Visual Abstract
Keywords: end stage kidney disease, peritoneal dialysis, prospective payment system
Abstract
Background and objectives
Peritoneal dialysis (PD) for ESKD is associated with similar mortality, higher quality of life, and lower costs compared with hemodialysis (HD), but has historically been underused. We assessed the effect of the 2011 Medicare prospective payment system (PPS) for dialysis on PD initiation, modality switches, and stable PD use.
Design, setting, participants, & measurements
Using US Renal Data System and Medicare data, we identified all United States patients with ESKD initiating dialysis before (2006–2010) and after (2011–2013) PPS implementation, and observed their modality for up to 2 years after dialysis initiation. Using logistic regression models, we examined the associations between PPS and early PD experience (any PD 1–90 days after initiation), late PD use (any PD 91–730 days after initiation), and modality switches (PD-to-HD or HD-to-PD 91–730 days after initiation). We adjusted for patient, dialysis facility, and regional characteristics.
Results
Overall, 619,126 patients with incident ESKD received dialysis at Medicare-certified facilities, 2006–2013. Observed early PD experience increased from 9.4% before PPS to 12.6% after PPS. Observed late PD use increased from 12.1% to 16.1%. In adjusted analyses, PPS was associated with increased early PD experience (odds ratio [OR], 1.51; 95% confidence interval [95% CI], 1.47 to 1.55; P<0.001) and late PD use (OR, 1.47; 95% CI, 1.45 to 1.50; P<0.001). In subgroup analyses, late PD use increased in part due to an increase in HD-to-PD switches among those without early PD experience (OR, 1.59; 95% CI, 1.52 to 1.66; P<0.001) and a decrease in PD-to-HD switches among those with early PD experience (OR, 0.92; 95% CI, 0.87 to 0.98; P=0.004).
Conclusions
More patients started, stayed on, and switched to PD after dialysis payment reform. This occurred without a substantial increase in transfers to HD.
Introduction
ESKD affects >700,000 Americans (1). Patients with ESKD who have not undergone kidney transplant can be treated with in-center hemodialysis (HD), home-based peritoneal dialysis (PD), or home HD. PD has been associated with a similar risk of mortality (2), but greater patient-reported quality of life (3–6), lower rates of complications (7,8), and lower societal costs (1,9–11) compared with HD. Nephrologists estimate that roughly half of patients with ESKD are good candidates for PD (12,13), and patients strongly prefer PD when given the option (5,6,14). Despite these advantages, only 7.6% of patients with ESKD were on PD in 2010 (1).
In 2011, the Centers for Medicare and Medicaid Services (CMS) implemented the ESKD prospective payment system (PPS), which altered payment for dialysis treatment by bundling dialysis, medications, and ancillary services into a single payment, adjusted for patient- and facility-level characteristics (15). The PPS also provided a training add-on for home dialysis. Because PD has historically been associated with lower costs than HD (10,11,16), dialysis facility revenues under the PPS were expected to increase by $330 per month for PD and decrease by $117 per month for in-center HD (17). Thus, it was anticipated that the PPS would increase supply and use of PD across the country.
The proportion of facilities offering PD increased modestly after the PPS, from 36% in 2006 to 42% in 2013 (18). Recent studies have found that PD use in the first 3–4 months after dialysis initiation increased after the PPS, regardless of age, sex, race, and ethnicity (18–22). Collectively, these studies show an initial upward trend in PD use. However, the sustainability of this trend over the long-term, and the factors contributing to it, are unknown. The PPS may contribute to other dialysis use patterns—increased use of PD more than 90 days after dialysis initiation, increased rates of HD-to-PD switches, and decreased rates of PD-to-HD switches—that have not been examined.
The objective of this study is to assess the effect of the PPS on PD use between 2006 and 2013 beyond the first few months after dialysis initiation. We extend the effort of recent studies (19–22) by comparing PD use up to 2 years after initiation and dialysis modality switches, before and after PPS implementation. We hypothesize that the PPS increased not only early PD experience, but also late PD use, by increasing PD initiation at the outset, increasing HD-to-PD switches, and decreasing PD-to-HD switches over time. Our results will improve our understanding of the PPS’s effect on an underused and clinically equivalent dialysis modality.
Materials and Methods
Study Design, Population, and Data Sources
We conducted a retrospective cohort study of all United States patients diagnosed with ESKD between 2006 and 2013. The pre-PPS period comprised years 2006–2010. The post-PPS period comprised years 2011–2013. Patients were excluded if they had missing demographic information, died within 90 days of initiating dialysis, recovered kidney function within 180 days of initiating dialysis, had no record of modality type by day 90, or had missing or invalid ZIP codes (Supplemental Figure 1). Patients enrolled in Medicare Advantage at dialysis initiation were also excluded, because their health care use claims were not publicly available when this study was conducted.
Patient demographic and clinical characteristics were from the US Renal Data System (USRDS) CMS Medical Evidence Report (CMS Form 2728) (23), which is completed whenever a patient with ESKD begins or re-enters care at a dialysis facility. Dialysis modality data were from the USRDS Treatment History files, which are on the basis of a combination of Medicare claims and provider-reported data (23). Dialysis facility characteristics were from the Annual Facility Survey (CMS Form 2744) of all Medicare-approved dialysis facilities. We merged these survey data with the Medicare Provider of Service dataset, which contains additional information on geographic location, to track dialysis facility ownership changes and improve the accuracy of facility characteristics (24). Hospital census data came from the American Hospital Association’s Annual Hospital Survey. County-level demographic statistics from the Area Health Resource File were converted to the ZIP code level. All ZIP code-level data were aggregated to generate market-level statistics for each year of the study period. We defined markets as hospital referral regions, which approximate the area within which patients may travel for tertiary care and monthly PD maintenance visits (25–27).
Measurement
Patient use was observed from dialysis initiation until death, kidney transplant, or the end of a 2-year observation period. We examined three outcomes to assess the effect of the PPS on PD use. Early PD experience was defined as a dichotomous indicator of any PD use versus no PD use within the first 90 days of initiating dialysis. We did not require the conventional “60-day rule” (60 consecutive days of stable treatment modality) for this outcome, so that we could assess whether the PPS led to any change in PD initiation—even short-lived—rather than stable treatment (23). The second outcome was late PD use, a dichotomous indicator of stable PD use (at least 60 consecutive days) between 91 days and 2 years after initiating dialysis (23). The third outcome was a dichotomous indicator of switch from one modality to the other 91 days to 2 years after dialysis initiation, with stable treatment on the switched modality. In this outcome, patients were stratified according to early PD experience: among patients with early PD experience, we assessed PD-to-HD switches; among patients without early PD experience, we assessed HD-to-PD switches.
