Abstract
Nowadays, there is still no effective drug with small side effects for acute lung injury. Monomer of herb is promising for anti-inflammation therapy. In this study, we first investigated the possible effects of isochlorogenic acid A in acute lung injury induced by LPS. The effects of oxidant stress, pulmonary edema and inflammation factors induced by LPS were inhibited by isochlorogenic acid A. Levels of lung active markers and inflammation factors induced by LPS were reversed by isochlorogenic acid A. Isochlorogenic acid A inhibited the cell apoptosis induced by LPS. The protein expressions of Nf-κB/NLRP3 signaling pathway induced by LPS were inhibited by isochlorogenic acid A. In summary, isochlorogenic acid A reversed the effects induced by LPS in acute lung injury and is a promising monomer for therapy of acute lung injury, providing references for future research on therapy of acute lung injury.
Keywords: Isochlorogenic acid A, acute lung injury, LPS, inflammation, oxidant stress
Introduction
Acute lung injury as a high-risk common disease has aroused many attentions [1-4]. The clinical features of lung injury are presented as capillary leakage, progressive refractory hypoxemia, decreased dynamic lung compliance and noncardiogenic pulmonary edema [5]. Acute lung injury with the injuries of alveolar epithelial cells and capillary endothelial cells caused by various direct and indirect injury factors has a high fatality rate. It is pressing to develop new drugs for treatment of acute lung injury.
LPS exists in outer membrane of gram-negative bacteria and has simulative effect on cells which is associated with inflammation reactions. LPS caused alveolar epithelial cells injury, resulting in proinflammatory cytokines release. Therefore, acute lung injury model was usually constructed by using LPS. As reported that the number of inflammatory cells and inflammatory cytokines in bronchoalveolar lavage fluid were increased by LPS [6]. In this study, acute lung injury induced by LPS in mice was constructed as the research object.
Inflammation and ROS caused by oxidative stress are the major causes of many diseases such as diabetes, atherosclerosis and so forth. Inflammation induced by oxidative stress was identified as the critical factors of acute lung injury as well [7,8]. Many traditional Chinese medicines have efficiently anti-inflammation effects. Quercetin was reported to have anti-inflammation effect in ARPE-19 Cells [9]. Trans-Cinnamaldehyde was reported to exert anti-inflammation effect in rat model of osteoarthritis [10]. Honeysuckle as one of traditional Chinese medicine with many pharmacological functions including anti-inflammation effect, anti-oxidant and promotion of lipid and glucose metabolism has been the research hotpot [11,12]. Moreover, the components of herbs are complexed, it’s valuable to explore the active ingredient that works efficiently in specific disease. Isochlorogenic acid A (IAA) is the bioactive constituent of honeysuckle and isochlorogenic acid A is also named 3, 5-dicaffeinic quininic acid A. Whether isochlorogenic acid A as the main monomeric compound has anti-inflammation effect in acute lung injury is pending. In this study, we first investigated the effects of isochlorogenic acid A on acute lung injury induced by LPS and the possible mechanism within it.
Material and method
Animals and treatment
BALB/C mice were purchased from animal experiment center and the mice were housed in the environment at 23±2°C with humidity of 55±5%. All the mice were given free access to food and water. The mice (n=10 per group) were randomly divided into six groups including control group, IAA group, LPS treatment group and LPS induced group pretreated with different concentrations of IAA. After the mice were anesthetized using sodium pentobarbital, LPS (5 mg/kg) was injected into the mice. IAA was injected into abdominal cavity of the mice by pretreatment with the concentration of 5 mg, 10 mg, 20 mg. The mice were sacrificed by cervical dislocation and the tissues of lung were surgically exposed. Part of the blood samples were centrifugated for 10 min to get the supernatants for detection and the remaining blood samples were stored by frozen.
Histopathology
The tissues of upper right lung lobe in the different groups were taken out and fixed by 4% formaldehyde for 48 h. Then ethyl alcohol was used for dehydration. Then it was paraffin-embedded and sliced. The slices were processed by HE staining. Neutral gum was used to seal the slices. Pathological changes in lung tissue were then assessed.
