Abstract
Aims
Cumulative consumption of alcohol and variations of alcohol intake by age are unknown in chronic pancreatitis (CP) patients in North America. This study summarizes the lifetime drinking history (LDH) by physician attribution of alcohol etiology, smoking status and sex in persons with CP.
Methods
We analyzed data on 193 CP participants who completed the LDH questionnaire in the North American Pancreatitis Continuation and Validation Study (NAPS2-CV). We collected data on frequency of drinking and drinks per drinking day for each drinking phase of their lives. We examined differences in total number of alcoholic drinks and weight of ethanol consumed by physician’s assessment of CP etiology, sex and smoking status. We also compared intensity of drinking in 20, 30 and 40s by timing of CP diagnosis.
Results
Persons diagnosed with alcoholic CP consumed median of 34,488 drinks (interquartile range 18,240–75,024) prior to diagnosis of CP, which occurred earlier than in persons with CP of other etiology (47 vs. 52 years). Cumulative drinking was greater in male vs. female patients. Male CP patients with a diagnosis of CP before the age of 45 drank more intensely in their 20s as compared to those with later onset of disease. Current smoking was prevalent (67%) among those diagnosed with alcoholic CP. Twenty-eight percent of patients without physician attribution of alcohol etiology reported drinking heavily in the past.
Conclusions
Lifetime cumulative consumption of alcohol and prevalence of current smoking are high in persons diagnosed with alcoholic pancreatitis. Intense drinking in early years is associated with earlier manifestation of the disease.
INTRODUCTION
Chronic pancreatitis (CP) newly affects 4–8 persons per 100,000 people annually (Yadav and Whitcomb, 2010; Yadav et al., 2011b; Yadav and Lowenfels, 2013). Consequences of CP after diagnosis include recurrent or persistent pain, episodes of acute pancreatitis, exocrine insufficiency, malabsorption, pancreatic cancer and even death (Ammann, 2006; Anderson et al., 2016).
Alcohol is the most common etiology for CP (Frulloni et al., 2009; Lankisch et al., 2009; Yadav et al., 2009; Yadav and Whitcomb, 2010; Romagnuolo et al., 2016). Heavy drinking is associated with increased risk of CP in a dose–response fashion with ≥4 drinks/day on average leading to 2- to 3-fold risk of CP (Yadav et al., 2007; Yadav and Whitcomb, 2010). Most studies on the relationship between alcohol and pancreatitis have relied on retrospective recall of recent history of drinking (Nordback et al., 2005, 2009; Pelli et al., 2008), of highest drinking period in a lifetime (Yadav et al., 2009) or of average consumption during certain period (Frulloni et al., 2009). For conditions like CP, in which disease progression may be influenced by long-term exposure to exogenous toxic factors, an understanding of the lifetime and cumulative exposure to alcohol is of importance. However, studies on lifetime alcohol intake are few and are limited only to subjects identified to have alcohol etiology and are not generalizable to the US population.
Previous studies in Japan and Iceland examining lifetime alcohol intake have noted greater total consumption and lower age of drinking initiation among males with alcoholic pancreatitis (Masamune et al., 2013; Juliusson et al., 2018). What these studies do not describe are how the level of drinking changes over the life course and how lifetime drinking is reflected in physician-determined etiology of CP. It is conceivable that the amount and pattern of alcohol consumption may vary over time within an individual and may influence susceptibility to pancreatitis. Moreover, CP is a complex disorder, in which factors other than alcohol, such as smoking, genetic mutations and autoimmunity, also play an important role in disease development. Previous studies evaluating lifetime drinking have focused only on patients with alcoholic pancreatitis, thereby limiting our ability to understand the full spectrum of alcohol consumption among all patients with CP. Such information will be important to fully realize the role of alcohol in this disorder.
We undertook a study to describe lifetime alcohol consumption and their patterns in persons with CP of all etiologies in the USA, to compare lifetime drinking history (LDH) in persons with physician-diagnosed alcoholic CP vs. those with CP of other purported causes, to compare LDH by sex and smoking status and to assess the correlation between period of maximum drinking in life with cumulative alcohol consumption.
METHOD
Study population
The study population comes from the North American Pancreatitis Study 2 Continuation and Validation study, a prospectively ascertained cohort of 521 subjects with a diagnosis of CP confirmed by computed tomography scan, magnetic resonance imaging or cholangiopancreatography, endoscopic ultrasound or histology. Recruitment took place between 2008 and 2012 at 13 clinical centers, coordinated by University of Pittsburgh (Conwell et al., 2017). The present study focused on subjects enrolled from five centers where LDH questionnaire was administered as part of data collection. Due to racial differences in susceptibility to pancreatitis based on alcohol and genetic factors (Lowenfels et al., 1999; Yadav et al., 2011a, 2012; Phillips et al., 2018), we limited our analyses in this study to only white subjects, which was the most common race observed.
