Abstract
Lung cancer is the leading cause of cancer-related deaths worldwide, but effective therapeutics is limited. This study aims to identify novel anticancer strategy from a Food and Drug Administration (FDA)-approved drug library consisting of 528 compounds. Benzethonium Chloride (BZN), a FDA-approved drug for anti-infective, was found to markedly induce apoptosis and inhibit proliferation and colony formation ability of lung cancer cells in dose- and time-dependent manners. BZN also enhanced the sensitivity of lung cancer cells to gefitinib, the first-line treatment strategy for selected lung cancer patients. Furthermore, BZN significantly delayed the growth of tumor xenografts in nude mice by increasing apoptosis and decreasing Ki-67 proliferation index, without obvious toxic effects to the vital organs of animals. Mechanistically, quantitative proteomics coupled with bioinformatics analyses and a series of functional assays demonstrated that BZN induced cell cycle arrest at G1 phase, and this was associated with an increase in p38-mediated phosphorylation at threonine 286 (T286) and accelerated degradation of cyclin D1. Our findings provide the first evidence that BZN could be a promising therapeutic agent in lung cancer treatment.
Keywords: Lung cancer, benzethonium chloride, cell cycle arrest, cyclin D1 degradation, gefitinib sensitivity, drug repurposing
Introduction
The incidence of lung cancer has dramatically increased over the last few decades and more than 2 million new cases are estimated to be diagnosed in 2018 worldwide [1,2]. Non-small cell lung cancer (NSCLC) accounts for 85% of all cases of lung cancer and about 10% to 15% are small cell lung cancer (SCLC) [3]. Patients with lung cancer often present with advanced disease at initial diagnosis [4,5]. At present, surgery remains the primary treatment option for lung cancer, and chemotherapy and radiotherapy are also used as adjuvant therapy to improve the treatment outcome. However, resistance of tumors to chemotherapy often occurs and many patients still relapse with more aggressive tumor phenotypes. Limited therapeutic options, chemoresistance and rapid metastasis lead to poor prognosis in lung cancer patients [6]. Therefore, development of novel treatment strategies is urgently needed for this lethal disease.
To find new uses for existing drugs outside the scope of the original indication, also known as drug repositioning, is an efficient method for obtaining more therapeutic drugs. A recent study reported that disulfiram, an old alcohol-aversion drug, can bind and immobilize NPL4 and trigger a heat-shock response, induce a complex cellular phenotype leading to cell death [7]. In present study, a drug library consisting of FDA-approved 528 small inhibitors was used to screen the compounds with anticancer property. We found that Benzethonium chloride (BZN), a quaternary ammonium salt, exerted a significant suppressive effect on tumorigenesis of lung cancer cells. BZN, a well-known surface antifungal and antibacterial compound, has been approved by FDA for use in various anti-infective products and inhibits the response of acetylcholine in neuronal nicotinic acetylcholine receptors in a competitive and non-competitive manner [8]. However, the effect of BZN in cancer remains unclear and in particular, its anticancer function has not been reported in lung cancer. Here, in vitro and in vivo experiments were carried out to study whether BZN could be a new treatment option for lung cancer.
Quantitative proteomics provides a powerful tool to uncover the action mechanisms of anticancer compounds including BZN [9-11], which remains to be elucidated. Here, Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC)-mass spectrometry (MS) coupled bioinformatics suggested that BZN may inhibit lung cancer cell proliferation through causing dysregulated cell cycle control. Cell cycle dysregulation is a common characteristic of human cancers that leads to uncontrolled cell proliferation and tumorigenesis. Cyclin D1 is a critical regulator of G1 to S phase transition, and plays an important role in development and progression of cancer [12]. Overexpression of cyclin D1 is frequently observed in various cancers [13], and deregulated protein degradation of cyclin D1 is one of the main reasons being responsible for the increased levels of cyclin D1 in cancer cells [14,15]. Therefore, enhancement of cyclin D1 degradation may offer a useful strategy for therapeutic intervention [16]. The mechanism involved in the regulation of cyclin D1 stability has been well studied that phosphorylation of two key sites, threonine residue 286 (T286) and threonine residue 288 (T288), participate in cyclin D1 degradation. Cyclin D1 can be phosphorylated at T286 for its protein degradation by p38 and extracellular regulated protein kinase 2 (ERK2) [16,17], and dual specificity tyrosine-phosphorylation-regulated kinase 1B (mirk/Dyrk1b) phosphorylate cyclin D1 at T288 [18,19]. In this study, we investigated whether BZN affects cell cycle progression through p38-mediated regulation of cyclin D1 protein phosphorylation and degradation in lung cancer cells.
Materials and methods
Cell lines and drugs
The human lung cancer cell lines A549 and H1299 (ATCC, Rockville, MD, USA) were cultured in DMEM medium (Life Technologies, Gaithersburg, MD, USA) with 10% fetal bovine serum (FBS; Life Technologies) at 37°C in 5% CO2. Benzethonium chloride, cycloheximide and MG132 obtained from Selleck Chemicals (Huston, TX, USA), and Gefitinib (Cayman Chemicals, Ann Arbor, MI, USA) were dissolved in dimethyl sulfoxide (DMSO).
WST-1 assay
Cell viability was measured using WST-1 assay (Beyotime, Jiangsu, China) as described previously [20]. Cells were seeded in 96-well plates and treated with indicated drugs at various concentrations for different time point. WST-1 was added and incubated at 37°C for 2 h, and then absorbance was read on an automated microplate spectrophotometer (BioTek Instruments, Vermont, USA) at 450 nm.
Colony formation assay
Colony formation assay was performed as described previously [21]. Cells were seeded in 6-well plates and cultured with BZN (up to 20 μM) for 14 days. After washing and fixation, the cells were stained with 1% crystal violet for 5 min. All statistical data were acquired from three independent experiments.
Annexin V-FITC/PI staining assay
Cells were treated with BZN (up to 20 μM) for 48 h, and the cell apoptosis was measured by using Annexin V-FITC/PI Apoptosis Detection Kit (KeyGen, Jiangsu, USA) [22]. Cells were suspended in binding buffer, stained with Annexin V-FITC and PI for 15 min at room temperature in dark. Apoptotic cells were analyzed using a BD Accuri C6 Analyzer (BD Biosciences, San Diego, CA, USA).
