Abstract
Phosphoglycerate kinase 1 (PGK1) is an essential enzyme in the aerobic glycolysis pathway. PGK1 catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP and produces 3-phosphoglycerate and ATP. In addition to cell metabolism regulation, PGK1 is involved in multiple biological activities, including angiogenesis, autophagy and DNA repair. Because of its multi-faceted functions, PGK1’s involvement in cancer development is complicated. High intracellular expression of PGK1 leads to tumor cell proliferation. However, high extracellular expression of PGK1 suppresses cancer malignancy through a suppression of angiogenesis. PGK1 is also associated with chemoradiotherapy resistance and poor prognosis of cancer patients. In this manuscript, we summarize the influence of PGK1 and its post-translational modifications on cancer initiation and progression. PGK1-mediated drug resistance and potential small molecule inhibitors targeting PGK1 are discussed for their future clinical applications.
Keywords: PGK1, cancer, post-translational modification, drug resistance, small molecule inhibitors
Introduction
Phosphoglycerate kinase (PGK) is a major enzyme which catalyzes the formation of ATP in the aerobic glycolysis pathway. PGK exists in all organisms with high sequence conservation throughout evolution [1]. The two isoforms of PGK, PGK1 and PGK2, have similar functions and structures in human beings, encompassing 417 amino acids with 87-88% sequence identity and near a 45-kDa molecular mass [2]. However, PGK1 and PGK2 have different expression distribution. PGK2 encoded by an autosomal gene is only expressed during spermatogenesis, while PGK1 located on the X-chromosome is ubiquitously expressed in all cells [3].
PGK1 is the only enzyme encoded by an X-linked gene and involved in the first ATP-generating step of the glycolytic pathway [4]. PGK1 and pyruvate kinase M2 (PKM2) are the only two enzymes controlling ATP production during aerobic glycolysis in cancer cells [5]. PGK1 catalyzes a reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP, producing 3-phosphoglycerate (3-PG) and ATP: 1,3-BPG + ADP ⇌ 3-PG + ATP. PGK1 plays a rate-limiting role in coordinating energy production with biosynthesis and redox balance by controlling ATP and 3-PG levels [6].
The protein structure of PGK1 has been well understood [7]. It is a monomeric protein containing two similar sized Rossmann fold domains, corresponding to the N- and C-terminal respectively, which are connected by a hinge region through hydrophobic interactions and hydrogen bonds [8]. The N-terminal domain of PGK1 allows 1,3-BPG or 3-PG to bind, while the C-domain binds to the nucleotide substrate ADP. Once the two substrates are bounded, a hinge-bending motion occurs to bring domains and their bound substrates into a closed conformation for substrate contact [9].
PGK1 has other functions besides regulating glycolytic metabolism, including mediating autophagy initiation [10-13], DNA replication and repair in mammal cell nuclei [14]. An abnormal expression level of PGK1 is related to disease occurrence. PGK1 deficiency has linkage with parkinsonism, hereditary non-spherocytic haemolytic anaemia, neurological impairment and myopathy [15-18]. At present, it is no definite treatment for PGK deficiency. On the other hand, PGK1 is overexpressed in synovial tissues and blood of rheumatoid arthritis, suggesting it participates in pro-inflammation and synovial hyperplasia of the disease [19].
More noticeably, PGK1 mediates glycolysis that generates ATP for tumor cells especially under hypoxic conditions, which is related to development and progress of various cancers [20]. Recently PGK1 has gradually become a focused target in cancer research. However, up to now no review has systematically summarized the important functions and mechanisms of PGK1 in tumorigenesis. This paper reviews the role of PGK1 and its post-translational modifications (PTMs) in cancer initiation and progression, and prospects the application of PGK1 as a new diagnostic biomarker and therapeutic target in cancer and drug resistance.
Bioinformatics analyses of PGK1 expression with cancer prognosis
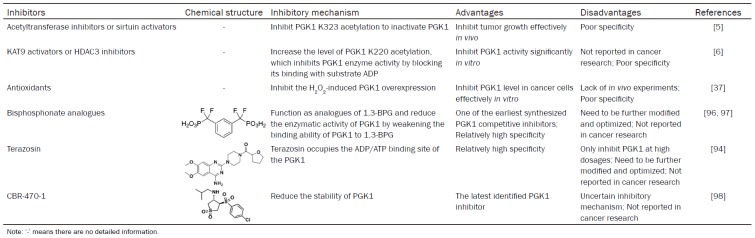
To understand associations of PGK1 expression level and prognostic value in human cancers, we have analyzed datasheet from several online databases, including Oncomine microarray database (http://www.oncomine.org) containing PGK1 mRNA level between tumors and normal tissues in multiple cancers. The parameter thresholds are set as follows according to the previous research: p-value: 1E-4; fold change: 2; gene rank: top 10%; analysis type: cancer vs. normal analysis; data type: mRNA [21]. The database contains a total of 353 unique analyses for PGK1. In 36 studies, PGK1 is ranked within the top 10% of all genes showing significant statistical differences, from which 33 cases of research data have confirmed a higher expression level of PGK1 in tumor than normal tissues. Although the mRNA expression level of PGK1 varies with the individual type of tumor, PGK1 is usually overexpressed in most cancers (Figure 1A).
Figure 1.
Bioinformatic analyses of the expression and prognosis of PGK1 in cancer. (A) The mRNA expression levels of PGK1 in various cancers are analyzed via the Oncomine database. The number in the colored cell represents the amount of cases that satisfy the thresholds. Cell coloring is determined by gene ranking. The more intense red (overexpression) or blue (underexpression) suggests a more highly significant upregulated or downregulated gene. (B-F) The relationship between the mRNA expression of PGK1 and the survival time of cancer patients is assessed using the HPA database online. High PGK1 mRNA expression predicts poor survival in breast cancer (B), head and neck cancer (C), cervical cancer (D), liver cancer (E), and pancreatic cancer (F).
Next, we assess the correlation between PGK1 expression and clinical outcomes of cancer patients by bioinformatics analysis on the Human Protein Atlas (HPA) database (https://www.proteinatlas.org/). It is an important tool for biomarker discovery [22]. The mRNA expression level of PGK1 is significantly associated with prognosis of patients in five different types of cancer. High PGK1 predicts poor survival in breast cancer, head and neck cancer, cervical cancer, liver cancer and pancreatic cancer (P < 0.001, Figure 1B-F).
PGK1-mediated tumor suppression mechanisms
Through database analysis, we find that PGK1 is overexpressed in most cancers and its overexpression indicates a poor prognosis in some specific cancer types. However, PGK1 is originally thought to be a tumor suppressor. It seems to play contradictory even opposite functions of the extracellular PGK1 compared with the intracellular PGK1 for cancer occurrence. Extracellular PGK1 can function as a disulfide reductase, which generates angiostatin by increasing the reduction of disulfide bonds in plasmin [23]. The formation of angiostatin from plasmin restricts tumor growth by suppressing angiogenesis [24]. Therefore, the extracellular PGK1 plays an inhibitory role in tumor growth and metastasis under certain conditions. For example, intraperitoneal administration of recombinant human PGK into fibrosarcoma-bearing mice and pancreatic tumor-bearing mice both cause an increase in plasma levels of angiostatin and a decrease in tumor angiogenesis and tumor growth [23]. Furthermore, PGK1 secreted into cellular matrix reduces VEGF level and enhances generation of angiostatin to inhibit angiogenesis in prostate cancer (PCa) [25]. Another recent study demonstrates that PGK1 expression is repressed by MVIH, a long noncoding RNA (lncRNA), which activates the tumor-induced angiogenesis in hepatocellular carcinoma (HCC) [26].
In addition to being a tumor suppressor by generating the angiogenesis inhibitor angiostatin, PGK1 can reduce the tumor growth of Lewis lung carcinoma by downregulating the expression of COX-2 and promoting anti-tumor immunity in vivo [27]. PGK1 comprises a nucleotide-binding domain (NBD) and two catalytic domains (CDI and CDII). Similar to full-length PGK1, both CDI and NBD domains of PGK1 can inhibit the growth of lung cancer cells in vivo. Regarding the molecular mechanism responsible for this, the NBD domain of PGK1 decreases COX-2 mRNA stability to inhibit COX-2 expression level and its metabolites PGE2 and TGF-β1 in lung cancer cells [28]. Glycolytic proteomic analysis of lung cancer tissues shows that poorly differentiated tumors have significantly lower PGK1 expression levels compared with moderate or highly-differentiated ones, suggesting PGK1 expression is downregulated during the development of lung cancer [29]. Moreover, PGK1 upregulation reduces the expression of cell surface urokinase-type plasminogen activator receptor (uPAR) and inhibits the uPAR-mediated cell proliferation and migration in H157 lung carcinoma cells [30]. PGK1 can also play a tumor suppressor role by enhancing the specific cytotoxic effects of immune cells on tumors. For instance, in HLA-A2+ colon cancer, PGK1 can be recognized as a tumor-associated antigen specifically by cytotoxic T lymphocyte to increase the production of IFN-γ in tumor-infiltrating T lymphocyte and enhance the killing effect of T lymphocyte on tumor [31].
On the other hand, PGK1 is notably downregulated in the highly metastatic gallbladder carcinoma (GBC) cell line GBC-SD18H compared to the poorly metastatic GBC-SD18L cells [32]. Decreased PGK1 expression is associated with poor prognosis in patients with GBC, which indicates PGK1 is a useful potential prognostic biomarker for GBC [33]. However, the specific molecular mechanism of how PGK1 exerts its anti-cancer function in GBC is not yet clear. Overall, PGK1 can serve as a tumor suppressor in some specific cancers, and PGK1-mediated tumor suppression mechanisms are summarized detailedly in Table 1.
Table 1.
PGK1-mediated tumor suppression mechanisms
| Cancer types | Molecular mechanisms | Function/clinical association | References |
|---|---|---|---|
| Fibrosarcoma | Promote the angiostatin formation | Inhibit tumor angiogenesis and tumor growth | [23] |
| Pancreatic cancer | [23] | ||
| Hepatocellular carcinoma | - | Inhibit tumor angiogenesis | [26] |
| Prostate cancer | Reduce VEGF secretion; Promote the angiostatin formation | Inhibit tumor angiogenesis | [25] |
| Lung cancer | Inhibit COX-2 expression; Promote anti-tumor immunity in vivo | Inhibit tumor growth | [27] |
| Inhibit COX-2 expression | Inhibit tumor growth | [28] | |
| - | Associated with prognosis of patients | [29] | |
| Reduce expression of uPAR | Inhibit cell proliferation and migration | [30] | |
| HLA-A2+ colon cancer | Recognized as a tumor-associated antigen specifically by cytotoxic T lymphocytes | Enhance the killing effect of T lymphocyte on tumor | [31] |
| Gallbladder carcinoma | - | Associated with prognosis of patients | [32] |
| Associated with prognosis of patients | [33] |
Note: ‘-’ means no detailed information.
