Visual Abstract
Keywords: acute renal failure, checkpoint inhibitors, onconephrology, acute kidney injury, acute interstitial nephritis, immune related adverse events, humans, male, creatinine, incidence, risk factors, proton pump inhibitors, proportional hazards models, renal dialysis, general hospitals, kidney function tests, glomerular filtration rate, nephrology, comorbidity, chronic renal insufficiency, nephritis, Massachusetts
Abstract
Background and objectives
Immune checkpoint inhibitor use in oncology is increasing rapidly. We sought to determine the frequency, severity, cause, and predictors of AKI in a real-world population receiving checkpoint inhibitors.
Design, setting, participants, & measurements
We included all patients who received checkpoint inhibitor therapy from May 2011 to December 2016 at Massachusetts General Hospital. Baseline serum creatinine, averaged 6 months before checkpoint inhibitor start date, was compared with all subsequent creatinine values within 12 months of starting therapy. AKI was defined by Kidney Disease: Improving Global Outcomes criteria for fold changes in creatinine from baseline. Sustained AKI events lasted at least 3 days and was our primary outcome. The cause of sustained AKI was determined by chart review. Cumulative incidence and subdistribution hazard models were used to assess the relationship between baseline demographics, comorbidities, and medications, and sustained AKI and potential checkpoint inhibitor–related AKI.
Results
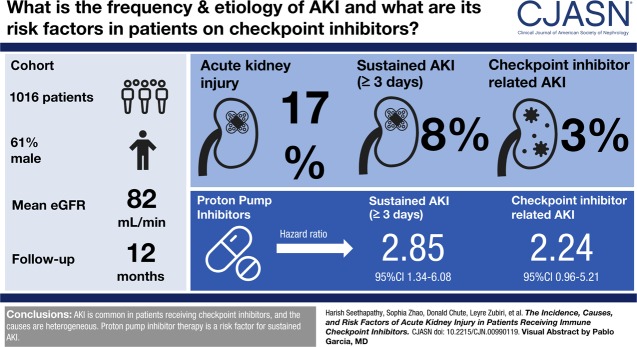
We included 1016 patients in the analysis. Average age was 63 (SD 13) years, 61% were men, and 91% were white. Mean baseline creatinine was 0.9 mg/dl (SD 0.4 mg/dl), and 169 (17%) had CKD (eGFR<60 ml/min per 1.73 m2) at baseline. A total of 169 patients (17%) experienced AKI, defined by an increase in creatinine at least 1.5 times the baseline within 12 months; 82 patients (8%) experienced sustained AKI and 30 patients (3%) had potential checkpoint inhibitor–related AKI. The first episode of sustained AKI occurred, on average, 106 days (SD 85) after checkpoint inhibitor initiation. Sixteen (2%) patients experienced stage 3 sustained AKI and four patients required dialysis. Proton pump inhibitor use at baseline was associated with sustained AKI.
Conclusions
AKI is common in patients receiving checkpoint inhibitor therapy. The causes of sustained AKI in this population are heterogenous and merit thorough evaluation. The role of PPI and other nephritis-inducing drugs in the development of sustained AKI needs to be better defined.
Introduction
Immune checkpoint inhibitors act by releasing the natural breaks on immune activation and enhancing the immune system’s ability to destroy tumor cells. Approved agents target checkpoint pathways mediated by cytotoxic T lymphocyte–associated antigen 4 (CTLA4), programmed cell death protein 1 (PD1), and programmed death ligand 1 (PDL1) (1–10). Checkpoint inhibitors have produced durable responses in a subset of patients with cancer, but the benefit comes at a cost. Unchecked activation of the immune system may cause multisystem, immune-related adverse events, which can be fatal (11,12). Acute interstitial nephritis (AIN) is the most common biopsy-proven diagnosis in patients on checkpoint inhibitors who develop AKI (13–16). The mechanism is not well defined. Checkpoint inhibitors may provoke unregulated T cell responses and proliferation in the tubulointerstitium; however, it is also possible that checkpoint inhibitors lead to loss of immune tolerance and activation of memory T cells previously primed by other haptens that cause AIN, including medications (12). Supporting the latter theory, two of the larger series found that 73% (14 of 19) of patients on immune checkpoint inhibitors with biopsy-proven AIN had exposure to other drugs associated with AIN, such as proton pump inhibitors (PPIs) or nonsteroidal anti-inflammatory drugs (NSAIDs) (13,14).
Currently, the American Society of Clinical Oncology guidelines recommend interrupting checkpoint inhibitor therapy and evaluating any patient whose serum creatinine rises 1.5-fold above baseline i.e., ≥stage 1 AKI (17). An empirical course of steroids is recommended for a patient with stage 2 AKI when an alternate cause cannot be identified. However, little is known about how common AKI is in the setting of checkpoint inhibitor use, nor how frequently AKI is attributed to checkpoint inhibitor use. We sought to determine the frequency, severity, cause, and predictors of AKI in a real-world population receiving checkpoint inhibitors.
Materials and Methods
Patient Population
This is a retrospective observational cohort study of all patients who received checkpoint inhibitors for malignancies at the Massachusetts General Hospital Cancer Center between May 2011 and December 2016. Patients received either (1) CTLA4 inhibitor (ipilimumab), (2) PD1 inhibitor (pembrolizumab and nivolumab), (3) PDL1 inhibitor (atezolizumab, avelumab, and durvalumab), or (4) a combination of CTLA4 and PD1 (ipilimumab and nivolumab). Initial administration date of the first checkpoint inhibitor administered, chosen as the exposure, was determined from oncology infusion billing records. The Research Patient Data Repository at Partners Healthcare System was queried to obtain laboratory data, medications, and diagnosis codes. Use of concomitant medications that have been reported to cause AIN, such as NSAIDs, allopurinol, and PPIs were recorded at the start date. We recorded exposure to potentially nephrotoxic chemotherapeutic agents (listed in Table 1) in the 6 months before or anytime during the 12-month study period.
Table 1.