Alcohol data
Of 261 white CP patients, 213 reported ever drinking. Of these, complete information on LDH was available for analysis on 193 participants (Skinner and Sheu, 1982). Although LDH relies on retrospective recall, the lifetime daily average drinking as assessed by LDH was found to have strong correlation with that assessed by the Michigan Alcoholism Screening Test and the Alcohol Use Inventory (Skinner and Sheu, 1982). LDH divides one’s lifetime into different drinking phases by age. Information is collected for each phase starting from the time an individual started drinking until the time of enrollment. For each drinking phase, the following data are collected: drinking days in a month, drinks per drinking day and whether the subject drank more than usual on some days, and if so how much. Each drink is considered to have an average alcohol content of 12 g. One drink is equivalent to one glass of wine, one can of beer and one shot of liquor. Independent of cumulative consumption, all NAPS2 subjects were also asked to quantify their alcohol consumption during the period of life he/she considered to represent their period of heaviest (maximum) drinking in life. Following definitions used by the National Health Interview Survey, we categorized patients as abstainers: no alcohol use or <20 drinks in lifetime; light drinkers: 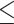 3 drinks/week; moderate drinkers: 4–7 drinks/week for females; 4–14 drinks/week for males; heavy drinkers: 8–27 drinks/week for females; 15–34 drinks/week for males; very heavy drinkers:
3 drinks/week; moderate drinkers: 4–7 drinks/week for females; 4–14 drinks/week for males; heavy drinkers: 8–27 drinks/week for females; 15–34 drinks/week for males; very heavy drinkers: 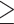 28 drinks/week for females and
28 drinks/week for females and 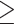 35 drinks/week for males using drinking information during the heaviest drinking period.
35 drinks/week for males using drinking information during the heaviest drinking period.
We describe alcohol intake until the diagnosis of CP in multiple ways. First, we summarized the intensity of consumption (average drinks on a drinking day) for each period reported by the patients and created a heat intensity chart of drinking by age. To quantify age-related drinking, we also summarized the median intensity of drinking at age 25, 35 and 45. We summarized duration of drinking at any level and duration of drinking spent at the maximum level. To understand the frequency of drinking, we also summarized the number of drinking days per month. Finally, we computed the cumulative consumption of alcohol over time for each subject until the diagnosis of CP. For this, we first calculated the total number of drinks consumed during each drinking phase by multiplying the number of drinks consumed on a drinking day with the number of days of drinking. We then added the total number of drinks consumed during each drinking phase to derive cumulative lifetime consumption as the number of drinks. Lastly, we summarized the participant’s response to a validated questionnaire designed for identifying at-risk drinking, called TWEAK (Chan et al., 1993) to assess alcoholism ‘in the months before getting pancreatitis’ (Yadav et al., 2009). TWEAK has demonstrated adequate sensitivity (83%) and specificity (91%) against DSM criteria for alcohol dependence (Cherpitel, 1999).
Covariates
Patient questionnaires were used to collect data on patient demographics, symptoms related to pancreatitis and smoking history. Smoking history was assessed in those who had ever smoked 100 cigarettes or more in their lifetime. For the purposes of the current analyses, we grouped patients into ever, former and current smoker at the time enrollment and quantified average smoking as packs/day. Patient-reported history of highest height and weight, as well as height and weight at enrollment, was used to assess peak body mass index (BMI) and BMI at enrollment. Physicians were instructed to assign etiology of pancreatitis to the most probable cause: alcohol, genetic, idiopathic, obstructive, autoimmune, most-necrotic, hypertriglyceridemia and other causes (e.g. trauma, post-ERCP). For analysis, etiologies other than alcohol were collapsed into a single ‘no alcohol’ category. CP of alcohol etiology by physician assessment will be heretofore abbreviated as ‘alcohol etiology CP’ or ‘alcoholic CP’.
Statistical analyses
We compared distribution of age, smoking status and measures of alcohol consumption by alcohol etiology and smoking status at enrollment. Within categories of alcohol etiology, we compared the covariate distribution by sex. We compared the distribution of continuous variables, such as age, intensity of drinking, BMI, number of drinks/day, duration of drinking, cumulative alcohol consumption by Wilcoxon rank sum test for two-way comparisons and Kruskal–Wallis test for three-way comparisons. We compared the distribution of categorical variables, including smoking status, current drinking status, TWEAK ≥3 and heavy drinking history (binary indicator for drinking more than 4 drinks on a drinking day) by Χ2 test.
The study population was then stratified by sex, alcohol etiology and smoking status (current vs. past or never smoker). Within each of the stratum, we compared cumulative number of drinks consumed over lifetime and number of drinks on a drinking day at ages 25, 35 and 45 by age of CP diagnosis (<45 vs. ≥45 years old) using Wilcoxon rank sum test.
The median cumulative number of drinks consumed over lifetime and the median cumulative number of drinks consumed during the heaviest drinking period were summarized and plotted on bar chart by category of intensity of drinking during the heaviest drinking period (low, moderate, heavy and very heavy definitions). We computed the correlation between the cumulative lifetime number of drinks and the category of intensity of drinking (as continuous variable with a range of 0–3) among males and females separately.
RESULTS
Overview of the study population
Of 193 patients with CP who provided information on LDH, 119 (62%) were male and 111 (58%) were assessed to have alcohol etiology of CP. Sixty (32%) patients were former smokers and 101 (52%) were current smokers, and 20 patients reported smoking to alleviate pain. Median age at CP diagnosis was 49, and average age of drinking initiation was 18 (Table 1). Nineteen percent of the cohort reported still consuming alcohol at the time of enrollment (Table 2). The median of the average number of drinks consumed on a drinking day during period of drinking was 5.1 drinks and that during the maximum drinking period was 6.5 drinks (Table 2). Median drinking duration was 25 years, of which a median of 6 years was spent during the heaviest drinking period. CP patients drank a median of 16,632 drinks (200 kg of alcohol) until CP diagnosis, but there was wide variation (Table 2).
Table 1.
