Abstract
Despite great efforts made in recent years, globally cardiovascular disease (CVD) remains the most common and devastating disease. Pharmacological, interventional and surgical treatments have proved to be only partly satisfactory for the majority of patients. A major underlying cause of poor prognosis is a high comorbidity rate between CVD and mental illness, which calls for the approaches of psychocardiology. As psychiatric disorders and CVD can influence each other bidirectionally, it is necessary to develop novel therapies targeting both systems simultaneously. Therefore, innovative stem cell (SC) therapy has become the most promising treatment strategy in psychocardiology. Bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs), among all different types of SCs, have drawn the most attention due to unique advantages in terms of ethical considerations, low immunogenicity and simplicity of preparation. In this review, we survey recent publications and clinical trials to summarize the knowledge and progress gained so far. Moreover, we discuss the feasibility of the clinical application of BM-MSCs in the area of psychocardiology.
Keywords: Psychocardiology, bone marrow-derived mesenchymal stem/stromal cells, inflammation, autonomic nervous system, platelet activation
Introduction
Epidemiology and concepts addressed by psychocardiology
Cardiovascular disease (CVD) is the leading global cause of mortality accounting for at least 16 million deaths annually [1]. In the USA alone, the financial burden on hospitals reached 196 billion USD in 2015 and is expected to exceed one trillion USD by 2030 [2]. Although significant advances in pharmacological, interventional and surgical treatments have been made during the last two decades, the population affected and mortality caused by CVD are still increasing with poor long-term prognosis for sufferers [2]. This is mainly caused by the complexity of CVD which involves multiple systemic dysfunctions. Among these, mental illness exhibits exceptionally high comorbidity rates with CVD. The concept of psychocardiology has been proposed as a new medical specialty aiming to unravel the entangled relationships between “heart” and “mind”, and so eventually improve the prognosis of those patients suffered from both CVD and psychiatric disorders [3].
Major depressive disorder (MDD), or depression, is one of the mental health disorders which draws particular attention because of its high comorbidity with CVD. It is reported that 15.9% of the patients who had experienced atrial fibrillation (AF) suffered from comorbid depression which resulted in a significantly increased incidence of intracranial bleeding [4]. For patients with unstable angina and ischemic heart disease (IHD), the occurrence rate of depression is as high as 41.4% and 45% respectively [5]. Recent meta-analysis has further revealed that several mental disorders including MDD (OR=2.52, P<0.0001), anxiety (OR=1.41, P<0.001), schizophrenia (OR=1.52, P<0.001) and post-traumatic stress disorder (PTSD) (OR=1.27, P<0.05) significantly increase the incidence of CVD [6].
The consequences of this comorbidity are severe. Mental health disorders not only decrease the quality of life in CVD patients, but also lead to significant increases in both short-term and long-term mortality [7-9]. Therefore, the need to develop novel therapies in psychocardiology is still tremendous and urgent.
Bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs)
The use of Stem Cells (SCs) has drawn much attention in the area of regenerative medicine because of its ability to regenerate damaged cardiac tissues and among all different types of SCs, BM-MSCs have already been well studied in animal and clinical research. In comparison with other SCs such as Embryonic Stem Cells (ESCs) and induced Pluripotent Stem Cells (iPSCs), BM-MSCs have several advantages including fewer ethical considerations, a minor risk of tumorigenicity and lower immunogenicity [10].
BM-MSCs are a subtype of non-hematopoietic stem cells localized in the bone marrow. They were first identified in 1976 and have proven to be capable of differentiating into adipocytes, chondrocytes, osteocytes and cardiomyocytes. Although they occupy only 0.001%-0.01% of the total monocytes in the bone marrow, they can be expanded over a million folds or 6 generations in vitro [11]. Since Friedenstein et al established the first method for isolating BM-MSCs, several techniques have been developed including a wholes defined by The International Society for Cellular Therapy (ISCT), all mesenchymal stem cells (MSCs) should be positive for CD105, CD73 and CD90 while being negative for CD34, CD45, CD11b/14 and CD19/79a [12]. Researchers also suggest that MSCs, especially BM-MSCs, also express several other surface markers such as CD13, CD26, CD29, CD105 and Stro-1 [13,14].
In 2002, Shake et al first observed the beneficial effects of BM-MSC transplantation in a swine Myocardial Infarct (MI) model where they discovered a significant increase in end diastolic/systolic wall thickness after autologous BM-MSCs transplantation [15]. Two years later, the first clinical trial was completed in 69 patients with Acute Myocardial Infarct (AMI). At the end of the 6 months follow-up period, patients receiving BM-MSCs transplantation showed compelling changes in terms of their cardiac functions. The Left Ventricular Ejection Fraction (LVEF) of patients was 67±3% in the BM-MSCs group and 54±5% in the control group [16]. Since then, BM-MSC therapy has been widely discussed in terms of the treatment for a broad range of cardiovascular diseases (see previous reviews for details [17,18]). However, none ever considered the potential applications of BM-MSC in psychocardiology. In this review, we discuss the feasibility of BM-MSC therapy in patients with both CVD and mental disorders by comprehensively summarizing possible effects of BM-MSC transplantation on underlying mechanisms of psychocardiological disease.
Mechanisms underlying the therapeutic effects of BM-MSCs in psychocardiology
Tissue regeneration
It is widely acknowledged that cell apoptosis and tissue necrosis are associated with the pathology of both CVD and psychiatric illness. Thus, the ability of BM-MSC to regenerate functional cardiomyocytes, endothelial cells, neurons and astrocytes is of great importance for its therapeutic effects in psychocardiological disorders (Figure 1).
Figure 1.
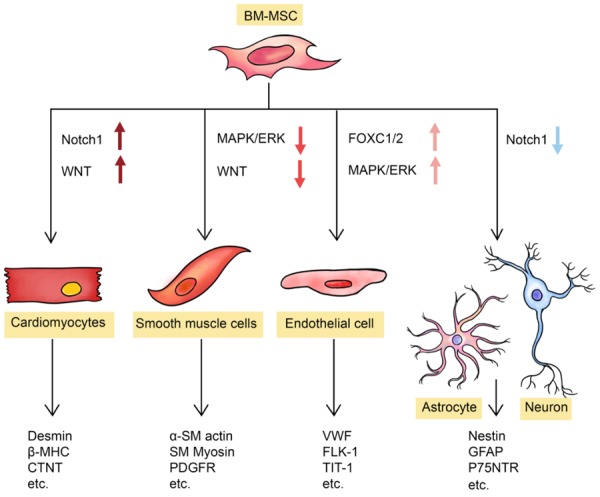
Regenerative abilities contribute to the application of BM-MSC in psychocardiological disease. Under different stimulations, BM-MSC can differentiate into cardiomyocytes via activation of Notch-1 and Wnt signaling pathways; into smooth muscle cells via inhibition of MAPK and Wnt signaling pathways; into endothelial cells via activation of FoxC and ERK signaling pathways; or into neural cells via inhibition of Notch-1 signaling pathway. The differentiated cells can express related biomarkers. Abbreviations: BM-MSC, bone marrow-derived mesenchymal stem cell.
In 1999, a research team from Keio University successfully generated cardiomyocytes from marrow stromal cells by 5-azacytidine (5-aza) treatment in vitro [19]. By now, several methodologies have been established to induce ex vivo/in vitro differentiation of BM-MSC into cardiomyocyte-like cells. These methodologies include aggregate co-culture, treatment with demethylating agents, incubation with growth factors and treatments with rehmannia glutinosa oligosaccharide [20-23]. Moreover, several research teams report that they have observed in vivo differentiation of BM-MSC into cardiac cells expressing multiple cardiac markers, such as desmin, β-MHC, β-actin, CTn-T and phospholamban, at almost the same levels seen in endogenous cardiomyocytes [24]. Molecular mechanisms underlying this differentiation involve the up-regulation of nuclear membrane proteins and transcription factors [25,26] which eventually activate downstream signal pathways such as Notch1 and WNT [27,28].
Besides cardiomyocytes, BM-MSCs were also shown to be able to differentiate into vessel smooth muscle (SM) cells and vascular endothelial cells. SM-like cells induced from BM-MSCs express SM proteins, including α-SM actin, PDGF-β receptor, SM myosin light chain and SM myosin heavy chain, at similar levels to those in freshly isolated SM cells. In addition, SM-like cells also exhibit identical electrophysiological features compared to SM cells [24]. On the other hand, expression of endothelial markers (vWF, Flk-1 and TIT1) can also be detected after, but not before, endothelial induction in BM-MSCs [24]. In vivo differentiation of BM-MSC into SM and endothelial cells was also observed, and more recent publications reveal that the inhibition of MAPK and WNT pathways result in differentiation into SM cells [29] while the activation of FOXC1/2 and ERK1/2 pathways contribute to the differentiation into endothelial cells [30,31].
Finally, BM-MSCs also show potential to differentiate into neuron-like cells which are able to express neural markers (Nestin, GFAP and DCX) and secrete multiple neurotrophic factors (BDNF, IGF-1 and FGF-2) in vitro [32]. Recently, different procedures have been established to generate specific subtypes of neural cells, such as Schwann cells and GABAergic neurons. Differentiated Schwann cells not only express neural markers (Nestin, GFAP and p75NTR), but also exhibit myelinating functions in vitro [33]. A culture medium consisting of retinoic acid (RA), ciliary neurotrophic factor (CNTF), and creatine was shown to be able to induce in vitro differentiation of GABAergic neurons from BM-MSC with enhanced expression of GAD1/2, VGAT, GABA and sy-naptophysin [34]. In addition, BM-MSC differentiation into neural cells was also detected in vivo both with and without chemical stimulations [35,36]. The mechanism regulating neural differentiation largely remains unknown, but it is believed to be associated with inhibition of Notch-1 signaling pathway [37].
Although differentiation into cardiomyocytes and neural cells was confirmed both in vitro and in vivo, whether the differentiation is directly leading the tissue regeneration is still in debate. Dai et al suggested that beneficial effects of BM-MSC transplantation in MI rats are caused by paracrine effects but not direct differentiation of introduced SCs [38]. Similarly, nerve regeneration following BM-MSC transplantation in a gastric denervation rat model was also demonstrated to result from secretion of neurotrophic factors but not direct differentiation of grafted BM-MSC [39]. Therefore, understanding these paracrine effects is of particular importance for the future application of BM-MSC in psychocardiological illness.
BM-MSCs secrete various growth factors and cytokines under certain conditions such as hypoxia, TNF stimulation and formation of cell-cell contacts [39-41]. These cytokines not only participate in angiogenesis and neurogenesis but also contribute to anti-apoptosis properties and endogenous regenerative activities. The detailed functions of each cytokine can be found in Figure 2 [42-67] as well as another review studying SC promoted angiogenesis [68]. Overall, the regenerative activity of BM-MSCs is one of the most fundamental predictors of their future application in psychocardiology.
Figure 2.
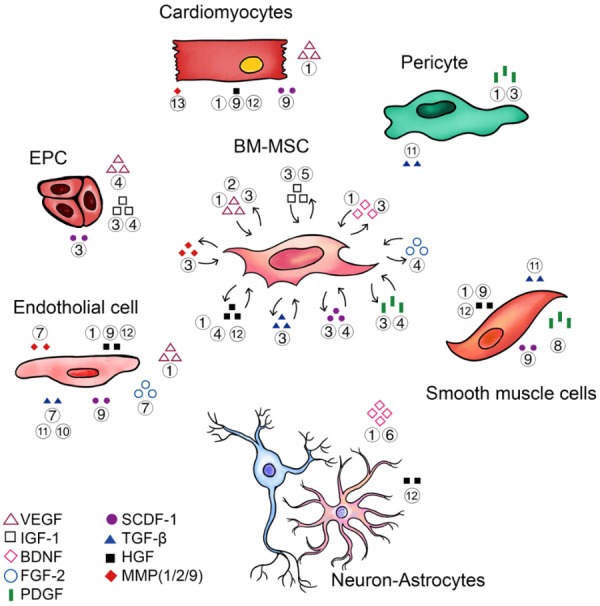
Functions of major cytokines and soluble factors secreted by BM-MSC. The growth factors and cytokines secreted by BM-MSC can act on both BM-MSC itself (autocrine) and other target cells (paracrine) which play essential roles during the development of psychocardiological disease. Figure legends: ① Anti-apoptosis; ② Activate endogenous BM-MSC; ③ Increase homing; ④ Promote proliferation; ⑤ Induce differentiation; ⑥ Increase neural plasticity; ⑦ Increase stability and integrity; ⑧ Inhibit over-proliferation of smooth muscle cells; ⑨ Anti-fibrosis; ⑩ Inhibition of migration; ⑪ Promote cell adherent; ⑫ Anti-oxidation; ⑬ Inhibit ventricular remodeling. Abbreviations: BM-MSC, bone marrow-derived mesenchymal stem cell; VEGF, Vascular Endothelial Growth Factor; EPC, Endothelial Progenitor Cell; IGF-1, Insulin-like Growth Factor 1; BDNF, Brain-Derived Neurotrophic Factor; FGF-2, Fibroblast Growth Factor 2; PDGF, Platelet-Derived Growth Factor; SCDF-1, Stromal Cell-Derived Factor 1; TGF-β, Transforming Growth Factor beta; HGF, Hepatocyte Growth Factor; MMP, Matrix Metalloproteinase.
