Abstract
CAR-T cell-based immunotherapy has shown great promise in clinical trials for the treatment of hematological malignancies. The majority of these trials utilize retroviral and lentiviral vectors to introduce CAR transgene. In spite of its satisfactory efficiency, the concerns about the potential carcinogenicity and complicated synthesis procedure restrict widespread clinical applications of viral vectors. Recent studies show that transposon-based gene transfer is a safer and simpler non-viral approach for stable transgene expression. Here, we developed an in house made polymeric nanomicelles carrier for piggyBac (PB) transposon delivery to primary T lymphocytes. The properties, transfection efficiency and toxicity of this carrier was analyzed. Results indicated that nanomicelles produced in our study were stable and reduction-sensitive. These micelles can completely condense DNA and mediate transfection with efficiency of average 30.2% with high cell viability (> 80%). Furthermore, incorporating piggyBac transposase elements into polyplexes promoted persistent expression of the transgene (up to 55%). At the end of culture, CAR-T cells mainly exhibited memory phenotype and consisted of CD3+CD8+ T cells. The cytotoxicity of these CAR-T cells was average 17% at 20:1 ratio. In conclusion, polymeric nanomicelles provide a flexible and safe method for gene delivery to T lymphocytes.
Keywords: PiggyBac transposon, polymeric nanomicelles, human T cells
Introduction
Adoptive transfer of T cells genetically engineered to express chimeric antigen receptors (CARs) is an attractive strategy for cancer immunotherapy. CAR redirects T-cell specificity to tumor-associated antigen in an MHC independent manner [1]. Impressive results have been achieved for the treatment of patients with B cell malignancies with such redirected T cells [2,3]. As CAR T-cell therapy advances to the later-phase clinical trials to be applicable for various patients, the transduction methods of T cell are under intensive debate. Common approaches to introduce CARs include viral vectors (i.e., retrovirus/lentivirus), and non-viral carriers such as DNA plasmids or mRNA [4]. Since extensive ex vivo T cell expansion is the prerequisite for adoptive cell transfer, genetic modifications must be stable and require vector integration. As such, transduction using chromosome-integrating virus vectors is superior to transient transfection of naked DNA plasmids or mRNA. In addition, the difficulties in large-scale production of pharmaceutical grade viral vectors limit their use in treating diseases, warranting the development of non-viral gene vectors [5].
Transposon system is a promising non-viral methodology for stable genetic modification. Compared to viral approach, this system is safer and simpler [6-8]. However, transposons cannot promote gene expression before they transpass cell membrane into cell nuclei. Most conventional non-viral gene delivery methods fail to efficiently transfect T lymphocytes. An electroporation system, e.g. nucleofection, has demonstrated the best efficacy so far. For example, PiggyBac transposons can mediate stable gene expression in 20-40% of primary T-cells without selection [9]. As nucleofection is dependent on expensive device and rigid procedures, its use is limited [10]. It is expected that these drawbacks will be soon overcome with rapid progresses in material sciences and nanotechnology [11].
Increasingly, polymeric carriers have been used in biomedical research as powerful tools for drug and gene delivery. Cationic polymers carriers are more attractive because of their controllable chemical diversity and shelf stability [12]. Early carriers such as poly (l-lysine) (PLL) and polyethylenimine (PEI) were studied for gene therapy of various cancers [13,14]. Polyethylene glycol (PEG), a biocompatible polymer, acts as the shell-forming, hydrophilic block in polymeric micelles. Due to its hydrophilicity, electrical neutrality, chain flexibility, absence of functional groups and immunogenicity, PEG is widely used in designing amphiphilic block copolymers [15]. In the current study, we developed a novel stimuli-sensitive cationic nanomicelles based on the block copolymer of poly (ethylene glycol)-poly (L-aspartate-Aminoethyldisulphide-g-Heptafluorobutyric) (mPEG-P (Asp-AED-g-HFB), denoted as PAEF, to delivery efficiently and release DNA to the expected sites. The synthesized polymeric carriers were characterized in terms of particle size, zeta potential, plasmid condensation ability, and protection against nuclease degradation as well as cytotoxicity. We also investigated the ability of nanomicelles to transfer transposon plasmid encoding CAR gene in T cells, laying the groundwork for the development of a low-cost and safe transfection method for CAR-T therapy.
