Abstract
Osteoarthritis (OA) is the most common degenerative joint disease. microRNAs (miRNAs) have been showen to act critical roles in several diseases including OA. However, the involvement and underlying mechanism of miR-137 in development of OA remains unkown. In our study, we firstly showed that IL-1β decreased the expression of miR-137 in the chondrocytes and we demonstrated that the miR-37 expression level was lower in the OA cases than in the control patients. Dual-luciferase reporter analysis was performed to confirm that ADAMTS-5 was a direct target gene of miR-137. Furthermore, we indicated that elevated expression of miR-137 decreased the protein expression of ADAMTS-5 in the chondrocytes. In additional, we showed that IL-1β induces the ADAMTS-5 expression in the chondrocytes. The ADAMTS-5 expression level was higher in the OA cases than in the control patients. We showed that the expression of ADAMTS-5 was negatively correlated with the miR-137 expression level in OA tissues. Overexpression of miR-137 suppressed cell growth, extracellular matrix (ECM) degradation and inflammation in chondrocytes. These preliminary data elucidated that miR-137 suppressed OA progression via inhibiting cell growth, inflammation and ECM degradation.
Keywords: Osteoarthritis, miR-137, ADAMTS-5, extracellular matrix
Introduction
Osteoarthritis is one age-correlated disorder of joint-bone that causes disability and pain in older and middle people worldwide [1-3]. As one degenerative illness, articular cartilage degeneration acts crucial roles in the development and pathogenesis of osteoarthritis [4-6]. The degeneration of articular cartilage owns to lack of balance of the extracellular matrix (ECM) components including proteoglycan and collagen [2,7,8]. A lot of factors conduce to articular cartilage degeneration such as strain, aging, obesity, inflammation, congenital malformation and trauma [9-11]. Thus, it is crucial to explore the regulatory mechanism and pathophysiology of osteoarthritis.
MicroRNAs (miRNAs) are non-coding, endogenous and small RNAs that modify protein coding gene expression via binding to 3’-UTR (untranslated region) of mRNA (messenger RNA), resulting in inhibition of enhancement or translation of target mRNA degradation [12-15]. Several studies suggested that miRNAs act crucial roles in diverse cellular and biological processes such as differentiation, apoptosis, proliferation and metabolism [16-20]. A number of miRNAs are found to be aberrantly altered in diverse diseases including neurological disorders, diabetes, heart failure, autoimmune disease, pulmonary hypertension, and cancer and disc degeneration [21-27]. Growing evidences also found that miRNAs act critical roles in the development of osteoarthritis [5,28,29]. A series of studies suggested that miR-137 played important functional roles in the development of several diseases [30-32]. For instances, Qi et al. [33] reported that the expression of miR-137 was downregulated in melanoma cell lines and tissues and miR-137 knockdown suppressed melanoma cell invasion and migration partly through regulating PIK3R3 expression. However, the involvement and underlying mechanism of miR-137 in development of OA remains unknown.
Here, we monitored miR-137 expression in the OA cases and normal control patients. We firstly showed that IL-1β decreased the expression of miR-137 in the chondrocytes and the miR-37 expression level was lower in the OA cases than in the control patients. Overexpression of miR-137 suppressed cell growth, ECM degradation and inflammation in chondrocytes.
Materials and methods
Human samples
The normal control cartilage tissues were contained from patients that were taken amputation without OA history or rheumatoid arthritis. OA cartilage tissues were collected from the OA patients that underwent total knee arthroplasty (AKT). Our study was approved by Ethics Informed consent of our hospital and informed consent was collected from all cases.
Cell culture and treatment
Chondrocytes were isolated from OA cartilage samples and cultured following to previous study [34]. These Chondrocytes cells were cultured in the Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. miR-137 mimic and miR-137 control miR-NC were obtained from Shanghai GenePharma (Shanghai, China) and was transfected into chondrocytes cells with Lipofectamine-2000 (Invitrogen, USA) according to information of manufacturer.