PGK1-invovled oncogenic signal pathways
As our previous bioinformatics analysis shows, PGK1 functions as an oncogene in most cases. We summarize several key PGK1-involved oncogenic signal pathways as follows (Table 2).
Table 2.
PGK1-invovled oncogenic signal pathways
| Cancer types | Signaling pathway/mechanisms | Function/clinical association | References |
|---|---|---|---|
| Hepatocellular carcinoma | HIF-1α/PGK1 pathway | Promote cell metastasis | [38] |
| MYC/PGK1 pathway | Induce glycolysis | [44] | |
| PGK1/AKT/mTOR pathway | Inhibit cell growth | [57] | |
| - | Associated with a poor outcome of patients | [70] | |
| Ovarian cancer | MYC/PGK1 pathway | Induce glycolysis | [45] |
| Brain cancer | HIF-1α/PGK1-induced activation of Notch pathway | Promote the tumorigenesis of GBM | [39] |
| PGK1/CXCR4 pathway | Promote tumor metastasis into the bone; Associated with a poor outcome of patients | [52] | |
| PGK1/CXCR4/β-catenin pathway | Promote the migration and invasion of glioma cells | [53] | |
| E2F1 binds to and activates the promoter of the PGK1 | Induce glycolysis; Promote tumor progression | [67] | |
| Breast cancer | HIF-1α/PGK1 pathway | Promote EMT process | [40] |
| MYC/PGK1 pathway | Promote cancer development | [46] | |
| miR-548a-3p represses the transcription factor SIX1-dependent transcription of PGK1 | Promote the Warburg effect | [59] | |
| PPARγ inhibits PGK1 gene transcription activity by targeting the consensus PPRE motif of PGK1 promoter region | Induce glycolysis | [68] | |
| Treatment with a specific HER-2 inhibitor Herceptin decreases the expression of PGK1 obviously | Inhibit cell growth | [69] | |
| Renal cancer | MYC/PGK1 pathway | Upregulated in RCC compared with adjacent normal kidney | [47] |
| Gastric cancer | PGK1/CXCR4/β-catenin pathway | Associated with a poor outcome of patients | [49] |
| PGK1/CXCR4/β-catenin pathway | Enhance the peritoneal dissemination and metastasis of gastric cancer | [50] | |
| PGK1/AKT/mTOR pathway | Promote the oncogenic properties of gastric cancer cell | [62] | |
| Prostate cancer | - | Enhance bone formation at the metastatic site in vivo | [54] |
| - | Induce glycolysis and promote the metastatic ability of prostate CTCs | [55] | |
| Colorectal cancer | miR-548c-5p recognizes the 3’-UTR of PGK1 and decreases the expression of PGK1 | Suppress cell proliferation | [58] |
| Upregulate EGR1 and CYR61 | Promote colon cancer metastasis | [65] | |
| Lung cancer | MetaLnc9-mediated PGK1/AKT/mTOR pathway | Promote cancer metastasis in vitro and in vivo | [61] |
| OTUB2-mediated PGK1/AKT/mTOR pathway | Promote the Warburg effect and tumorigenesis | [63] | |
| Rab11FIP2-mediated PGK1/AKT/mTOR pathway | Suppress tumor growth | [64] | |
| - | Associated with a poor outcome of patients | [73] | |
| Papillary thyroid cancer cell | SIRT6 increases the expression of PGK1 | Promote the Warburg effect and enhance tumor aggressiveness | [66] |
| Endometrial cancer | - | Associated with a poor outcome of patients | [74] |
| - | Associated with a poor outcome of patients | [75] | |
| Pancreatic cancer | - | Associated with a poor outcome of patients | [71] |
| - | Associated with a poor outcome of patients | [72] | |
| Oral squamous cell carcinoma | - | Associated with a poor outcome of patients | [76] |
Note: ‘-’ means there are no detailed information.
HIF-1α-PGK1 axis-induced glycolysis enhancement
Even under oxygen-sufficient conditions, cancer cells prefer glycolysis rather than mitochondrial oxidative phosphorylation for glucose metabolism during proliferation and metastasis, which is called Warburg effect. Hypoxia inducible factor 1α (HIF-1α) serves as a transcriptional factor to promote cell glycolysis for Warburg effect [34,35]. As an important ATP-generating enzyme in the glycolytic pathway, PGK1 is directly regulated by HIF-1α in many cancers. Earlier studies have shown that PGK1 is transcriptionally activated by HIF-1α in colorectal cancer cells and hepatoma cells under hypoxia stress [36]. In human colon carcinoma cells, PGK1 is identified as a potential biomarker for reflecting intracellular oxidative stress status, and antioxidants can inhibit the expression of HIF-1α and its downstream target protein PGK1 [37].
Moreover, PGK1 is relative with cancer cell metastatic ability due to HIF-1α/PGK1 mediated epithelial-mesenchymal transition (EMT) process. Higher expression levels of PGK1 and HIF-1α are detectable in high metastatic HCC cells HCCLM9 compared with MHCC97L cells with low metastasis [38]. Similarly, the upregulated HIF-1α and PGK1 activate the Notch pathway to promote progression of human glioblastoma (GBM) [39]. A high PGK1 expression indicates poor prognosis in patients with breast cancer, so PGK1 is a promising invasion promoter and survival biomarker for breast cancer [40]. In addition, PGK1 is significantly increased in HIF-1α-overexpressed leukemic cell K562. Overexpression of HIF-1α protects K562 cells from 1,4-benzoquinone-induced toxicity by inhibiting reactive oxygen species (ROS), apoptosis and enhancing glycolysis [41]. Generally, intracellular PGK1 production by tumor cells is enhanced under hypoxia, but its extracellular secretion is inhibited. This is responsible for the different functions of intracellular and extracellular PGK1 [20].
MYC-PGK1 pathway-mediated cell metabolism
Similar to HIF-1α, MYC is one of main regulators of the glycolytic switch observed in cancer cells [42,43]. PGK1 plays an important role in MYC-induced metabolic reprogramming, which leads to enhanced Warburg effect in a variety of cancers. MYC-dependent PGK1 induces the overexpression of key glycolysis enzymes GLUT4, HK2 and LDHA to accelerate glycolysis rate and produce large amounts of ATP and lactate, which promotes HCC proliferation and metastasis [44]. On the contrary, knockdown of endogenous MYC significantly reduces PGK1 levels, suggesting MYC is a major regulator in controlling PGK1 enzyme activity in HCC cells [5]. In ovarian cancer, Pim1-MYC-PGK1 pathway is activated to change cell metabolic process [45]. MYC is also recruited to activate PGK1 in the development of human breast cancer [46] and clear cell renal cell carcinoma (ccRCC) [47].
PGK1-CXCR4/CXCL12 pathway
A tight relationship between PGK1 and the CXCR4/CXCL12 (C-X-C chemokine receptor 4/C-X-C chemokine ligand 12) axis has been verified in several cancers. CXCR4 is overexpressed in many solid and hematologic cancers, and activates the downstream oncogenic signaling pathway by binding its ligand, CXCL12 [48]. In gastric cancer, tumor samples with peritoneal carcinomatosis have a higher mRNA expression of PGK1, CXCR4, CXCL12 and β-catenin compared with tissues without peritoneal carcinomatosis [49]. Moreover, PGK1 and CXCR4 are able to regulate each other via a co-stimulating way in gastric cancer cells, and overexpression of PGK1 increases the peritoneal dissemination and metastasis of gastric cancer by activating the CXCR4/CXCL12/β-catenin signaling pathway [50]. The ability of PGK1 to promote the progression and metastasis of gastric cancer is further demonstrated in a tumor mouse model [51]. PGK1, CXCR4 and β-catenin are all overexpressed in high metastatic HCC cells [38].
PGK1 is a biomarker for predicting glioma metastatic capacity and a promising therapy target for metastatic glioma. The upregulated PGK1 in neuroblastoma cells is positively correlated with tumor metastasis to bone marrow, and poor prognosis of patient [52]. PGK1 knockdown suppresses migration and invasion of glioma cells by decreasing CXCR4 and β-catenin [53]. Most studies have shown that intracellular PGK1 actually plays an oncogenic role in PCa. PGK1 improves bone formation at the metastatic site of PCa in vivo [54]. Circulating tumor cells (CTCs) are cancer cells derived from tumor origin or metastasis that release into the peripheral blood. High expression of PGK1 in prostate CTCs accelerates glycolysis process and increases the supply of energy to promote cell metastatic ability [55]. This indicates PGK1 seems to be a biomarker for prostate cancer metastasis. In addition, PGK1 induces fibroblasts towards the cancer-associated fibroblasts phenotype in tumor microenvironment of PCa. PGK1 upregulation in PCa-associated stromal cells stimulates CXCR4/CXCL12 signaling pathway to produce cytokines released into tumor microenvironment, thereby it contributes migration and invasion of PCa cells [56].
PGK1 functions regulated by non-coding RNA
In recent two years, the regulation of PGK1 roles by non-coding RNA has become a research hotspot. It seems PGK1 expression is regulated by several microRNAs (miRNAs). A negative correlation between miR-450b-3p and PGK1 protein is observed in HCC specimens using Pearson’s correlation analysis. The 3’-UTR of PGK1 contains a complementary sequence of miR-450b-3p, therefore PGK1 probably is a direct target of miR-450b-3p. Moreover, miR-450b-3p inhibits cell growth by downregulating PGK1 and PGK1-mediated AKT phosphorylation in HCC cells [57]. The miR-548c-5p suppresses colorectal cancer cell proliferation by recognizing the 3’-UTR of PGK1 to decrease the expression of PGK1 [58]. In breast cancer, transcription factor SIX1 promotes the Warburg effect and tumor growth in vitro and in vivo by binding to the promoter region of PGK1 and recruiting histone acetyltransferase HBO1 to induce the transcription of PGK1. While SIX1 is directly inhibited by miR-548a-3p, which leads to the decrease of PGK1 expression and aerobic glycolysis [59]. These evidences indicate miR-548a-3p downregulates PGK1 to suppress glycolysis.
In non-small cell lung cancer (NSCLC), the lncRNA LINC00963 (MetaLnc9) physically interacts with PGK1 to prevent PGK1 degradation by ubiquitination, which stimulates PGK1-activated oncogenic AKT/mTOR signaling pathway to promote cancer metastasis in vitro and in vivo [60,61]. Similarly, another a long non-coding RNA GBCDRlnc1 directly binds to PGK1 and inhibits PGK1 ubiquitination, and then the overexpression of PGK1 induces chemoresistance of GBC cancer cells by activating autophagy [13].