Flow cytometric cell cycle analysis
Cells were treated with BZN (up to 20 μM) for 48 h and the cell cycle was determined as described previously [23]. In brief, the cells were fixed and stained with propidium iodide (PI) staining buffer for 10 min at room temperature in dark. Cell cycle distribution was measured using a BD Accuri C6 Analyzer.
Western blot and immunoprecipitation
The whole cell lysates were prepared in lysis buffer (Cell Signaling Technology, Beverly, MA, USA). The BCA kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the protein concentration. The samples were loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and subsequently electrotransferred to a PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 5% nonfat milk for 1 h, the membrane was incubated with primary antibodies for 2 h at room temperature and washed three times for 10 min each with 1x Tris Buffered Saline with Tween (TBST). And then the membrane was incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. The reaction was visualized using Clarity Western ECL substrate (Bio-Rad, Hercules, CA, USA) and detected by exposure to autoradiographic film [24]. For immunoprecipitation assay, the detailed experimental procedure was described previously [25].
SILAC labeling and LC-MS/MS analysis
SILAC labeling was described previously [21,26]. The ‘light’ labeled A549 cells were treated with 10 μM BZN for 48 h, and the ‘heavy’ labeled A549 cells were treated with DMSO. A total of 500 μg ‘heavy’ and 500 μg ‘light’ proteins were mixed together, and protein digestion and MS analysis were performed as previously described [20]. Peptides were analyzed in Obitrap Fusion Lumos Tribird Mass Specutrometer (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Tumorigenicity in nude mice
Female BALB/c nude mice aged 6-8 weeks were maintained under standard conditions and cared for according to the institutional guidelines for animal care. All the animal experiments were approved by the Ethics Committee for Animal Experiments of Jinan University. The study was performed in accordance with the Declaration of Helsinki. A549 cells in equal volumes of PBS and Matrigel were subcutaneously injected into the flanks of mice to establish tumor xenografts [27]. When the tumor xenografts reached 5 mm in diameter, the mice were randomly divided into treatment and control groups. The treatment groups received oral gavage of BZN (10 mg/kg) or intraperitoneal injection of BZN (5 mg/kg) every two days, whereas the control group received vehicle only. The body weight and tumor size was measured every two days, and the tumor volume was calculated using the following equation: V = (length × width2)/2. At the end of the study, tumors, lungs, livers, and kidneys were collected [28]. Proliferative index was determined by immunohistochemistry with the use of Ki-67 antibody (Dako Diagnostics, Mississauga, ON, USA) [29].
Statistical analysis
All in vitro experiments were performed in triplicate on three independent experiments. All values were expressed as the means ± SD, and compared using Student’s t-test. P < 0.05 was considered statistically significant.
Results
Identification of potential anticancer drug with a FDA-approved drug library
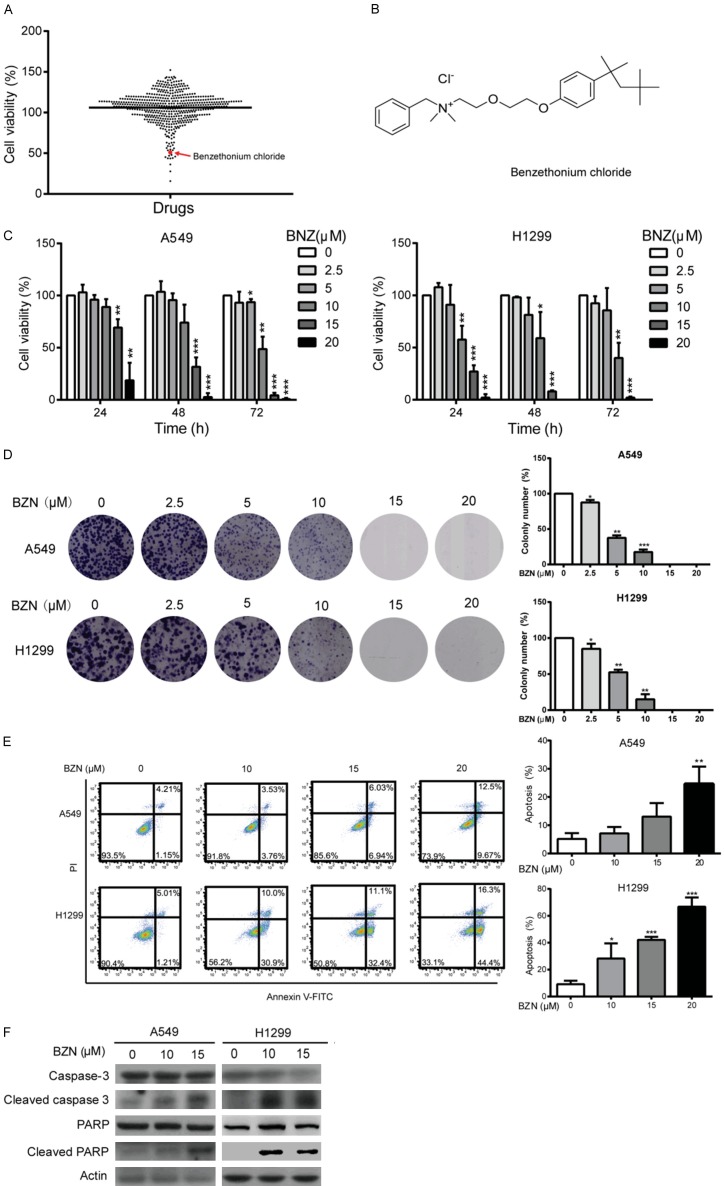
To screen novel anticancer compounds among the conventional drugs which have been used for other diseases, we took advantage of a compound library comprising of 528 FDA-approved drugs. A549 cells were treated with the 528 compounds or DMSO control for 48 h, and the cell viability was monitored to determine the effect of various drugs on NSCLC cell proliferation (Figure 1A). As displayed in Figure 1A, a total of 11 drugs exerted an obviously inhibitory effect (> 50%) on cancer cell growth (Table S1), including Epirubicin HCl and Fludarabine, the commonly used drugs for cancer treatment in clinic [30-33], suggesting that our screening strategy could be used to identify novel compounds with anticancer effects. Among the 11 candidates, BZN (Figure 1B), a well-known surface antifungal and antibacterial compound [8], which has not been linked to lung cancer treatment, attracted our interest for further study.
Figure 1.
