Abstract
Background.
Men with a low-risk prostate cancer (PCa) should consider observation, particularly active surveillance (AS), a monitoring strategy that avoids active treatment (AT) in the absence of disease progression.
Objective.
To determine clinical and decision-making factors predicting treatment selection.
Design.
Prospective cohort study.
Setting:
Kaiser Permanente Northern California (KPNC).
Patients.
Men newly diagnosed with low-risk prostate cancer between 2012-14 who remained enrolled in KPNC for 12 months following diagnosis.
Measurements.
We used surveys and medical record abstractions to measure sociodemographic and clinical characteristics and psychological and decision-making factors. Men were classified as being on observation if they did not undergo AT within 12 months of diagnosis. We performed multivariable logistic regression analyses.
Results.
The average age of the 1171 subjects was 61.5 (SD 7.2), 81% were white. Overall, 639 (57%) were managed with observation; in adjusted analyses, significant predictors of observation included awareness of low-risk status (OR 1.75; 95% CI, 1.04-2.94), knowing that observation was an option (3.62; 1.62-8.09), having concerns about treatment-related quality of life (1.21, 1.091.34), reporting a urologist recommendation for observation (8.20; 4.68-14.4), and having a lower clinical stage (T1c vs. T2a, 2.11; 1.16-3.84). Conversely, valuing cancer control (1.54; 1.37-1.72) and greater decisional certainty (1.66; 1.18-2.35) were predictive of AT.
Limitations.
Results may be less generalizable to other types of health care systems and to more diverse populations.
Conclusions.
Many participants selected observation and this was associated with tumor characteristics. However, non-clinical decisional factors also independently predicted treatment selection. Efforts to provide early decision support, particularly targeting knowledge deficits, and reassurance to men with low-risk cancers may facilitate better decision making and increase uptake of observation, particularly AS.
The advent of PSA testing in the late 1980s dramatically increased the number of men being diagnosed with and treated for localized prostate cancer. By 2005, PSA testing was estimated to have led more than a million additional U.S. men to undergo active treatment (AT) with radical prostatectomy or radiotherapy in attempt to cure the cancer.[1] However, mathematical modeling studies based on screening trial and autopsy data suggest that substantial proportions of PSA-detected cancers could be considered overdiagnosed because they would not have been diagnosed in the absence of screening.[2-4] Furthermore, many men with low-risk tumor characteristics and/or limited life expectancy were being aggressively treated, raising concerns about overtreatment.[5, 6]
Consequently, U.S. and European professional societies recommend observational strategies, particularly active surveillance (AS) for men with a low-risk prostate cancer.[7–10] Although definitions vary, low risk is usually defined as PSA < 10 ng/mL, Gleason stage < 7, clinical stage ≤ T2a, and minimal tumor burden on biopsy. AS monitoring protocols generally include routine PSA tests, digital rectal examinations, and periodic prostate biopsies (no more frequently than every 12 months). Although using rectal swabs to target antibiotics can reduce the risk of serious biopsy infections to less than 1%,[11] concerns about complications have led to recommendations for considering multiparametric prostate MRI as a component of AS.[12] AS enables men with a low-risk prostate cancer to avoid the potential harms of AT while still being able to receive timely curative treatment in the event of tumor progression. AS differs from watchful waiting (WW), which offers men, particularly those with limited life expectancy regardless of risk group or stage, only palliative treatment for disease progression, though both are considered observational strategies. Cohort studies suggest that managing low-risk cancers with AS substantially reduced the proportion of men undergoing AT, with negligible risk of prostate cancer mortality, particularly for very-low risk tumors.[13–18]
In the U.S., many factors have limited uptake of observation, including fee-for-service care models, the rapid dissemination of new technological interventions for treating cancer, and the pervasive cultural metaphor for waging war against cancer.[19, 20] However, the uptake of observation appears to be increasing over the past decade. Data from numerous U.S. practice settings show increasing proportions of men with low-risk disease avoiding initial AT, from 10% to 20% in the mid-2000s to 30% to 50% by 2015.[21–24] Data from Australia and Europe similarly show increasing uptake, particularly in Sweden, where 74% of men with a low-risk prostate cancer were explicitly being managed with AS in 2014.[25–27]
Making a decision to manage prostate cancer with observation is challenging, men must weigh the risks of cancer progression without treatment versus the risks of complications from undergoing potentially unnecessary treatment. While numerous studies have evaluated clinical and sociodemographic factors associated with selecting observation among men with low-risk cancers,[24, 28–41] few have conducted prospective, in-depth analyses of decision-making factors, including knowledge, decisional processes, and psychological influences. Guided by Zafar’s model of treatment decision making, which postulates that demographic, clinical, and decision-making factors influence cancer treatment selection, we prospectively evaluated these factors in a large cohort of men recently diagnosed with a low-risk prostate cancer.[42] We hypothesized that, after adjusting for demographic and clinical characteristics, we would find that receiving a physician recommendation, being more knowledgeable about prostate cancer and treatment options, having less anxiety and greater decisional certainty, and valuing the avoidance of treatment side effects more than controlling the cancer would independently predict selecting active surveillance.
METHODS
Subjects
The Patient REported outcomes for Prostate cARE (PREPARE) study cohort was assembled by enrolling consecutive Kaiser Permanente Northern California patients newly diagnosed with a low-risk prostate cancer from May 2012 through May 2014. Detailed descriptions of the study have been published elsewhere.[43] Briefly, inclusion criteria were: stage T2a or less, PSA < 10 ng/mL, and Gleason = 6; ability to provide informed consent; and English speaking. We excluded men who had already started treatment for prostate cancer; were not diagnosed from a prostate biopsy; had a previous prostate cancer diagnosis; or whose urologist refused us permission to contact the patients. We restricted the current analyses to the men who remained continuously enrolled in Kaiser Permanente Northern California for 12 months following their prostate cancer diagnosis.
