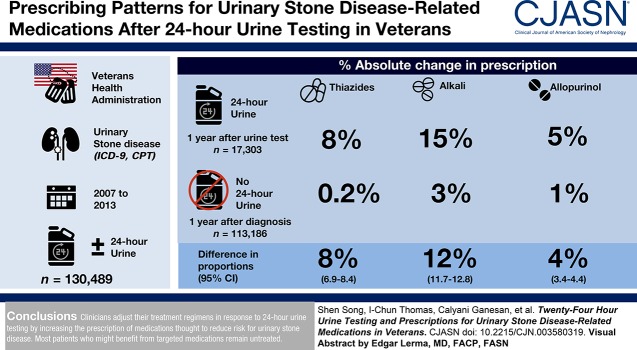
Visual Abstract
Keywords: kidney stones, 24-hour urine, urinary stone disease, Veterans Health Administration, medications, humans, citric acid, hypercalciuria, sodium chloride symporter inhibitors, allopurinol, veterans health, nephrologists, uric acid, calcium, urologists, secondary prevention, veterans, alkalies, urinary calculi, citrates, United States Department of Veterans Affairs, thiazides, cohort studies
Abstract
Background and objectives
Current guidelines recommend 24-hour urine testing in the evaluation and treatment of persons with high-risk urinary stone disease. However, how much clinicians use information from 24-hour urine testing to guide secondary prevention strategies is unknown. We sought to determine the degree to which clinicians initiate or continue stone disease–related medications in response to 24-hour urine testing.
Design, setting, participants, & measurements
We examined a national cohort of 130,489 patients with incident urinary stone disease in the Veterans Health Administration between 2007 and 2013 to determine whether prescription patterns for thiazide diuretics, alkali therapy, and allopurinol changed in response to 24-hour urine testing.
Results
Stone formers who completed 24-hour urine testing (n=17,303; 13%) were significantly more likely to be prescribed thiazide diuretics, alkali therapy, and allopurinol compared with those who did not complete a 24-hour urine test (n=113,186; 87%). Prescription of thiazide diuretics increased in patients with hypercalciuria (9% absolute increase if urine calcium 201–400 mg/d; 21% absolute increase if urine calcium >400 mg/d, P<0.001). Prescription of alkali therapy increased in patients with hypocitraturia (24% absolute increase if urine citrate 201–400 mg/d; 34% absolute increase if urine citrate ≤200 mg/d, P<0.001). Prescription of allopurinol increased in patients with hyperuricosuria (18% absolute increase if urine uric acid >800 mg/d, P<0.001). Patients who had visited both a urologist and a nephrologist within 6 months of 24-hour urine testing were more likely to have been prescribed stone-related medications than patients who visited one, the other, or neither.
Conclusions
Clinicians adjust their treatment regimens in response to 24-hour urine testing by increasing the prescription of medications thought to reduce risk for urinary stone disease. Most patients who might benefit from targeted medications remain untreated.
Introduction
Urinary stone disease or urolithiasis imposes a major health and economic burden in the United States. The prevalence of urolithiasis has been estimated to be 11% for men and 7% for women and continues to rise; prevalence is now twice as high as it was three decades ago (1,2). The burden of urolithiasis is further compounded by a high likelihood of recurrence, estimated to be 50% within 5–10 years of first stone occurrence and 75% in 20 years (3,4). Therefore, secondary prevention strategies that aim to reduce risk for urinary stone disease hold the promise of diminishing the health and economic consequences of this disease.
Twenty-four-hour urine testing is the current standard for diagnosing urinary abnormalities responsible for stone recurrence and for guiding specific dietary and pharmacologic interventions for secondary prevention of urolithiasis. The clinical value of 24-hour urine testing is highlighted by practice guidelines from the American Urological Association and the European Association of Urology, both of which recommend 24-hour urine testing for evaluating and treating people who are high-risk or interested first-time stone formers and recurrent stone formers (5,6). However, clinicians use 24-hour urine testing in only a minority of patients with urinary stone disease (7–9). Milose et al. (7) estimated the frequency of 24-hour urine testing to be between 7% and 8% in patients who are high-risk first-time stone formers. Dauw et al. (8) showed that only 16% of stone formers receive a repeat 24-hour urine test within 6 months of their initial test.
We sought to determine whether clinicians initiate or continue stone-related medications in response to 24-hour urine testing by examining a national cohort of patients cared for within the Veterans Health Administration (VHA). We used a national cohort of patients in the VHA because it provides researchers the ability to link diagnostic claims, laboratory test results, and pharmacy prescriptions. In addition, these patients have similar access to medical care across the country, thus reducing detection bias that may be present in more fragmented civilian health care systems. We hypothesized that clinicians appropriately adjust their practice patterns by increasing prescriptions of medications that may reduce risk for urolithiasis (thiazides, alkali, and allopurinol).