Benzethonium chloride (BZN) inhibites lung cancer cell proliferation. A. A549 cells were treated with the 528 FDA-approved drugs separately, and cell viability was determined by WST-1 assay. B. BZN structure diagram. C. WST-1 assay was performed to detect cell viability in the A549 and H1299 cells treated with different concentrations of BZN for up to 72 h. D. The A549 and H1299 cells treated with BZN (up to 20 μM) were compared for colony formation ability. E. A549 and H1299 were treated with BZN (up to 20 μM) for 48 h, and apoptotic cells were detected by flow cytometry. Note that BZN significantly increased the percentage of apoptotic cells in a dose-dependent manner. F. Western blotting was performed to compare expression levels of caspase-3, cleaved caspase-3, PARP and cleaved PARP. Bars, SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared with DMSO-treated cells.
Benzethonium chloride inhibits lung cancer cell proliferation
To study the anti-proliferation effect of BZN on lung cancer cells, A549 and H1299 cells were treated with various concentrations of BZN for up to 72 h. As shown in Figure 1C, BZN markedly suppressed the cell viability in dose- and time-dependent manners. Moreover, we conducted colony formation assay to further determine the effects of BZN on the growth of lung cancer cells. The results showed that BZN significantly reduced colony formation ability of A549 and H1299 cells (Figure 1D), indicating that BZN has potently inhibitory effect on lung cancer cell proliferation.
Benzethonium chloride triggers apoptosis in lung cancer cells
We next used Annexin V-FITC/PI double staining assay to detect whether BZN has effect on apoptosis in lung cancer cells. The flow cytometry data showed that the A549 and H1299 cells treated with BZN exerted an increase in percentage of apoptotic cells in a dose-dependent manner (Figure 1E). Consistently, increased expressions of cleaved caspase-3 and cleaved PARP, the active form of caspase-3 and PARP, were observed upon BZN treatment in A549 and H1299 cells (Figure 1F), confirming that BZN can induce apoptosis to inhibit cell growth in lung cancer cells.
Quantitative proteomics suggests the involvement of cell cycle control in action mechanisms of benzethonium chloride in cancer cells
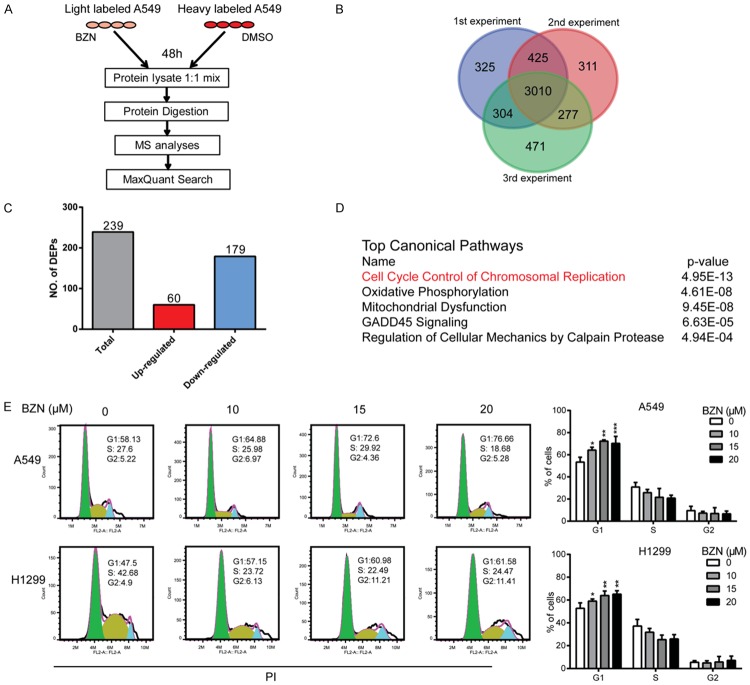
To gain insights into the mechanism in anticancer effect of BZN, SILAC-based quantitative proteomics was used to identify the differentially expressed proteins in the BZN-treated lung cancer cells. “Light”-labeled and “Heavy”-labeled A549 cells were treated with 10 μM BZN and DMSO for 48 h, respectively, and then lysated and mixed for mass spectrometry (MS) analysis (Figure 2A). Here, a total of 5123 proteins were identified and quantified, among which 3010 proteins were all detected in the triplicate samples (Figure 2B). A total of 239 proteins were found to be markedly regulated by BZN (fold change ≥ 1.5 and P-value ≤ 0.05) (Table S2), including 60 upregulated and 179 downregulated proteins, respectively (Figure 2C), which were uploaded to Ingenuity pathway analysis (IPA) for functional characterization of the signaling that the 239 proteins may participate in [34]. The results suggested that cell cycle control ranks first among the predicted top five canonical pathways, with a p value of 4.95 × 10-13 (Figure 2D). To confirm the prediction, cell cycle distribution was determined in the BZN-treated A549 and H1299 by flow cytometry, and the data showed that BZN induced a significant cell cycle arrest at G1 phase (Figure 2E). Collectively, these data indicated that BZN may affect the process of G1 to S transition to inhibit lung cancer tumorigenesis.
Figure 2.
Quantitative proteomics suggests the involvement of cell cycle control in action mechanisms of benzethonium chloride in cancer cells. A. Experimental process of the identification of BZN-regulated proteins by SILAC-based quantitative proteomics experiment. B. Venn diagram showing the identification of overlapped proteins in three biological replicates. C. Differentially expressed proteins with fold change ≥ 1.5 and P ≤ 0.05 in the BZN-treated A549 cells. D. The top five canonical pathways from the IPA analysis of the differentially expressed proteins in BZN-treated cells. E. A549 and H1299 cells were treated with BZN (up to 20 μM) for 48 h, and cell cycle distribution was analyzed by flow cytometry. Note that BZN arrested cells at G1 phase. Bars, SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared with DMSO-treated cells.
Benzethonium chloride accelerates protein degradation of cyclin D1 through ubiquitin-proteasome pathway
To further dissect the molecular mechanisms how BZN induces G1 cell cycle arrest, expression levels of several key regulators of G1 cell cycle were examined by Western blot. As shown in Figure 3A, decreased expressions of cyclin D1, cyclin E1, CDK4 and CDK6 were observed. Cyclin D1 is the most sensitive and important cyclin protein in G1 phase, so we focused on the regulation of cyclin D1 by BZN [14,19].
Figure 3.
