Abstract
Purpose: To determine if the viability of random pattern dorsal skin flaps in rats could be improved with local injection of exosomes derived from bone marrow mesenchymal stem cells (BMSCs). Methods: 30 adult male SD rats (weight 200-250 g) were randomly divided into experimental and control groups. Exosomes were isolated from the 4th generation of BMSCs. Experimental group were treated with local injection of exosomes suspension while control group were treated with saline solution in the same way. McFarlane-type flaps (9.0 × 3.0 cm) were then operated in the dorsum of all. On the seventh postoperative day, the percentage of viability area was measured and the blood flow was detected by laser doppler. Then rats were sacrificed and flaps were cut off for further check. Results: Compared with controls, the exosomes increased the area of survival (P<0.05). Hematoxylin and eosin (HE) staining of zone II showed higher microvascular density (P<0.05) and better angiogenesis (P<0.05) in the exosomes group. Similarly, the blood flow of exosomes was better than the control group according to laser doppler imaging (P<0.05). And the result of immunohistochemistry and western blot showed that the exosomes group had significantly higher VEGF and CD34 expression compared to the controls (P<0.05). Conclusions: Local injection of BMSCs exosomes was effective to attenuate necrosis of the McFarlane-type flaps in rats.
Keywords: Random skin flaps, SD rat, mesenchymal stem cells, exosomes
Introduction
In the clinic, random skin flaps have become one of the common techniques for repairing tissue defects. However, the necrosis of distal part of the random skin flaps remains challenging and limits its clinical application. Flap necrosis is caused primarily by inadequate blood perfusion or ischemia-reperfusion (I/R) [1]. How to boost the success rate of random skin flap transfer is one of the focus issues discussed in the academic fields.
Exosomes contain a large number of protein molecules, mRNA, miRNA rRNA, lncRNA and DNA [2]. As mediators of intercellular communication, exosomes can play an important role in intercellular transmission under physiological and pathological conditions, including antigen presentation, transmission of infectious agents, and tumor immune regulation, molecular markers, drug loading and other aspects of research and application [3]. Exosome’s main function is to carry the intercellular signal exchange [4] Though many kinds of cells could secrete exosomes, mesenchymal stem cell (MSC) was the most prolific exosomes’ producer of all and has the capacity to mass produce exosomes [5]. As Ruenn’s report, exosomes secreted by MSC were identified as cardioprotective component in myocardial ischemia/reperfusion injury [6]. Similarly, MSC-derived exosomes were also observed enhancing wound healing [7], promote healing of diabetic skin defects [8], hepatoprotective effect in liver injury [9], improve functional recovery after traumatic brain injury [10] and so on. These results highlight the important value of exosome as mediator of tissue repair.
Therefore, we tried to determine if the viability of random pattern dorsal skin flaps in rats could be improved with local injection of exosomes derived from BMSCs.
Methods
Materials
SD rats (approved by the Animal Experimental Center of Wenzhou Medical University. No. SCXK [ZJ] 2005-0019). DMEM(1X)+GlutaMAXTM-1 Dulbecco’s Modified Eagle Medium (1859228, GIBICO); FBS (04-001-1A, BioInd); Exosome extraction kit (E1340, Weihui Biology); Trypsin/EDTA (T1300, Solarbio); Penicillin-Streptomycin Solution (100X, P1400, Solarbio); BCA Protein Assay Kit (CW0014S, CWBIO); Rabbit Anti-CD63 antibody (25682, PROTEINTECH); Mouse Monoclonal Anti-GAPDH (TA-08, ZSGB-BIO); Peroxidase-Conjugated Goat anti-Mouse IgG (H+L) (ZB-2305, ZSGB-BIO); Peroxidase-Conjugated Goat anti-Rabbit IgG (H+L) (ZB-3201, ZSGB-BIO); Anti-CD34 antibody (ab185732, abcam); VEGF Antibody (19003-1-AP, Proteintech); ECL Western Blotting Substrate (PE0010, Solarbio); PVDF membranes (Roche Applied Science, Indianapolis); Malondialdehyde (MDA) assay kit (A003-1-2, NJJC); Total Superoxide Dismutase (T-SOD) assay kit (A001-1-2, NJJC).