Materials and methods
Nanomicelle and plasmid
The block copolymer, mPEG-P (Asp-AED-g-HFB) (PAEF), was synthesized via serial reactions (Figure S1). The morphology of the blank micelles was examined using an Atomic Force Microscope (AFM). The granule diameter of the micelles was determined at 25°C using a Nano-ZSE equipment (Malvern). FDA labeled blank nanomicelle was co-cultured with jurkat cells and recorded under fluorescence microscope. The piggyBac transposon and transposase plasmids (PB513A-1, PB210PA-1) were purchased from System Biosciences and purified by standard techniques using EndoFree kits (Invitrogen). EGFRvIII CAR was cloned into transposon vector via EcoRI/XbaI restriction endonuclerase sites as previously described [16]. The sequence of EGFRvIII CAR was confirmed using DNA sequencing analysis.
Preparation of DNA-nanomicelle polyplex
The plasmid DNA (pDNA) and nanomicelle solution were mixed at different N/P ratios (the ratio between cationic polymers and DNA, w/w), and then the mixture was kept at room temperature for 30 min prior to use. The plasmid DNA was loaded into nanomicelles through electrostatic interactions.
Agarose gel electrophoresis
The DNA binding ability of nanomicelle was evaluated by agarose gel retardation assay. The complexes were prepared at various N/P ratios ranged from 2.5 to 20. In order to detect the encapsulation efficiency of the nanomicelle, the complexes were prepared at 10, 15, 20 N/P ratios and digested by EcoRI/XbaI restriction endonuclerase. The in vitro pDNA release ability of complex was also analyzed. Reducing regent DTT was utilized and incubated with complex for 1.5 h at room temperature. These samples were loaded into individual wells of 1% agarose gel and separated at 120 V for 40 min. And resulting plasmid migration pattern was revealed by ultraviolet imaging system (Bio Rad).
Measurements of polyplex size and zeta potential
The nanomicelle/pDNA complexes were prepared at various N/P ratios that ranged from 5 to 100. The amount of pDNA for each sample was 0.5 μg. The micelle size and zeta potential of each sample were conducted in triplicate at room temperature.
T cells in vitro transfection
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque gradient centrifugation (Solarbio) and monocytes were removed by adherent plastics. T cells were collected and activated utilizing anti-human CD3/CD28 beads at the 1:1 beads to cell ratio for 48 h. The beads were then removed. About 5 × 105 activated cells were counted and cultured in 250 µL transfection medium in 24-well plate until transfection. The plasmid DNA (1.5 μg CAR-transposon and 0.5 μg transposase) was first diluted in ddH2O, and then the cationic polymers were added at an optimal N/P ratio to a final volume of 50 µL. This mixture was vortexed for 12 s and incubated at room temperature for 30 min. Then, the complex solution was added to cells. Four hours later, half of the transfection medium was removed and additional 750 µL pre-warmed fresh medium containing 300 IU IL-2 (Peprotech) was supplemented. After electroporations, the transfection efficiency was evaluated by a flow cytometer (FACS canto II) at 48 h after transfection. Non-transfected cells were used for setting the auto-fluorescence baseline.
Cytotoxicity assay
T cells were prepared and treated the same as transfection assay. Forty-eight hour after gene transfection, T cells were counted and transferred to 96-well plates with 1 × 104 cells/well. CCK-8 solution (10 μL, Beyotime) was added to each well and incubated at 37°C in 5% CO2 for 4 h. The absorbance at 450 nm were obtained by Synergy H1 Hybrid Reader (BioTek, Germany) after 4 h incubation. All experiments were conducted in triplicate.
Optimization of transfection conditions
To determine the optimal cell status for transfection, non-activated or activated cells were transfected for 24 h or 48 h in different medium. The effects of cell number and plasmid dosage on transfection efficiency were also evaluated. Complex used in all experiments was prepared at the N/P ratio of 40. Percentage of positively transfected cells was detected by flow cytometry and fluorescence microscope. Cell mortality was calculated by Cellometer Auto2000 (Nexcelom Biosciences).
CAR-T cells expansion, subset and function analysis
After transfection, T cells were transferred to human recombinant EGFRvIII antigen/anti-CD28 antibody (EGFRvIII/CD28, 5 μg/mL) coated 24-well plates. Percentage of positively transfected cells was examined by flow cytometry on day 0, day 2 and day 12. To detect phenotype and subset, CAR-T cells were stained with mouse anti-human CD4 Percp, CD8 PE, CD62L PE, CCR7 Percp, CD45RO PE/Cy7 and CD45RA APC/Cy7 on day 12 (Biolegend). All samples were analyzed with canto II (BD Biosciences), and data were processed by FlowJo software. Furthermore, cytotoxicity measurements were performed using LDH-CytoxTM Assay Kit (Biolegend).