Wet/Dry weight ratios of lung tissues
After the mice were killed, the right main bronchus in different groups were ligatured. The middle lobe of right lung was taken out. Surface moisture were removed by absorbent paper. Then the tissues were weighed and wet weight was recorded. Then the lung tissues were subjected to the drying oven by setting the temperature as 60°C until the weight of the tissues don’t change anymore. The weight after drying was recorded as the dry weight. Lung edema was evaluated by the ratio of wet/dry weight.
Lung active markers and inflammation factors evaluation
SPA and SPD as the active markers were evaluated by corresponding kits according to the instructions of manufacture. The levels of inflammation factors in the blood samples of the mice were detected by the corresponding assay kits.
Evaluation on the oxidant stress in the lung injury induced by LPS
MDA, LDH and SOD as the markers of oxidant stress were detected via using the corresponding kits. The alveolar lavage fluid was collected to detect the LDH level. The lung tissues in different group were collected respectively to detect the levels of MDA and SOD.
Cell apoptosis evaluated by tunel staining assay
The tunel assay kit was used to detect cell apoptosis based on the extensive DNA degradation during the cell apoptosis. Tunel detection liquid were prepared according to the instruction of manufacture. 50 μL tunel solution was added at 37°C. The cell apoptosis was observed under a fluorescence microscope.
Western blot
The samples extracted from lung tissues in different groups were subjected to 10% SDS-PAGE for separation and then electrotransfered to PVDF membrane. Then, the membranes were blocked with 5% skimmed milk. The membranes were incubated with primary antibody overnight and then secondary antibody (1:1000 dilution) for two hours.
Statistics analysis
The results presented herein were mean ± SD. SPSS software and Graph prism were applied to process data. One-way analysis of variance was used to evaluate the statistical significance.
Results
Isochlorogenic acid A attenuated the lung lesions
As shown in Figure 1A, there is no sign of lesion in control group. While, the group with LPS injection presented severely lesions in lung with a large quantity of inflammatory cells. The alveolar walls were thicker and alveolar vascular is blocked in LPS-induced group. Nearly no lesion existed in the group treated with IAA only. After pretreated with IAA, the lesions were attenuated significantly in LPS-induced lung injury in a dose dependent manner, indicating that IAA possessed a protective effect in LPS-induced lung injury.
Figure 1.
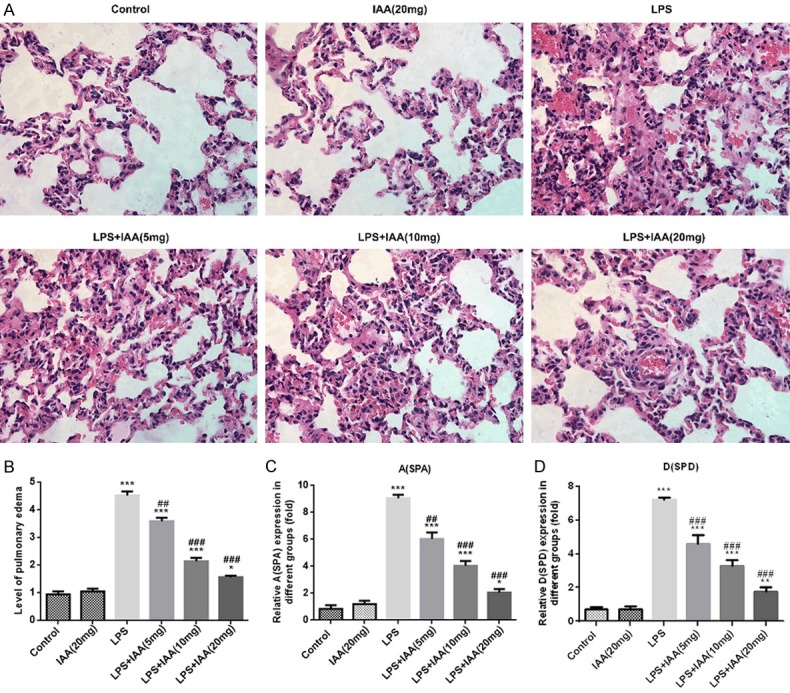
The images of histopathology in different groups (A); The Wet/Dry weight ratios of lung tissues in different groups (B); Expressions of surfactant protein A (SPA) (C) and surfactant protein D (SPD) (D) in different groups (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. control group; ##P < 0.01 and ###P < 0.001 vs. LPS group).