Primary culture and identification of BMSCs
BMSCs were isolated and cultured primarily by whole bone marrow culture method. All cells were cultured in DMEM containing 20% FBS and Penicillin-Streptomycin (100 U/ml) at a humidified atmosphere of 37°C and 5% CO2. A 3-week-old male SD rat weighted 48 g were suppliers of BMSCs. Bone marrow was obtained from bilateral femurs of the rat and added into the cultures. After 6 days of the initial culture, a few of primary culture cells began to adhere to the bottom of culture bottle. The culture medium was first replaced in the 9th day and the first subculture was carried out in the 14th day. Cells were passaged by trypsin at a ratio of 1:2 when expanded to 80% confluence.
Extraction of exosomes
The 4th generation of cells were used for the experiments. When expended to 90%, cell-culture medium were changed to DMEM and Penicillin-Streptomycin (100 U/ml) without FBS. After 2 days’ starvation treatment by serum-free medium, the medium was centrifuged for 10 min at 2000 g and 4°C, then the supernatant liquid was used to purify exosomes with the exosomes extraction kit.
After separation and purification by exosomes extraction kit, the protein content of exosomes suspension was determined by the BCA protein assay kit. Exosomes suspension was diluted to 50 μg/ml by phosphate buffer saline.
Identification of exosomes
After separation and purification, 20 μl exosomes suspension was dripped in a sample copper net. Keeping for 1 minute before dried by filter paper. Then the copper net was negative stained by 3% hosphotungstic acid (PH=6.8) at 25°C for 5 minutes. After dried by incandescent lights, the sample copper net was observed by transmission electron microscope. And the characteristic proteins of exosomes including CD9, CD63, TSG101 were analyzed by western blot analysis.
Surgical procedure
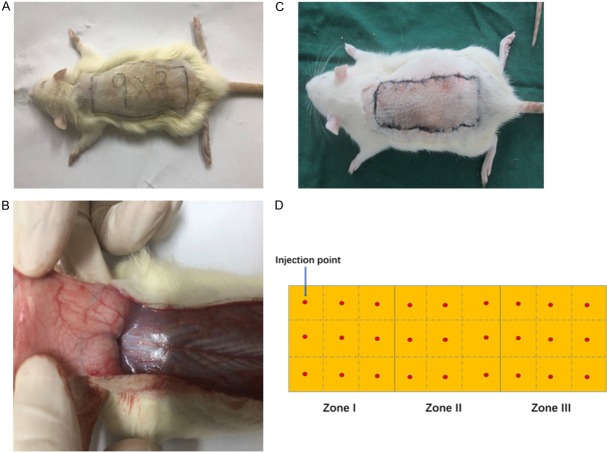
30 SD rats (216 ± 13 g), in accordance with the random number table, were randomly divided into experimental group and control group, 15 rats each. All rats were anaesthetized with intraperitoneal injections of chloral hydrate (300 mg/kg body weight). The dorsal hairs was removed with a electronic shaver and the skin was disinfected with iodophor and alcohol. According to McFarlane-type flap [11], Size of 9.0 × 3.0 cm rectangular area was marked in the dorsum of each one (Figure 2A). Each flap was divided into three equal sized regions, the proximal zone (I), the middle zone (II), and the distal zone (III) for the interest of appraisal. In the experimental group, each flap was injected with 2.7 ml exosomes suspension while phosphate buffer saline (2.7 ml) was injected into each flap in the control group. The injection sites were shown in Figure 2D, each site for 0.1 ml, and the injections were performed evenly at the level of deep dermis. Then caudally pedicled dorsal skin flaps were performed. The flap was then incised with scalpel, being elevated in a plane superficial to the deep fascia. Perforated vessels at the flap bases were ligatured to create completely random vascular patterns (Figure 2B). After controlling any bleeding, the skin flap was sutured back by 4-0 silk (Figure 2C).
