenterol 2016;50:33–9. [DOI] [PubMed] [Google Scholar]
- 144.Collin P, Vilska S, Heinonen PK, et al. Infertility and coeliac disease. Gut 1996;39:382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meloni GF, Dessole S, Vargiu N, et al. The prevalence of coeliac disease in infertility. Hum Reprod 1999;14:2759–61. [DOI] [PubMed] [Google Scholar]
- 146.Machado AP, Silva LR, Zausner B, et al. Undiagnosed celiac disease in women with infertility. J Reprod Med 2013;58:61–6. [PubMed] [Google Scholar]
- 147.Grode LB, Agerholm IE, Humaidan P, et al. Unrecognised coeliac disease among men and women undergoing fertility treatment: A screening study. United European Gastroenterol J 2018;6:1477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Juneau CR, Franasiak JM, Goodman LR, et al. Celiac disease is not more prevalent in patients undergoing in vitro fertilization and does not affect reproductive outcomes with or without treatment: a large prospective cohort study. Fertil Steril 2018;110:437–42. [DOI] [PubMed] [Google Scholar]
- 149.Zugna D, Richiardi L, Akre O, et al. A nationwide population-based study to determine whether coeliac disease is associated with infertility. Gut 2010;59:1471–5. [DOI] [PubMed] [Google Scholar]
- 150.Zugna D, Richiardi L, Akre O, et al. Celiac disease is not a risk factor for infertility in men. Fertil Steril 2011;95:1709–13 e1–3. [DOI] [PubMed] [Google Scholar]
- 151.Lasa JS, Zubiaurre I, Soifer LO. Risk of infertility in patients with celiac disease: a meta-analysis of observational studies. Arq Gastroenterol 2014;51:144–50. [DOI] [PubMed] [Google Scholar]
- 152.Bedwal RS, Bahuguna A. Zinc, copper and selenium in reproduction. Experientia 1994;50:626–40. [DOI] [PubMed] [Google Scholar]
- 153.Morris JS, Adjukiewicz AB, Read AE. Coeliac infertility: an indication for dietary gluten restriction? Lancet 1970;1:213–4. [DOI] [PubMed] [Google Scholar]
- 154.Tersigni C, Castellani R, de Waure C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update 2014;20:582–93. [DOI] [PubMed] [Google Scholar]
- 155.Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353–6. [DOI] [PubMed] [Google Scholar]
- 156.Moleski SM, Lindenmeyer CC, Veloski JJ, et al. Increased rates of pregnancy complications in women with celiac disease. Ann Gastroenterol 2015;28:236–40. [PMC free article] [PubMed] [Google Scholar]
- 157.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology 2005;129:454–63. [DOI] [PubMed] [Google Scholar]
- 158.Berry N, Basha J, Varma N, et al. Anemia in celiac disease is multifactorial in etiology: A prospective study from India. JGH Open 2018;2:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mahadev S, Laszkowska M, Sundstrom J, et al. Prevalence of Celiac Disease in Patients With Iron Deficiency Anemia-A Systematic Review With Meta-analysis. Gastroenterology 2018;155:374–82 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Di Sabatino A, Brunetti L, Carnevale Maffe G, et al. Is it worth investigating splenic function in patients with celiac disease? World J Gastroenterol 2013;19:2313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bullen AW, Hall R, Gowland G, et al. Hyposplenism, adult coeliac disease, and autoimmunity. Gut 1981;22:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood 2007;109:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corazza GR, Zoli G, Di Sabatino A, et al. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol 1999;94:391–7. [DOI] [PubMed] [Google Scholar]
- 164.Corazza GR, Lazzari R, Frisoni M, et al. Splenic function in childhood coeliac disease. Gut 1982;23:415–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Di Sabatino A, Rosado MM, Cazzola P, et al. Splenic hypofunction and the spectrum of autoimmune and malignant complications in celiac disease. Clin Gastroenterol Hepatol 2006;4:179–86. [DOI] [PubMed] [Google Scholar]
