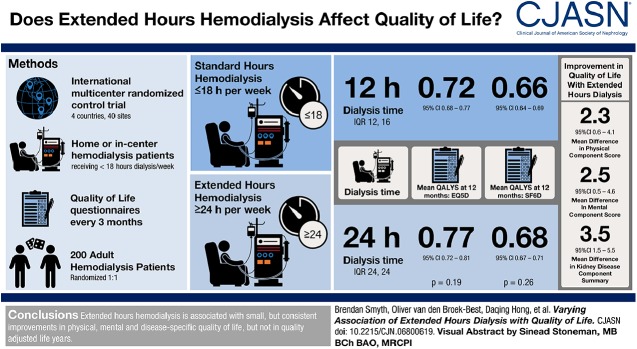
Visual Abstract
Keywords: quality of life, chronic hemodialysis, randomized controlled trials, humans, renal dialysis, quality-adjusted life years, confidence intervals, New Zealand, patient reported outcome measures, home hemodialysis, kidney diseases, kidney, Australia, China, Canada
Abstract
Background and objectives
Little is known about the effect of changes in dialysis hours on patient-reported outcome measures. We report the effect of doubling dialysis hours on a range of patient-reported outcome measures in a randomized trial, overall and separately for important subgroups.
Design, setting, participants, & measurements
The A Clinical Trial of IntensiVE Dialysis trial randomized 200 participants to extended or standard weekly hours hemodialysis for 12 months. Patient-reported outcome measures included two health utility scores (EuroQOL-5 Dimensions-3 Level, Short Form-6 Dimension) and their derived quality-adjusted life year estimates, two generic health scores (Short Form-36 Physical Component Summary, Mental Component Summary), and a disease-specific score (Kidney Disease Component Score). Outcomes were assessed as the mean difference from baseline using linear mixed effects models adjusted for time point and baseline score, with interaction terms added for subgroup analyses. Prespecified subgroups were dialysis location (home- versus institution-based), dialysis vintage (≤6 months versus >6 months), region (China versus Australia, New Zealand, Canada), and baseline score (lowest, middle, highest tertile). Multiplicity-adjusted P values (Holm–Bonferroni) were calculated for the main analyses.
Results
Extended dialysis hours was associated with improvement in Short Form-6 Dimension (mean difference, 0.027; 95% confidence interval [95% CI], 0.00 to 0.05; P=0.03) which was not significant after adjustment for multiple comparisons (Padjusted=0.05). There were no significant differences in EuroQOL-5 Dimensions-3 Level health utility (mean difference, 0.036; 95% CI, −0.02 to 0.09; P=0.2; Padjusted=0.2) or in quality-adjusted life years. There were small positive differences in generic and disease-specific quality of life: Physical Component Summary (mean difference, 2.3; 95% CI, 0.6 to 4.1; P=0.01; Padjusted=0.04), Mental Component Summary (mean difference, 2.5; 95% CI, 0.5 to 4.6; P=0.02; Padjusted=0.05) and Kidney Disease Component Score (mean difference, 3.5; 95% CI, 1.5 to 5.5; P=0.001; Padjusted=0.005). The results did not differ among predefined subgroups or by baseline score.
Conclusions
The effect of extended hours hemodialysis on patient-reported outcome measures reached statistical significance in some but not all measures. Within each measure the effect was consistent across predefined subgroups. The clinical importance of these differences is unclear.
Introduction
In addition to a substantially higher mortality rate (1), dialysis-dependent patients also have poorer health-related quality of life than the general population (2,3). Within dialysis cohorts, poorer quality of life correlates with the risk of death and hospitalization (4,5). In observational studies, quality of life is worse with more severe CKD (3) and is consistently worse in patients treated with dialysis versus kidney transplantation (6,7), leading to the hypothesis that treatments that more adequately replace kidney clearance may improve both clinical outcomes and quality of life. Observational studies support the hypothesis that increased dose, frequency, or duration of hemodialysis improves quality of life (8–10); however, randomized trials in this field have yielded conflicting results (11–14).
Multiple tools for measuring quality of life exist, including both generic instruments designed for use in any population, and disease-specific instruments designed for use in particular populations. In addition, some instruments permit calculation of health utility, a measure of “the preference for, or desirability of, a specific level of health status or specific health outcome,” which can be used in health economic analysis to estimate quality-adjusted life years (QALYs) (15). These instruments were not originally designed for use in trials and their development did not include assessments of their responsiveness to interventions. Despite this, they are commonly used to assess the effect of an intervention on quality of life (16). When utilized as a study outcome, quality of life fits within the larger class of patient-reported outcome measures. Such measures are now a mandated consideration for drug approval by the US Food and Drug Administration (FDA) (17).
A Clinical Trial of IntensiVE (ACTIVE) Dialysis was an international, multicenter, randomized, open-label, blinded end point-assessment clinical trial where participants were randomized to extended hours hemodialysis (≥24 hours per week) versus standard hours (≤18 hours per week) (18). Extended hours hemodialysis had no effect on the primary end point of EuroQOL-5 Dimension-3 Level (EQ-5D-3L) measured health utility but was associated with improvement in Short Form-36 (SF-36) Physical Component Summary (PCS) and Mental Component Summary (MCS) (19). In this analysis of secondary outcomes, we aimed to describe the effect of extended hours hemodialysis on all patient-reported outcomes measured in the ACTIVE Dialysis study and to identify any differences between key patient subgroups or by baseline quality of life.
Materials and Methods
The design and primary results of the ACTIVE Dialysis trial have been reported previously (18,19). In brief, patients aged 18 years or older, receiving maintenance hemodialysis (home- or institution-based) for <18 hours per week were recruited. The study interventions were standard hours hemodialysis (defined as ≤18 hours per week) or extended hours hemodialysis (defined as ≥24 hours per week). A minimum of three dialysis sessions per week was specified and all other treatment was in accordance with routine clinical practice as determined by the individual sites. Participants were randomized (1:1) via a web-based system. Randomization was aided by a minimization strategy to ensure balanced allocation according to dialysis vintage (≤6 or >6 months), dialysis location (home or institution), and region (China versus Australia, Canada, and New Zealand). Quality-of-life questionnaires were administered at 3-month intervals via telephone by a blinded interviewer during the intervention period. Interpreter services were available if required.