Isochlorogenic acid A attenuated the pulmonary edema in LPS-induced acute lung injury
Compared to the control group and IAA group, wet/dry weight ratio of lung tissues was higher in LPS-induced group than that in any other group (Figure 1B). Whilst the wet/dry weight ratio was decreased by the IAA pretreatment in the LPS induced lung injury in a dose dependent manner, suggesting that IAA had the protective effect on LPS induced pulmonary edema.
Isochlorogenic acid A attenuated the expressions of active markers in LPS-induced acute lung injury
Surfactant protein A (SPA) and surfactant protein D (SPD) are active markers in lung injury [13,14]. The results showed that the levels of SPA and SPD were higher in LPS group than that in any other group (Figure 1C and 1D). After the pretreatment of IAA, the levels of SPA and SPD induced by LPS were decreased by IAA in a dose dependent level. The pro-inflammatory enzymes cyclooxygenase 2 (COX2) and inducible nitric oxide synthase (iNOS) that contributed to inflammation were assessed as well. As shown in Figure 2, the levels of COX2 and iNOS were highest in LPS induced group among all the groups. While, expressions of COX2 and iNOS induced by LPS were reduced by IAA in a dose dependent manner. All the results described above demonstrated that isochlorogenic acid A reduced the expressions of active markers induced by LPS in acute lung injury.
Figure 2.
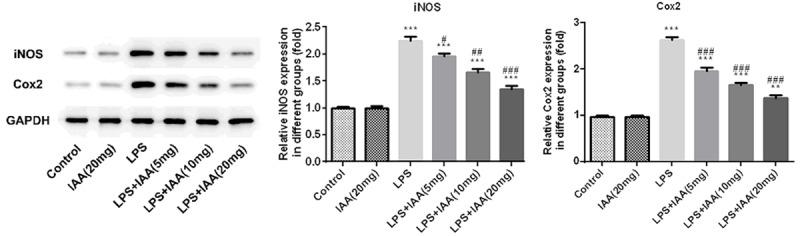
The relative expressions of COX2 and iNOS in different groups (**P < 0.01 and ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. LPS group).
Isochlorogenic acid A attenuated inflammation and the oxidant stress induced by LPS in acute lung injury
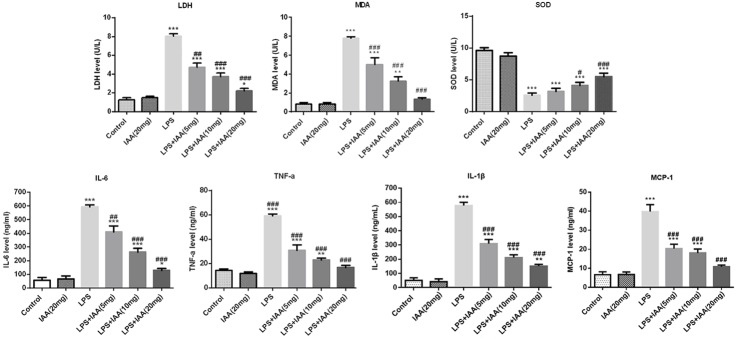
MDA, LDH and SOD as the indicators of oxidant stress were evaluated herein. As shown in Figure 3, the levels of MDA and LDH were higher in LPS induced group than that in any other group. While the SOD level was the lowest in LPS induced group. The MDA, LDH and SOD levels induced by LPS were reversed by isochlorogenic acid A in a dose dependent manner. IL-6, TNFα, IL-1β and MCP-1 as indicative markers of inflammation were evaluated. Consistent with the previous studies, the inflammatory markers were higher in LPS induced group than that in any other group [15,16]. While the enhanced expressions of IL-6, TNFα, IL-1β and MCP-1 were reversed by IAA in a dose dependent level (Figure 3). All the results indicated that IAA pretreatment alleviated the oxidant stress and inflammation in LPS induced acute lung injury.