Procedures
We surveilled the Kaiser Permanente Northern California electronic pathology system weekly to identify all men with histologic evidence of a prostate cancer diagnosis and reviewed the electronic medical record to confirm eligibility criteria. After ensuring that the urologist had informed the patient of the diagnosis, we notified the urologist of our intent to enroll the patient and gave him/her one week to indicate any disagreement. We then mailed an invitation letter to eligible men. Trained research assistants contacted patients to conduct the baseline telephone assessment within 30 days of a patient being informed of his prostate cancer diagnosis. The baseline interview required about 30 minutes and participants received a $20 gift card. The institutional review boards of the Kaiser Foundation Research Institute (IRB 00000401) and Georgetown University (IRB 2011-442) approved the study.
Baseline Measures
Sociodemographic and clinical characteristics
We obtained self-reported demographic characteristics through the telephone interview, including age, race/ethnicity, marital status, education, employment, and income. We used the Elixhauser Comorbidity Index to calculate a comorbidity score, based on the compilation of 30 individual health conditions noted in the electronic medical record, from one year pre-diagnosis to 60 days post-diagnosis.[44] We also asked participants about prostate cancer diagnoses and deaths among family members. We used items from the Patient-Reported Outcomes Measurement System to ask about physical function and overall health.[45, 46] Higher scores indicated better physical function and overall health. We used the Expanded Prostate Cancer Index instrument to assess urinary, sexual, and bowel function.[47] We abstracted medical record information on diagnosis date, PSA levels, biopsy results, and clinical stage.
Decision making
Knowledge of risk category and treatment options
We assessed whether men were aware of their cancer risk category and asked those responding “yes” to classify their risk as “low,” “intermediate,” or “high.” Responses of “low-risk” were considered correct; we considered responses of “do not know” to be incorrect because guidelines highlight the need for patients to understand their risk group in order to facilitate treatment decisions.[7] Based on our previously developed scales,[48, 49] we used 5 items to assess participants’ knowledge about prostate cancer and treatment (Table 2). Response choices were “true,” “false,” or “don’t know,” with “don’t know” scored as incorrect.
Table 2.
Baseline Comparisons of Observation and Active Treatment Participants: Psychological and Decision-Making Factors
| Variable, No. (%) | Categories | Observation (n=639) | Active Treatment (n=478) | P value |
|---|---|---|---|---|
| Knowledge | ||||
| Aware of low-risk status | Yes | 580 (90.8) | 403 (84.3) | < 0.001 |
| Knowledge items | ||||
| Usually, prostate cancer grows very quickly compared to other types of cancer | Incorrect | 580 (90.8) | 435 (91.0) | 0.98 |
| Most men diagnosed with prostate cancer die of something other than prostate cancer | Correct | 578 (90.5) | 422 (88.3) | 0.18 |
| Men with low risk prostate cancer can choose to be monitored closely by their doctors, rather than receive surgery or radiation | Correct | 624 (97.7) | 434 (90.8) | < 0.001 |
| Loss of sexual function is a common side effect of prostate cancer treatment | Correct | 474 (74.2) | 375 (78.5) | 0.12 |
| Urinary incontinence (e.g., leaking urine) is a rare side effect of prostate cancer treatment | Incorrect | 394 (61.7) | 293 (61.3) | 0.85 |
| Treatment discussions | ||||
| Discussed with a radiation oncologist | Yes | 67 (10.5) | 139 (29.1) | < 0.001 |
| Discussed with primary care provider | Yes | 52 (8.1) | 52 (10.9) | 0.12 |
| Discussed with a urologist | Yes | 535 (83.7) | 439 (91.8) | < 0.001 |
| Urology recommendation | < 0.001 | |||
| Observation alone | Yes | 228 (35.7) | 20 (4.2) | |
| Active treatment alone | Yes | 64 (10.0) | 165 (34.5) | |
| Other recommendations | Yes | 243 (38.0) | 257 (53.8) | |
| Urology discussions | ||||
| When the urologist gave you his or her treatment or management recommendation, did he/she encourage you to ask questions or express your concerns? | Yes, definitely | 376 (58.8) | 287 (60.0) | 0.54 |
| Did the urologist involve you as much as you wanted in the decision-making process? | Yes, definitely | 484 (75.7) | 392 (82.0) | 0.62 |
| Other discussions | ||||
| Discussed prostate cancer treatment or management options with spouse or partner | Yes | 512 (80.1) | 412 (86.2) | < 0.01 |
| Discussed prostate cancer treatment or management options with friend | Yes | 383 (59.9) | 336 (70.3) | < 0.001 |
| Decision-making preferences | ||||
| Degner decision control preferences | Prefers to leave decision to doctor/prefers doctor consider my opinion/considers doctor’s opinion | 396 (62.0) | 328 (68.6) | < 0.0001 |
| Prefers shared decision making | 165 (25.8) | 73 (15.3) | ||
| Prefers to make an independent decision | 78 (12.2) | 76 (15.9) | ||
| Health concerns | ||||
| Treatment-related quality of life, “very important” | Categorical | |||
| Avoiding possible problems with sexual function and intimacy | 378 (59.2) | 252 (52.7) | 0.03 | |
| Avoiding possible problems with bowel function | 470 (73.6) | 347 (72.6) | 0.66 | |
| Avoiding possible problems with urinary function | 508 (79.5) | 369 (77.2) | 0.28 | |
| Side effects experienced by friends, acquaintances, or family members who have been treated for prostate cancer | 148 (23.2) | 138 (28.9) | 0.03 | |
| Avoiding major surgery | 300 (46.9) | 193 (40.4) | 0.02 | |
| Avoiding radiation exposure | 256 (40.1) | 155 (32.4) | < 0.01 | |
| The length of the time required to complete treatment or time needed for recovery following the treatment | 210 (32.9) | 188 (39.3) | 0.03 | |