Baseline characteristics of patients receiving immune checkpoint inhibitor therapy
| Characteristics | All Patients | No Sustained AKI | Sustained AKI | Immune Checkpoint Inhibitor–Related AKI |
|---|---|---|---|---|
| Mean±SD or n (%) | ||||
| No. of patients | 1016 | 934 | 82 | 30 |
| Age, yr | 63±13 | 63±14 | 63±12 | 65±12 |
| Baseline creatinine, mg/dL | 0.9±0.3 | 0.9±0.4 | 0.9±0.3 | 0.9±0.3 |
| eGFR, ml/min | 82±22 | 82±22 | 85±20 | 83±22 |
| Men | 616 (61) | 566 (61) | 50 (61) | 18 (60) |
| Women | 400 (39) | 368 (39) | 32 (39) | 12 (40) |
| Race | ||||
| White | 920 (91) | 844 (90) | 76 (93) | 27 (90) |
| Black | 19 (2) | 19 (2) | 0 | 0 |
| Hispanic | 16 (1) | 13 (1) | 3 (4) | 2 (7) |
| Asian | 27 (3) | 25 (3) | 2 (2) | 0 |
| Other/unknown | 34 (3) | 33 (4) | 1 (1) | 1 (3) |
| Cirrhosis | 17 (2) | 15 (2) | 2 (2) | 0 |
| Hypertensiona | 513 (50) | 463 (50) | 50 (61)a | 22 (73) |
| Diabetes | 171 (17) | 156 (17) | 15 (18) | 7 (23) |
| Drugs | ||||
| NSAIDs | 358 (35) | 332 (36) | 26 (32) | 13 (43) |
| Allopurinol | 74 (7) | 68 (7) | 6 (7) | 1 (3) |
| PPIsa | 607 (60) | 549 (59) | 58 (71)a | 23 (77) |
| H2 blockers | 396 (39) | 367 (39) | 29 (35) | 12 (40) |
| ACE/ARB | 403 (40) | 364(39) | 39 (48) | 15 (50) |
| Baseline kidney function (eGFR group) | ||||
| <60 ml/min per 1.73 m2 | 169 (17) | 159 (17) | 10 (12) | 4 (15) |
| 60–90 ml/min per 1.73 m2 | 441 (43) | 406 (43) | 35 (43) | 10 (39) |
| ≥90 ml/min per 1.73 m2 | 406 (40) | 369 (40) | 37 (45) | 12 (46) |
| Immune checkpoint inhibitor class | ||||
| PD1 agents | 701 (69) | 650 (69) | 51 (62) | 16 (53) |
| CTLA4 agents | 249 (24) | 223 (24) | 26 (32) | 12 (40) |
| PDL1 agents | 37 (4) | 34 (4) | 3 (4) | 1 (4) |
| Combined therapy | 29 (3) | 27 (3) | 2 (2) | 1 (3) |
| Prior exposure to nephrotoxic chemotherapya | 309 (30) | 276 (30) | 33 (40)a | 10 (33) |
| Malignancy | ||||
| Melanoma | 438 (43) | 396 (42) | 42 (51) | 18 (60) |
| Lung | 310 (30) | 293 (31) | 17 (21) | 6 (20) |
| Head and neck | 58 (6) | 53 (6) | 5 (6) | 3 (10) |
| Luminal | 38 (4) | 34 (4) | 4 (5) | 0 |
| Liquid | 36 (3) | 33 (4) | 3 (4) | 0 |
| Glioblastoma multiforme | 29 (3) | 26 (3) | 3 (4) | 1 (3) |
| Hepatobiliary | 23 (2) | 19 (2) | 4 (5) | 0 |
| Renal cell carcinoma | 26 (3) | 23 (2) | 3 (3) | 2 (7) |
| Other | 58 (6) | 57 (6) | 1 (1) | 0 |
The baseline characteristics for “All patients” are shown as a percentage of the overall cohort n=1016. For the outcomes, sustained AKI and immune checkpoint inhibitor–related AKI, the percentage of events in each subgroup is presented. First sustained AKI event was specified as the outcome in each patient. Comorbid conditions, including hypertension and cirrhosis, were determined by International Classification of Diseases, Ninth or Tenth Revision codes appearing at least twice in the electronic medical record. Diagnosis of diabetes was determined by either a hemoglobin A1c ≥6.5% or by prescription of a glucose-lowering medication and a diagnosis code for diabetes. Other than the race being unknown in a few patients, there were no missing demographic or comorbidities data. NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton pump inhibitors; H2, Histamine H2-receptor Antagonists; ACE/ARB, Angiotensin Converting Enzyme inhibitors/Angiotensin Receptor Blockers; PD1, Programmed cell death protein 1; CTLA4, Cytotoxic T lymphocyte–associated antigen 4; PDL1, programmed death ligand 1; Combined, ipilimumab (CTLA4) and nivolumab (PD1).
In univariable models comparing demographic and clinical characteristics of patients who experienced sustained AKI with those who did not, only baseline proton pump inhibitor exposure (0.03), nephrotoxic chemotherapy exposure (0.04), and hypertension (0.05) were significant to a P value of <0.10. These were included in the multivariable model for sustained AKI along with other clinically important variables that were determined a priori to be exposures of interest. Nephrotoxic chemotherapies included carboplatin, cisplatin, oxaliplatin, gemcitabine, capecitabine, cyclophosphamide, methotrexate, topotecan, irinotecan, vemurafenib, and bortezomib.