Materials and Methods
Data and Study Population
The Veterans Affairs Palo Alto Health Care System Research and Development Committee and Stanford University Institutional Review Board approved this study. We used inpatient and outpatient data from the VHA for the calendar years 2007 through 2013 to identify patients with urinary stone disease. We defined urinary stone disease in patients when they had one or more inpatient International Classification of Diseases, Ninth Revision (ICD-9) codes for kidney stones; two or more outpatient ICD-9 codes for kidney stones; or one or more Current Procedural Terminology codes for kidney stone procedures (Supplemental Table 1), as described previously (10). We limited our analytic cohort to patients who were free from a stone diagnosis or procedure in the 2 years before entry into the cohort to capture how clinicians respond to a new stone event. Each patient entered the cohort once, at the time of his or her first qualification as a stone former during the observation period. We used the national VHA Corporate Data Warehouse to abstract individual demographic information. We used Veterans Integrated Service Network codes to identify geographic regions (Supplemental Table 2). We used clinic stop codes to identify the type of stone specialty follow-up visit (nephrology or urology) as well as nutrition/dietitian clinic visits (Supplemental Table 3).
We identified the fraction of stone formers who completed a 24-hour urine collection from time of entry into the observation period through 2014. We used Logical Observation Identifiers Names and Codes to define a 24-hour urine collection as the presence of a 24-hour urine measurement for calcium, citrate, oxalate, or sulfate (Supplemental Table 4). We used VHA class codes to identify the following medication categories: thiazide or thiazide-type diuretics (hereon referred to as “thiazides”), alkali, and allopurinol (Supplemental Table 5). We defined the following classes of medications: (1) thiazides as prescription for hydrochlorothiazide, chlorthalidone, or indapamide; and (2) alkali therapy as prescription for potassium citrate, sodium bicarbonate, sodium citrate, or potassium bicarbonate. A medication was included for analysis if it was found to be an active and filled prescription in a patient’s health record during the specified time period.
Primary Outcomes
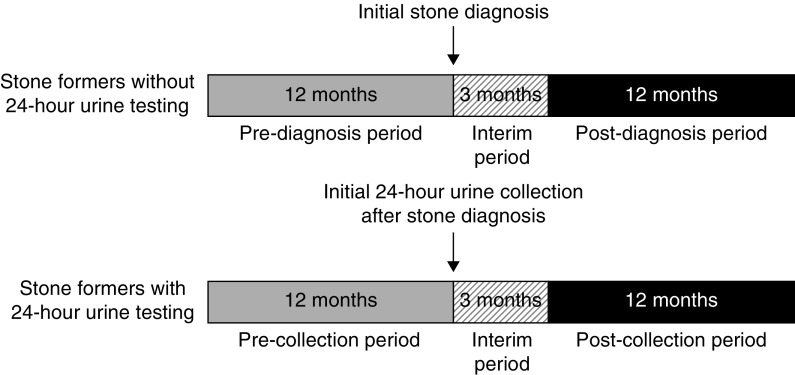
For Veterans with urinary stone disease who completed a 24-hour urine collection, we compared the difference in proportions of filled prescription of medications before and after the date of the first 24-hour urine collection. We also compared prescription of medications before and after the date of a 24-hour urine analyte that is specific to each medication class (Supplemental Table 6). For example, we analyzed the following: (1) whether prescription of thiazides changed after a 24-hour urine calcium measurement, (2) whether prescription of alkali changed after a 24-hour urine citrate measurement, and (3) whether prescription of allopurinol changed after a 24-hour urine uric acid measurement (Supplemental Table 6). We instituted a 3-month interim period after the first 24-hour urine collection to allow sufficient time for 24-hour urine results to become available and to avoid potential misclassification of prescribed medications that might have been automatically refilled before an ordering clinician could interpret 24-hour urine test results. We then compared filled prescription of medications 1 year before and 1 year after this 3-month interim period. For patients who did not complete a 24-hour urine collection, we compared filled prescription of medications 1 year before the date of initial stone diagnosis and 1 year after an analogous 3-month interim period (Figure 1).
Figure 1.
Comparison of observation periods between stone formers without and with 24-hour urine testing. For stone formers who did not complete 24-hour urine testing, we used the date of the initial stone diagnosis as a reference for comparison for the date of the initial 24-hour urine test in stone formers who completed 24-hour urine testing. We then compared filled prescriptions in the observation periods between stone formers with and without 24-hour urine testing.
Analyses of Specific Drugs and Biochemical Indications
The proportion of each urine analyte that qualified for a 24-hour urine collection was variable (Supplemental Table 7). We tested how clinicians might respond to 24-hour urine abnormalities by examining the proportion of patients with 24-hour urine abnormalities and who filled the following prescriptions below. For each prescription class, we also fit logistic regression models to test for the presence of effect modification by age, sex, or the presence of osteoporosis.