PGK1/AKT/mTOR pathway
PGK1 mediated AKT/mTOR pathway is commonly activated in cancers. In addition to the two examples mentioned above [57,60,61], PGK1 interacts with other targets to involve in AKT/mTOR pathway to promote tumorigenesis. Overexpression of gankyrin alleviates cellular oxidative stress and increases the oncogenic properties of gastric cancer cell by activating PGK1/AKT/mTOR pathway [62]. In NSCLC cells, OTUB2 directly binds to U2AF2 and reduces its ubiquitination, thus stabilizing the protein expression of U2AF2 [63]. Then, U2AF2 enhances the Warburg effect and tumorigenesis via the PGK1/AKT/mTOR signaling pathway. Moreover, the combined evaluation of OTUB2, U2AF2 and PGK1 is more accurate to predict prognosis for patients. A high expression of OTUB2, U2AF2 and PGK1 is significantly correlated with worse prognosis in NSCLC patients [63]. Furthermore, Rab11FIP2 can interact with PGK1 and improve its degradation in NSCLC cells, resulting in inactivation of the oncogenic PGK1/AKT/mTOR signaling pathway to suppress tumor growth [64].
Other PGK1-mediated signaling pathways
PGK1 also promotes tumorigenesis through other specific signal transduction pathways, which are not common and occur only in some specific tumors. For instance, PGK1 upregulates the expression of EGR1, a metastasis-related factor, to enhance colon cancer metastasis. On the other hand, PGK1 positively regulates CYR61 and its downstream transcription factors FOS and JUN to promote the metastasis of colon cancer [65]. In papillary thyroid cancer cell, SIRT6 enhances tumor aggressiveness by upregulating the expression of key Warburg effect genes including PGK1, PKM2, GLUT1, HK2, LDHA, ENO1 and GAPDH [66]. Moreover, E2F1 directly binds to the promoter of PGK1, which results in the promotion of aerobic glycolysis and tumor progression of neuroblastoma [67]. In addition, PPARγ inhibits PGK1 gene transcription activity by targeting the consensus PPRE motif of PGK1 promoter region, leading to the reduced glycolysis of breast cancer cells [68]. Besides, the overall expression of PGK1 is much higher in HER-2/neu-positive breast cancer compared with that in HER-2/neu-negative breast cancer, and treatment with a specific HER-2 inhibitor Herceptin decreases the expression of PGK1 obviously in breast cancer cells [69].
Clinical significance of PGK1 expression in cancer
As the incidence of cancer rises continuously, the need to develop highly sensitive and specific biomarkers for early stage diagnosis and treatment of cancer has become an important priority. It is a trend that liquid biopsy will replace traditional tumor tissue biopsy in the future. PGK1 is a secretory protein, therefore the abnormal expression of PGK1 can be detected conveniently not only in tumor tissues, but also in peripheral blood and saliva of patients.
Several studies have demonstrated that PGK1 has potential to be used independently as a diagnostic, prognostic, and treatment biomarker in various cancer. For example, it is confirmed that the high serum level of PGK1 is tightly associated with HCC early relapse and worse prognosis, and the serum level of PGK1 is complementary with alpha-fetoprotein (AFP) to further enhance the sensitivity and specificity for predicting the recurrence of HCC [70]. On the other hand, the serum levels of PGK1 are significantly higher in pancreatic cancer patients than those in healthy controls [71], and high PGK1 level predicts poor prognosis in patients with pancreatic cancer [72]. Similarly, elevated levels of PGK1 in serum and tumor tissue are also significantly linked with poor outcome in patients with lung adenocarcinoma [73]. In addition, the expression of PGK1 in endometrial cancer is higher compared to normal samples, and it is significantly correlated with tumor grade [74]. Another research also indicates that high PGK1 expression correlates positively with FIGO stage, histological grade, lymph node status, and survival time of patients with endometrial cancer [75]. Moreover, the prognosis of oral squamous cell carcinoma (OSCC) patients can be predicted by detecting the expression of PGK1 in tumor tissues and saliva samples. Patients with upregulated PGK1 in the invasive tumor front (ITF) of OSCC have a higher risk of local relapse and worse survival, and the low expression of PGK1 in saliva samples from OSCC patients is associated with lymph node metastasis and advanced clinical stage [76]. In conclusion, PGK1 exhibits a good ability to predict diagnosis and prognosis in many cancers, but the underlying molecular mechanisms need to be further clarified in the future.
PTMs of PGK1 in cancer
Several somatic variants of PGK1 have been identified in cancer cells, being summarized on the database COSMIC (http://cancer.sanger.ac.uk) [77]. The nucleotide mutations in the 3-PG binding region, hinge region and ADP binding region of PGK1 have important effects on its own catalytic activity and conformational stability [7]. Besides, nucleotide mutations probably have influences on protein PTMs. Therefore, PTMs of PGK1 have related to occurrence and development of tumors. Previous studies have confirmed several PTMs of PGK1, including acetylation, phosphorylation, ubiquitination and succinylation, play an important role in regulating tumor metabolism and growth (Table 3).
Table 3.
Post-translational modifications of PGK1
| PTMs | PTM sites | Function | Cancer types | Possible pathways and mechanisms | Regulators | References |
|---|---|---|---|---|---|---|
| Acetylation | K323 | Oncogenic role | HCC | Enhance PGK1 enzymatic activity for glycolytic metabolism. | PCAF: interact with and acetylate PGK1; SIRT7: bind to PGK1 and reduce its acetylation | [5] |
| K220 | Decrease glycolytic ATP production and cellular redox state | - | Inhibit PGK1 enzyme activity by blocking its binding with substrate ADP. | KAT9: acetylate and inactivate PGK1; HDAC3: deacetylate and activate PGK1 | [6] | |
| K388 | Oncogenic role | GBM | Lead to the activation of VPS34-Beclin1-ATG14L complex required for autophagosome formation to promote tumor growth. | Glutamine deprivation and hypoxia result in ARD1-dependent PGK1 K388 acetylation | [10] | |
| Phosphorylation | S203 | Oncogenic role | GBM | Phosphorylate and activate PDHK1, leading to decrease of mitochondrial pyruvate metabolism and increase of glycolysis. | Hypoxia, activation of EGFR, and K-Ras/B-Raf mutation all induce ERK-dependent phosphorylation of PGK1 at S203 | [79] |
| T243 | Oncogenic role | GBM | Reduce the 3-PG affinity of PGK1 to facilitate the equilibrium of the PGK1-catalyzed reaction toward glycolysis. | M2 macrophages can secrete IL-6 to increase PDPK1-dependent PGK1 T243 phosphorylation | [81] | |
| Ubiquitination | Lysine | Tumor suppressor role | GBC | Enhance the ubiquitin degradation of PGK1. | GBCDRlnc1 | [13] |
| NSCLC | MetaLnc9 | [60] | ||||
| NSCLC | Rab11FIP2 | [64] | ||||
| Succinylation | Lysine | Oncogenic role | RCC | PGK1 lysine succinylation is upregulated in RCC tissues compared with adjacent normal tissues. | - | [82] |
Note: ‘-’ means there are no detailed information.
Acetylation of PGK1
PGK1 is acetylated at K323 in liver cancer, which is correlated with poor survival of HCC patients [5]. Acetylation at the K323 site of PGK1 is considered as a significant regulatory mechanism for promoting PGK1 enzymatic activity, cell metabolism, glucose uptake and tumorigenesis. K323 acetylation is modulated by the upstream regulators PCAF and SIRT7 [5]. PCAF is a major acetyltransferase that interacts with and acetylates PGK1, while SIRT7 is a deacetylase which binds to PGK1 and reduces its acetylation. In conclusion, acetylation of PGK1 at K323 increases PGK1 enzyme activity and promotes liver cancer cell proliferation and tumorigenesis.
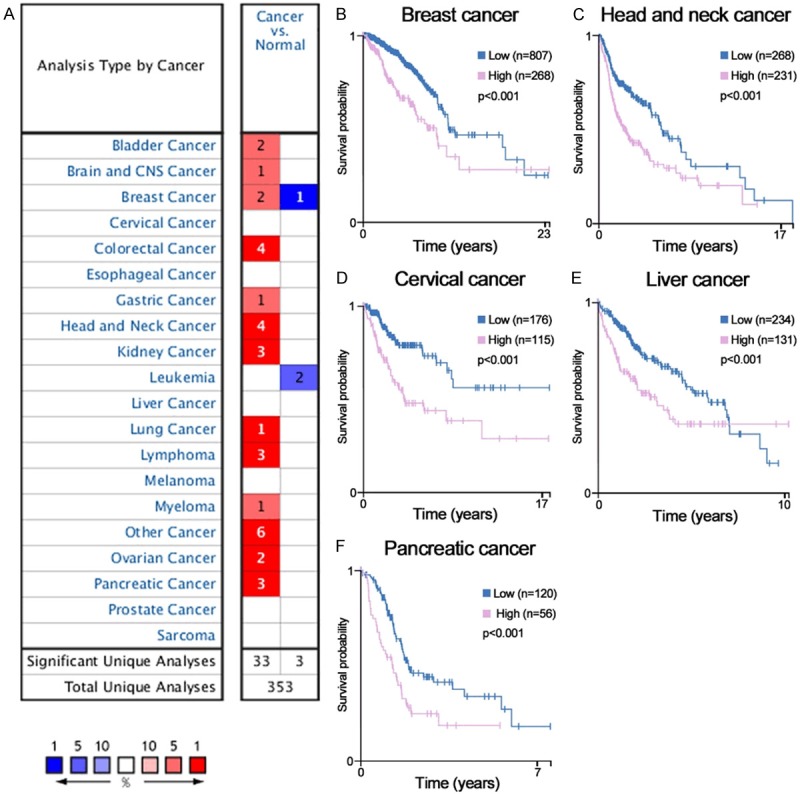
Acetylation of specific residues has different biochemical roles. For example, another research reports that PGK1 is acetylated at K220, and this acetylation inhibits PGK1 enzyme activity by blocking its binding with substrate ADP [6]. KAT9 is a potential acetyltransferase of PGK1, which improves the PGK1 K220 acetylation level and decreases PGK1 activity. On the other hand, HDAC3 is identified as the deacetylase of PGK1 that deacetylates and activates PGK1. Moreover, insulin-induced PI3K/AKT/mTOR pathway increases HDAC3 S424 phosphorylation, which promotes PGK1-HDAC3 interaction and PGK1 K220 deacetylation to stimulate PGK1 activity. In general, insulin treatment can promote glucose metabolism, glycolytic ATP production and redox state of cells via HDAC3-mediated deacetylation and activation of PGK1 (Figure 2A). By now, it has not yet been explored whether K220 acetylation of PGK1 plays an essential role in tumorigenesis.
Figure 2.