Quality-of-Life Measurement
Five distinct quality-of-life measures were obtained in the ACTIVE Dialysis trial (Table 1). The primary outcome was EQ-5D-3L health utility (19,20). EQ-5D-3L produces a health utility score derived from general United Kingdom (UK) population health-state preferences, ranging from −0.594 to 1, where death and perfect health are assigned scores of zero and one, respectively (21,22). It has been studied in a variety of populations and is available in validated English and Chinese language versions (22–24). The generic and kidney-disease specific quality of life measured in this study were collected using Kidney Disease Quality of Life-Short Form version 1.3 (KDQOL-SF) (25). KDQOL-SF consists of the 36 items of SF-36, plus 43 kidney-disease specific items. SF-36 has been validated in a large number of populations and medical conditions, facilitating the definition of population norms and quality-of-life comparisons (22,26,27). It results in two summary scores, PCS and MCS, standardized against a reference population with a predefined mean score of 50 and SD of 10 (26). The mean of the 11 domains of the kidney-disease specific items of KDQOL-SF are summarized as the Kidney Disease Component Summary (25). KDQOL-SF and Kidney Disease Component Summary have been validated in patients on maintenance dialysis and are widely used (28,29). Using responses to SF-36, a health utility score known as Short Form-6 Dimension can be derived which, using preference-based weightings obtained from a representative sample of the UK population, results in a single measure of health utility ranging from 0.301 to 1 (30). UK preference weights for both EQ-5D-3L and Short Form-6 Dimension have been used widely, including in China (31), and were chosen to ensure a uniform standard across the four participating countries and facilitate comparison with previous studies.
Table 1.
Overview of quality of life measures in ACTIVE Dialysis
| Type of Measure | Health Utility | Generic | Disease Specific | ||
|---|---|---|---|---|---|
| Name of measure | EuroQOL-5 Dimension | Short Form-6 Dimension | Physical Component Summary | Mental Component Summary | Kidney Disease Component Summary |
| Source instrument | EuroQOL-5 Dimension-3 Level | Kidney Disease Quality of Life-Short Form | |||
| Domains | Mobility, self-care, usual activities, pain/discomfort, anxiety/depression | Physical functioning, role limitations, social functioning, bodily pain, mental health, vitality | Physical functioning, role limitations, (physical) bodily pain, general health perceptions | Vitality, social functioning, role limitations, (emotional) mental health | Symptoms/problems effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, dialysis staff encouragement, patient satisfaction |
| Range | −0.594 to 1 | 0.301–1 | 0–100; normalized to mean 50, SD of 10 | 0–100; normalized to mean 50, SD of 10 | 0–100 |
Statistical Analyses
Results and baseline characteristics are reported as frequencies and percentages for categorical variables, mean and SD for normally distributed variables, and median and interquartile range (IQR) when non-normally distributed. Comparisons of baseline characteristics were made using Pearson chi-squared test, Wilcoxon rank-sum test, or two-sample t test, as appropriate. The sample size of 200 was designed to provide >90% power to detect a baseline adjusted difference of 0.10 in EQ-5D-3L at 12 months. The assumptions behind this estimate are provided elsewhere (19). The treatment effect was analyzed as a mean difference between groups using a linear mixed effects model. Three prespecified subgroups were defined (in keeping with the prespecified statistical analysis plan and primary analyses [19,32]): dialysis vintage (≤6 months versus >6 months), intended location of dialysis at randomization (home versus institution), and country (China versus Australia, Canada, and New Zealand). An additional subgroup analysis examined the possibility a differential effect by baseline score by dividing each cohort into equal thirds (tertiles) on the basis of quality-of-life score at baseline (lowest, middle, and highest). Subgroup effects were explored using a mixed linear model (as described above) incorporating an interaction between treatment allocation and the relevant subgroup. All models were adjusted for time point and baseline score, with a random intercept per participant and an exchangeable covariance structure (19). No imputation was made for missing values in the primary analyses. Standardized effect size was calculated as the ratio of the mean difference and baseline SD according to the method described by Cohen (33). By convention, an effect size ≤0.2 is not considered clinically important, an effect size >0.2 to ≤0.5 is considered small, >0.5 to ≤0.8 is considered moderate, and >0.8 is considered large. QALYs were estimated from Short Form-6 Dimension and EQ-5D-3L after the imputation of zero for all subsequent visits for participants who died and then imputation of remaining missing data, such that each participant had a complete set of 12 months of observations (see below for imputation method). Mean patient QALYs were compared between treatment groups by t test. Two sensitivity analyses of the treatment effect estimates were performed. Firstly, using preference weights from a Chinese population for EQ-5D-3L (34) and Short Form-6 Dimension (35), and secondly, after imputation of missing values using multiple imputation with chained equations. Quality-of-life scores at all time points were included in the multiple imputation with chained equation models along with age, sex, study visit, treatment allocation, prespecified subgroups (see above), history of diabetes, and history of any cardiovascular disease. Forty imputations were performed with confirmation of convergence. All analyses were performed on an intention to treat basis. P values<0.05 were considered significant. To explore the robustness of the main effects of extended hours dialysis on each of the five quality-of-life scores (EQ-5D-3L, Short Form-6 Dimension, PCS, MCS, and Kidney Disease Component Summary), adjusted P values were determined post hoc using the Holm–Bonferroni method (see Supplemental Material) (36). Statistical analyses were performed using Stata/IC 15.1 (StataCorp).
Post Hoc Meta-Analysis of Randomized Trials of Intensive Hemodialysis
To place the ACTIVE Dialysis findings in the context of previous randomized trials of intensive hemodialysis, a meta-analysis of the three prior studies was performed using the reported mean difference in SF-36 PCS and MCS (11–13,37,38). Effect estimates and 95% confidence intervals (95% CIs) were pooled in a random effects model as described by DerSimonian and Laird (see Supplemental Material) (39).
Registration and Ethics Approval
The clinical trial was registered at Clinicaltrials.gov (identifier: NCT00649298) and was approved by the Harbor Human Research Ethics Committee of Northern Sydney Central Coast Health, New South Wales, Australia, and local authorities as required. It was conducted in adherence with the Declaration of Helsinki and all patients provided written informed consent.