Determination of AKI Events
Laboratory studies obtained as a part of routine care before, during, and after checkpoint inhibitor treatment were analyzed. Baseline creatinine was determined by averaging all serum creatinine measurements in the 6 months before therapy initiation. Patients without a baseline creatinine or on dialysis were excluded. eGFR was calculated using the CKD Epidemiology Collaboration equation (18). AKI was defined as a ≥1.5-fold increase in creatinine from baseline within 12 months of checkpoint inhibitor initiation (19–21). The Kidney Disease: Improving Global Outcomes criteria were used to grade AKI severity by fold change in creatinine from baseline (22,23). To evaluate events most likely to be associated with checkpoint inhibitor toxicity, our primary outcome was “sustained AKI” episodes rather than “transient AKI” episodes. Sustained AKI meant the creatinine remained ≥1.5 times the baseline for at least 3 days and could occur in an inpatient or outpatient setting. Urinalyses obtained within 1 week of meeting criteria for sustained AKI were reviewed. All cases of sustained AKI were chart reviewed by two nephrologists (H.S. and M.E.S.). A third nephrologist (F.C.) resolved diagnostic disagreements (n=4). The cause of sustained AKI was divided into four categories: potential checkpoint inhibitor–related AKI, hemodynamic AKI/acute tubular necrosis (ATN), urinary tract obstruction, and AKI of undetermined cause. “Potential checkpoint inhibitor–related AKI” was defined as AKI attributed to checkpoint inhibitor on the basis of kidney biopsy or subspecialist evaluation, or unexplained sustained AKI experienced at the same time as another immune-related adverse event; these patients did not have evidence for an alternative cause for AKI, such as hemodynamic AKI/ATN or obstruction. “Hemodynamic AKI/ATN” included AKI that occurred in the context of dehydration (poor oral intake, diarrhea, vomiting), tumor lysis syndrome, septic or ischemic ATN, or nephrotoxin exposure. “Obstructive AKI” included all causes of confirmed bilateral ureteral or urinary outlet obstruction. The cause of sustained AKI was listed as “undetermined cause” if the patient did not undergo specific diagnostic workup, did not have another concurrent immune-related adverse event, and did not have checkpoint inhibitor therapy interrupted or receive steroids. Potential checkpoint inhibitor–related AKI was our secondary outcome.
Statistical Analyses
Baseline characteristics were described using means and SD for continuous variables, and counts and percentages for categorical variables. Baseline characteristics of patients who had sustained AKI and those who did not were compared using a t test, chi-squared test, or Fisher exact test, as appropriate. Rates of AKI events were calculated and stratified by checkpoint inhibitor type and by malignancy type.
Survival analyses were conducted to evaluate the effect of baseline risk factors and medication use on the first occurrence of sustained AKI as well as potential checkpoint inhibitor–related AKI. Data were censored at the time of death, loss of follow-up, or at the end of the 12-month observation period, whichever happened first. Death was determined from the electronic medical records or assumed when all laboratory studies ceased. Given that patients who died were no longer at risk of developing sustained AKI, we generated figures for the cumulative incidence function (CIF) for the outcomes, taking into account the competing risk of death (24). Specifically:
where T is time, and D represents the type of events that occurred. When the kth event is sustained AKI, CIF is the probability (Pr) of sustained AKI before time t and before a competing event of death. Fine and Gray subdistribution hazard function was used to estimate the risk of sustained AKI in patients with advanced malignancies given their baseline characteristics (25,26). Adjusted hazard ratios (aHR) for first occurrences of sustained AKI with 95% confidence intervals (95% CIs) are reported. Age, sex, race, checkpoint inhibitor class, and baseline eGFR groups were chosen a priori as exposures of interest for the multivariable regression analysis. In addition, baseline variables with a P value <0.1 in the univariable analysis were included. Since the proportional hazard assumption does not hold for baseline PPI, we added an interaction term between time (before or after 2.5 months of follow-up) and baseline PPI in the model. Descriptive analyses were repeated for our secondary outcome, the first occurrence of checkpoint inhibitor–related AKI; multivariable survival analyses was not performed for this outcome because of the small number of events.
All analyses were performed using SAS version 9.4 and STATA version 13. A two-sided P value of <0.05 was considered to indicate statistical significance. The Institutional Review Board at Partners Healthcare System approved this study.
Results
Between May 2011 and December 2016, 1843 patients were started on checkpoint inhibitors. 1016 patients had at least one creatinine measured in the 6-month baseline period, had at least one creatinine measured within 12 months after starting checkpoint inhibitor therapy, and were not on dialysis at baseline (Figure 1). The average age was 63 years (SD 13), 61% were men, and 91% were white. The mean baseline creatinine was 0.9 mg/dl (SD, 0.3 mg/dl), and 169 (17%) had eGFR<60 ml/min per 1.73 m2 (Table 1). Patients had, on average, ten (SD 11) creatinine measurements in the 6-month baseline period.
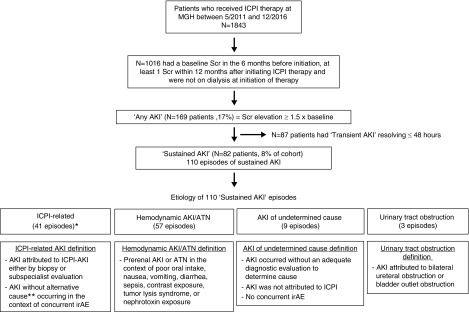
Figure 1.
Patient flow and causes of sustained AKI events. Eighty-two patients (8.1% of the total cohort n=1016) experienced 110 episodes of sustained AKI between them. *The 41 immune checkpoint inhibitor–related sustained AKI events occurred in 30 patients, (3% incidence). **Patient did not have sepsis, nephrotoxin exposure, or a hemodynamic cause, and they did not improve with an intravenous fluid challenge. ICPI, immune checkpoint inhibitor; irAE, immune-related adverse event; MGH, Massachusetts General Hospital; Scr, serum creatinine.
Incidence of AKI, Sustained AKI, and Potential Immune Checkpoint Inhibitor–Related Sustained AKI, and Mortality
A total of 169 patients (17%) experienced an AKI event within the 12-month study period; 87 patients (9%) only experienced transient AKI (lasting ≤48 hours). A total of 82 patients (8% of the total cohort) experienced a sustained AKI event that lasted at least three consecutive days. There were no cases of potential checkpoint inhibitor–related AKI in patients with transient AKI events (Supplemental Figure 1, Supplemental Table 1). The rate of AKI per 100 person-years was 28.7 (95% CI, 24.7 to 33.3) for any AKI, 13.4 (95% CI, 10.8 to 16.6) for sustained AKI and 4.8 (95% CI, 3.4 to 6.9) for potential checkpoint inhibitor–related AKI.