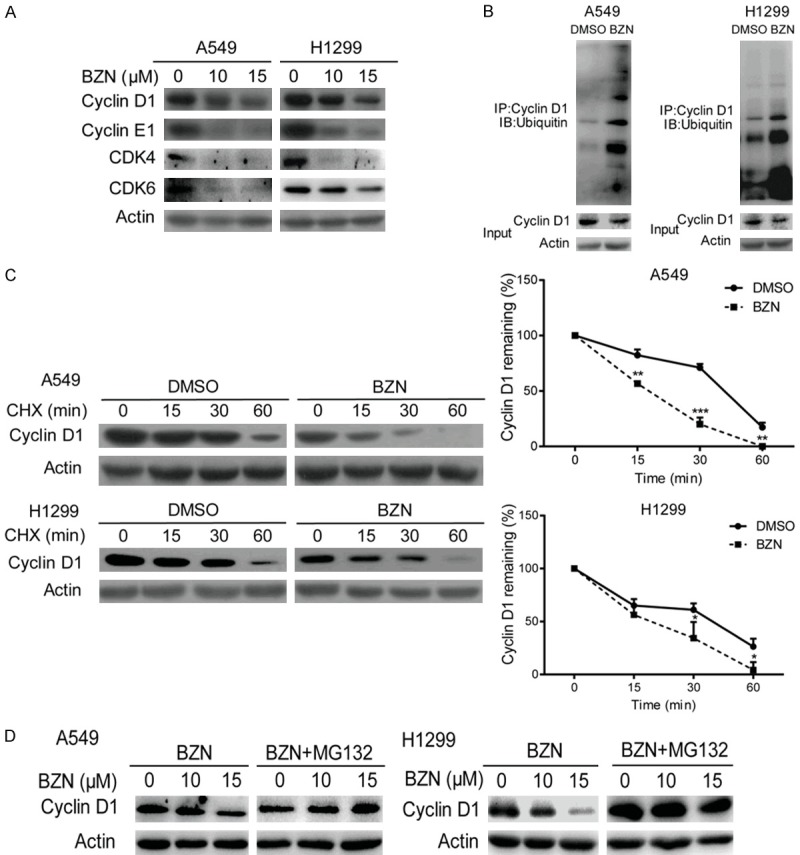
Benzethonium chloride accelerates protein degradation of cyclin D1 through ubiquitin-proteasome pathway. A. Expression levels of the G1 phase specific cyclin and CDK proteins were compared in BZN-treated A549 and H1299 cells by western blot. B. Immunoprecipitation assay was performed in the BZN-treated lung cancer cells, and ubiquitin expression was examined in the cyclin D1 immunoprecipitates. C. After pretreatment with cycloheximide (CHX; 50 μg/mL) for 12 h, A549 and H1299 cells were exposed to 10 μM BZN, and the cell lysates were collected at the indicated time points and compared for cyclin D1 expression using Western blotting. Cyclin D1 signals were quantified by densitometry, and the degradation rate was shown as the ratio of cyclin D1 level at each time point to the respective original level (0 min). D. MG132 could rescue the inhibitory effect of BZN on cyclin D1 expression. Bars, SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared with DMSO-treated cells.
We next investigated whether BZN could affect protein degradation of cyclin D1. The interaction of cyclin D1 and ubiquitin was compared in the lung cancer cells with or without BZN treatment by immunoprecipitation, and a significant increase in the binding of ubiquitin to cyclin D1 was observed in both A549 and H1299 cells (Figure 3B), suggesting enhanced protein degradation of cyclin D1 upon BZN treatment. Moreover, following a 12 h pretreatment with cycloheximide (CHX), which could inhibit protein synthesis, the cells were exposed to 10 μM BZN. The cell lysates were collected at the indicated time points and compared for cyclin D1 expression using Western blotting. As shown in Figure 3C, cyclin D1 protein degradation was accelerated in the BZN-treated lung cancer cells compared with the control cells, suggesting that exposure of cancer cells to BZN leads to degradation of BZN protein. Furthermore, the inhibitory effect of BZN on cyclin D1 expression could be rescued by a proteasome inhibitor MG132 (Figure 3D), which strongly supports that BZN induces G1 cell cycle arrest through promoting ubiquitin-mediated proteasomal degradation of cyclin D1.
Benzethonium chloride activates p38 signaling to phosphorylate cyclin D1 at T286
The threonine residue 286 and 288 of cyclin D1 are the key points about the stability. p38 and ERK2 have also been known to regulate cyclin D1 stability by phosphorylating T286, and the phosphorylation of T288 was regulated by mirk/Dyrk1b [16,17]. Therefore, we examined the phosphorylation of T286 and T288 residue after BZN treatment, and the data showed that the phosphorylation of T286 was increased but there was no change in T288 phosphorylation (Figure 4A). Since phosphorylation of p38, but not ERK2, was significantly regulated in the BZN-treated lung cancer cells (Figure 4B), these observations suggested that BZN could increase ubiquitination degradation of cyclin D1 through activated p38 to phosphorylation T286, thereby inhibiting the growth of lung cancer cells (Figure 4C).
Figure 4.
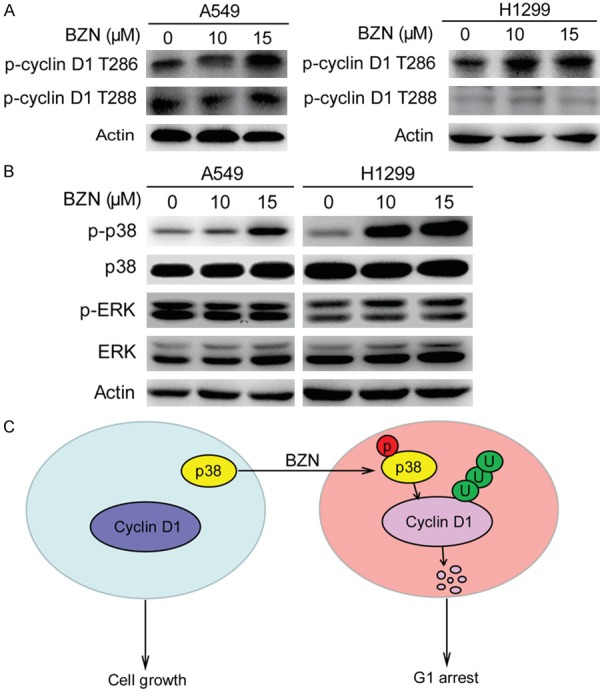
Benzethonium chloride activates p38 signaling to phosphorylate cyclin D1 at T286. A. Comparison of p-cyclin D1 T286 and p-cyclin D1 T288 expression in the BZN-treated A549 and H1299 cells. B. BZN markedly increased p-p38, but did not change p-ERK. C. Schematic diagram summarizing the mechanisms how BZN inhibits lung cancer cell proliferation.
