Visual Abstract
Keywords: humans, pregnancy, female, atypical hemolytic uremic syndrome, complement membrane, HELLP syndrome, complement C9, eculizumab, complement pathway, alternative, pre-eclampsia, hypertension, malignant, fibrin, antibodies, monoclonal, humanized, complement system proteins, complement activation, thrombotic microangiopathies, recurrence, fluorescent antibody technique, endothelial cells
Abstract
Background and objectives
Atypical hemolytic uremic syndrome is a form of thrombotic microangiopathy caused by dysregulation of the alternative complement pathway. There is evidence showing complement activation in other thrombotic microangiopathies. The aim of this study was to evaluate complement activation in different thrombotic microangiopathies and to monitor treatment response.
Design, setting, participants, & measurements
Complement activation was assessed by exposing endothelial cells to sera or activated-patient plasma—citrated plasma mixed with a control sera pool (1:1)—to analyze C5b-9 deposits by immunofluorescence. Patients with atypical hemolytic uremic syndrome (n=34) at different stages of the disease, HELLP syndrome (a pregnancy complication characterized by hemolysis, elevated liver enzymes, and low platelet count) or severe preeclampsia (n=10), and malignant hypertension (n=5) were included.
Results
Acute phase atypical hemolytic uremic syndrome–activated plasma induced an increased C5b-9 deposition on endothelial cells. Standard and lower doses of eculizumab inhibited C5b-9 deposition in all patients with atypical hemolytic uremic syndrome, except in two who showed partial remission and clinical relapse. Significant fibrin formation was observed together with C5b-9 deposition. Results obtained using activated-plasma samples were more marked and reproducible than those obtained with sera. C5b-9 deposition was also increased with samples from patients with HELLP (all cases) and preeclampsia (90%) at disease onset. This increase was sustained in those with HELLP after 40 days, and levels normalized in patients with both HELLP and preeclampsia after 6–9 months. Complement activation in those with malignant hypertension was at control levels.
Conclusions
The proposed methodology identifies complement overactivation in patients with atypical hemolytic uremic syndrome at acute phase and in other diseases such as HELLP syndrome and preeclampsia. Moreover, it is sensitive enough to individually assess the efficiency of the C5 inhibition treatment.
Introduction
Thrombotic microangiopathies (TMAs) include a group of disorders that share clinical features, such as a the triad of microangiopathic hemolytic anemia, thrombocytopenia, and organ damage (1,2). Atypical hemolytic uremic syndrome (aHUS) is a TMA primarily caused by a lack of regulation of the alternative complement pathway. New evidence suggests that a number of TMAs other than aHUS could also be associated with complement overactivation (3,4).
In both inherited or acquired aHUS there is an assembly of the membrane-attack complex leading to endothelial cell inflammation, activation, and injury (5,6). The introduction of eculizumab, an mAb which blocks terminal complement activation, significantly improved the natural progression of the disease (7,8). Lifelong treatment with this drug is often considered in inherited aHUS. The ability to monitor treatment efficiency and patient recovery, with appropriate biologic markers, could help in decision making regarding treatment continuation.
aHUS occurs as dysregulation of the alternative complement pathway (9,10) and the presence of complement-amplifying conditions, resulting in endothelial damage (11,12). Some complement-amplifying conditions are sufficient to lead to TMAs (5). This phenomenon could play a role in pregnancy-related complications such as preeclampsia and HELLP (a pregnancy complication characterized by hemolysis, elevated liver enzymes, and low platelet count) syndrome (13,14), and in association with certain drugs (15), tumors (16), autoimmune diseases (17,18), malignant hypertension (19), and transplant-associated TMAs (20,21).
With this expanding spectrum of complement-mediated diseases, knowledge about a person’s complement status could identify patients who might benefit from complement-targeting therapies. In this regard, some successful efforts have been made in aHUS (22–24). However, the approaches proposed have not been tested in severe preeclampsia or HELLP syndrome. The aim of this study was to modify the assay developed by Noris et al. (22) to detect complement overactivation in patients in different situations: those with acute aHUS, those under C5 inhibitor treatment to monitor eculizumab treatment efficiency, and those with other more prevalent disorders such as pregnancy-related complications and malignant hypertension.
Materials and Methods
Study Population and Sample Collection
A total of 34 patients with aHUS were enrolled from 15 different European hospitals. We obtained 11 samples in the acute phase of the disease (four of them with postpartum onset; Table 1), 20 samples from stable patients (complete remission) treated with regular doses of eculizumab, ten stable patients treated with lower doses or for longer intervals than those specified in the data sheet (Supplemental Table 1), five samples from patients in remission without eculizumab treatment, and four asymptomatic carriers of the mutation (Supplemental Table 2). Genetic analysis including the detection of genetic variants, risk haplotypes, anti-CFH antibodies, and serum levels of complement components and regulatory proteins from patients with aHUS are described in Supplemental Table 3. To evaluate test specificity, we also included five patients with different CKD stages due to autosomal dominant polycystic kidney disease (Supplemental Table 4).
Table 1.
Clinical parameters and complement activation markers in incident patients with atypical hemolytic uremic syndrome
| Patient | Genetic Variants | Disease Phase (Trigger) | Creatinine (0.3–1.3 mg/dl) | Hemoglobin (12–17 g/dl) | Platelets (130–400 109/L) | Lactate Dehydrogenase (<234 U/L) | Haptoglobin (0.3–1.8 g/L) | Serum C3 (0.8–1.9 g/L) | Serum C4 (0.1–0.5 g/L) | Serum 50% Hemolytic Complement (28–60 U/ml) | Serum C5b-9 Deposits (Mean±SEM) | Activated-Plasma C5b-9 Deposits (Mean±SEM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | MCP: (c.478G>T) p.Val160Phe | Acute (none) | 4.9 | 8.7 | 117 | 317 | N.D. | 1.0 | N.D. | 55 | 3.2±1.5a | N.A. |
| Patient 2 | C3: c.3125G>A MCP: c.1148C>T | Acute (respiratory infection) | 3.9 | 9.4 | 7 | 2430 | N.D. | 0.8 | 0.3 | 72 | 2.5±1.2a | N.A. |
| Remissionb | 0.8 | 12.1 | 259 | 146 | 1.1 | 0.8 | N.D. | N.D. | 1.4±0.9ac | N.A. | ||
| Patient 3 | No | Acute (none) | 5 | 8.5 | 196 | 415 | N.D. | 0.7 | 0.1 | 28 | 1.9±1a | N.A. |