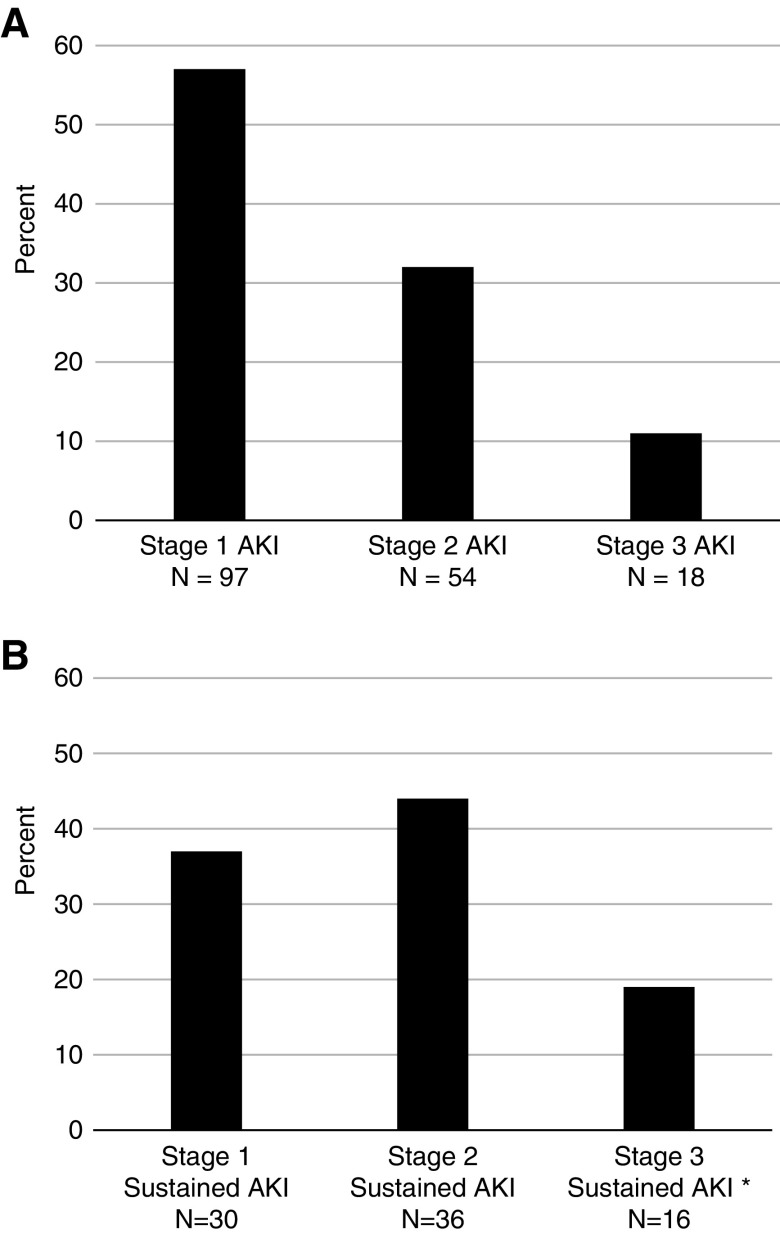
The distributions of AKI stages among patients with any AKI and sustained AKI are shown in Figure 2, A and B. Of the 82 patients with sustained AKI, 19 had multiple sustained AKI events, resulting in a total of 110 sustained AKI events (Figure 1). The first episode of sustained AKI occurred, on average, 106 days (SD 85) after checkpoint inhibitor initiation. A total of 56 events (51%) occurred in the context of inpatient hospitalization, whereas 54 events (49%) were managed exclusively in the outpatient setting. Only 26% were evaluated by a nephrologist.
Figure 2.
Proportion of AKI stages among patients experiencing any and sustained AKI. (A) Any AKI: 169 patients (16.6% of the total cohort n=1016) experienced an AKI event within 12 months of immune checkpoint inhibitor start date. Among these patients, the highest grade of AKI experienced was stage 1 (1.5–2 times the baseline creatinine) in 97 (57%) patients, stage 2 (two to three times the baseline creatinine) in 54 (32%) patients, and stage 3 (more than three times the baseline creatinine) in 18 (11%) patients. (B) Sustained AKI: 82 patients (8% of the total cohort n=1019) experienced a sustained AKI event within 12 months of immune checkpoint inhibitor start date. Among these patients, the highest grade of AKI experienced was stage 1 (1.5–2 times the baseline creatinine) in 30 (37%) patients, stage 2 (two to three times the baseline creatinine) in 36 (44%) patients, and stage 3 (more than three times the baseline creatinine) in 16 (19%) patients. *Of the 16 patients with stage 3 AKI, four required dialysis.
Of the 110 sustained AKI events, chart review determined that 41 (37%) events were potentially checkpoint inhibitor–related, and these occurred in 30 patients (3% incidence) (Figure 1). Fourteen (47%) patients had AKI stage 1, 11 (37%) had stage 2, and five (16%) had stage 3; only one patient (3%) required dialysis. Of the remaining 52 patients with sustained AKI, the majority experienced hemodynamic AKI/ATN. Urinary tract obstruction and AKI due to an undetermined cause were rare (Figure 1).
The majority of patients with potential checkpoint inhibitor–related AKI had a concurrent immune related adverse event (26 of 30 patients, 87%), with 13 (47%) experiencing immune-mediated toxicity in multiple other organs. Thyroiditis and colitis were the most common coexisting immune-related adverse events (Supplemental Table 2). The first episode of potential checkpoint inhibitor–related AKI occurred 105 days (SD 81) after therapy initiation. Only 12 patients with potential checkpoint inhibitor–related AKI (40%) saw a nephrologist in consultation. Urinalysis was obtained in 23 patients (77%), of which ten (43%) demonstrated leukocyturia (>5 white blood cells/high power field). No patients had substantial proteinuria (all were ≤1+ on urinary dipstick). Only one patient underwent a kidney biopsy, which showed AIN; of note, this patient had 0–2 white blood cells seen on urinalysis obtained at the time of kidney biopsy. A total of 21 (70%) patients received high-dose steroids (at least 0.5 mg/kg per day). Seventeen (57%) were rechallenged with checkpoint inhibitors. Deidentified case summaries of the 30 patients with potential checkpoint inhibitor–related sustained AKI are shown in in Supplemental Table 3.