Benzethonium chloride sensitizes lung cancer cells to gefitinib
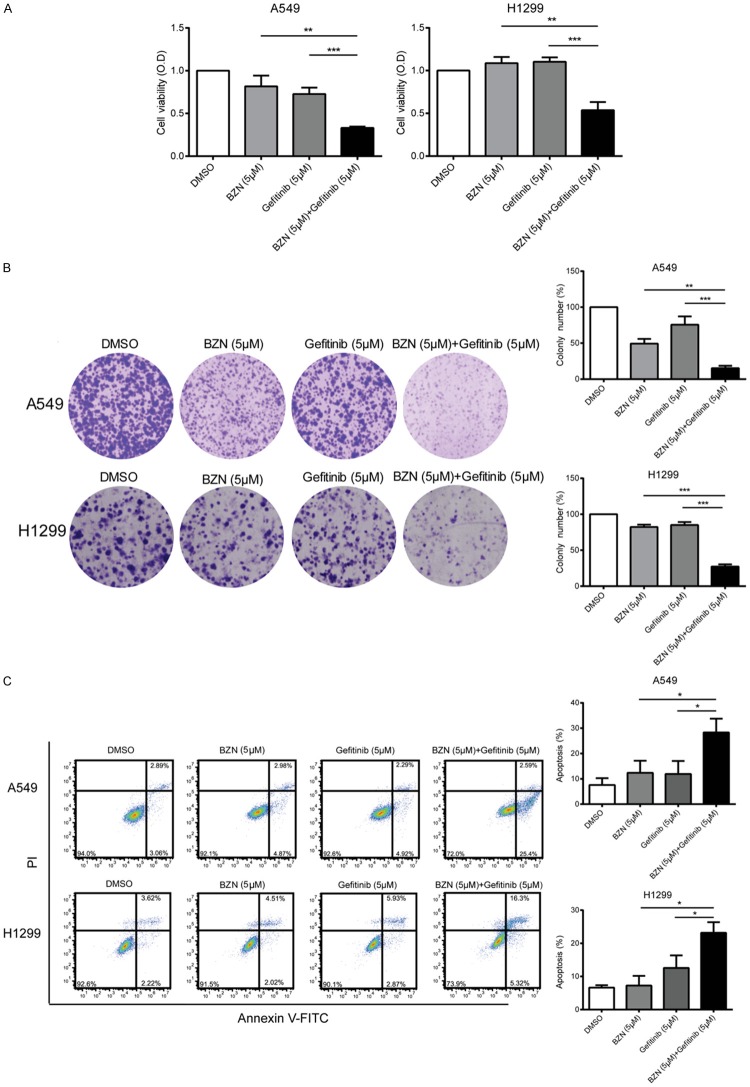
Given that BZN induces G1 cell cycle arrest, we postulated that it could be used as a drug sensitizer in cancer treatment. Gefitinib is a famous standard-of-care treatment in NSCLC patients with EGFR mutations [35,36]. Unfortunately, most patients inevitably recur due to drug resistance [35,37,38]. Here, we detected whether combing BZN with gefitinib could have a synergistic effect to improve treatment efficacy. As shown in Figure 5A, although low dose of BZN or gefitinib alone has no or modest effect on cell viability in lung cancer cells, a combination of BZN and gefitinib exerted stronger inhibitory effect on lung cancer cell proliferation, whereas the same conclusion was obtained in colony formation assay (Figure 5B). Moreover, the combination of low dose BZN and gefitinib could significantly induce apoptosis in lung cancer cells, compared with the cells treated with BZN or gefitinib alone (Figure 5C). Together, BZN can enhance the sensitivity of lung cancer cells to gefitinib.
Figure 5.
Benzethonium chloride sensitizes lung cancer cells to gefitinib. A. A549 and H1299 were treated with DMSO, BZN (5 μM), Gefitinib (5 μM) or combination of BZN and gefitinib, respectively, for 72 h, and cell viability was determined by WST-1 assay. B. Colony formation assay showed that BZN significantly enhanced the sensitivity of lung cancer cells to gefitinib. C. The percentage of apoptotic cells was detected in the cells treated with DMSO, BZN, gefitinib or the combination. Bars, SD; *P < 0.05; **P < 0.01; ***P < 0.001 compared with gefitinib-treated cells.
Benzethonium chloride suppresses the tumor growth in vivo
We next evaluated the therapeutic potential of BZN in nude mice. BZN was intraperitoneally injected or orally administrated to the nude mice bearing subcutaneous tumor xenografts, and tumor volume and body weight of mice were monitored. The results showed that the tumor growth was markedly suppressed by BZN treatment (Figure 6A and 6B). The Ki-67 proliferation index in the tumor xenografts was determined, and the results revealed that BZN inhibited tumor growth via decreased cell proliferation (Figure 6C). As shown in Figure 6D, western blot analysis indicated that the expression of p-p38, p-Cyclin D1 and cleaved caspase-3 were up-regulated in the tumor xenograft treated with BZN (Figure 6D), which was consistent with in vitro experiments. In addition, the weight of mice was no significant change between the treatment and control groups (Figure 6E). Histological examination of vital organs, including lungs, livers and kidneys, did not reveal any overt changes in morphology (Figure 6F). These data suggested that BZN could inhibit the tumor growth and with low toxicity in vivo.
Figure 6.