Immunosuppressive effects
Elevated inflammatory responses are detected in patients with CVD as well as patients with mental illness [69]. A recent meta-analysis indicated that depression is associated with significant increases in levels of multiple cytokines including interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP) [70]. Higher concentrations of such pro-inflammatory markers not only suggest an increased inflammatory state which is known to participate in the development of CVD [71-73], but also forebode increased risks of major cardiovascular adverse events (MCAE) and even cardiac death. For example, a higher serum level of IL-6 (range from 2.08 pg/mL to 3.9 pg/mL) results in risk ratios (RR) of all-cause mortality and cardiovascular mortality at 1.49 (95% CI 1.33-1.67) and 1.69 (95% CI 1.27-2.25) respectively. On the other hand, higher levels of CRP are associated with a RR of 2.03 (95% CI 1.65-2.50) for cardiovascular mortality, and 1.75 (95% CI 1.55-1.98) for all-cause mortality [74]. Meanwhile, elevated baseline levels of CRP and IL-6 are also positively associated with cognitive symptoms of depression after full adjustments [75]. The importance of inflammation management in psychocardiology can also be corroborated by the fact that several widely used drugs in the treatment of CVD and mental illness exhibit anti-inflammatory properties. Metoprolol, a representative of β-blockers, were shown to inhibit the activation of neutrophils, and thereby rescue cardiac function in MI mice and patients [76]. Similarly, fluoxetine, a selective serotonin reuptake inhibitor (SSRI) commonly used as an antidepressant, has been shown to be able to inhibit the activation of microglia, the innate immune cells of the central nervous system (CNS) [77]. In a recent meta-analysis reduced serum levels of IL-6, TNF-α and IL-1β were also observed after SSRI treatment in patients with MDD [78]. Although which cytokines are involved in SSRIs induced anti-inflammatory responses are still under debate [79], there seems no doubt that immunoregulation by SSRIs plays an essential role in their modulation of neurobehavioral functions. Nowadays, increasing interest is being focused on developing therapeutics targeting the immune system and related signaling pathways in both cardiology and psychology [80-83].
Although the exact molecular mechanisms underlying the interaction of CVD, mental health disorders and inflammation are not completely understood, it is widely accepted that 5-hydroxytryptamine (5-HT), which is also known as serotonin, is one of the most important mediators (Figure 3). As a neurotransmitter in the CNS, 5-HT regulates a wide range of neurological activities including happiness, cognition, learning and memory. Abnormal levels of 5-HT, 5-HT transporter (SERT) and 5-HT receptors are believed to be a major cause of mental health disorders and are considered to be the main targets for the treatment [84-87]. Meanwhile, 5-HT also acts as a vasoconstrictor or a vasodilator under pathological or physiological circumstances in the peripheral circulatory system [88]. In particular, its vasoconstrictive ability is believed to be a major contributor to the development of several cardiovascular diseases including hypertension [89], heart failure (HF) [90] and atherosclerosis [91]. Roles for 5-HT in the immune system have been thoroughly presented in other reviews [92,93]. Briefly, 5-HT can be synthesized by tryptophan hydroxylase 1 (TPH 1) in multiple immune cells including mast cells [94] and T helper (Th) cells [95]. The release of 5-HT from platelets and other cells can further exert immunostimulatory functions through the activation of various 5-HT receptors while the blockage of such receptors results in immunosuppression [92]. On the other hand, pro-inflammatory cytokines, such as Interferon-γ (IFN-γ) and TNF-α, can induce increased activity of indoleamine 2,3-dioxygenase (IDO), which degrades tryptophan into kynurenine [96]. This inflammation-driven kynurenine metabolism is considered to be a primary pathologic pathway leading to depression. Degradation of tryptophan not only increases the formation of neurotoxic metabolites, but also contributes directly to a decline in the level of 5-HT, which can only be synthesized from tryptophan, and thereby promotes depressive symptoms [97-99].
Figure 3.
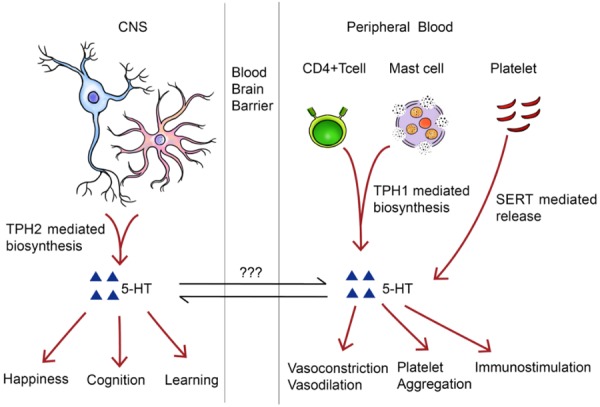
Brief introduction of the importance of 5-HT in the central nerve system (CNS) and peripheral blood system (PBS). The 5-HT is synthesized by Tph2 and Tph1 in the CNS and PBS respectively. In addition, the 5-HT can also be released from platelet. Inside the CNS, 5-HT functions as a neurotransmitter during the physiological process in happiness, cognition and learning. In the circulatory blood, 5-HT is a hormone which can regulate vascular tone, induce platelet aggregation and trigger immunological responses. Abbreviations: BM-MSC, bone marrow-derived mesenchymal stem cell; 5-HT, 5-hydroxytryptamine, TPH, tryptophan hydroxylase; SERT, serotonin transporter.
The immunoregulatory effects of BM-MSCs can’t be overemphasized when considering their applications in psychocardiology. Firstly, BM-MSC can inhibit the activation of lymphocytes and B cells through direct cell-cell interaction mainly mediated by integration of programmed cell death protein 1 (PD-1) and Programmed cell death 1 ligand 1 (PD-L1). Transplantation of BM-MSCs results in increased proliferation of Th2 and Treg with a decrease in numbers of Th1 and Th17 cells, which suggests down-regulation of immune responses. Silencing PD-L1 in BM-MSCs significantly attenuated the above immunosuppressive effects [100]. Similarly, the PD1/PDL1 pathway is also important for inhibitory effects on B cells which lead to dramatic reductions in secretion of IgM and IgG [101]. Interestingly, recent studies also show that BM-MSCs are able to secret PDL1 and thereby inhibit CD4+ T cells through the AKT-FOXO3 signaling pathway [102].
Although there is no doubt that PD-1/PD-L1 induced reticence plays essential roles in BM-MSC effects, most research has still been focused on their paracrine activities. Soluble factors from BM-MSCs or BM-MSC extracellular vesicles can act on both innate and adaptive immune cells. Prostaglandin E2 (PGE2), IL-6 and granulocyte macrophage colony stimulating factor (GM-CSF) released by BM-MSCs can suppress the differentiation of macrophage subtype 1 (M1) from immature macrophages and promotes differentiation towards an immunosuppressive phenotype (macrophage subtype 2, M2) [103,104]. This polarization from M1 to M2 is associated with increased expression of CD206 and decreased expression of CD86 on the cell membrane [105], as well as elevated secretion of anti-inflammatory cytokines (IL-10) and reduced secretion of proinflammatory cytokines (TNF-α) [106]. In addition to macrophages, IL-6 also inhibits apoptosis of neutrophils, and prevents the respiratory burst which generates reactive oxygen species (ROS) [107]. Moreover, mast cells, which produce histamine and heparin during infection and allergy, are also under the control of BM-MSC secreted PGE2. Activation of prostanoid receptor EP4 results in decreased levels of degranulation and reduced release of TNF-α from mast cells [108]. Nature killer (NK) cells, as a major element of innate immunity, are targets of BM-MSC secreted factors as well. Evidence suggested that indoleamine 2,3-dioxygenase (IDO) [109], PGE2 [109], TGF-β [110] together with human leucocyte antigen-G5 (HLA-G5) [111] contribute to suppress cytotoxic effects of NK cells. On the other hand, dendritic cells (DCs) can also be regulated by IL-6 and PGE2 [112], finally resulting in down-regulation of DC markers (CD40&CD83), reduced expression levels of pro-inflammatory markers (TNF-α, IFN-γ, IL-12p70 and MIP-1) [113,114], enhanced expression of immunoregulatory factors (IL-6&IL-10) [115], and the inhibition of migration of DCs [116]. Furthermore, similarly soluble PD-L1, IDO [109], PGE2 [109,117] and HLA-G5 [111] also exhibit anti-inflammatory functions through the polarization from Th1 and Th17 to Treg. In addition to the proteins and metabolites mentioned above, at least 49 micro-RNAs were identified as being enriched in exosomes secreted from BM-MSC including miR21-5p, miR142-3p, miR223-3p, and miR126-3p [118]. Some of these micro-RNAs work together and regulate target immune cells. For example, miR21-5p down-regulates CCR7 expression on DC cells and limits their migratory ability [118]. MiR146a-5p targets the gene IRAK1 which plays a crucial part in Toll-like Receptors (TLR) mediated inflammatory reactions [119]. Overall, BM-MSCs display remarkable anti-inflammatory effects which give great promise for their future application in psychocardiology (Figure 4).
Figure 4.
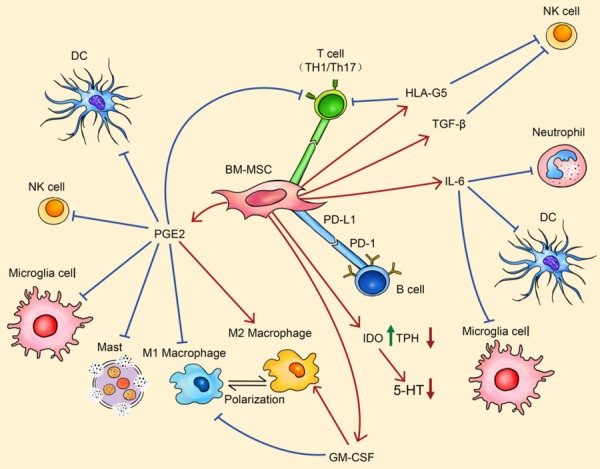
BM-MSC regulates the immune system. By PD-1 mediated cell-cell contact, BM-MSC can inhibit the activation of adaptive immune cells. By secreting PGE2, HLA-G5, TGF-β, IL-6 and GM-CSF, BM-MSC can inhibit the activation of almost every type of immune cells. The regulatory effects can be observed by a pro-inflammatory to anti-inflammatory cell polarization (such as M1 macrophage to M2 macrophage). Meanwhile, IDO secreted from BM-MSC can down-regulate the biosynthesis of 5-HT. Arrows in red indicate stimulative effects while arrows in blue show inhibitory effects. Abbreviations: BM-MSC, bone marrow-derived mesenchymal stem cell; PD-L1, programmed death ligand 1; PD-1, programmed death 1; DC, dendritic cells; NK, natural killer; PGE2, prostaglandin E2; HLA-G5, human leucocyte antigen G5; TGF-β, transforming growth factor-β; IL-6, interleukin 6; IDO, indoleamine 2,3-dioxygenase; TPH, tryptophan hydroxylase; 5-HT, 5-hydroxytryptamine; GM-CSF, granulocyte macrophage colony stimulating factor.
It should also be acknowledged that BM-MSCs also show the potential to regulate levels of 5-HT in the CNS and the peripheral circulatory system, through mechanisms that are not fully recognized. Ali et al have suggested that BM-MSC transplantation increases concentrations of 5-HT in the cortex and midbrain in a rodent brain injury model [120]. An avian model also revealed increased 5-HTR1A expression and decreased TPH-1 expression after the transplantation of BM-MSC [121]. Additionally, IDO released from BM-MSCs may also be involved in the modulation of the 5-HT system via altered tryptophan metabolism.