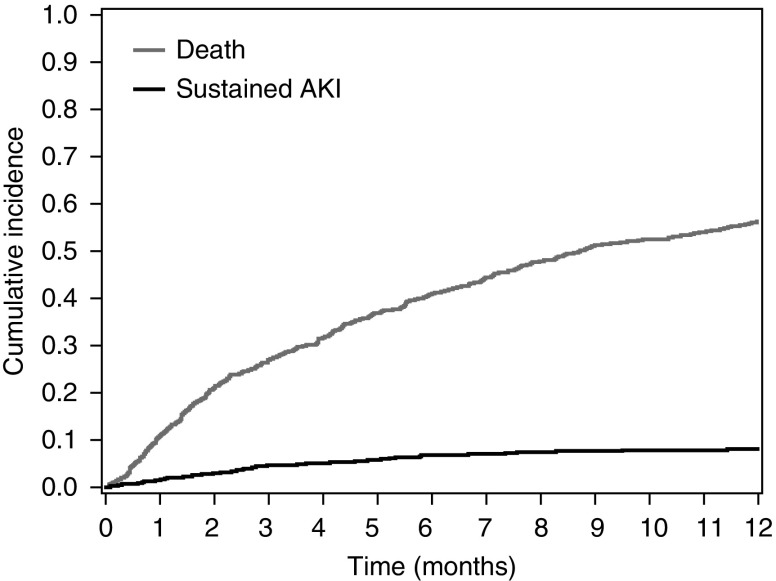
Overall 1-year mortality was extremely high in this cohort with advanced malignancies. Approximately 55% of the cohort died within the 1-year follow-up period. Supplemental Table 4 displays the timing of death by month follow-up. Of the 82 patients with sustained AKI, 54 (67%) died in the follow-up period and death occurred a median of 22 days (interquartile range, 6–84) after the sustained AKI episode. Figure 3 shows the cumulative incidence of death and sustained AKI in our cohort.
Figure 3.
Cumulative incidence curve for death and sustained AKI. Patients were censored at the time of sustained AKI or their last serum creatinine measurement, which served as a proxy for death if laboratory testing suddenly ceased. The breakdown of sustained AKI by those who died and those who were alive at 1 year is shown in Supplemental Figure 4.
Predictors of Sustained AKI and Potential Checkpoint Inhibitor–Related AKI
There were no significant differences in the distribution of age, sex, or race between those who experienced an episode of sustained AKI versus those who did not (Table 1). Baseline eGFR was not associated with the risk of sustained AKI (Table 2). When cumulative incidence rates of sustained AKI and time to immune checkpoint inhibitor–related AKI were evaluated by baseline eGFR, we did not detect a statistical difference between the three eGFR groups (Supplemental Figure 2, A and B). The rate per 100 person-years was 10.0 (95% CI, 5.4 to 18.7) for those with eGFR<60 ml/min per 1.73 m2, 12.5 (95% CI, 9.0 to 17.5) for those with eGFR 60–90 ml/min per 1.73 m2, and 15.8 (95% CI, 11.4 to 21.8) for those with baseline eGFR>90 ml/min per 1.73 m2.
Table 2.
Multivariable Fine and Gray subdistribution hazard regression of sustained AKI
| Characteristics | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Age | 0.99 | 0.98 to 1.02 | 0.89 |
| Men | 1.10 | 0.69 to 1.75 | 0.69 |
| Race (nonwhite versus white) | 0.68 | 0.29 to 1.60 | 0.38 |
| Hypertension | 1.57 | 0.97 to 2.55 | 0.07 |
| Baseline PPI exposure (before follow-up time of 2.5 mo)a | 0.82 | 0.40 to 1.67 | 0.58 |
| Baseline PPI exposure (after follow-up time of 2.5 mo)a | 2.85 | 1.34 to 6.08 | 0.007 |
| Nephrotoxic chemotherapy exposure | 1.52 | 0.95 to 2.44 | 0.08 |
| Immune checkpoint inhibitors class | |||
| CTLA4 versus PD1 | 1.85 | 1.05 to 3.27 | 0.21 |
| PDL1 versus PD1 | 1.38 | 0.43 to 4.45 | |
| Combined versus PD1 | 1.17 | 0.26 to 5.23 | |
| Baseline eGFR group | |||
| 60–90 versus ≥90 ml/min per 1.73 m2 | 0.88 | 0.51 to 1.53 | 0.52 |
| <60 versus ≥90 ml/min per 1.73 m2 | 0.65 | 0.31 to1.36 |
In this multivariable model, baseline demographics (age, race, sex), immune checkpoint inhibitor group, and baseline eGFR were selected a priori for inclusion. Additionally, baseline variables with a P value <0.1 in the univariable model (Table 1) were included. Medication exposure was defined by inclusion in the active medication list at the time that immune checkpoint inhibitor therapy began. PD1 inhibitors were used as the reference group for the immune checkpoint inhibitors class comparison in this model because they were associated with the lowest risk of sustained AKI. Normal eGFR ≥90 ml/min per 1.73 m2 was chosen as reference for eGFR groups. Time to first sustained AKI event (n=82) was the outcome. 95% CI, 95% confidence interval, PPI, proton pump inhibitor, CTLA4, cytotoxic T lymphocyte–associated antigen 4; PD1, Programmed cell death protein 1; PDL1, programmed death ligand 1; Combined, ipilimumab (CTLA4) and nivolumab (PD1).
Interaction of PPI and follow-up time P value =0.01. Derived from the main effect of PPI and the interaction effect between PPI and follow-up time.