| Cancer control, “very important” | Categorical | |||
| Wanting the cancer removed from your body | 332 (52.0) | 381 (79.7) | < 0.001 | |
| The need to feel that you are doing something active to treat the prostate cancer | 342 (53.5) | 332 (69.5) | < 0.001 | |
| Worry that you might regret your treatment or management decision | 167 (26.1) | 111 (23.2) | 0.26 | |
| Doing everything you can to increase the overall quality of your life | 555 (86.9) | 430 (90.0) | 0.13 | |
| Doing everything you can to increase the length of your life | 476 (74.5) | 399 (83.5) | < 0.001 | |
| Wanting to be cured of the cancer | 468 (73.2) | 434 (90.8) | < 0.001 | |
| Treatment burden, “very important” | Categorical | |||
| Inconvenience or burden to your partner or family during treatment/ management and recovery | 232 (36.3) | 165 (34.5) | 0.40 | |
| Concerns about out-of-pocket costs of the treatment or management approach | 155 (24.3) | 107 (22.4) | 0.45 | |
| Psychological variables | ||||
| PROMIS anxiety, mean (SD) | Continuous | 51.0 (8.5) | 53.7 (8.8) | < 0.001 |
| PROMIS depression, mean (SD) | Continuous | 48.3 (8.4) | 50.3 (9.3) | < 0.001 |
| PROMIS social-role function, mean (SD) | Continuous | 56.7 (8.1) | 57.1 (7.5) | 0.34 |
| Prostate-cancer related anxiety, “quite a bit/very much” | Categorical | |||
| I sometimes worry about dying before my time | 89 (13.9) | 86 (18.0) | 0.07 | |
| I worry about what my doctor will find next | 104 (16.3) | 77 (16.1) | 0.93 | |
| I worry that changes in my medical condition will not be detected early | 113 (17.7) | 66 (13.8) | 0.08 | |
| I live in fear that my PSA will rise | 96 (15.0) | 56 (11.7) | 0.11 | |
| I am confident that my cancer can be kept under control | 442 (66.9) | 385 (80.5) | < 0.001 | |
| Decisional certainty | ||||
| SURE scale, total score of 4 | Categorical | 349 (54.6) | 305 (63.8) | 0.002 |
| SURE items, “yes” | Categorical | |||
| Do you feel sure about the best choice for you? | 438 (68.5) | 337 (70.5) | 0.48 | |
| Do you know the risks and benefits of each option? | 492 (77.0) | 405 (84.7) | 0.002 | |
| Are you clear about which benefits and risks matter most to you? | 496 (77.6) | 425 (88.9) | < 0.001 | |
| Do you have enough support and advice to make a choice? | 532 (83.3) | 417 (87.2) | 0.06 | |
PROMIS, Patient-Reported Outcomes Measurement System.
Treatment discussions with physicians, family, and friends
We asked participants to report the physicians (by specialty) with whom they had discussed treatment. The majority of subjects discussed treatment just with a urologist and we further characterized these discussions. We asked about specific treatment recommendations and used items from the Physician Decision Making Style scale to elicit whether the participant felt encouraged by the urologist to ask questions about treatment recommendations and were involved as much as they wanted in the decision-making process.[50] Response options were “yes, definitely,” “yes, somewhat,” or “no.” We also asked participants whether they discussed treatment with a spouse/partner or friends and whether these persons expressed a preference for observation.
Decisional processes and psychological variables
We used the Degner Control Preference Scale to assess men’s preferences regarding making treatment decisions with clinicians (Table 2).[51] We asked men about the health concerns influencing their treatment decision, including cancer control, treatment-related quality of life, and treatment burdens (Table 2). We used Patient-Reported Outcomes Measurement System items to assess anxiety, depression, and social-role function.[45, 46] Higher anxiety and depression scores indicated poorer mental health, while higher social-role scores indicated better social function. We assessed prostate cancer-related anxiety with five items from the Cancer Control Subscale of the Health Worry Scale (Table 2).[52] The five-point response categories ranged from ‘not at all’ to ‘very much.’ Higher scores indicated greater prostate health worry.
Decisional certainty
We measured decisional certainty with the SURE Test,[53] a four-item version of the Decisional Conflict Scale (Table 2).[54] Response categories were “yes” = 1 and “no” = 0. Scores ≤ 3 are considered to indicate decisional conflict.
Treatment classification
We reviewed medical records through 12 months following prostate cancer diagnosis to identify receipt of radical prostatectomy, external beam radiotherapy, brachytherapy, or hormone therapy as well as PSA testing and biopsies. We classified men who received any treatments as being in the (AT) group unless they first underwent a surveillance biopsy (we classified the three such men as selecting observation). We classified men who did not receive any AT during this time frame as being on observation. We could not clearly distinguish AS from WW with only 12 months of follow-up data because subjects may have undergone initial surveillance testing after that date.
Statistical Analyses
We assessed baseline differences between the AT and observation groups across sociodemographic and clinical characteristics using two-sided chi-square and Fisher’s exact tests for categorical variables and t-tests for continuous variables (Table 1). We further assessed group differences on decision-making factors, including knowledge, decisional processes, and psychological variables (Table 2). We performed multivariable logistic regression analyses to evaluate the decisional factors predicting being on observation and included in the model all baseline variables with bivariate associations of p < 0.10. We also adjusted for tumor characteristics, race, ethnicity, marital status, education, family history, and comorbidity because these variables have previously been reported to be associated with treatment selection.
Table 1.