Statistical analysis
All statistical analyses in this study were performed with SPSS software (version 21.0). Transfection efficiency, key factors affecting transfection efficiency and cell phenotype were assessed from data collected from three independent experiments. Data were analyzed by Student’s t-test when comparing two sets of data. P-values less than 0.05 were considered statistically significant. Data was presented as mean ± standard deviation.
Results
Characterization of nanomicelles
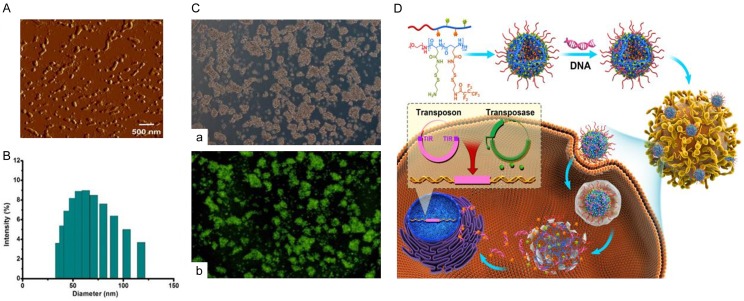
Several factors affect the physical and chemical properties of nanomicelles, and these factors subsequently impact their stability, loading ability of DNA and the transfection efficiency. In the AFM photograph (Figure 1A), a spherical and polydisperse nature of the representative nanomicelle was obvious, which might facilitate their cell uptake. The size of these nanoparticles were also measured (Figure 1B). Result indicated that the blank polymers exhibited an averaged size of 62.7 nm. Due to their unique structure, polymeric micelles can penetrate plasma membrane using endocytosis. As shown in Figure 1C, FDA labeled blank nanomicelle could be successfully uptaken by jurkat cell lines.
Figure 1.
The nanomicelle can be successfully taken up into cells by endocytosis. A. Nanomicelles exhibited spherical and polydisperse nature and recorded by AFM. B. The size distribution of the blank micelles was examined by granularity analyzer, and the average size was 62.7 nm. C. Jurkat cell lines were co-incubated with FDA labeled blank nanomicelles for 48 h. The endocytosis efficiency was detected by fluorescent microscope. a. White light. b. Fluorescence. D. Schematic outline of transferring plasmid DNA to T cells by nanocarriers. The polyplexes loaded with plasmid DNA were uptaken into cells by endocytosis. DNA was released from the polyplex under reductive environment and diffused into the nucleus.
The mechanism of plasmid DNA delivery to T cells by nanocarriers
Endocytosis and escape from endocytic vesicles are two important steps for gene delivery by nanomicelles. When nanomicelles are close to a cell, the interactions between the micelles and the cell membrane generate forces from different origins. This causes the membrane wrapping of the nanoparticles followed by cellular uptake. These interactions depend on the shape and size of nanoparticles, the biomechanical properties of the cell membrane, and the local environment of the cells. To improve DNA-release properties, a reduction-sensitive polymeric micelles by introducing disulfide bond linkages was designed. Generally, cellular redox status is regulated by reductive glutathione (GSH), which is produced intracellularly and maintained at mM concentrations in the cytosol and subcellular compartments. In extracellular matrix, however, rapid enzymatic degradation limits GSH concentrations to mM levels. Owing to the significant difference (100-fold to 1000-fold), disulfide linker can be quickly cleaved inside cells (enriched GSH), and DNA released from disassembled nanomicelles [17]. In this work, the reduction-sensitive nanomicelle was developed to achieve DNA site-specific release. DNA molecules were complexed in the interlayer of micelle by means of electrostatic interaction with amino groups which were linked to the midblock by a reducible disulfide bond. Polyplexes were formulated with an excess positive charge to promote electrostatic binding to the negative cell membrane. Highly cationic polyplexes were taken up into cells by interaction with surface proteoglycans, followed by internalization via endocytosis. The genetic material was released from polyplexes into the cytoplasm, and diffused to the nucleus for gene expression of interest (Figure 1D). Here, both piggyBac transposon and super piggyBac transposase were condensed into nanomicelle. The transposase may bind to two inverted terminal repeats (ITRs) of the transposon, and precisely cut the transgene out of the plasmid, inserting the transgene into genome of T cells.