Several typical post-translational modifications of PGK1. A. KAT9 is a potential acetyltransferase of PGK1, which increases the K220 acetylation level and inhibits PGK1 enzyme activity by blocking its binding with substrate ADP. On the other hand, insulin-induced PI3K/AKT/mTOR pathway increases S424 phosphorylation of HDAC3, which promotes PGK1-HDAC3 interaction and PGK1 deacetylation at K220 to stimulate PGK1 activity. Finally, this HDAC3-dependent PGK1 deacetylation increases the production of glycolytic ATP, 3-PG and NADPH in cells. B. Hypoxia and glutamine deprivation inhibit mTOR-dependent ARD1 phosphorylation at S228, which enables ARD1 to interact with PGK1 for PGK1 acetylation. Next, the acetylated PGK1 binds to and phosphorylates Beclin1 at S30, which leads to activation of the VPS34-Beclin1-ATG14L complex for autophagosome formation. C. Signals (hypoxia, activation of EGFR, and K-Ras/B-Raf mutation) induce ERK-dependent phosphorylation of PGK1 at S203. Phosphorylated PGK1 is transported to mitochondria under the regulation of TOM complex and PIN1. Mitochondrial PGK1 functions as a protein kinase to phosphorylate and activate PDHK1, which further leads to phosphorylation and inactivation of PDH complex. This process results in the decrease of mitochondrial pyruvate metabolism and ROS generation and the enhancement of glycolysis in cytoplasm, and ultimately promotes GBM cell proliferation and tumorigenesis. D. M2-polarized macrophages secrete IL-6 to increase PDPK1-dependent PGK1 phosphorylation in GBM cells. This phosphorylation can reduce the 3-PG affinity of PGK1, which facilitates the equilibrium of the PGK1-catalyzed reaction toward glycolysis.
Autophagy is essential for maintaining cell homeostasis. Many cancer cells upregulate autophagy to promote survival under stress stimulation, including hypoxia, starvation and metabolic pressure [78]. Recent studies have found that acetylation at the K388 site of PGK1 promotes the initial stage of autophagy to promote tumor growth in U87 GBM cells [10-12]. More importantly, GBM patients with high PGK1 K388 acetylation expression have a significantly shorter survival compared with those with low expression of PGK1 K388 acetylation. However, the involvement of PGK1 acetylation in the regulation of autophagy is very complex. First, hypoxia and glutamine deprivation inhibit mTOR and mTOR-dependent S228 phosphorylation of ARD1, which enables ARD1 to interact with PGK1 for PGK1 K388 acetylation [10]. Next, the acetylated PGK1 binds to and phosphorylates Beclin1 at S30, which leads to activation of the VPS34-Beclin1-ATG14L complex required for autophagosome formation. In a word, PGK1 functions as a protein kinase and phosphorylates Beclin1 to induce autophagy and tumorigenesis in GBM (Figure 3B).
Figure 3.
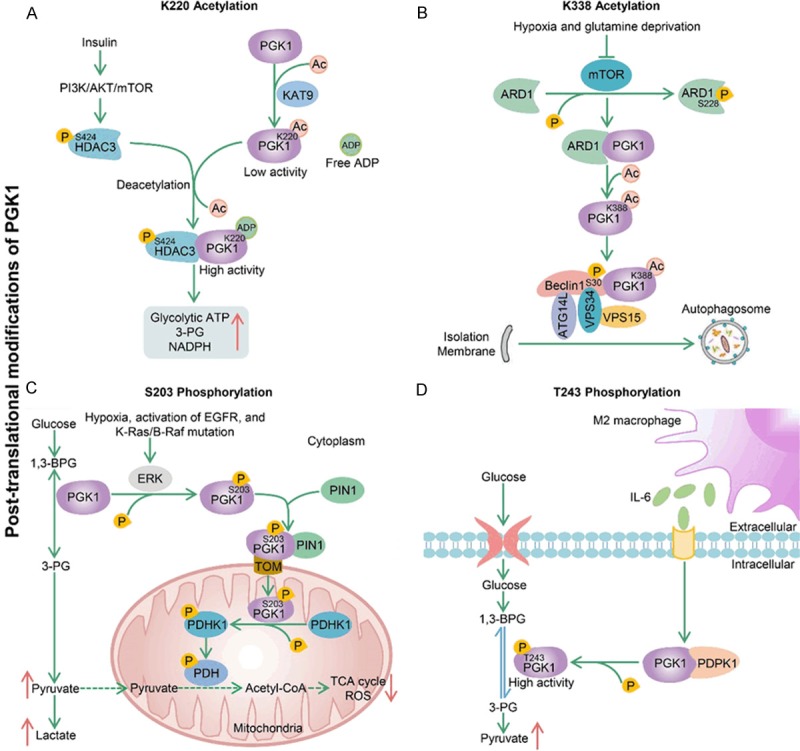
PGK1 involvement in drug resistance. A. Sustained drug treatment leads to tumor starvation and intratumoral hypoxia. B. P-glycoprotein encoded by MDR1 induces multidrug resistance through the ATP-dependent efflux of lipophilic anticancer drugs. C. PGK1 induces chemoresistance of cancer cells by upregulating autophagy. D. PGK1 facilities chemoresistance by triggering HSP90/ERK pathway mediated DNA repair and methylation. E. PGK1 causes drug resistance by scavenging ROS and inhibiting ROS-stimulated apoptosis.
Phosphorylation of PGK1
In addition to acetylation, phosphorylation at the S203 site of PGK1 also plays a significant role in promoting cancer cell metabolism and tumorigenesis of GBM cells [79,80]. High expression of PGK1 S203 phosphorylation independently predicts poor prognosis for GBM patients. A possible underlying mechanism of PGK1 S203 phosphorylation promoting tumor growth is proposed. First of all, multiple factors including hypoxia, EGFR activation, and K-Ras/B-Raf mutation all can induce ERK-dependent phosphorylation of PGK1 at S203 [79]. Secondly, PIN1 binds to and cis-trans isomerizes the pS203P204 motif of phosphorylated PGK1, which makes the 38-QRIKAA-43 residues of PGK1 exposed on protein surface so that they can be recognized by the TOM (translocase of the outer mitochondrial membrane) complex for mitochondrial translocation of PGK1. Following the mitochondrial PGK1 functions as a protein kinase to phosphorylate and activate PDHK1, which further leads to phosphorylation and inactivation of PDH complex. This process results in the decrease of mitochondrial pyruvate metabolism and ROS generation and the enhancement of glycolysis in cytoplasm, and ultimately promotes GBM cell proliferation and tumorigenesis (Figure 3C).
Another interesting finding from a recent study suggests that PGK1 T243 phosphorylation is necessary for M2 macrophage-mediated glycolysis and proliferation of GBM cells [81]. M2-polarized macrophages secrete IL-6 to increase PDPK1-dependent PGK1 phosphorylation at T243 in GBM cells. This phosphorylation reduces the affinity of PGK1 with 3-PG, which facilitates the equilibrium of the PGK1-catalyzed reaction toward glycolysis, thereby enhancing cell proliferation (Figure 3D). In addition, T243 phosphorylation of PGK1 is associated with macrophage infiltration, grading and prognosis of GBM patients [81]. To summarize, this study provides a new strategy for treatment of GBM, which is to cut off the connection between M2 macrophages and GBM cells by suppressing the T243 phosphorylation of PGK1.
Ubiquitination of PGK1
Two examples mentioned above have indicated that ubiquitination of PGK1 is of great significance in suppressing the tumor growth and metastasis of NSCLC [60,61,64]. In addition, ubiquitin degradation of PGK1 inhibits autophagy-associated chemoresistance of GBC cancer cells [13]. However, ubiquitin modification sites of PGK1 are not confirmed in these findings. In conclusion, promoting degradation of PGK1 by increasing its ubiquitination may be an effective strategy for PGK1-targeted cancer therapies.
Succinylation of PGK1
More and more types of PTMs on PGK1 have been discovered recently, and succinylation is one example. PGK1 and its lysine succinylation are both upregulated in RCC tissues compared with matched adjacent normal tissues by quantitative global proteome and lysine succinylome analyses [82]. However, the specific molecular mechanism of PGK1 succinylation in RCC has not been elucidated.
With the development of multiple omics profiling technologies, more PTMs of PGK1 may be uncovered to regulate cell metabolism and growth. However, all previous studies mentioned above only focus one PTM of PGK1. Actually, several PTMs of PGK1 probably interplay together on physiological and pathological conditions. Therefore, considering the crosstalk between different PTM, the combination of individual PTM on PGK1 protein surface can create a PTM code to regulate cell behavior. This will pave a new way to design drugs for interference tumorigenesis and cancer progression.
PGK1 with anticancer drug resistance
Drug resistance of cancer cells is one of the main obstacles in clinical cancer treatment, and its mechanism is complex and diverse. Generally, drug resistance of cancer cells is induced by many factors, such as enhancement of cell anti-apoptotic ability, improved DNA damage repair ability and upregulation of ATP-dependent drug transport protein expression. A large amount of clinical and experimental evidence shows that the high intracellular PGK1 plays a significant role in drug resistance (Table 4).
Table 4.
The essential role of PGK1 in drug and radiation resistance
| Cancer types | Aberrant expression | Drugs or radiation | Underlying pathways | References |
|---|---|---|---|---|
| Leukemia | ↑ | 1,4-benzoquinone | PGK1 can protect K562 cells from 1,4-benzoquinone-induced toxicity by scavenging ROS and inhibiting ROS-stimulated apoptosis. | [41] |
| Astrocytoma | ↑ | Radiation | Not mentioned. | [83] |
| Ovarian cancer | ↑ | Cisplatin | Not mentioned. | [84] |
| Gastric cancer | ↑ | 5-fluorouracil and mitomycin-C | Not mentioned. | [85] |
| Breast cancer | ↑ | Paclitaxel | Sustained chemotherapy drugs treatment leads to tumor starvation and intratumoral hypoxia, inducing the expression of HIF-1α and its downstream target gene PGK1. | [86] |
| Osteogenic sarcoma | ↑ | Paclitaxel, vincristine, adriamycin and mitoxantrone | PGK1 induces a multidrug resistant phenotype through an MDR-1 independent mechanism. | [88] |
| Gallbladder carcinoma | ↑ | Doxorubicin | PGK1 induces chemoresistance of cancer cells by upregulating autophagy. | [13] |
| Endometrial cancer | ↑ | Cisplatin | PGK1 facilities cisplatin chemoresistance by triggering HSP90/ERK pathway mediated DNA repair and methylation. | [92] |
| - | ↑ | H2O2 | PGK1 can protect RAW 264.7 cells from H2O2-induced cell death by enhancing the chaperone activity of HSP90. | [94] |
| - | ↑ | Menadione | PGK1 knockdown induces higher susceptibility to menadione-induced cell death by producing a mass of ROS. | [6] |
↑: PGK1 expression is upregulated in drug or radiation resistant cells/PGK1 promotes the drug or radiation resistance of cells, -: The study is not carried out in cancer cells.
Reducing the expression of PGK1 will be a new strategy to overcome drug resistance in the future. For example, PGK1 is upregulated in radioresistant astrocytomas [83] and cisplatin-resistant ovarian cancer [84]. Moreover, inhibition of PGK1 increases the sensitivity of gastric adenocarcinoma cells to chemotherapeutic drugs 5-fluorouracil and mitomycin-C [85]. Although the exact molecular mechanisms for PGK1-invovled drug resistance are not clearly clarified in the above research.