- 166.Reddy D, Salomon C, Demos TC, et al. Mesenteric lymph node cavitation in celiac disease. AJR Am J Roentgenol 2002;178:247. [DOI] [PubMed] [Google Scholar]
- 167.Simons M, Scott-Sheldon LAJ, Risech-Neyman Y, et al. Celiac Disease and Increased Risk of Pneumococcal Infection: A Systematic Review and Meta-Analysis. Am J Med 2018;131:83–9. [DOI] [PubMed] [Google Scholar]
- 168.Bai JC, Ciacci C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J Clin Gastroenterol 2017;51:755–68. [DOI] [PubMed] [Google Scholar]
- 169.O’Grady JG, Stevens FM, Harding B, et al. Hyposplenism and gluten-sensitive enteropathy. Natural history, incidence, and relationship to diet and small bowel morphology. Gastroenterology 1984;87:1326–31. [PubMed] [Google Scholar]
- 170.Trewby PN, Chipping PM, Palmer SJ, et al. Splenic atrophy in adult coeliac disease: is it reversible? Gut 1981;22:628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Anania C, Olivero F, Spagnolo A, et al. Immune response to vaccines in children with celiac disease. World J Gastroenterol 2017;23:3205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahishali E, Boztas G, Akyuz F, et al. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci 2008;53:2156–9. [DOI] [PubMed] [Google Scholar]
- 173.Godkin A, Davenport M, Hill AV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology 2005;41:1383–90. [DOI] [PubMed] [Google Scholar]
- 174.Nemes E, Lefler E, Szegedi L, et al. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics 2008;121:e1570–6. [DOI] [PubMed] [Google Scholar]
- 175.Castillo NE, Vanga RR, Theethira TG, et al. Prevalence of abnormal liver function tests in celiac disease and the effect of a gluten-free diet in the US population. Am J Gastroenterol 2015;110:1216–22. [DOI] [PubMed] [Google Scholar]
- 176.Sainsbury A, Sanders DS, Ford AC. Meta-analysis: Coeliac disease and hypertransaminasaemia. Aliment Pharmacol Ther 2011;34:33–40. [DOI] [PubMed] [Google Scholar]
- 177.Majumdar K, Sakhuja P, Puri AS, et al. Coeliac disease and the liver: spectrum of liver histology, serology and treatment response at a tertiary referral centre. J Clin Pathol 2017. [DOI] [PubMed]
- 178.Hoffmanova I, Sanchez D, Tuckova L, et al. Celiac Disease and Liver Disorders: From Putative Pathogenesis to Clinical Implications. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kaukinen K, Halme L, Collin P, et al. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology 2002;122:881–8. [DOI] [PubMed] [Google Scholar]
- 180.Mounajjed T, Oxentenko A, Shmidt E, et al. The liver in celiac disease: clinical manifestations, histologic features, and response to gluten-free diet in 30 patients. Am J Clin Pathol 2011;136:128–37. [DOI] [PubMed] [Google Scholar]
- 181.Reilly NR, Lebwohl B, Hultcrantz R, et al. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol 2015;62:1405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Narciso-Schiavon JL, Schiavon LL. To screen or not to screen? Celiac antibodies in liver diseases. World J Gastroenterol 2017;23:776–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Abenavoli L, Milic N, De Lorenzo A, et al. A pathogenetic link between non-alcoholic fatty liver disease and celiac disease. Endocrine 2013;43:65–7. [DOI] [PubMed] [Google Scholar]
- 184.Piacentini M, Baiocchini A, Del Nonno F, et al. Non-alcoholic fatty liver disease severity is modulated by transglutaminase type 2. Cell Death Dis 2018;9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vici G, Belli L, Biondi M, et al. Gluten free diet and nutrient deficiencies: A review. Clin Nutr 2016;35:1236–41. [DOI] [PubMed] [Google Scholar]