Figure 2.
A. Size of 9.0 × 3.0 cm rectangular area was marked in the dorsum of rat; B. Perforated vessels at the flap bases were ligatured to create completely random vascular patterns; C. The skin flap was sutured back by silk; D. Injection sites of dorsal flap.
Assessment of survival areas
Flaps were photographed on the 7th postoperative day, and surviving areas were measured by superimposition of photographs on graph paper. The percentages of survival area was determined as: extent of survival area × 100/total area (survival and necrosis) × 100%.
Laser doppler imaging
Skin flaps were observed by Laser Doppler System on the 7th postoperative day, laser Doppler perfusion imaging was obtained using a laser Doppler instrument (Moor Instruments, Axminster, UK) in a warm and quiet environment under anesthesia, and doppler pictures were captured for evaluation of the blood flow.
Tissue edema measurement
Tissue edema was reflected by the percentage of water content. After euthanizing rat and collecting samples, each flap was cut off and weighed, then dehydrated in an autoclave at 50°C and weighed again until the weight was stabilized for 24 hours. The percentage of water content was calculated as: (initial weight - constant weight)/initial weight × 100%.
HE staining and microvascular density measure
Two samples (0.5 cm × 0.5 cm) of central flap tissue were collected from each area of each flap and fixed in 4% paraformaldehyde (pH 7.4) for 48 h. After gradually dehydration and routinely paraffin embedding, slices (4 μm) were stained with hematoxylin and eosin and examined by light microscopy. We evaluated the thickness of granulation tissue and whether edema and neutrophil infiltration were evident. We measured microvascular density (MVD) as follows. First, we microscopically identified the most vascularized areas under low magnification (40 ×). Next, we counted vessels (at 400 ×) in five random microscopic fields each 0.152 mm2 in area, thus 0.44 mm in diameter. We calculated microvascularity per unit area (mm2) as an indicator of microvascular density.
Immunohistochemistry
Slides were blocked with TBS containing 10% goat serum and 1% BSA for 2 hours at room temperature. Then incubated in antibody solution (diluted anti-CD34 antibody solution to 1:100; anti-VEGF antibody solution to 1:100) for 12 hours at 4°C. All slices were warmed to 37°C for 45 min and washed with TBS. Next, CD34 slides were incubated in goat anti-rabbit antibody solution (diluted to 1:1000) for 50 minutes at room temperature while VEGF slides were incubated in goat anti-rat antibody solution (diluted 1:50) for 50 minutes at room temperature. After washing with TBS, slides were incubated in 3,3 N-diaminobenzidine tetrahydrochloride (DAB) solution for 5 min. The most intensely stained areas were identified under low magnification, and then vessels in five fields of each slice were viewed under higher magnification (400 ×). Observation parameters (white balance, aperture, shutter speed, and time) were held constant. Images were saved using Image-Pro Plus software, version 6.0 (Media Cybernetics, Rockville, MD) and the integral absorbance (IA) values were used as indicators of CD34 expression levels.
Western blot
Tissue samples (0.5 cm × 0.5 cm) were separated from the border of zone II/III of each flap. 5 samples in each group were processed by extracting proteins with lysis buffer. BCA Protein Assay Kit was used to determine the concentration of protein. Proteins were separated by 12% polyacrylamide gel electrophoresis and electro-transferred into PVDF membranes. After blocking with 5% (w/v) nonfat milk, the membranes were incubated with primary antibodies at 4°C for 10 hours. After washed by TBST at room temperature, membranes were incubated with secondary antibodies for an hour. Then immunoreactive proteins were visualized and quantified by Image Laboratory 3.0 software (Bio-Rad Laboratories Inc, Hercules, CA, USA).