Figure 3.
The levels of inflammation indicators and the oxidant stress indicators in different groups (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. LPS group).
Isochlorogenic acid A attenuated apoptosis of epithelial cells induced by LPS in acute lung injury
The tunel assay was used to evaluate the epithelial cell apoptosis. As seen in Figure 4, the cell apoptosis in LPS group was more obviously than that in any other group. Isochlorogenic acid A pretreatment attenuated the cell apoptosis induced by LPS. The cell apoptosis was decreased with the IAA increasing, indicating that IAA had anti-apoptosis effect in LPS induced lung injury. For further evidence, the apoptosis-associated proteins were evaluated including bax, cleaved caspase 3 and Bcl-2. Bcl-2 as anti-apoptosis protein in LPS group were the lowest than that in any other group (Figure 5). The levels of cleaved caspase 3 and bax, which are pro-apoptotic proteins, were on the contrary to bcl-2. The level of bcl-2, cleaved caspase 3 and bax induced by LPS were reversed by IAA.
Figure 4.
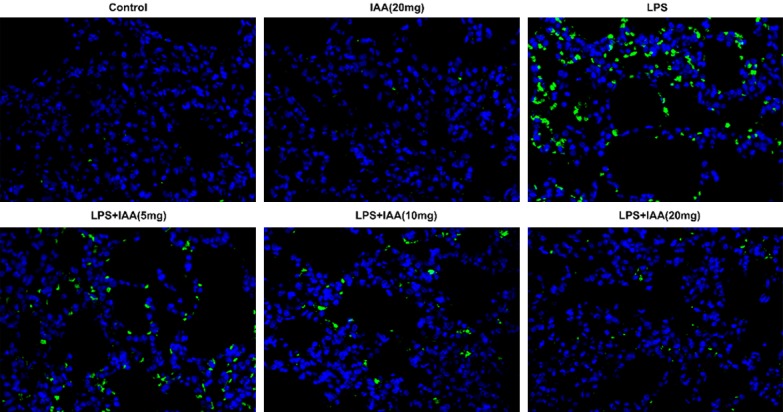
The images of cell apoptosis in different groups.
Figure 5.
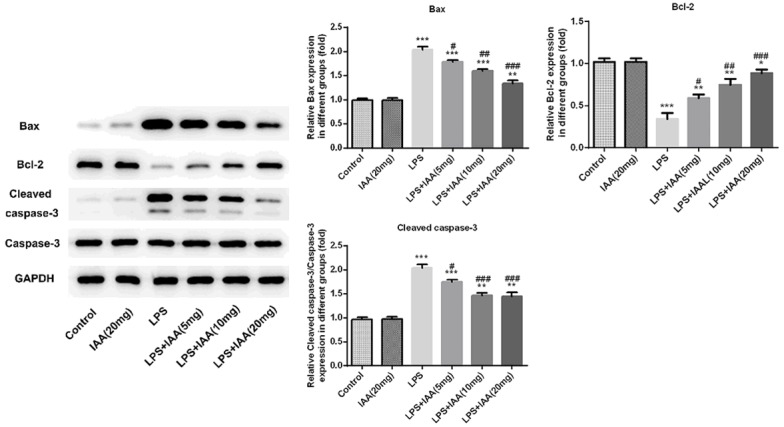
The expressions of Bax, cleaved caspase 3 and Bcl-2 in different groups (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. LPS group).