- 186.Bascunan KA, Vespa MC, Araya M. Celiac disease: understanding the gluten-free diet. Eur J Nutr 2017;56:449–59. [DOI] [PubMed] [Google Scholar]
- 187.Dumic I, Martin S, Salfiti N, et al. Deep Venous Thrombosis and Bilateral Pulmonary Embolism Revealing Silent Celiac Disease: Case Report and Review of the Literature. Case Rep Gastrointest Med 2017;2017:5236918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ludvigsson JF, West J, Card T, et al. Risk of stroke in 28,000 patients with celiac disease: a nationwide cohort study in Sweden. J Stroke Cerebrovasc Dis 2012;21:860–7. [DOI] [PubMed] [Google Scholar]
- 189.Goodwin FC, Beattie RM, Millar J, et al. Celiac disease and childhood stroke. Pediatr Neurol 2004;31:139–42. [DOI] [PubMed] [Google Scholar]
- 190.Emilsson L, Smith JG, West J, et al. Increased risk of atrial fibrillation in patients with coeliac disease: a nationwide cohort study. Eur Heart J 2011;32:2430–7. [DOI] [PubMed] [Google Scholar]
- 191.De Marchi S, Chiarioni G, Prior M, et al. Young adults with coeliac disease may be at increased risk of early atherosclerosis. Aliment Pharmacol Ther 2013;38:162–9. [DOI] [PubMed] [Google Scholar]
- 192.Demir AM, Kuloglu Z, Yaman A, et al. Carotid intima-media thickness and arterial stiffness as early markers of atherosclerosis in pediatric celiac disease. Turk J Pediatr 2016;58:172–9. [DOI] [PubMed] [Google Scholar]
- 193.Ludvigsson JF, James S, Askling J, et al. Nationwide cohort study of risk of ischemic heart disease in patients with celiac disease. Circulation 2011;123:483–90. [DOI] [PubMed] [Google Scholar]
- 194.Emilsson L, Lebwohl B, Sundstrom J, et al. Cardiovascular disease in patients with coeliac disease: A systematic review and meta-analysis. Dig Liver Dis 2015;47:847–52. [DOI] [PubMed] [Google Scholar]
- 195.Patwari AK, Anand VK, Kapur G, et al. Clinical and nutritional profile of children with celiac disease. Indian Pediatr 2003;40:337–42. [PubMed] [Google Scholar]
- 196.Nelson EW, Ertan A, Brooks FP, et al. Thrombocytosis in patients with celiac sprue. Gastroenterology 1976;70:1042–4. [PubMed] [Google Scholar]
- 197.Ungprasert P, Wijarnpreecha K, Tanratana P. Risk of venous thromboembolism in patients with celiac disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:1240–5. [DOI] [PubMed] [Google Scholar]
- 198.Azzam NA, Al Ashgar H, Dababo M, et al. Mesentric vein thrombosis as a presentation of subclinical celiac disease. Ann Saudi Med 2006;26:471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Malhi RK, Dhami A, Malhi NS, et al. Central retinal vein occlusion revealing celiac disease: The first report of two cases from India. Indian J Ophthalmol 2018;66:1315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dogan M, Peker E, Akbayram S, et al. Cerebral venous sinus thrombosis in 2 children with celiac disease. Clin Appl Thromb Hemost 2011;17:466–9. [DOI] [PubMed] [Google Scholar]
- 201.Afredj N, Guessab N, Nani A, et al. Aetiological factors of Budd-Chiari syndrome in Algeria. World J Hepatol 2015;7:903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Daum S, Ipczynski R, Schumann M, et al. High rates of complications and substantial mortality in both types of refractory sprue. Eur J Gastroenterol Hepatol 2009;21:66–70. [DOI] [PubMed] [Google Scholar]
- 203.Saibeni S, Bottasso B, Spina L, et al. Assessment of thrombin-activatable fibrinolysis inhibitor (TAFI) plasma levels in inflammatory bowel diseases. Am J Gastroenterol 2004;99:1966–70. [DOI] [PubMed] [Google Scholar]
- 204.Volta U, Caio G, Tovoli F, et al. Gut-liver axis: an immune link between celiac disease and primary biliary cirrhosis. Expert Rev Gastroenterol Hepatol 2013;7:253–61. [DOI] [PubMed] [Google Scholar]