Antiplatelet properties
Thrombogenesis induced by platelet activation and aggregation, as is well-known, is the leading cause of the development of multiple CVD and major adverse cardiovascular events (MACE) [122]. Markers of platelet activation usually include the increased synthesis of the αIIb-β3 complex, P-selectin, Annexin V, CD62p and platelet factor 4 (PF4). Compared to healthy controls, patients with MDD were shown to have increased levels of PF4 whilst total platelet counts were unchanged [123]. Other research illustrates that subjects with depressive symptoms have a higher percentage of circulating CD62p positive platelets [123]. Similar results were consistently reported by different groups [124,125]. A more recent study which included 26 CAD patients discovered a positive correlation between depressive symptom severity and platelet factor abundance [126]. Therefore, platelet activation can be considered to be a link between cardiovascular disease and major depression [127]. Besides this, activated platelets can recruit and mobilize multiple immune cells, including neutrophils [128] and macrophages [129,130], which further damage the blood vessels and so contribute to worsen prognosis [131].
In addition, the activation of platelets is also associated with the release of 5-HT and the activation of the 5-HTR2A in the platelet membrane. It is well known that 5-HT leads to platelet aggregation [132], and can be used as a predictor for cardiac events [133] for dozens of years. On the other hand, recent studies have further confirmed the role of platelets in psychocardiology. For example, Peitl et al found that patients with depression had impaired 5-HT storage [134] while Williams et al confirmed increased 5-HTR2A levels in platelets in such patients [135]. Multiple SSRIs, on the other hand, show anti-platelet functions. In ADP induced aggregation tests, impedance was reduced by 23% and 29% on treatment with escitalopram and nortriptyline respectively [136]. However the latest publications evaluating the anti-platelet capacity of all types of SSRIs, have drawn opposing conclusions [137,138] indicating that more research with enlarged sample size may be required. Furthermore, even should their anti-platelet effects be confirmed, whether SSRIs are able to reduce the incidence rates of MACE would still be contentious. For instance, after analyzing the data from 238,963 patients with depression, Coupland et al expressed the opinion that a reduced risk of MI can be detected in SSRIs users [139]. Opposing conclusions were reached by Iasella et al who claimed that SSRI patients shared higher MACE risk than patients on placebo treatments (HR 1.21, 95% CI 1.02-1.43, P=0.030) [140].
We speculate that therapeutic effects of BM-MSC transplantation on platelet activity may occur through the inflammatory and serotonergic pathways mentioned before. Moreover, the ability of BM-MSC transplantation to regenerate endotheliocytes can also contribute to its anti-platelet outcome as damaged vessel endothelium is a major contributor to 5-HT induced platelet aggregation. Furthermore, emerging evidence suggests a direct inhibitory effect via interaction between CD73 and CD39 which are expressed on the membranes of BM-MSCs and platelets respectively [141]. This cell-cell interaction based inhibitory effect is of great importance as BM-MSCs, unlike MSCs from other origins, do not express podoplanin, which is a ligand of the C-type lectin-like receptor (CLEC-2). The tissue-specific expression of adhesions molecules results in tissue-specific hematoblastic reactions whereby BM-MSCs decrease platelet aggregation while umbilical cord MSCs promote platelet aggregation [142].
Regulation of autonomic nervous system (ANS)
The balance of sympathetic and vagal activity controls a wide range of cardiovascular outputs including heart rate and blood pressure. Abnormal increases in sympathetic activity cause many adverse cardiovascular events [143,144]. Recent studies even suggest that electrocardiographic indicators of elevated sympathetic activity can predict sudden cardiac death in patients with MI and HF [145,146]. Catecholamines (epinephrine and norepinephrine), used as molecular markers for sympathetic activity, were also shown to be raised in patients with CVD [147] as well as patients with mental disorders [69]. The over-accumulation of epinephrine and norepinephrine may also be seen as a consequence of the activation of the hypothalamic-pituitary-adrenal (HPA) axis, which is also dominated by the ANS. Importantly, other biomarkers of stimulation of the HPA axis, such as aldosterone and cortisol/corticosterone, are found to be higher in the serum of patients with depressive symptoms [148-150]. On the other hand, an increase in cortisol is associated with significantly increased mortality [151]. Alternately, imbalances in the ANS can raise the potential to develop other independent cardiovascular risk factors such as hyperlipidemia, obesity and insulin tolerance.
Besides the ANS, the activity of the HPA-axis is also associated with 5-HT signaling pathways. In vivo animal experiments suggest that transduction of HPA-axis responses relies on 5-HTR1A and 5-HTR2A, but is independent of 5-HTR2C [152,153]. By using a social isolation rhesus macaque model, Sorenson et al demonstrated that short allele (ss) SERT genotype is linked to impair HPA-axis function and finally results in elevated levels of cortisol [154]. Accordingly, effects of SERT and its methylation on cortisol release were further confirmed in humans [155,156]. These discoveries answered the question regarding the involvement of 5-HT in the regulation of the HPA-axis raised 20 years ago when scientists, for the first time, observed an effect of the SSRI-citalopram on HPA-axis regulation in rats [157]. Additionally, superexcitation of the HPA-axis normally leads to pro-inflammatory responses. This can be verified by positive correlations between levels of cortisol/norepinephrine and levels of multiple inflammatory markers including TNF-α, IL-6, IL-10 and CRP [158-160]. Interestingly, although 5-HT is also a primary mediator of inflammation, it seems that this HPA-axis-induced immune response is not associated with 5-HT metabolism [161], but is affected by glucocorticoid receptor pathways [162], adrenergic receptor signaling [163] and the status of ATP-sensitive potassium channels [164]. This may partly explain why high concentrations of cortisol do not directly cause abnormal platelet functions in hypercortisolaemic patients [165].
Apart from the HPA-axis, a decrease in heart rate viability (HRV), another marker of a disabled ANS, is also detected in psychocardiological disease. HRV, which consists of a series of parameters including SDNN, SDANN, RMSSD, PLVAR10 and LF/HF, is considered to reflect the ability of the heart to deal with physiological drives, a decreased HRV indicates an excessive enhancement of sympathetic activity. It has been observed that MDD patients have significantly reduced RMSSD compared with healthy controls [166]. Similar results were observed in patients with other mental illness such as schizophrenia [167], bipolar disorder [167] and anxiety [168]. A decline in HRV is a well-known predictor for poor prognosis of CVD, development of MACE and even cardiac death [169,170]. Notably, when compared to MI patients without depression, depressed MI patients seem to have more significantly decreased HRV [171]. Strong links have also been suggested between HRV and inflammation. In patients with juvenile dermatomyositis, SDNN, pNN50 and RMSSD are all negatively correlated to levels of hsCRP [172]. In the CARLA study which included 1671 participants from the general population, multiple HRV parameters were shown to be negatively associated with levels of hsCRP, sTNF-R1 and IL-6 [170]. Besides this, the observed effect of SSRIs on HRV also indicates a potential engagement of 5-HT metabolism [173].
Tissue regenerative, anti-inflammatory and anti-platelet properties may all contribute to the therapeutic effects of BM-MSC in terms of ANS modulation in animal models with ANS dysfunctions [174]. Moreover, BM-MSC transplantation has been shown to decrease the levels of norepinephrine and corticosterone in a rat brain injury model [120] and a diabetic rat model [175] respectively. Similar effects were also detected in cardiomyopathy rats where the expression of CYP11B2, the aldosterone synthase, was significantly inhibited at the mRNA level after the transplantation [176]. In addition to modulation of the HPA-axis, BM-MSCs also appeared able to boost HRV both in animal experiments [177] and clinical trials [178]. Overall, we conclude that the modulatory effects of BM-MSC transplantation on the ANS considerably contribute to its potential applications in psychocardiology.
Other factors
Many other factors may also participate in the development of psychocardiological illness and can be regard as targets of BM-MSC therapy. Lower level of BDNF is proved to be associated with multiple metal disorders [179-182] and it shows direct regulatory effects on 5-HT metabolic genes SERT and TPH-1 [183]. As well as neurological activities, such as neurogenesis and neural plasticity, BDNF also plays essential roles within the cardiovascular system. It not only promotes the development of cardiovascular organs during embryogenesis, but also exhibits anti-apoptotic, anti-fibrosis and anti-inflammatory properties on endotheliocytes and cardiomyocytes [184]. Therefore, the ability to secret BDNF, as mentioned above and shown in Figure 2, may further increase the utility of BM-MSC transplantation in psychocardiological disease. Besides this, BM-MSCs are capable of managing oxidative stress, which is actively involved in the development of mental illness [185] and CVD [186,187], through different signaling pathways [188,189].
In addition to above physiological and pathological mechanisms, recent studies suggest that genetic and epigenetic changes also contribute significantly to the link between mental illness and CVD. So far, at least eight single nucleotide polymorphisms (SNPs) have been identified as being associated with both psychiatric disorders and CVD [190-194] (Table 1). While these changes can’t be reversed by pharmacological and surgical treatments, they may be alleviated by the introduction of BM-MSCs carrying other polymorphic forms.
Table 1.
Single nucleotide polymorphisms in psychocardiology
| SNP Access No. | Genes | Alleles | Functions related to psychocardiology |
|---|---|---|---|
| rs3917010 | VCAM1 | A>C | Increased risk of MI; predict depressive symptoms in CVD patients |
| rs1324072 | CR1 | C>G | Predict depressive symptoms in CVD patients |
| rs1424386 | CHRM2 | G>A | |
| rs2239106 | CACNA1C | A>T | |
| rs216856 | vWF | T>C | |
| rs216873 | T>C | ||
| rs3125 | HTR2A | C>G/T | Predict depressive symptoms in CVD patients; increase in suicidal ideation; risk of bipolar disorder; risk of MDD |
| rs6265 | BDNF | C>T | Increase incidence of cardiovascular events; increase anxiety; increase suicidality |
Abbreviations: VCAM1, Vascular Cell Adhesion Molecule 1; CR1, Cannabinoid Receptor 1; CHRM2, Vholinergic Receptor Muscarinic 2; CACNA1C, Calcium Voltage-gated Channel subunit alpha1 C; vWF, von Willebrand Factor; HTR2A, 2-HT receptor 2A; BDNF, Brain-Derived Neurotrophic Factor.
Evidence from pre-clinical and clinical trials
A tremendous number of research articles have extensively discussed the usefulness of BM-MSC transplantation in treatments of CVDs (please find details in previous reviews [17,195,196]). In this review, we have briefly summarized the main outcomes of 7 clinical trials reported since 2015 which can be found in Table 2 [197-203]. Generally, recent promising results indicate potential applications for BM-MSC transplantation in several cardiac diseases. However, its utility in psychocardiological disease is not fully understood as none of the above trials considered the impact of transplantation on mental health status, such as depressive and anxiety-like behaviours.
Table 2.
Clinical trials using BM-MSC in cardiac diseases
| Disease | Sample size* | Injection method | Source | Cell number | Follow-up time | Main outcomes | Reference |
|---|---|---|---|---|---|---|---|
| IHF | 37/18 | Myocardial | Autologous | 107-108 | 6 months | Reduced LVESV; Improved LVEF, stroke volume of and myocardial mass. | [198] |
| ICM | 30/0 | Myocardial | Allogeneic | 2*107/108 | 1 year | Reduced scar size; Improved LVEF in 108 group; Improved NYHA class. | [200] |
| CHF | 120/231 | Myocardial | Autologous | 6*108 | 1 year | Reduced LVESV and LVEDV. | [202] |
| ICM | 10/0 | Myocardial | Autologous | 6*107 | 1 year | Improvements in LVEF, LVESV, 6-min walk test and NYHA functional class. | [199] |
| AMI | 8/8 | Intravenous | Allogeneic | 6*107 | 2 years | No significant differences. | [197] |
| DCM | 37/0 | Myocardial | Allogeneic & Autologous | 9*107 | 1 year | Increased EF, 6-min walk distance and decreased MLHFQ score in Allo-group. | [201] |
| DCM | 17/20 | Intracoronary | Autologous | 5*108 | 1 year | Improved LVEF, NYHA class and myocardial perfusion. | [203] |
Sample size was shown as: numbers in experimental group/numbers in control group.
Abbreviations: AMI, Acute Myocardial Infarction; IHF, Ischaemic Heart Failure; LVESV, LV end-systolic volume; LVEDV, LV end-diastolic volume; ICM, Ischemic Cardiomyopathy; NYHA, New York Heart Association; CHF, Congestive Heart Failure; DCM, Dilated Cardiomyopathy; MLHFQ, Minnesota Living With Heart Failure Questionnaire.