Isochlorogenic acid A inhibited the relevant protein expressions in Nf-κB/NLRP3 signal pathway
Nf-κB and NLRP3 signal pathways are both involved in inflammation of acute lung injury. In order to investigate the possible protective mechanism of isochlorogenic acid A in LPS-induced lung injury. The relevant protein expressions in Nf-κB/NLRP3 signal pathway were investigated. As shown in Figure 6, the expressions of p-p65, NLRP3, ASC and cleaved caspase 1 were higher in LPS group than that in any other group. Whilst the relevant proteins induced by LPS mentioned above were inhibited by isochlorogenic acid A in a dose dependent manner.
Figure 6.
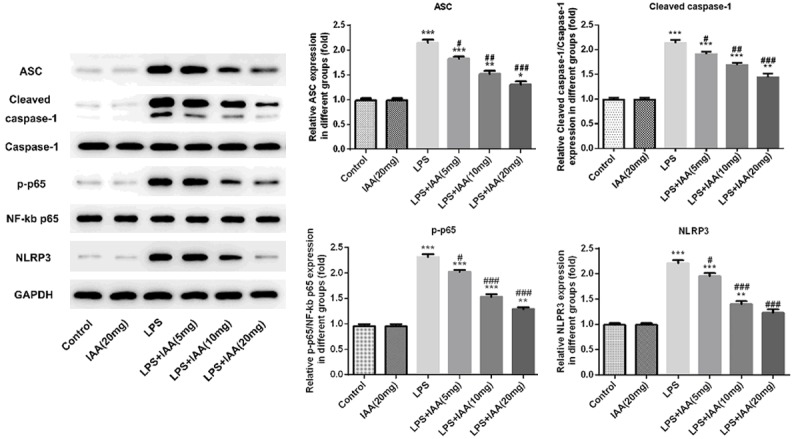
The expressions of relevant proteins in Nf-κB/NLRP3 signal pathway in different groups (*P < 0.05, **P < 0.01 and ***P < 0.001 vs. control group; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. LPS group).
Discussion
Acute lung injury accounts for over 40% of mortality and even after treatment, the five-year survival rate is lower than 50% [17]. There is a pressing need for new drug development which possesses high curative effect, low toxicity, and nearly no side effects. As known that inflammation and oxidant stress are the major causes of many diseases such as diabetes, atherosclerosis and acute lung injury. Nowadays, some Chinese medicine monomers were confirmed as promising agents for anti-inflammation and anti-oxidative stress therapy in varieties of tissue injuries. As reported, lonicera caerulea berry extracts have inhibiting effects on lipopolysaccharide-induced inflammation and oxidative stress [18]. Lonicera japonica as the same family is the dried flower bud or flower at first opening of honeysuckle and its relatives which possesses similar effect. Lonicera japonica thunb was reported to play pivotal roles in the clinical treatment of traditional Chinese medicine [19]. Wherein, the active ingredient which plays major effect is still unknown. There are many compounds existing in traditional Chinese medicine. Study on the exactly major active compound is essential for avoiding the side effects induced by other ingredients in Chinese herb. Isochlorogenic acid A is the major ingredient of lonicera japonica. Whether isochlorogenic acid A has anti-inflammation and anti-oxidant stress effects in acute lung injury is still unknown. In this study, acute lung injury induced by LPS was constructed as the pathological model. As shown by the results, the lesions were more severely, which is consistent with previous studies [20,21]. The levels of inflammation and oxidant stress induced by LPS were higher (Figure 3), which was the further evidence for the successfully establishment of acute lung injury model.
After the pathological model of acute lung injury induced by LPS was successfully constructed, the effects of isochlorogenic acid A on acute lung injury induced by LPS was investigated. The lung lesions as showed by Figure 1 were attenuated by isochlorogenic acid A. The inflammation and the oxidant stress induced by LPS were alleviated by isochlorogenic acid A as well. The inflammation and oxidant stress were the major causes of lung injury [22,23]. Isochlorogenic acid A exerted anti-inflamation and antioxidant stress effects on acute lung injury induced by LPS, indicating that isochlorogenic acid A is a promising chinese medicine monomer in protecting acute lung injury. In addition, the pulmonary edema and apoptosis of epithelial cells induced by LPS were attenuated by isochlorogenic acid A. The active markers including SPA, SPD, COX2 and iNOS stimulated by LPS were decreased by isochlorogenic acid A, further confirming that isochlorogenic acid A have the protective effects on acute lung injury. The attenuative effect of isochlorogenic on LPS induced lung injury was significant in a dose dependent manner.