SOD activity and MDA content test
Tissue samples (0.5 cm × 0.5 cm) were separated from the border of zone II/III of each flap. Samples were weighed, homogenized, and diluted to 10% (v/v) in an ice bath. SOD activity was determined by the Hydroxylamine method: 0.1 ml homogenate was added into 1.4 ml solution of xanthine oxidase and then kept in the incubation at 37°C for 40 min. Then, 2.0 ml of developer was added for shade selection at 550 nm following a 10 min incubation. MDA content was determined via reaction with thiobarbituric acid (TBA): 0.1 ml homogenate was mixed with 0.1 ml dehydrated alcohol, 0.1 ml TBA, and 4.0 ml developer, and bathed in water at 95°C for 40 min. After cooled to room temperature and centrifuged at 2500 g for 10 min. The absorbance of supernatant was detected at 532 nm.
Statistical analysis
All results are expressed as means ± standard deviations. Statistical evaluations were carried out with the aid of SPSS version 20.0 software. Graphs were constructed using GraphPad Prism version 7.0. A P value <0.05 was regarded statistically significant. The significance difference between two groups was tested via analysis of t-test.
Results
BMSCs’ culture and Identification of exosomes
The 4th generation of BMSCs under light microscope was showed in Figure 1A. The exosomes looked similar to the round under transmission electron microscope (Figure 1B), the diameters of which were mostly in the 80~100 nm range (Figure 1C). And CD9, CD63, TSG101 were further detected by western blot (Figure 1D).
Figure 1.
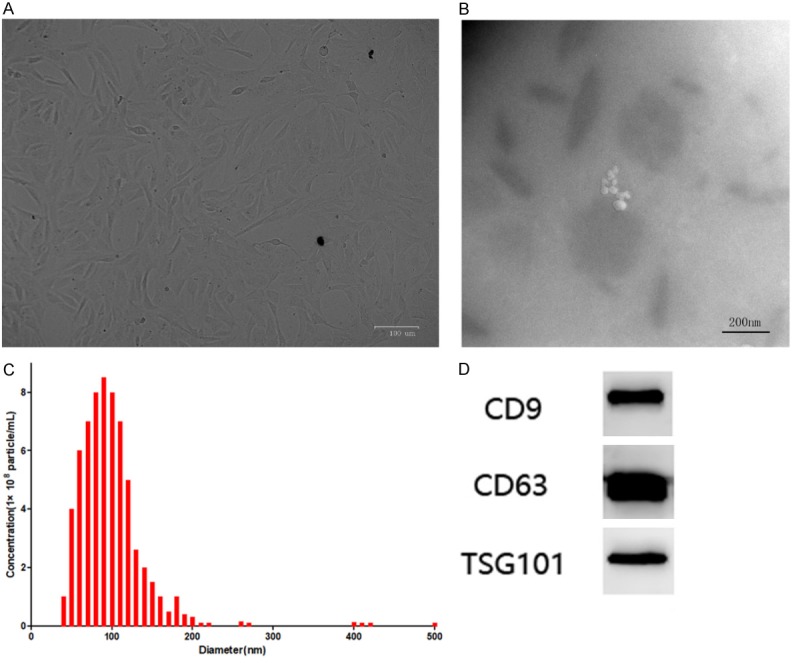
A. The 4th generation of BMSCs under light microscope; B. Scanning electron microscope photograph of BMSCs derived exosomes; C. The diameters of exosomes were mostly in the 80~100 nm range; D. Western blot result of exosomes’ marker protein.