Although a regulatory effect on mental status can’t be observed directly in CVD patients, it can be implied in animals with other conditions. Tsyb et al first described anti-depressive effects of BM-MSCs in a brain trauma rat model through use of plus maze tests [204]. Moreover, in a Flinders sensitive line (FSL) depression rat model, left lateral ventricle injection of BM-MSCs significantly improved performance in forced swim tests (FST) and dominant-submissive relationship (DSR) tests [32]. Similar results were also generated in a subarachnoid hemorrhage (SAH) rat model where depressive behaviors, which were assessed by sucrose preference test (SPT), were reversed by BM-MSC [205]. Additionally, anti-depressive effects of BM-MSCs were further confirmed in traumatic brain injury (TBI) rodent models by two separate research groups [206,207]. However, it should be particularly noticed that the anti-depressive effects of BM-MSC may rely heavily on the methodology adopted. Coquery et al have demonstrated that although intrahippocampal transplantation of BM-MSCs promoted neural plasticity, it failed to rescue depressive behaviours in a rat depression model [208]. Overall, we suggest that an effect on mental health is reliably observed with appropriate transplantation methods, but it is still too early to compel its clinical application in psychiatric disorders at this point.
Conclusion
CVD and mental health disorders are two types of disease that worldwide affect the largest populations. Comorbidity of these diseases leads to a significantly worse prognosis which calls for the concept of psychocardiology. From a sociological perspective, mental disorders can increase the incidence of CVD by influencing daily activities. Depressed patients are less involved in regular exercise [209], and regularly adopt unbalanced diets [210]. Besides the well-known cardiovascular risk factor, obesity, physical inactivity has also been shown to be an independent predictor of cardiac death [211,212]. In return, CVD patients are more susceptible to mental illness as a result of economic stress. Biologically, the development of psychocardiological disease is associated with dysfunctions of multiple systems, including the immune system, 5-HT metabolisms, platelet aggregation, the ANS, etc.
In this review, we summarize the ability of BM-MSC transplantation to control almost all biological aspects of psychocardiological illness (Figure 5). Pre-clinical and clinical results also suggest the effectiveness of BM-MSC transplantation in treating both CVD and mental disorders. All together, we propose that BM-MSC therapy is the most promising methodology for treatment of these interwined disorders. However, future research and trials are urgently needed as our current understanding does not match the requirements to apply BM-MSC transplantation clinically. We suggest that future research should be conducted focusing on at least 3 questions: 1) What are the effects of BM-MSCs in psychocardiological disease animal models (such as a post-MI depression model) [213]? 2) What exact mechanisms underlie the therapeutic effects seen in the above models? 3) What effects of BM-MSCs are seen in patients with CVD and mental illness in terms of both cardiovascular and behavioural performance?
Figure 5.
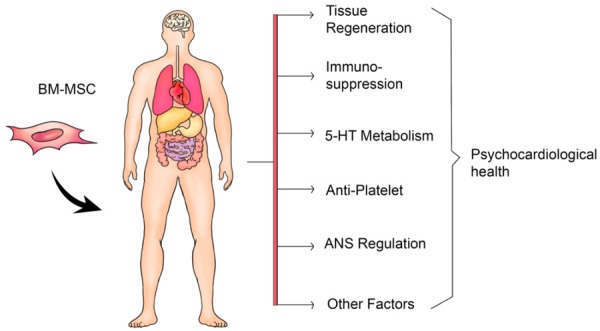
Overview of BM-MSC transplantation in the treatment of psychocardiological disease. BM-MSC exerts therapeutic effects by regenerating tissues, repressing immunological responses, regulating 5-HT biosynthesis, inhibiting platelet aggregation, balancing ANS. Abbreviations: BM-MSC, bone marrow-derived mesenchymal stem cell; 5-HT, 5-hydroxytryptamine; ANS, autonomic nervous system.
Acknowledgements
The authors thank Dr. Robert Jackson for polishing the language and Ms. Qian Zhang for the graphing.
Disclosure of conflict of interest
None.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Jefferson JW. Psychocardiology: meeting place of heart and mind. Psychosomatics. 1985;26:841–842. doi: 10.1016/S0033-3182(85)72774-0. [DOI] [PubMed] [Google Scholar]
- 4.Dodson JA, Petrone A, Gagnon DR, Tinetti ME, Krumholz HM, Gaziano JM. Incidence and determinants of traumatic intracranial bleeding among older veterans receiving warfarin for atrial fibrillation. JAMA Cardiol. 2016;1:65–72. doi: 10.1001/jamacardio.2015.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teply RM, Packard KA, White ND, Hilleman DE, DiNicolantonio JJ. Treatment of depression in patients with concomitant cardiac disease. Prog Cardiovasc Dis. 2016;58:514–528. doi: 10.1016/j.pcad.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa-Chhetri N, Fornaro M, Gallicchio D, Collantoni E, Pigato G, Favaro A, Monaco F, Kohler C, Vancampfort D, Ward PB, Gaughran F, Carvalho AF, Stubbs B. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–180. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appleton KM, Woodside JV, Arveiler D, Haas B, Amouyel P, Montaye M, Ferrieres J, Ruidavets JB, Yarnell JW, Kee F, Evans A, Bingham A, Ducimetiere P, Patterson CC PRIME study group. A role for behavior in the relationships between depression and hostility and cardiovascular disease incidence, mortality, and all-cause mortality: the prime study. Ann Behav Med. 2016;50:582–591. doi: 10.1007/s12160-016-9784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Shiga T, Omori H, Tatsumi F, Nishimura K, Hagiwara N. Depression and outcomes in Japanese outpatients with cardiovascular disease - a prospective observational study. Circ J. 2016;80:2482–2488. doi: 10.1253/circj.CJ-16-0829. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Korhonen K, Moustgaard H, Silventoinen K, Martikainen P. Pre-existing depression predicts survival in cardiovascular disease and cancer. J Epidemiol Community Health. 2018;72:617–622. doi: 10.1136/jech-2017-210206. [DOI] [PubMed] [Google Scholar]
- 10.Muller P, Lemcke H, David R. Stem cell therapy in heart diseases - cell types, mechanisms and improvement strategies. Cell Physiol Biochem. 2018;48:2607–2655. doi: 10.1159/000492704. [DOI] [PubMed] [Google Scholar]
- 11.El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, Bellusci S. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21:166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Kim T, Lemaster JE, Chen F, Li J, Jokerst JV. Photoacoustic imaging of human mesenchymal stem cells labeled with prussian blue-poly(l-lysine) nanocomplexes. ACS Nano. 2017;11:9022–9032. doi: 10.1021/acsnano.7b03519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin SS, He DQ, Luo D, Wang Y, Yu M, Guan B, Fu Y, Li ZX, Zhang T, Zhou YH, Wang CY, Liu Y. A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano. 2019;13:6581–6595. doi: 10.1021/acsnano.9b00489. [DOI] [PubMed] [Google Scholar]
- 15.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 16.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther. 2017;8:242. doi: 10.1186/s13287-017-0697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolati S, Yousefi M, Mahdipour M, Afrasiabi Rad A, Pishgahi A, Nouri M, Jodati AR. Mesenchymal stem cell and bone marrow mononuclear cell therapy for cardiomyopathy: from bench to bedside. J Cell Biochem. 2019;120:45–55. doi: 10.1002/jcb.27531. [DOI] [PubMed] [Google Scholar]
- 19.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanabdali R, Saadat A, Fazilah M, Bazli KF, Qazi RE, Khalid RS, Hasan Adli DS, Moghadamtousi SZ, Naeem N, Khan I, Salim A, Shamsuddin SA, Mohan G. Promoting effect of small molecules in cardiomyogenic and neurogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Drug Des Devel Ther. 2016;10:81–91. doi: 10.2147/DDDT.S89658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XH, Du HW, Guo XH, Wang SW, Zhou RB, Li Y, Li ZB, Zhao YS, Zhu QL. Rehmannia glutinosa oligosaccharide induces differentiation of bone marrow mesenchymal stem cells into cardiomyocyte-like cells. Genet Mol Res. 2016;15 doi: 10.4238/gmr15047795. [DOI] [PubMed] [Google Scholar]
- 22.Lee MO, Jung KB, Jo SJ, Hyun SA, Moon KS, Seo JW, Kim SH, Son MY. Modelling cardiac fibrosis using three-dimensional cardiac microtissues derived from human embryonic stem cells. J Biol Eng. 2019;13:15. doi: 10.1186/s13036-019-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szaraz P, Gratch YS, Iqbal F, Librach CL. In vitro differentiation of human mesenchymal stem cells into functional cardiomyocyte-like cells. J Vis Exp. 2017 doi: 10.3791/55757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Zheng H, Wang Y, Lian F, Hu Z, Xue S. Nesprin-1 has key roles in the process of mesenchymal stem cell differentiation into cardiomyocyte-like cells in vivo and in vitro. Mol Med Rep. 2015;11:133–142. doi: 10.3892/mmr.2014.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yannarelli G, Pacienza N, Montanari S, Santa-Cruz D, Viswanathan S, Keating A. OCT4 expression mediates partial cardiomyocyte reprogramming of mesenchymal stromal cells. PLoS One. 2017;12:e0189131. doi: 10.1371/journal.pone.0189131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding R, Jiang X, Ha Y, Wang Z, Guo J, Jiang H, Zheng S, Shen Z, Jie W. Activation of Notch1 signalling promotes multi-lineage differentiation of c-Kit(POS)/NKX2.5(POS) bone marrow stem cells: implication in stem cell translational medicine. Stem Cell Res Ther. 2015;6:91. doi: 10.1186/s13287-015-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X, Pan B, Zhou H, Liu L, Lv T, Zhu J, Huang X, Tian J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J Biomed Sci. 2017;24:29. doi: 10.1186/s12929-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Zhang T, Shan S, Wang S, Bian W, Ren T, Yang D. MiR-124 regulates transforming growth factor-beta1 induced differentiation of lung resident mesenchymal stem cells to myofibroblast by repressing Wnt/beta-catenin signaling. Dev Biol. 2019;449:115–121. doi: 10.1016/j.ydbio.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi Y, Chosa N, Sawada S, Kondo H, Yaegashi T, Ishisaki A. VEGF-C and TGF-beta reciprocally regulate mesenchymal stem cell commitment to differentiation into lymphatic endothelial or osteoblastic phenotypes. Int J Mol Med. 2016;37:1005–1013. doi: 10.3892/ijmm.2016.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu W, Li X. Vascular stem/progenitor cells: functions and signaling pathways. Cell Mol Life Sci. 2018;75:859–869. doi: 10.1007/s00018-017-2662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2010;15:1164–1175. doi: 10.1038/mp.2009.110. [DOI] [PubMed] [Google Scholar]
- 33.Cai S, Tsui YP, Tam KW, Shea GK, Chang RS, Ao Q, Shum DK, Chan YS. Directed differentiation of human bone marrow stromal cells to fate-committed schwann cells. Stem Cell Reports. 2017;9:1097–1108. doi: 10.1016/j.stemcr.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M, Taheri T, Hassoun HK. Creatine enhances transdifferentiation of bone marrow stromal cell-derived neural stem cell into GABAergic neuron-like cells characterized with differential gene expression. Mol Neurobiol. 2017;54:1978–1991. doi: 10.1007/s12035-016-9782-9. [DOI] [PubMed] [Google Scholar]