The ability of nanomicelle to load and release DNA in vitro
The complex formation of nanomicelle with DNA was evaluated by gel retardation and encapsulation assay at various N/P ratio (Figure 2A). Condensed and encapsulated DNA by micelles remained at the top of the agarose gel at N/P ratio of 20, suggesting DNA was successfully entrapped into micelles. In contrast, unconjugated or digested DNA run into the gel as well as those with low N/P ratios due to their unstable states migrate to the positive pole. In addition, in vitro pDNA release study was also conducted in lysosome-mimicking environment with high concentration DTT at N/P = 20 (Figure 2B). Results indicated that under 10 mM DTT, pDNA was successfully released from nanopolymer as shown in lane 3.
Figure 2.
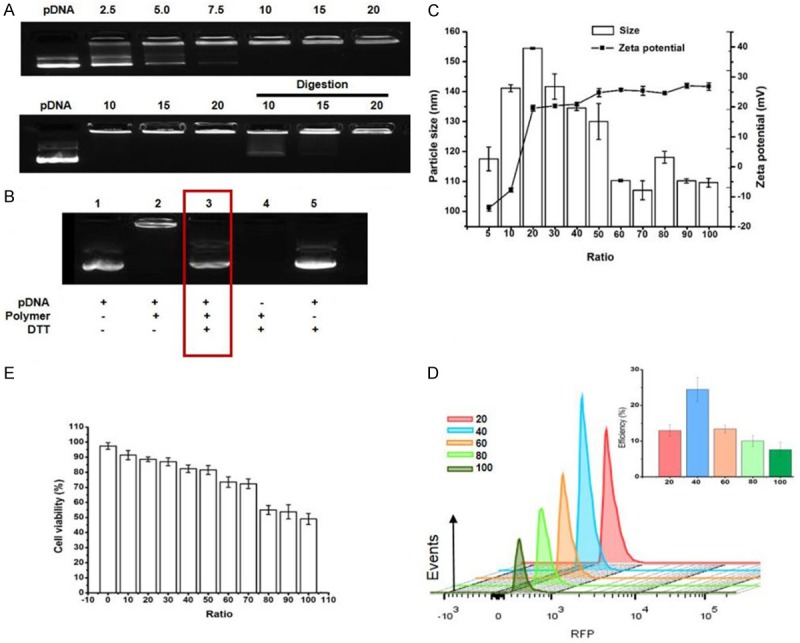
The nanomicelle can condense and site-specific release of DNA. A. The agarose gel electrophoretic was utilized to detect retardation, encapsulation of pDNA after complexing with nanopolymer at various N/P ratios. B. The plasmid was released from the polyplex after 10 mM DTT was added in lane 3 (N/P = 20). C. The zeta potential and particle size of polyplex formed at different N/P ratios was measured by granularity analyzer (n = 3). D. In vitro transfection efficiency was detected by flow cytometry at various N/P ratios (n = 3). E. Cell viability of T cells co-incubated with various complexes was detected by CCK-8 kit (n = 3). Events number was set to 10,000 in flow cytometry of the picture.
The N/P ratio may play an important role in affecting nanopolymer diameter, thus further influencing the transfection efficiency and cytotoxicity of carriers. As shown in Figure 2C, as the N/P ratios increased from 20 to 100, the zeta potential increased to +26.8 from +19.6 mV, whereas the complex size decreased to 109.6 from 154.5 nm. At the N/P ratio of 40, the polyplex showed a relatively high zeta potential (+20.9 mV) and small diameter (134.5 nm), which were considered appropriate for further biological studies.
Evaluation of transgene expression and cell viability
The transfection efficiencies varied at different N/P ratios were evaluated by flow cytometry (Figure 2D). A low N/P ratio would yield physically unstable micelle/DNA complexes which result, resulting in poor transfection efficiency due to low amounts of DNA delivery, while those too stable complexes also led to the same problem if N/P ratio was too high because DNA cannot be released. Results indicated that the micelle/pDNA complexes formed at the N/P ratio of 40 attained the highest values (24.5% ± 3.3%). To evaluate the influence of complex on cell viability, CCK-8 assay was utilized (Figure 2E). Results showed that the cytotoxicity of the complexes gradually increased with increasing N/P ratios. At the N/P ratio of 40, the viability of cells incubated with the micelle/DNA complexes was 82.3% ± 2.5%. The viability of control cells cultured without transfection was 97.5% ± 2.2%.