Gross morphology and edema
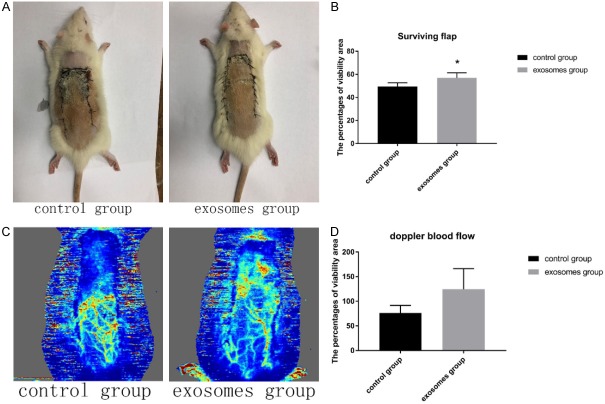
On the first day, all flaps were swollen to some extent, and distal zone III was dark purple in color, but without obvious necrosis. On the third day, zone II and zone III in both control and experimental groups exhibited reddish-brown focal or patchy necrosis, with congestion. On the seventh day, most of these necrotic parts had fused, scabbed, and hardened. The boundaries between necrotic and surviving regions were clearly demarcated. Surviving flap portions grew fine hair but the necrotic regions became hard, dark, and glabrous (Figure 3A). The percentages of viability area in the exosomes group was 56.9 ± 4.4%, significantly higher (P<0.05) than 49.5 ± 3.1% in the control group (Figure 3B). As for edema measurement, extent of flap edema was reflected by percentage of water, the percentage of water in the exosomes group was 61.2 ± 4.7%, significantly lower (P<0.05) than that value in 56. 3 ± 6.9% in the control group (Figure 4B).
Figure 3.
A. The dorsal photograph of SD rats on the seventh day; B. Bar graph of viability area percentages on the seventh day; C. Imaging of Laser doppler on the seventh day; D. Bar graph of doppler blood flow.
Figure 4.
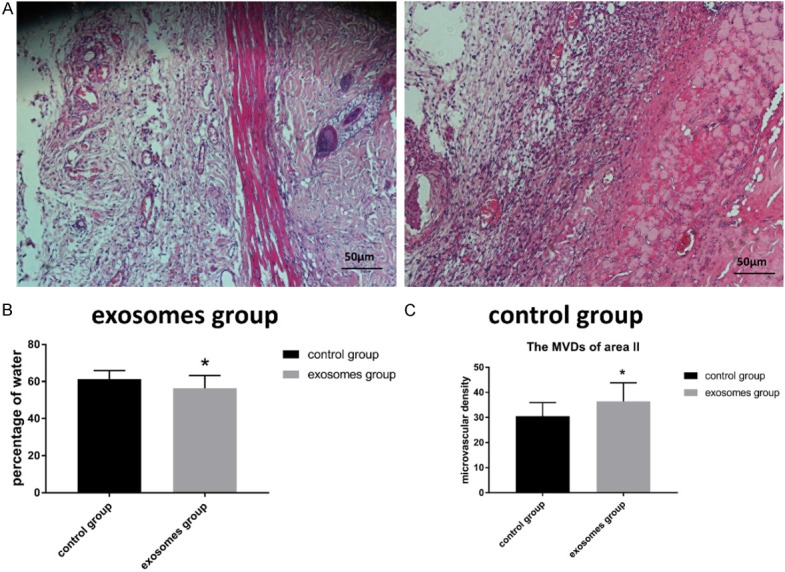
A. HE photograph at 100-fold; B. Bar graph of flap tissue’s water percentages; C. Bar graph of microvascular density.
Laser doppler imaging
7 days after operative, doppler pictures (Figure 3C) showed obviously richer blood supply in the distal part of skin flaps. In the zone II, the blood flow of control group was 76.21 ± 15.46, and the blood flow of exosomes group was 124.57 ± 41.75 (Figure 3D). This difference had statistical significance (P<0.05).
Histological structure
Seven days after operation, similarly degeneration and necrosis of tissue were observed in flap zone I in both group. In flap zone II, the exosomes group exhibited greater proliferation of fibroblasts, less edema, more diffuse neutrophil infiltration, and more neovascularization compared to the control group (Figure 4A). The MVDs of zone II in the exosomes groups were 36.4 ± 7.4/mm2, significantly higher than 30.5 ± 5.4/mm2 in the control group (P<0.05) (Figure 4C).