- 35.Bao C, Wang Y, Min H, Zhang M, Du X, Han R, Liu X. Combination of ginsenoside Rg1 and bone marrow mesenchymal stem cell transplantation in the treatment of cerebral ischemia reperfusion injury in rats. Cell Physiol Biochem. 2015;37:901–910. doi: 10.1159/000430217. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XM, Ma J, Sun Y, Yu BQ, Jiao ZM, Wang D, Yu MY, Li JY, Fu J. Tanshinone IIA promotes the differentiation of bone marrow mesenchymal stem cells into neuronal-like cells in a spinal cord injury model. J Transl Med. 2018;16:193. doi: 10.1186/s12967-018-1571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu Q, Zhuang H, Fan J, Wang X, Xu G. Wogonin induces retinal neuron-like differentiation of bone marrow stem cells by inhibiting Notch-1 signaling. Oncotarget. 2017;8:28431–28441. doi: 10.18632/oncotarget.16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 39.Lin R, Ding Z, Ma H, Shi H, Gao Y, Qian W, Shi W, Sun Z, Hou X, Li X. In vitro conditioned bone marrow-derived mesenchymal stem cells promote De Novo functional enteric nerve regeneration, but not through direct-transdifferentiation. Stem Cells. 2015;33:3545–3557. doi: 10.1002/stem.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heo SK, Noh EK, Gwon GD, Kim JY, Jo JC, Choi Y, Koh S, Baek JH, Min YJ, Kim H. LIGHT (TNFSF14) increases the survival and proliferation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2016;11:e0166589. doi: 10.1371/journal.pone.0166589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang T, Zhu L, Gao W, Gong M, Ren J, Yao H, Wang K, Shi D. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio. 2017;7:1722–1736. doi: 10.1002/2211-5463.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makhoul G, Jurakhan R, Jaiswal PK, Ridwan K, Li L, Selvasandran K, Duong M, Schwertani A, Cecere R. Conditioned medium of H9c2 triggers VEGF dependent angiogenesis by activation of p38/pSTAT3 pathways in placenta derived stem cells for cardiac repair. Life Sci. 2016;153:213–221. doi: 10.1016/j.lfs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Ge Q, Zhang H, Hou J, Wan L, Cheng W, Wang X, Dong D, Chen C, Xia J, Guo J, Chen X, Wu X. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol Med Rep. 2018;17:1667–1675. doi: 10.3892/mmr.2017.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Y, Wu Y, Zhao R, Zhang K, Midgley AC, Kong D, Li Z, Zhao Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials. 2019;204:13–24. doi: 10.1016/j.biomaterials.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 45.Hou J, Peng X, Wang J, Zhang H, Xia J, Ge Q, Wang X, Chen X, Wu X. Mesenchymal stem cells promote endothelial progenitor cell proliferation by secreting insulin-like growth factor-1. Mol Med Rep. 2017;16:1502–1508. doi: 10.3892/mmr.2017.6741. [DOI] [PubMed] [Google Scholar]
- 46.Cai H, Wu FY, Wang QL, Xu P, Mou FF, Shao SJ, Luo ZR, Zhu J, Xuan SS, Lu R, Guo HD. Self-assembling peptide modified with QHREDGS as a novel delivery system for mesenchymal stem cell transplantation after myocardial infarction. FASEB J. 2019;33:8306–8320. doi: 10.1096/fj.201801768RR. [DOI] [PubMed] [Google Scholar]
- 47.Luo H, Xu C, Liu Z, Yang L, Hong Y, Liu G, Zhong H, Cai X, Lin X, Chen X, Wang C, Nanwen Z, Xu W. Neural differentiation of bone marrow mesenchymal stem cells with human brain-derived neurotrophic factor gene-modified in functionalized self-assembling peptide hydrogel in vitro. J Cell Biochem. 2019;120:2828–2835. doi: 10.1002/jcb.26408. [DOI] [PubMed] [Google Scholar]
- 48.Zhang SJ, Song XY, He M, Yu SB. Effect of TGF-beta1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur Rev Med Pharmacol Sci. 2016;20:899–905. [PubMed] [Google Scholar]
- 49.Lejkowska R, Kawa MP, Pius-Sadowska E, Rogińska D, Łuczkowska K, Machaliński B, Machalińska A. Preclinical evaluation of long-term neuroprotective effects of BDNF-engineered mesenchymal stromal cells as intravitreal therapy for chronic retinal degeneration in Rd6 mutant mice. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim W, Barron DA, San Martin R, Chan KS, Tran LL, Yang F, Ressler SJ, Rowley DR. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proc Natl Acad Sci U S A. 2014;111:16389–16394. doi: 10.1073/pnas.1407097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chabot V, Dromard C, Rico A, Langonné A, Gaillard J, Guilloton F, Casteilla L, Sensebé L. Urokinase-type plasminogen activator receptor interaction with beta1 integrin is required for platelet-derived growth factor-AB-induced human mesenchymal stem/stromal cell migration. Stem Cell Res Ther. 2015;6:188. doi: 10.1186/s13287-015-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cieslik KA, Trial J, Entman ML. Mesenchymal stem cell-derived inflammatory fibroblasts promote monocyte transition into myeloid fibroblasts via an IL-6-dependent mechanism in the aging mouse heart. FASEB J. 2015;29:3160–3170. doi: 10.1096/fj.14-268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preda MB, Rosca AM, Tutuianu R, Burlacu A. Pre-stimulation with FGF-2 increases in vitro functional coupling of mesenchymal stem cells with cardiac cells. Biochem Biophys Res Commun. 2015;464:667–673. doi: 10.1016/j.bbrc.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 54.Kalomoiris S, Cicchetto AC, Lakatos K, Nolta JA, Fierro FA. Fibroblast growth factor 2 regulates high mobility group A2 expression in human bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2016;117:2128–2137. doi: 10.1002/jcb.25519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nazari M, Ni NC, Ludke A, Li SH, Guo J, Weisel RD, Li RK. Mast cells promote proliferation and migration and inhibit differentiation of mesenchymal stem cells through PDGF. J Mol Cell Cardiol. 2016;94:32–42. doi: 10.1016/j.yjmcc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Okada K, Kawao N, Yano M, Tamura Y, Kurashimo S, Okumoto K, Kojima K, Kaji H. Stromal cell-derived factor-1 mediates changes of bone marrow stem cells during the bone repair process. Am J Physiol Endocrinol Metab. 2016;310:E15–23. doi: 10.1152/ajpendo.00253.2015. [DOI] [PubMed] [Google Scholar]
- 57.Shi XF, Wang H, Xiao FJ, Yin Y, Xu QQ, Ge RL, Wang LS. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem Biophys Res Commun. 2016;470:670–677. doi: 10.1016/j.bbrc.2016.01.084. [DOI] [PubMed] [Google Scholar]
- 58.Bernardi M, Agostini F, Chieregato K, Amati E, Durante C, Rassu M, Ruggeri M, Sella S, Lombardi E, Mazzucato M, Astori G. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J Transl Med. 2017;15:90. doi: 10.1186/s12967-017-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tari K, Atashi A, Kaviani S, AkhavanRahnama M, Anbarlou A, Mossahebi-Mohammadi M. Erythropoietin induces production of hepatocyte growth factor from bone marrow mesenchymal stem cells in vitro. Biologicals. 2017;45:15–19. doi: 10.1016/j.biologicals.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Yang Z, Wu B, Jia S, Zhao Y, Hou R, Liu X, Wang X, Chen L, Yang X, Lei D, Wang L. The mechanically activated p38/MMP-2 signaling pathway promotes bone marrow mesenchymal stem cell migration in rats. Arch Oral Biol. 2017;76:55–60. doi: 10.1016/j.archoralbio.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Qiu B, Wang J, Yao Y, Wang C, Liu J. Effects of BDNF-transfected BMSCs on neural functional recovery and synaptophysin expression in rats with cerebral infarction. Mol Neurobiol. 2017;54:3813–3824. doi: 10.1007/s12035-016-9946-7. [DOI] [PubMed] [Google Scholar]
- 62.Chen R, Cai X, Liu J, Bai B, Li X. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 2018;215:31–42. doi: 10.1016/j.lfs.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 63.Iso Y, Usui S, Toyoda M, Spees JL, Umezawa A, Suzuki H. Bone marrow-derived mesenchymal stem cells inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia after arterial injury in rats. Biochem Biophys Rep. 2018;16:79–87. doi: 10.1016/j.bbrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee EJ, Hwang I, Lee JY, Park JN, Kim KC, Kim GH, Kang CM, Kim I, Lee SY, Kim HS. Hepatocyte growth factor improves the therapeutic efficacy of human bone marrow mesenchymal stem cells via RAD51. Mol Ther. 2018;26:845–859. doi: 10.1016/j.ymthe.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Li C, Tang Y, Huang Y, Cheng Q, Huang X, Zhao F, Hao C, Feng D, Xu J, Han J, Tang S, Liu W, Yue S, Luo Z. NMDA receptor activation inhibits the antifibrotic effect of BM-MSCs on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315:L404–L421. doi: 10.1152/ajplung.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makarevich PI, Dergilev KV, Tsokolaeva ZI, Boldyreva MA, Shevchenko EK, Gluhanyuk EV, Gallinger JO, Menshikov MY, Parfyonova YV. Angiogenic and pleiotropic effects of VEGF165 and HGF combined gene therapy in a rat model of myocardial infarction. PLoS One. 2018;13:e0197566. doi: 10.1371/journal.pone.0197566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sassoli C, Pierucci F, Tani A, Frati A, Chellini F, Matteini F, Vestri A, Anderloni G, Nosi D, Zecchi-Orlandini S, Meacci E. Sphingosine 1-phosphate receptor 1 is required for MMP-2 function in bone marrow mesenchymal stromal cells: implications for cytoskeleton assembly and proliferation. Stem Cells Int. 2018;2018:5034679. doi: 10.1155/2018/5034679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;310:H455–465. doi: 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adibfar A, Saleem M, Lanctot KL, Herrmann N. Potential biomarkers for depression associated with coronary artery disease: a critical review. Curr Mol Med. 2016;16:137–164. doi: 10.2174/1566524016666160126144143. [DOI] [PubMed] [Google Scholar]
- 70.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018;23:733–758. doi: 10.1007/s10741-018-9716-x. [DOI] [PubMed] [Google Scholar]
- 72.Cocco G, Jerie P, Amiet P, Pandolfi S. Inflammation in heart failure: known knowns and unknown unknowns. Expert Opin Pharmacother. 2017;18:1225–1233. doi: 10.1080/14656566.2017.1351948. [DOI] [PubMed] [Google Scholar]
- 73.Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 74.Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, Wan Q, He R, Wang Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, Que J, Gadad BS, Trivedi MH, Kelsoe JR, Lu L. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0474-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 76.García-Prieto J, Villena-Gutiérrez R, Gómez M, Bernardo E, Pun-García A, García-Lunar I, Crainiciuc G, Fernández-Jiménez R, Sreeramkumar V, Bourio-Martínez R, García-Ruiz JM, Del Valle AS, Sanz-Rosa D, Pizarro G, Fernández-Ortiz A, Hidalgo A, Fuster V, Ibanez B. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun. 2017;8:14780. doi: 10.1038/ncomms14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alboni S, Poggini S, Garofalo S, Milior G, El Hajj H, Lecours C, Girard I, Gagnon S, Boisjoly-Villeneuve S, Brunello N, Wolfer DP, Limatola C, Tremblay MÈ, Maggi L, Branchi I. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun. 2016;58:261–271. doi: 10.1016/j.bbi.2016.07.155. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 79.Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Debowska W, Mosiolek A, Waszkiewicz N, Szulc A. Effect of antidepressant treatment on peripheral inflammation markers - a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:217–226. doi: 10.1016/j.pnpbp.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 80.Ayoub KF, Pothineni NVK, Rutland J, Ding Z, Mehta JL. Immunity, inflammation, and oxidative stress in heart failure: emerging molecular targets. Cardiovasc Drugs Ther. 2017;31:593–608. doi: 10.1007/s10557-017-6752-z. [DOI] [PubMed] [Google Scholar]