Thiazides.
We defined hypercalciuria as urine calcium excretion >200 mg/d (11). We compared the proportion of patients who filled prescriptions for thiazides in groups stratified by level of 24-hour calcium excretion: ≤200, 201–400, and >400 mg calcium per day. Because thiazides are commonly prescribed for hypertension, we conducted a separate sensitivity analysis in which we excluded Veterans with a concomitant diagnosis of hypertension.
Alkali Therapy.
We defined hypocitraturia as urine citrate excretion of <400 mg/d (11). We compared the proportion of patients who filled prescriptions for alkali therapy in groups stratified by level of 24-hour urine citrate excretion: ≤200, 201–400, and >400 mg citrate per day.
Allopurinol.
For the primary analysis, we included patients with gout. In a secondary analysis, we excluded stone formers with a concomitant diagnosis of gout because allopurinol is also commonly prescribed for gout prevention. We then stratified the remaining stone formers by level of 24-hour urine uric acid excretion. We compared the proportion of patients who filled prescriptions for allopurinol in patients with urine uric acid excretion of >800 or ≤800 mg/d (11,12).
Statistical Analysis
When comparing groups by biochemical features, we used the t test to compare continuous variables, and the chi-squared test to compare categoric variables. We compared the difference in proportions of the population specified for each medication class. Because we performed comparisons with three classes of medications (thiazides, alkali therapy, and allopurinol), we considered two-tailed P values <0.016 (0.05/3) as statistically significant. All analyses were performed within the VA VINCI platform using SAS and SAS Enterprise Guide 7.1 (Cary, NC).
Results
We identified a cohort of 130,489 patients with urinary stone disease from 2007 through 2013 and found that 17,303 patients (13%) completed at least one 24-hour urine collection (Table 1). Patients who completed 24-hour urine testing were less likely to be affected by hypertension or cardiovascular disease and equally likely to be affected by gout.
Table 1.
Characteristics of United States veterans with incident urinary stone disease from 2007 to 2013
| Characteristics | Without 24-h Urine Testing (N=113,186; 87%) | With 24-h Urine Testing (N=17,303; 13%) |
|---|---|---|
| Age (mean±SD, yr) | 61±14 | 58±12 |
| Sex (N, %) | ||
| Male | 107,383 (95%) | 16,042 (93%) |
| Female | 5803 (5%) | 1261 (7%) |
| Race (N, %) | ||
| White | 87,541 (77%) | 13,803 (80%) |
| Black | 12,540 (11%) | 1521 (9%) |
| Other or unknown | 13,105 (12%) | 1979 (11%) |
| Laboratory data | ||
| BMI (mean±SD, kg/m2) | 29.9±6.2 | 30.7±6.3 |
| eGFR (mean±SD, ml/min per 1.73m2) | 73±25 | 77±23 |
| Creatinine (mean±SD, mg/dl) | 1.3±2.7 | 1.7±1.4 |
| Potassium (mean±SD, mEq/L) | 4.2±0.5 | 4.2±1.1 |
| Sodium (mean±SD, mEq/L) | 139±4 | 139±6 |
| Bicarbonate (mean±SD, mEq/L) | 27±4 | 27±3 |
| Calcium (mean±SD, mg/dl) | 9.3±1.4 | 10.2±1.6 |
| Albumin (mean±SD, g/dl) | 4.1±15.1 | 4.1±1.2 |
| Comorbidities (N, %) | ||
| Hypertension | 76,811 (68%) | 10,944 (63%) |
| Gout | 6467 (6%) | 1005 (6%) |
| Osteoporosis | 2530 (2%) | 484 (3%) |
| Osteopenia | 2259 (2%) | 441 (3%) |
| Diabetes mellitus | 42,214 (37%) | 6690 (39%) |
| CKD | 3769 (3%) | 430 (2%) |
| Cardiovascular disease | 9608 (8%) | 947 (5%) |
| Baseline medications (N, %) | ||
| Thiazide or thiazide-like diuretics | 19,188 (17%) | 2815 (16%) |
| Alkali therapy | 1000 (1%) | 192 (1%) |
| Allopurinol | 5861 (5%) | 890 (5%) |
| Geographic region (N, %) | ||
| Midwest | 27,962 (25%) | 4795 (28%) |
| Northeast | 18,737 (17%) | 2244 (13%) |
| Southeast | 43,521 (38%) | 5314 (31%) |
| West | 22,966 (20%) | 4950 (28%) |
BMI, body mass index.