| Remissiond | 4.4 | 9.2 | 492 | 194 | N.D. | 0.9 | 0.2 | 7 | 0.5±0.5c | N.A. | ||
| Patient 4 | MCP: exon 6, heterozygosis (c.800_820Del) p.Thr267Asn273del CFH: exon 6, heterozygosis (c.292C>T) p.Leu98Phe | Acute (urinary infection) | 8.6 | 10.9 | 73 | 1353 | N.D. | 0.6 | 0.2 | 24 | 1.7±0.8a | 5.6±0.2a |
| Remissione | 6 | 12.7 | 158 | 516 | 1.29 | 0.8 | 0.3 | 9 | 0.6±0.5c | 0.3±0.1c | ||
| Patient 5 | CFH: exon 19, heterozygosis (c.2850G>T) p.Gln950 | Acute (postpartum) | 2.4 | 5.8 | 61 | 1678 | N.D. | N.D. | N.D. | N.D. | 2±0.8a | 4.9±0.1a |
| Remissione | 0.8 | 11.7 | 305 | 247 | 0.5 | 0.9 | 0.2 | N.D. | 0.1±0.1c | 0.1±0.18c | ||
| Patient 6 | No | Acute (postpartum) | 4.1 | 6.7 | 74 | 1781 | 0.2 | 1.4 | 0.5 | 21 | 1.6±0.4a | 5.9±0.4a |
| Remissione | 0.8 | 10.9 | 268 | 179 | 1.12 | 0.8 | 0.2 | 12 | 0.8±0.1c | 0.3±0.1c | ||
| Patient 7 | No | Acute (postpartum) | 1.6 | 13.0 | 93 | 266 | 0.3 | 1.1 | 0.3 | N.D. | 2.7±0.4a | 4.1±0.3a |
| Partial remissione | 1.5 | 13.5 | 115 | 251 | 1.1 | 0.9 | 0.2 | N.D. | 2.7±0.5a | 2.4±0.1a | ||
| Patient 8 | CFI: exon 5, heterozygosis (c.739T>G) p.Cys247Gly | Acute (pancreatic cancer) | 1.9 | 8.3 | 40 | 2407 | N.D. | 1.7 | 0.2 | 55 | 2.8±0.5a | 3.2±0.2a |
| Remissione | 1.3 | 12.3 | 290 | 399 | 0.3 | 1.0 | 0.2 | 16 | 1.1±0.6 | 0.5±0.1c | ||
| Patient 9 | CFH: heterozygosis (c.3514G>T) p.Glu172Stop | Acuteb (cocaine) | 7.2 | 7.1 | 105 | 682 | 0.3 | 0.8 | 0.2 | 41 | N.A. | 13.1±0.2a |
| Remissione | 2.2 | 13.0 | 197 | 304 | 1.3 | 0.7 | 0.2 | 8 | N.A. | 0.78±0.1c | ||
| Patient 10 | CFH: heterozygosis (c.3514G>T) p.Glu172Stop | Acute (cocaine) | 12.2 | 5.9 | 44 | 3214 | N.D. | N.D. | N.D. | N.D. | N.A. | 10.9±0.3a |
| Remissione | 1.3 | 12.2 | 207 | 349 | 0.8 | 0.8 | 0.2 | 11 | N.A. | 1.1±0.2c | ||
| Patient 11 | CFHR1::CFH, hybrid gene (loss of signal in exon 6 of CFHR1 and gain of signal exon 23 of CFH) | Acute (postpartum) | 4.2 | 9.8 | 114 | 1649 | 0.1 | 0.4 | 0.1 | 29 | N.A. | 6.4±0.2a |
| Remissione | Hemodialysis | 11.3 | 214 | 430 | 1.2 | 1.0 | 0.4 | 6 | N.A. | 0.8±0.1c |
Statistical analysis was performed with raw data using the t test for paired samples. N.D., not done; N.A., sample not available.
Values statistically different than control values. (P<0.05).
Plasma exchange treatment.
Values statistically different than acute phase values. (P<0.05).
Plasma exchange and eculizumab treatment.
Eculizumab treatment.
Three patients with HELLP syndrome, seven with severe preeclampsia (Table 2), and ten healthy pregnant women matched by gestational age were included in the study (Supplemental Table 5). Finally, five patients with a clinical diagnosis of malignant hypertension were also included (Table 3). Disease definitions and diagnosis details are provided in Supplemental Material. Control samples were obtained from healthy individuals. Genetic analysis was not performed on patients with diseases other than aHUS in this study, which is a limitation of our work.
Table 2.
Clinical parameters and complement profile in patients with HELLP syndrome and severe preeclampsia
| Patient | Disease Phase | Creatinine (0.3–1.3 mg/dl) | Hemoglobin (14–18 g/dl) | Platelets (130–400 109/L) | Proteinuria (mg/gr) | Blood Pressure (mm Hg) | Ankle Edema | Glutamic Oxaloacetic Transaminase (5–40 U/L) | Glutamic Pyruvic Transaminase (5–40 U/L) | Serum C3 (0.8–1.9 g/L) | Serum C4 (0.1–0.5 g/L) | Serum 50% Hemolytic Complement (28–60 U/mL) | Activated-Plasma C5b-9 Deposits (Mean±SEM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-1 | Acute (26 wk of pregnancy) | 0.6 | 8.3 | 100 | 452 | 177/111 | No | 73 | 65 | 1.3 | 0.3 | 43.5 | 8.7±1a |
| Quarantine | 0.6 | 10 | 125 | 110 | 120/82 | No | 14 | 26 | 0.9 | 0.3 | 42 | 3.8±0.2a | |
| 7 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.D. | No | N.D. | N.D. | 1.0 | 0.3 | 32 | 1.2±0.1 | |
| H-2 | Acute (37.5 wk of pregnancy) | 2.8 | 11.2 | 63 | 6434 | 220/120 | Yes | 864 | 433 | 0.6 | 0.1 | 25 | 15.4±0.5a |
| Quarantine | 0.9 | 11.7 | 351 | 216 | 115/80 | No | 99 | 276 | 1.3 | 0.6 | 48.5 | 9.1±0.2a | |
| 8 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.D. | No | N.D. | N.D. | 1.3 | 0.3 | 49 | 1.1±0.1 | |
| H-3 | Acute (34 wk of pregnancy) | 1.1 | 10.2 | 55 | 910 | 180/118 | Yes | 1628 | 847 | 1.2 | 0.1 | 14 | 7.8±1a |
| Quarantine | 0.6 | 11.9 | 382 | 145 | 116/78 | No | 37 | 71 | 1.8 | 0.4 | 50.5 | 6±1.2a | |
| 9 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.D. | No | N.D. | N.D. | 1.6 | 0.3 | 50 | 1.2±0.2 | |
| PE-1 | Acute (24.6 wk of pregnancy) | 0.5 | 10.6 | 147 | 1059 | 161/84 | No | 44 | 36 | 13 | 0.3 | 45 | 2.3±0.8a |
| Quarantine | 0.7 | 13.6 | 211 | 71 | 133/87 | No | 23 | 18 | 1.4 | 0.5 | 42 | 0.9±0.1 | |
| 6/9 mo from delivery | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | |
| PE-2 | Acute (26 wk of pregnancy) | 0.6 | 12.5 | 222 | 638 | 170/110 | No | 19 | 30 | 1.3 | 0.1 | 49 | 2.4±0.8a |
| Quarantine | 0.8 | 12.3 | 306 | 50 | 118/69 | No | 20 | 40 | 1.6 | 0.4 | 53 | 0.6±0.2 | |
| 9 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.A. | No | N.D. | N.D. | 1.1 | 0.3 | 48 | 0.8±0.2 | |
| PE-3 | Acute (35.2 wk of pregnancy) | 1.0 | 11.7 | 135 | 2036 | 147/90 | No | 65 | 51 | 0.6 | 0.2 | 27 | 3.9±0.1a |
| Quarantine | 1.0 | 12.0 | 296 | N.D. | 109/56 | No | 21 | 28 | 1.5 | 0.4 | 58 | 1.5±0.1a | |
| 7 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.D. | No | N.D. | N.D. | 1.3 | 0.4 | 59 | 0.6±0.1 | |
| PE-4 | Acute (34.4 wk of pregnancy) | 0.6 | 13.2 | 83 | 1102 | 160/110 | No | 25 | 21 | 1.8 | 0.3 | 63 | 1.5±0.14a |
| Quarantine | 0.6 | 14.0 | 104 | 222 | 98/55 | No | 25 | 47 | 1.5 | 0.3 | 39 | 1.0±0.1 | |
| 6 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.D. | No | N.D. | N.D. | 1.5 | 0.3 | 39 | 0.7±0.1 | |
| PE-5 | Acute (34 wk of pregnancy) | 1.2 | 12.1 | 201 | 298 | 174/99 | Yes | 160 | 286 | 1.1 | 0.2 | 52 | 2.6±0.12a |
| Quarantine | 0.6 | 12.2 | 289 | 55 | 113/58 | No | 20 | 27 | 1.5 | 0.3 | 57 | 1.5±1a | |
| 6/9 mo from delivery | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | |
| PE-6 | Acute (33.4 wk of pregnancy) | 0.6 | 14.3 | 117 | 5028 | 139/87 | No | 74 | 110 | 1.1 | 0.2 | 48 | 1.1±0.1 |
| Quarantine | 0.5 | 15.5 | 282 | 253 | 123/76 | No | 27 | 46 | 1.3 | 0.3 | 52.5 | 0.8±0.1 | |
| 9 mo from delivery | N.D. | N.D. | N.D. | N.D. | N.D. | No | N.D. | N.D. | 1.09 | 0.19 | 38.5 | 1.2±0.1 | |
| PE-7 | Acute (34 wk of pregnancy) | 0.6 | 10.2 | 282 | 2024 | 170/100 | Yes | 37 | 19 | 1.6 | 0.4 | 58 | 2.1±0.1a |
| Quarantine | 0.5 | 11.9 | 371 | 63 | 106/63 | No | 22 | 15 | 1.5 | 0.3 | 56 | N.D. | |
| 6/9 mo from delivery | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
Statistical analysis was performed with raw data using the t test for paired samples. H-1, patient 1 with HELLP; N.D., not done; PE-1, patient 1 with preeclampsia; N.A., not available.