In order to explicit the possible mechanism, Nf-κB/NLRP3 signal pathway as the common inflammation relevant signal pathway were investigated herein. As shown by the results, the relevant proteins of Nf-κB/NLRP3 signal pathway induced by LPS were decreased by isochlorogenic acid A, indicating that Nf-κB/NLRP3 signal pathway is suppressed by isochlorogenic acid A.
Taken together, all the results indicated that isochlorogenic acid A inhibited the effect induced by LPS in acute lung injury via inhibiting Nf-κB/NLRP3 signal pathway and was a promising Chinese monomer which may be developed into monomer drug for therapy of acute lung injury.
Conclusion
In this study, as shown by the results, isochlorogenic acid A possessed effects of anti-inflammation, anti-oxidant stress and anti-proptosis in LPS induced lung injury, providing a new avenue for acute lung injury and paving the way for future research.
Disclosure of conflict of interest
None.
References
- 1.Liu ZJ, Zhong J, Zhang M, Chen ZH, Wang JY, Chen HY, Wang XQ, Zhang B. The alexipharmic mechanisms of five licorice ingredients involved in CYP450 and Nrf2 pathways in paraquat-induced mice acute lung injury. Oxid Med Cell Longev. 2019;2019:7283104. doi: 10.1155/2019/7283104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu G, Mei H, Chen M, Qin S, Li K, Zhang W, Chen T. Protective effect of agmatine against hyperoxia-induced acute lung injury via regulating lncRNA gadd7. Biochem Biophys Res Commun. 2019;516:68–74. doi: 10.1016/j.bbrc.2019.04.164. [DOI] [PubMed] [Google Scholar]
- 3.Tu GW, Ju MJ, Zheng YJ, Hao GW, Ma GG, Hou JY, Zhang XP, Luo Z, Lu LM. CXCL16/CXCR6 is involved in LPS-induced acute lung injury via P38 signalling. J Cell Mol Med. 2019;23:5380–5389. doi: 10.1111/jcmm.14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Cao Y, Liu Y, Zhang X, Ji F, Li J, Zou Y. PIM1 inhibitor SMI-4a attenuated lipopolysaccharide-induced acute lung injury through suppressing macrophage inflammatory responses via modulating p65 phosphorylation. Int Immunopharmacol. 2019;73:568–574. doi: 10.1016/j.intimp.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Caruso M, Alamo A, Crisafulli E, Raciti C, Fisichella A, Polosa R. Adenosine signaling pathways as potential therapeutic targets in respiratory disease. Expert Opin Ther Targets. 2013;17:761–772. doi: 10.1517/14728222.2013.795220. [DOI] [PubMed] [Google Scholar]
- 6.Huang X, Zhu J, Jiang Y, Xu C, Lv Q, Yu D, Shi K, Ruan Z, Wang Y. SU5416 attenuated lipopolysaccharide-induced acute lung injury in mice by modulating properties of vascular endothelial cells. Drug Des Devel Ther. 2019;13:1763–1772. doi: 10.2147/DDDT.S188858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Lv H, Li H, Ci X, Peng L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathwayspathways. Cell Commun Signal. 2019;17:62. doi: 10.1186/s12964-019-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X, Liu R, Zhang Z, Chen Z, He J, Liu Y. Mitochondrial division inhibitor 1 attenuates mitophagy in a rat model of acute lung injury. Biomed Res Int. 2019;2019:2193706. doi: 10.1155/2019/2193706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng SC, Huang WC, S Pang JH, Wu YH, Cheng CY. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20122957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia T, Gao R, Zhou G, Liu J, Li J, Shen J. Trans-cinnamaldehyde inhibits IL-1β-stimulated inflammation in chondrocytes by suppressing NF-κB and p38-JNK pathways and exerts chondrocyte protective effects in a rat model of osteoarthritis. Biomed Res Int. 2019;2019:4039472. doi: 10.1155/2019/4039472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurgoński A, Juśkiewicz J, Zduńczyk Z. An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition. 