Our analysis controlled for baseline patient and regional characteristics that might influence dialysis modality choice. Patient baseline demographic characteristics included age, sex, race, Hispanic ethnicity, United States region, employment status, and insurance status. Using patient and facility ZIP codes, we calculated patient distance to their nearest dialysis facility (which may or may not offer PD) and relative distance to the nearest PD facility (25). Patient clinical characteristics included cause of ESKD, receipt of pre-ESKD nephrology care, baseline kidney function (28), baseline body mass index, and comorbid conditions.
Regional characteristics included dialysis facility composition (e.g., proportion of freestanding, for-profit, and urban facilities), demographics (e.g., proportion of urban residents, and per capita income in the general population), hospital density (number of hospital beds per 100,000 in the hospital referral region) (18,29), and dialysis facility competition (30–32). Competition was calculated using the Herfindahl–Hirschman index, where facilities affiliated with the same chain were considered a single firm in each market (30,31,33).
Analysis
Multiple logistic regression models were fit to examine changes in outcomes between the pre-PPS and post-PPS periods, adjusting for patient and regional characteristics. We used discontinuity regression models to model time of dialysis initiation in years (2006–2013), with the PPS beginning in 2011. Unlike previous studies that modeled 2009–2010 as a separate transitional period (19,21), we included these years in the pre-PPS period to conservatively assess the effect of the fully implemented PPS. For the modality switch outcome, two separate models were fit: one for the subgroup with any early PD experience, and one for the subgroup without early PD experience (i.e., only early HD experience). Patients were censored at death or transplant. We included all aforementioned covariates in our models to address potential confounding.
To assess the overall PPS effect (odds ratio [OR]) for each of the outcomes, we estimated the differences in early PD experience, late PD use, and modality switches between the pre-PPS and post-PPS periods, averaging over the years in each of the periods. Predicted probabilities over the pre-PPS and post-PPS periods, as well as for individual years, were estimated using the appropriate intercept and slope parameters, with fixed values of all covariates centered at means (see the Supplemental Material). Caution should be used when inferring these probabilities to population parameters.
Patients who started dialysis toward the end of the pre-PPS period (2008–2010) may have had a substantial portion of their dialysis use spill over into the post-PPS period. In sensitivity analysis, we excluded 2008–2010 from the pre-PPS period to assess the effect of this phenomenon. This study was approved by the Institutional Review Board of Duke University.
Results
Descriptive Characteristics
Overall, we identified 619,126 patients with incident ESKD who received dialysis at Medicare-certified facilities: 387,115 in the pre-PPS era and 232,011 in the post-PPS era (Table 1). Before the PPS, mean age was 61, 57% of patients were men, and 64% were white. A majority of patients were unemployed (87%), lived in urban areas (79%), and had consulted with a nephrologist before their ESKD diagnosis (60%). The cause of ESKD was diabetes for almost half of the patients (47%). These characteristics remained generally unchanged after the PPS.
Table 1.
Characteristics of patients with incident ESKD in the United States, by Medicare prospective payment system policy period and early peritoneal dialysis experience
| Characteristic | Overall N=619,126 | Prepolicy Cohort: 2006–2010 N=387,115 | Postpolicy Cohort: 2011–2013 N=232,011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HD n=350,619 | PDa n=36,496 | HD n=202,837 | PDa n=29,174 | |||||||
| Cohort characteristics | ||||||||||
| Days observed, mean (SD) | 615 | (198) | 607 | (204) | 625 | (183) | 622 | (194) | 641 | (172) |
| Censored, N (%) | 244,833 | (40) | 145,418 | (42) | 18,589 | (51) | 69,641 | (34) | 11,235 | (39) |
| Patient characteristics | ||||||||||
| Age, mean (SD) | 61 | (16) | 61 | (15) | 55 | (17) | 61 | (15) | 55 | (16) |
| Male, N (%) | 353,727 | (57) | 199,025 | (57) | 20,479 | (56) | 117,419 | (58) | 16,804 | (58) |
| Race, N (%) | ||||||||||
| White | 396,992 | (64) | 220,586 | (63) | 26,101 | (72) | 129,824 | (64) | 20,481 | (70) |
| Black | 185,293 | (30) | 109,967 | (31) | 7953 | (22) | 60,855 | (30) | 6518 | (22) |
| Other | 36,841 | (6) | 20,066 | (6) | 2442 | (7) | 12,158 | (6) | 2175 | (8) |
| Hispanic ethnicity, N (%) | 87,414 | (14) | 48,649 | (14) | 4382 | (12) | 30,452 | (15) | 3931 | (14) |
| Employed (full- or part-time), N (%) | 77,597 | (13) | 38,433 | (11) | 10,143 | (28) | 20,962 | (10) | 8059 | (28) |