Results
Two hundred participants were randomized to conventional or extended hours hemodialysis from 40 sites across four countries between 2009 and 2013. The baseline characteristics were similar between the two groups (Table 2). Sixteen patients did not complete 12 months of the intervention because of withdrawal of consent (four patients), transplantation (five patients), or death (seven patients). Over the 12-month intervention period, the median weekly dialysis hours in the extended hours group (24 hours; IQR, 24–24) was twice that of the standard hours group (12 hours; IQR, 12–16). The median number of dialysis sessions per week was 3.0 (IQR, 3.0–3.0) in the standard treatment group and 3.0 (IQR, 3.0–4.0) in the extended hours group. Nocturnal dialysis comprised part or all of weekly dialysis for 15% of participants (averaged over all follow-up time points) in the extended hours group versus 5% in the conventional hours group. The proportion of patients dialyzing at home in the extended hours group averaged 21% and the standard hours group averaged 19%. Over the duration of the study and after excluding those participants who died or were lost to follow-up, an average of 90% of expected scores were available in the standard hours group and 89% in the extended hours group (see Supplemental Material). Four participants (2%) did not have any follow-up values and were excluded from the main analysis.
Table 2.
Baseline participant characteristics
| Characteristics | Standard (n=100) | Extended (n=100) |
|---|---|---|
| Age in yr | 52 (12) | 52 (13) |
| Men | 70 | 69 |
| Primary cause of kidney disease | ||
| Diabetic kidney disease | 34 | 27 |
| Hypertension/vascular nephrosclerosis | 11 | 11 |
| Glomerulonephritis | 34 | 41 |
| Other or unknown | 21 | 21 |
| Comorbidity | ||
| Diabetes mellitus | 39 | 34 |
| Hypertension | 85 | 82 |
| Cardiovascular disease | 32 | 33 |
| Country | ||
| Australia | 28 | 30 |
| New Zealand | 4 | 3 |
| Canada | 6 | 5 |
| China | 62 | 62 |
| Dialysis vintage in yr, median (IQR) | 2.63 (0.97–6.74) | 2.43 (0.67–5.04) |
| >6 mo | 81 | 80 |
| ≤6 mo | 19 | 20 |
| Intended dialysis site during study | ||
| Home | 25 | 26 |
| Institution | 75 | 74 |
| Dialysis session time | ||
| Daytime only | 94 | 96 |
| Nocturnal or combination of both | 6 | 4 |
| Quality-of-life scores | ||
| EQ-5D-3L, median (IQR) [n] | 0.796 (0.689–1.000) [100] | 0.814 (0.689–1.000) [99] |
| Short Form-6 Dimension [n] | 0.682 (0.115) [94] | 0.677 (0.129) [97] |
| Physical Component Summary [n] | 40.0 (9.4) [93] | 39.5 (9.9) [97] |
| Mental Component Summary [n] | 50.1 (10.5) [93] | 48.3 (11.0) [97] |
| Kidney Disease Component Summary [n] | 66.6 (12.5) [100] | 66.0 (13.9) [100] |
Data are number (percentage) or mean (SD) unless otherwise stated. EQ-5D-3L, EuroQOL-5 Dimension-3 Level; IQR, interquartile range; PCS, Physical Component Summary; MCS, Mental Component Summary.
Effect of Extended Hours Dialysis on Health Utility and QALYs
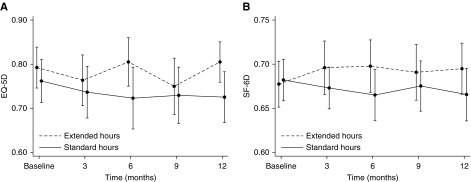
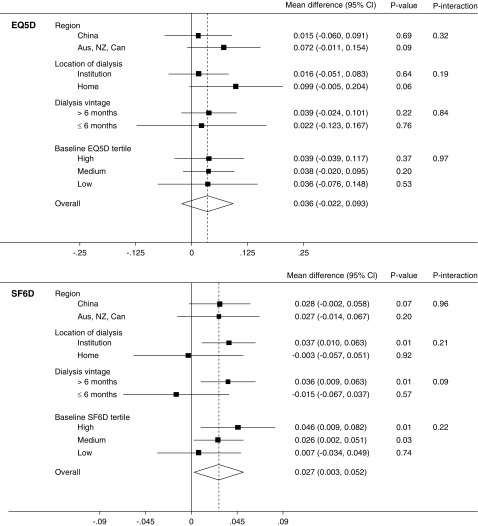
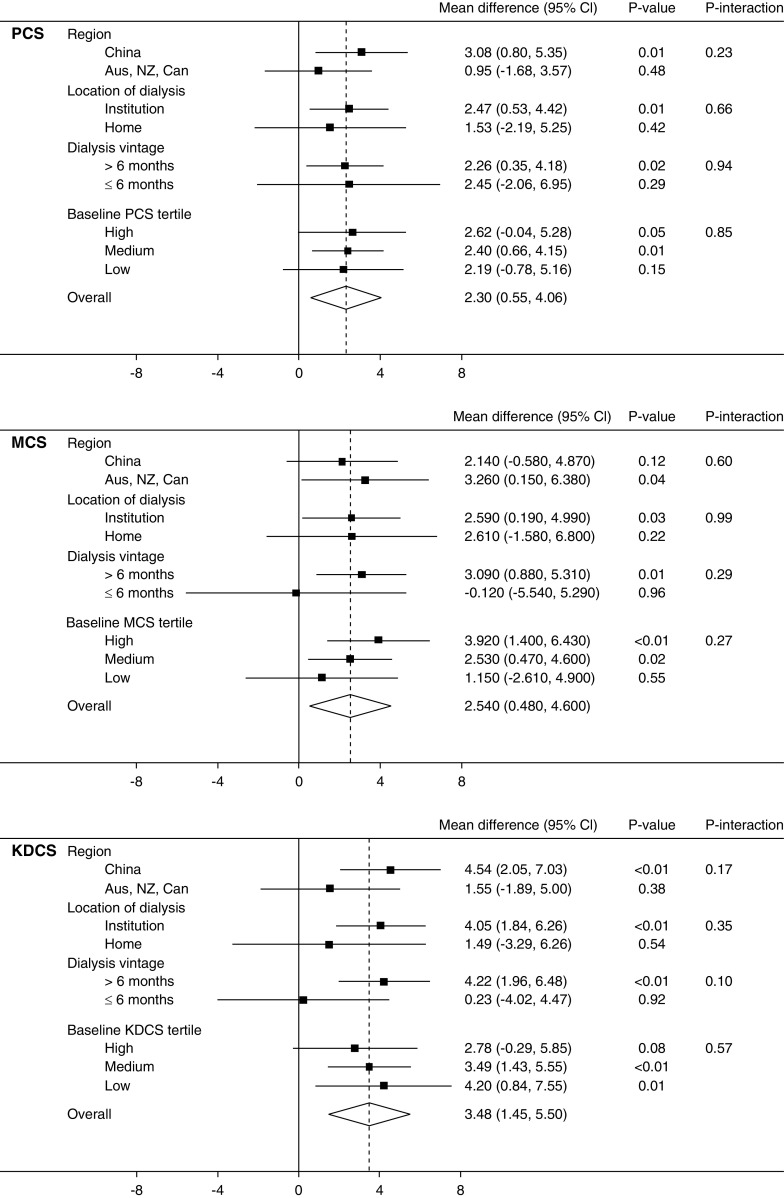
There was no statistically significant mean difference in EQ-5D-3L health utility (0.036; 95% CI, −0.02 to 0.09; P=0.2; Padjusted=0.2) (19). In contrast, health utility improved significantly with extended dialysis when measured by Short Form-6 Dimension (mean difference, 0.027; 95% CI, 0.00 to 0.05; P=0.03); however, this difference was not significant after adjustment for multiple comparisons (Padjusted=0.05) (Figure 1). Similar results were obtained using Chinese preference weightings and imputation of missing values (Supplemental Material). Mean QALYs over the 12 months of the study by EQ-5D-3L were 0.72 (95% CI, 0.68 to 0.77) in the standard hours group and 0.77 (95% CI, 0.72 to 0.81) in the extended hours group. Corresponding QALYs measured by Short Form-6 Dimension were 0.66 (95% CI, 0.64 to 0.69) and 0.68 (95% CI, 0.67 to 0.71). Neither difference was significant (P=0.19 and P=0.26). For the 100 participants randomized to extended hours dialysis, this represents a total gain in QALYs measured by EQ-5D-3L of 4.49 (95% CI, −2.17 to 11.16; P=0.19) and 2.19 (95% CI, −1.51 to 5.90; P=0.26) by Short Form-6 Dimension. Per participant, this equates to 0.045 (95% CI, −0.02 to 0.11) or 0.022 (95% CI −0.02 to 0.06) QALYs, equivalent to 16.4 (95% CI, −7.9 to 40.7) or 8.0 (95% CI, −5.5 to 21.5) days of life with full health (measured by EQ-5D-3L and Short Form-6 Dimension, respectively). The effect of extended hours dialysis on health utility as measured by each score was consistent among the prespecified subgroups and between participants in the highest, middle, and lowest tertiles of baseline score (Figure 2).
Figure 1.
Mean (95% CIs) health utility over time. (A) EuroQOL-5-Dimension-3 Level (EQ5D), mean difference 0.036; 95% confidence interval, 0.02 to 0.09; P=0.2; Padjusted=0.2. (B) Short Form-6 Dimension (SF-6D), mean difference 0.027; 95% confidence interval, 0.00 to 0.05; P=0.03; Padjusted=0.05. Mean scores at each time point are presented in the Supplemental Material.
Figure 2.
Effect of extended hours dialysis on health utility by subgroups. Mean difference between treatment groups from linear mixed effects model of health utility and subgroup, adjusted by time point and baseline score and with an interaction between subgroup and treatment group. Zero represents no effect of the intervention, positive values reflect benefit. Aus, Australia; Can, Canada; NZ, New Zealand; EQ5D, EuroQOL-5 Dimension-3 Level; SF6D, Short-Form 6-Dimension.
Effect of Extended Hours Dialysis on Generic and Disease-Specific Quality of Life
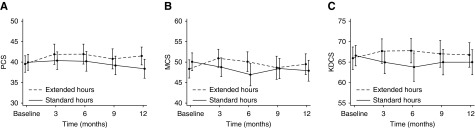
Both generic measures of quality of life significantly improved with extended hours dialysis with a mean difference in PCS of 2.3 (95% CI, 0.6 to 4.1; P=0.01; Padjusted=0.04) and MCS of 2.5 (95% CI, 0.5 to 4.6; P=0.02; Padjusted=0.05) (Figure 3). Similarly, disease-specific quality of life as measured by Kidney Disease Component Summary also improved significantly (3.5; 95% CI, 1.5 to 5.5; P=0.001; Padjusted=0.005) (Figure 3). Imputation of missing values did not change these conclusions. As with health utility, no significant interactions were found between treatment group and baseline tertile or subgroup (Figure 4), suggesting that the positive effect of extended hours dialysis on both generic and disease-specific quality of life was similar between subpopulations.
Figure 3.
Mean (95% CIs) generic and disease-specific quality of life over time. (A) Physical Component Summary (PCS). Mean difference, 2.3; 95% confidence interval (95% CI), 0.6 to 4.1; P=0.01; Padjusted=0.04. (B) Mental Component Summary (MCS). Mean difference, 2.5; 95% CI, 0.5 to 4.6; P=0.02; Padjusted=0.05. (C) Kidney Disease Component Summary (KDCS). Mean difference, 3.5, 95% CI, 1.5 to 5.5; P=0.001; Padjusted=0.005. Mean scores at each time point are presented in the Supplemental Material.
Figure 4.
Effect of extended hours dialysis on generic and disease-specific quality of life by subgroups. Mean difference between treatment groups from linear mixed effects model of health utility and subgroup, adjusted by time point and baseline score and with an interaction between subgroup and treatment group. Zero represents no effect of the intervention, positive values reflect benefit. Aus, Australia; Can, Canada; PCS, Physical Component Summary; MCS, Mental Component Summary; KDCS, Kidney Disease Component Summary; NZ, New Zealand; SF6D, Short-Form 6-Dimension.