Values statistically different than control values.
Table 3.
Clinical parameters, complement activation markers, and evolution in patients with malignant hypertension
| Patient | Clinical Parameters | Complement Parameters | Treatment and Evolution | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Pressure (mm Hg) | Hypertensive Retinopathya | Creatinine (0.3–1.3 mg/dl) | Platelets (130–400 109/L) | Hemoglobin (12–17 g/dl) | Lactate Dehydrogenase (<234 U/L) | Haptoglobin (0.3–1.8 g/L) | ADAMTS-13 Activity (%) | Serum C3 (0.8–1.9 g/L) | Serum C4 (0.1–0.5 g/L) | Serum 50% Hemolytic Complement (28–60 U/ml) | Activated-Plasma C5b-9 Deposits (Mean±SEM) | Anti-hypertensives drugs | Other Treatment | Response | Dialysis | |
| MH-1 | 180/95 | Grade 3 | 19.2 | 67 | 4.8 | 601 | 0.6 | 64 | 0.8 | 0.4 | 53.5 | 0.7±0.1 | 2 | No | No | Yes |
| MH-2 | 204/115 | Grade 3 | 38.8 | 76 | 6.9 | 833 | N.D. | 54 | 1.0 | 0.3 | 58 | 0.8±0.2 | 3 | No | Hematologic | Yes |
| MH-3 | 170/90 | Grade 4 | 23 | 110 | 8.3 | 384 | N.D. | 85 | 1 | 0.2 | 49.5 | 0.9±0.1 | 3 | Steroids; eculizumab | Hematologic | Yes |
| MH-4 | 240/170 | Grade 3 | 11.8 | 97 | 10.7 | 2095 | 0.01 | 46 | 1 | 0.3 | 54.5 | 0.6±0.2 | 2 | No | No | Yes |
| MH-5 | 190/120 | Grade 3 | 13.1 | 126 | 9.2 | 491 | 2.6 | N.D. | 1.4 | 0.4 | 57 | 1.1±0.1 | 5 | No | Hematologic | Yes |
ADAMTS-13, a disintegrin and metalloproteinase with thrombospondin motifs 13; MH-1, patient 1 with malignant hypertension; N.D., not done.
Hypertension-related retinopathy classification according to the classification of Keith, Wagener, and Barker (51).
This study was approved by the ethics committee of Hospital Clinic and conformed to the ethical guidelines of the Helsinki Declaration. Participants or their legal guardians provided informed written consent before sample collection. Serum and plasma samples were obtained by centrifugation of nonanticoagulated blood and citrated blood (3000 × g, 15 minutes), respectively, before 6 hours after extraction in all cases. All samples were aliquoted and stored at −80°C until they were used, avoiding freeze/thaw cycles.
Serum C3, C4, and 50% Hemolytic Complement Levels
Factors C3 and C4 were quantified in a Siemens Dade Behring BN II Nephelometer and its corresponding kits. Complement activity (50% hemolytic complement, CH50) was measured using the Autokit CH50 (Wako).
C5b-9 Fluorescence Imaging
To evaluate complement activation, a modification of the technique described by Noris et al. (22) was used. With the aim of enhancing complement deposition, we proposed to take advantage of the interaction between coagulation and complement cascades and we added control sera to patient citrated plasma (1:1) to obtain activated plasma. The human dermal microvascular endothelial cell line (American Type Culture Collection) (25) was seeded on glass coverslips and used confluent. Cells were washed with test medium (HBSS without calcium or magnesium, 0.5% BSA; Life Technologies) and activated or not with 10 μM ADP (Sigma-Aldrich) (10 minutes, 37°C). Cells were then incubated (4 hours) with activated plasma diluted with test medium (1:2). Control samples were obtained by mixing healthy plasma from donors with pooled sera from controls. Cultures were then washed and fixed. For C5b-9 immunostaining, cells were treated with 2% BSA (1 hour) and incubated with a rabbit anti–human complement C5b-9 complex (Calbiochem), followed by Alexa594-conjugated goat anti-rabbit secondary antibody (Life Technologies) and 4′,6-diamidino-2-phenylindole. Micrographs were captured by fluorescent microscopy (Leica DM4000B) through a video camera (Leica DFC310FX) and analyzed using Fiji (ImageJ) (26). A total of 20 photographs were randomly obtained from each preparation. The area covered by C5b-9 deposits was calculated and expressed as the average fold increase of each condition versus control. All samples were tested at least three times.
Statistical Analysis
Levels of the percentage of area covered by C5b-9 deposits were calculated as mean±SEM. Statistical analysis (SPSS) was performed with raw data using the t test for paired samples. Results were considered statistically significant when P<0.05. However, in the article results are expressed as the fold increase of covered surface with respect to the control.
Results
Activated Plasma from Patients with Atypical Hemolytic Uremic Syndrome Is More Efficient in Inducing C5b-9 Deposition on Endothelial Cells than Sera
ADP-activated endothelial cells were exposed to sera (22) or activated plasma from 11 different patients with aHUS in acute phase (patient 1 to patient 11) and during remission (patient 2 to patient 6 and patient 8 to patient 11) (Table 1).