Thiazides
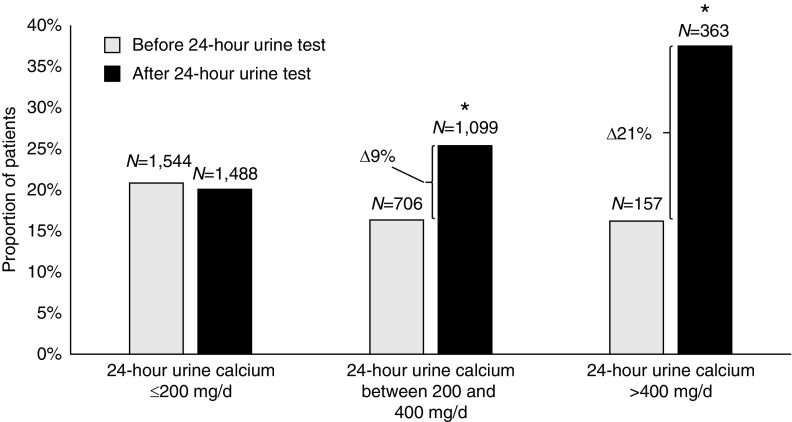
The proportion of patients prescribed thiazides was similar between the two groups of stone formers before 24-hour urine testing or stone diagnosis (16% versus 17%) (Table 1). For patients who completed 24-hour urine testing, prescriptions for thiazides increased significantly after a 24-hour urine test (8% absolute increase, P<0.001; Table 2). In contrast, among patients who did not complete 24-hour urine testing, prescriptions for thiazides did not change (0.2% absolute increase; Table 2). The absolute difference in proportions of patients who were prescribed thiazides in the two groups of stone formers were also the same if we considered whether a 24-hour urine calcium test was performed (Supplemental Table 6). Indeed, thiazides were significantly more likely to be prescribed after a 24-hour urine test in patients who excreted a higher level of urine calcium (Figure 2). In patients with 24-hour urine calcium of ≤200, 201–400, and >400 mg/d, the absolute change in the proportion of patients prescribed thiazides was −1%, 9%, and 21%, respectively (P=0.06, <0.001, and <0.001 for corresponding comparisons). In a sensitivity analysis excluding patients with hypertension, we found similar trends (Supplemental Figure 1). We also found no evidence of effect modification in the prescription of thiazides by age, sex, or history of osteoporosis.
Table 2.
Prescription of medications that affect urinary stone recurrence during the first year after incident stone diagnosis
| Medication | Without 24-h Urine Test (N=113,186; 87%) | With 24-h Urine Test (N=17,303; 13%) | Percentage Difference in Proportions (95% CI)a |
|---|---|---|---|
| Participants receiving medication during first year after diagnosis (N, %) | |||
| Thiazides | 19,372 (17%) | 4172 (24%) | 7 (6.3 to 7.7) |
| Alkali | 4882 (4%) | 2918 (17%) | 13 (12.0 to 13.1) |
| Allopurinol | 7526 (7%) | 1817 (11%) | 4 (3.4 to 4.3) |
| Difference from baseline in proportion of participants receiving medication (% absolute change) | |||
| Thiazides | 0.2 | 8 | 8 (6.9 to 8.4) |
| Alkali | 3 | 15 | 12 (11.7 to 12.8) |
| Allopurinol | 1 | 5 | 4 (3.4 to 4.4) |
Percentage difference in proportions is the proportion of patients with active prescription of medication in 1 yr after stone diagnosis or 24-h urine test minus the proportion of patients with active prescription of medication in 1 yr before stone diagnosis or 24-h urine test.
Figure 2.
Thiazide prescriptions increase in the proportion of patients who excrete a higher level of urine calcium. Thiazide prescriptions in stone formers with 24-hour urine test, before and after 24-hour urine testing, stratified by level of 24-hour urine calcium excretion. *Difference in proportions before and after 24-hour urine test were significant at P<0.001. Δ, change.