Distribution of demographic factors in persons with CP, by alcohol etiology, smoking status
| Variable | All | Alcohol etiology | No alcohol etiology | P value | Never smoker | Past smoker | Current smoker | P value * |
|---|---|---|---|---|---|---|---|---|
| Number | 193 | 111 | 82 | 32 | 60 | 101 | ||
| Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | |||
| Age at enrollment (years) | 52 (44, 59) | 52 (46, 57) | 54 (41, 64) | 0.3366 | 56 (43, 64) | 58 (47, 66) | 50 (43, 55) | 0.0001 |
| Age at CP diagnosis (years) | 49 (40, 57) | 47 (43, 54) | 52 (39, 60) | 0.1278 | 53 (39, 59) | 57 (49, 64) | 47 (40, 51) | 0.0001 |
| Current smoker at enrollment | 101 (52%) | 74 (66.7%) | 27 (32.9%) | <0.0001 | — | — | — | |
| Former smoker at enrollment | 60 (31%) | 27 (24.3%) | 33 (40.2%) | — | — | — | ||
| Smoking to alleviate pain | 20 (11%) | 11 (10%) | 9 (11%) | 0.7753 | 0 | 2 (3.5%) | 18 (18%) | 0.0014 |
| Average intensity of smoking (packs per day) | 0 (0, 1) | 1 (0, 1) | 0 (0, 1) | 0.0022 | 0 (0, 0) | 1 (0, 1) | 1 (0, 1) | <0.0001 |
| Highest BMI ever (kg/m2) | 29 (25.39, 33.66) | 28.73 (25.1, 33.75) | 29.15 (25.39) | 0.6516 | 30.34 (26.93, 35.23) | 30.87 (26.62, 33.91) | 28.13 (24.69, 32.77) | 0.0690 |
| BMI at enrollment (kg/m2) | 24.39 (21.71, 28) | 23.96 (20.77, 27.37) | 24.74 (22.24, 28.8) | 0.0963 | 27.32 (23.72, 31.1) | 25.37 (22.24, 29.35) | 23.29 (20.64, 26.11) | 0.0002 |
* P values for smoking refers to global Χ2 tests for difference in the distribution of covariates by smoking status.
Table 2.
Distribution alcohol-related variables by alcohol etiology and smoking status in persons with CP
| Variable | All | Alcohol etiology | No alcohol etiology | P value | Never smoker | Past smoker | Current smoker | P value |
|---|---|---|---|---|---|---|---|---|
| Number | 193 | 111 | 82 | 32 | 60 | 101 | ||
| Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | Median (IQR) or N (%) | |||
| Age when drinking started (years) | 18 (16, 21) | 20 (16, 21) | 20 (18, 21) | 0.0003 | 20 (17.5, 22) | 18 (17.5, 21) | 18 (16, 21) | 0.0299 |
| Current drinker | 36 (19%) | 19 (17%) | 17 (21%) | 0.5240 | 5 (16%) | 15 (25%) | 16 (16%) | 0.3147 |
| TWEAK score of ≥3 prior to CP development | 106 (55%) | 92 (82.9%) | 14 (17.1%) | <0.0001 | 13 (40.6%) | 23 (38.3%) | 70 (69.3%) | <0.0001 |
| Average no. of drinks per day during periods of drinking | 2 (0.4, 4.7) | 4 (2.14, 7.8) | 0.4 (0.13, 1.26) | <0.0001 | 0.8 (0.2, 2.78) | 0.9 (0.165, 3.03) | 3.1 (1.15, 5.62) | 0.0002 |
| History of heavy or very heavy drinking based on maximum lifetime drinking period | 125 (64.8%) | 102 (91.9%) | 23 (28.1%) | <0.0001 | 15 (46.9%) | 30 (50%) | 80 (79.2%) | 0.0005 |
| No. of drinking days per month during the maximum drinking period | 15 (4, 30) | 30 (17, 30) | 4 (2, 9) | <0.0001 | 8 (3.5, 27.5) | 8.5 (3, 25) | 30 (8, 30) | <0.0001 |
| No. of drinks on a drinking day during maximum drinking period | 5.1 (2.8, 8.4) | 6.9 (4.42, 11.24) | 2.8 (1.76, 5.16) | <0.0001 | 3.3 (1.98, 5.55) | 3.8 (2, 7.77) | 6.5 (4, 9.33) | 0.2701 |
| Drinking duration (years) | 25 (13, 25) | 28 (19, 35) | 17.5 (6, 35) | 0.0051 | 24.5 (11, 37) | 27 (13.5, 41) | 25 (13, 32) | 0.6 |
| Duration spent at maximum drinking level in years | 6 (3, 13) | 7 (3, 15) | 5 (2, 11) | 0.04 | 5 (3, 17) | 5.5 (3, 10) | 6 (3, 11) | 0.0003 |
| Cumulative lifetime alcohol consumption No. of drinks kilograms |
16632 (2484, 46752) 199.6 (29.8, 561) |
34488 (18240, 75024) 413.9 (218.9, 900.29) |
1968 (600, 6156) 23.6 (7.2, 73.872) |
<0.0001 | 6024 (1644, 24144) 72.3 (19.73, 289.73) |
5052 (1002, 28944) 60.6 (12.02, 347.33) |
29736 (6960, 60336) 356.8 (83.52, 724.03) |
<0.0001 |
| Cumulative alcohol consumption in kg per kg maximum weight |
2.1 (0.36, 6.53) | 5.1 (2.3, 10.42) | 0.3 (0.089, 0.72) | <0.0001 | 0.6 (0.22, 2.94) | 0.6 (0.17, 3.86) | 3.9 (0.8, 8.84) | <0.0001 |
Comparisons by alcohol etiology, smoking status and sex
Persons with CP of alcohol etiology were more likely to smoke currently (67%) as compared to those with CP due to other etiology (33%) (Table 1). Most patients with CP of alcohol etiology (83%) had a TWEAK score of ≥3, indicating the presence of alcohol use disorder per DSM criteria (Cherpitel, 1999), as compared to those without alcohol etiology (17%) (Table 2). Average number of drinks per day during periods of drinking (4 vs. 0.4 drinks/day), average number of drinks per drinking day during periods of drinking (6.9 vs. 2.8 drinks) and number of drinks on a drinking day during maximum drinking period (10 vs. 3.9 drinks) were all significantly higher for CP patients with alcohol etiology as compared to those without alcohol etiology (Table 2). Moreover, the number of days spent drinking during their maximum period of time was higher for CP patients with alcohol etiology (30 days) than for persons without alcohol etiology (4 days) (Table 2). Persons with CP of alcohol etiology reported drinking considerably more over a lifetime (34,488 drink, 414 kg of alcohol) as compared to those who were deemed to have CP due to other etiology (1968 drinks, 24 kg of alcohol) (Table 2).