Baseline Comparisons of Observation and Active Treatment Participants: Sociodemographic and Clinical Characteristics
| Variable, No. (%) | Categories | Observation (n=639) | Active Treatment (n=478) | P value |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age, y, at diagnosis, mean (SD) | Continuous | 62.4 (7.13) | 60.3 (7.33) | <.0001 |
| Age, y, at diagnosis | ≤ 60 | 224 (35.1) | 215 (45.0) | < 0.001 |
| 60-69 | 329 (51.5) | 226 (47.3) | ||
| 70+ | 86 (13.5) | 37 (7.7) | ||
| Race | White | 518 (81.1) | 387 (81.1) | 0.65 |
| Black | 75 (11.7) | 62 (13.0) | ||
| Other | 46 (7.2) | 29 (6.1) | ||
| Ethnicity | Hispanic | 61 (9.5) | 57 (11.9) | 0.20 |
| Marital status | Married | 510 (79.8) | 401 (83.9) | 0.09 |
| Education | ≤ High school | 122 (19.1) | 91 (19.0) | 0.11 |
| College | 336 (52.6) | 279 (58.4) | ||
| Grad school | 177 (27.7) | 108 (22.6) | ||
| Employment | Employed | 383 (59.9) | 276 (57.7) | 0.29 |
| Annual income, USD | ≤ $75,000 | 217 (34.0) | 151 (31.6) | 0.49 |
| $75,001-125,000 | 204 (31.9) | 145 (30.3) | ||
| $125,001+ | 172 (26.9) | 149 (31.2) | ||
| Clinical characteristics | ||||
| Elixhauser comorbidity score | 0 | 214 (33.5) | 163 (34.1) | 0.92 |
| 1 | 178 (27.9) | 128 (26.8) | ||
| 2+ | 247 (38.7) | 187 (39.1) | ||
| Personal history of cancer | Yes | 42 (6.6) | 36 (7.5) | 0.53 |
| First-degree relative diagnosed with prostate cancer | Yes | 166 (26.0) | 158 (33.1) | 0.01 |
| Relative(s) died of prostate cancer | Yes | 65 (10.2) | 88 (18.4) | < 0.001 |
| Health function measures, mean (SD) | ||||
| EPIC urinary function | Continuous | 93.9 (13.1) | 94.8 (12.2) | 0.24 |
| EPIC sexual function | Continuous | 69.1 (28.0) | 70.2 (28.2) | 0.52 |
| EPIC bowel function | Continuous | 92.9 (13.2) | 93.4 (11.2) | 0.51 |
| PROMIS physical function | Continuous | 54.7 (8.1) | 55.9 (7.5) | 0.02 |
| PROMIS overall health | Continuous | 2.3 (1.0) | 2.3 (1.0) | 0.53 |
| Tumor characteristics | ||||
| Prostate-specific antigen value, ng/mL, mean, (SD) | Continuous | 6.1 (1.8) | 6.1 (1.8) | 0.94 |
| Prostate-specific antigen value, ng/mL | Categorical | 0.25 | ||
| ≤ 4 | 65 (10.2) | 57 (11.9) | ||
| 4.1-5.9 | 262 (41.0) | 190 (39.7) | ||
| 6.0-7.9 | 220 (32.4) | 146 (30.5) | ||
| ≥ 8.0 | 92 (14.4) | 85 (17.8) | ||
| Percent positive biopsy cores | < 10% | 294 (46.0) | 102 (21.3) | < 0.001 |
| 10-25% | 201 (31.6) | 137 (28.7) | ||
| >25% | 144 (22.5) | 239 (50.0) | ||
| Clinical stage | T1c | 596 (93.3) | 429 (89.7) | 0.04 |
AS, active surveillance; AT, active treatment; EPIC, Expanded Prostate Cancer Index; PROMIS, Patient-Reported Outcomes Measurement System.
When modeling the health concern items, we evaluated three domains treatment-related quality of life, cancer control, and treatment burden (Table 2). Treatment burden variables were not predictive of treatment selection. We used Cronbach’s alpha to select a parsimonious set of items for the other two domains. We found the highest Cronbach’s alphas when including three items for quality of life (alpha = 0.63) and four for cancer control (alpha = 0.74). Higher scores indicated having greater concern about these domains. To address multiple comparisons, we used a Bonferroni correction for the p-values from the regression analysis and considered results with two-side P-values < 0.004 to be statistically significant. We performed analyses with SAS version 9.3.
The funding agency had no role in the design, conduct, or reporting of the study.
RESULTS
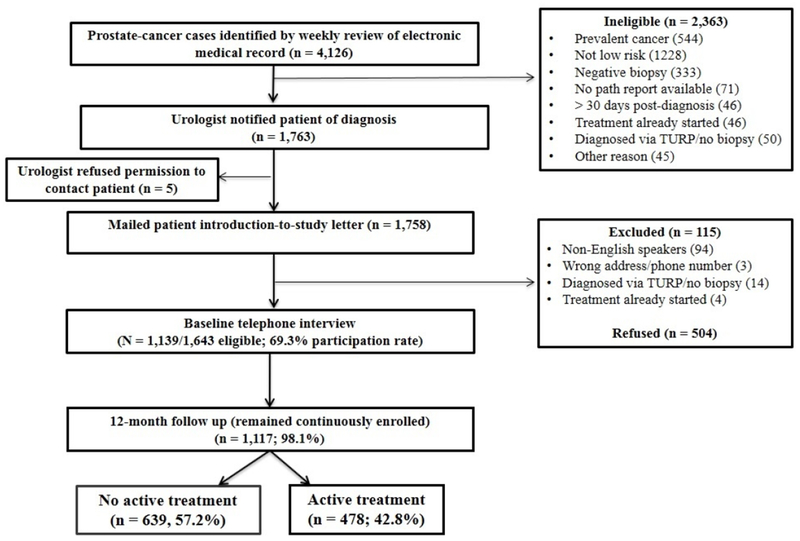
We identified the 4,126 men with newly diagnosed prostate cancer during the study period, which included all 1,643 (39.8%) meeting eligibility criteria for low-risk prostate cancer (Figure 1). We approached all of these eligible subjects for participation and enrolled 1,139 (69.3%). Overall, 1117 (98.1%) subjects remained continuously enrolled in Kaiser Permanente Northern California for 12 months following diagnosis. Participants were significantly more likely than non-participants to be white; we found no differences for age, ethnicity, comorbidities, PSA or Gleason score (data not shown). We conducted baseline interviews a median of 25 days (range 9-100) after diagnosis. Overall, 639 (57.2%) men were on observation and 478 (42.8%) men underwent AT during the 12-month period following diagnosis. During this time period, 164 (25.7%) observation subjects underwent a surveillance biopsy and 614 (96.1%) had at least one surveillance PSA test. By 24 months, all observation subjects underwent surveillance PSA testing and 66.9% underwent a surveillance biopsy.
Figure 1.
Study flow chart.