Optimization of key parameters for T cell transfection
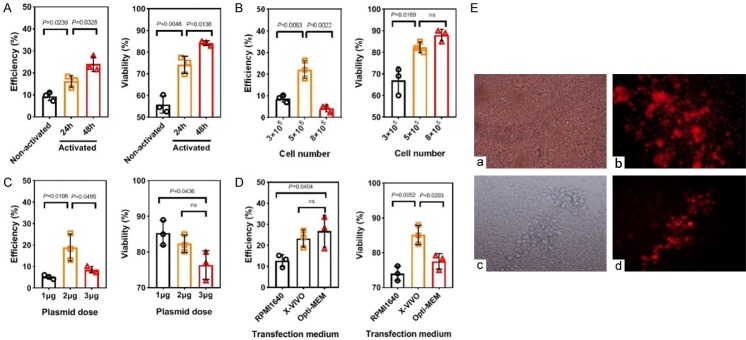
Unlike viruses, nanopolymers do not have an active mechanism for conveying DNA into the nucleus. The transfection efficiency is dependent on the cell cycle. Therefore, we first examined whether T cell activation affects transfection efficiency. Freshly isolated T cells were stimulated with anti-human CD3/CD28 beads for different time (24 h or 48 h). Unstimulated or stimulated cells (5 × 105) were transfected using total 2 μg plasmids. Results showed that transfection efficiency and viability increased along with extended activation time. PBLs stimulated for 48 h showed the highest efficiency and cell viability (Figure 3A).
Figure 3.
Activation time, cell number, plasmid input and medium are key factors affecting transfection efficiency and cytotoxicity of the polyplexes. A-D. Effects of different T cell activation time, initial cell number, plasmid input and medium on transfection and cell viability was detected by flow cytometry and cell analyzer, respectively (N/P = 40, n = 3). E. About 5 × 105 cells per well in a 24-well plate were transfected with total dose of 2 µg of DNA in X-VIVO medium 48 h after activation. Transfection efficiency was observed under this condition by fluorescence microscope. a, c. White light. b, d. Red fluorescence; a, b. × 20. c, d. × 40. Events number was set to 10,000 in flow cytometry of the picture. ns = not significant.
In addition, initial cell number and plasmid concentration are important factors affecting transfection efficiency. Therefore, we investigated the effects of cell and plasmid input during transfection. T cells stimulated for 48 h were divided into groups with different cell concentrations (3 × 105, 5 × 105 and 8 × 105 cells/well) and transfected using 2 μg of pDNA. Results indicated that higher cell densities (8 × 105) did not increase transfection efficiency and cell viability, but a little improvement was observed compared to the 3 × 105 group (Figure 3B). Activated T cells were then divided into three groups with each containing 5 × 105 cells and transfected with 1 μg, 2 μg, or 3 μg plasmids. Results showed that higher dose (3 μg) showed lower transfection efficiencies and cell viability (Figure 3C). Furthermore, serum-free medium X-VIVO was a better transfection medium, most likely due to the lower nanopolymer concentration (N/P ratio) required for polyplex delivery, thus contributed to post-transfection survival of cells (Figure 3D). Representative transfection results detected by fluorescence microscopy were shown in Figure 3E.
Evaluation of CAR-T cells transduction efficiency, phenotype and function after expansion
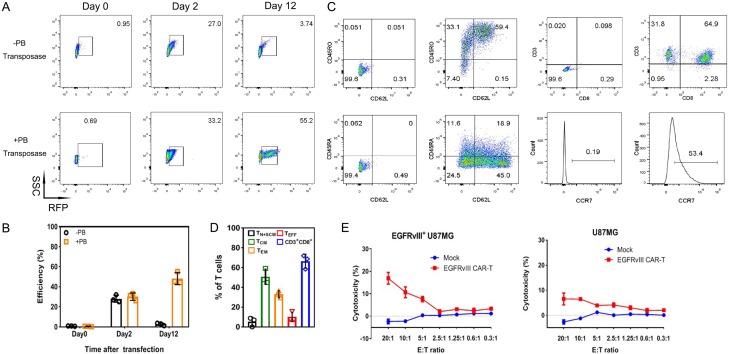
Since effective expression of CAR gene is prerequisite for adoptive cell treatment, the RFP positive cells were enriched after transfection. The CAR-transposon plasmid with or without transposase were delivered into T lymphocytes by polymer. Then, T cells were transferred to EGFRvIII/CD28 coated 24-well plates (Figure 4A and 4B). Results indicated that the expression of report gene in co-transfect group exhibited the average transfection efficiency of 30.2% on day 2, similar to T cells transfected only with transposons (28.1%). However, co-transduced lymphocytes was gradually increased and showed the maximum RFP expression (55.2%) at the end of the culture, while the expression in T cells transfected only with transposons rapidly reduced to 2.7%. These results showed that foreign gene can be successfully transfected into T lymphocytes in the absence of transposase, but not integrated in T cell genome. Transposable elements played an important role in PB transposition. Furthermore, co-transfected T cells can be enriched by human EGFRvIII antigen.