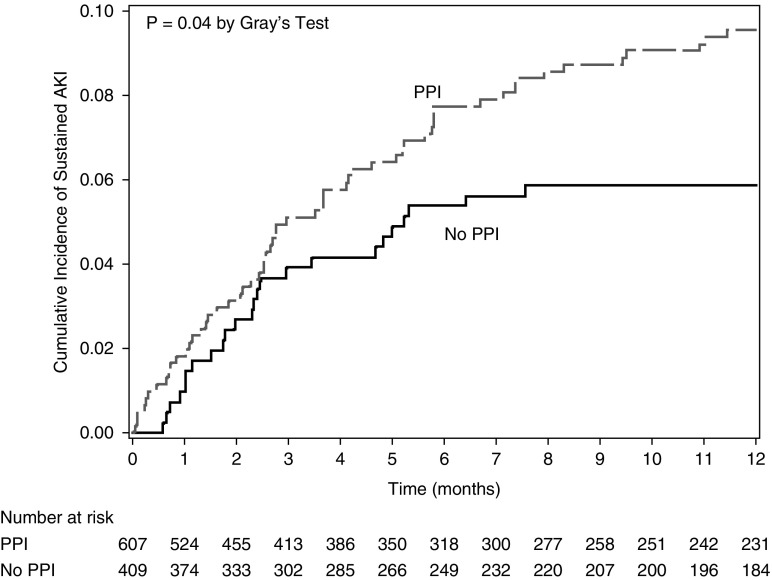
PPI use was associated with sustained AKI in the univariable comparison (Table 1; P=0.03). A similar trend was detected between PPI and potential checkpoint inhibitor–related AKI in an unadjusted analysis (hazard ratio, 2.24; 95% CI, 0.96 to 5.21; P=0.06). Evaluation of the multivariable model, which accounted for the interaction with time, demonstrated that exposure to PPI was a strong risk factor for sustained AKI after 2.5 months of follow-up (aHR, 2.85; 95% CI, 1.34 to 6.08; P<0.007) (Table 2). Figure 4 shows the cumulative incidence of sustained AKI in PPI users versus nonusers at the time of beginning checkpoint inhibitor therapy. The rate per 100 person-years was 17 (95% CI, 13 to 21) for those prescribed PPI at baseline, compared with 9 (95% CI, 6 to 14) for those not on PPI. Other medications associated with AIN, such as allopurinol and NSAIDs, and other gastric acid–lowering medications (H2 blockers) were not significantly associated with either sustained AKI or potential checkpoint inhibitor–related AKI (Table 1). The risk of sustained AKI in patients who had exposure to nephrotoxic chemotherapy did not reach statistical significance (aHR, 1.52; 95% CI, 0.95 to 2.44; P=0.08) (Table 1).
Figure 4.
Cumulative incidence curve for sustained AKI by PPI use. There was a significant difference in the cumulative incidence of sustained AKI by baseline PPI, which began after 2.5 months of follow- up. There was a significant interaction of PPI and follow-up time (P=0.01). Data were censored at the time of death, loss of follow-up, or at the end of the 12-month observation period, whichever happened first. A breakdown of events (death, AKI) is provided in Supplemental Table 5C.
With regard to the classes of checkpoint inhibitors used, PD1 use was most common. A total of 701 patients (69%) received nivolumab (n=433) or pembrolizumab (n=269), 249 (24%) received a CTLA4 inhibitor (ipilimumab), 37 (4%) received a PDL1 inhibitor (atezolizumab n=15, avelumab n=2, or durvalumab n=20), and 29 (3%) received combination therapy (ipilimumab and nivolumab). Frequency of sustained AKI was numerically higher, but not statistically significant, in patients receiving CTLA4 therapy (10%) compared with patients receiving PD1 inhibitors (7%), PDL1 inhibitors (8%), or combined therapy (7%) (Table 2). Figure 5 shows the proportion of grades of sustained AKI and potential checkpoint inhibitor–related AKI by checkpoint inhibitor class. Although there were changes in the annual trends in which checkpoint inhibitors were administered, AKI rates remained stable (Supplemental Table 5).
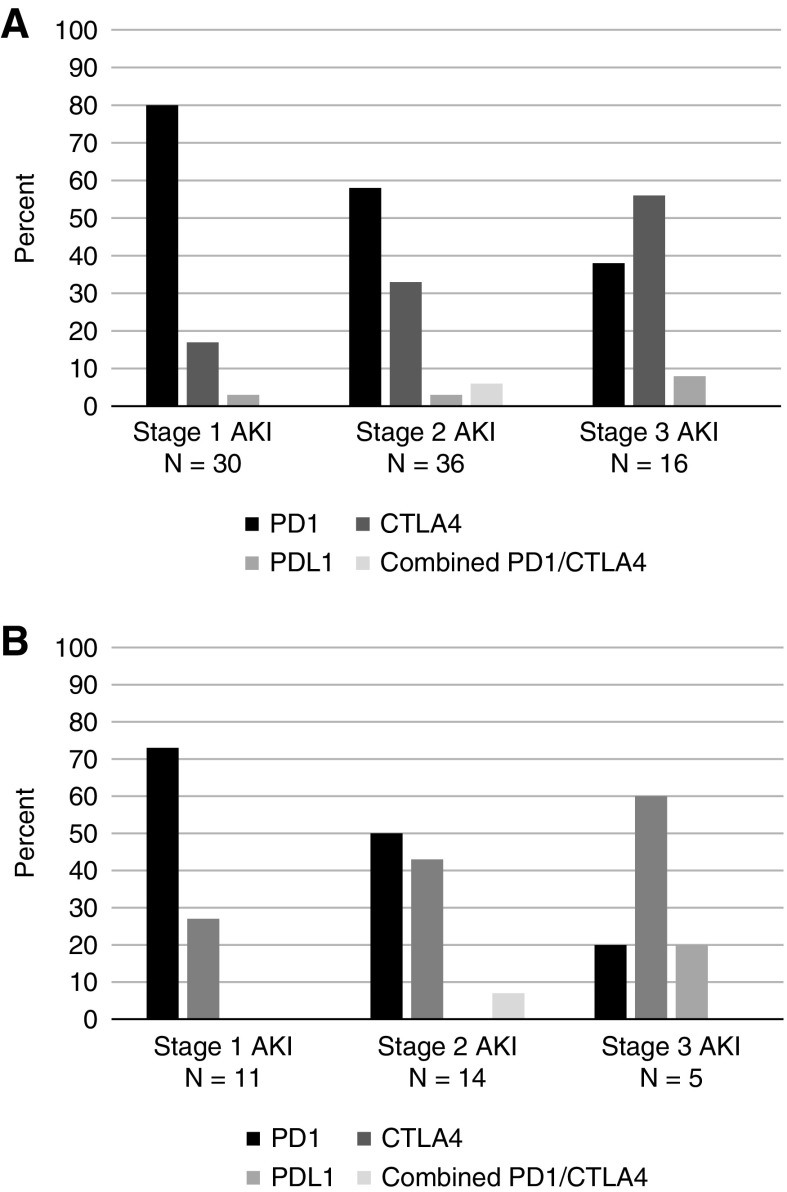
Figure 5.