In breast cancer patients who undergo paclitaxel chemotherapy, patients with high expression of PGK1 have a shorter overall survival than those with low level of PGK1 [86]. It indicates that high PGK1 expression may be the key reason responsible for poor prognosis of breast cancer patients treated with paclitaxel. A hypothesis about the mechanism of PGK1 promoting drug resistance is proposed in this research. The sustained chemotherapy drug treatment leads to tumor starvation and intratumoral hypoxia, supporting the selection of resistant cell clones adapted to the deficiency of oxygen and nutrients. Therefore, response to hypoxia, PGK1 and related target gene of HIF-1α are initiated to increase glycolysis in drug-resistant cells (Figure 3A).
The human multidrug resistance 1 (MDR1) gene encodes the plasma membrane P-glycoprotein that pumps lipophilic anticancer drugs out of the cancer cell and lead to drug resistance [87]. Overexpression of PGK1 induces a multidrug resistance phenotype in human osteogenic sarcoma cells, but the expression level of MDR1 has no change with the overexpression of PGK1 [88]. This suggests the generation of drug resistance phenotype induced by PGK1 is independent on MDR1 (Figure 3B).
Moreover, accumulating evidences suggest autophagy is also contributed to the resistance of cancer cells to anticancer drugs [89]. PGK1 has been demonstrated to play an essential role in modulating autophagy initiation [10-13]. Therefore, PGK1 seems to induce chemoresistance of cancer cells by upregulating autophagy (Figure 3C). For example, the latest study shows that knockdown of PGK1 can inhibit the autophagic flux of doxorubicin (Dox)-resistant GBC cancer cells at initial stage, which enhances the sensitivity of Dox-resistant GBC cancer cells to Dox in vitro and in vivo [13].
Tumor cells exposed to chemotherapy agents often improve global DNA hypermethylation, and this drug-induced DNA hypermethylation can cause drug resistance by inactivating the genes whose products are needed for chemotherapy agents to kill cancer cells [90]. Moreover, DNA hypermethylation can be induced by overexpression of DNA methyltransferase (DNMTs), mainly DNMT1, DNMT3A and DNMT3B [91]. Recent studies have found that PGK1 promotes the expression of DNA repair-associated proteins (FOSL1, c-JUN, and POLD1) and DNA methylation-related enzymes (DNMT1, DNMT3A and DNMT3B) through the HSP90/ERK pathway. And this eventually enhances the chemoresistance of endometrial cancer cells to cisplatin [92,93] (Figure 3D). Another research also indicates that PGK1 can activate HSP90 to enhance stress tolerance [94].
Moreover, drug-induced apoptosis is linked with the enhanced levels of ROS, and a prolonged drug treatment reduces ROS to make cells resistant to drugs [97]. PGK1 knockdown cells show higher susceptibility to ROS-induced cell death [6], and PGK1 upregulation protects K562 cells from 1,4-benzoquinone-induced toxicity by inhibiting ROS-stimulated apoptosis [41]. These results indicate that PGK1 has ability to scavenge ROS for drug resistance (Figure 3E).
Potential inhibitors of PGK1
Considering close associations of PGK1 with carcinogenesis and drug resistance, the development of PGK1 inhibitors is of great value for anticancer therapy. Due to lack of promising lead compounds, the research on small molecule inhibitors of PGK1 is comparatively weak by now [5]. We summarize several potential PGK1 inhibitors and compare their advantages and disadvantages in Table 5.
Table 5.
Potential inhibitors for PGK1
Current strategies to inhibit PGK1 in cancer are often through an indirect approach. The enzymatic activity of PGK1 is inhibited by modulating PTMs of PGK1. For example, K323 acetylation of PGK1 is thought to promote the enzymatic activity of PGK1, so the use of acetyltransferase inhibitors or sirtuin activators can repress PGK1 function effectively [5]. Similarly, KAT9 activators or HDAC3 inhibitors can increase the level of PGK1 K220 acetylation, which inhibits PGK1 enzyme activity by blocking its binding with substrate ADP [6]. Moreover, several antioxidants, including α-tocopherol, butylated hydroxytoluene and Trolox, can inhibit the increase of PGK1 protein expression induced by hydrogen peroxide [37]. However, the specificity of these indirect approaches is controversial, mainly because PGK1 is not the only target of these inhibitors.
PGK1 catalyzes the conversion of 1,3-BPG and ADP into 3-PG and ATP, and this important catalytic reaction in glycolysis requires the binding of PGK1 to substrates (1,3-BPG and ADP). Therefore, the design of inhibitors for PGK1 has generally focused on analogues of the natural substrate 1,3-BPG. Bisphosphonate analogues of 1,3-BPG are one of the earliest synthesized PGK1 competitive inhibitors [96,97]. These bisphosphates can competitively bind to PGK1, which weakens the binding ability of PGK1 to 1,3-BPG, thus greatly reducing the enzymatic activity of PGK1. Terazosin is also considered as a potential inhibitor of PGK1. Terazosin can significantly inhibit PGK1 at high dosages (2.5-25 μM) [97]. Co-crystal structure data from PGK1 with terazosin suggests that the terazosin-binding site overlaps with the ADP/ATP binding site of the PGK1. Therefore, terazosin is supposed to be a competitive inhibitor of PGK1. Recently, a small-molecule compound, CBR-470-1, has been identified as a new novel inhibitor of PGK1 [98]. However, the specific mechanism of CBR-470-1 inhibiting PGK1 is unclear. So far, whether CBR-470-1, bisphosphonates, terazosin or their derivatives, the effects of these PGK1 inhibitors on cancer cells have not been reported. That is to say, whether they can inhibit the growth of tumors by inhibiting PGK1 needs to be further evaluated by experiments.
In brief, there is still a long way to develop novel PGK1 inhibitors for clinical cancer treatment. Future strategies for developing PGK1 inhibitors may be through virtual screening (i.e., similarity search, pharmacophore model, shape-based model and molecular docking) based on the X-ray structure of PGK1/terazosin complex [99]. Meanwhile, designing strong inhibitors for PGK1 and then seeking to modify and optimize them is also a trend in the future.
Future perspective
Cancer is considered as a metabolic disease. Therefore, it is important to understand metabolic related genes in tumors. Even under oxygen-sufficient conditions, cancer cells prefer glycolysis than mitochondrial oxidative phosphorylation for energy acquisition during proliferation and metastasis. As a key enzyme in glycolysis, PGK1 catalyzes the reversible conversion of 1,3-BPG to 3-PG, generating one molecule of ATP to meet the energy requirements for rapid growth of cancer cells. PGK1 and PKM2 are the only two enzymes that generate ATP during aerobic glycolysis in cancer cells. However, the enzyme activity of PKM2 in cancer cells is very low [100]. Low activity PKM2 can increase the accumulation of upstream glycolysis metabolites, which can be converted into nucleic acids, amino acids and lipids through corresponding pathways for biosynthesis. Therefore, it can be inferred that PGK1 controls a considerable part of ATP synthesis in cancer cells and plays an important role in promoting tumor growth.
However, under hypoxic conditions, PGK1 production by tumor cells is enhanced, but its secretion is inhibited [20]. In addition, patients with stronger PGK1 expression in the ITF of OSCC have a higher risk of local relapse and worse survival, but the low expression of PGK1 in saliva samples from OSCC patients is associated with lymph node metastasis and advanced clinical stage [76]. These contradictions may imply that the functions of intracellular and extracellular PGK1 are different. Extracellular PGK1 can serve as a disulfide reductase, which can generate angiostatin by promoting the reduction of disulfide bonds in plasmin. Regulation of glucose metabolism (Figure 4A) and angiogenesis (Figure 4B) are the two main mechanisms of PGK1-mediated tumor growth and migration. High levels of PGK1 are essential for tumor growth but inhibit angiogenesis when released extracellularly. The functional role and regulatory mechanism of PGK1 in cancer progression are still not particularly clear. How to make a balance and utilize the important role of PGK1 in glycolysis and angiogenesis remains to be further studied for targeted cancer therapy.
Figure 4.
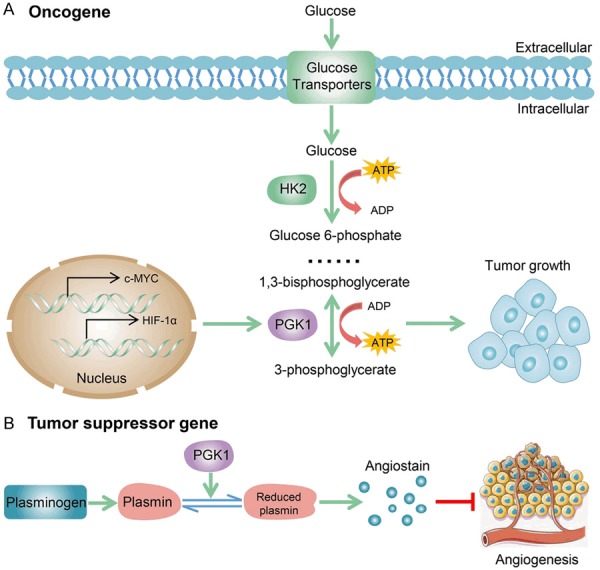
Molecular mechanisms of PGK1-mediated glucose metabolism and angiogenesis. A. PGK1 has an essential role in metabolic reprogramming induced by c-MYC and HIF-1α, leading to an enhanced Warburg effect and tumorigenesis. B. Extracellular PGK1 serves as a disulfide reductase, which can promote the reduction of disulfide bonds in plasmin to generate angiostatin for angiogenesis inhibition.
PGK1 secretion into cell extracellular space provides a convenient way of liquid biopsy. The abnormal level of PGK1 is detectable conveniently not only in tumor tissues, but also in peripheral blood and saliva of patients. Therefore, as a promising diagnostic and prognostic biomarker, PGK1 has a good application prospect in liquid biopsy. Combined detection of PGK1 and other tumor markers can significantly improve the sensitivity and specificity of diagnosis. For example, the high serum level of PGK1 is closely related with HCC early relapse and worse prognosis, and the serum PGK1 level is complementary to monitor AFP for improving the sensitivity and specificity of predicting HCC recurrence [70].
PGK1 is considered to be a drug resistance-related protein in cancer cells. High expression of PGK1 protein may promote drug resistance through complicate molecular mechanisms. Therefore, predicting sensitivity to chemotherapeutic drugs by detecting the expression of PGK1 before commencing chemotherapy will be beneficial to those patients who are unlikely to respond to drugs, and will protect patients from unnecessary drug toxicity. In addition, decreasing the expression of PGK1 will be a new strategy to overcome drug resistance in the future. The combination of PGK1 inhibitors and chemotherapeutic drugs may be effective in reversing drug resistance and improving therapeutic effect.