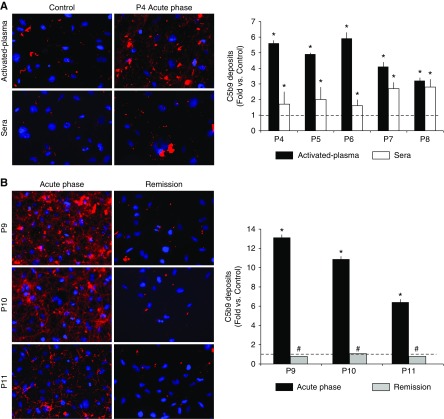
Serum samples obtained in acute phase induced more C5b-9 deposition on endothelial cells than control sera (fold increases of 3.2±1.5 for patient 1, P=0.01; 2.5±1.2 for patient 2, P<0.001; 1.9±1 for patient 3, P=0.02; 1.7±0.8 for patient 4, P=0.03; 2±0.8 for patient 5, P=0.04; 1.6±0.4 for patient 6, P=0.04; 2.7±0.4 for patient 7, P=0.03; and 2.8±0.5 for patient 8, P=0.03; versus control). Serum samples obtained during remission induced C5b-9 deposits equivalent or even lower than control sera (fold increase versus control of 1.4±0.9 for patient 2, P=0.001; 0.5±0.5 for patient 3, P<0.001; 0.6±0.5 for patient 4, P=0.008; 0.1±0.1 for patient 5, P=0.004; 0.8±0.1 for patient 6, P=0.03; and 1.1±0.6 for patient 8, P=0.01; for all samples compared with results at acute phase), except for patient 7. This patient had partial remission and C5b-9 deposits remained statistically higher than in controls (2.7±0.5 versus control, P=0.04).
Experiments were run in parallel using both serum and activated plasma from patients with aHUS (patient 4 to patient 8) obtained during the acute phase and remission. Activated plasma from the acute phase induced a marked C5b-9 deposition on endothelial cells (5.6±0.2 for patient 4, 4.9±0.1 for patient 5, 5.9±0.4 for patient 6, 4.1±0.3 for patient 7, and 3.2±0.2 for patient 8, versus control; P<0.001 for all) (Figure 1A). Levels of C5b-9 returned to control levels when we used samples obtained from four out of five patients in remission (0.3±0.1 for patient 4, 0.2±0.1 for patient 5, 0.3±0.1 for patient 6, and 0.5±0.1 for patient 8, versus control; P<0.001 for all versus onset) (Table 1). The activated plasma from patient 7 at partial remission induced an increased C5b-9 deposition (2.4±0.1 versus control, P<0.001). Although the results obtained from sera from patients with aHUS reached statistical significance, the values obtained with activated plasma were much more notable and consistent. The coefficient of variation of the results obtained using the sera from the five patients at acute phase (52%, 74%, 74%, 31%, and 30%) was significantly higher than those obtained with activated plasma of the same five patients (9%, 11%, 10%, 10%, and 18%) (Supplemental Figure 1). These coefficients of variation were obtained analyzing 20 micrographs taken from each processed sample.
Figure 1.
Atypical hemolytic uremic syndrome (aHUS)–activated plasma induces C5b-9 deposition on microvascular endothelial cells with a coefficient of variation significantly lower than that obtained using aHUS serum. (A) Representative microscopy image of C5b-9 deposition staining (red) on endothelial cells (4′,6-diamidino-2-phenylindole–stained nuclei, blue) induced by activated plasma or sera from a control individual and a patient with aHUS in the acute phase (patient 4, P4). Bar diagram represents quantification of C5b-9 deposits expressed as fold increase of the covered surface with respect to the control, from five different patients with aHUS in the acute phase (P4 to P8). Black bars represent the results obtained with activated plasma and white bars with serum. The dotted line represents control values. (B) Representative microscopy images of staining of C5b-9 deposition on endothelial cells (induced by activated plasma from the acute phase and at remission in three patients: P9 to P11). Bar diagram represents C5b-9 quantification, black bars represent results from the acute phase, and gray bars represent remission. All values at the acute phase were statistically higher than in control samples (*P<0.05) and all values at remission are statistically lower than those at the acute phase (#P<0.05). Statistical analysis was performed with raw data using the t test for paired samples.
Activated plasma from three additional patients with aHUS was evaluated. C5b-9 deposits showed fold increases of 13.1±0.2 for patient 9, 10.9±0.3 for patient 10, and 6.4±0.2 for patient 11 (P<0.001 for all values versus control) at acute phase, and 0.8±0.1 for patient 9, 1.1±0.2 for patient 10, and 0.8±0.1 for patient 11 during remission (P<0.001 for all values versus acute phase) (Figure 1B). Of note, experiments with activated plasma from patient 9 after five daily plasma exchanges showed a positive effect in reducing complement activation, although it was unable to avoid it completely (6.9±0.4 versus control, P<0.001 versus control and versus acute phase). This finding showed a clear relation with clinical outcome (hematologic improvement without kidney function recovery). After eculizumab initiation, the patient showed both hematologic and kidney responses, and C5b-9 deposits were lower than in control.
Moreover, C5b-9 deposits induced by activated plasma from five patients with aHUS in remission without treatment and asymptomatic-mutation carriers on ADP-stimulated and -nonstimulated cells were also performed. Our results showed no differences when using both type of cells (Supplemental Table 2).
Effect of Standard and Lower Doses of Eculizumab on C5b-9 Deposition on Endothelial Cells
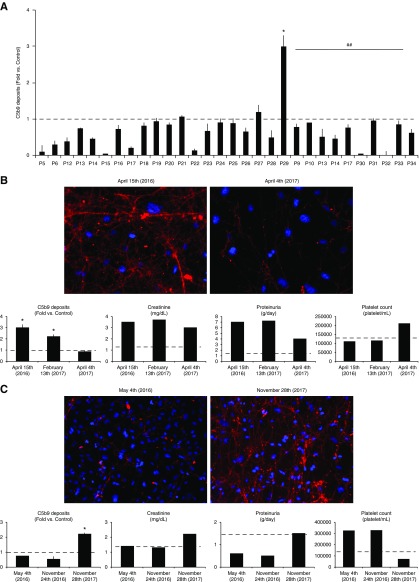
Complement activation, measured by C5b-9 deposits on activated endothelial cells, was at control levels or even lower when we analyzed the effect of activated-plasma samples from patients with aHUS who were treated at regular doses of eculizumab (n=20) or lower doses (n=10) (Figure 2A).
Figure 2.
C5b-9 deposits on activated-endothelial cells are at control level or even lower when analyzing the effect of activated-plasma samples from aHUS patients treated at regular doses of eculizumab or lower doses. Identification of two patients with partial remision and clinical relapse. (A) Bar diagrams show the results of C5b-9 deposition on endothelial cells of activated plasma from 20 patients with aHUS (P5 to P29) who were treated at regular doses of eculizumab (according to weight in pediatric patients; 900 mg/wk for 4 weeks as induction therapy for adults, and 1200 mg every 2 weeks thereafter as maintenance). All values resulted in control levels except in patient 29, in whom they were significantly higher (*). Bar diagram also shows, under the continuous line and ## symbol, the results from ten patients with aHUS who were treated at lower doses or for longer intervals than those specified in the data sheet. Activated plasma from those patients induced C5b-9 deposition at similar or even lower levels than control activated plasma. The dotted line represents control values. Statistical analysis was performed with raw data using the t test for paired samples. (B) Results from the C5b-9 deposition assay allowed the identification of a patient who was receiving an underdose of eculizumab and allowed for a consequent dose adjustment. The left image corresponds to a representative image of C5b-9 deposits induced by activated plasma from patient 29 which was obtained on April 15, 2016. At that time point, C5b-9 deposition was statistically higher (*P<0.05) than control levels as seen in the bar diagrams. The right image corresponds to activated plasma from the same patient obtained on April 4, 2016, after several eculizumab dose changes, showing a negative result. Statistical analysis was performed with raw data using the t test for paired samples. The results from C5b-9 assays at three different time points corresponding to different eculizumab guidelines are quantified in the bar diagrams along with creatinine (mg/dl), proteinuria (g/d), and platelet count (platelet/ml). (C) Results from the C5b-9 deposition assay confirmed the identification of a patient with aHUS recurrence (left image) when C5b-9 deposition was higher than control levels (bar diagrams), and complete clinical recovery (right image) after the start of eculizumab treatment. Bar diagrams show C5b-9 quantification, creatinine (mg/dl), proteinuria (g/d), and platelet count (platelet/ml) at different time points.