We compared baseline sociodemographic and clinical variables between men on observation and those selecting AT in Table 1. Men on observation were significantly older than those selecting AT, less likely to have first-degree relatives diagnosed with prostate cancer or any relatives dying from prostate cancer, and had lower scores on the Patient-Reported Outcomes Measurement System physical function scale. We found no significant differences by race, ethnicity, marital status, education, or comorbidity. The AT group had significantly higher proportions of men with > 25% positive biopsy cores and with stage T2a cancers.
We compared decision-making factors between the two treatment groups in Table 2. Men on observation were significantly more likely than those selecting AT to be aware of both their low-risk status and the option for observation and to prefer shared decision making. They were also more likely to report having received a urologist’s recommendation for observation and less likely to have discussed treatment with a radiation oncologist, spouse/partner, or friend. Men on observation had less anxiety and depression on Patient-Reported Outcomes Measurement System scales than those selecting AT, but were less confident that their cancer could be kept under control. Men on observation were more concerned about treatment-related quality of life, while men selecting AT valued taking active steps to remove and cure cancer and increase their length--though not quality--of life. These men also reported more decisional certainty and were more likely to prefer assuming responsibility for decision making.
Results from the multivariable model are shown in Table 3. After adjusting for sociodemographic and clinical variables (only having a PSA ≥ 8 ng/mL was significantly associated with not selecting observation), we found that the psychological and decision-making factors predicting a greater likelihood of selecting observation included awareness of observation as a treatment option, receiving an unequivocal recommendation for observation from a urologist, and concerns about treatment-related quality of life. Valuing cancer control, discussing treatment with a radiation oncologist, and reporting more decisional confidence predicted a lower likelihood of selecting observation.
Table 3.
Results from Multivariable Logistic Regression Model Used to Determine Psychological and Decision-Making Factors Predicting Selecting Observation
| Variable | Odds Ratio (95% CI) | Overall P value |
|---|---|---|
| General psychological variables | ||
| PROMIS anxiety (per 0.5 SD change) | 0.86 (0.74 to 1.00) | 0.05 |
| PROMIS depression (per 0.5 SD change) | 0.97 (0.84 to 1.12) | 0.71 |
| Prostate cancer-related anxiety | ||
| I sometimes worry about dying before my time | 0.82 (0.49 to 1.35) | 0.43 |
| I worry that changes in my medical condition will not be detected early | 0.58 (0.36 to 0.96) | 0.03 |
| I am confident that my cancer can be kept under control | 1.42 (0.96 to 2.11) | 0.08 |
| Knowledge | ||
| Risk level (reference: Not aware of low-risk status/don’t know risk) | 0.03 | |
| Aware of low risk | 1.79 (1.05 to 3.03) | |
| Men with low risk prostate cancer can choose to be monitored closely by their doctors to rather than receive surgery or radiation (reference: Incorrect) | < 0.001 | |
| Correct | 3.91 (1.75 to 8.74) | |
| Treatment discussions | ||
| Discussed prostate cancer treatment or management options with radiation oncologist (reference: No) | < 0.0001 | |
| Yes | 0.42 (0.27 to 0.63) | |
| Urology recommendation (reference: Other) | < 0.0001 | |
| Observation alone | 8.18 (4.69 to 14.3) | |
| Active treatment alone | 0.55 (0.37 to 0.83) | |
| No urologist treatment discussion | 2.34 (1.40 to 3.92) | |
| Discussed prostate cancer treatment or management options with spouse or partner (reference: No/not applicable) | 0.67 | |
| Yes | 1.14 (0.62 to 2.11) | |
| Discussed prostate cancer treatment or management options with a friend (reference: No/not applicable) | 0.01 | |
| Yes | 0.73 (0.52 to 1.03) | |
| Health concerns | ||
| Treatment-related quality of life (per 1-unit change) | 1.20 (1.10 to 1.34) | < 0.001 |
| Cancer control (per 1-unit change) | 0.66 (0.59 to 0.74) | < 0.0001 |
| Decision-making preferences | ||
| Control preference scale (reference: shared decision making) | 0.58 | |
| Prefers to leave decision to doctor/have doctor consider my opinion/consider doctor’s opinion | 0.80 (0.53 to 1.22) | |
| Prefers to make an independent decision | 0.83 (0.48 to 1.45) | |
| Decisional certainty | ||
| SURE Scale (reference: score = 4) | 0.003 | |
| Score = 0 to 3 | 1.68 (1.19 to 2.37) | |
Data adjusted for age, race, ethnicity, marital status, education, comorbidity, physical function, family history of prostate cancer mortality, and tumor characteristics (PSA level, clinical stage, percent positive prostate biopsies). PROMIS, Patient-Reported Outcomes Measurement System.
DISCUSSION
Nearly 60% of our cohort of men diagnosed with a low-risk prostate cancer was on observation during the 12 months following diagnosis. We identified clinical variables significantly associated with observation, including having relatively less aggressive tumor characteristics and not having relatives who died from prostate cancer. However, decisional and psychological factors were also independently associated with treatment decisions, including knowledge of treatment options, treatment discussions, concerns about quality of life and cancer control, and decisional certainty.
Our observed proportion of men deferring AT (57%) is higher than previously reported in community practice, Surveillance, Epidemiology, and End Result Program, or Veterans Health Administration data.[21, 55];Ritch, 2015 #235;Weiner, 2015 #65;Loppenberg, 2018 #239;Loeb, 2018 #255} However, we have more recent data and previous studies consistently showed an increasing proportion of men with low-risk cancers deferring AT over time. The associations between tumor characteristics and observation accord with previous reports.[22, 32, 37, 38, 41, 56] Importantly, we also found that decision-making factors independently predicted treatment selection. Men on observation were more aware than men selecting AT that observation was a treatment option. Although knowledge of risk status was a significant predictor only on bivariate analysis, others have shown that men were comfortable with AS if they perceived that their cancer was not life threatening.[30, 35, 57, 58] In addition, many men with low-risk cancers have reported not being offered AS or else incorrectly considered AS to be a palliative approach.[31, 33, 34]
Our participants on observation were more concerned about treatment-related quality of life than those selecting AT; men are often reported to select AS particularly to avoid adverse effects on urinary and sexual function.[30, 31, 35, 36] Meanwhile, men selecting AT placed a higher value on cancer control and were more likely to report having a relative who died from prostate cancer. The themes of needing to remove or cure cancer, anxiety about not actively intervening, and fear of disease progression are frequently cited as determinants for undergoing AT.[29, 33, 36, 59]
Our findings suggest the potential benefit of systematically informing all newly diagnosed men about the natural history of low-risk prostate cancers, the distinction between AS and WW, the rigor of the AS monitoring protocol, and results from other studies showing that undergoing AS does not preclude receiving effective curative therapy. However, men may need further assurance that their cancer is low risk, including from repeating a prostate biopsy, performing magnetic resonance imaging studies, or undergoing validated molecular or genomic analyses.