Alkali Therapy
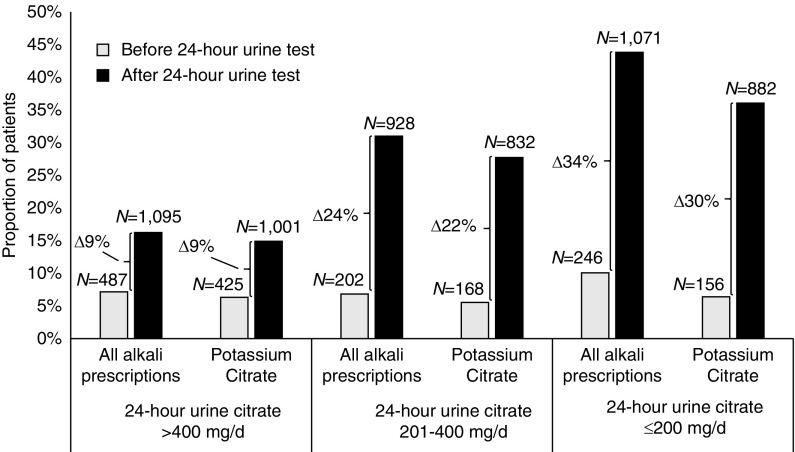
Baseline use of alkali therapy was similar between the two groups of stone formers before 24-hour urine testing or stone diagnosis (1% versus 1%, Table 1). For patients who completed 24-hour urine testing, prescriptions for alkali therapy increased significantly after a 24-hour urine test (15% absolute increase, P<0.001; Table 2). For patients who did not complete 24-hour urine testing, prescriptions for alkali therapy also increased after stone diagnosis, but the increase was much lower in magnitude (3% absolute increase, P<0.001; Table 2). The absolute difference in proportions of patients who were prescribed alkali therapy were also the same if we considered whether a 24-hour urine citrate test was performed (Supplemental Table 6). Alkali therapy was significantly more likely to be prescribed after a 24-hour urine test in patients who excreted a lower level of urine citrate (Figure 3). In patients with 24-hour urine citrate of >400, 201–400, and ≤200 mg/d, the absolute change in the proportion of patients prescribed alkali was 9%, 24%, and 34%, respectively (P<0.001 for all comparisons). Potassium citrate, a common form of alkali therapy, was also significantly more likely to be prescribed after a 24-hour urine test in patients who excreted a lower level of urine citrate (Figure 3). In patients with 24-hour urine citrate of >400, 201–400, and ≤200 mg/d, the absolute change in the proportion of patients prescribed potassium citrate was 9%, 22%, and 30%, respectively (P<0.001 for all comparisons). We found no evidence of effect modification in the prescription of alkali therapy by age, sex, or history of osteoporosis.
Figure 3.
Alkali prescriptions increase in the proportion of patients who excrete a lower level of urine citrate. Alkali prescriptions in stone formers with 24-hour urine test, before and after 24-hour urine testing, stratified by level of 24-hour urine citrate excretion. Differences in proportions before and after 24-hour urine test were significant for all levels of 24-hour urine citrate excretion at P<0.001. Δ, change.
Allopurinol
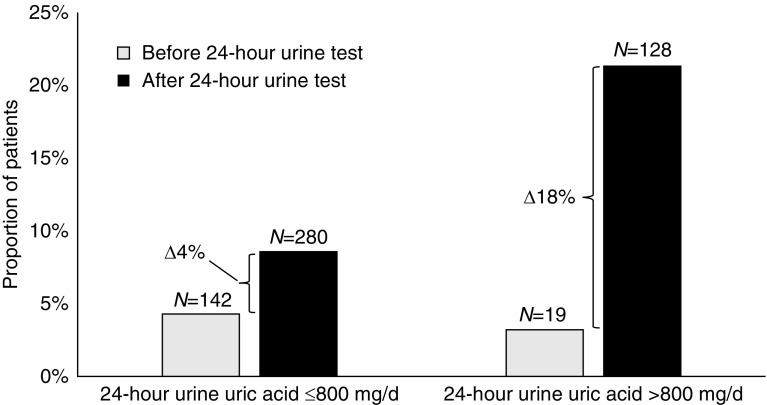
Baseline use of allopurinol was similar in patients before 24-hour urine testing or stone diagnosis (5% versus 5%; Table 1). The proportion of patients prescribed allopurinol increased significantly among patients who completed a 24-hour urine test compared with those who did not (5% increase versus 1% increase; P<0.001, Table 2), even though the prevalence of a gout was similar in both groups (6% versus 6%, P=0.6; Table 1). The absolute difference in proportions of patients who were prescribed allopurinol was also similar if we considered whether a 24-hour urine uric acid test was performed (Supplemental Table 6). Prescriptions for allopurinol increased significantly after a 24-hour urine test in patients with hyperuricosuria, even after excluding patients with gout (4% increase if urine uric acid ≤800 mg/d, 18% increase if urine uric acid >800 mg/d, P<0.001 for both comparisons; Figure 4). We found no evidence of effect modification in the prescription of allopurinol by age, sex, or history of osteoporosis.
Figure 4.
Allopurinol prescriptions increase in the proportion of patients who excrete a higher level of urine uric acid. Allopurinol prescriptions in stone formers with 24-hour urine test and without gout, before and after 24-hour urine testing, stratified by level of 24-hour urine uric acid excretion. Differences in proportions before and after 24-hour urine test were significant for both levels of 24-hour urine uric acid excretion at P<0.001. Δ, change.