Prospective C5b-9 Deposition on Endothelial Cells To Titrate Eculizumab Dosage in Patients with Atypical Hemolytic Uremic Syndrome: Case Report 1
A 20-year-old woman (patient 29) with an MCP mutation (c.-325A>C) manifested aHUS in 2012. The patient recovered kidney function after 12 plasma-exchange sessions. She started eculizumab treatment in June 2014 after clinical and histologic disease recurrence. Despite initial improvement, she developed severe hypertension and progressive proteinuria with impaired kidney function (Figure 2B). CH50 and C5 free levels measured 14 days after eculizumab administration were undetectable, with correct, and even higher than normal, free eculizumab levels (740 μg/ml). However, at the same point, the patient had low C3 (71 mg/dl) and high sC5b-9 levels (471 ng/ml) (27). Analysis of C5b-9 deposition on endothelial cells showed increased complement activation when the patient was receiving a standard eculizumab dosage (3±0.3 versus control, P=0.01) and even when the time between doses was reduced to 10 days (2.2±0.1 versus control, P=0.01). C5b-9 deposits only reached control levels after the eculizumab dose was increased up to 1500 mg every 14 days (0.8±0.2 versus control), accompanied by better BP control, a recovery in C3 levels (84 mg/dl), persistent reduction of CH50 (11 mg/ml), a decrease in proteinuria, and stabilization of kidney function (Figure 2B).
C5b-9 Deposition Confirms Clinical Relapse in Patients with Atypical Hemolytic Uremic Syndrome with an Interruption in Eculizumab Treatment: Case Report 2
A 36-years-old woman (patient 13) was diagnosed with aHUS in 2004. She was treated with plasma exchange (>100 sessions) and achieved hematologic and kidney remission. She developed aHUS relapse in 2013 and started eculizumab treatment with an excellent response. In July 2017, after a period of lower eculizumab doses, she stopped treatment. She had normal C5b-9 deposits during eculizumab treatment (0.7±0.1 with standard doses and 0.5±0.2 with lower doses versus control). However, in November 2017 she developed a second aHUS relapse. An activated-plasma sample induced increased C5b-9 deposition on endothelial cells (2.2±0.1 versus control, P=0.03; Figure 2C). Early eculizumab introduction allowed complete clinical recovery.
Significant Complement Activation Shown by HELLP and Preeclampsia, but Not Malignant Hypertension
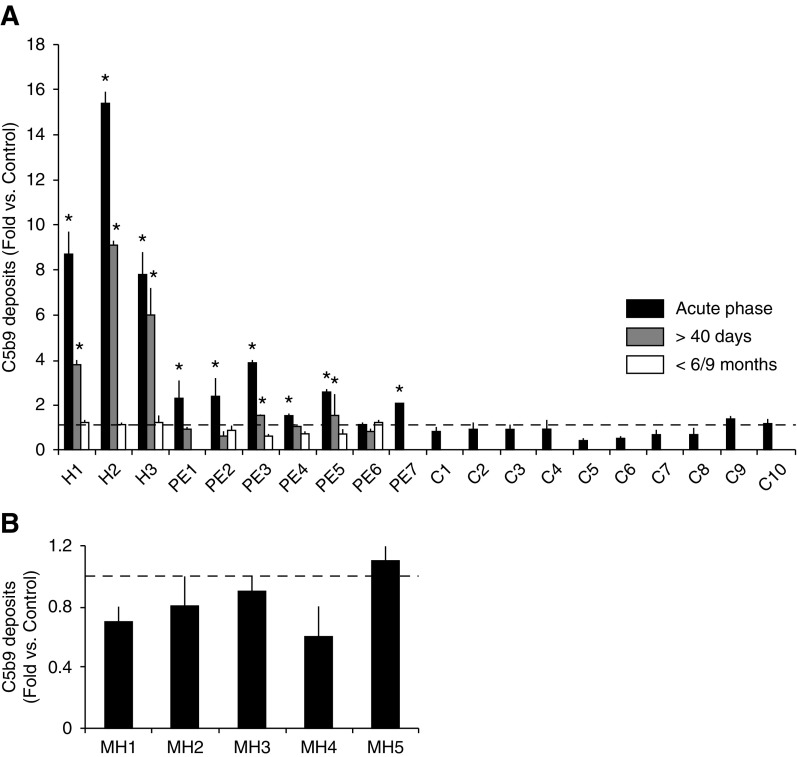
The C5b-9 deposition on endothelial cells was analyzed in activated plasma from patients with HELLP syndrome and preeclampsia at the acute phase, 40 days after delivery and 6–9 months after onset (Table 2). Activated-plasma samples from patients with malignant hypertension at the acute phase were also tested (Table 3).
The baseline and perinatal outcomes of the pregnant study population are shown in Table 4. All patients with HELLP syndrome showed marked complement activation at the acute phase (8.7±1 for patient 1 with HELLP, 15.4±0.5 for patient 2 with HELLP, and 7.8±1 for patient 3 with HELLP versus control, P<0.001 for all values) that was still increased after 40 days (3.8±0.2 for patient 1 with HELLP, 9.1±0.20 for patient 2 with HELLP, and 6±1.2 for patient 3 with HELLP versus control, P<0.001 for all values), and normalized after 6–9 months. All except one patient with preeclampsia also showed increased complement activation, but only two were still positive after 40 days. All patients with preeclampsia showed normal complement activity after 6–9 months (Figure 3A). Ten control pregnant women were analyzed and they showed normal complement levels (Supplemental Table 5).
Table 4.
Characteristics and perinatal outcomes of the study populations
| Characteristics and Outcomes | Control (n=10) | Severe Preeclampsia/HELLP Syndrome (n=10) |
|---|---|---|
| Characteristics | ||
| Age (yr) | 33±4 | 33±5 |
| White (%) | 50 | 60 |
| Nulliparity (%) | 50 | 60 |
| Gestational age at inclusion (wk) | 37±4 | 33±6 |
| Perinatal outcomes | ||
| Gestational age at delivery (wk) | 40 (2) | 34 (8) |
| Male gender (%) | 30 | 78 |
| Birth weight (g) | 3383 (188) | 1794 (1180) |
| Birthweight percentile | 47 (40) | 3 (31) |
| Cesarean section (%) | 10 | 90 |
| Umbilical cord artery pH | 7.2±0.1 | 7.2±0.1 |
| 5 min Apgar score | 10 (0) | 10 (1) |
Data are shown as percentages, mean±SD, or median (interquartile range).
Figure 3.