Immunohistochemistry
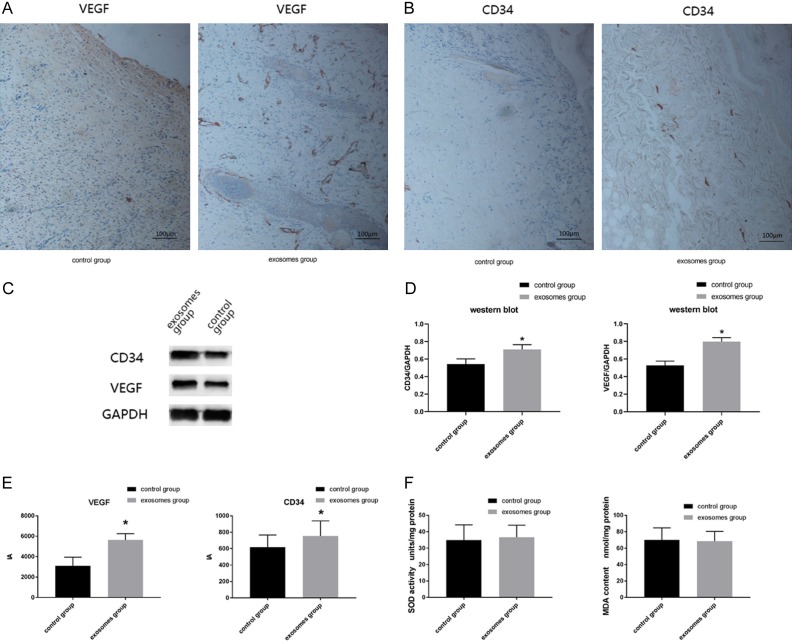
According to IA results of immunohistochemistry (Figure 5E), a statistic difference (P<0.05) was detected in VEGF expression: the IA value of the exosomes group was 5631.1 ± 627.5 and that of the control group was 3105.0 ± 835.3. Similarly, the CD34 IA value of exosomes group (753.7 ± 184.1) was significantly higher than that of the control group (618.4 ± 147.1).
Figure 5.
A. Immunohistochemistry of VEGF at 40-fold; B. Immunohistochemistry of CD34 at 40-fold; C. Western blot result of VEGF and CD34; D. Western blot analysis of VEGF and CD34; E. Bar graph of immunohistochemistry result; F. Bar graph of SOD activity and MDA content.
Western blot
According to western blot results (Figure 5C), the VEGF/GAPDH ratio of the exosomes group was 0.709 ± 0.054, significantly higher than that ratio of the control group (0.543 ± 0.059). And the CD34/GAPDH ratio of the exosomes group (0.798 ± 0.046) was also significantly higher than the control group (0.528 ± 0.048) (Figure 5D) (P<0.05).
SOD activity and MDA content test
The SOD activity was 34.87 ± 9.34 units/mg protein in the exosomes group and 36.69 ± 7.31 in the control group (Figure 5F). While he MDA content was 70.16 ± 14.62 nmol/mg protein in the exosomes group versus 68.63 ± 11.80 nmol/mg protein in the control group (Figure 5F). Neither the SOD activity nor MDA content showed statistic significant difference.