Regional Variation in Response to 24-hour Urine Data
The proportion of stone formers with documented metabolic abnormalities by 24-hour urine testing and who were prescribed stone-related medications was generally similar in direction and magnitude across the Northeast, Southeast, Midwest, and West regions (Table 3). Absolute increases in thiazide prescriptions ranged between 7% and 15% for patients with urine calcium >200 mg/d; absolute increases in alkali prescriptions ranged between 26% and 32% for patients with urine citrate ≤400 mg/d; and absolute increases in allopurinol prescriptions ranged between 3% and 6% for patients (excluding gout) with urine uric acid >800 mg/d. Although the differences by region were statistically significant, in part related to our large sample size, we did not consider them to be clinically meaningful.
Table 3.
Prescription of medications that affect urinary stone recurrence during the first yr after incident stone diagnosis, among patients who completed at least one 24-h urine test, by geographic region
| Geographic Region | Thiazide Prescriptions | Alkali Prescriptions | Allopurinol Prescriptionsa | |||
|---|---|---|---|---|---|---|
| 24-h Urine Calcium >200 mg/d (n=5292) | 24-h Urine Citrate ≤400 mg/d (n=5433) | 24-h Urine Uric Acid >800 mg/d (n=601) | ||||
| Participants Receiving Medication during First Year after Diagnosis (N, %) | Difference from Baseline in Proportion of Participants Receiving Medication (%) | Participants Receiving Medication during First Year after Diagnosis (N, %) | Difference from Baseline in Proportion of Participants Receiving Medication (%) | Participants Receiving Medication during First Year after Diagnosis (N, %) | Difference from Baseline in Proportion of Participants Receiving Medication (%) | |
| Midwest | 424 (30%) | 15 | 559 (37%) | 29 | 97 (11%) | 6 |
| Northeast | 149 (25%) | 7 | 252 (35%) | 27 | 26 (6%) | 4b |
| Southeast | 353 (27%) | 10 | 545 (36%) | 26 | 78 (8%) | 4 |
| West | 536 (27%) | 11 | 643 (39%) | 32 | 79 (8%) | 3 |
All P values for changes in prescriptions were significant at <0.001 except where marked. All P values for differences in changes in prescriptions between regions were significant at <0.001.
Excluding patients with gout.
P value 0.009.
Urinary Stone Disease Specialty Care
In our cohort, 57% of stone formers visited a urologist, 11% visited a nephrologist, 11% visited both a nephrologist and urologist, and 21% visited neither specialist in the 6 months after a 24-hour urine collection (Table 4). Use of stone-related medications differed significantly depending on whom patients visited after a 24-hour urine collection. In patients with hypercalciuria, prescriptions for thiazides increased by 11% if they visited a urologist, 17% if they visited a nephrologist, 24% if they visited both a urologist and nephrologist, and only 4% if they saw neither a urologist nor a nephrologist (Table 4). We observed similar trends for how alkali or allopurinol prescriptions differed depending on whom stone formers visited after a 24-hour urine collection for patients with hypocitraturia or hyperuricosuria, respectively (Table 4). Finally, we examined whether stone formers who completed a 24-hour urine test were more likely to be referred for dietary counseling. We found that 19% of patients who completed 24-hour urine testing visited a nutritionist or dietitian compared with 12% of patients who did not.
Table 4.
Prescription of medications that affect urinary stone recurrence during the first year after incident stone diagnosis, among patients who completed at least one 24-h urine test, by subspecialty clinic visits within 6 mo of 24-h urine test
| Clinic Visit Type | Total Patients with 24-h Urine Tests (n=17,303) | Thiazide Prescriptions | Alkali Prescriptions | Allopurinol Prescriptionsa | |||
|---|---|---|---|---|---|---|---|
| 24-h Urine Calcium >200 mg/d (n=5292) | 24-h Urine Citrate ≤400 mg/d (n=5433) | 24-h Urine Uric Acid >800 mg/d (n=601) | |||||
| Participants with Clinic Visit within 6 mo of 24-h Urine Test (N, %) | Participants Receiving Medication during First Year after Diagnosis (N, %) | Difference from Baseline in Proportion of Participants Receiving Medication (%) | Participants Receiving Medication during First Year after Diagnosis (N, %) | Difference from Baseline in Proportion of Participants Receiving Medication (%) | Participants Receiving Medication during First Year after Diagnosis (N, %) | Difference from Baseline in Proportion of Participants Receiving Medication (%) | |
| Urology | 9927 (57%) | 834 (26%) | 11 | 1044 (34%) | 28 | 133 (7%) | 4 |
| Nephrology | 1887 (11%) | 171 (37%) | 17 | 366 (48%) | 35 | 54 (15%) | 5b |
| Both | 1870 (11%) | 214 (42%) | 24 | 435 (54%) | 40 | 53 (12%) | 6 |
| Neither | 3619 (21%) | 243 (21%) | 4 | 154 (19%) | 13 | 40 (8%) | 3 |
All P values were significant at <0.001 except where marked.