- 81.Ooi BK, Chan KG, Goh BH, Yap WH. The role of natural products in targeting cardiovascular diseases via Nrf2 pathway: novel molecular mechanisms and therapeutic approaches. Front Pharmacol. 2018;9:1308. doi: 10.3389/fphar.2018.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medina-Rodriguez EM, Lowell JA, Worthen RJ, Syed SA, Beurel E. Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front Neurosci. 2018;12:547. doi: 10.3389/fnins.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu CH, Zhang GZ, Li B, Li M, Woelfer M, Walter M, Wang L. Role of inflammation in depression relapse. J Neuroinflammation. 2019;16:90. doi: 10.1186/s12974-019-1475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ries AS, Hermanns T, Poeck B, Strauss R. Serotonin modulates a depression-like state in drosophila responsive to lithium treatment. Nat Commun. 2017;8:15738. doi: 10.1038/ncomms15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneider I, Kugel H, Redlich R, Grotegerd D, Burger C, Burkner PC, Opel N, Dohm K, Zaremba D, Meinert S, Schroder N, Strassburg AM, Schwarte K, Schettler C, Ambree O, Rust S, Domschke K, Arolt V, Heindel W, Baune BT, Zhang W, Dannlowski U, Hohoff C. Association of serotonin transporter gene AluJb methylation with major depression, amygdala responsiveness, 5-HTTLPR/rs25531 polymorphism, and stress. Neuropsychopharmacology. 2018;43:1308–1316. doi: 10.1038/npp.2017.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daut RA, Fonken LK. Circadian regulation of depression: a role for serotonin. Front Neuroendocrinol. 2019;54:100746. doi: 10.1016/j.yfrne.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei YB, McCarthy M, Ren H, Carrillo-Roa T, Shekhtman T, DeModena A, Liu JJ, Leckband SG, Mors O, Rietschel M, Henigsberg N, Cattaneo A, Binder EB, Aitchison KJ, Kelsoe JR. A functional variant in the serotonin receptor 7 gene (HTR7), rs7905446, is associated with good response to SSRIs in bipolar and unipolar depression. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0397-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watts SW. Oh, the places you’ll go! My many colored serotonin (apologies to Dr. Seuss) Am J Physiol Heart Circ Physiol. 2016;311:H1225–H1233. doi: 10.1152/ajpheart.00538.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mouraret N, Houssaini A, Abid S, Quarck R, Marcos E, Parpaleix A, Gary-Bobo G, Dubois-Rande JL, Derumeaux G, Boczkowski J, Delcroix M, Blasco MA, Lipskaia L, Amsellem V, Adnot S. Role for telomerase in pulmonary hypertension. Circulation. 2015;131:742–755. doi: 10.1161/CIRCULATIONAHA.114.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selim AM, Sarswat N, Kelesidis I, Iqbal M, Chandra R, Zolty R. Plasma serotonin in heart failure: possible marker and potential treatment target. Heart Lung Circ. 2017;26:442–449. doi: 10.1016/j.hlc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Sugiura T, Dohi Y, Yamashita S, Hirowatari Y, Fujii S, Ohte N. Serotonin in peripheral blood reflects oxidative stress and plays a crucial role in atherosclerosis: novel insights toward holistic anti-atherothrombotic strategy. Atherosclerosis. 2016;246:157–160. doi: 10.1016/j.atherosclerosis.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 92.Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100–114. doi: 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:48. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nowak EC, de Vries VC, Wasiuk A, Ahonen C, Bennett KA, Le Mercier I, Ha DG, Noelle RJ. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med. 2012;209:2127–2135. doi: 10.1084/jem.20120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y, Leon-Ponte M, Pingle SC, O’Connell PJ, Ahern GP. T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta Physiol (Oxf) 2015;213:860–867. doi: 10.1111/apha.12470. [DOI] [PubMed] [Google Scholar]
- 96.Banzola I, Mengus C, Wyler S, Hudolin T, Manzella G, Chiarugi A, Boldorini R, Sais G, Schmidli TS, Chiffi G, Bachmann A, Sulser T, Spagnoli GC, Provenzano M. Expression of indoleamine 2,3-dioxygenase induced by IFN-gamma and TNF-alpha as potential biomarker of prostate cancer progression. Front Immunol. 2018;9:1051. doi: 10.3389/fimmu.2018.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vancassel S, Capuron L, Castanon N. Brain kynurenine and BH4 pathways: relevance to the pathophysiology and treatment of inflammation-driven depressive symptoms. Front Neurosci. 2018;12:499. doi: 10.3389/fnins.2018.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. 2016;14:665–673. doi: 10.2174/1570159X14666151208113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dantzer R. Role of the kynurenine metabolism pathway in inflammation-induced depression: preclinical approaches. Curr Top Behav Neurosci. 2017;31:117–138. doi: 10.1007/7854_2016_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen D, Tang P, Liu L, Wang F, Xing H, Sun L, Jiang Z. Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle. 2018;17:858–867. doi: 10.1080/15384101.2018.1442624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. Interferon-gamma-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62:2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 102.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35:766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McClain-Caldwell I, Vitale-Cross L, Mayer B, Krepuska M, Boyajian M, Myneni V, Martin D Genomics and Computational Biology Core. Marko K, Nemeth K, Mezey E. Immunogenic potential of human bone marrow mesenchymal stromal cells is enhanced by hyperthermia. Cytotherapy. 2018;20:1437–1444. doi: 10.1016/j.jcyt.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. doi: 10.1186/s13287-018-1039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Sayed M, El-Feky MA, El-Amir MI, Hasan AS, Tag-Adeen M, Urata Y, Goto S, Luo L, Yan C, Li TS. Immunomodulatory effect of mesenchymal stem cells: cell origin and cell quality variations. Mol Biol Rep. 2019;46:1157–1165. doi: 10.1007/s11033-018-04582-w. [DOI] [PubMed] [Google Scholar]
- 106.Ding J, Chen B, Lv T, Liu X, Fu X, Wang Q, Yan L, Kang N, Cao Y, Xiao R. Bone marrow mesenchymal stem cell-based engineered cartilage ameliorates polyglycolic acid/polylactic acid scaffold-induced inflammation through M2 polarization of macrophages in a pig model. Stem Cells Transl Med. 2016;5:1079–1089. doi: 10.5966/sctm.2015-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Munir H, Luu NT, Clarke LS, Nash GB, McGettrick HM. Comparative ability of mesenchymal stromal cells from different tissues to limit neutrophil recruitment to inflamed endothelium. PLoS One. 2016;11:e0155161. doi: 10.1371/journal.pone.0155161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J, Kuwabara A, Kamio Y, Hu S, Park J, Hashimoto T, Lee JW. Human mesenchymal stem cell-derived microvesicles prevent the rupture of intracranial aneurysm in part by suppression of mast cell activation via a PGE2-dependent mechanism. Stem Cells. 2016;34:2943–2955. doi: 10.1002/stem.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Loisel S, Dulong J, Menard C, Renoud ML, Meziere N, Isabelle B, Latour M, Bescher N, Pedeux R, Bertheuil N, Flecher E, Sensebé L, Tarte K. Brief report: proteasomal indoleamine 2,3-dioxygenase degradation reduces the immunosuppressive potential of clinical grade-mesenchymal stromal cells undergoing replicative senescence. Stem Cells. 2017;35:1431–1436. doi: 10.1002/stem.2580. [DOI] [PubMed] [Google Scholar]
- 110.Qu M, Yuan X, Liu D, Ma Y, Zhu J, Cui J, Yu M, Li C, Guo D. Bone marrow-derived mesenchymal stem cells attenuate immune-mediated liver injury and compromise virus control during acute hepatitis B virus infection in mice. Stem Cells Dev. 2017;26:818–827. doi: 10.1089/scd.2016.0348. [DOI] [PubMed] [Google Scholar]
- 111.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 112.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 113.Bacskai I, Mázló A, Kis-Tóth K, Szabó A, Panyi G, Sarkadi B, Apáti Á, Rajnavölgyi É. Mesenchymal stromal cell-like cells set the balance of stimulatory and inhibitory signals in monocyte-derived dendritic cells. Stem Cells Dev. 2015;24:1805–1816. doi: 10.1089/scd.2014.0509. [DOI] [PubMed] [Google Scholar]
- 114.Laranjeira P, Gomes J, Pedreiro S, Pedrosa M, Martinho A, Antunes B, Ribeiro T, Santos F, Domingues R, Abecasis M, Trindade H, Paiva A. Human bone marrow-derived mesenchymal stromal cells differentially inhibit cytokine production by peripheral blood monocytes subpopulations and myeloid dendritic cells. Stem Cells Int. 2015;2015:819084. doi: 10.1155/2015/819084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59:325–333. doi: 10.1007/s00125-015-3808-0. [DOI] [PubMed] [Google Scholar]
- 116.Dong L, Chen X, Shao H, Bai L, Li X, Zhang X. Mesenchymal stem cells inhibited dendritic cells via the regulation of STAT1 and STAT6 phosphorylation in experimental autoimmune uveitis. Curr Mol Med. 2018;17:478–487. doi: 10.2174/1566524018666180207155614. [DOI] [PubMed] [Google Scholar]
- 117.Rozenberg A, Rezk A, Boivin MN, Darlington PJ, Nyirenda M, Li R, Jalili F, Winer R, Artsy EA, Uccelli A, Reese JS, Planchon SM, Cohen JA, Bar-Or A. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl Med. 2016;5:1506–1514. doi: 10.5966/sctm.2015-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reis M, Mavin E, Nicholson L, Green K, Dickinson AM, Wang XN. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front Immunol. 2018;9:2538. doi: 10.3389/fimmu.2018.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma H, Zhang S, Xu Y, Zhang R, Zhang X. Analysis of differentially expressed microRNA of TNF-alpha-stimulated mesenchymal stem cells and exosomes from their culture supernatant. Arch Med Sci. 2018;14:1102–1111. doi: 10.5114/aoms.2017.70878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ali EHA, Ahmed-Farid OA, Osman AAE. Bone marrow-derived mesenchymal stem cells ameliorate sodium nitrite-induced hypoxic brain injury in a rat model. Neural Regen Res. 2017;12:1990–1999. doi: 10.4103/1673-5374.221155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pinkas A, Turgeman G, Tayeb S, Yanai J. An avian model for ascertaining the mechanisms of organophosphate neuroteratogenicity and its therapy with mesenchymal stem cell transplantation. Neurotoxicol Teratol. 2015;50:73–81. doi: 10.1016/j.ntt.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Spiliopoulos S, Pastromas G. Current status of high on-treatment platelet reactivity in patients with coronary or peripheral arterial disease: mechanisms, evaluation and clinical implications. World J Cardiol. 2015;7:912–921. doi: 10.4330/wjc.v7.i12.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zahn D, Petrak F, Franke L, Hägele AK, Juckel G, Lederbogen F, Neubauer H, Norra C, Uhl I, Herpertz S. Cortisol, platelet serotonin content, and platelet activity in patients with major depression and type 2 diabetes: an exploratory investigation. Psychosom Med. 2015;77:145–155. doi: 10.1097/PSY.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 124.Can MM, Guler G, Guler E, Ozveren O, Turan B, DiNicolantinio JJ, Kipshidze N, Serebruany V. Enhanced platelet reactivity in pediatric depression: an observational study. Blood Coagul Fibrinolysis. 2015;26:731–735. doi: 10.1097/MBC.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 125.Ormonde do Carmo MB, Mendes-Ribeiro AC, Matsuura C, Pinto VL, Mury WV, Pinto NO, Moss MB, Ferraz MR, Brunini TM. Major depression induces oxidative stress and platelet hyperaggregability. J Psychiatr Res. 2015;61:19–24. doi: 10.1016/j.jpsychires.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 126.Mazereeuw G, Herrmann N, Xu H, Blanchard AP, Figeys D, Oh PI, Bennett SA, Lanctôt KL. Platelet activating factors are associated with depressive symptoms in coronary artery disease patients: a hypothesis-generating study. Neuropsychiatr Dis Treat. 2015;11:2309–2314. doi: 10.2147/NDT.S87111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ozturk HM, Ozturk S, Yetkin E. Linkage between cardiovascular diseases and major depression: contribution of platelet cells. Psychiatry Res. 2017 doi: 10.1016/j.psychres.2017.11.079. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 128.Bromberger T, Klapproth S, Rohwedder I, Zhu L, Mittmann L, Reichel CA, Sperandio M, Qin J, Moser M. Direct Rap1/Talin1 interaction regulates platelet and neutrophil integrin activity in mice. Blood. 2018;132:2754–2762. doi: 10.1182/blood-2018-04-846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laffont B, Corduan A, Rousseau M, Duchez AC, Lee CH, Boilard E, Provost P. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost. 2016;115:311–323. doi: 10.1160/TH15-05-0389. [DOI] [PubMed] [Google Scholar]
- 130.Okubo K, Kurosawa M, Kamiya M, Urano Y, Suzuki A, Yamamoto K, Hase K, Homma K, Sasaki J, Miyauchi H, Hoshino T, Hayashi M, Mayadas TN, Hirahashi J. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med. 2018;24:232–238. doi: 10.1038/nm.4462. [DOI] [PubMed] [Google Scholar]
- 131.Mauler M, Herr N, Schoenichen C, Witsch T, Marchini T, Hardtner C, Koentges C, Kienle K, Ollivier V, Schell M, Dorner L, Wippel C, Stallmann D, Normann C, Bugger H, Walther P, Wolf D, Ahrens I, Lammermann T, Ho-Tin-Noé B, Ley K, Bode C, Hilgendorf I, Duerschmied D. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation. 2019;139:918–931. doi: 10.1161/CIRCULATIONAHA.118.033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mammadova-Bach E, Mauler M, Braun A, Duerschmied D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets. 2018;29:541–548. doi: 10.1080/09537104.2018.1478072. [DOI] [PubMed] [Google Scholar]