| Urban residential status, N (%) | 492,730 | (80) | 280,062 | (80) | 27,499 | (75) | 162,771 | (80) | 22,398 | (77) |
| Distance to nearest dialysis facility, mean miles (SD)b | 4 | (7) | 4 | (7) | 6 | (12) | 3 | (6) | 5 | (12) |
| Relative distance to nearest PD facility, mean miles (SD)b | 5 | (12) | 5 | (12) | 5 | (14) | 4 | (11) | 4 | (11) |
| Region, N (%) | ||||||||||
| South | 261,662 | (42) | 146,864 | (42) | 16,248 | (45) | 85,340 | (42) | 13,210 | (45) |
| Midwest | 133,346 | (22) | 76,494 | (22) | 7903 | (22) | 42,869 | (21) | 6080 | (21) |
| Northeast | 104,349 | (17) | 61,278 | (18) | 4850 | (13) | 34,754 | (17) | 3467 | (12) |
| West | 119,769 | (19) | 65,983 | (19) | 7495 | (21) | 39,874 | (20) | 6417 | (22) |
| Insurance, N (%)c | ||||||||||
| Medicare | 325,987 | (53) | 184,971 | (53) | 13,323 | (37) | 115,525 | (57) | 12,168 | (42) |
| Medicaid | 173,113 | (28) | 100,723 | (29) | 5909 | (16) | 61,600 | (30) | 4881 | (17) |
| Department of Veterans Affairs | 14,176 | (2) | 7764 | (2) | 512 | (1) | 5411 | (3) | 489 | (2) |
| Employer group | 164,044 | (27) | 91,041 | (26) | 16,747 | (46) | 44,232 | (22) | 12,024 | (41) |
| Other | 121,901 | (20) | 72,005 | (21) | 6963 | (19) | 37,840 | (19) | 5093 | (18) |
| None | 55,415 | (9) | 31,739 | (9) | 3270 | (9) | 17,519 | (9) | 2887 | (10) |
| Cause of ESKD, N (%) | ||||||||||
| Diabetes | 289,664 | (47) | 165,317 | (47) | 14,928 | (41) | 97,049 | (48) | 12,370 | (42) |
| Hypertension | 179,257 | (29) | 101,296 | (29) | 8762 | (24) | 61,497 | (30) | 7702 | (26) |
| GN | 56,086 | (9) | 29,656 | (9) | 6259 | (17) | 15,501 | (8) | 4670 | (16) |
| Other/Missing | 94,119 | (15) | 54,350 | (16) | 6547 | (18) | 28790 | (14) | 4432 | (15) |
| Unknown | ||||||||||
| Comorbidities, N (%)d | ||||||||||
| Hypertension | 538,007 | (87) | 301,935 | (86) | 31,457 | (86) | 178,880 | (88) | 25,735 | (88) |
| Diabetes | 341,783 | (55) | 193,327 | (55) | 16,730 | (46) | 117,329 | (58) | 14,397 | (49) |
| Congestive heart failure | 185,094 | (30) | 113,070 | (32) | 6331 | (17) | 60,985 | (30) | 4708 | (16) |
| Atherosclerotic heart disease | 114,870 | (19) | 71,700 | (20) | 5252 | (14) | 34,577 | (17) | 3341 | (12) |
| Peripheral vascular disease | 75,976 | (12) | 47,210 | (14) | 3232 | (9) | 23,456 | (12) | 2078 | (7) |
| Pre-ESKD nephrology care, N (%) | ||||||||||
| Yes | 375,642 | (61) | 201,886 | (58) | 29,641 | (81) | 120,448 | (59) | 23,667 | (81) |
| No | 171,393 | (28) | 106,635 | (30) | 5001 | (14) | 55,766 | (28) | 3991 | (14) |
| Unknown | 72,091 | (12) | 42,098 | (12) | 1854 | (5) | 26,623 | (13) | 1516 | (5) |
| BMI, kg/m2, mean (SD) | 29 | (8) | 29 | (8) | 29 | (7) | 30 | (8) | 29 | (7) |
| eGFR, ml/min per 1.73 m2, mean (SD)e | 10 | (6) | 10 | (6) | 10 | (5) | 10 | (6) | 10 | (5) |
| Market characteristics (hospital referral region) | ||||||||||
| Dialysis facility composition, mean (SD) | ||||||||||
| % offering PD in 2006f | 39 | (15) | 38 | (15) | 40 | (16) | 41 | (15) | 43 | (15) |
| % freestanding facilities | 90 | (15) | 89 | (16) | 90 | (15) | 91 | (13) | 92 | (12) |
| % for profit | 82 | (20) | 81 | (20) | 82 | (21) | 85 | (18) | 85 | (18) |
| % chain-affiliated | 85 | (16) | 83 | (17) | 84 | (17) | 86 | (15) | 88 | (13) |
| % urban location | 77 | (24) | 77 | (24) | 74 | (24) | 78 | (23) | 75 | (24) |
| Dialysis market competition, mean (SD)g | 39 | (18) | 39 | (18) | 41 | (19) | 39 | (18) | 41 | (18) |
| Hospital density, mean (SD)h | 301 | (117) | 306 | (121) | 303 | (112) | 293 | (113) | 290 | (107) |
| % urban general population, mean (SD) | 81 | (16) | 81 | (16) | 78 | (16) | 81 | (16) | 79 | (16) |
| Per capita income, $1000s, mean (SD) | 40 | (99) | 39 | (8) | 38 | (8) | 43 | (9) | 42 | (9) |
HD, hemodialysis; PD, peritoneal dialysis; BMI, body mass index; GN, glomerulonephritis.
PD modality at any point during the 2-yr follow-up period.
Absolute distance to nearest dialysis facility and relative distance (difference) between distance to nearest dialysis facility and distance to nearest dialysis facility that offers PD.
Insurance status is not mutually exclusive. Patients can have multiple sources of insurance coverage.
Other comorbidities included chronic obstructive pulmonary disease, cerebrovascular disease, inability to ambulate, inability to transfer, other cardiac disease, cancer, drug dependence, and tobacco use, not shown here but described in Supplemental Table 1.
Calculation of eGFR is on the basis of the Chronic Kidney Disease Epidemiology Collaboration formula.
PD includes continuous ambulatory PD, continuous cyclic PD, and other PD.
Measured using the Herfindahl–Hirschman index of dialysis market competition. Higher values correspond to less competition, i.e., more monopolistic markets.
Hospital density is defined as the number of community hospital beds per 100,000 people in a hospital referral region.
The proportion of for-profit facilities in markets increased from 81% pre-PPS to 85% post-PPS, and the proportion of facilities affiliated with chains increased from 83% to 87% (18).