Unfortunately, due to the lack of promising lead compounds, the research on small molecule inhibitors of PGK1 is relatively weak. The small molecule inhibitors of PGK1 have some shortcomings, such as poor activity, low druggability and bad specificity, which bring great challenges to drug discovery and development. At present, PGK1-specific inhibitor is still not available [5]. Some antioxidants, acetyltransferase inhibitors and sirtuin activators have been shown to significantly reduce the enzyme activity of PGK1 to inhibit tumor growth [5,37]. However, the specificity of these inhibitors is controversial, mainly because PGK1 is not the only target of them. On the other hand, some competitive inhibitors of PGK1, such as bisphosphonates, terazosin and their derivatives can specifically inhibit PGK1 in vitro. Despite this, the effects of these PGK1 competitive inhibitors on cancer cells have not been reported. In the future, a large number of in vivo experiments are needed to study whether they have inhibitory effects on tumors and whether they have toxic and side effects on the body. At the same time, proper levels of PGK1 expression are essential for normal cell function, and PGK1 deficiency has been associated with multiple diseases [15-18]. It can be inferred that PGK1 inhibitors have some toxic and side effects on normal cells. Therefore, it is urgent to construct a targeted drug delivery system to allow PGK1 inhibitors targeting cancer cells.
Moreover, several somatic variants of PGK1 have been identified in cancer cells, but more detailed experiments are needed to explore the function of mutated PGK1 in cancer cells. Meanwhile, multiple PTMs of PGK1 are found to be able to regulate tumor metabolism and growth. The crosstalk between various PTMs of PGK1 in the development of cancer deserves a further study. In addition, how to design drugs according to the functions of PGK1 PTMs is also a difficult problem.
Overall, PGK1 has great value in the diagnosis, treatment and prognosis of various types of cancer (Figure 5). It is of great significance to develop therapeutic drugs targeting PGK1 in the future.
Figure 5.
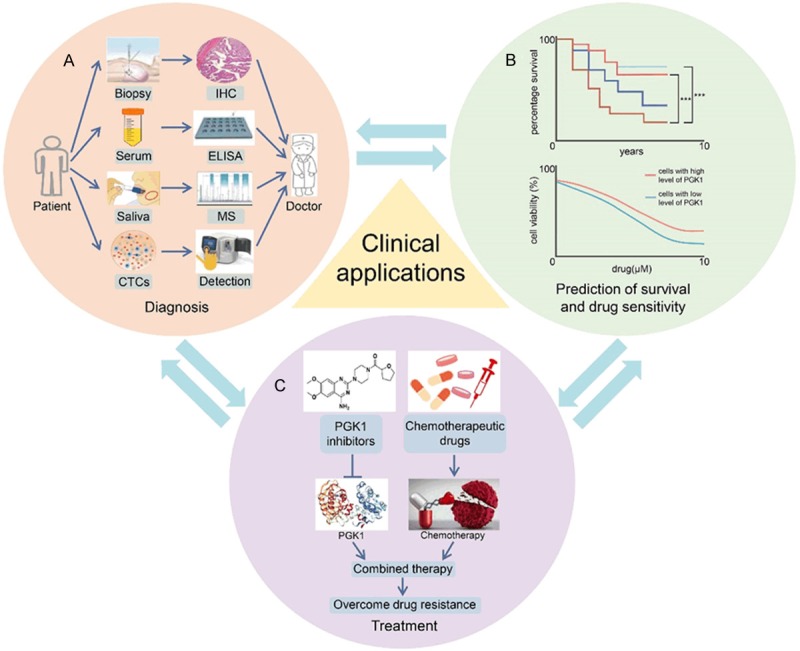
Potential clinical value of PGK1 for diagnosis, treatment and prognosis of cancers. A. Monitoring PGK1 in clinical samples such as tissue, serum, saliva and circulating tumor cells (CTCs) is helpful for doctors to diagnose and treat patients better. B. PGK1 level is related to patient survival and drug sensitivity. C. Combined therapy with PGK1 inhibitors and chemotherapeutic drugs in the treatment of cancer significantly overcome drug resistance.
Acknowledgements
This work was financially supported by the grants from National Natural Sciences Foundation of China (31961143005), the Health & Family Planning Commission of Sichuan Province (17ZD045) and Chengdu Science and Technology Program (2017-GH02-00062-HZ).
Disclosure of conflict of interest
None.
Abbreviations
- PGK
phosphoglycerate kinase
- PKM2
pyruvate kinase M2
- 1,3-BPG
1,3-bisphosphoglycerate
- 3-PG
3-phosphoglycerate
- PTM
post-translational modification
- HPA
human protein atlas database
- PCa
prostate cancer
- lncRNA
long noncoding RNA
- HCC
hepatocellular carcinoma
- NBD
nucleotide-binding domain
- CD
catalytic domain
- uPAR
urokinase-type plasminogen activator receptor
- GBC
gallbladder carcinoma
- HIF-1α
hypoxia inducible factor 1α
- EMT
epithelial-mesenchymal transition
- GBM
glioblastoma
- ROS
reactive oxygen species
- ccRCC
clear cell renal cell carcinoma
- CXCR4
C-X-C chemokine receptor 4
- CXCL12
C-X-C chemokine ligand 12
- CTCs
circulating tumor cells
- NSCLC
non-small cell lung cancer
- AFP
alpha-fetoprotein
- OSCC
oral squamous cell carcinoma
- ITF
invasive tumor front
- TOM
translocase of the outer mitochondrial membrane
- MDR1
multidrug resistance 1
- Dox
doxorubicin
- DNMTs
DNA methyltransferase
References
- 1.McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- 2.Chiarelli LR, Morera SM, Bianchi P, Fermo E, Zanella A, Galizzi A, Valentini G. Molecular insights on pathogenic effects of mutations causing phosphoglycerate kinase deficiency. PLoS One. 2012;7:e32065. doi: 10.1371/journal.pone.0032065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, McCarrey JR, Eddy EM, O’Brien DA. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod. 2010;82:136–145. doi: 10.1095/biolreprod.109.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowler MW. Conformational dynamics in phosphoglycerate kinase, an open and shut case? FEBS Lett. 2013;587:1878–1883. doi: 10.1016/j.febslet.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Zhu W, Qin J, Chen M, Gong L, Li L, Liu X, Tao Y, Yin H, Zhou H, Zhou L, Ye D, Ye Q, Gao D. Acetylation of PGK1 promotes liver cancer cell proliferation and tumorigenesis. Hepatology. 2017;65:515–528. doi: 10.1002/hep.28887. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Jiang B, Zhang T, Liu L, Wang Y, Wang Y, Chen X, Lin H, Zhou L, Xia Y, Chen L, Yang C, Xiong Y, Ye D, Guan KL. Insulin and mTOR pathway regulate HDAC3-mediated deacetylation and activation of PGK1. PLoS Biol. 2015;13:e1002243. doi: 10.1371/journal.pbio.1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorillo A, Petrosino M, Ilari A, Pasquo A, Cipollone A, Maggi M, Chiaraluce R, Consalvi V. The phosphoglycerate kinase 1 variants found in carcinoma cells display different catalytic activity and conformational stability compared to the native enzyme. PLoS One. 2018;13:e0199191. doi: 10.1371/journal.pone.0199191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valentini G, Maggi M, Pey AL. Protein stability, folding and misfolding in human PGK1 deficiency. Biomolecules. 2013;3:1030–1052. doi: 10.3390/biom3041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks RD, Blake CC, Evans PR, Haser R, Rice DW, Hardy GW, Merrett M, Phillips AW. Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme. Nature. 1979;279:773–777. doi: 10.1038/279773a0. [DOI] [PubMed] [Google Scholar]
- 10.Qian X, Li X, Cai Q, Zhang C, Yu Q, Jiang Y, Lee JH, Hawke D, Wang Y, Xia Y, Zheng Y, Jiang BH, Liu DX, Jiang T, Lu Z. Phosphoglycerate kinase 1 phosphorylates beclin1 to induce autophagy. Mol Cell. 2017;65:917–931. doi: 10.1016/j.molcel.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariosa AR, Klionsky DJ. A novel role for a glycolytic pathway kinase in regulating autophagy has implications in cancer therapy. Autophagy. 2017;13:1091–1092. doi: 10.1080/15548627.2017.1321723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian X, Li X, Lu Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy. 2017;13:1246–1247. doi: 10.1080/15548627.2017.1313945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Q, Wang S, Jin L, Weng M, Zhou D, Wang J, Tang Z, Quan Z. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18:82. doi: 10.1186/s12943-019-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popanda O, Fox G, Thielmann HW. Modulation of DNA polymerases alpha, delta and epsilon by lactate dehydrogenase and 3-phosphoglycerate kinase. Biochim Biophys Acta. 1998;1397:102–117. doi: 10.1016/s0167-4781(97)00229-7. [DOI] [PubMed] [Google Scholar]
- 15.Morales-Briceño H, Ha AD, London K, Farlow D, Chang FCF, Fung VSC. Parkinsonism in PGK1 deficiency implicates the glycolytic pathway in nigrostriatal dysfunction. Parkinsonism Relat Disord. 2019;64:319–323. doi: 10.1016/j.parkreldis.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Echaniz-Laguna A, Nadjar Y, Béhin A, Biancalana V, Piraud M, Malfatti E, Laforêt P. Phosphoglycerate kinase deficiency: a nationwide multicenter retrospective study. J Inherit Metab Dis. 2019;42:803–808. doi: 10.1002/jimd.12087. [DOI] [PubMed] [Google Scholar]
- 17.Hogrel JY, Ledoux I, Béhin A. Hyperammonaemia following exercise may also reveal PGK1 deficiency. J Clin Pathol. 2019;72:452. doi: 10.1136/jclinpath-2019-205750. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E. PGK deficiency. Br J Haematol. 2007;136:3–11. doi: 10.1111/j.1365-2141.2006.06351.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Yan X, Li X, Zheng Y, Li S, Chang X. PGK1, a glucose metabolism enzyme, may play an important role in rheumatoid arthritis. Inflamm Res. 2016;65:815–825. doi: 10.1007/s00011-016-0965-7. [DOI] [PubMed] [Google Scholar]
- 20.Daly EB, Wind T, Jiang XM, Sun L, Hogg PJ. Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim Biophys Acta. 2004;1691:17–22. doi: 10.1016/j.bbamcr.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Qiao L, Xie N, Shi Y, Liu N, Wang J. Expression and prognosis analyses of the Tob/BTG antiproliferative (APRO) protein family in human cancers. PLoS One. 2017;12:e0184902. doi: 10.1371/journal.pone.0184902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontén F, Schwenk JM, Asplund A, Edqvist PH. The human protein atlas as a proteomic resource for biomarker discovery. J Intern Med. 2011;270:428–446. doi: 10.1111/j.1365-2796.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 23.Lay AJ, Jiang XM, Kisker O, Flynn E, Underwood A, Condron R, Hogg PJ. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408:869–873. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- 24.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Wang J, Dai J, Jung Y, Wei CL, Wang Y, Havens AM, Hogg PJ, Keller ET, Pienta KJ, Nor JE, Wang CY, Taichman RS. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2007;67:149–159. doi: 10.1158/0008-5472.CAN-06-2971. [DOI] [PubMed] [Google Scholar]