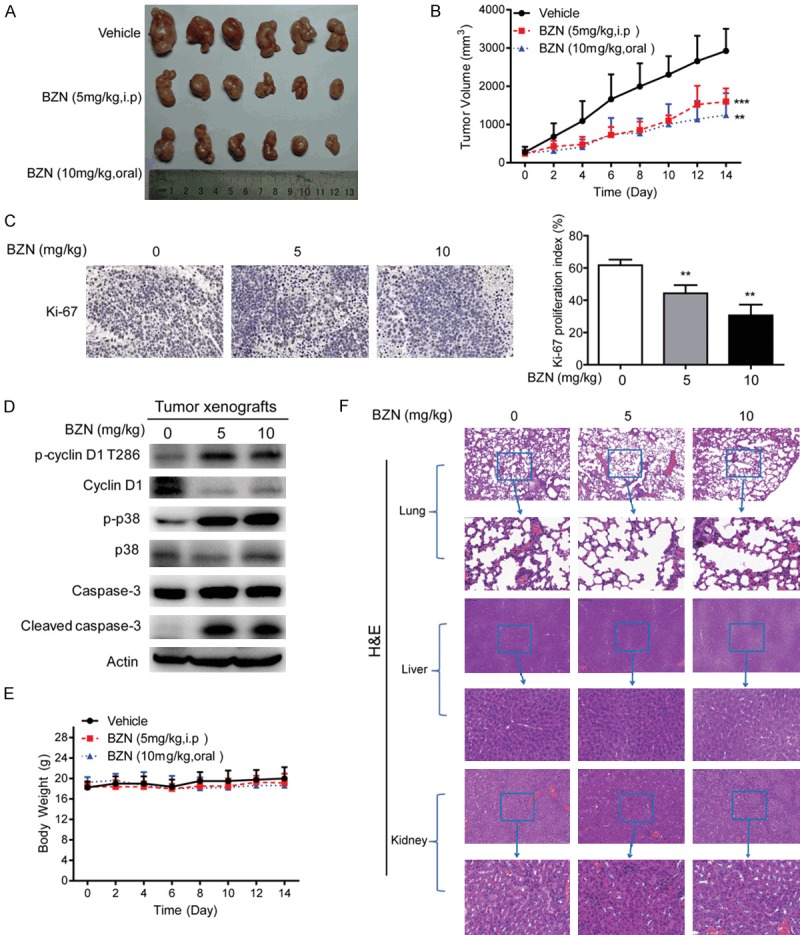
Benzethonium chloride suppresses lung cancer tumorigenesis in vivo. A549 cells were subcutaneously injected into nude mice to establish tumor xenograft. When tumor reached ~0.5 cm diameter, the mice were randomized into three groups to receive oral gavage of BZN (10 mg/kg), intraperitoneal injection of BZN (5 mg/kg) or vehicle control every two days, respectively. A. Image of tumors in control group and BZN treatment groups. B. Tumor curves showing the suppressive effect of BZN on growth of A549-derived tumor xenografts (n = 6). C. Immunohistochemical analysis of Ki-67 proliferation index in tumor xenografts treated with BZN and vehicle. D. Western blot was performed to compare expression levels of p-cyclin D1 T286, cyclin D1, p-p38, p38, caspase-3 and cleaved caspase-3 in the tumor xenograft treated with BZN and vehicle, respectively. E. Body weight of nude mice during the experimental period. F. Hematoxylin and eosin (H&E) staining of the lungs, livers and kidneys collected from treatment and control groups. Bars, SD; **P < 0.01; ***P < 0.001 compared with control group.
Discussion
The discovery of new drugs is a long process that requires high investment but faces various unknown risks. Since the clinical drug development success rate is very low in recent years, there is an emerging recognition that identification of new indications for the existing drugs could be a relatively low-cost and efficient strategy for development of novel anticancer reagents [39]. Mifepristone (MIF), a drug regularly used for abortion, has been reported to have anti-tumor activity in multiple hormone-dependent cancers, including luminal type breast cancer [40]. Celecoxib, a drug for rheumatoid arthritis, has been reported to suppress cutaneous squamous cell carcinoma cell (CSCC) migration [41]. In this study, we provide the first evidence that BZN, a FDA-approved drug for anti-infective products, induces apoptosis and suppresses proliferation and tumorigenesis of lung cancer. Actually, our findings were corroborated by the recent studies that BZN can activate endoplasmic reticulum (ER) stress and reduce proliferation in head and neck cancer (HNSCC) [8]. The National Cancer Institute/NIH Developmental Therapeutics Program on 60 human cancer cell lines revealed that BZN has broad-range antitumor activity [42]. However, there was no related research about the use of BZN in lung cancer. Our study here suggests that BZN could be a promising therapeutic agent in lung cancer treatment.
Increasing therapeutic agents have been identified to induce cyclin D1 degradation in vitro [43,44]. Cyclin D1 is a critical regulator of cell cycle progression. Together with its binding partners CDK4 and CDK6, cyclin D1 form active complexes that promote G1 to S phase transition by phosphorylating and inactivating the Rb [45]. Overexpression of cyclin D1 is frequently observed in various cancers and correlates to cancer development and progression. Cyclin D1 is a very unstable protein with a short half-life, indicating that enhancement of cyclin D1 protein degradation may offer a useful strategy for therapeutic intervention. Previous report suggests that knockdown of ubiquitin-specific proteases 2 (USP2), a specific deubiquitinase to stabilize cyclin D1, can destablize cyclin D1 and induce growth arrest in human cancer cells [46]. In addition, the ablation of cyclin D1 can shut off the growth of human breast cancers with activated Neu-Ras pathway [46,47]. In this study, we demonstrated that BZN can suppress lung cancer tumorigenesis. Mechanistically, BZN treatment activates p38 signaling to phosphorylate cyclin D1 at T286 and enhances the interaction between ubiquitin and cyclin D1, therefore promoting cyclin D1 degradation to induce cell cycle arrest and inhibit cancer cell proliferation. Our findings here further support solid evidence on the rationale of cyclin D1 as a therapeutic target in cancer treatment.
Lung cancer is a deadly disease with the highest lethality rate among all cancers. Overexpression or mutations of epidermal growth factor receptor (EGFR) is largely responsible for development and progression of NSCLC [48]. Gefitinib, a tyrosine kinase inhibitor (TKI), has been shown to significantly improve the prognosis of patients and extensively used as the first line therapy in advanced NSCLC patients with EGFR mutations [49]. Unfortunately, most patients inevitably relapse because of resistance [50]. Therefore, it is urgent to improve efficacy of gefitinib treatment. In this study, we found that the combination of BZN and gefitinib significantly induced apoptosis and exerted stronger inhibitory effects on cell proliferation in lung cancer cells (Figure 5). Our results imply that BZN is a promising medicine to improve gefitinib efficacy in NSCLC.