Comparison of the Effect on Different Instruments as Assessed by Effect Size
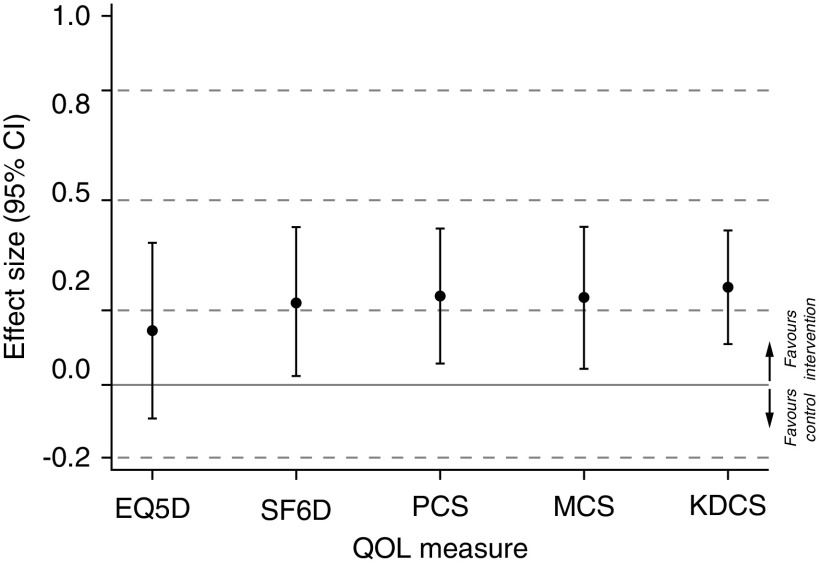
Each of the five measures demonstrated a similar effect size of approximately 0.2 SDs (Figure 5, Table 3). However, the effect of extended hours dialysis on EQ-5D-3L was not significant and the point estimate of 0.15 (95% CI, −0.09 to 0.39; P=0.2) did not reach the level of a minimally important difference. In contrast, the point estimates for effects on Short Form-6 Dimension (0.22; 95% CI, 0.02 to 0.43; P=0.03), PCS (0.24; 95% CI, 0.06 to 0.42; P=0.01), MCS (0.24; 95% CI, 0.04 to 0.43; P=0.05), and Kidney Disease Component Summary (0.26; 95% CI, 0.11 to 0.42; P=0.001) were all within the range regarded as defining an important effect of small size, although the 95% CIs included a clinically unimportant positive effect of ≤0.2.
Figure 5.
Standardized effect of extended hours dialysis on quality of life measures. The y-axis represents the effect size defined by the ratio of mean difference to the baseline SD of each quality-of-life score. Effect size categorized as <0.2 not important; 0.2 to <0.5 small, 0.5 to <0.8 moderate; and ≥0.8 large. KDCS, Kidney Disease Component Summary; QOL, quality of life; SF6D, Short-Form 6-Dimension; EQ5D, EuroQOL 5-Dimensions 3-Level; PCS, Physical Component Summary; MCS, Mental Component Summary.
Table 3.
Mean difference and standardized effect size over 12 months between extended and standard hemodialysis
| Quality-of-Life Score | N/200 | Mean Difference | P Value Unadjusted/Adjusteda | Baseline SD | Standardized Effect Size |
|---|---|---|---|---|---|
| EQ-5D-3L | 194 | 0.036 (−0.02 to 0.09) | 0.2/0.2 | 0.239 | 0.15 (−0.09 to 0.39) |
| Short Form-6 Dimension | 182 | 0.027 (0.00 to 0.05) | 0.05/0.05 | 0.122 | 0.22 (0.02 to 0.43) |
| PCS | 180 | 2.3 (0.6 to 4.1) | 0.01/0.04 | 9.6 | 0.24 (0.06 to 0.42) |
| MCS | 180 | 2.5 (0.5 to 4.6) | 0.02/0.05 | 10.8 | 0.24 (0.04 to 0.43) |
| Kidney Disease Component Summary | 192 | 3.5 (1.5 to 5.5) | 0.001/0.005 | 13.2 | 0.26 (0.11 to 0.42) |
EQ-5D-3L, EuroQOL-5 Dimension-3 Level; PCS, Physical Component Summary; MCS, Mental Component Summary. Mean scores at each time point are presented in the Supplemental Material.
Adjusted by Holm–Bonferroni method (see Supplemental Material).
Meta-Analysis of Physical and Mental Quality of Life with Intensive Hemodialysis
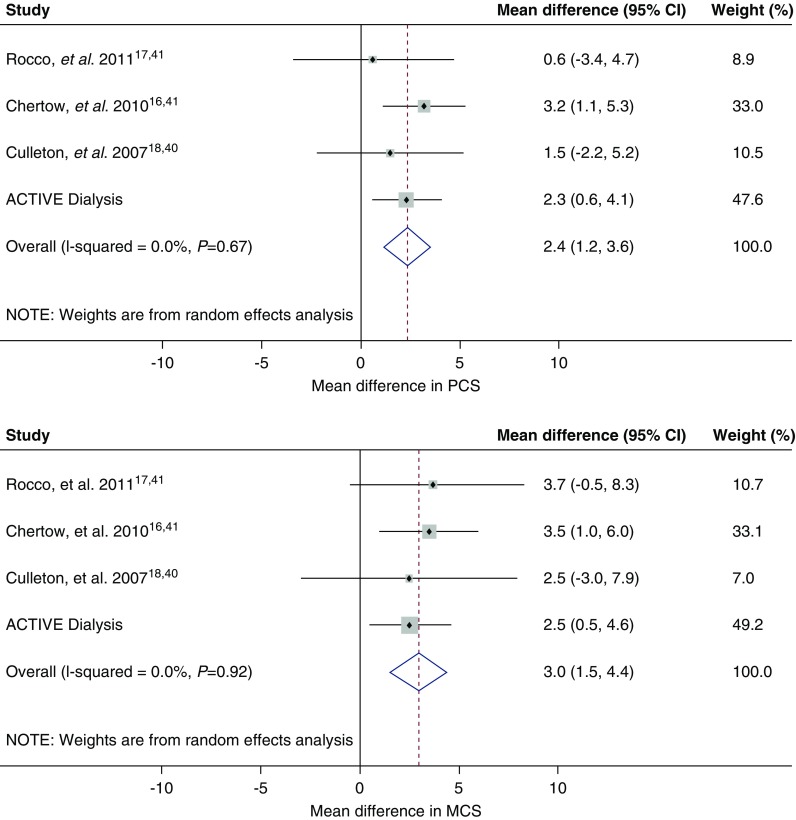
When our study was considered together with the three previous randomized trials of frequent hemodialysis (11–13,37,38), intensive hemodialysis was seen to be associated with a small but consistent improvement in physical and mental quality of life as measured by PCS (overall difference, 2.4; 95% CI, 1.2 to 3.6; I2=0.0%; Pheterogeneity=0.67) and by MCS (overall difference, 3.0; 95% CI, 1.5 to 4.4; I2=0.0%; Pheterogeneity=0.92) (Figure 6).
Figure 6.
Mean change in physical and mental quality of life with intensive hemodialysis on physical and mental quality of life: meta-analysis of four trials. Mean difference in short form-36 physical and mental quality of life. MCS, Mental Component Summary; PCS, Physical Component Summary.
Discussion
Extended hours dialysis, delivered predominantly during the day and over three sessions per week, is associated with improvements in generic and disease-specific quality of life and with health utility as measured by Short Form-6 Dimension. These effects were consistent across subgroups defined by patient characteristics and by baseline scores. This study is the first to report an improvement in health utility with extended hours dialysis, although only with one of the two instruments tested. The beneficial effect on Short Form-6 Dimension was robust to imputation of missing values, but not to adjustment for multiple testing.