2013;29:898–902. doi: 10.1016/j.nut.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Cho IJ, Kim JW, Lee SK, Ku SK, Lee HJ. Evaluation of in vitro anti-oxidant and anti-inflammatory activities of Korean and Chinese Lonicera caerulea. Nutr Res Pract. 2018;12:486–493. doi: 10.4162/nrp.2018.12.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benfante A, Messina R, Paterno A, Scichilone N. Serum surfactant protein D and exhaled nitric oxide as biomarkers of early lung damage in systemic sclerosis. Minerva Med. 2018;109:71–78. doi: 10.23736/S0026-4806.17.05285-5. [DOI] [PubMed] [Google Scholar]
- 14.Coya JM, Akinbi HT, Saenz A, Yang L, Weaver TE, Casals C. Natural anti-infective pulmonary proteins: in vivo cooperative action of surfactant protein SP-A and the lung antimicrobial peptide SP-BN. J Immunol. 2015;195:1628–1636. doi: 10.4049/jimmunol.1500778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao L, Meng D, Yang F, Song H, Tang D. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;487:194–200. doi: 10.1016/j.bbrc.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Kong Q, Xia Z, Zhan L, Duan W, Song X. Penehyclidine hydrochloride alleviates lipopolysaccharideinduced acute lung injury in rats: potential role of caveolin-1 expression upregulation. Int J Mol Med. 2019;43:2064–2074. doi: 10.3892/ijmm.2019.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah A, Liu H, Graham SH. Inflammation in ischemic stroke: mechanisms, consequences and possible drug targets. CNS Neurol Disord Drug Targets. 2014;13:1378–1396. doi: 10.2174/1871527313666141023094720. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li B, Zhu J, Zhang Q, Zhang X, Li L, Ma Y, Meng X. Lonicera caerulea berry extract suppresses lipopolysaccharide-induced inflammation via toll-like receptor and oxidative stress-associated mitogen-activated protein kinase signaling. Food Funct. 2016;7:4267–4277. doi: 10.1039/c6fo00627b. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Yin L, Zhang Y, Huang X, Zhao F, Cui X, Shi L, Xu L. Study on anti-inflammatory efficacy and correlative ingredients with pharmacodynamics detected in acute inflammation rat model serum from Caulis Lonicerae japonicae. Phytomedicine. 2016;23:597–610. doi: 10.1016/j.phymed.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Song S, Hu Z, Zhang Z, Li Y, Yan C, Li Z, Tang H. Activation of epac alleviates inflammation and vascular leakage in LPS-induced acute murine lung injury. Biomed Pharmacother. 2017;96:1127–1136. doi: 10.1016/j.biopha.2017.11.110. [DOI] [PubMed] [Google Scholar]
- 21.Cheng N, Liang Y, Du X, Ye RD. Serum amyloid A promotes LPS clearance and suppresses LPS-induced inflammation and tissue injury. EMBO Rep. 2018;19 doi: 10.15252/embr.201745517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang HX, Liu SJ, Tang XL, Duan GL, Ni X, Zhu XY, Liu YJ, Wang CN. H2S attenuates LPS-induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cell Physiol Biochem. 2016;40:1603–1612. doi: 10.1159/000453210. [DOI] [PubMed] [Google Scholar]
- 23.Gerin F, Sener U, Erman H, Yilmaz A, Aydin B, Armutcu F, Gurel A. The effects of quercetin on acute lung injury and biomarkers of inflammation and oxidative stress in the rat model of sepsis. Inflammation. 2016;39:700–705. doi: 10.1007/s10753-015-0296-9. [DOI] [PubMed] [Google Scholar]