Figure 4.
T cells can be stably modified through nanocarrier with piggyBac transposon. A. Representative results of RFP-expression detection by flow cytometry. T cells only transfected with transposon were used as control (-transposase). B. Co-transfection of plasmids promoted persistent transgene expression (n = 3). C. Representative results of cell phenotype analysis by flow cytometry. D. CAR-T cells mainly exhibited memory phenotype and consisted of CD3+CD8+ T cells at the end of culture (n = 3). TN+SCM: CD45RA+CD45RO-CD62+CCR7+, TCM: CD45RA-CD45RO+CD62+CCR7+, TEM: CD45RA-CD45RO+CD62-CCR7-, TEFF: CD45RA+CD45RO-CD62-CCR7-. E. Functional CAR-T cells were produced, and cytotoxicity was measured based on LDH release. Non-transfected T cells, EGFRvIII-negative U87 cells was used as effector or target cell control, respectively. Events number was set to 10,000 in flow cytometry of the picture.
On day 12, immune phenotype of CAR-T cells was detected by flow cytometry (Figure 4C and 4D). Results indicated that T cells maintained memory phenotype after expansion. Less-differentiated cells consisted of TN, TSCM and TCM accounted for (56.2 ± 2.3)% of T cells. This cell population was critical for in vivo expansion, survival, and long-term persistence [18]. Besides, a large majority of the cultured cells were CD3+CD8+ T cell subset (66.4 ± 7.1)%, which was highly related to the cytotoxicity of T cells. To determine whether the EGFRvIII CAR-T cells can recognize EGFRvIII-positive U87 cells, a cytotoxicity detection assay based on LDH release was performed (Figure 4E). After 4 h co-culture with target cells, an enhancement in the cytotoxicity was detected as an increase in E:T ratio. The killing ability was average 17% at 20:1 ratio. No evident killing activity was found between T cells in different groups toward EGFRvIII-negative U87 cells.
Discussion
Adoptive cell therapy using chimeric antigen receptor (CAR)-engineered T cells has emerged as a very promising approach to combating cancer. T cells modified with CARs ex vivo provide both targeting specificity and T cell activation upon cancer cell recognition. To date, CAR-T therapy has generated extraordinarily clinical responses against some hematological tumors [19,20]. Most clinical trials utilize viral vectors such as retroviruses, lentiviruses and adeno-associated viruses (AAVs) to deliver CAR genes. These viruses are highly efficient and able to integrate into the host genome, resulting in permanent CAR expression. However, viral vectors have some limitations, including limited DNA carrying capacity, difficult to prepare in large scale, and high cost. Non-viral vectors have the potential to address some of these limitations.
Many studies have suggested that the piggyBac transposon system is a straightforward and safe tool for genetic modification of T cells [21,22]. PiggyBac is a highly active transposon derived from the cabbage looper moth. It mediates gene transfer through a “cut and paste” mechanism whereby the transposase integrates the transposon segment into the genome of the target cell(s) of interest [23]. Until now, piggyBac has been used to modify human T cells with a variety of purposes [24]. In this study, CAR gene expression cassette was inserted between PB transposon inverted terminal repeats. Then, transposon and hyperactive transposase were co-transfected to T cells for stable expression of transgene. In addition, implementation of gene transposition also depends on effective transfection method. Lonza Nucleofector II electroporation system was broadly used for T cell modification. However, the high cost of Lonza proprietary electroporation kits and the reliance on manufacturer reagents and devices may limit the application of this technology for large-scale experiments.