Early PD Experience and Late PD Use
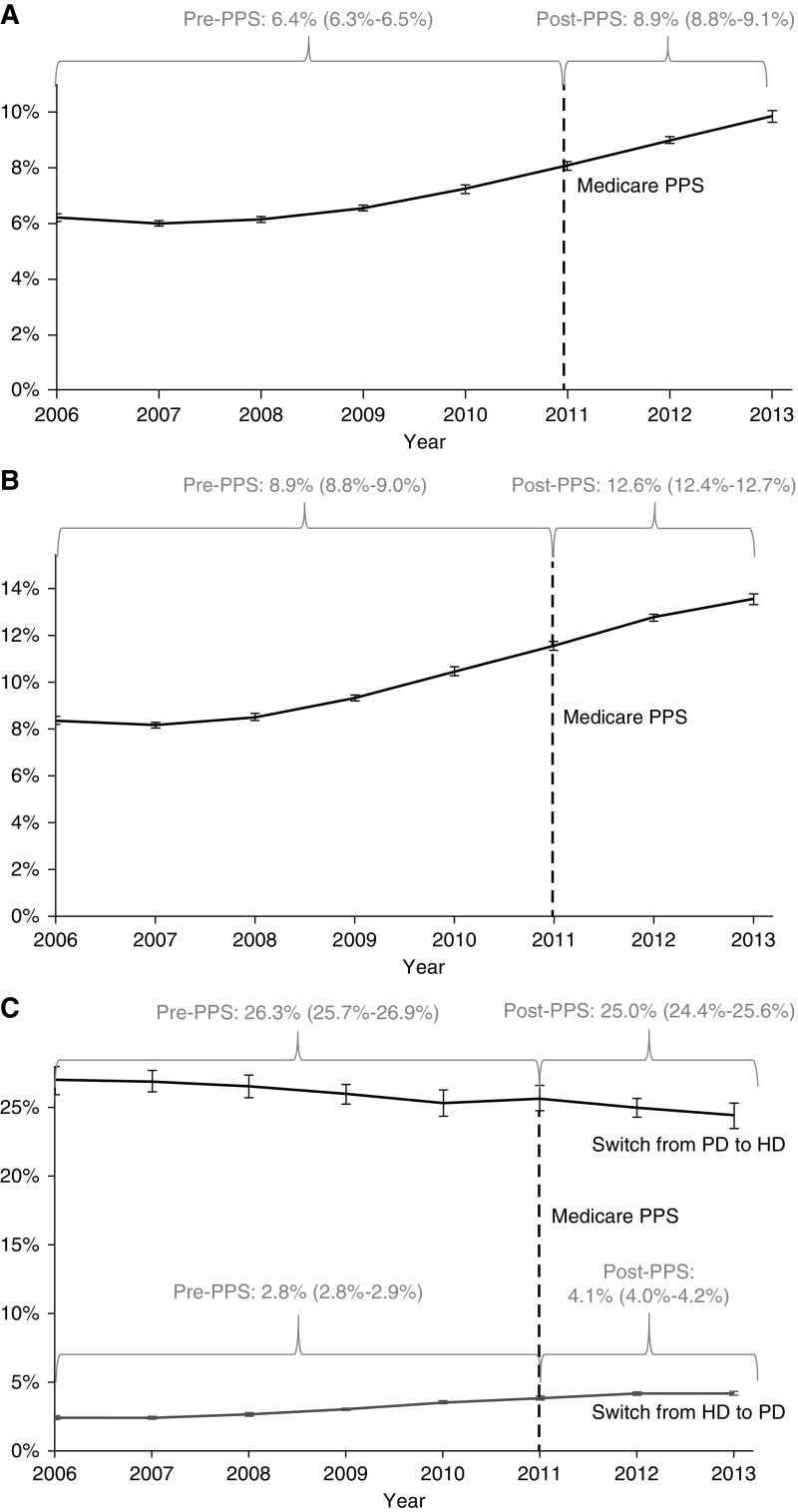
In the pre-PPS period, an average of 9.4% of patients (36,496 of 387,115) had early PD experience (Figure 1, Panel A). In the post-PPS period, an average of 12.6% of patients (29,174 of 232,011) had early PD experience (Figure 1, Panel B), a 3.2% increase. In adjusted analysis, the PPS was associated with increased odds of early PD experience, compared with the pre-PPS period (OR, 1.51; 95% confidence interval [95% CI], 1.47 to 1.55; P<0.001) (Table 2). The estimated proportion of patients with early PD experience increased from 6.2% (95% CI, 6.0 to 6.3) in 2006 to 9.8% (95% CI, 9.6 to 10.0) in 2013 (Figure 2, Panel A).
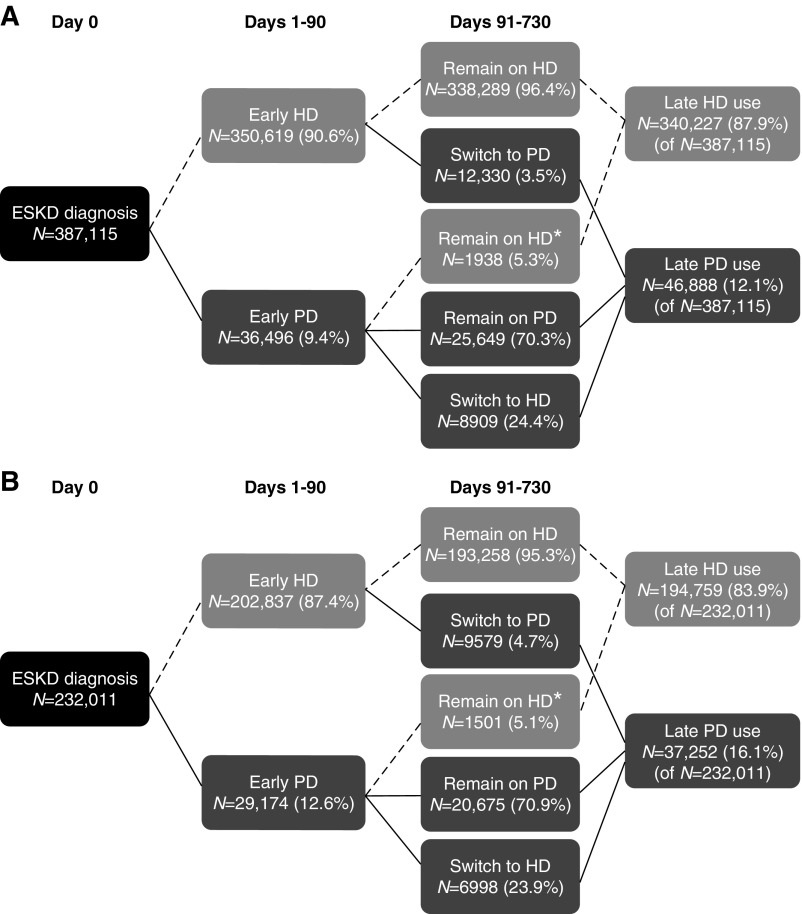
Figure 1.
Unadjusted early PD experience, late PD use, and switches from HD to PD increased after implementation of the 2011 PPS. (Panel A) Use before PPS (2006–2010), N=387,115. (Panel B) Use after PPS (2011–2013), N=232,011. After ESKD diagnosis, patients could initiate dialysis with PD or HD. After the 90-day initiation period, patients could remain on their initial dialysis modality, or switch to the other modality. A minority of patients who attempted PD in the first 90 days but were identified as HD users at the start of the late use observation period (91–730 days after dialysis initiation) remained on HD during this period. Numbers of patients and percentages are presented. Percentages may not add up to 100%, due to rounding. HD, hemodialysis; PD, peritoneal dialysis; PPS, Medicare prospective payment system for dialysis.
Table 2.