- 205.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology 1999;117:297–303. [DOI] [PubMed] [Google Scholar]
- 206.Conti L, Lahner E, Galli G, et al. Risk Factors Associated with the Occurrence of Autoimmune Diseases in Adult Coeliac Patients. Gastroenterology Research and Practice 2018;2018:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Danielsson O, Lindvall B, Hallert C, et al. Increased prevalence of celiac disease in idiopathic inflammatory myopathies. Brain Behav 2017;7:e00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Villalta D, Girolami D, Bidoli E, et al. High prevalence of celiac disease in autoimmune hepatitis detected by anti-tissue tranglutaminase autoantibodies. J Clin Lab Anal 2005;19:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Volta U, De Franceschi L, Molinaro N, et al. Frequency and significance of anti-gliadin and anti-endomysial antibodies in autoimmune hepatitis. Dig Dis Sci 1998;43:2190–5. [DOI] [PubMed] [Google Scholar]
- 210.Sun X, Lu L, Yang R, et al. Increased Incidence of Thyroid Disease in Patients with Celiac Disease: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0168708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Elfstrom P, Montgomery SM, Kampe O, et al. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab 2008;93:3915–21. [DOI] [PubMed] [Google Scholar]
- 212.Ludvigsson JF, Ludvigsson J, Ekbom A, et al. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care 2006;29:2483–8. [DOI] [PubMed] [Google Scholar]
- 213.Elfstrom P, Montgomery SM, Kampe O, et al. Risk of primary adrenal insufficiency in patients with celiac disease. J Clin Endocrinol Metab 2007;92:3595–8. [DOI] [PubMed] [Google Scholar]
- 214.Ciacci C, Cavallaro R, Iovino P, et al. Allergy prevalence in adult celiac disease. J Allergy Clin Immunol 2004;113:1199–203. [DOI] [PubMed] [Google Scholar]
- 215.Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol 2017;176:100–6. [DOI] [PubMed] [Google Scholar]
- 216.Ludvigsson JF, Elfstrom P, Broome U, et al. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol 2007;5:63–9 e1. [DOI] [PubMed] [Google Scholar]
- 217.Cataldo F, Marino V, Ventura A, et al. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut 1998;42:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ludvigsson JF, Rubio-Tapia A, Chowdhary V, et al. Increased risk of systemic lupus erythematosus in 29,000 patients with biopsy-verified celiac disease. J Rheumatol 2012;39:1964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Laszkowska M, Roy A, Lebwohl B, et al. Nationwide population-based cohort study of celiac disease and risk of Ehlers-Danlos syndrome and joint hypermobility syndrome. Dig Liver Dis 2016;48:1030–4. [DOI] [PubMed] [Google Scholar]
- 220.Marild K, Stordal K, Hagman A, et al. Turner Syndrome and Celiac Disease: A Case-Control Study. Pediatrics 2016;137:e20152232. [DOI] [PubMed] [Google Scholar]
- 221.Marild K, Stephansson O, Grahnquist L, et al. Down syndrome is associated with elevated risk of celiac disease: a nationwide case-control study. J Pediatr 2013;163:237–42. [DOI] [PubMed] [Google Scholar]
- 222.Fuchs V, Kurppa K, Huhtala H, et al. Delayed celiac disease diagnosis predisposes to reduced quality of life and incremental use of health care services and medicines: A prospective nationwide study. United European Gastroenterol J 2018;6:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pulido O, Zarkadas M, Dubois S, et al. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can J Gastroenterol 2013;27:449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fuchs V, Kurppa K, Huhtala H, et al. Factors associated with long diagnostic delay in celiac disease. Scand J Gastroenterol 2014;49:1304–10. [DOI] [PubMed] [Google Scholar]