Discussion
Exosomes, as important mediators of intercellular communication, could mediate autocrine, paracrine, and endocrine effects, which might be exploited therapeutically [12]. It was commonly recognized that paracrine took the most important role in vivo, and that the generation of exosomes was a critical parameter in their ability to modify the function of host cells and tissues [13]. Exosomes were not only themselves therapeutic agents, but vehicles for targeted drug delivery and therapy [14]. Various studies have proven the potentially therapeutic effect on acute myocardial infarction [15], acute kidney injury [16], traumatic brain injury [17], focal cerebral ischemia [18], liver injury [9], pulmonary hypertension [19], necrotizing enterocolitis [20], cutaneous wound healing [21] and etc. In view of the necrosis of random skin flap was still unsolved problem in clinic, and the actual functions of MSCs derived exosomes in random skin flap model had not been rigorously studied, we logically presume exosomes might exert protection against flap necrosis at the design stage. We isolated exosomes from BMSCs and observed them by electron microscope. And common exosomes’ marker namely CD9, CD63 and TSG101 [22-24] were detected by western blot. Thus both the morphology and characteristic proteins were consistent with former reported MSCs derived exosomes [25]. The aim of this study was to evaluate the effect of local injection of BMSCs derived exosomes in rats dorsal skin flaps. As was shown by the result, the exosomes group was observed with significantly larger viability area (P<0.05).
We observed angiogenesis educed by BMSCs exosomes. HE staining of zone II showed higher microvascular density (P<0.05) and better angiogenesis (P<0.05) in the exosomes group. Similarly, on the 7th postoperative day, the blood flow of exosomes was better than the control group according to laser doppler imaging (P<0.05). Former researches had proved that BMSCs exosomes could enhance angiogenesis In vitro and in vivo [26]. Further studies showed MSCs exosomes function as paracrine effectors of angiogenesis, and the medium dose of MSC exosomes (10 μg/mL) effectively induced significant tubule formation [27]. As former studies reported, the enhancement of angiogenesis might be associated with the Wnt4/β-catenin pathway [28] or nuclear factor kappa-B signaling [27].
Regarding water content as metric of edema, we calculated the water content of each flap by continuously dehydration and weight. The exosomes group showed lessened flap edema (P<0.05). Better blood circulation might also give an account of slighter edema we observed. And alleviation of edema might feed back to circulation.
As the result of immunohistochemistry and WB, VEGF was found significantly increased with local injection of exosomes (P<0.05). VEFG was generally accepted as a factor with the ability of enhance the survival of the ischemic skin flaps [29]. VEGF was proven to induce angiogenesis and enhance skin paddle survival in musculocutaneous flap [30]. In mouse tumor xenograft model, MSC exosomes enhanced expression of VEGF by activating signal-regulated kinase1/2 (ERK1/2) pathway [31]. Opposite to our observation, in breast cancer model, MSCs derived exosomes significantly suppress angiogenesis by down-regulating VEGF expression [32]. VEGF itself had great capability of angiogenic [33], so we guess VEGF might partly participate in this process of angiogenesis and protection. Similarly, the exosomes group also showed higher CD34 expression than controls (P<0.05). Peripheral circulative CD34+ cells, an endothelial/hematopoietic progenitor-enriched cell population, had been proven to promote angiogenes-is [34,35]. CD34+ cells were mobilized into peripheral blood and homed to ischemic tissue to stimulate angiogenesis [36]. Consequently, the IA of CD34 cells might indicate the extent of tissue repair and angiogenesis [37].
Ischemia/reperfusion was a important element of necrosis of random skin flap [1]. In the process of I/R, the generation of reactive oxygen species (ROS) occurs was an important element. Superoxide produced under physiologic conditions under physiologic conditions is neutralized by SOD, a major defender against superoxide [38]. Overproduced ROS overwhelmed antioxidant defenses injured cellular components, which was called oxidative stress [39]. And MDA, the end product of lipid peroxidation, was one of signs of peroxide damage. In our former research, SOD activity and MDA content were used to evaluate the level of oxidative stress [40]. But as shown in Figure 5F, no statistical significance was found. In other words, the results obtained in this study do not support the protection have significant correlation with oxidative stress or I/R.
All these results of our study indicated the protective effective against flap necrosis of BMSCs-exosomes’ local injection might relate to angiogenesis and reducing edema. We speculate that the BMSCs exosomes could stimulate angiogenesis by the paracrine effect. VEGF might participate in this process of protection. But the concrete mechanism was still uncertain. More studies are required to clarify this problem and pave the way for exosomes’ application.