- 133.Williams MS, Ziegelstein RC, McCann UD, Gould NF, Ashvetiya T, Vaidya D. Platelet serotonin signaling in patients with cardiovascular disease and comorbid depression. Psychosom Med. 2019;81:352–362. doi: 10.1097/PSY.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peitl V, Vidrih B, Karlović Z, Getaldić B, Peitl M, Karlović D. Platelet serotonin concentration and depressive symptoms in patients with schizophrenia. Psychiatry Res. 2016;239:105–110. doi: 10.1016/j.psychres.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Williams MS, Ziegelstein RC, McCann UD, Gould NF, Ashvetiya T, Vaidya D. Platelet serotonin signaling in patients with cardiovascular disease and comorbid depression. Psychosom Med. 2019;81:352–362. doi: 10.1097/PSY.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flock A, Zobel A, Bauriedel G, Tuleta I, Hammerstingl C, Hofels S, Schuhmacher A, Maier W, Nickenig G, Skowasch D. Antiplatelet effects of antidepressant treatment: a randomized comparison between escitalopram and nortriptyline. Thromb Res. 2010;126:e83–87. doi: 10.1016/j.thromres.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 137.Dawood T, Barton DA, Lambert EA, Eikelis N, Lambert GW. Examining endothelial function and platelet reactivity in patients with depression before and after SSRI therapy. Front Psychiatry. 2016;7:18. doi: 10.3389/fpsyt.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aleksovski B, Neceva V, Vujovic V, Manusheva N, Rendevski V, Novotni A, Filipce A, Spasovska A, Sofijanova A, Aleksovski V, Gjorgoski I. SSRI-reduced platelet reactivity in non-responding patients with life-long recurrent depressive disorder: detection and involved mechanisms. Thromb Res. 2018;165:24–32. doi: 10.1016/j.thromres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 139.Coupland C, Hill T, Morriss R, Moore M, Arthur A, Hippisley-Cox J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ. 2016;352:i1350. doi: 10.1136/bmj.i1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iasella CJ, Kreider MS, Huang L, Coons JC, Stevenson JM. Effect of selective serotonin reuptake inhibitors on cardiovascular outcomes after percutaneous coronary intervention: a retrospective cohort study. Clin Drug Investig. 2019;39:543–551. doi: 10.1007/s40261-019-00776-7. [DOI] [PubMed] [Google Scholar]
- 141.Netsch P, Elvers-Hornung S, Uhlig S, Klüter H, Huck V, Kirschhöfer F, Brenner-Weiß G, Janetzko K, Solz H, Wuchter P, Bugert P, Bieback K. Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine. Stem Cell Res Ther. 2018;9:184. doi: 10.1186/s13287-018-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sheriff L, Alanazi A, Ward LSC, Ward C, Munir H, Rayes J, Alassiri M, Watson SP, Newsome PN, Rainger GE, Kalia N, Frampton J, McGettrick HM, Nash GB. Origin-specific adhesive interactions of mesenchymal stem cells with platelets influence their behavior after infusion. Stem Cells. 2018;36:1062–1074. doi: 10.1002/stem.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol. 2017;2:278–284. doi: 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuo TB, Lai CT, Chen CY, Lee GS, Yang CC. Unstable sleep and higher sympathetic activity during late-sleep periods of rats: implication for late-sleep-related higher cardiovascular events. J Sleep Res. 2013;22:108–118. doi: 10.1111/j.1365-2869.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto H, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kondo T, Ozaki T, Seo M, Sato Y, Ikeda I, Fukuhara E, Abe M, Nakamura J, Fukunami M. Prediction of sudden cardiac death in patients with chronic heart failure by regional washout rate in cardiac MIBG SPECT imaging. J Nucl Cardiol. 2019;26:109–117. doi: 10.1007/s12350-017-0913-0. [DOI] [PubMed] [Google Scholar]
- 146.Rizas KD, McNitt S, Hamm W, Massberg S, Kääb S, Zareba W, Couderc JP, Bauer A. Prediction of sudden and non-sudden cardiac death in post-infarction patients with reduced left ventricular ejection fraction by periodic repolarization dynamics: MADIT-II substudy. Eur Heart J. 2017;38:2110–2118. doi: 10.1093/eurheartj/ehx161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN Val-HeFT Investigators. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 148.Murck H, Braunisch MC, Konrad C, Jezova D, Kircher T. Markers of mineralocorticoid receptor function: changes over time and relationship to response in patients with major depression. Int Clin Psychopharmacol. 2019;34:18–26. doi: 10.1097/YIC.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 149.Kofman YB, Eng ZE, Busse D, Godkin S, Campos B, Sandman CA, Wing D, Yim IS. Cortisol reactivity and depressive symptoms in pregnancy: the moderating role of perceived social support and neuroticism. Biol Psychol. 2019 doi: 10.1016/j.biopsycho.2019.01.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jia Y, Liu L, Sheng C, Cheng Z, Cui L, Li M, Zhao Y, Shi T, Yau TO, Li F, Chen L. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J Nerv Ment Dis. 2019;207:271–276. doi: 10.1097/NMD.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 151.Munoz MJ, Kumar RG, Oh BM, Conley YP, Wang Z, Failla MD, Wagner AK. Cerebrospinal fluid cortisol mediates brain-derived neurotrophic factor relationships to mortality after severe TBI: a prospective cohort study. Front Mol Neurosci. 2017;10:44. doi: 10.3389/fnmol.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pilar-Cuéllar F, Vidal R, Díaz Á, Garro-Martínez E, Linge R, Castro E, Haberzettl R, Fink H, Bert B, Brosda J, Romero B, Crespo-Facorro B, Pazos Á. Enhanced stress response in 5-HT1AR overexpressing mice: altered HPA function and hippocampal long-term potentiation. ACS Chem Neurosci. 2017;8:2393–2401. doi: 10.1021/acschemneuro.7b00156. [DOI] [PubMed] [Google Scholar]
- 153.Prabhakar V, Gupta D, Kanade P, Radhakrishnan M. Diabetes-associated depression: the serotonergic system as a novel multifunctional target. Indian J Pharmacol. 2015;47:4–10. doi: 10.4103/0253-7613.150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sorenson AN, Sullivan EC, Mendoza SP, Capitanio JP, Higley JD. Serotonin transporter genotype modulates HPA axis output during stress: effect of stress, dexamethasone test and ACTH challenge. Transl Dev Psychiatry. 2013;1:21130. doi: 10.3402/tdp.v1i0.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schepers R, Keulers EH, Markus CR. Effects of 5-HTTLPR genotype and cognitive rumination on long-term cortisol reactivity measured in human hair. Stress. 2019;22:221–227. doi: 10.1080/10253890.2018.1553945. [DOI] [PubMed] [Google Scholar]
- 156.Alexander N, Illius S, Stalder T, Wankerl M, Muehlhan M, Kirschbaum C. Serotonin transporter gene methylation predicts long-term cortisol concentrations in hair. Psychoneuroendocrinology. 2019;106:179–182. doi: 10.1016/j.psyneuen.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 157.Jensen JB, Jessop DS, Harbuz MS, Mork A, Sánchez C, Mikkelsen JD. Acute and long-term treatments with the selective serotonin reuptake inhibitor citalopram modulate the HPA axis activity at different levels in male rats. J Neuroendocrinol. 1999;11:465–471. doi: 10.1046/j.1365-2826.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 158.Majidi J, Kosari-Nasab M, Salari AA. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res Bull. 2016;120:1–13. doi: 10.1016/j.brainresbull.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 159.Straub RH, Detert J, Dziurla R, Fietze I, Loeschmann PA, Burmester GR, Buttgereit F. Inflammation is an important covariate for the crosstalk of sleep and the HPA axis in rheumatoid arthritis. Neuroimmunomodulation. 2017;24:11–20. doi: 10.1159/000475714. [DOI] [PubMed] [Google Scholar]
- 160.Osborne S, Biaggi A, Chua TE, Du Preez A, Hazelgrove K, Nikkheslat N, Previti G, Zunszain PA, Conroy S, Pariante CM. Antenatal depression programs cortisol stress reactivity in offspring through increased maternal inflammation and cortisol in pregnancy: the psychiatry research and motherhood - depression (PRAM-D) study. Psychoneuroendocrinology. 2018;98:211–221. doi: 10.1016/j.psyneuen.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karu N, McKercher C, Nichols DS, Davies N, Shellie RA, Hilder EF, Jose MD. Tryptophan metabolism, its relation to inflammation and stress markers and association with psychological and cognitive functioning: tasmanian chronic kidney disease pilot study. BMC Nephrol. 2016;17:171. doi: 10.1186/s12882-016-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martins CS, Elias D, Colli LM, Couri CE, Souza MC, Moreira AC, Foss MC, Elias LL, de Castro M. HPA axis dysregulation, NR3C1 polymorphisms and glucocorticoid receptor isoforms imbalance in metabolic syndrome. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2842. [DOI] [PubMed] [Google Scholar]
- 163.Henriquez AR, Snow SJ, Schladweiler MC, Miller CN, Dye JA, Ledbetter AD, Richards JE, Mauge-Lewis K, McGee MA, Kodavanti UP. Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation. Toxicol Appl Pharmacol. 2018;339:161–171. doi: 10.1016/j.taap.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao XJ, Zhao Z, Yang DD, Cao LL, Zhang L, Ji J, Gu J, Huang JY, Sun XL. Activation of ATP-sensitive potassium channel by iptakalim normalizes stress-induced HPA axis disorder and depressive behaviour by alleviating inflammation and oxidative stress in mouse hypothalamus. Brain Res Bull. 2017;130:146–155. doi: 10.1016/j.brainresbull.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 165.Świątkowska-Stodulska R, Mital A, Wiśniewski P, Babińska A, Skibowska-Bielińska A, Sworczak K. Assessment of platelet function in endogenous hypercortisolism. Endokrynol Pol. 2015;66:207–213. doi: 10.5603/EP.2015.0014. [DOI] [PubMed] [Google Scholar]
- 166.Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F. A meta-analysis of heart rate variability in major depression. Psychol Med. 2019;49:1948–1957. doi: 10.1017/S0033291719001351. [DOI] [PubMed] [Google Scholar]
- 167.Quintana DS, Westlye LT, Kaufmann T, Rustan OG, Brandt CL, Haatveit B, Steen NE, Andreassen OA. Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatr Scand. 2016;133:44–52. doi: 10.1111/acps.12498. [DOI] [PubMed] [Google Scholar]
- 168.O’Neil A, Taylor CB, Hare DL, Thomas E, Toukhsati SR, Oldroyd J, Scovelle AJ, Oldenburg B. The relationship between phobic anxiety and 2-year readmission after acute coronary syndrome: what is the role of heart rate variability? J Affect Disord. 2019;247:73–80. doi: 10.1016/j.jad.2018.12.078. [DOI] [PubMed] [Google Scholar]
- 169.Sessa F, Anna V, Messina G, Cibelli G, Monda V, Marsala G, Ruberto M, Biondi A, Cascio O, Bertozzi G, Pisanelli D, Maglietta F, Messina A, Mollica MP, Salerno M. Heart rate variability as predictive factor for sudden cardiac death. Aging (Albany NY) 2018;10:166–177. doi: 10.18632/aging.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Medenwald D, Swenne CA, Loppnow H, Kors JA, Pietzner D, Tiller D, Thiery J, Nuding S, Greiser KH, Haerting J, Werdan K, Kluttig A. Prognostic relevance of the interaction between short-term, metronome-paced heart rate variability, and inflammation: results from the population-based CARLA cohort study. Europace. 2017;19:110–118. doi: 10.1093/europace/euv333. [DOI] [PubMed] [Google Scholar]
- 171.Wilkowska A, Rynkiewicz A, Wdowczyk J, Landowski J, Cubala WJ. Heart rate variability and incidence of depression during the first six months following first myocardial infarction. Neuropsychiatr Dis Treat. 2019;15:1951–1956. doi: 10.2147/NDT.S212528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barth Z, Nomeland Witczak B, Schwartz T, Gjesdal K, Flato B, Koller A, Sanner H, Sjaastad I. In juvenile dermatomyositis, heart rate variability is reduced, and associated with both cardiac dysfunction and markers of inflammation: a cross-sectional study median 13.5 years after symptom onset. Rheumatology (Oxford) 2016;55:535–543. doi: 10.1093/rheumatology/kev376. [DOI] [PubMed] [Google Scholar]