Urologists’ treatment recommendations were highly influential, particularly when suggesting only observation. Studies have consistently noted the importance of the urologist’s recommendation, particularly when the physician-patient relationship was strong.[30, 31, 33, 59–65] Men selecting AS were very influenced by their perceptions of the urologist’s expertise and their trust that the urologist was recommending the best treatment option. Unfortunately, we did not measure trust in our survey. There is a potential downside to the urologist’s influence because eligible patients may not necessarily learn about or be supported in selecting AS. A recent Veterans Health Administration study found that urologist’s recommendations for managing low-risk prostate cancer trumped patient preferences and that decisions were often made without eliciting the patient’s values.[34] A population-based cohort study found marked variation in the proportion of urologists whose low-risk cancer patients were managed with observation, ranging from 4.5% to 64%, and other studies have shown marked variation in deferring AT across geographic areas and health care systems.[38, 39, 41, 66] Having a discussion with a radiation oncologist was highly associated with undergoing active treatment. These consultations were likely being arranged for men who were not interested in AS but were unwilling to undergo or not being offered surgery.[67]
These data suggest potentially unwarranted practice variation in managing men with low-risk cancers. In turn, this may be driven by clinicians’ failure to fully engage men in a decision-making process that informs them about the disease and treatment options and incorporates the patient’s preferences and values. Shared decision making, particularly supported by decision aids, has been shown to increase knowledge, better engage patients in decision making, and reduce decisional conflict.[68] We saw the importance of health concerns in shaping decisions, particularly related to cancer control and treatment complications. However, some of the cancer control concerns raised by men selecting AT may have reflected being unaware of having a potentially indolent cancer and the option of undergoing observation.
Most men reported discussing treatment with a spouse/partner and the majority also discussed treatment with friends. Studies have shown that the support of family and friends for observation strongly influences treatment selection.[30, 31, 57, 69, 70] However, spouses/partners, particularly of younger men, and friends with prostate cancer generally supported AT. Although less than 10% of subjects reported that a spouse/partner or friend expressed a preference for observation, this was highly associated with selecting this option (data not shown). The strong influence of family and friends highlights the need for shared decision-making processes that engage more than just the patient.
Our study had limitations and strengths. AS is defined by following a monitoring protocol, but our survey questions did not distinguish between AS and WW because we wanted to clearly contrast AT and observation. However, the high proportion of men undergoing surveillance procedures, including biopsies, by 24 months suggest that we had assembled an AS cohort. Kaiser Permanente Northern California is an integrated health care system that has a lower proportion of Hispanics than the general population. While our results might be less generalizable to different health care delivery systems and to more diverse populations, we are better able to isolate the effects of decisional processes and psychological variables on treatment selection given that Kaiser Permanente Northern California clinicians are salaried providers. Additional strengths of the study included having a large cohort of men with low-risk cancer who were systematically surveyed at a uniform point soon after diagnosis and then prospectively followed using comprehensive electronic medical record data.
The U.S. Preventive Services Task Force’s guideline shifted last year from recommending against prostate cancer screening to supporting individualized decision making, partly because the increasing uptake of AS could mitigate harms from overdiagnosis.[71] However, men with low-risk prostate cancers face challenges in selecting observation, particularly AS. Our results suggest the potential value of offering decision-making support soon after cancer diagnosis that provides men with accurate and complete information, which may be shared with family and friends, about the natural history of their cancer and the rationale, protocols, and evidence for observation. Men may also require further clinical confirmation of having a low-risk cancer as well as interventions to reduce overall anxiety to help them be more comfortable with selecting observation. As uptake of AS increases worldwide, though, further research is needed to identify the optimal surveillance protocols, to assess long-term adherence with observation and monitoring protocols, and to follow trends in prostate cancer mortality.
Acknowledgments
We gratefully acknowledge the men who participated in the study and the Kaiser Permanente Northern California research staff (Socorro Caglia, Carol Rabello, and Erica Kerezsi).
Financial support for this study was provided entirely by the National Cancer Institute, R01 CA 155578-01 (KL Taylor and S Van Den Eeden, multiple PIs). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Dr. Hoffman is also supported by the National Cancer Institute Cancer Center Support Grant P030 CA086862
Footnotes
The work has been presented, in part, at the 2018 meetings of the American Society of Preventive Medicine and the Society of General Internal Medicine.
Declaration of Conflicting Interests
None to report.