Excluding patients with gout.
P value 0.16.
Discussion
In this study we used a large national cohort of Veterans to specifically test whether clinicians initiate or continue medications for prevention of urinary stone disease in response to 24-hour urine testing. We found that clinicians are more likely to prescribe thiazides, alkali therapy, or allopurinol for stone formers who complete 24-hour urine testing. Moreover, clinicians are more likely to prescribe these medications if a 24-hour urine test detects an elevated metabolic risk parameter for urinary stone disease. These findings suggest that clinicians do respond to results obtained from 24-hour urine testing and adjust their prescription patterns for the prevention of urinary stone disease.
However, we also show that clinicians could be more cognizant of initiating or continuing medications that decrease urolithiasis risk in patients with abnormal 24-hour urine tests. For example, only 38% of patients with severe hypercalciuria (urine calcium excretion >400 mg/d) were prescribed thiazides—drugs that are well known to decrease urinary calcium excretion. Similarly, only 44% of patients with severe hypocitraturia (urine citrate excretion ≤200 mg/d) were prescribed alkali therapy. Reasons for the lower-than-expected use of these medications are unclear. Patient factors such as unwillingness to initiate new medications, inability to tolerate side effects of medications (e.g., hypotension and/or hyperuricemia with thiazides or gastrointestinal discomfort with potassium citrate), or contraindications (e.g., hyponatremia with thiazides or hyperkalemia with potassium citrate) may play a role. Some patients may have opted for dietary and lifestyle modifications only. For example, some providers may have recommended a reduction in dietary sodium rather than a prescription with a thiazide as a strategy to reduce urine calcium excretion. In support of this possibility, we found that patients who completed 24-hour urine testing were more likely to visit a nutritionist or dietitian compared with those who did not. Although we are unable to determine the exact reason(s) for these visits, we can assume that at least a proportion of these were related to dietary counseling for stone prevention.
We considered possible geographic or provider factors underlying the lower-than-expected use of medications which are believed to decrease risk for urolithiasis. However, we found no clinically meaningful regional differences that could explain the limited use of these medications (in other words, there appears to be underutilization of potentially effective medications for stone prevention across the VHA system). We also found that prescriptions of stone-related medications differed significantly depending on whether patients visited a specialist after a 24-hour urine collection. Approximately one in five patients who completed 24-hour urine testing did not visit a urologist or nephrologist within 6 months of a 24-hour urine test; these stone formers had the lowest utilization of stone-related medications. These findings could reflect the relative lack of patient follow-up visits, denying stone specialists the opportunity to respond. Alternatively, patients who completed 24-hour urine testing could have returned to see primary care physicians or nonphysician providers who might be less inclined to respond to 24-hour urine testing. In contrast, patients who visited both a urologist and nephrologist within 6 months of 24-hour urine collection were the group most likely to be prescribed stone-related medications, suggesting that multidisciplinary care with urologists and nephrologists for high-risk stone formers could represent an approach toward improving medical (and ultimately surgical) management of urinary stone disease.
This study has several strengths. The VHA is well suited for evaluating the practice of 24-hour urine testing in prevention of urolithiasis. The VHA is an integrated health care system, which enabled us to link prescription patterns across this nationwide stone cohort with a broad and diverse array of patients. Veterans receiving care in the VHA are older, more likely to be men, and have higher rates of comorbid illnesses compared with the general patient population, all attributes that place them at higher risk for urinary stone disease (1). The VHA also provides full access to laboratory results, allowing us to compare use of stone prevention medications stratified by 24-hour urine results—a feature not possible in other large data sets devoid of laboratory and prescription data, where claims are used exclusively. Finally, clinicians in the VHA do not require prior authorization from insurance companies to order 24-hour urine collections, which could reduce detection bias that may be present in civilian health care systems.
This study has several limitations. First, because the majority of the VHA study population consists of men, we may not be able to fully generalize these results to populations with higher proportions of women. Second, we defined a 24-hour urine collection for the purpose of determining risk for urinary stone disease to be any one of four urinary parameters (calcium, oxalate, citrate, or sulfate). Whereas the objective of this approach was to maximize specificity for evaluation and/or prevention of urinary stone disease, it is possible that some of these tests, particularly 24-hour urine calcium, were ordered for other indications. It is also possible that we did not capture data from all VHA facilities that send 24-hour urine samples to an outside laboratory for processing. To address this concern, we queried 24-hour urine data from our local VHA facility, which sends out 24-hour urine samples to an outside laboratory. We confirmed that 24-hour urine calcium, oxalate, citrate, and sulfate were recorded in the VHA, but we acknowledge that we do not know if this would be the case for all VHA facilities. Third, the use of ICD-9 codes to capture patients with urinary stone disease may overestimate prevalence of symptomatic stone disease because some patients may be asymptomatic stone formers. Fourth, we did not capture medical care for patients stone formers who received care outside the VHA. Fifth, we were unable to abstract information on stone composition. Finally, we were not able to determine from VHA data the specific reasons why clinicians prescribed or discontinued stone-related medications. Although we suggest that clinicians respond to 24-hour urine testing by initiating or continuing medications that reduce stone risk, we cannot exclude the possibility that clinicians happen to order 24-hour urine testing in Veterans with more severe stone disease and who happen to be on medical therapy. We also cannot exclude the possibility that a clinician may have considered a previous 24-hour urine test, outside our observation period, in his or her decision to prescribe a stone-related medication. Alternatively, we were limited by the difficulty in surmising from aggregate data what were the individual reasons that led to discontinuation, or lack of initiation, of medications for stone prevention.