- 26.Yuan SX, Yang F, Yang Y, Tao QF, Zhang J, Huang G, Yang Y, Wang RY, Yang S, Huo XS, Zhang L, Wang F, Sun SH, Zhou WP. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 27.Tang SJ, Ho MY, Cho HC, Lin YC, Sun GH, Chi KH, Wang YS, Jhou RS, Yang W, Sun KH. Phosphoglycerate kinase 1-overexpressing lung cancer cells reduce cyclooxygenase 2 expression and promote anti-tumor immunity in vivo . Int J Cancer. 2008;123:2840–2848. doi: 10.1002/ijc.23888. [DOI] [PubMed] [Google Scholar]
- 28.Ho MY, Tang SJ, Ng WV, Yang W, Leu SJ, Lin YC, Feng CK, Sung JS, Sun KH. Nucleotide-binding domain of phosphoglycerate kinase 1 reduces tumor growth by suppressing COX-2 expression. Cancer Sci. 2010;101:2411–2416. doi: 10.1111/j.1349-7006.2010.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy JP, Pinto DM. Targeted proteomic analysis of glycolysis in cancer cells. J Proteome Res. 2011;10:604–613. doi: 10.1021/pr100774f. [DOI] [PubMed] [Google Scholar]
- 30.Shetty S, Ganachari M, Liu MC, Azghani A, Muniyappa H, Idell S. Regulation of urokinase receptor expression by phosphoglycerate kinase is independent of its catalytic activity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L591–598. doi: 10.1152/ajplung.00319.2004. [DOI] [PubMed] [Google Scholar]
- 31.Shichijo S, Azuma K, Komatsu N, Ito M, Maeda Y, Ishihara Y, Itoh K. Two proliferation-related proteins, TYMS and PGK1, could be new cytotoxic T lymphocyte-directed tumor-associated antigens of HLA-A2+ colon cancer. Clin Cancer Res. 2004;10:5828–5836. doi: 10.1158/1078-0432.CCR-04-0350. [DOI] [PubMed] [Google Scholar]
- 32.Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP, Cheng Y, Cheng DQ, Weng WH, Wu XS, Fei XZ, Quan ZW, Li JY, Li SG, Liu YB. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009;281:71–81. doi: 10.1016/j.canlet.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, Gao J, Yang J, Cao Y, Jiang L, Li M, Zhang Y, Zhou J, Liu Y. Down-regulated phosphoglycerate kinase 1 expression is associated with poor prognosis in patients with gallbladder cancer. Medicine (Baltimore) 2015;94:e2244. doi: 10.1097/MD.0000000000002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohwer N, Zasada C, Kempa S, Cramer T. The growing complexity of HIF-1α’s role in tumorigenesis: DNA repair and beyond. Oncogene. 2013;32:3569–3576. doi: 10.1038/onc.2012.510. [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 36.Kress S, Stein A, Maurer P, Weber B, Reichert J, Buchmann A, Huppert P, Schwarz M. Expression of hypoxia-inducible genes in tumor cells. J Cancer Res Clin Oncol. 1998;124:315–320. doi: 10.1007/s004320050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang CH, Lee IA, Ha YR, Lim J, Sung MK, Lee SJ, Kim JS. PGK1 induction by a hydrogen peroxide treatment is suppressed by antioxidants in human colon carcinoma cells. Biosci Biotechnol Biochem. 2008;72:1799–1808. doi: 10.1271/bbb.80079. [DOI] [PubMed] [Google Scholar]
- 38.Ai J, Huang H, Lv X, Tang Z, Chen M, Chen T, Duan W, Sun H, Li Q, Tan R, Liu Y, Duan J, Yang Y, Wei Y, Li Y, Zhou Q. FLNA and PGK1 are two potential markers for progression in hepatocellular carcinoma. Cell Physiol Biochem. 2011;27:207–216. doi: 10.1159/000327946. [DOI] [PubMed] [Google Scholar]
- 39.Irshad K, Mohapatra SK, Srivastava C, Garg H, Mishra S, Dikshit B, Sarkar C, Gupta D, Chandra PS, Chattopadhyay P, Sinha S, Chosdol K. A combined gene signature of hypoxia and notch pathway in human glioblastoma and its prognostic relevance. PLoS One. 2015;10:e0118201. doi: 10.1371/journal.pone.0118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu D, He C, Wei J, Zhang Z, Luo Y, Tan H, Ren C. PGK1 is a potential survival biomarker and invasion promoter by regulating the HIF-1α-mediated epithelial-mesenchymal transition process in breast cancer. Cell Physiol Biochem. 2018;51:2434–2444. doi: 10.1159/000495900. [DOI] [PubMed] [Google Scholar]
- 41.Sun R, Meng X, Pu Y, Sun F, Man Z, Zhang J, Yin L, Pu Y. Overexpression of HIF-1a could partially protect K562 cells from 1,4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol In Vitro. 2019;55:18–23. doi: 10.1016/j.tiv.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859–862. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie H, Tong G, Zhang Y, Liang S, Tang K, Yang Q. PGK1 drives hepatocellular carcinoma metastasis by enhancing metabolic process. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Deng Y, Zhu J, Duan Y, Weng W, Wu X. Pim1 promotes cell proliferation and regulates glycolysis via interaction with MYC in ovarian cancer. Onco Targets Ther. 2018;11:6647–6656. doi: 10.2147/OTT.S180520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu D, Aka JA, Wang R, Lin SX. 17beta-hydroxysteroid dehydrogenase type 5 is negatively correlated to apoptosis inhibitor GRP78 and tumor-secreted protein PGK1, and modulates breast cancer cell viability and proliferation. J Steroid Biochem Mol Biol. 2017;171:270–280. doi: 10.1016/j.jsbmb.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Tang SW, Chang WH, Su YC, Chen YC, Lai YH, Wu PT, Hsu CI, Lin WC, Lai MK, Lin JY. MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett. 2009;273:35–43. doi: 10.1016/j.canlet.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 48.Walenkamp AME, Lapa C, Herrmann K, Wester HJ. CXCR4 ligands: the next big hit? J Nucl Med. 2017;58(Suppl 2):77S–82S. doi: 10.2967/jnumed.116.186874. [DOI] [PubMed] [Google Scholar]
- 49.Zieker D, Königsrainer I, Traub F, Nieselt K, Knapp B, Schillinger C, Stirnkorb C, Fend F, Northoff H, Kupka S, Brücher BL, Königsrainer A. PGK1 a potential marker for peritoneal dissemination in gastric cancer. Cell Physiol Biochem. 2008;21:429–436. doi: 10.1159/000129635. [DOI] [PubMed] [Google Scholar]
- 50.Zieker D, Königsrainer I, Tritschler I, Löffler M, Beckert S, Traub F, Nieselt K, Bühler S, Weller M, Gaedcke J, Taichman RS, Northoff H, Brücher BL, Königsrainer A. Phosphoglycerate kinase 1 a promoting enzyme for peritoneal dissemination in gastric cancer. Int J Cancer. 2010;126:1513–1520. doi: 10.1002/ijc.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zieker D, Königsrainer I, Weinreich J, Beckert S, Glatzle J, Nieselt K, Bühler S, Löffler M, Gaedcke J, Northoff H, Mannheim JG, Wiehr S, Pichler BJ, von Weyhern C, Brücher BL, Königsrainer A. Phosphoglycerate kinase 1 promoting tumor progression and metastasis in gastric cancer - detected in a tumor mouse model using positron emission tomography/magnetic resonance imaging. Cell Physiol Biochem. 2010;26:147–154. doi: 10.1159/000320545. [DOI] [PubMed] [Google Scholar]
- 52.Ameis HM, Drenckhan A, von Loga K, Escherich G, Wenke K, Izbicki JR, Reinshagen K, Gros SJ. PGK1 as predictor of CXCR4 expression, bone marrow metastases and survival in neuroblastoma. PLoS One. 2013;8:e83701. doi: 10.1371/journal.pone.0083701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang JB, Xiang YS, Liu LH, Wang XY. Phosphoglycerate kinase 1 gene knockdown suppresses the migration and invasion of glioma cells. Int J Clin Exp Pathol. 2016;9:9068–9076. [Google Scholar]
- 54.Jung Y, Shiozawa Y, Wang J, Wang J, Wang Z, Pedersen EA, Lee CH, Hall CL, Hogg PJ, Krebsbach PH, Keller ET, Taichman RS. Expression of PGK1 by prostate cancer cells induces bone formation. Mol Cancer Res. 2009;7:1595–1604. doi: 10.1158/1541-7786.MCR-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Cao S, Situ B, Zhong J, Hu Y, Li S, Huang J, Xu J, Wu S, Lin J, Zhao Q, Cai Z, Zheng L, Wang Q. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J Exp Clin Cancer Res. 2018;37:127. doi: 10.1186/s13046-018-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Ying G, Wang J, Jung Y, Lu J, Zhu J, Pienta KJ, Taichman RS. Characterization of phosphoglycerate kinase-1 expression of stromal cells derived from tumor microenvironment in prostate cancer progression. Cancer Res. 2010;70:471–480. doi: 10.1158/0008-5472.CAN-09-2863. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Chen Z, Zhuang W, Wang Z, Xiao W, Don W, Li X, Chen X. MicroRNA-450b-3p inhibits cell growth by targeting phosphoglycerate kinase 1 in hepatocellular carcinoma. J Cell Biochem. 2019;120:18805–18815. doi: 10.1002/jcb.29196. [DOI] [PubMed] [Google Scholar]
- 58.Ge J, Li J, Na S, Wang P, Zhao G, Zhang X. miR-548c-5p inhibits colorectal cancer cell proliferation by targeting PGK1. J Cell Physiol. 2019;234:18872–18878. doi: 10.1002/jcp.28525. [DOI] [PubMed] [Google Scholar]
- 59.Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, Li Y, You W, Dong Q, Hong T, Yan Z, Jin S, Wang T, Zhao W, Mai H, Huang J, Han X, Ji Q, Song Q, Yang C, Zhao S, Xu X, Ye Q. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33:368–385. e7. doi: 10.1016/j.ccell.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 60.De Mello RA, Aguiar PN, Tadokoro H, Farias-Vieira TM, Castelo-Branco P, de Lima Lopes G, Pozza DH. MetaLanc9 as a novel biomarker for non-small cell lung cancer: promising treatments via a PGK1-activated AKT/mTOR pathway. J Thorac Dis. 2018;10(Suppl 17):S2076–S2078. doi: 10.21037/jtd.2018.04.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu T, Zhao Y, Hu Z, Li J, Chu D, Zhang J, Li Z, Chen B, Zhang X, Pan H, Li S, Lin H, Liu L, Yan M, He X, Yao M. MetaLnc9 facilitates lung cancer metastasis via a PGK1-activated AKT/mTOR pathway. Cancer Res. 2017;77:5782–5794. doi: 10.1158/0008-5472.CAN-17-0671. [DOI] [PubMed] [Google Scholar]