Proportion of KDIGO stages of sustained AKI and immune checkpoint inhibitor-related AKI by immune checkpoint inhibitor type. (A) Sustained AKI: A total of 80% of stage 1 AKI occurred in patients receiving PD1 inhibitors (nivolumab, pembrolizumab), whereas 56% of stage 3 AKI occurred in patients receiving the CTLA4 inhibitor ipilimumab. (B) Immune checkpoint inhibitor-related AKI: A total of 73% of stage 1 AKI occurred in patients receiving PD1 inhibitors (nivolumab, pembrolizumab), whereas 60% of stage 3 AKI occurred in patients receiving the CTLA4 inhibitor ipilimumab.
Incidence of AKI (any, sustained, and potential checkpoint inhibitor–related AKI) did not statistically differ by malignancy type (Supplemental Figure 3); however, this study was not powered to detect differences in the rates of AKI by malignancy type.
Discussion
This is the largest retrospective cohort study evaluating AKI in patients receiving checkpoint inhibitors for cancer. We evaluated more than 1000 patients receiving a variety of different checkpoint inhibitors for a wide range of malignancy types and found that AKI and sustained AKI events were common (17% and 8%, respectively) within 12 months of initiating therapy. This is the first report to define the incidence of checkpoint inhibitor–related AKI using a consistent approach and definition; we found that it affects 3% of patients and occurs a median of 15 weeks after starting therapy. This is slightly higher than the reported treatment-related incidence of 2% from clinical trials data and consistent with the previously described timing of AKI (13,15). As the clinical spectrum of checkpoint inhibitor use continues to grow, the study of associated toxicities becomes increasingly important (27). This and other reports all confirm that there are no consistent symptoms, nor urinary findings, to facilitate a noninvasive diagnosis of immune checkpoint inhibitor–related AIN (13,14,28). None of our patients had significant proteinuria and we suspect that the majority of potential immune checkpoint inhibitor–related events were secondary to varying degrees of tubular and interstitial inflammation and injury. However, it is important to note that there have been recent reports of glomerular diseases, including lupus nephritis, vasculitis, and podocytopathies, and consideration of these diagnostic possibilities is warranted should significant proteinuria be detected in patients on checkpoint inhibitors (15,29–31). Given the risks associated with kidney biopsy, clinicians are often left with the dilemma of whether to empirically treat for checkpoint inhibitor–induced AIN without a definitive diagnosis. However, a misdiagnosis of AIN and empirical corticosteroid treatment are not without risk and may compromise treatment of the underlying cancer. Although some studies suggest the treatment of immune-related events with high-dose steroids does not adversely affect cancer outcomes, others have shown higher mortality with the use of high-dose steroids in patients with immune-related hypophysitis resulting from immunotherapy (32,33). As such, establishing the cause of AKI to the greatest extent of certainty possible is vital.
Baseline characteristics, such as age, race, sex, malignancy type, or baseline eGFR, were not associated with sustained AKI or checkpoint inhibitor–related AKI; therefore, the use of these agents should not be withheld in patients with CKD who are otherwise good candidates for therapy, with the caveat that very few patients in this cohort had an eGFR<30 ml/min per 1.73 m2. In this cohort, we did not find a statistically significant association between sustained AKI events and checkpoint inhibitor type; however, it should be noted that only a small number (n=29, 3%) were treated with combination therapy. Our cohort included patients with many tumor types, and the overall 1-year mortality was high.
PPI use at the time of checkpoint inhibitor initiation was a risk factor for sustained AKI after prolonged exposure to checkpoint therapy. This correlates with the typical timing of checkpoint inhibitor–related AKI reported in this and other cohorts, which occurs, on average, 2–4 months after starting therapy (13,15). If other datasets validate PPI use as a risk factor, it may have important treatment implications. For instance, it may be beneficial to switch patients without a strong indication for PPIs to H2 blocker therapy before starting checkpoint inhibitors. In our cohort, PPI use was also more common in patients experiencing potential checkpoint inhibitor–related AKI. Other case series have noted a high proportion of patients with checkpoint-inhibitor induced AIN were on culprit AIN medications (including PPIs and NSAIDs) at the time of diagnosis; it is possible that checkpoint inhibitors may induce loss of tolerance of memory T cells that have previously been primed to a drug or other hapten. Drug hapten–specific T cell responses that drive hypersensitivity reactions affecting the skin and internal organs are regulated by PD1 and CTLA4 pathways (34); blocking these pathways may inadvertently lead to activation of T cells to drug antigens. Further study into the mechanism of checkpoint inhibitor–related AIN is desperately needed, and may provide insight into AIN from other common causes.