Recently, nanomaterials have been widely used as gene and drug delivery vehicles in cancer therapy [25,26]. Various nanocarriers including nanoparticles, liposomes, micelles, and dendrimers have opened up new possibilities for the genetic modification of mammalian cells. Here, a novel stimuli-sensitive cationic nanomicelle was produced, which consisted of a PEG corona to stabilize the nanoparticles, reduction-sensitive interlayer to complex DNA, and a reduction-sensitive fluorocarbon core to increase the nanomicelle’s stability to traverse the lipid bilayers of cells, and to facilitate DNA’s endosomal escape [27]. The unique structural design was expected to endow the micelle with DNA-release behavior in response to the in vivo microenvironment. In other words, the micelle should be stable in an environment with low GSH concentration (e.g. bloodstream) but disassemble to rapidly release DNA inside cell with enriched GSH (Figure 1D). Results demonstrated these blank nanomicelles showed values of positive zeta potential, suggesting that they tend to repel each other and not come together and thus representing a nice long-term stability under tested conditions. The agarose gel assay showed that the positively charged nanomicelle could thoroughly neutralize the negative charge of pDNA, which made it possible to deliver the pDNA into cells and protect plasmid DNA from enzymatic degradation. In the reductant-enriched environment (10 mM DTT), disulfide linkage in the polymer structure was broken, leading to the quick release of pDNA from the copolymer.
Cell uptake and endocytosis are key factors for the nanocarrier transfection. Usually, submicron size particles (100 nm~1.0 μm) are efficiently taken up by the cells. In addition, nanomicelles with high zeta potential attach are easier to negatively charge cell membranes but also lead to high cytotoxicity. In this study, the polyplex formed at N/P 40 possessed a relatively small size (about 134 nm) and a moderate positive charge (about +20.9 mV), which was verified to reach highest transfection efficiency of T cells with more than 80% cell viability. Therefore, polyplex formed at N/P = 40 was used in further experiment. Next, we investigated other common factors that could possibly affect T cell transfection efficiencies, including, (a) different cell physical profile and activation status, (b) the input cell number, (c) the initial amount of DNA. Under our experimental conditions, transfected 5 × 105 T cells in X-vivo medium with a total dose of 2 µg pDNA at 48 h post-activation achieved the optimal RFP expression (~33%). Although modest transfection efficiency, nanopolymer could be a useful tool for the screening experiments in the development of new chimeric antigen receptors. Having a straightforward and efficient method to test different CAR constructs instead of creating a new viral vector for each one, could significantly reduce the time. Importantly, no difference was observed in transfection efficiency on day 2 when nanomicelles were loaded with one or two plasmids, which was consistent with previous study [28]. And the flexible cargo loading of those nanomicelles makes it attractive for ex vivo gene delivery applications.
Then, purified EGFRvIII antigen was selected with the aim to specifically activate EGFRvIII CAR expressing T cells. As such, the positive ratio of foreign gene in co-transfected T cells increased to 55% at the end of culture. In addition, CAR-T cells mainly exhibited memory phenotype and consisted of cytotoxic CD8+ T cells after expansion. Thus, CAR-T cells displayed increasing cytotoxicity against EGFRvIII-positive U87 cells as E:T ratio increased.