- 173.Kemp AH, Fráguas R, Brunoni AR, Bittencourt MS, Nunes MA, Dantas EM, Andreão RV, Mill JG, Ribeiro AL, Koenig J, Thayer JF, Benseñor IM, Lotufo PA. Differential associations of specific selective serotonin reuptake inhibitors with resting-state heart rate and heart rate variability: implications for health and well-being. Psychosom Med. 2016;78:810–818. doi: 10.1097/PSY.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 174.Na Kim H, Yeol Kim D, Hee Oh S, Sook Kim H, Suk Kim K, Hyu Lee P. Feasibility and efficacy of intra-arterial administration of mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy. Stem Cells Transl Med. 2017;6:1424–1433. doi: 10.1002/sctm.16-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Monnerat-Cahli G, Trentin-Sonoda M, Guerra B, Manso G, Ferreira AC, Silva DL, Coutinho DC, Carneiro-Ramos MS, Rodrigues DC, Cabral-da-Silva MC, Goldenberg RC, Nascimento JH, Campos de Carvalho AC, Medei E. Bone marrow mesenchymal stromal cells rescue cardiac function in streptozotocin-induced diabetic rats. Int J Cardiol. 2014;171:199–208. doi: 10.1016/j.ijcard.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 176.Yu Q, Li Q, Na R, Li X, Liu B, Meng L, Liutong H, Fang W, Zhu N, Zheng X. Impact of repeated intravenous bone marrow mesenchymal stem cells infusion on myocardial collagen network remodeling in a rat model of doxorubicin-induced dilated cardiomyopathy. Mol Cell Biochem. 2014;387:279–285. doi: 10.1007/s11010-013-1894-1. [DOI] [PubMed] [Google Scholar]
- 177.de Morais Sdel B, da Silva LE, Lataro RM, Silva CA, de Oliveira LF, de Carvalho EE, Simões MV, da Silva Meirelles L, Fazan R Jr, Salgado HC. Mesenchymal stem cells improve heart rate variability and baroreflex sensitivity in rats with chronic heart failure. Stem Cells Dev. 2015;24:2181–2192. doi: 10.1089/scd.2014.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramireddy A, Brodt CR, Mendizabal AM, DiFede DL, Healy C, Goyal V, Alansari Y, Coffey JO, Viles-Gonzalez JF, Heldman AW, Goldberger JJ, Myerburg RJ, Hare JM, Mitrani RD. Effects of transendocardial stem cell injection on ventricular proarrhythmia in patients with ischemic cardiomyopathy: results from the POSEIDON and TAC-HFT trials. Stem Cells Transl Med. 2017;6:1366–1372. doi: 10.1002/sctm.16-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peng S, Li W, Lv L, Zhang Z, Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med. 2018;26:127–136. [PubMed] [Google Scholar]
- 180.Shen Z, Zhu J, Yuan Y, Ren L, Qian M, Lin M, Cai M, Zhang Z, Shen X. The roles of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) in predicting treatment remission in a Chinese Han population with generalized anxiety disorder. Psychiatry Res. 2019;271:319–324. doi: 10.1016/j.psychres.2018.08.111. [DOI] [PubMed] [Google Scholar]
- 181.Zhu G, Sun X, Yang Y, Du Y, Lin Y, Xiang J, Zhou N. Reduction of BDNF results in GABAergic neuroplasticity dysfunction and contributes to late-life anxiety disorder. Behav Neurosci. 2019;133:212–224. doi: 10.1037/bne0000301. [DOI] [PubMed] [Google Scholar]
- 182.Ryan KM, Dunne R, McLoughlin DM. BDNF plasma levels and genotype in depression and the response to electroconvulsive therapy. Brain Stimul. 2018;11:1123–1131. doi: 10.1016/j.brs.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 183.Yu H, Lv D, Shen M, Zhang Y, Zhou D, Chen Z, Wang C. BDNF mediates the protective effects of scopolamine in reserpine-induced depression-like behaviors via up-regulation of 5-HTT and TPH1. Psychiatry Res. 2019;271:328–334. doi: 10.1016/j.psychres.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 184.Pius-Sadowska E, Machalinski B. BDNF - a key player in cardiovascular system. J Mol Cell Cardiol. 2017;110:54–60. doi: 10.1016/j.yjmcc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 185.Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, Doligez N, Rivat C, Do KQ, Bernard C, Benoliel JJ, Becker C. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 2017;22:1701–1713. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- 186.Fontana J, Zima M, Vetvicka V. Biological markers of oxidative stress in cardiovascular diseases: after so many studies, what do we know? Immunol Invest. 2018;47:823–843. doi: 10.1080/08820139.2018.1523925. [DOI] [PubMed] [Google Scholar]
- 187.Xuan Y, Gao X, Holleczek B, Brenner H, Schottker B. Prediction of myocardial infarction, stroke and cardiovascular mortality with urinary biomarkers of oxidative stress: results from a large cohort study. Int J Cardiol. 2018;273:223–229. doi: 10.1016/j.ijcard.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 188.Ahmed ESA, Ahmed NH, Medhat AM, Said UZ, Rashed LA, Abdel Ghaffar ARB. Mesenchymal stem cells targeting PI3K/AKT pathway in leukemic model. Tumour Biol. 2019;41:1010428319846803. doi: 10.1177/1010428319846803. [DOI] [PubMed] [Google Scholar]
- 189.Laporte C, Tubbs E, Cristante J, Gauchez AS, Pesenti S, Lamarche F, Cottet-Rousselle C, Garrel C, Moisan A, Moulis JM, Fontaine E, Benhamou PY, Lablanche S. Human mesenchymal stem cells improve rat islet functionality under cytokine stress with combined upregulation of heme oxygenase-1 and ferritin. Stem Cell Res Ther. 2019;10:85. doi: 10.1186/s13287-019-1190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nasibullin TR, Timasheva YR, Sadikova RI, Tuktarova IA, Erdman VV, Nikolaeva IE, Sabo J, Kruzliak P, Mustafina OE. Genotype/allelic combinations as potential predictors of myocardial infarction. Mol Biol Rep. 2016;43:11–16. doi: 10.1007/s11033-015-3933-3. [DOI] [PubMed] [Google Scholar]
- 191.Ching-López A, Cervilla J, Rivera M, Molina E, McKenney K, Ruiz-Perez I, Rodriguez-Barranco M, Gutiérrez B. Epidemiological support for genetic variability at hypothalamic-pituitary-adrenal axis and serotonergic system as risk factors for major depression. Neuropsychiatr Dis Treat. 2015;11:2743–2754. doi: 10.2147/NDT.S90369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jiang R, Babyak MA, Brummett BH, Hauser ER, Shah SH, Becker RC, Siegler IC, Singh A, Haynes C, Chryst-Ladd M, Craig DM, Williams RB. Brain-derived neurotrophic factor rs6265 (Val66Met) polymorphism is associated with disease severity and incidence of cardiovascular events in a patient cohort. Am Heart J. 2017;190:40–45. doi: 10.1016/j.ahj.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim MJ, Avinun R, Knodt AR, Radtke SR, Hariri AR. Neurogenetic plasticity and sex influence the link between corticolimbic structural connectivity and trait anxiety. Sci Rep. 2017;7:10959. doi: 10.1038/s41598-017-11497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schosser A, Carlberg L, Calati R, Serretti A, Massat I, Spindelegger C, Linotte S, Mendlewicz J, Souery D, Zohar J, Montgomery S, Kasper S. The impact of BDNF polymorphisms on suicidality in treatment-resistant major depressive disorder: a european multicenter study. Int J Neuropsychopharmacol. 2017;20:782–787. doi: 10.1093/ijnp/pyx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Poglajen G, Zemljic G, Frljak S, Cerar A, Andročec V, Sever M, Černelč P. Stem cell therapy in patients with chronic nonischemic heart failure. Stem Cells Int. 2018;2018:6487812. doi: 10.1155/2018/6487812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ward MR, Abadeh A, Connelly KA. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Transl Med. 2018;7:543–550. doi: 10.1002/sctm.17-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chullikana A, Majumdar AS, Gottipamula S, Krishnamurthy S, Kumar AS, Prakash VS, Gupta PK. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17:250–261. doi: 10.1016/j.jcyt.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 198.Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sorensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur Heart J. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 199.Guijarro D, Lebrin M, Lairez O, Bourin P, Piriou N, Pozzo J, Lande G, Berry M, Le Tourneau T, Cussac D, Sensebe L, Gross F, Lamirault G, Huynh A, Manrique A, Ruidavet JB, Elbaz M, Trochu JN, Parini A, Kramer S, Galinier M, Lemarchand P, Roncalli J. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: results of the MESAMI 1 pilot trial. Int J Cardiol. 2016;209:258–265. doi: 10.1016/j.ijcard.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 200.Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM, Mushtaq M, Golpanian S, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Valasaki K, Pujol MV, Ghersin E, Miki R, Delgado C, Abuzeid F, Vidro-Casiano M, Saltzman RG, DaFonseca D, Caceres LV, Ramdas KN, Mendizabal A, Heldman AW, Mitrani RD, Hare JM. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study) Circ Res. 2017;121:1279–1290. doi: 10.1161/CIRCRESAHA.117.311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro-Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G CHART Investigators. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the congestive heart failure cardiopoietic regenerative therapy (CHART-1) study. Eur J Heart Fail. 2017;19:1520–1529. doi: 10.1002/ejhf.898. [DOI] [PubMed] [Google Scholar]
- 203.Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, Wang X, Hu D. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58:238–244. doi: 10.1536/ihj.16-328. [DOI] [PubMed] [Google Scholar]
- 204.Tsyb AF, Roshal LM, Konoplyannikov AG, Souchkevitch GN, Verkhovskii YG, Shevchuk AS, Pavlova LN, Zhavoronkov LP, Semenova JB, Yuzhakov VV, Kolganova OI, Lushnikova GA, Ananyeva EP. Evaluation of psychoemotional status of rats after brain injury and systemic transplantation of mesenchymal stem cells. Bull Exp Biol Med. 2007;143:539–542. doi: 10.1007/s10517-007-0174-z. [DOI] [PubMed] [Google Scholar]
- 205.Nijboer CH, Kooijman E, van Velthoven CT, van Tilborg E, Tiebosch IA, Eijkelkamp N, Dijkhuizen RM, Kesecioglu J, Heijnen CJ. Intranasal stem cell treatment as a novel therapy for subarachnoid hemorrhage. Stem Cells Dev. 2018;27:313–325. doi: 10.1089/scd.2017.0148. [DOI] [PubMed] [Google Scholar]
- 206.Darkazalli A, Ismail AA, Abad N, Grant SC, Levenson CW. Use of human mesenchymal stem cell treatment to prevent anhedonia in a rat model of traumatic brain injury. Restor Neurol Neurosci. 2016;34:433–441. doi: 10.3233/RNN-150628. [DOI] [PubMed] [Google Scholar]
- 207.Mishra SK, Rana P, Khushu S, Gangenahalli G. Therapeutic prospective of infused allogenic cultured mesenchymal stem cells in traumatic brain injury mice: a longitudinal proton magnetic resonance spectroscopy assessment. Stem Cells Transl Med. 2017;6:316–329. doi: 10.5966/sctm.2016-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Coquery N, Blesch A, Stroh A, Fernandez-Klett F, Klein J, Winter C, Priller J. Intrahippocampal transplantation of mesenchymal stromal cells promotes neuroplasticity. Cytotherapy. 2012;14:1041–1053. doi: 10.3109/14653249.2012.694418. [DOI] [PubMed] [Google Scholar]
- 209.Zhang C, Xue Y, Zhao H, Zheng X, Zhu R, Du Y, Zheng J, Yang T. Prevalence and related influencing factors of depressive symptoms among empty-nest elderly in Shanxi, China. J Affect Disord. 2019;245:750–756. doi: 10.1016/j.jad.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 210.Gibson-Smith D, Bot M, Brouwer IA, Visser M, Penninx BWJH. Diet quality in persons with and without depressive and anxiety disorders. J Psychiatr Res. 2018;106:1–7. doi: 10.1016/j.jpsychires.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 211.Finocchiaro G, Papadakis M, Dhutia H, Cole D, Behr ER, Tome M, Sharma S, Sheppard MN. Obesity and sudden cardiac death in the young: clinical and pathological insights from a large national registry. Eur J Prev Cardiol. 2018;25:395–401. doi: 10.1177/2047487317751291. [DOI] [PubMed] [Google Scholar]
- 212.Doukky R, Mangla A, Ibrahim Z, Poulin MF, Avery E, Collado FM, Kaplan J, Richardson D, Powell LH. Impact of physical inactivity on mortality in patients with heart failure. Am J Cardiol. 2016;117:1135–1143. doi: 10.1016/j.amjcard.2015.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu M, Liu J, Zhang L, Geng Q, Ge Y. Antidepressant-like effects of ginseng fruit saponin in myocardial infarction mice. Biomed Pharmacother. 2019;115:108900. doi: 10.1016/j.biopha.2019.108900. [DOI] [PubMed] [Google Scholar]