References
- [1].Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst. 2009; 101(19):1325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009; 101(6):374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002; 94(13):981–90. [DOI] [PubMed] [Google Scholar]
- [4].Bell KJ, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. International journal of cancer. 2015; 137(7):1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shao YH, Albertsen PC, Roberts CB, Lin Y, Mehta AR, Stein MN, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/ml. Archives of internal medicine. 2010; 170(14):1256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Roberts CB, Albertsen PC, Shao YH, Moore DF, Mehta AR, Stein MN, et al. Patterns and correlates of prostate cancer treatment in older men. The American journal of medicine. 2011; 124(3):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol. 2018; 199(3):683–90. [DOI] [PubMed] [Google Scholar]
- [8].Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D’Amico AV, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014; 12(5):686–718. [DOI] [PubMed] [Google Scholar]
- [9].Briganti A, Fossati N, Catto JWF, Cornford P, Montorsi F, Mottet N, et al. Active Surveillance for Low-risk Prostate Cancer: The European Association of Urology Position in 2018. European urology. 2018; 74(3):357–68. [DOI] [PubMed] [Google Scholar]
- [10].Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. European urology. 2017; 71(4):618–29. [DOI] [PubMed] [Google Scholar]
- [11].Zembower TR, Maxwell KM, Nadler RB, Cashy J, Scheetz MH, Qi C, et al. Evaluation of targeted antimicrobial prophylaxis for transrectal ultrasound guided prostate biopsy: a prospective cohort trial. BMC Infect Dis. 2017; 17(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J Urol. 2018. [DOI] [PubMed] [Google Scholar]
- [13].Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015; 33(3):272–7. [DOI] [PubMed] [Google Scholar]
- [14].Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011; 29(16):2185–90. [DOI] [PubMed] [Google Scholar]
- [15].van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. European urology. 2009; 55(1):1–8. [DOI] [PubMed] [Google Scholar]
- [16].Eggener SE, Mueller A, Berglund RK, Ayyathurai R, Soloway C, Soloway MS, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009; 181(4):1635–41; discussion 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Newcomb LF, Thompson IM Jr., Boyer HD, Brooks JD, Carroll PR, Cooperberg MR, et al. Outcomes of Active Surveillance for Clinically Localized Prostate Cancer in the Prospective, Multi-Institutional Canary PASS Cohort. J Urol. 2016; 195(2):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term Results of Active Surveillance in the Goteborg Randomized, Population-based Prostate Cancer Screening Trial. European urology. 2016; 70(5):760–6. [DOI] [PubMed] [Google Scholar]
- [19].Zietman A Active Surveillance in Low-Risk Prostate Cancer: When Will We Pay It More Than Just Lip Service? 2014. [cited 2019 July 11]Available from: https://www.ascopost.com/issues/august-15-2014/active-surveillance-in-low-risk-prostate-cancer-when-will-we-pay-it-more-than-just-lip-service/
- [20].McGinley L For new cancer treatments, less is more. 2018. [cited 2019 July 11]Available from: https://www.washingtonpost.com/national/health-science/for-new-cancer-treatments-less-is-more/2018/09/09/5b71ab96-95c1-11e8-a679-b09212fb69c2_story.html?utm_term=.497a67bcdeff
- [21].Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015; 314(1):80–2. [DOI] [PubMed] [Google Scholar]
- [22].Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol. 2015; 193(1):95–102. [DOI] [PubMed] [Google Scholar]
- [23].Loeb S, Byrne N, Makarov DV, Lepor H, Walter D. Use of Conservative Management for Low-Risk Prostate Cancer in the Veterans Affairs Integrated Health Care System From 2005–2015. JAMA. 2018; 319(21):2231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. European urology. 2015; 67(1):44–50. [DOI] [PubMed] [Google Scholar]
- [25].Weerakoon M, Papa N, Lawrentschuk N, Evans S, Millar J, Frydenberg M, et al. The current use of active surveillance in an Australian cohort of men: a pattern of care analysis from the Victorian Prostate Cancer Registry. BJU international. 2015; 115 Suppl 5:50–6. [DOI] [PubMed] [Google Scholar]
- [26].Huland H, Graefen M. Changing Trends in Surgical Management of Prostate Cancer: The End of Overtreatment? European urology. 2015; 68(2): 175–8. [DOI] [PubMed] [Google Scholar]
- [27].Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol. 2017; 3(10):1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dall’Era MA. Patient and disease factors affecting the choice and adherence to active surveillance. Current opinion in urology. 2015; 25(3):272–6. [DOI] [PubMed] [Google Scholar]
- [29].van den Bergh RC, Korfage IJ, Bangma CH. Psychological aspects of active surveillance. Current opinion in urology. 2012; 22(3):237–42. [DOI] [PubMed] [Google Scholar]
- [30].Davison BJ, Oliffe JL, Pickles T, Mroz L. Factors influencing men undertaking active surveillance for the management of low-risk prostate cancer. Oncol Nurs Forum. 2009; 36(1):89–96. [DOI] [PubMed] [Google Scholar]
- [31].Gorin MA, Soloway CT, Eldefrawy A, Soloway MS. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011; 77(3):588–91. [DOI] [PubMed] [Google Scholar]
- [32].Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013; 190(5):1742–9. [DOI] [PubMed] [Google Scholar]
- [33].Xu J, Neale AV, Dailey RK, Eggly S, Schwartz KL. Patient perspective on watchful waiting/active surveillance for localized prostate cancer. J Am Board Fam Med. 2012; 25(6):763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Scherr KA, Fagerlin A, Hofer T, Scherer LD, Holmes-Rovner M, Williamson LD, et al. Physician Recommendations Trump Patient Preferences in Prostate Cancer Treatment Decisions. Med Decis Making. 2017; 37(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Volk RJ, McFall SL, Cantor SB, Byrd TL, Le YC, Kuban DA, et al. ‘It’s not like you just had a heart attack’: decision-making about active surveillance by men with localized prostate cancer. Psycho-oncology. 2014; 23(4):467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Anandadas CN, Clarke NW, Davidson SE, O’Reilly PH, Logue JP, Gilmore L, et al. Early prostate cancer--which treatment do men prefer and why? BJU international. 2011; 107(11):1762–8. [DOI] [PubMed] [Google Scholar]
- [37].Ritch CR, Graves AJ, Keegan KA, Ni S, Bassett JC, Chang SS, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015; 193(3):801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moschini M, Fossati N, Sood A, Lee JK, Sammon J, Sun M, et al. Contemporary Management of Prostate Cancer Patients Suitable for Active Surveillance: A North American Population-based Study. Eur Urol Focus. 2016. [DOI] [PubMed] [Google Scholar]