We compared detailed alcohol consumption history, including average number of drinks on a drinking day, duration of drinking and cumulative alcohol consumption by sex within each stratum of alcohol etiology. Among CP patients with alcohol etiology, males reported higher average number of drinks per day (4.5 drinks/day) and higher number of drinks on a drinking day (7.3 drinks/day) during periods of drinking than women (2.9 drinks/day and 5.6 drinks/day, respectively; Supplementary Table 1 is available at Alcohol and Alcoholism online). However, the number of drinks on a drinking day during maximum drinking period was similar between males (10.2 drinks/day) and females (8.4 drinks/days) with CP of alcohol etiology (Supplementary Table 1 is available at Alcohol and Alcoholism online). Cumulative lifetime alcohol consumption was higher for men with CP of alcohol etiology (46,776 drinks, 561 kg of alcohol) compared to women counterparts (28,320 drinks or 340 kg of alcohol) (Supplementary Table 1 is available at Alcohol and Alcoholism online). Considering that men may tolerate more alcohol due to larger body weight, we also compared the per-weight difference in alcohol consumption between men and women. The difference in cumulative alcohol consumption per kg of weight (highest ever in lifetime) showed borderline significance between men and women (6.55 kg alcohol per kg body weight in men vs. 4.11 kg alcohol per kg body weight in women, P = 0.056) (Supplementary Table 1 is available at Alcohol and Alcoholism online).
Age at diagnosis of CP was higher for never smokers and past smokers as compared to current smokers (53 vs. 57 vs. 47) (Table 1). Former and current smokers started drinking at an earlier age than their counterparts (18 vs. 20) (Table 1). Smoking to alleviate pain was more common among current smokers than former smokers (18 vs 3.5%) (Table 1). Current smokers reported drinking on a daily basis (30 days of drinking/month) during the maximum drinking period, as opposed to former and never smokers who drank 8–8.5 drinking days/month during their maximum drinking period (Table 2). CP patients who currently smoke consumed a median of 29,736 drinks (356.8 kg of alcohol), nearly 20-fold greater than that consumed by former and never smokers (5052 drinks or 60.6 kg of alcohol and 6024 drinks or 72.3 kg of alcohol, respectively) (Table 2). Sex-specific comparisons within smoking categories are available in Supplementary Material 2 at Alcohol and Alcoholism online.
Correlation between cumulative drinking and drinking intensity at maximum drinking period
Figure 1 presents cumulative number of drinks consumed during lifetime (Fig. 1A) and during the heaviest drinking period in life (Fig. 1B) by category of drinking intensity on a drinking day based on the heaviest drinking period. Cumulative lifetime drinks increased with the drinking category for males and females. Correlation coefficient between cumulative lifetime number of drinks and drinking behavior during the period of heaviest drinking in life was r = 0.77 for males and r = 0.86 for females. The correlation coefficient between cumulative number of drinks consumed during heaviest drinking period in life and the drinking category was r = 0.75 for males and r = 0.75 for females.
Fig. 1.
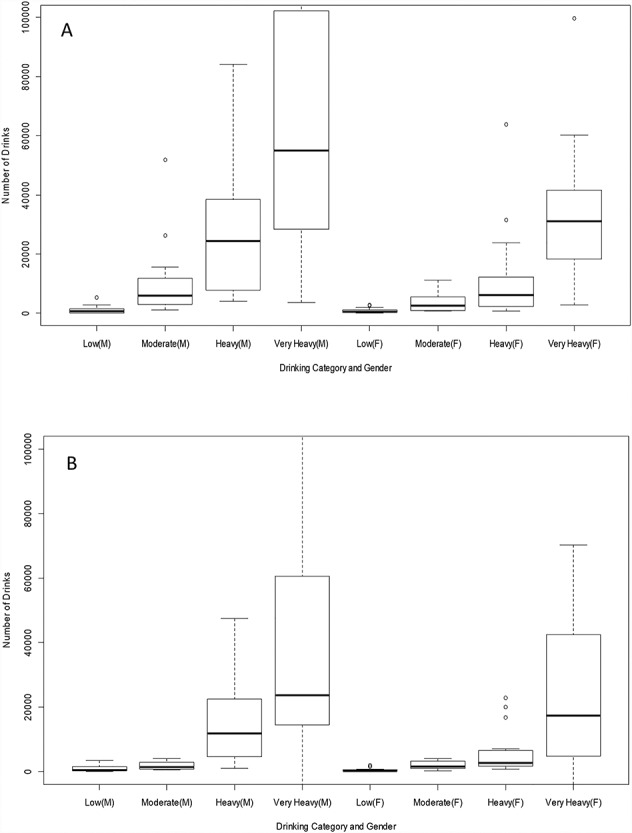
Cumulative number of drinks consumed over lifetime and during period of heaviest drinking by drinking category during the heaviest drinking period. (A) Cumulative number of drinks during lifetime by category of drinking intensity on a drinking day based on the heaviest drinking period. ‘M’ denotes males and ‘F’ denotes females. Correlation coefficient r = 0.77 for males and r = 0.86 for females. (B) Cumulative number of drinks during period of heaviest drinking by drinking category during the heaviest drinking period. Correlation coefficient r = 0.75 for males and r = 0.75 for females.