Observed and adjusted results for early PD experience, late PD use, HD-to-PD switches, and PD-to-HD switches
| Outcome | Pre-PPS (2006–2010) | Post-PPS (2011–2013) | Post-PPS versus Pre-PPS OR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| Observed N (%) | Adjusted % (95% CI)a | Observed N (%) | Adjusted % (95% CI)a | |||
| Full sample | N=387,115 | N=232,011 | ||||
| Early PD experience | 36,496 (9.4) | 6.4 (6.3 to 6.5) | 29,174 (12.6) | 8.9 (8.8 to 9.1) | 1.51 (1.47 to 1.55) | <0.001 |
| Late PD use | 46,888 (12.1) | 8.9 (8.8 to 9.0) | 37,252 (16.1) | 12.6 (12.4 to 12.7) | 1.47 (1.45 to 1.50) | <0.001 |
| Subgroup without early PD experience | n=350,619 | n=202,837 | ||||
| Switch from HD to PDb | 12,330 (3.5) | 2.8 (2.8 to 2.9) | 9579 (4.7) | 4.1 (4.0 to 4.2) | 1.59 (1.52 to 1.66) | <0.001 |
| Subgroup with early PD experience | n=36,496 | n=29,174 | ||||
| Switch from PD to HDc | 8909 (24.4) | 26.3 (25.7 to 26.9) | 6998 (23.9) | 25.0 (24.4 to 25.6) | 0.92 (0.87 to 0.98) | 0.004 |
PPS, Medicare prospective payment system for dialysis; OR, odds ratio; 95% CI, 95% confidence interval; PD, peritoneal dialysis; HD, hemodialysis.
Estimated adjusted percentages and associated 95% CIs for all outcomes for the pre-PPS and post-PPS periods were estimated from models that included all covariates from Table 1. All adjusted estimates use the appropriate intercept and slope parameters, with fixed values of all covariates centered at means. Caution should be used when inferring these probabilities to population parameters.
Fewer than 0.3% of these patients (n=631 pre-PPS, n=699 post-PPS) actually did try PD in the first 90 days but switched to HD before day 90 and then switched back to PD after day 90.
One patient in the pre-PPS period actually initiated dialysis with HD, but switched to PD before day 90, and then switched back to HD after day 90.
Figure 2.
Adjusted results: Estimated rates of early PD experience, late PD use and switches from HD to PD increased after the PPS, while switches from PD to HD declined modestly. (Panel A) Early PD experience reflects any PD use in the first 90 days of dialysis initiation and increased over the study period. (Panel B) Late PD use reflects stable PD use (≥60 consecutive days) in days 91–730 after dialysis initiation and also increased during 2006–2013. (Panel C) Dialysis modality switches among the subgroup of patients without early PD experience (i.e., only early HD experience), who switched to PD in days 91–730 after dialysis initiation, increased during 2006–2013. The subgroup of patients with early PD experience, who later switched to HD in days 91–730 after dialysis initiation, decreased during 2006–2013. A minority of patients with early PD experience were HD users at the start of the late use period and remained on HD days 91–730 after dialysis initiation; however, this misclassification did not affect our results. All estimates were generated from predicted probabilities over the pre- and post-PPS periods and in individual years, using appropriate intercept and slope parameters with fixed values of covariates centered at mean values. Note: The error bars in the figure panels display annual model-estimated PD use rate 95% confidence intervals which, in some cases, are small. HD, hemodialysis; PD, peritoneal dialysis; PPS, Medicare prospective payment system for dialysis.
Late PD use increased from 12.1% (46,888 of 287,115) in the pre-PPS period (Figure 1, Panel A) to 16.1% (37,252 of 232,011) in the post-PPS period (Figure 1, Panel B), a 4.0% increase. In adjusted analysis, the PPS was associated with increased odds of late PD use, compared with the pre-PPS period (OR, 1.47; 95% CI, 1.45 to 1.50; P<0.001) (Table 2). The estimated proportion of patients with late PD use increased from 8.4% (95% CI, 8.2 to 8.6) in 2006 to 13.5% (95% CI, 13.3 to 13.8) in 2013 (Figure 2, Panel B). When we restricted the pre-PPS period to years 2006–2007 (the post-PPS period remained 2011–2013), the PPS effect for late PD use increased modestly to 1.60.
Subgroup Analysis: Dialysis Modality Switches
Modality switches could lead to an increase in late PD use in two ways: (1) increased switches from HD to PD, and (2) decreased switches from PD to HD. Overall, 6.1% of patients (5.5% pre-PPS, 7.1% post-PPS) made at least one modality switch throughout the observation period (Figure 1). Only 1.2% of patients switched modalities more than once. Overall, mean (median) time to switch after the day 90 threshold was 219 (169) days.
Among patients without early PD experience, switches from HD to PD increased from 3.5% (12,330 of 350,619) pre-PPS to 4.7% (9579 of 202,837) post-PPS (Figure 1, Panels A and B). Mean (median) time to switch after the day 90 threshold was 192 (134) days in the pre-PPS period and 178 [123] days in the post-PPS period. In adjusted analysis, the PPS was associated with increased odds of HD-to-PD switches (OR, 1.59; 95% CI, 1.52 to 1.66; P<0.001) (Table 2). The estimated mean proportion of patients who switched from HD to PD increased from 2.4% (95% CI, 2.3 to 2.5) in 2006 to 4.2% (95% CI 4.1 to 4.4) in 2013 (Figure 2, Panel C).
Among patients with early PD experience, PD-to-HD switches decreased modestly, from 24.4% (8909 of 36,496) in the pre-PPS period to 24.0% (6998 of 29,174) in the post-PPS period (Figure 1, Panels A and B). Mean time to switch after the day 90 threshold was 264 (239) days in the pre-PPS period and 269 (246) days in the post-PPS period. In adjusted analysis, the PPS was associated with decreased odds of PD-to-HD switches (OR, 0.92; 95% CI, 0.87 to 0.98; P=0.004) (Table 2). The estimated mean proportion of patients who switched from PD to HD decreased from 27.0% (95% CI, 25.9 to 28.1) in 2006 to 24.4% (95% CI, 23.5 to 25.3) in 2013 (Figure 2, Panel C).