Our study has several limitations. Although the cohort is large, it was sourced from a single health care system and was a predominantly white population, raising concerns about the generalizability to other populations. Another limitation was the retrospective nature of the data collection. It is possible that patients had AKI events managed at hospitals outside our health care network, resulting in an underestimation of AKI frequency. We only included patients who had at least one creatinine measured in the 12-month follow-up period to ensure that patients getting the majority of their care outside our health care system were not included in the analysis. Furthermore, on average, our cohort had >15 creatinine measurements in the 12 months after starting checkpoint inhibitors, suggesting they were followed closely. The ability to determine the timeline and acuity of AKI precisely may have been affected in the patients with sustained AKI who were not admitted to the hospital; in these cases, laboratory tests were not performed daily. Because this was a retrospective cohort, concluding with administrations that took place until the end of 2016, PDL1 use and combination checkpoint inhibitor therapy were less commonly used than in current practice. Since we used the first checkpoint inhibitor administered as the exposure, we may have misattributed some AKIs because some patients switched to a second checkpoint inhibitor later in their treatment course. However, chart review of the 110 episodes of sustained AKI confirmed that only seven episodes (6%) had exposure to a second checkpoint inhibitor class before the sustained AKI episode. Our primary model was for sustained AKI and any associations with potential checkpoint inhibitor–related AKI is mainly descriptive and should be interpreted in the context of a limited number of cases. Finally, there was limited phenotyping of some cases of potential checkpoint inhibitor–related AKI, with only one undergoing biopsy; this is likely because of low nephrology referral and limited understanding of spectrum of the effects of immune checkpoint inhibitors on the kidney, especially given that the report by Cortazar et al. (13) first describing 13 cases of checkpoint inhibitor–related AIN was only published in 2016, the final year of this cohort study.
Nephrologists must be aware of the high frequency of sustained AKI after checkpoint inhibitor therapy and the chances that such events are checkpoint inhibitor–related (approximately one in three). With a high frequency of AKI events being related to prerenal azotemia or ATN, a thorough assessment is required to rule out other causes of AKI before starting immunosuppression. Accurately diagnosing these events, including the use of kidney biopsy when needed, will help ensure appropriate management of patients with AKI after checkpoint inhibitor administration.
Disclosures
Dr. Cortazar reports that he has served as a consultant for ChemoCentryx and Momenta Pharmaceuticals. Dr. Mooradian reports receiving an honorarium from AstraZeneca. Dr. Sise reports receiving research grant funding from EMD Serono and Gilead Sciences and personal fees from positions on the scientific advisory boards at AbbVie, Gilead, and Merck, outside the submitted work. Dr. Sullivan reports receiving personal fees from serving as a consultant or on advisory boards of Amgen, Array, Compugen, Genentech, Merck, Novartis, and Replimmune. All of the remaining authors have nothing to disclose.
Funding
Dr. Sullivan is supported by grants from Amgen and Merck.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Acute Kidney Injury with Immune Checkpoint Inhibitors: A Push beyond Case Reports,” on pages 1679–1681.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00990119/-/DCSupplemental.
Supplemental Table 1: Baseline characteristics of patients receiving immune checkpoint inhibitor therapy grouped by duration of AKI
Supplemental Table 2: Incidence and types of concurrent immune related adverse events in patients with potential immune checkpoint inhibitor-related acute kidney injury
Supplemental Table 3: De-identified case summaries of patients with ‘potential immune checkpoint inhibitor related AKI’.
Supplemental Table 4A, 4B, 4C, 4D: Events of sustained AKI, ICPI AKI and Death by eGFR group and baseline PPI use.
Supplemental Table 5: Incidence of ‘sustained AKI’ and ‘potential ICPI related AKI’ by year and drug type.
Supplemental Figure 1A and 1B: Incidence of transient AKI events by KDIGO stage and etiology of stage 2-3 transient AKI events.
Figure 2A, 2B. Cumulative Incidence curve for sustained AKI and ICPI AKI by eGFR group
Supplemental Figure 3A, 3B, 3C. Incidence of ‘any AKI’, ‘sustained AKI’, ‘ICPI AKI’ by malignancy type
Supplemental Figure 4: Cumulative Incidence curve for sustained AKI by Vital Status.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD: Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33: 1889–1894, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR: Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627–1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators: Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803–1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823–1833, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R: Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387: 1909–1920, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P: Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 17: 1374–1385, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, Fishman MN, Zhang J, Srinivas S, Parikh J, Antal J, Jin X, Gupta AK, Ben Y, Hahn NM: Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 3: e172411, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG: PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 379: 341–351, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, Sidlow R, Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Sury K, Perazella MA, Shirali AC: Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol 14: 571–588, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, Varga A, Malka D, Leary A, Michels J, Michot JM, Marabelle A, Lambotte O, Amoura Z, Soria JC, Kaaki S, Quellard N, Goujon JM, Brocheriou I: Renal toxicities associated with pembrolizumab. Clin Kidney J 12: 81–88, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network: Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36: 1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA: Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, Szczech L: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 62: 849–859, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S: Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 23. National Cancer Institute: Division of Cancer Treatment & Diagnosis: CTCAE guidelines, version 5.0. 2018. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed June 1, 2018.
- 24.Austin PC, Lee DS, Fine JP: Introduction to the analysis of survival data in the presence of competing risks. Circulation 133: 601–609, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 26.Lau B, Cole SR, Gange SJ: Competing risk regression models for epidemiologic data. Am J Epidemiol 170: 244–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators: Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378: 2078–2092, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Murakami N, Motwani S, Riella LV: Renal complications of immune checkpoint blockade. Curr Probl Cancer 41: 100–110, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadel F, El Karoui K, Knebelmann B: Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 361: 211–212, 2009 [DOI] [PubMed] [Google Scholar]
- 30.van den Brom RR, Abdulahad WH, Rutgers A, Kroesen BJ, Roozendaal C, de Groot DJ, Schröder CP, Hospers GA, Brouwer E: Rapid granulomatosis with polyangiitis induced by immune checkpoint inhibition. Rheumatology (Oxford) 55: 1143–1145, 2016 [DOI] [PubMed] [Google Scholar]
- 31. Gao B, Lin N, Wang S, Wang Y: Minimal change disease associated with anti-PD1 immunotherapy: A case report. BMC Nephrol 19: 156, 2018. [DOI] [PMC free article] [PubMed]
- 32.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM, Panageas KS, Wolchok JD, Chapman PB: Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 33: 3193–3198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, Sullivan RJ: High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124: 3706–3714, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Gibson A, Faulkner L, Lichtenfels M, Ogese M, Al-Attar Z, Alfirevic A, Esser PR, Martin SF, Pirmohamed M, Park BK, Naisbitt DJ: The effect of inhibitory signals on the priming of drug hapten-specific T cells that express distinct Vβ receptors. J Immunol 199: 1223–1237, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.