Variations in drinking intensity over time
Figure 2 displays intensity of drinking by age for each individual represented in the study population separated by men and women and by physician assessed alcohol etiology. Each bar represents the length of self-reported drinking duration until CP diagnosis, and the colors note the number of drinks typically consumed on a drinking day during each phase of drinking. Several observations were noted: Extent of drinking varied between individuals at each age range in their lives and also at the time of CP. Those with consistent heavy drinking (red and yellow bars) showed earlier onset of CP. Males show higher intensity of drinking as compared to women with alcoholic pancreatitis. Some persons with no alcohol etiology also drank for long durations, but less intensely (long green and blue bars). Among patients with similar average level of drinking per drinking day in the twenties, the time to onset of CP varied. Among persons with alcoholic CP, there were a number of patients who have had little to only moderate levels of drinking prior to CP diagnosis (grey, blue bars prior to CP onset), and in some the period of heavy drinking was separated from the diagnosis of CP by many years. Among persons without alcohol etiology, there were a few patients who reported heavy drinking in the past (presence of yellow or red bars in the past).
Fig. 2.
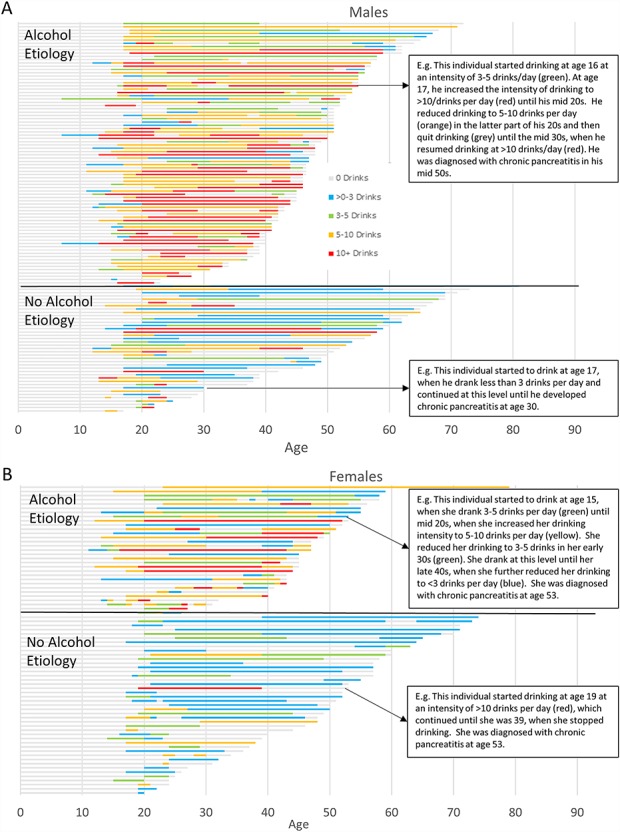
Heat map of drinking intensity by age based on average number of drinks on a drinking day in CP patients. (A) Male patients with CP and (B) female patients with CP. Intensity of drinking by age for each individual represented in the study population separated by men and women and by physician assessed alcohol etiology. Each bar represents the length of self-reported drinking duration until CP diagnosis, and the colors note the number of drinks typically consumed on a drinking day during each phase of drinking.
Table 3 presents cumulative consumption of alcohol and number of drinks consumed per drinking day at successive decades of life stratified by age of diagnosis of CP (<45 vs. ≥45 years), sex and smoking status at the time of enrollment. Among currently smoking males with CP of alcohol etiology, those diagnosed prior to 45 years of age had lower cumulative alcohol consumption (45,666 drinks) as compared to those diagnosed after 45 years of age (59,316 drinks), but they had higher intensity of drinking at age 25 (9.0 drinks/drinking day) as compared to those diagnosed with alcoholic CP later in life (6.3 drinks/drinking day) (Table 3). The difference in drinking intensity at age 25 between early- and late-onset CP reached marginal statistical significance (P = 0.064) when the smoking categories were combined.
Table 3.