Factors Associated with PD Use
A number of patient characteristics were associated with PD use (Supplemental Tables 2 and 3). Black patients had almost half the odds of using PD compared with white patients (early PD experience, OR, 0.59; 95% CI, 0.57 to 0.60; late PD use, OR, 0.58; 95% CI, 0.57 to 0.60). Patients with nephrology care before ESKD onset had higher odds of early PD experience (OR, 3.01; 95% CI, 2.94 to 3.09) and late PD use (OR, 2.09; 95% CI, 2.04 to 2.13) than patients who had no pre-ESKD nephrology care. Patients with Medicaid had lower odds of late PD use (OR, 0.60; 95% CI, 0.59 to 0.62) compared with non-Medicaid beneficiaries, whereas patients with private insurance had higher odds of late PD use (OR, 1.39; 95% CI, 1.35 to 1.43) than those without private insurance. Patients living in the northeast United States tended to have lower odds of both early PD experience (OR, 0.71; 95% CI, 0.69 to 0.73) and late PD use (OR, 0.73; 95% CI, 0.71 to 0.75) compared with patients living in the southern United States. PD use did not vary meaningfully by urban/rural region or PD facility characteristics (Supplemental Tables 2 and 3).
Discussion
The primary aim of our study was to evaluate the effect of the PPS on PD use. In the initial years after Medicare payment reform, late PD use increased significantly, as more patients initiated dialysis with PD and more patients switched from HD to PD. Our results suggest that Medicare’s PPS for dialysis may be achieving one of its intended goals in the initial years of payment reform implementation.
Recent studies evaluated the effect of the PPS on dialysis modality in the first 3–4 months after dialysis initiation (19–22) in sample sizes ranging from 18,346 (20) to 717,604 (19), and found that short-term PD use increased after 2011. CMS only starts monitoring clinical outcomes for patients with ESKD (e.g., mortality and hospitalizations) starting 3 months after dialysis initiation (23), so understanding changes in PD use in the longer term is an important mechanism for achieving policy goals.
Only one previous study evaluated HD-to-PD switches in the first year after dialysis initiation (20). Modality switching is an important pathway to late PD use. Thus, by examining late dialysis use as well as trends in modality switches, our findings provide a more complete and nuanced understanding of the effects of the PPS. We found that modality changes contributed to an increase in overall PD use. Specifically, increased switching from HD to PD signals providers’ response to payment reform: promoting use of home-based modalities among patients who had initiated or were established on HD, despite the costs associated with switching modalities (e.g., opportunity cost of HD occupancy, training). The observed growth in early PD experience and late PD use, and the small reduction in PD-to-HD switches, suggests that the PPS did not induce inappropriate referrals to PD. This finding highlights the potential for more growth in PD use in the coming years. The use of home-assisted PD, for instance, may further increase PD use among patients who face barriers to self-care (34–36). Altogether, our results suggest that as the PPS era moves forward, PD use could continue to increase not only at dialysis initiation, but also months or years afterward, as patient awareness of and education on treatment modalities improve and as providers become more experienced in managing patients on PD.
Prior research found that the increase in short-term PD use has not been homogenous across the ESKD population (18,21,32). Although our study did not explicitly evaluate interactions between the PPS effect and patient demographics, we did find that PD use generally varied according to race, geography, and access to specialty care. Late PD use was lower among patients on Medicaid compared with patients not on Medicaid and lower among black patients compared with white patients. This latter finding is consistent with recent reports that black and Hispanic patients are less likely to initiate PD and more likely to switch from PD to HD, compared with white and Asian counterparts (37). Patients without pre-ESKD nephrology care before dialysis initiation, likely due to poor access to specialty care, had lower odds of PD use compared with those who met with a nephrologist. Such disparities may be related to insufficient patient education about modality types, lower reimbursement rates for Medicaid patients, and/or misaligned physician and facility financial incentives. As evidence mounts that payment reform can alter the availability and use of PD, policies should be developed to support outreach and education on dialysis for underserved patients with known CKD. Additionally, more efforts are needed to recognize CKD earlier among these patients and refer them to nephrologists before ESKD development.
Although PD use did increase, it is likely that an even greater proportion of patients could benefit from PD. In many European countries, >20% of patients with ESKD receive PD (1). Three reasons may explain the American lag in PD use, despite PPS implementation. First, recent work has shown that PD availability has increased modestly at dialysis facilities (18), with less than half of facilities offering PD. Second, PD providers were temporarily plagued by shortages in PD solutions in 2015 (38), likely limiting patients’ ability to initiate or switch to PD that year. These shortages were not included in our model, because they occurred outside of our study period. Third, many nephrologists do not have sufficient training, knowledge, or comfort managing patients on PD, so they may be less likely to offer it as an option (39). Recent work has shown that although it is well known that most patients prefer home-based dialysis modalities (5,6,14), a majority do not receive sufficient education about them before initiating treatment, and end up receiving the dialysis modality ordered by their physician (40). The decision about dialysis modality is likely to be strongly influenced by physician preference and experience with the technique. Future research should evaluate the effect of individual physicians or physician groups on modality choice. Although we did not explicitly assess technique survival, it will also be important to monitor associated changes between increased patient and provider use and PD technique survival (41,42).
Although many factors are involved in dialysis modality decisions, our results imply that the realignment of financial incentives may have played a role in the overall increase in PD use. Others have shown similar trends (19,43), signaling anticipation of changes as early as 2008 (i.e., passage of PPS legislation). Before 2010, the average operating margin for PD was lower than that of HD (17), which may partly explain the dominance of in-center HD in the United States compared with other high-income countries (1). When Medicare developed the PPS, the expectation was that operating margins would increase for PD (from roughly -$185 to $201 per patient-month) relative to HD (from roughly $76 to $86 per patient-month) (17). These values may have changed after 2012 legislation that mandated a gradual decrease in Medicare reimbursement for dialysis in 2014–2018 (44), and may change even further after recent federal proposals to implement additional incentives for increasing provider referrals for home dialysis (45). Future research should monitor the sensitivity of PD use trends to fluctuations in payment.
Our study has several limitations. First, it used an observational pre/post study design. Nearly all dialysis facilities were exposed to and fully implemented the PPS at the same time, so we could not compare our cohort with patients who were not exposed to payment reform. Additionally, other policies implemented around the same time as the PPS, including the Food and Drug Administration’s black box warning on epoetin labels (15) and education initiatives for nephrologists and nephrology fellows (39), may have had a simultaneous effect on PD use. We found that the rise in PD use started earlier than 2011, so it is likely that these other policies—implemented before the PPS—also played a role in this trend. Second, our study relied on the quality and accuracy of USRDS and CMS data. Dialysis facilities and physicians are required to submit demographic and medical data only when patients are first enrolled. Changes in employment status, insurance status, and comorbidities over time, for example, are not reliably documented. Moreover, a significant number of records contain missing baseline clinical characteristics (e.g., serum albumin, A1c) that may influence modality choice. Third, data on modality use may not have been recorded as frequently for patients without Medicare, so we may have underestimated the number of brief, early periods of PD use in our model. Fourth, despite all attempts to adjust for factors to minimize bias, unmeasured confounders were not controlled for and causality cannot be proven. Finally, results may not generalize to Medicare Advantage enrollees, who constitute roughly one-third of all Medicare beneficiaries and 11% of patients initiating dialysis (46). A recent study showed that patients with ESKD who were enrolled in Medicare Advantage at the time of dialysis initiation tended to disenroll from Medicare Advantage within a year, typically because their intensive health care needs were not adequately met (47). Because policymakers anticipate continued growth in Medicare Advantage, differences in modality choice between patients with traditional Medicare and Medicare Advantage should be examined.