- [39].Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary Nationwide Patterns of Active Surveillance Use for Prostate Cancer. JAMA Intern Med. 2015; 175(9):1569–71. [DOI] [PubMed] [Google Scholar]
- [40].Maurice MJ, Zhu H, Abouassaly R. A hospital-based study of initial observation for low-risk prostate cancer and its predictors in the United States. Can Urol Assoc J. 2015; 9(3-4):E193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parikh RR, Kim S, Stein MN, Haffty BG, Kim IY, Goyal S. Trends in active surveillance for very low-risk prostate cancer: do guidelines influence modern practice? Cancer Med. 2017; 6(10):2410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP. Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer. 2009; 17(2):117–27. [DOI] [PubMed] [Google Scholar]
- [43].Taylor KL, Hoffman RM, Davis KM, Luta G, Leimpeter A, Lobo T, et al. Treatment Preferences for Active Surveillance versus Active Treatment among Men with Low-Risk Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2016; 25(8):1240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998; 36(1):8–27. [DOI] [PubMed] [Google Scholar]
- [45].DeWalt DA, Rothrock N, Yount S, Stone AA, Group PC. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007; 45(5 Suppl 1):S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007; 45(5 Suppl 1):S22–31. [DOI] [PubMed] [Google Scholar]
- [47].Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000; 56(6):899–905. [DOI] [PubMed] [Google Scholar]
- [48].Taylor KL, Davis JL 3rd, Turner RO, Johnson L, Schwartz MD, Kerner JF, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006; 15(11):2179–88. [DOI] [PubMed] [Google Scholar]
- [49].Taylor KL, Williams RM, Davis K, Luta G, Penek S, Barry S, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med. 2013; 173(18):1704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Arora NK, Weaver KE, Clayman ML, Oakley-Girvan I, Potosky AL. Physicians’ decision-making style and psychosocial outcomes among cancer survivors. Patient Educ Couns. 2009; 77(3):404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997; 29(3):21–43. [PubMed] [Google Scholar]
- [52].Clark JA, Inui TS, Silliman RA, Bokhour BG, Krasnow SH, Robinson RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003; 21:3777–84. [DOI] [PubMed] [Google Scholar]
- [53].Legare F, Kearing S, Clay K, Gagnon S, D’Amours D, Rousseau M, et al. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010; 56(8):e308–14. [PMC free article] [PubMed] [Google Scholar]
- [54].O’Connor A User Manual-Decisional Conflict Scale (10-item question format). 1993. 2010 February 17, 2017]Available from: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- [55].Avila M, Becerra V, Guedea F, Suarez JF, Fernandez P, Macias V, et al. Estimating preferences for treatments in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2015; 91(2):277–87. [DOI] [PubMed] [Google Scholar]
- [56].Loppenberg B, Friedlander DF, Krasnova A, Tam A, Leow JJ, Nguyen PL, et al. Variation in the use of active surveillance for low-risk prostate cancer. Cancer Cytopathol. 2018; 124(1):55–64. [DOI] [PubMed] [Google Scholar]
- [57].Davison BJ, Goldenberg SL. Patient acceptance of active surveillance as a treatment option for low-risk prostate cancer. BJU international. 2011; 108(11): 1787–93. [DOI] [PubMed] [Google Scholar]
- [58].van den Bergh RC, van Vugt HA, Korfage IJ, Steyerberg EW, Roobol MJ, Schroder FH, et al. Disease insight and treatment perception of men on active surveillance for early prostate cancer. BJU international. 2010; 105(3):322–8. [DOI] [PubMed] [Google Scholar]
- [59].Pickles T, Ruether JD, Weir L, Carlson L, Jakulj F. Psychosocial barriers to active surveillance for the management of early prostate cancer and a strategy for increased acceptance. BJU international. 2007; 100(3):544–51. [DOI] [PubMed] [Google Scholar]
- [60].Orom H, Homish DL, Homish GG, Underwood W, 3rd. Quality of physician-patient relationships is associated with the influence of physician treatment recommendations among patients with prostate cancer who chose active surveillance. Urol Oncol. 2014; 32(4):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].van den Bergh RC, Essink-Bot ML, Roobol MJ, Wolters T, Schroder FH, Bangma CH, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009; 115(17):3868–78. [DOI] [PubMed] [Google Scholar]
- [62].Bellardita L, Rancati T, Alvisi MF, Villani D, Magnani T, Marenghi C, et al. Predictors of health-related quality of life and adjustment to prostate cancer during active surveillance. European urology. 2013; 64(1):30–6. [DOI] [PubMed] [Google Scholar]
- [63].Mader EM, Li HH, Lyons KD, Morley CP, Formica MK, Perrapato SD, et al. Qualitative insights into how men with low-risk prostate cancer choosing active surveillance negotiate stress and uncertainty. BMC Urology. 2017; 17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lyons KD, Li HH, Mader EM, Stewart TM, Morley CP, Formica MK, et al. Cognitive and Affective Representations of Active Surveillance as a Treatment Option for Low-Risk Prostate Cancer. Am J Mens Health. 2017; 11(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].O’Callaghan C, Dryden T, Hyatt A, Brooker J, Burney S, Wootten AC, et al. ‘What is this active surveillance thing?’ Men’s and partners’ reactions to treatment decision making for prostate cancer when active surveillance is the recommended treatment option. Psychooncology. 2014; 23(12): 1391–8. [DOI] [PubMed] [Google Scholar]
- [66].Hoffman KE, Niu J, Shen Y, Jiang J, Davis JW, Kim J, et al. Physician variation in management of low-risk prostate cancer: a population-based cohort study. JAMA Intern Med. 2014; 174(9):1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kim SP, Gross CP, Nguyen PL, Smaldone MC, Shah ND, Karnes RJ, et al. Perceptions of Active Surveillance and Treatment Recommendations for Low-risk Prostate Cancer: Results from a National Survey of Radiation Oncologists and Urologists. Med Care. 2014; 52(7):579–85. [DOI] [PubMed] [Google Scholar]
- [68].Stacey D, Legare F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017; 4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Penson DF. Factors influencing patients’ acceptance and adherence to active surveillance. J Natl Cancer Inst Monogr. 2012; 2012(45):207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns. 2012; 87(3):369–74. [DOI] [PubMed] [Google Scholar]
- [71].Preventive US Services Task Force, Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018; 319(18):1901–13. [DOI] [PubMed] [Google Scholar]