- 62.Huang B, Cai W, Wang Q, Liu F, Xu M, Zhang Y. Gankyrin drives malignant transformation of gastric cancer and alleviates oxidative stress via mTORC1 activation. Oxid Med Cell Longev. 2018;2018:9480316. doi: 10.1155/2018/9480316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Cheng D, Zhu M, Yu H, Pan Z, Liu L, Geng Q, Pan H, Yan M, Yao M. OTUB2 stabilizes U2AF2 to promote the Warburg effect and tumorigenesis via the AKT/mTOR signaling pathway in non-small cell lung cancer. Theranostics. 2019;9:179–195. doi: 10.7150/thno.29545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong W, Li H, Wu X. Rab11-FIP2 suppressed tumor growth via regulation of PGK1 ubiquitination in non-small cell lung cancer. Biochem Biophys Res Commun. 2019;508:60–65. doi: 10.1016/j.bbrc.2018.11.108. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad SS, Glatzle J, Bajaeifer K, Bühler S, Lehmann T, Königsrainer I, Vollmer JP, Sipos B, Ahmad SS, Northoff H, Königsrainer A, Zieker D. Phosphoglycerate kinase 1 as a promoter of metastasis in colon cancer. Int J Oncol. 2013;43:586–590. doi: 10.3892/ijo.2013.1971. [DOI] [PubMed] [Google Scholar]
- 66.Yu W, Yang Z, Huang R, Min Z, Ye M. SIRT6 promotes the Warburg effect of papillary thyroid cancer cell BCPAP through reactive oxygen species. Onco Targets Ther. 2019;12:2861–2868. doi: 10.2147/OTT.S194256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang E, Wang J, Hong M, Zheng L, Tong Q. Valproic acid suppresses Warburg effect and tumor progression in neuroblastoma. Biochem Biophys Res Commun. 2019;508:9–16. doi: 10.1016/j.bbrc.2018.11.103. [DOI] [PubMed] [Google Scholar]
- 68.Shashni B, Sakharkar KR, Nagasaki Y, Sakharkar MK. Glycolytic enzymes PGK1 and PKM2 as novel transcriptional targets of PPARγ in breast cancer pathophysiology. J Drug Target. 2013;21:161–174. doi: 10.3109/1061186X.2012.736998. [DOI] [PubMed] [Google Scholar]
- 69.Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics. 2005;4:1686–1696. doi: 10.1074/mcp.M400221-MCP200. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Chen H, Wu X, Sun Y, Wang Y, Zeng Y, Chen G, Liu X, Xing X, Zhao B, Liu J. The serum proteomics tracking of hepatocellular carcinoma early recurrence following radical resection. Cancer Manag Res. 2019;11:2935–2946. doi: 10.2147/CMAR.S190561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang TL, Liang Y, Chien KY, Yu JS. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics. 2006;6:2259–2272. doi: 10.1002/pmic.200500345. [DOI] [PubMed] [Google Scholar]
- 72.Zhou Y, Cui J, Du H. Autoantibody-targeted TAAs in pancreatic cancer: a comprehensive analysis. Pancreatology. 2019;19:760–768. doi: 10.1016/j.pan.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Chen G, Gharib TG, Wang H, Huang CC, Kuick R, Thomas DG, Shedden KA, Misek DE, Taylor JM, Giordano TJ, Kardia SL, Iannettoni MD, Yee J, Hogg PJ, Orringer MB, Hanash SM, Beer DG. Protein profiles associated with survival in lung adenocarcinoma. Proc Natl Acad Sci U S A. 2003;100:13537–13542. doi: 10.1073/pnas.2233850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Townsend MH, Ence ZE, Felsted AM, Parker AC, Piccolo SR, Robison RA, O’Neill KL. Potential new biomarkers for endometrial cancer. Cancer Cell Int. 2019;19:19. doi: 10.1186/s12935-019-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo S, Xiao Y, Li D, Jiang Q, Zhu L, Lin D, Jiang H, Chen W, Wang L, Liu C, Fang W, Lin L. PGK1 and GRP78 overexpression correlates with clinical significance and poor prognosis in Chinese endometrial cancer patients. Oncotarget. 2017;9:680–690. doi: 10.18632/oncotarget.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carnielli CM, Macedo CCS, De Rossi T, Granato DC, Rivera C, Domingues RR, Pauletti BA, Yokoo S, Heberle H, Busso-Lopes AF, Cervigne NK, Sawazaki-Calone I, Meirelles GV, Marchi FA, Telles GP, Minghim R, Ribeiro ACP, Brandão TB, de Castro G Jr, González-Arriagada WA, Gomes A, Penteado F, Santos-Silva AR, Lopes MA, Rodrigues PC, Sundquist E, Salo T, da Silva SD, Alaoui-Jamali MA, Graner E, Fox JW, Coletta RD, Paes Leme AF. Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat Commun. 2018;9:3598. doi: 10.1038/s41467-018-05696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, Stefancsik R, Harsha B, Kok CY, Jia M, Jubb H, Sondka Z, Thompson S, De T, Campbell PJ. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45:D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, He J, Hunter T, Wang L, Lu Z. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–719. doi: 10.1016/j.molcel.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X, Zheng Y, Lu Z. PGK1 is a new member of the protein kinome. Cell Cycle. 2016;15:1803–1804. doi: 10.1080/15384101.2016.1179037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Yu G, Chu H, Wang X, Xiong L, Cai G, Liu R, Gao H, Tao B, Li W, Li G, Liang J, Yang W. Macrophage-associated PGK1 phosphorylation promotes aerobic glycolysis and tumorigenesis. Mol Cell. 2018;71:201–215. doi: 10.1016/j.molcel.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Zhang N, Gao R, Yang J, Zhu Y, Zhang Z, Xu X, Wang J, Liu X, Li Z, Li Z, Gong D, Li J, Bi J, Kong C. Quantitative global proteome and lysine succinylome analyses reveal the effects of energy metabolism in renal cell carcinoma. Proteomics. 2018;18:e1800001. doi: 10.1002/pmic.201800001. [DOI] [PubMed] [Google Scholar]
- 83.Yan H, Yang K, Xiao H, Zou YJ, Zhang WB, Liu HY. Over-expression of cofilin-1 and phosphoglycerate kinase 1 in astrocytomas involved in pathogenesis of radioresistance. CNS Neurosci Ther. 2012;18:729–736. doi: 10.1111/j.1755-5949.2012.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lincet H, Guével B, Pineau C, Allouche S, Lemoisson E, Poulain L, Gauduchon P. Comparative 2D-DIGE proteomic analysis of ovarian carcinoma cells: toward a reorientation of biosynthesis pathways associated with acquired platinum resistance. J Proteomics. 2012;75:1157–1169. doi: 10.1016/j.jprot.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 85.Schneider CC, Archid R, Fischer N, Bühler S, Venturelli S, Berger A, Burkard M, Kirschniak A, Bachmann R, Königsrainer A, Glatzle J, Zieker D. Metabolic alteration-overcoming therapy resistance in gastric cancer via PGK-1 inhibition in a combined therapy with standard chemotherapeutics. Int J Surg. 2015;22:92–98. doi: 10.1016/j.ijsu.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 86.Sun S, Liang X, Zhang X, Liu T, Shi Q, Song Y, Jiang Y, Wu H, Jiang Y, Lu X, Pang D. Phosphoglycerate kinase-1 is a predictor of poor survival and a novel prognostic biomarker of chemoresistance to paclitaxel treatment in breast cancer. Br J Cancer. 2015;112:1332–1339. doi: 10.1038/bjc.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, Remião F. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1–123. doi: 10.1016/j.pharmthera.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Duan Z, Lamendola DE, Yusuf RZ, Penson RT, Preffer FI, Seiden MV. Overexpression of human phosphoglycerate kinase 1 (PGK1) induces a multidrug resistance phenotype. Anticancer Res. 2002;22:1933–1941. [PubMed] [Google Scholar]
- 89.Huang Z, Zhou L, Chen Z, Nice EC, Huang C. Stress management by autophagy: implications for chemoresistance. Int J Cancer. 2016;139:23–32. doi: 10.1002/ijc.29990. [DOI] [PubMed] [Google Scholar]
- 90.Si X, Liu Y, Lv J, Ding H, Zhang XA, Shao L, Yang N, Cheng H, Sun L, Zhu D, Yang Y, Li A, Han X, Sun Y. ERα propelled aberrant global DNA hypermethylation by activating the DNMT1 gene to enhance anticancer drug resistance in human breast cancer cells. Oncotarget. 2016;7:20966–20980. doi: 10.18632/oncotarget.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanai Y, Hirohashi S. Alterations of DNA methylation associated with abnormalities of DNA methyltransferases in human cancers during transition from a precancerous to a malignant state. Carcinogenesis. 2007;28:2434–2442. doi: 10.1093/carcin/bgm206. [DOI] [PubMed] [Google Scholar]
- 92.Zhou JW, Tang JJ, Sun W, Wang H. PGK1 facilities cisplatin chemoresistance by triggering HSP90/ERK pathway mediated DNA repair and methylation in endometrial endometrioid adenocarcinoma. Mol Med. 2019;25:11. doi: 10.1186/s10020-019-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Y, Zhai L, Ma S, Zhang C, Zhao L, Li N, Xu Y, Zhang T, Guo Z, Zhang H, Xu P, Zhao X. Down-regulation of RIP3 potentiates cisplatin chemoresistance by triggering HSP90-ERK pathway mediated DNA repair in esophageal squamous cell carcinoma. Cancer Lett. 2018;418:97–108. doi: 10.1016/j.canlet.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 94.Chen X, Zhao C, Li X, Wang T, Li Y, Cao C, Ding Y, Dong M, Finci L, Wang JH, Li X, Liu L. Terazosin activates Pgk1 and Hsp90 to promote stress resistance. Nat Chem Biol. 2015;11:19–25. doi: 10.1038/nchembio.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Machida K. Pluripotency transcription factors and metabolic reprogramming of mitochondria in tumor-initiating stem-like cells. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caplan NA, Pogson CI, Hayes DJ, Blackburn GM. Novel bisphosphonate inhibitors of phosphoglycerate kinase. Bioorg Med Chem Lett. 1998;8:515–520. doi: 10.1016/s0960-894x(98)00059-6. [DOI] [PubMed] [Google Scholar]
- 97.Kotsikorou E, Sahota G, Oldfield E. Bisphosphonate inhibition of phosphoglycerate kinase: quantitative structure-activity relationship and pharmacophore modeling investigation. J Med Chem. 2006;49:6692–6703. doi: 10.1021/jm0604833. [DOI] [PubMed] [Google Scholar]
- 98.Bollong MJ, Lee G, Coukos JS, Yun H, Zambaldo C, Chang JW, Chin EN, Ahmad I, Chatterjee AK, Lairson LL, Schultz PG, Moellering RE. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature. 2018;562:600–604. doi: 10.1038/s41586-018-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia J, Feng B, Shao Q, Yuan Y, Wang XS, Chen N, Wu S. Virtual screening against phosphoglycerate kinase 1 in quest of novel apoptosis inhibitors. Molecules. 2017;22 doi: 10.3390/molecules22061029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ledford H. Metabolic quirks yield tumour hope. Nature. 2014;508:158–159. doi: 10.1038/508158a. [DOI] [PubMed] [Google Scholar]