Activated plasma from patients with HELLP syndrome and preeclampsia at onset induced an increase C5b-9 deposition on endothelial cells. (A) Quantification of C5b-9 deposits on endothelial cells show that three HELLP syndrome patients (H1 to H3) showed marked complement activation in the acute phase (black bars) that was still increased after 40 days (gray bars), and normalized after 6–9 months (white bars). All except one patient with preeclampsia (PE) also showed increased complement activation in the acute phase, but only two were still positive after 40 days. All patients with preeclampsia analyzed had normal complement activity after 6–9 months. Ten healthy pregnant women were also analyzed (C1 to C10), obtaining normal C5b-9 deposits. The dotted line represents control values. *P<0.05 represents values statistically higher than control samples. Statistical analysis was performed with raw data using the t test for paired samples. (B) Bar diagrams show results from C5b-9 deposition induced by activated plasma from five patients with malignant hypertension (MH1 to MH5). All patients in the acute phase showed normal complement activity, represented by the dotted line. Statistical analysis was performed with raw data using the t test for paired samples.
Activated plasma from patients with malignant hypertension (n=5) induced normal complement deposition (Figure 3B). After adequate BP control, clinical microangiopathic signs partially resolved (Table 3).
Circulating Complement Protein Levels
Soluble forms of C3, C4, and CH50 were analyzed in serum samples of all patients during both the acute phase and clinical remission. Circulating levels of these proteins were normal in most patients regardless of clinical status (see Supplemental Material).
Discussion
The deposition of the membrane-attack complex on endothelial cells after exposure to sera from patients with aHUS in the acute phase indicates that the dysregulation of the alternative complement pathway plays a primordial pathogenic role in this disorder, as described by Noris et al. (22,23). We modified this previously described technique by exposing endothelial cells to plasma samples from patients with aHUS with the aim of reducing variability in the results obtained. C5b-9 deposition occurred together with fibrin formation and was more notable, reproducible, and highly consistent than that obtained using serum. Deposition of C5b-9 showed an excellent correlation with aHUS clinical stages. The evaluation of C5b-9 deposition allowed monitoring patient response to the treatment, enabling the identification of partial remission and clinical relapse. Our results indicate complement hyperactivation could also be crucial in the pathogenesis of HELLP syndrome and severe preeclampsia, but not in malignant hypertension. These findings could have potential diagnostic and therapeutic implications.The development of techniques verifying the underlying disease mechanism of aHUS (28,29) is crucial to allow early etiological treatment. In this scenario, Noris et al. (22) evaluated C5b-9 deposition on endothelial cells using serum from patients with aHUS and showed that deposits were present on unstimulated cells exposed to sera from patients during acute phase and this normalized at remission; whereas using stimulated cells they could also detect asymptomatic mutation carriers. We confirmed these results in acute aHUS, but we encountered a very high coefficient of variation. We had no success when trying to reproduce the method of Gavriilaki et al. (24). With the aim of reducing interobserver variation, we proceeded to validate C5b-9 deposition on endothelial cells with activated plasma from patients with aHUS. The results followed the same tendency as those observed with sera, but C5b-9 deposits occurred together with fibrin formation on the cell surface. Importantly, we did not find differences when evaluating C5b-9 deposits induced by patients with acute aHUS in remission without treatment, and asymptomatic mutation carriers over stimulated and nonstimulated cells, meaning that this modification is not useful for the identification of asymptomatic mutation carriers (Supplemental Figure 2) (29). The potential bias involved in providing normal complement regulator proteins to the patient’s sample should be overcome because results were always compared with control activated plasma. In addition, circulating levels of C3, C4, and CH50 were also measured, but failed to predict either aHUS diagnosis or clinical status. Finally, patients with CKD unrelated to TMA presented C5b-9 deposits at control levels (Supplemental Figure 3).
Eculizumab is the only medication approved for aHUS management. A complement functional test for monitoring the eculizumab administration schedule has been used to evaluate patients with aHUS in the stable phase (30,31). Other forms of monitoring have been published with promising results (32,33). We evaluated a total of 30 samples from 27 patients with aHUS treated with eculizumab. All patients achieving clinical remission had C5b-9 deposits at control levels or lower, independently of dosage and therapeutic schedule. Of note, in patient 9 (who received five daily plasma exchanges with resolution of hemolysis but without kidney improvement), C5b-9 deposition still occurred, although it decreased with respect to the acute phase. After eculizumab initiation, this patient achieved complete clinical recovery, with C5b-9 deposits lower than in controls. In addition, C5b-9 deposition assessment in patient 29 allowed the identification of underdosing, and was useful to titrate the necessary dose for clinical remission. Finally, patient 13 had aHUS relapse after 4 months of stopping eculizumab, and C5b-9 evaluation confirmed the clinical suspicion.
Regarding pregnancy-related complications, our results showed intense complement activation in those with preeclampsia and HELLP syndrome during the acute phase, which was persistent 40 days after delivery in all HELLP cases, despite clinical remission. Normal complement activity was observed 6–9 months later. These results are in agreement with those of previous studies suggesting that a dysregulation of the complement pathway may play an important role in its pathogenesis. Serum and urinary C5a and C5b-9 concentrations are elevated in hypertensive mothers (34,35), and preeclampsia has been described as a multifactorial disease with an angiogenic imbalance as an essential underlying mechanism that could be related to complement activation (35,36). There is experimental evidence indicating that complement activation induces the production of angiogenic molecules and C5a release by monocytes (37,38). Additionally, aberrant decidual angiogenesis appears to trigger the activation of the complement pathway. This potential key role for the complement system in the pathogenesis of HELLP and preeclampsia offers new opportunities for early prediction, monitoring, and therapy. Eculizumab has been used during pregnancy without apparent adverse outcomes for the mother or the fetus (39,40), and in vitro studies suggest a beneficial effect of eculizumab in HELLP (41).
Clinical diagnosis of malignant hypertension is based on the presence of very high BP levels and grade 3/4 hypertensive retinopathy. Clinical reports and short case series indicate the possibility that some malignant hypertension with TMA signs could be complement mediated (42,43). Etiological differentiation between malignant hypertension and aHUS is crucial to initiate an etiological treatment. These results indicate that complement activity was normal in the acute phase and after BP control in patients with malignant hypertension. In contrast, when exploring samples from five out of the 11 patients with aHUS (who had grade 3 or 4 hypertensive retinopathy) who were evaluated at the acute phase, C5b-9 deposits were significantly high. Although we included a low number of patients with malignant hypertension in this study, these results validate recently published data (44) where C5b-9 deposits on endothelial cells in the setting of severe hypertension differentiated patients with complement dysregulation from those with mechanical stress as the cause of disease. It will be crucial in future investigations to know if patients diagnosed with HELLP, preeclampsia, and malignant hypertension are carriers of complement mutations.
This complement-activity assay is based on the existence of multiple links between the complement and the clotting system (45), as it could be derived from the inhibitory experiments carried out (see Supplemental Material). C5b-9 deposition on the endothelial cell surface occurs together with fibrin formation, even in control situations, and strongly depends on thrombin generation (Supplemental Figure 4). This observation is in line with others reporting that complement activation is attenuated by thrombin inhibitors (46,47). The inhibition of the reaction by blocking coagulation Factor XII suggests that serine proteases in the coagulation system are able to activate the complement cascade independently of the pathways established to date (48). Moreover, our assay seems to be independent of the extrinsic coagulation pathway because the inhibition of the tissue factor by a specific antibody had no effect on C5b-9 deposition (Supplemental Figure 4) (49). Finally, our reaction strongly depends on the presence of calcium because it does not occur when using nonactivated plasma or when the activated plasma is obtained from blood with EDTA (Supplemental Figure 4). This could be because EDTA presents a much higher binding constant than citrate (50). By adding the serum of healthy donors to the plasma samples of patients, we overcame the partial complement inhibition due to calcium chelation and reduced the variability observed in complement deposition when using sera.