Acknowledgements
The work was supported by the National Key R & D Program of China (2017YFA0505100), The National Natural Science Foundation of China (31570828, 31770888, 81803551, 81773085) and Guangdong Natural Science Research Grant (2016A030313838).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 3.Njatcha C, Farooqui M, Kornberg A, Johnson DE, Grandis JR, Siegfried JM. STAT3 cyclic decoy demonstrates robust antitumor effects in non-small cell lung cancer. Mol Cancer Ther. 2018;17:1917–1926. doi: 10.1158/1535-7163.MCT-17-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li LJ, Chen DF, Wu GF, Guan WJ, Zhu Z, Liu YQ, Gao GY, Qin YY, Zhong NS. Incidence and risk of thromboembolism associated with bevacizumab in patients with non-small cell lung carcinoma. J Thorac Dis. 2018;10:5010–5022. doi: 10.21037/jtd.2018.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Zhang X, Vidaurre I, Cai R, Sha W, Schally AV. Inhibition of experimental small-cell and non-small-cell lung cancers by novel antagonists of growth hormone-releasing hormone. Int J Cancer. 2018;142:2394–2404. doi: 10.1002/ijc.31308. [DOI] [PubMed] [Google Scholar]
- 6.Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 7.Skrott Z, Mistrik M, Andersen KK, Friis S, Majera D, Gursky J, Ozdian T, Bartkova J, Turi Z, Moudry P, Kraus M, Michalova M, Vaclavkova J, Dzubak P, Vrobel I, Pouckova P, Sedlacek J, Miklovicova A, Kutt A, Li J, Mattova J, Driessen C, Dou QP, Olsen J, Hajduch M, Cvek B, Deshaies RJ, Bartek J. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature. 2017;552:194–199. doi: 10.1038/nature25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayess HM, Xi Y, Garshott DM, Brownell AL, Yoo GH, Callaghan MU, Fribley AM. Benzethonium chloride activates ER stress and reduces proliferation in HNSCC. Oral Oncol. 2018;76:27–33. doi: 10.1016/j.oraloncology.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li YJ, Huang XH, Zheng CC, Yin XF, Li B, He QY. Liensinine perchlorate inhibits colorectal cancer tumorigenesis by inducing mitochondrial dysfunction and apoptosis. Food Funct. 2018;9:5536–5546. doi: 10.1039/c8fo01137k. [DOI] [PubMed] [Google Scholar]
- 10.Xu WW, Zheng CC, Huang YN, Chen WY, Yang QS, Ren JY, Wang YM, He QY, Liao HX, Li B. Synephrine hydrochloride suppresses esophageal cancer tumor growth and metastatic potential through inhibition of Galectin-3-AKT/ERK signaling. J Agric Food Chem. 2018;66:9248–9258. doi: 10.1021/acs.jafc.8b04020. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Dephoure N, Sun H, Zhang H, Fan F, Liu J, Ning X, Dai S, Liu B, Gao M, Fu S, Gygi SP, Zhou C. Proteomic profiling of paclitaxel treated cells identifies a novel mechanism of drug resistance mediated by PDCD4. J Proteome Res. 2015;14:2480–2491. doi: 10.1021/acs.jproteome.5b00004. [DOI] [PubMed] [Google Scholar]
- 12.McKay JA, Douglas JJ, Ross VG, Curran S, Murray GI, Cassidy J, McLeod HL. Cyclin D1 protein expression and gene polymorphism in colorectal cancer. Aberdeen colorectal initiative. Int J Cancer. 2000;88:77–81. doi: 10.1002/1097-0215(20001001)88:1<77::aid-ijc12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Santra MK, Wajapeyee N, Green MR. F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature. 2009;459:722–725. doi: 10.1038/nature08011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JT, Shan J, Gu W. Targeting the degradation of cyclin D1 will help to eliminate oncogene addiction. Cell Cycle. 2010;9:857–858. doi: 10.4161/cc.9.5.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–1628. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- 16.Okabe H, Lee SH, Phuchareon J, Albertson DG, McCormick F, Tetsu O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS One. 2006;1:e128. doi: 10.1371/journal.pone.0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 18.Zou Y, Ewton DZ, Deng X, Mercer SE, Friedman E. Mirk/dyrk1B kinase destabilizes cyclin D1 by phosphorylation at threonine 288. J Biol Chem. 2004;279:27790–27798. doi: 10.1074/jbc.M403042200. [DOI] [PubMed] [Google Scholar]
- 19.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Yu RY, Zhang J, Zhang WX, Huang ZH, Hu HF, Li YL, Li B, He QY. Inhibition of Nrf2 enhances the anticancer effect of 6-O-angeloylenolin in lung adenocarcinoma. Biochem Pharmacol. 2017;129:43–53. doi: 10.1016/j.bcp.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zhang J, Huang ZH, Huang XH, Zheng WB, Yin XF, Li YL, Li B, He QY. Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis. 2017;8:e2876. doi: 10.1038/cddis.2017.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng WB, Li YJ, Wang Y, Yang J, Zheng CC, Huang XH, Li B, He QY. Propafenone suppresses esophageal cancer proliferation through inducing mitochondrial dysfunction. Am J Cancer Res. 2017;7:2245–2256. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Y, Yang J, Xu WW, Wang Y, Zheng CC, Li B, He QY. KCTD12 promotes tumorigenesis by facilitating CDC25B/CDK1/Aurora A-dependent G2/M transition. Oncogene. 2017;36:6177–6189. doi: 10.1038/onc.2017.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu WW, Li B, Zhao JF, Yang JG, Li JQ, Tsao SW, He QY, Cheung ALM. IGF2 induces CD133 expression in esophageal cancer cells to promote cancer stemness. Cancer Lett. 2018;425:88–100. doi: 10.1016/j.canlet.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Xu WW, Guan XY, Qin YR, Law S, Lee NP, Chan KT, Tam PY, Li YY, Chan KW, Yuen HF, Tsao SW, He QY, Cheung AL. Competitive binding between Id1 and E2F1 to Cdc20 regulates E2F1 degradation and thymidylate synthase expression to promote esophageal cancer chemoresistance. Clin Cancer Res. 2016;22:1243–1255. doi: 10.1158/1078-0432.CCR-15-1196. [DOI] [PubMed] [Google Scholar]
- 26.Hu HF, Xu WW, Wang Y, Zheng CC, Zhang WX, Li B, He QY. Comparative proteomics analysis identifies Cdc42-Cdc42BPA signaling as prognostic biomarker and therapeutic target for colon cancer invasion. J Proteome Res. 2018;17:265–275. doi: 10.1021/acs.jproteome.7b00550. [DOI] [PubMed] [Google Scholar]