Conclusions
The results indicated the local injection of exosomes derived from BMSCs was effective to attenuate range of necrosis in McFarlane-type flap.
Acknowledgements
This study was supported by the Zhejiang Medical and Health Science and Technology Plan Project (No. 2017KY480) and National Natural Science Foundation of China (No. 81701928).
Disclosure of conflict of interest
None.
Abbreviations
- MSC
mesenchymal stem cell
- I/R
ischemia/reperfusion
- MVD
microvascular density
- PBS
phosphate buffer saline
- HE
Hematoxylin and eosin
- IA
integral absorbance
- WB
western blot
- VEGF
VEGF vascular endothelial growth factor
- SOD
superoxide dismutase
- MDA
malondialdehyde
- TBA
thiobarbituric acid
- ROS
reactive oxygen species
References
- 1.Krammer CW, Ibrahim RM, Hansen TG, Sørensen JA. The effects of epinephrine and dobutamine on skin flap viability in rats: a randomized double-blind placebo-controlled study. J Plast Reconstr Aesthet Surg. 2015;68:113–9. doi: 10.1016/j.bjps.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 2.Yates L, Norbury C, Gilbert RC. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damania A, Jaiman D, Teotia AK, Kumar A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res Ther. 2018;9:31. doi: 10.1186/s13287-017-0752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–67. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mcfarlane RM, Deyoung G, Henry RA. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast Reconstr Surg. 1965;35:177–82. doi: 10.1097/00006534-196502000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Samir EA, Imre MG, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 13.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–60. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016;113:170–5. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–43. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aliotta JM, Pereira M, Wen S, Dooner MS, Del TM, Papa E, Goldberg LR, Baird GL, Ventetuolo CE, Quesenberry PJ. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–30. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51:942–7. doi: 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–68. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 23.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:1–15. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.H Rashed M, Bayraktar E, K Helal G, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C. Exosomes from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtiö J, Nolta JA. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4:513–522. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padubidri A, Browne E Jr. Effect of vascular endothelial growth factor (VEGF) on survival of random extension of axial pattern skin flaps in the rat. Ann Plast Surg. 1996;37:604–11. doi: 10.1097/00000637-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Fischer K, Komorowska-Timek E, Guo M, Cui D, Dorsett-Martin W, Buncke HJ, Lineaweaver WC. Improvement of skin paddle survival by application of vascular endothelial growth factor in a rat TRAM flap model. Ann Plast Surg. 2001;46:314–319. doi: 10.1097/00000637-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Przemyslaw L, Goldman CK, Raffi G, Kevin C, Maria S. Enhancement of epigastric skin flap survival by adenovirus-mediated VEGF gene therapy. Plast Reconstr Surg. 2002;109:1986–93. doi: 10.1097/00006534-200205000-00031. [DOI] [PubMed] [Google Scholar]
- 34.Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, Kubo S, Kawamoto A, Asahara T, Kurosaka M, Kuroda R. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26:819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]
- 35.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Liu L, Zhang M, Bergeron A, Cui Z, Dong JF, Zhang J. Correlation of CD34+ cells with tissue angiogenesis after traumatic brain injury in a rat model. J Neurotrauma. 2009;26:1337–44. doi: 10.1089/neu.2008.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiroki F, Hiromi F, Shinsuke C, Keiko T, Zhonghua Q, Yukiko K, Breyer MD, Harris RC, Yuichiro Y, Takamune T. Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J Am Soc Nephrol. 2009;20:1303. doi: 10.1681/ASN.2008080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai L, Huang W, Lin D. Effects of traditional Chinese medicine Shuxuetong injection on random skin flap survival in rats. ScientificWorldJournal. 2014;2014:816545. doi: 10.1155/2014/816545. [DOI] [PMC free article] [PubMed] [Google Scholar]