Statistical significance aside, it is critical to understand the clinical importance of observed changes in patient-reported outcome measures. The gain in health utility after kidney transplantation has been variably estimated to be from 0.04 to as high as 0.33 (40), well above the gains observed in this study. There are multiple possible approaches to determine the minimal clinically important difference (MCID) for a given score in a given population, with no agreed “gold-standard” method (33). The approach chosen in this study (Cohen’s d) uses baseline variation among participants as the yardstick against which any change is measured. This simple method has been shown to accord with MCID derived by other methods (33,41), and in the present context is consistent with previous work estimating the MCID in the PCS, MCS, and Kidney Disease Component Summary to be between three to five points (42,43). Yet cautious interpretation is warranted, given the uncertainty inherent in estimation of MCID, and the wide confidence intervals surrounding the estimated effect size. Overall, the gain in quality of life with extended hours dialysis in the ACTIVE Dialysis study is small at best.
Three other randomized trials have assessed the effect of standard compared with increased dialysis time on quality of life (11–13,37,38). Meta-analysis of the SF-36 data from all four studies provides evidence that more intensive dialysis schedules are associated with improvement in both physical and mental quality of life, although the magnitude of benefit is likely to be small. The lack of between-study heterogeneity despite the differences between each study intervention suggests common mechanisms at play; however, bias due to within-study factors (e.g., cognitive dissonance affecting self-reported quality of life) or publication bias cannot be excluded. Culleton et al. (13) also reported significant improvements in two of the four subdomains of Kidney Disease Component Summary selected for testing a priori (effects of kidney disease, 8.6; 95% CI, 2.0 to 15.2 and burden of kidney disease, 9.4; 95% CI, 1.3 to 17.5), but no difference in EQ-5D-3L health utility (0.05; 95% CI, −0.07 to 0.17). Neither health utility nor disease-specific quality of life were reported by the Frequent Hemodialysis Network group (11,12,37,38).
Although EQ-5D-3L and Short Form-6 Dimension produced similar numeric results in the ACTIVE Dialysis study with substantially overlapping confidence intervals, the differences between them were sufficient to support differing interpretations of the efficacy of extended hours dialysis. Differences between EQ-5D-3L and Short Form-6 Dimension have been remarked upon in a variety of contexts (44–46). In general, mean values of Short Form-6 Dimension tend to be higher than EQ-5D-3L and the range and variation is lower (44) (although mean Short Form-6 Dimension utility was lower in our cohort). There is also evidence that Short Form-6 Dimension may be more responsive to small changes in health states at the upper end of the scale (45), which may be related to the ceiling effect reported for EQ-5D-3L. The magnitude of these differences appears to vary depending on the disease state of the studied population (44). After this trial was designed, a five-level version of EQ-5D was released (permitting the respondent to grade their response using five, rather than three, levels of severity or impairment) with a view to increasing the discriminatory capacity of the instrument (47). Conceptual differences also exist: although both instruments have analogous highly correlated domains for pain, mental health, and functional limitations, the Short Form-6 Dimension domains “social functioning” and “vitality” are not well represented in the EQ-5D-3L descriptive system, and the mobility domain from EQ-5D-3L does not have a clear counterpart in the Short Form-6 Dimension. Finally, the standard preference-weights assigned to each health state are derived via different methodologies (time trade-off for EQ-5D-3L versus standard gamble for Short Form-6 Dimension) despite both using cohorts of random sampled individuals from the general UK population (46). Patient-reported outcome measures are likely to grow in importance (48), at least in part driven by the prominence given to them in recent statements by the FDA (17) and with the recommendation that KDQOL-SF (36-item version), from which Short Form-6 Dimension can be derived, be used routinely as part of the Centers for Medicare and Medicaid Services review of dialysis unit performance (49). In this climate, the differences between various measures may have important consequences. A deeper understanding of the responsiveness of existing patient-reported outcome measures to interventions and of the clinical meaning of measured score changes is clearly needed.
The strengths of this study include the use of multiple validated measures of quality of life at repeated intervals over 12 months and delivered by blinded interviewers, limiting observation bias. The study also included participants from multiple sites in four countries and dialyzing both at home and at an institution, meaning these findings are potentially generalizable to a variety of settings. However, as the intervention was delivered predominantly by increasing session duration over three sessions per week, the results may be less relevant to patients achieving extended hours by performing more frequent dialysis sessions. The ACTIVE Dialysis study cohort was also younger and had a higher baseline PCS than the general United States hemodialysis population (50). This study has a number of other limitations. With regard to health utility, although the main treatment effects were not different when preference weights from China were used, it should be noted that health state preference valuations do vary by country, which limits the generalizability of these results. Furthermore, although the response rates were good throughout the study, it is possible that complete data would have altered the conclusions. Unavoidably, study participants could not be blinded, meaning their self-assessment of quality of life could have been affected by prior expectations. The sample size of 200 is relatively small, which limits the power of the subgroup analysis, meaning that we can conclude only that there is no evidence of subgroup differences. Thus it remains possible that certain patient subgroups do benefit disproportionately from more intensive dialysis and further study is required to clarify this issue. Furthermore, the present results do not alter the need to individualize dialysis therapy, as particular clinical characteristics, along with patient preference or circumstance may still favor longer or more frequent sessions. Additional research, including qualitative studies, could also consider the role of the dialysis unit as a social or support network for patients suffering a chronic disease as this may have contributed to some of the observed improvements in quality-of-life scores. Finally, it remains possible that greater differences in quality of life would accrue over time if the (still unproven) benefits of increased dialysis on cardiovascular health and mortality were to manifest. Larger and longer studies are required to answer this question.
In conclusion, this secondary analysis of the ACTIVE Dialysis randomized, clinical trial suggests that extended hours hemodialysis is associated with an improvement in a variety of important patient-reported outcome measures, including both generic and kidney-disease specific quality of life as well as, potentially, in health utility as measured by Short Form-6 Dimension, but not by EQ-5D-3L. There were no significant differences in response among key patient subgroups. Given the small magnitude of benefit and the secondary nature of the analysis, this study cannot provide conclusive evidence of a clinically meaningful improvement in quality of life with increased dialysis hours. However, our results suggest that the additional time spent on dialysis is unlikely to be associated with worse quality of life, an important consideration that should encourage further research to identify the true effect of extended hours dialysis on long-term mortality and morbidity.