Cumulative number of drinks over lifetime and intensity of drinking at successive decades in life prior to diagnosis of CP by age of onset of CP and sex
| Alcohol etiology and smoking category | Alcohol consumption variables | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| Age < 45 at CP diagnosis | Age ≥ 45 at CP diagnosis | P value | Age < 45 at CP diagnosis | Age ≥ 45 at CP diagnosis | P value | ||
| Alcohol etiology only | |||||||
| Current smoker | N: | 15 | 30 | 9 | 13 | ||
| Cumulative no. of drinks over lifetime: | 46800 (29496, 67440) | 59316 (25680, 102852) | 0.7467 | 18240 (10344, 29736) | 31584 (11328, 41796) | 0.3605 | |
| Drinks/drinking day at age 25: | 9.46 (6, 14) | 6.295 (4, 8) | 0.1274 | 2 (0, 3.33) | 5 (4, 8.67) | 0.1144 | |
| Drinks/drinking day at age 35: | 5.705 (0, 9) | 6.195 (4.86, 10.83) | 0.2788 | 5.67 (0, 6) | 4 (2, 8.67) | 0.8833 | |
| Drinks/drinking day at age 45: | N/A | 3.635 (0, 6) | N/A | 5 (0, 7) | |||
| Past OR never smoker | N: | 5 | 21 | 1 | 5 | ||
| Cumulative no. of drinks over lifetime: | 26244 (23376, 73080) | 31428 (14712, 95640) | 0.8468 | 2796 (2796, 2796) | 31344 (29640, 38064) | 0.3333 | |
| Drinks/drinking day at age 25: | 10.33 (9.35, 13.2) | 5.11 (3, 8) | 0.4412 | 0 (0, 0) | 4.22 (3, 5.59) | 0.3333 | |
| Drinks/drinking day at age 35: | 5.165 (0, 11.465) | 6 (3.5, 8.33) | 0.7398 | 0 (0, 0) | 5.59 (3, 6) | 0.1667 | |
| Drinks/drinking day at age 45: | N/A | 3.5 (0, 6) | N/A | 3.17 (2, 3.6) | |||
| Non-alcohol etiology | |||||||
| Current smoker | N: | 5 | 6 | 5 | 7 | ||
| Cumulative no. of drinks over lifetime: | 3072 (1152, 6960) | 11064 (2928, 32640) | 0.3290 | 288 (48, 960) | 3456 (2736, 13344) | 0.0177 | |
| Drinks/drinking day at age 25: | 2 (1, 6) | 3 (0, 6.4) | 0.7619 | 0 (0, 0.75) | 0 (0, 3) | 0.4273 | |
| Drinks/drinking day at age 35: | 0 (0, 1) | 0 (0, 3.12) | 0.9242 | 0.75 (0, 1.5) | 2 (0, 6) | 0.5 | |
| Drinks/drinking day at age 45: | N/A | 1.56 (0, 6) | N/A | 0 (0, 2) | |||
| Past OR never smoker | N: | 6 | 16 | 6 | 18 | ||
| Cumulative no. of drinks over lifetime: | 2988 (168, 7752) | 10320 (3876, 27840) | 0.1335 | 1764 (528, 2688) | 1116 (576, 2484) | 0.7211 | |
| Drinks/drinking day at age 25: | 0 (0, 2) | 2.5 (2, 6.325) | 0.1277 | 0.5 (0, 6) | 2 (0, 3) | 0.7117 | |
| Drinks/drinking day at age 35: | 0 (0, 0) | 2 (1.085, 6.215) | 0.0062 | 0 (0, 6) | 1 (0, 2) | 0.6895 | |
| Drinks/drinking day at age 45: | N/A | 2 (1, 5.5) | N/A | 1 (0, 2) | |||
DISCUSSION
In this analysis of 193 persons with CP from North America, we found that consumption of alcohol over lifetime was high and variable among patients with CP, with higher consumption for men vs. women and in current smokers vs. never and former smokers. Importantly, we observed that male patients with earlier onset of CP drank more intensely in their 20s as compared to those with later onset of disease and that concomitant smoking in the presence of heavy drinking was associated with earlier manifestation of disease. Of interest to clinicians, we noted that some patients without physician assessment of alcohol etiology had a history of heavy drinking and that current smoking was highly prevalent among those diagnosed with alcoholic CP, despite current abstinence from alcohol.
We found that persons with alcohol etiology of CP had high cumulative consumption of alcohol of 34,488 drinks or 357 kg of alcohol prior to diagnosis of CP. Cumulative drinking was greater for male patients (46,776 drinks, 561 kg of alcohol) than for female patients (28,320 drinks, 340 kg of alcohol). The cumulative total consumption in our population is higher than that reported in a study from Iceland but lower than that reported from Japan. In contrast to our study consisting solely of CP patients of all etiologies, these studies included patients with acute or chronic alcoholic pancreatitis. In the Iceland study, 45 male patients with alcoholic pancreatitis who were recruited from a list of recent hospitalized cases drank 35,360 drinks over median duration of 38 years, and female patients drank 19,890 over a median duration of 32 years. However, there was no difference between lifetime number of drinks for CP as opposed to acute pancreatitis patients (31,910 vs. 30,790 drinks, respectively) (Juliusson et al., 2018). Similarly, a Japanese study also found that average consumption of alcohol per day and the frequency of drinking were similar for CP patients as compared to acute pancreatitis patients (Masamune et al., 2013). The cumulative volume of alcohol consumed by female and male alcoholic CP patients as reported in the Japanese study was 1416 l of ethanol for males and 1049 l for females. Assuming ethanol density of 0.789 g/cm3, the reported volumes are equivalent to 1117 kg of ethanol and 829 kg of ethanol, which is higher than cumulative consumption reported for our male and female alcoholic CP patients. Japanese CP patients reported higher average consumption of alcohol per day (8 drinks/day for males and 7 drinks/day for females) as compared to our CP population (4.5 drinks/day for males and 2.9 drinks/day for females). This is despite the fact that the prevalence of hazardous drinking (five or more drinks per day; 13% in males and 3.4% in females) is lower in Japan (Higuchi et al., 2007) than reported in the USA: 18% men and 5% of women (Hilton, 1987). The fact that Japanese male CP patient reported higher consumption in 40s and 50s than in the 20s and 30s may also have contributed to higher cumulative consumption than in our male CP patients whose consumption was higher in the 20s and 30s and had lower age of diagnosis of CP (median 48 years) than in the Japanese male patients with alcoholic CP (mean 55 years) (Supplementary Material 3 is available at Alcohol and Alcoholism online).