In conclusion, our study suggests that clinicians use information from 24-hour urine testing and adjust their practice patterns by increasing prescriptions of medications believed to reduce risk in stone formers. In spite of our observations that relevant clinical data changes provider practice patterns in patients with urinary stone disease, the fact remains that the majority of stone formers with urine abnormalities predisposing them to a high risk of stone recurrence are not prescribed stone-related medications that could reduce that risk. Acquisition of additional observational or interventional evidence evaluating the use of stone-related medications to prevent stone recurrence, implementation of multidisciplinary care with urologists and nephrologists for patients who are high-risk stone formers, and determination of the optimal use of urine testing for diagnosis of metabolic abnormalities could improve the care of Veterans and non-Veterans with urinary stone disease.
Disclosures
Dr. Chertow reports personal fees from a position on a trial steering committee from Akebia, Amgen, Ardelyx, AstraZeneca, Gilead, and Sanifit; from a position on a data safety monitoring board at Angion, Bayer, and ReCor; from a position on an advisory board at DiaMedica, Reata, and Vertex; and from a position on the Board of Directors at Satellite Healthcare. Dr. Chertow also reports stock options from Ardelyx, Cricket, Durect, DxNow, and Outset. Dr. Conti, Dr. Elliott, Dr. Ganesan, Dr. Leppert, Dr. Liao, Dr. Pao, Dr. Sohlberg, Dr. Song, and Mrs. Thomas have nothing to disclose.
Funding
Dr. Chertow was supported by the National Institutes of Health Midcareer Investigator Award in Patient-Oriented Research (K24 DK085446). Dr. Song was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (5 F32 DK118801).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Gaps in Care among Veterans with Urinary Stone Disease,” on pages 1690–1691.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03580319/-/DCSupplemental.
Supplemental Table 1. All diagnostic codes.
Supplemental Table 2. Geographic region codes.
Supplemental Table 3. Clinic visit codes.
Supplemental Table 4. Laboratory codes.
Supplemental Table 5. Pharmacy codes.
Supplemental Table 6. Change in prescriptions for stone formers without or with a specific 24-hour urine test.
Supplemental Table 7. Number and proportion of each 24-hour urine analyte measured in stone formers with 24-hour urine test.
Supplemental Figure 1. Thiazide diuretic prescriptions in stone formers without hypertension, before and after 24-hour urine testing, stratified by 24-hour urine calcium.
References
- 1.Scales CD Jr., Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Sutherland JW, Parks JH, Coe FL: Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab 11: 267–269, 1985 [PubMed] [Google Scholar]
- 4.Trinchieri A, Ostini F, Nespoli R, Rovera F, Montanari E, Zanetti G: A prospective study of recurrence rate and risk factors for recurrence after a first renal stone. J Urol 162: 27–30, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR; American Urological Assocation : Medical management of kidney stones: AUA guideline. J Urol 192: 316–324, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Bultitude M, Smith D, Thomas K: Contemporary management of stone disease: The New EAU Urolithiasis Guidelines for 2015. Eur Urol 69: 483–484, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Milose JC, Kaufman SR, Hollenbeck BK, Wolf JS Jr., Hollingsworth JM: Prevalence of 24-hour urine collection in high risk stone formers. J Urol 191: 376–380, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Dauw CA, Alruwaily AF, Bierlein MJ, Asplin JR, Ghani KR, Wolf JS Jr., Hollingsworth JM: Provider variation in the quality of metabolic stone management. J Urol 193: 885–890, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Ellison JS, Kaufman SR, Kraft KH, Wolf JS Jr., Hollenbeck BK, Hollingsworth JM: Underuse of 24-hour urine collection among children with incident urinary stones: A quality-of-care concern? Urology 84: 457–461, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Litwin MS, Saigal CS, editors: Urologic Diseases in America, Washington, DC, US Government Printing Office, US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 11.Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Ettinger B, Tang A, Citron JT, Livermore B, Williams T: Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 315: 1386–1389, 1986 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.