In conclusion, measurement of C5b-9 deposits on endothelial cells is a useful tool to explore the overactivation of the complement system, not only in aHUS but also in other diseases, such as HELLP and preeclampsia. Moreover, this technique could be useful to monitor the efficiency of eculizumab treatment in individual patients with aHUS, allowing for follow-up of treatment response, exploration of optimal dosage, and identification of potential recurrences. Our approach offers consistent results that, if confirmed in larger cohorts, could open the door to the evaluation of the role of complement activation in the pathogenesis of other TMAs that may eventually benefit from C5 inhibition and monitor treatment response in these patients.
Disclosures
Dr. Ariceta reports advisory and educational activities for Advicenne, Alexion Pharmaceuticals, Chiesi, Kyowa Kirim, and Orphan Europe, and a position as Member of the Scientific Board of the International Registry for Atypical Hemolytic Uremic Syndrome sponsored by Alexion Pharmaceuticals, all outside of the submitted work. Dr. Blasco reports fees from an advisory board position and symposium speaker honoraria from Alexion Pharmaceuticals outside of the submitted work. Dr. Carreras reports receiving a grant and personal fees from Jazz Pharmaceuticals outside of the submitted work. Dr. Cao reports personal fees from Alexion and AstraZeneca and nonfinancial support from Genzyme outside of the submitted work. Dr. Diaz-Ricart reports receiving a grant and personal fees from Jazz Pharmaceuticals outside of the submitted work. Dr. Espinosa reports fees from an advisory board position and speaker fees from Alexion Pharmaceuticals outside of the submitted work. Dr. Fraga-Rodriguez reports speaker honoraria from Alexion Pharmaceuticals outside the submitted work. Dr. Huerta reports fees from an advisory board position and speaker fees from Alexion Pharmaceuticals outside of the submitted work. Dr. Manrique reports personal fees from Alexion Pharmaceuticals and nonfinancial support from Amgen and Vifor Pharma outside of the submitted work. Dr. Morales reports personal fees from Alexion Pharmaceuticals and Vifor Fresenius outside of the submitted work. Dr. Palomo reports speaker fees from Jazz Pharmaceuticals outside of the submitted work. Dr. Poch reports speaker fees from Alexion, Fresenius, and Otsuka outside of the submitted work. Dr. Praga reports receiving a grant from Alexion Pharmaceuticals and personal fees from Fresenius, Otsuka, and Retrophin outside of the submitted work. The remaining authors have nothing to disclose.
Funding
This work was partially supported by the Spanish Kidney Research Network (Instituto de Salud Carlos III [ISCIII]-RETIC, Red de Investigación Renal [REDinREN] RD16/0009/0023), Fundació Miarnau (Spain), Jazz Pharmaceuticals (IST-16-10355), German Jose Carreras Leukaemia Foundation (DJCLS 11R/2016 and DJCLS 03R/2019), ISCIII, Spanish Government (Fondo de Investigación en Salud, PI19/00888 ISCIII; Integrated Project in Health Institutes, PIE15/00027; and Technology Development Projects in Health 2016, DTS16/00133), Centres de Recerca de Catalunya Programme of the Generalitat de Catalunya, Generalitat de Catalunya (2017-SGR671), the European Regional Development Funds (Fondo Europeo de Desarrollo Regional [FEDER]), ISCIII/FEDER (PI13/02502, PICI14/00350, and PI16/01685), Autonomous Region of Madrid (S2017/BMD-3673), RedInRen (RD12/0021/0029), “La Caixa” Foundation (LCF/PR/GN14/10270005), ISCIII (CM16/00142) integrated in Plan Nacional de I+D+I and cofunded by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa,” Cerebra Foundation for the Brain Injured Child (Carmarthen, Wales, United Kingdom), Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) 2017 SGR grant number 1531, and a grant from “Fundació Dexeus Mujer.”
Supplementary Material
Acknowledgments
The authors would like to thank Professor Santiago Rodríguez de Córdoba, from Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Científicas (Madrid, Spain), and Professor Karin Dahan from Institut de Pathologie et Génétique at Goselies (Belgium), for the genetic information kindly provided.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Monitoring Complement Activation: The New Conundrum in Thrombotic Microangiopathies,” on pages 1682–1683.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05830519/-/DCSupplemental.
Supplemental Table 1. Clinical parameters and complement activation markers in aHUS patients treated with eculizumab.
Supplemental Table 2. Clinical parameters and complement activation markers in aHUS patients without eculizumab treatment and asymptomatic mutation carriers.
Supplemental Table 3. Genetic and molecular analyses in aHUS patients.
Supplemental Table 4. Kidney function and complement parameters in ADPKD patients.
Supplemental Table 5. Complement parameters in pregnancy controls.
Supplemental Figure 1. Activated-plasma and sera induction of C5b9 deposits on endothelial cells.
Supplemental Figure 2. Asymptomatic mutation carrier’s activated-plasma induction of C5b-9 deposits on activated or non-activated endothelial cells.
Supplemental Figure 3. Autosomal dominant polycystic kidney disease patients activated-plasma induction of C5b-9 deposits on endothelial cells.
Supplemental Figure 4. Blockade of C5b-9 deposition on endothelial cells by eculizumab and intrinsic coagulation pathway inhibitors.