In summary, our study found that more patients are now starting, staying on, and switching to PD than before the PPS was implemented, achieving a goal of payment reform. This growth in PD use occurred without a substantial increase in transfers to HD, achieving a secondary goal of payment reform. PD use may have accelerated since 2013, as dialysis providers have developed more experience managing patients on PD. It will be important to evaluate this trend in the longer-term to determine whether, when, and where payment reform encourages dialysis use that is more closely aligned with patient preference and clinical appropriateness.
Disclosures
Dr. Coffmann, Dr. Maciejewski, and Dr. Wang report receiving grant funding from the US Department of Veterans Affairs Health Services Research and Development Service (Maciejewski, RCS-10-391) and the Durham Center of Innovation to Accelerate Discovery & Practice Transformation (CIN 13-410) at the Durham VA Health Care System, outside of the submitted work. Dr. Maciejewski reports stock ownership in Amgen due to his spouse's employment. Dr. Coffman, Dr. Hirth, Dr. Lee, Dr. Maciejewski, and Dr. Wang report receiving funding from the National Institutes of Health outside of the submitted work. Ms. Sanders and Dr. Sloan have nothing to disclose.
Funding
Dr. Coffman, Dr. Hirth, Dr. Lee, Dr. Maciejewski, Ms. Sanders, and Dr. Wang were supported by a grant from NIDDK of the National Institutes of Health (R01DK097165). Dr. Sloan was supported by a US Department of Veterans Affairs Office of Academic Affiliation postdoctoral fellowship (TPH 21-000).
Supplementary Material
Acknowledgments
The authors thank Dr. Clarissa Diamantidis, Dr. Julia Scialla, Dr. Ebony Boulware, Ms. Kathryn Sleeman, and the anonymous reviewers for scientific counsel and helpful comments on this manuscript, and Ms. JaNell Wylie and Ms. Nikita Shah for research assistance.
Preliminary findings were presented at the 2018 Annual Meetings of AcademyHealth (June 24, 2018, Seattle, WA) and the American Society of Nephrology (October 25, 2018, San Diego, CA).
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] or the US Department of Veterans Affairs), the University of Michigan, or Duke University.
All authors made substantial contributions to the conception and design of this study. Dr. Wang acquired the data. Ms. Sanders and Dr. Coffman analyzed the data. All authors made substantial contributions to interpretation of the data. Dr. Sloan drafted the article. All authors revised it critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
An earlier version of this study was presented at the American Society of Nephrology Kidney Week Meeting in October 2018 in San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Public Policy and Patient Choice of Dialysis Modality,” on pages 1677–1678.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05910519/-/DCSupplemental.
Supplemental Statistical Model Information.
Supplemental Table 1. Characteristics of patients with incident ESKD, by policy period and dialysis modality for the first 90 days.
Supplemental Tables 2. Odds ratio estimates from logistic regression model for all covariates for outcomes early PD experience, late PD use, HD-to-PD switches, and PD-to-HD switches.
Supplemental Table 3. Estimated PPS effect and model parameters from logistic regression models for outcomes early PD experience, late PD use, HD-to-PD switch, and PD-to-HD switch.
Supplemental Figure 1. Patient sampling frame.
References
- 1.US Renal Data System : USRDS 2017 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 2.Wong B, Ravani P, Oliver MJ, Holroyd-Leduc J, Venturato L, Garg AX, Quinn RR: Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis 71: 344–351, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Queeley GL, Campbell ES: Comparing treatment modalities for end-stage renal disease: A meta-analysis. Am Health Drug Benefits 11: 118–127, 2018 [PMC free article] [PubMed] [Google Scholar]
- 4.Tong A, Lesmana B, Johnson DW, Wong G, Campbell D, Craig JC: The perspectives of adults living with peritoneal dialysis: Thematic synthesis of qualitative studies. Am J Kidney Dis 61: 873–888, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Walker RC, Hanson CS, Palmer SC, Howard K, Morton RL, Marshall MR, Tong A: Patient and caregiver perspectives on home hemodialysis: A systematic review. Am J Kidney Dis 65: 451–463, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Walker RC, Morton RL, Palmer SC, Marshall MR, Tong A, Howard K: A discrete choice study of patient preferences for dialysis modalities. Clin J Am Soc Nephrol 13: 100–108, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen KL, Zhang R, Huang Y, Chen SC, Blagg CR, Goldfarb-Rumyantzev AS, Hoy CD, Lockridge RS Jr., Miller BW, Eggers PW, Kutner NG: Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: A USRDS study. Kidney Int 76: 984–990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramar P, Ahmed AT, Wang Z, Chawla SS, Suarez MLG, Hickson LJ, Farrell A, Williams AW, Shah ND, Murad MH, Thorsteinsdottir B: Effects of different models of dialysis care on patient-important outcomes: A systematic review and meta-analysis. Popul Health Manag 20: 495–505, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Liu FX, Walton SM, Leipold R, Isbell D, Golper TA: Financial implications to Medicare from changing the dialysis modality mix under the bundled prospective payment system. Perit Dial Int 34: 749–757, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sennfält K, Magnusson M, Carlsson P: Comparison of hemodialysis and peritoneal dialysis--a cost-utility analysis. Perit Dial Int 22: 39–47, 2002 [PubMed] [Google Scholar]
- 11.Shih YC, Guo A, Just PM, Mujais S: Impact of initial dialysis modality and modality switches on Medicare expenditures of end-stage renal disease patients. Kidney Int 68: 319–329, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Mendelssohn DC, Mullaney SR, Jung B, Blake PG, Mehta RL: What do American nephologists think about dialysis modality selection? Am J Kidney Dis 37: 22–29, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Stack AG: Determinants of modality selection among incident US dialysis patients: Results from a national study. J Am Soc Nephrol 13: 1279–1287, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Devoe DJ, Wong B, James MT, Ravani P, Oliver MJ, Barnieh L, Roberts DJ, Pauly R, Manns BJ, Kappel J, Quinn RR: Patient education and peritoneal dialysis modality selection: A systematic review and meta-analysis. Am J Kidney Dis 68: 422–433, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services: End stage renal disease (ESRD) prospective payment system (PPS), 2018. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ESRDpayment/index.html. Accessed August 8, 2018