Acknowledgements
This work was funded by National Natural Science Foundation of China (No. 81772670, 81600775 and 21504082).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Wu MR, Jusiak B, Lu TK. Engineering advanced cancer therapies with synthetic biology. Nat Rev Cancer. 2019;19:187–195. doi: 10.1038/s41568-019-0121-0. [DOI] [PubMed] [Google Scholar]
- 2.Salter AI, Pont MJ, Riddell SR. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood. 2018;131:2621–2629. doi: 10.1182/blood-2018-01-785840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipanee J, VandenDriessche T, Chuah MK. Transposons: moving forward from preclinical studies to clinical trials. Hum Gene Ther. 2017;28:1087–1104. doi: 10.1089/hum.2017.128. [DOI] [PubMed] [Google Scholar]
- 5.Lin G, Zhang H, Huang L. Smart polymeric nanoparticles for cancer gene delivery. Mol Pharm. 2015;12:314–321. doi: 10.1021/mp500656v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Canedo A, Bonamino MH. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J Transl Med. 2016;14:288. doi: 10.1186/s12967-016-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ptáčková P, Musil J, Štach M, Lesný P, Němečková Š, Král V, Fábry M, Otáhal P. A new approach to CAR T-cell gene engineering and cultivation using piggyBac transposon in the presence of IL-4, IL-7 and IL-21. Cytotherapy. 2018;20:507–520. doi: 10.1016/j.jcyt.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Saha S, Woodard LE, Charron EM, Welch RC, Rooney CM, Wilson MH. Evaluating the potential for undesired genomic effects of the piggyBac transposon system in human cells. Nucleic Acids Res. 2015;43:1770–1782. doi: 10.1093/nar/gkv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakazawa Y, Huye LE, Dotti G, Foster AE, Vera JF, Manuri PR, June CH, Rooney CM, Wilson MH. Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes. J Immunother. 2009;32:826–836. doi: 10.1097/CJI.0b013e3181ad762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Lytton-Jean AK, Chen Y, Love KT, Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman M, Peng CG, Charisse K, Borodovsky A, Manoharan M, Donahoe JS, Truelove J, Nahrendorf M, Langer R, Anderson DG. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dizaj SM, Jafari S, Khosroushahi AY. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res Lett. 2014;9:252. doi: 10.1186/1556-276X-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Nouri F, Sadeghpour H, Heidari R, Dehshahri A. Preparation, characterization, and transfection efficiency of low molecular weight polyethylenimine-based nanoparticles for delivery of the plasmid encoding CD200 gene. Int J Nanomedicine. 2017;12:5557–5569. doi: 10.2147/IJN.S140734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Li Z, Zhou T, Zhang J, Xia H, Li H, He J, He S, Wang L. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. Int J Nanomedicine. 2015;10:6027–6037. doi: 10.2147/IJN.S90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, Zhao YY, Zhao LM, Cui J, Gupta P, Wong AJ, Han SY. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol. 2013;6:33. doi: 10.1186/1756-8722-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Cheng D, Yin T, Chen W, Lin Y, Chen J, Li R, Shuai X. Copolymer of poly (ethylene glycol) and poly (L-lysine) grafting polyethylenimine through a reducible disulfide linkage for siRNA delivery. Nanoscale. 2014;6:1732–1740. doi: 10.1039/c3nr05024f. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, Liu H, Creighton CJ, Gee AP, Heslop HE, Rooney CM, Savoldo B, Dotti G. Closely related T-memory stem cells correlate with in vivo expansion of CAR. CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurgens B, Clarke NS. Evolution of CAR T-cell immunotherapy in terms of patenting activity. Nat Biotechnol. 2019;37:370–375. doi: 10.1038/s41587-019-0083-5. [DOI] [PubMed] [Google Scholar]
- 20.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao S, Jiang E, Chen S, Gu Y, Shangguan AJ, Lv T, Luo L, Yu Z. PiggyBac transposon vectors: the tools of the human gene encoding. Transl Lung Cancer Res. 2016;5:120–125. doi: 10.3978/j.issn.2218-6751.2016.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita D, Nishio N, Saito S, Tanaka M, Kawashima N, Okuno Y, Suzuki S, Matsuda K, Maeda Y, Wilson MH, Dotti G, Rooney CM, Takahashi Y, Nakazawa Y. Enhanced expression of anti-CD19 chimeric antigen receptor in piggybac transposon-engineered T cells. Mol Ther Methods Clin Dev. 2018;8:131–140. doi: 10.1016/j.omtm.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tipanee J, Chai YC, VandenDriessche T, Chuah MK. Preclinical and clinical advances in transposon-based gene therapy. Biosci Rep. 2017;37 doi: 10.1042/BSR20160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodard LE, Wilson MH. PiggyBac-ing models and new therapeutic strategies. Trends Biotechnol. 2015;33:525–533. doi: 10.1016/j.tibtech.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Kievit FM, Zhang M. Nanoparticles for cancer gene therapy: recent advances, challenges, and strategies. Pharmacol Res. 2016;114:56–66. doi: 10.1016/j.phrs.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Wang CE, Stayton PS, Pun SH, Convertine AJ. Polymer nanostructures synthesized by controlled living polymerization for tumor-targeted drug delivery. J Control Release. 2015;219:345–354. doi: 10.1016/j.jconrel.2015.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang LH, Wu DC, Xu HX, You YZ. High DNA-binding affinity and gene-transfection efficacy of bioreducible cationic nanomicelles with a fluorinated core. Angew Chem Int Ed Engl. 2016;55:755–759. doi: 10.1002/anie.201508695. [DOI] [PubMed] [Google Scholar]
- 28.Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS, Stephan MT. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.