References
- 1.George JN, Nester CM: Syndromes of thrombotic microangiopathy. N Engl J Med 371: 654–666, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Fervenza FC: Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 40: 416–421, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Román E, Mendizábal S, Jarque I, de la Rubia J, Sempere A, Morales E, Praga M, Ávila A, Górriz JL: Secondary thrombotic microangiopathy and eculizumab: A reasonable therapeutic option. Nefrologia 37: 478–491, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Cavero T, Rabasco C, López A, Román E, Ávila A, Sevillano Á, Huerta A, Rojas-Rivera J, Fuentes C, Blasco M, Jarque A, García A, Mendizabal S, Gavela E, Macía M, Quintana LF, María Romera A, Borrego J, Arjona E, Espinosa M, Portolés J, Gracia-Iguacel C, González-Parra E, Aljama P, Morales E, Cao M, Rodríguez de Córdoba S, Praga M: Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant 32: 466–474, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokiranta TS: HUS and atypical HUS. Blood 129: 2847–2856, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T: Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 36: 673–681, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C: Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89: 701–711, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Fakhouri F, Hourmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, Menne J, Minetti EE, Provôt F, Rondeau E, Ruggenenti P, Weekers LE, Ogawa M, Bedrosian CL, Legendre CM: Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: A single-arm, open-label trial. Am J Kidney Dis 68: 84–93, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez de Córdoba S, Hidalgo MS, Pinto S, Tortajada A: Genetics of atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 40: 422–430, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Vieira-Martins P, El Sissy C, Bordereau P, Gruber A, Rosain J, Fremeaux-Bacchi V: Defining the genetics of thrombotic microangiopathies. Transfus Apheresis Sci 54: 212–219, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G: Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asif A, Nayer A, Haas CS: Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: Case reports and a review of the evidence for treatment with eculizumab. J Nephrol 30: 347–362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufano A, Coppola A, Maruotti GM, Martinelli P, Cerbone AM, Di Minno G: HELLP syndrome and its relation with the antiphospholipid syndrome. Blood Transfus 12: 114–118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbor M, Johnson A, Harris F, Makanjoula D, Shehata H: Pregnancy-associated atypical haemolytic uraemic syndrome in the postpartum period: A case report and review of the literature. Obstet Med 4: 83–85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN: Drug-induced thrombotic microangiopathy: A systematic review of published reports. Blood 125: 616–618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton JM, George JN: Microangiopathic hemolytic anemia and thrombocytopenia in patients with cancer. J Oncol Pract 12: 523–530, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Song D, Wu LH, Wang FM, Yang XW, Zhu D, Chen M, Yu F, Liu G, Zhao MH: The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res Ther 15: R12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Pintó I, Espinosa G, Cervera R: Catastrophic APS in the context of other thrombotic microangiopathies. Curr Rheumatol Rep 17: 482, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Mathew RO, Nayer A, Asif A: The endothelium as the common denominator in malignant hypertension and thrombotic microangiopathy. J Am Soc Hypertens 10: 352–359, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Dhakal P, Bhatt VR: Is complement blockade an acceptable therapeutic strategy for hematopoietic cell transplant-associated thrombotic microangiopathy? Bone Marrow Transplant 52: 352–356, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Garg N, Rennke HG, Pavlakis M, Zandi-Nejad K: De novo thrombotic microangiopathy after kidney transplantation. Transplant Rev (Orlando) 32: 58–68, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, Tripodo C, Bettoni S, Donadelli R, Valoti E, Tedesco F, Amore A, Coppo R, Ruggenenti P, Gotti E, Remuzzi G: Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 124: 1715–1726, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galbusera M, Noris M, Gastoldi S, Bresin E, Mele C, Breno M, Cuccarolo P, Alberti M, Valoti E, Piras R, Donadelli R, Vivarelli M, Murer L, Pecoraro C, Ferrari E, Perna A, Benigni A, Portalupi V, Remuzzi G: An Ex Vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis 74: 56–72, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Gavriilaki E, Yuan X, Ye Z, Ambinder AJ, Shanbhag SP, Streiff MB, Kickler TS, Moliterno AR, Sperati CJ, Brodsky RA: Modified Ham test for atypical hemolytic uremic syndrome. Blood 125: 3637–3646, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ: HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99: 683–690, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A: Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subías Hidalgo M, Martin Merinero H, López A, Anter J, García SP, Ataúlfo Gonzalez-Fernández F, Forés R, Lopez-Trascasa M, Villegas A, Ojeda E, Rodríguez de Córdoba S: Extravascular hemolysis and complement consumption in Paroxysmal Nocturnal Hemoglobinuria patients undergoing eculizumab treatment. Immunobiology 222: 363–371, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Campistol JM, Arias M, Ariceta G, Blasco M, Espinosa L, Espinosa M, Grinyó JM, Macía M, Mendizábal S, Praga M, Román E, Torra R, Valdés F, Vilalta R, Rodríguez de Córdoba S: An update for atypical haemolytic uraemic syndrome: Diagnosis and treatment. A consensus document. Nefrologia 35: 421–447, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, Coppo R, Emma F, Johnson S, Karpman D, Landau D, Langman CB, Lapeyraque AL, Licht C, Nester C, Pecoraro C, Riedl M, van de Kar NC, Van de Walle J, Vivarelli M, Frémeaux-Bacchi V; HUS International : An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 31: 15–39, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Volokhina EB, van de Kar NC, Bergseth G, van der Velden TJ, Westra D, Wetzels JF, van den Heuvel LP, Mollnes TE: Sensitive, reliable and easy-performed laboratory monitoring of eculizumab therapy in atypical hemolytic uremic syndrome. Clin Immunol 160: 237–243, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Ardissino G, Tel F, Sgarbanti M, Cresseri D, Giussani A, Griffini S, Grovetto E, Possenti I, Perrone M, Testa S, Paglialonga F, Messa P, Cugno M: Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome: An update. Pediatr Nephrol 33: 457–461, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Wehling C, Amon O, Bommer M, Hoppe B, Kentouche K, Schalk G, Weimer R, Wiesener M, Hohenstein B, Tönshoff B, Büscher R, Fehrenbach H, Gök ÖN, Kirschfink M: Monitoring of complement activation biomarkers and eculizumab in complement-mediated renal disorders. Clin Exp Immunol 187: 304–315, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puissant-Lubrano B, Puissochet S, Congy-Jolivet N, Chauveau D, Decramer S, Garnier A, Huart A, Kamar N, Ribes D, Blancher A: Alternative complement pathway hemolytic assays reveal incomplete complement blockade in patients treated with eculizumab. Clin Immunol 183: 1–7, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB: Urinary excretion of C5b-9 in severe preeclampsia: Tipping the balance of complement activation in pregnancy. Hypertension 62: 1040–1045, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Guseh SH, Feinberg BB, Dawood HY, Yamamoto HS, Fichorova RN, Burwick RM: Urinary excretion of C5b-9 is associated with the anti-angiogenic state in severe preeclampsia. Am J Reprod Immunol 73: 437–444, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, Kellems RE, Xia Y: Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension 60: 712–721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Kong LR, Ge Q, Lu YY, Hong MN, Zhang Y, Ruan CC, Gao PJ: Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J Cell Mol Med 22: 1034–1046, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andries G, Karass M, Yandrapalli S, Linder K, Liu D, Nelson J, Pawar R, Chugh S: Atypical hemolytic uremic syndrome in first trimester pregnancy successfully treated with eculizumab. Exp Hematol Oncol 6: 4, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly RJ, Höchsmann B, Szer J, Kulasekararaj A, de Guibert S, Röth A, Weitz IC, Armstrong E, Risitano AM, Patriquin CJ, Terriou L, Muus P, Hill A, Turner MP, Schrezenmeier H, Peffault de Latour R: Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 373: 1032–1039, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Vaught AJ, Gavriilaki E, Hueppchen N, Blakemore K, Yuan X, Seifert SM, York S, Brodsky RA: Direct evidence of complement activation in HELLP syndrome: A link to atypical hemolytic uremic syndrome. Exp Hematol 44: 390–398, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai HM: Does anticomplement therapy have a role in the management of malignant hypertension? J Clin Hypertens (Greenwich) 18: 359–360, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timmermans SAMEG, Abdul-Hamid MA, Vanderlocht J, Damoiseaux JGMC, Reutelingsperger CP, van Paassen P; Limburg Renal Registry : Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int 91: 1420–1425, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Timmermans SAMEG, Abdul-Hamid MA, Potjewijd J, Theunissen ROMFIH, Damoiseaux JGMC, Reutelingsperger CP, van Paassen P; Limburg Renal Registry : C5b9 formation on endothelial cells reflects complement defects among patients with renal thrombotic microangiopathy and severe hypertension. J Am Soc Nephrol 29: 2234–2243, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Brückner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M: Molecular intercommunication between the complement and coagulation systems. J Immunol 185: 5628–5636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD: Complement and coagulation: Strangers or partners in crime? Trends Immunol 28: 184–192, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B: Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: Possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 51: 1779–1784, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M: Interaction between the coagulation and complement system. Adv Exp Med Biol 632: 71–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD: Interactions between coagulation and complement--their role in inflammation. Semin Immunopathol 34: 151–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keowmaneechai E, McClements DJ: Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCl2. J Agric Food Chem 50: 7145–7153, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Keith NM, Wagener HP, Barker NW: Some different types of essential hypertension: their course and prognosis. Am J Med Sci 268: 336–345, 1974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.