Data Sharing Statement
The deidentified participant data and a data dictionary used in this analysis will be made available from the time of publication of this manuscript to those able to provide a scientifically valid research proposal and analysis plan, and who are willing to sign a Data Sharing agreement. Interested parties may contact Dr. Jardine at mjardine@georgeinstitute.org.au.
Disclosures
Dr. Cass reports receiving unrestricted grants from Astellas and Novartis for the Indigenous Patient Voices Symposium at the Australia and New Zealand Society of Nephrology Annual Scientific Meeting and outside the submitted work. Dr. Chan reports receiving consultant fees from Baxter and NxStage and a grant from the Medtronic Investigator Initiated Grant Program outside of the submitted work. Dr. Gray reports receiving a travel grant from Amgen Australia and speaker fees from Baxter Healthcare. Dr. Perkovic reports receiving honoraria for scientific presentations and for speaking at science symposia from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Mitsubishi Tanabe, Novo Nordisk, Pfizer, and Servier; fees from an advisory board member position as with Astellas, AstraZeneca, Bristol-Myers Squibb, Dimerix, Durect, Eli Lilly, Gilead, Merck, Metavant, Mundipharma, Novartis, Novo Nordisk, Pharmalink, Relypsa, and Tricida; and fees from a steering committee position with AbbVie, Boehringer Ingelheim, GSK, Janssen, Novartis, Novo Nordisk, and Tricida. Dr. Perkovic also reports receiving a personal Senior Research Fellowship and a program grant from the National Health and Medical Research Council; author agreement fees from UpToDate; and clinical trial support from AbbVie, Janssen, Pfizer, and Tricida outside of the submitted work. Dr. Smyth reports receiving supported conference travel from Roche. Dr. de Zoysa, Dr. Gallagher, Dr. Hong, Dr. Howard, Dr. Jardine, Dr. Lin, Dr. Rogers, Dr. van den Broek-Best, Dr. Xu, Dr. Zhang, and Dr. Zuo have nothing to disclose.
Funding
Dr. Gallagher is supported a peer-reviewed government grant from the National Health and Medical Research Council. Dr. Jardine is supported by a Medical Research Future Fund Career Development Fellowship. Dr. Perkovic is supported by advisory board fees from Baxter International. Dr. Smyth is supported by an Australian Government Research Training Program Scholarship through the University of Sydney. Dr. Xu is supported by a grant from the Fourth Hospital Affiliated to Hebei Medical University. The ACTIVE Dialysis study was funded by grants from the National Health and Medical Research Council of Australia (APP571045 and APP358395) and an unrestricted grant from Baxter International. The funders had no role in the study design, collection, analysis and interpretation of data, writing the report, and the decision to submit for publication.
Supplementary Material
Acknowledgments
Dr. Smyth performed the data analysis and cowrote the manuscript with Dr. van den Broek-Best. Dr. Hong and Dr. Howard contributed to study design and critically reviewed the analysis plan. Dr. Rogers developed and directed the data analysis. Dr. Zuo, Dr. Gray, Dr. de Zoysa, Dr. Chan, Dr. Lin, Dr. Zhang, and Dr. Xu led key study sites, collected data, and provided critical review of the manuscript. Dr. Cass, Dr. Gallagher, and Dr. Perkovic contributed to study design, funding acquisition, and study conduct and critically reviewed the manuscript. Dr. Jardine conceived of and led the study, supervised data analysis, and critically reviewed the manuscript. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work.
Footnotes
B.S. and O.V.d.B.-B. are co-primary authors.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “How Extended Hemodialysis Treatment Time Can Affect Patient Quality of Life,” on pages 1687–1689.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06800619/-/DCSupplemental.
Supplemental Table 1. Missing value summary.
Supplemental Table 2. Mean quality-of-life scores over the study duration.
Supplemental Figure 1. Participant flow.
References
- 1.United States Renal Data System: 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States, 2015, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Available at: http://www.usrds.org/adr.aspx. Accessed June 11, 2019
- 2.Kimmel PL, Patel SS: Quality of life in patients with chronic kidney disease: Focus on end-stage renal disease treated with hemodialysis. Semin Nephrol 26: 68–79, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S: Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 10: 71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacson E Jr., Xu J, Lin S-F, Dean SG, Lazarus JM, Hakim RM: A comparison of SF-36 and SF-12 composite scores and subsequent hospitalization and mortality risks in long-term dialysis patients. Clin J Am Soc Nephrol 5: 252–260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sexton DJ, Lowney AC, O’Seaghdha CM, Murphy M, O’Brien T, Casserly LF, McQuillan R, Plant WD, Eustace JA, Kinsella SM, Conlon PJ: Do patient-reported measures of symptoms and health status predict mortality in hemodialysis? An assessment of POS-S Renal and EQ-5D. Hemodial Int 20: 618–630, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Liem YS, Bosch JL, Hunink MG: Preference-based quality of life of patients on renal replacement therapy: A systematic review and meta-analysis. Value Health 11: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein FO, Schiller B, Daoui R, Gehr TW, Kraus MA, Lea J, Lee Y, Miller BW, Sinsakul M, Jaber BL: At-home short daily hemodialysis improves the long-term health-related quality of life. Kidney Int 82: 561–569, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Heidenheim AP, Muirhead N, Moist L, Lindsay RM: Patient quality of life on quotidian hemodialysis. Am J Kidney Dis 42[Suppl]: 36–41, 2003 [DOI] [PubMed] [Google Scholar]
- 10.McPhatter LL, Lockridge RS Jr., Albert J, Anderson H, Craft V, Jennings FM, Spencer M, Swafford A, Barger T, Coffey L: Nightly home hemodialysis: Improvement in nutrition and quality of life. Adv Ren Replace Ther 6: 358–365, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocco MV, Lockridge RS Jr., Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, Kliger A, Eggers P, Briggs J, Hostetter T, Narva A, Star R, Augustine B, Mohr P, Beck G, Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, Mackrell J, Wiggins K, Sherer S, Weiss B, Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M, Tran T, West J, Unruh M, Keene R, Schlarb J, Chan C, McGrath-Chong M, Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C, Eknoyan G, Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E, Rocco M, Miller B, Riley J, Schuessler R, Lockridge R, Pipkin M, Peterson C, Hoy C, Fensterer A, Steigerwald D, Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E, Chan C, McGrath-Chong M, Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D, Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E, Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Pierratos A, Chan W, Regozo K, Kwok S; Frequent Hemodialysis Network (FHN) Trial Group : The effects of frequent nocturnal home hemodialysis: The Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 80: 1080–1091, 2011. 21775973 [Google Scholar]