- 27.Xu WW, Li B, Guan XY, Chung SK, Wang Y, Yip YL, Law SY, Chan KT, Lee NP, Chan KW, Xu LY, Li EM, Tsao SW, He QY, Cheung AL. Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression. Nat Commun. 2017;8:14399. doi: 10.1038/ncomms14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Xu WW, Han L, Chan KT, Tsao SW, Lee NPY, Law S, Xu LY, Li EM, Chan KW, Qin YR, Guan XY, He QY, Cheung ALM. MicroRNA-377 suppresses initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Oncogene. 2017;36:3986–4000. doi: 10.1038/onc.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Tsao SW, Chan KW, Ludwig DL, Novosyadlyy R, Li YY, He QY, Cheung AL. Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance-implications for IGF-II and IGF-IR-targeted therapy. Clin Cancer Res. 2014;20:2651–62. doi: 10.1158/1078-0432.CCR-13-2735. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Xue X, Zhang B, Cao H, Kong F, Jiang W, Li J, Sun D, Guo R. Enhanced antitumor activity and attenuated cardiotoxicity of epirubicin combined with paeonol against breast cancer. Tumour Biol. 2016;37:12301–12313. doi: 10.1007/s13277-016-5088-9. [DOI] [PubMed] [Google Scholar]
- 31.An L, Li DD, Chu HX, Zhang Q, Wang CL, Fan YH, Song Q, Ma HD, Feng F, Zhao QC. Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and Notch reversal. Pharmacol Res. 2017;124:105–115. doi: 10.1016/j.phrs.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Nitsche M, Christiansen H, Lederer K, Griesinger F, Schmidberger H, Pradier O. Fludarabine combined with radiotherapy in patients with locally advanced NSCLC lung carcinoma: a phase I study. J Cancer Res Clin Oncol. 2012;138:1113–1120. doi: 10.1007/s00432-012-1185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow KU, Kim SZ, von Neuhoff N, Schlegelberger B, Stilgenbauer S, Wunderle L, Cordes HJ, Bergmann L. Clinical efficacy of immunochemotherapy with fludarabine, epirubicin and rituximab in the treatment for chronic lymphocytic leukaemia and prolymphocytic leukaemia. Eur J Haematol. 2011;87:426–433. doi: 10.1111/j.1600-0609.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu WT, Wang Y, Zhang J, Ye F, Huang XH, Li B, He QY. A novel strategy of integrated microarray analysis identifies CENPA, CDK1 and CDC20 as a cluster of diagnostic biomarkers in lung adenocarcinoma. Cancer Lett. 2018;425:43–53. doi: 10.1016/j.canlet.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 35.Mao J, Ma L, Shen Y, Zhu K, Zhang R, Xi W, Ruan Z, Luo C, Chen Z, Xi X, Chen S. Arsenic circumvents the gefitinib resistance by binding to P62 and mediating autophagic degradation of EGFR in non-small cell lung cancer. Cell Death Dis. 2018;9:963. doi: 10.1038/s41419-018-0998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Xie S, He B. Effect of EGFR gene polymorphism on efficacy of chemotherapy combined with targeted therapy for non-small cell lung cancer in Chinese patients. Am J Cancer Res. 2019;9:619–627. [PMC free article] [PubMed] [Google Scholar]
- 37.Yi H, Li S, Li H, Wang P, Zheng H, Cheng X. Gefitinib induces non-small cell lung cancer H1650 cell apoptosis through downregulating tumor necrosis factor-related apoptosis-inducing ligand expression levels. Oncol Lett. 2018;16:4768–4772. doi: 10.3892/ol.2018.9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X, Jiang J, Zhu J, He N, Tan J. HOXA4-regulated miR-138 suppresses proliferation and gefitinib resistance in non-small cell lung cancer. Mol Genet Genomics. 2018;294:85–93. doi: 10.1007/s00438-018-1489-3. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Li PK, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett. 2014;349:8–14. doi: 10.1016/j.canlet.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu R, Shi P, Nie Z, Liang H, Zhou Z, Chen W, Chen H, Dong C, Yang R, Liu S, Chen C. Mifepristone suppresses basal triple-negative breast cancer stem cells by down-regulating KLF5 expression. Theranostics. 2016;6:533–544. doi: 10.7150/thno.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong T, Yu Y, Yang B, Lin M, Huang JW, Cheng B, Ji C. Celecoxib suppresses cutaneous squamous-cell carcinoma cell migration via inhibition of SDF1-induced endocytosis of CXCR4. Onco Targets Ther. 2018;11:8063–8071. doi: 10.2147/OTT.S180472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yip KW, Mao X, Au PY, Hedley DW, Chow S, Dalili S, Mocanu JD, Bastianutto C, Schimmer A, Liu FF. Benzethonium chloride: a novel anticancer agent identified by using a cell-based small-molecule screen. Clin Cancer Res. 2006;12:5557–5569. doi: 10.1158/1078-0432.CCR-06-0536. [DOI] [PubMed] [Google Scholar]
- 43.Alao JP, Stavropoulou AV, Lam EW, Coombes RC, Vigushin DM. Histone deacetylase inhibitor, trichostatin a induces ubiquitin-dependent cyclin D1 degradation in MCF-7 breast cancer cells. Mol Cancer. 2006;5:8. doi: 10.1186/1476-4598-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Q, Sekula D, Muller R, Freemantle SJ, Dmitrovsky E. Uncovering residues that regulate cyclin D1 proteasomal degradation. Oncogene. 2007;26:5098–5106. doi: 10.1038/sj.onc.1210309. [DOI] [PubMed] [Google Scholar]
- 45.Tian XP, Jin XH, Li M, Huang WJ, Xie D, Zhang JX. The depletion of PinX1 involved in the tumorigenesis of non-small cell lung cancer promotes cell proliferation via p15/cyclin D1 pathway. Mol Cancer. 2017;16:74. doi: 10.1186/s12943-017-0637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan J, Zhao W, Gu W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell. 2009;36:469–476. doi: 10.1016/j.molcel.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 48.Liang S, Lin M, Niu L, Xu K, Wang X, Liang Y, Zhang M, Du D, Chen J. Cetuximab combined with natural killer cells therapy: an alternative to chemoradiotherapy for patients with advanced non-small cell lung cancer (NSCLC) Am J Cancer Res. 2018;8:879–891. [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res. 2014;4:411–435. [PMC free article] [PubMed] [Google Scholar]
- 50.Guerard M, Robin T, Perron P, Hatat AS, David-Boudet L, Vanwonterghem L, Busser B, Coll JL, Lantuejoul S, Eymin B, Hurbin A, Gazzeri S. Nuclear translocation of IGF1R by intracellular amphiregulin contributes to the resistance of lung tumour cells to EGFR-TKI. Cancer Lett. 2018;420:146–155. doi: 10.1016/j.canlet.2018.01.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.