In addition to alcohol etiology, the intensity of drinking varied over time in persons with CP, and the patterns varied according to sex, age of onset and smoking status. Although not statistically significant, male alcoholic pancreatitis patients who developed CP before the age of 45 had greater intensity of drinking per drinking day at age 25, but lower cumulative number of drinks as compared to those who developed CP on or after 45 years of age. Female patients with alcoholic CP diagnosed <45 years of age also had lower cumulative consumption of alcohol than those diagnosed on or after 45 years of age. Exposure to alcohol and smoking reduced the age at diagnosis of CP. These data suggest that cumulative consumption of alcohol as well as intensity of drinking (and smoking) plays an important role in individual susceptibility and age at clinical manifestation of CP. In other words, the injury that alcohol imparts on the pancreas is not necessarily uniform and rather may depend on the extent of episodic drinking and how frequently and in what amount alcohol is consumed. The presence of other co-factors, such as smoking and genetic susceptibility, will further impact susceptibility and clinical manifestation of the disease (Singhvi and Yadav, 2018).
About one-fourth of CP patients without alcohol etiology reported drinking heavily throughout or at different times in their life prior to diagnosis. Without a clear guideline for attributing etiology of pancreatitis to alcohol, physicians are left applying their best guesses on the cause of pancreatitis. It is particularly challenging to determine the role of heavy drinking in the past when patients have not consumed alcohol in recent history and equally challenging to evaluate the role of consistent non-heavy drinking on pancreatitis. Further investigations on the risk of pancreatitis in persons who reduce intake after a period of heavy drinking and in persons who report consistent light-to-moderate drinking are warranted.
Persons with alcoholic CP have by and large stopped drinking by the time CP had been diagnosed. Only ~20% reported current drinking at the time of enrollment, but they did not stop smoking. The prevalence of current smoking was 67%, some of whom were smoking to alleviate pain. This has an important public health implication, considering that 1-pack of cigarette smoking per day increases the risk of pancreatic cancer by two fold (Zou et al., 2014). Because CP patients are already at much higher risk of pancreatic cancer (Malka et al., 2002), reduction in use of tobacco products should be considered a priority in addition to quit alcohol. Pain management recommendations should also include advice on tobacco reduction and cessation and provide alternatives for alleviating pain. It is unknown whether the patients were smoking products other than tobacco or were using electronic nicotine delivery systems. Given this, emerging use of vaporizers and marijuana products also warrants research on their potential impact on outcomes of CP. Of note, recent study on cannabis use among hospitalized pancreatitis patients demonstrated potential inverse association between cannabis use and alcohol-associated pancreatitis (Adejumo et al., 2019).
Our study has a few limitations. First, we relied on patients own memory to recall the lifetime exposure to alcohol, which is subject to misclassification. A nationwide study on the recall of alcohol intake in the past 10 years not only found strong correlation between recalled and previously reported exposure (r = 0.70; Liu et al., 1996) but also found that current heavy drinkers tended to underestimate their previous drinking. Thus, actual cumulative consumption of alcohol may be higher than estimated in the current analyses. Secondly, we had limited number of observations on female patients with alcoholic CP. This is due to the fact that there are generally fewer female CP patients and that they are less likely to have CP of alcohol etiology. Considering that drinking prevalence is increasing in the USA with more rapid increase among women (Grant et al., 2017), further studies on patterns of drinking in female patients with alcoholic pancreatitis are warranted. Lastly, our study population was limited to those with CP. This limits our ability to understand how exposure to alcohol differs in patients diagnosed with CP from those with acute or recurrent acute pancreatitis, which are often the initial manifestations of CP, and whether and how exposure to alcohol affects the natural course in such patients.
Despite the limitations, our study sheds light on the pathophysiology and clinical management of alcoholic CP. Importantly, we determined the extent and variability in lifetime cumulative consumption of alcohol that leads to risk of CP. We found that those with intense drinking in the 20s have earlier onset pancreatitis, exposure to alcohol and smoking reduced the age at clinical manifestation of CP and that smoking is highly prevalent among CP patients.
Conflict of Interest Statement
David C. Whitcomb, MD, PhD is a consultant for AbbVie, Regeneron and Ariel Precision Medicine and may have equity in Ariel Precision Medicine. The other authors declare no relevant conflicts.