- 13.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Unruh M, Benz R, Greene T, Yan G, Beddhu S, DeVita M, Dwyer JT, Kimmel PL, Kusek JW, Martin A, Rehm-McGillicuddy J, Teehan BP, Meyer KB; HEMO Study Group : Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int 66: 355–366, 2004 [DOI] [PubMed] [Google Scholar]
- 15.National Information Center on Health Services Research and Health Care Technology (NICHSR) : Glossary of Frequently Encountered Terms in Health Economics: U.S. National Library of Medicine, 2018. Available at: https://www.nlm.nih.gov/nichsr/edu/healthecon/glossary.html. Accessed June 11, 2019
- 16.Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP: Reporting and interpretation of SF-36 outcomes in randomised trials: Systematic review. BMJ 338: a3006, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food Drug Administration : Plan for Issuance of Patient-Focused Drug Development Guidance under 21st Century Cures Act Title III, Rockville, MD, FDA, 2017 [Google Scholar]
- 18.Jardine MJ, Zuo LI, Gray NA, de Zoysa J, Chan CT, Gallagher MP, Howard K, Hertier S, Cass A, Perkovic V; ACTIVE Dialysis Steering Committee : Design and participant baseline characteristics of ‘A Clinical Trial of IntensiVE Dialysis’: The ACTIVE Dialysis Study. Nephrology (Carlton) 20: 257–265, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Jardine MJ, Zuo L, Gray NA, de Zoysa JR, Chan CT, Gallagher MP, Monaghan H, Grieve SM, Puranik R, Lin H, Eris JM, Zhang L, Xu J, Howard K, Lo S, Cass A, Perkovic V; ACTIVE Dialysis Steering Committee; Paul : A trial of extending hemodialysis hours and quality of life. J Am Soc Nephrol 28: 1898–1911, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EuroQol Group : EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 16: 199–208, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Szende A, Oppe M, Devlin N: EQ-5D Value Sets: Inventory, Comparative Review and User Guide, Dordrecht, The Netherlands, Springer, 2007 [Google Scholar]
- 22.Németh G: Health related quality of life outcome instruments. Eur Spine J 15[Suppl 1]: S44–S51, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Reenen M, Oppe M: EQ-5D-3L User Guide, Version 5.1, Dordrecht, The Netherlands, EuroQOL Research Foundation, 2015 [Google Scholar]
- 24.Rabin R, de Charro F: EQ-5D: A measure of health status from the EuroQol group. Ann Med 33: 337–343, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Kallich JD, Mapes DL, Coons SJ, Amin N, Carter WB, Caren K: Kidney Disease Quality of Life Short Form (KDQOL-SFTM), Version 1.3: A Manual for Use and Scoring, Santa Monica, CA, RAND, 1997 [Google Scholar]
- 26.Ware JE, Jr.: SF-36 health survey update. Spine (Phila Pa 1976) 25: 3130–3139, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Wight JP, Edwards L, Brazier J, Walters S, Payne JN, Brown CB: The SF36 as an outcome measure of services for end stage renal failure. Qual Health Care 7: 209–221, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT; NECOSAD-study group : Validation of the KDQOL-SF: A dialysis-targeted health measure. Qual Life Res 11: 437–447, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Mapes DL, Bragg-Gresham JL, Bommer J, Fukuhara S, McKevitt P, Wikström B, Lopes AA: Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44[Suppl 2]: 54–60, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Brazier JE, Roberts J: The estimation of a preference-based measure of health from the SF-12. Med Care 42: 851–859, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Gong Y, Wu J, Zhang S, Yin X, Dong X, Li W, Cao S, Mkandawire N, Lu Z: Chinese version of the EQ-5D preference weights: Applicability in a Chinese general population. PLoS One 11: e0164334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The ACTIVE Dialysis Steering Committee: ACTIVE Dialysis Statistical Analysis Plan, The George Institute, 2014. Available at: http://www.georgeinstitute.org.au/projects/active-dialysis-a-clinical-trial-of-intensive-dialysis. Accessed June 11, 2019
- 33.Crosby RD, Kolotkin RL, Williams GR: Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol 56: 395–407, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Liu GG, Wu H, Li M, Gao C, Luo N: Chinese time trade-off values for EQ-5D health states. Value Health 17: 597–604, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Lam CL, Brazier J, McGhee SM: Valuation of the SF-6D health states is feasible, acceptable, reliable, and valid in a Chinese population. Value Health 11: 295–303, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Holm S: A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979 [Google Scholar]
- 37.Manns BJ, Walsh MW, Culleton BF, Hemmelgarn B, Tonelli M, Schorr M, Klarenbach S; Alberta Kidney Disease Network : Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney Int 75: 542–549, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, Tarallo M, Finkelstein FO, Kimmel PL; FHN Trials Group : Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. Am J Kidney Dis 61: 748–758, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 40.Wyld M, Morton RL, Hayen A, Howard K, Webster AC: A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 9: e1001307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D: Determining clinically important differences in health status measures: A general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 15: 141–155, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339–349, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Leaf DE, Goldfarb DS: Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int 75: 15–24, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Brazier J, Roberts J, Tsuchiya A, Busschbach J: A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ 13: 873–884, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Longworth L, Bryan S: An empirical comparison of EQ-5D and SF-6D in liver transplant patients. Health Econ 12: 1061–1067, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Grieve R, Grishchenko M, Cairns J: SF-6D versus EQ-5D: Reasons for differences in utility scores and impact on reported cost-utility. Eur J Health Econ 10: 15–23, 2009 [DOI] [PubMed] [Google Scholar]
- 47.EuroQol Research Foundation : EQ-5D-5L - About, Dordrecht, The Netherlands, EuroQol Research Foundation, 2017. Available at: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Accessed June 11, 2019 [Google Scholar]
- 48.Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, Crowe S, Van Biesen W, Winkelmayer WC, O’Donoghue D, Tam-Tham H, Shen JI, Pinter J, Larkins N, Youssouf S, Mandayam S, Ju A, Craig JC; SONG-HD Investigators : Establishing core outcome domains in hemodialysis: Report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop. Am J Kidney Dis 69: 97–107, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peipert JD, Hays RD: Using patient-reported measures in dialysis clinics. Clin J Am Soc Nephrol 12: 1889–1891, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraus MA, Fluck RJ, Weinhandl ED, Kansal S, Copland M, Komenda P, Finkelstein FO: Intensive hemodialysis and health-related quality of life. Am J Kidney Dis 68[Suppl 1]: S33–S42, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.