- 16.US Renal Data System : 2015 Annual Data Report and Researcher’s Guide to the USRDS Database, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 17.Hornberger J, Hirth RA: Financial implications of choice of dialysis type of the revised Medicare payment system: An economic analysis. Am J Kidney Dis 60: 280–287, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Wang V, Coffman CJ, Sanders LL, Lee SD, Hirth RA, Maciejewski ML: Medicare’s new prospective payment system on facility provision of peritoneal dialysis. Clin J Am Soc Nephrol 13: 1833–1841, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin E, Cheng XS, Chin KK, Zubair T, Chertow GM, Bendavid E, Bhattacharya J: Home dialysis in the prospective payment system era. J Am Soc Nephrol 28: 2993–3004, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez JJ, Zhao B, Qureshi S, Winkelmayer WC, Erickson KF: Health insurance and the use of peritoneal dialysis in the United States. Am J Kidney Dis 71: 479–487, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turenne M, Baker R, Pearson J, Cogan C, Mukhopadhyay P, Cope E: Payment reform and health disparities: Changes in dialysis modality under the new Medicare dialysis payment system. Health Serv Res 53: 1430–1457, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Thamer M, Kshirsagar O, Zhang Y: Impact of the end stage renal disease prospective payment system on the use of peritoneal dialysis. Kidney Int Rep 2: 350–358, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Renal Data System : 2016 Researcher’s Guide to the USRDS Database, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016 [Google Scholar]
- 24.Centers for Medicare & Medicaid Services : Provider of service files (years 2006–2013). Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Downloadable-Public-Use-Files/Provider-of-Services/index.html. Accessed February 5, 2015
- 25.Wang V, Maciejewski ML, Coffman CJ, Sanders LL, Lee SD, Hirth R, Messana J: Impacts of geographic distance on peritoneal dialysis utilization: Refining models of treatment selection. Health Serv Res 52: 35–55, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dartmouth Atlas of Health Care Working Group : Geographic Boundary Files: Hospital Referral Region, NH, Lebanon, Center for the Evaluative Clinical Sciences, 2003 [Google Scholar]
- 27.Wennberg JE, Cooper MA: The Dartmouth Atlas of Health Care in the United States, Chicago, IL, American Hospital Association, 1999 [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang V, Lee SY, Patel UD, Maciejewski ML, Ricketts TC: Longitudinal analysis of market factors associated with provision of peritoneal dialysis services. Med Care Res Rev 68: 537–558, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Hirth RA, Held PJ, Orzol SM, Dor A: Practice patterns, case mix, Medicare payment policy, and dialysis facility costs. Health Serv Res 33: 1567–1592, 1999 [PMC free article] [PubMed] [Google Scholar]
- 31.Pozniak AS, Hirth RA, Banaszak-Holl J, Wheeler JR: Predictors of chain acquisition among independent dialysis facilities. Health Serv Res 45: 476–496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang V, Lee SY, Patel UD, Weiner BJ, Ricketts TC, Weinberger M: Geographic and temporal trends in peritoneal dialysis services in the United States between 1995 and 2003. Am J Kidney Dis 55: 1079–1087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapter 21: Government regulation - principal regulatory mechanisms. In : The Economics of Health and Health Care, 3rd Ed., edited by Folland S, Goodman AC, and Stano M, Upper Saddle River, NJ, Prentice-Hall, Inc., 2001, pp 493–496 [Google Scholar]
- 34.Dimkovic N, Aggarwal V, Khan S, Chu M, Bargman J, Oreopoulos DG: Assisted peritoneal dialysis: What is it and who does it involve? Adv Perit Dial 25: 165–170, 2009 [PubMed] [Google Scholar]
- 35.Franco MR, Fernandes N, Ribeiro CA, Qureshi AR, Divino-Filho JC, da Glória Lima M: A Brazilian experience in assisted automated peritoneal dialysis: A reliable and effective home care approach. Perit Dial Int 33: 252–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ: Home care assistance and the utilization of peritoneal dialysis. Kidney Int 71: 673–678, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Shen JI, Erickson KF, Chen L, Vangala S, Leng L, Shah A, Saxena AB, Perl J, Norris KC: Expanded prospective payment system and use of and outcomes with home dialysis by race and ethnicity in the United States. Clin J Am Soc Nephrol 14: 1200–1212, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seaborg E: Peritoneal dialysis fluid shortage disrupted growth of popular therapy. ASN Kidney News 7: 1–3, 2015 [Google Scholar]
- 39.Rope RW, Pivert KA, Parker MG, Sozio SM, Merell SB: Education in nephrology fellowship: A survey-based needs assessment. J Am Soc Nephrol 28: 1983–1990, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song MK, Lin FC, Gilet CA, Arnold RM, Bridgman JC, Ward SE: Patient perspectives on informed decision-making surrounding dialysis initiation. Nephrol Dial Transplant 28: 2815–2823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N: Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 13: 90–103, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Perl J, Wald R, Bargman JM, Na Y, Jassal SV, Jain AK, Moist L, Nessim SJ: Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J Am Soc Nephrol 7: 1145–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirth RA, Turenne MN, Wheeler JR, Nahra TA, Sleeman KK, Zhang W, Messana JA: The initial impact of Medicare’s new prospective payment system for kidney dialysis. Am J Kidney Dis 62: 662–669, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Centers for Medicare & Medicaid Services (CMS); Department of Health and Human Services : Medicare Program; End-stage renal disease prospective payment system, quality incentive program, and durable medical equipment, prosthetics, orthotics, and supplies. 79 Fed Reg 66119 (final rule November 6, 2014) [PubMed]
- 45.Centers for Medicare & Medicaid Services; Department of Health and Human Services: Medicare program: Specialty care models to improve quality of care and reduce expenditures. 84 Fed Reg 34478 (proposed rule July 18, 2019) [Google Scholar]
- 46.US Renal Data System : USRDS 2017 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 47.Li Q, Trivedi AN, Galarraga O, Chernew ME, Weiner DE, Mor V: Medicare advantage ratings and voluntary disenrollment among patients with end-stage renal disease. Health Aff (Millwood) 37: 70–77, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.