Supplementary Material
Acknowledgements
Grant Support: This research was partly supported by the NIH DK061451 (D.C.W.), DK077906 (D.Y.), U01 DK108306 (D.C.W., D.Y.) and U01DK108314 (C.Y.J.). This publication was made possible in part by Grant Number UL1 RR024153 and UL1TR000005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (University of Pittsburgh. PI, Steven E. Reis, MD). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- Adejumo AC, Akanbi O, Adejumo KL, Bukong TN. (2019) Reduced risk of alcohol-induced pancreatitis with cannabis use. Alcohol Clin Exp Res 43:277–86. [DOI] [PubMed] [Google Scholar]
- Ammann RW. (2006) Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly 136:166–74. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Akshintala V, Albers KM, et al. (2016) Mechanism, assessment and management of pain in chronic pancreatitis: recommendations of a multidisciplinary study group. Pancreatology 16:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Pristach EA, Welte JW, Russell M. (1993) Use of the TWEAK test in screening for alcoholism/heavy drinking in three populations. Alcohol Clin Exp Res 17:1188–92. [DOI] [PubMed] [Google Scholar]
- Cherpitel CJ. (1999) Screening for alcohol problems in the U.S. general population: a comparison of the CAGE and TWEAK by gender, ethnicity, and services utilization. J Stud Alcohol 60:705–11. [DOI] [PubMed] [Google Scholar]
- Conwell DL, Banks PA, Sandhu BS, et al. (2017) Validation of demographics, etiology, and risk factors for chronic pancreatitis in the USA: a report of the North American pancreas study (NAPS) group. Dig Dis Sci 62:2133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frulloni L, Gabbrielli A, Pezzilli R, et al. (2009) Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis 41:311–7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, et al. (2017) Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on alcohol and related conditions. JAMA Psychiat 74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Maesato H, Osaki Y. (2007) Japan: alcohol today. Addiction 102:1849–62. [DOI] [PubMed] [Google Scholar]
- Hilton ME. (1987) The presence of alcohol in four social situations: survey results from 1964 and 1984. Int J Addict 22:487–95. [DOI] [PubMed] [Google Scholar]
- Juliusson SJ, Nielsen JK, Runarsdottir V, et al. (2018) Lifetime alcohol intake and pattern of alcohol consumption in patients with alcohol-induced pancreatitis in comparison with patients with alcohol use disorder. Scand J Gastroenterol 1–7, Jun;53(6):748–754. [DOI] [PubMed] [Google Scholar]
- Lankisch PG, Breuer N, Bruns A, et al. (2009) Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol 104:2797–805quiz 2806. [DOI] [PubMed] [Google Scholar]
- Liu S, Serdula MK, Byers T, et al. (1996) Reliability of alcohol intake as recalled from 10 years in the past. Am J Epidemiol 143:177–86. [DOI] [PubMed] [Google Scholar]
- Lowenfels AB, Maisonneuve P, Grover H, et al. (1999) Racial factors and the risk of chronic pancreatitis. Am J Gastroenterol 94:790–4. [DOI] [PubMed] [Google Scholar]
- Malka D, Hammel P, Maire F, et al. (2002) Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 51:849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune A, Kume K, Shimosegawa T. (2013) Sex and age differences in alcoholic pancreatitis in Japan: a multicenter nationwide survey. Pancreas 42:578–83. [DOI] [PubMed] [Google Scholar]
- Nordback I, Pelli H, Lappalainen-Lehto R, et al. (2009) The recurrence of acute alcohol-associated pancreatitis can be reduced: a randomized controlled trial. Gastroenterology 136:848–55. [DOI] [PubMed] [Google Scholar]
- Nordback I, Pelli H, Lappalainen-Lehto R, Sand J. (2005) Is it long-term continuous drinking or the post-drinking withdrawal period that triggers the first acute alcoholic pancreatitis? Scand J Gastroenterol 40:1235–9. [DOI] [PubMed] [Google Scholar]
- Pelli H, Lappalainen-Lehto R, Piironen A, et al. (2008) Risk factors for recurrent acute alcohol-associated pancreatitis: a prospective analysis. Scand J Gastroenterol 43:614–21. [DOI] [PubMed] [Google Scholar]
- Phillips AE, Larusch J, Greer P, et al. (2018) Known genetic susceptibility factors for chronic pancreatitis in patients of European ancestry are rare in patients of African ancestry. Pancreatology. Volume 18, Issue 5, July 2018, Pages 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnuolo J, Talluri J, Kennard E, et al. (2016) Clinical profile, etiology, and treatment of chronic pancreatitis in North American women: analysis of a large multicenter cohort. Pancreas 45:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi A, Yadav D. (2018) Myths and realities about alcohol and smoking in chronic pancreatitis. Curr Opin Gastroenterol 34:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. (1982) Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Stud Alcohol 43:1157–70. [DOI] [PubMed] [Google Scholar]
- Yadav D, Hawes RH, Brand RE, et al. (2009) Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, Lowenfels AB. (2013) The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, Muddana V, O'connell M. (2011a) Hospitalizations for chronic pancreatitis in Allegheny County, Pennsylvania, USA. Pancreatology 11:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, O'connell M, Papachristou GI. (2012) Natural history following the first attack of acute pancreatitis. Am J Gastroenterol 107:1096–103. [DOI] [PubMed] [Google Scholar]
- Yadav D, Papachristou GI, Whitcomb DC. (2007) Alcohol-associated pancreatitis. Gastroenterol Clin N Am 36:219–38, vii. [DOI] [PubMed] [Google Scholar]
- Yadav D, Timmons L, Benson JT, et al. (2011b) Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 106:2192–9. [DOI] [PubMed] [Google Scholar]
- Yadav D, Whitcomb DC. (2010) The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 7:131–45. [DOI] [PubMed] [Google Scholar]
- Zou L, Zhong R, Shen N, et al. (2014) Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer 50:193–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