RNA extraction and quantitative real-time PCR
Total RNAs of tissues or cells were isolated by using TRIzol Reagent (Life Technologies). mRNA and miRNA expression was determined with qRT-PCR. qRT-PCR analysis was performed by using SYBR RT-PCR Reagent (Takara) and stem-loop RT primers on the ABI PRISM 7900 (Applied Biosystems, Foster City, USA). U6 and GAPDH were performed as the internal control for miRNA and mRNA expression respectively. These primers which used in this study were shown as following: miR-137, forward, 5’-GTGACGGGTATTCTTGGGT-3’ and reverse 5’-GACTACGCGTATTCTTAAGCAA-3’ and U6, forward, 5’-CGCTTCGGCAGCACATATAC-3’ and reverse 5’-TTCACGAATTTGCGTGTCAT-3’ and GAPDH forward, 5’-GGAATCCACTGGCGTCTTCA-3’ and reverse 5’-GGTTCACGCCCATCACAAAC.
CCK-8 assay
Cell growth was measured by exploiting CCK-8 (Cell Counting Assay Kit-8) (Dojindo, Gaithersburg, MD) following to protocol of manufacturer. Cells were cultured in the 96-well plate and then transfected with miR-137 mimics. These cells were detected at the 24, 48 and 72 hours respectively. Ten mL CCK-8 kit was added into each 96-well and there cells continued to incubate for 2 hours. The absorbance at 450 nm was measured with microplate reader.
Western blot
Cell lysates were established in the RIPA buffer and the concentration of protein was measured with BCA kit (Pierce). Cell protein lysate was separated with 12% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with non-fat milk with 2 hours, the membranes were incubated with anti-ADAMTS-5 and anti-GAPDH overnight. These membranes were detected by chemiluminescent detection (ECL) kit. GAPDH was used as the internal control.
Dual luciferase activity assay
The human ADAMTS-5 3’UTR and mutant 3’UTR luciferase reporter vector was cloned into 3’UTR region of the pMIR-Report vector (Promega). Cell were cultured in the 24-well plate and co-transfected with miR-137 mimic or scramble, ADAMTS-5 3’UTR or mutant ADAMTS-5 3’UTR by using Lipofectamine 2000 (Invitrogen) according to protocol of manufacturer. After 24 hours, the luciferase activity was determined by using Dual-Luciferase Reporter System (Promega) following to the instructions of manufacturer.
Statistical analysis
Data were expressed as means ± SD (standard deviation). SPSS statistical (Inc., Chicago, USA) was used to analyze the significant difference. Statistically difference was detected with Two-tailed Student’s t test. P < 0.05 was indicated as statistically significant.
Results
IL-1β decreased the expression of miR-137 in the chondrocytes
Firstly, we study the expression of miR-137 in the chondrocytes after treatment of inflammatory mediator IL-1. Our result showed that IL-1β (10 ng/ml) suppressed the miR-137 expression in the chondrocytes at the time-dependently (Figure 1A). In additional, we demonstrated that the miR-137 expression level was downregulated after treatment with 0 (as a control), 5, 10 and 20 ng/ml IL-1β (Figure 1B).
Figure 1.
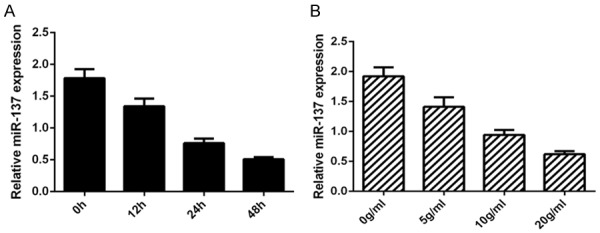
IL-1β decreased the expression of miR-137 in the chondrocytes. A. The expression of miR-137 was detected by qRT-PCR assay and U6 was used as the internal control. B. The miR-137 expression level was downregulated after treatment with 0 (as a control), 5, 10 and 20 ng/ml IL-1β.
miR-137 expression was downregulated in the OA issues
Next, we determined the expression level of miR-137 in the OA cases and normal control patients. Our data suggested that the miR-37 expression level was lower in the OA cases than in the control patients (Figure 2).
Figure 2.
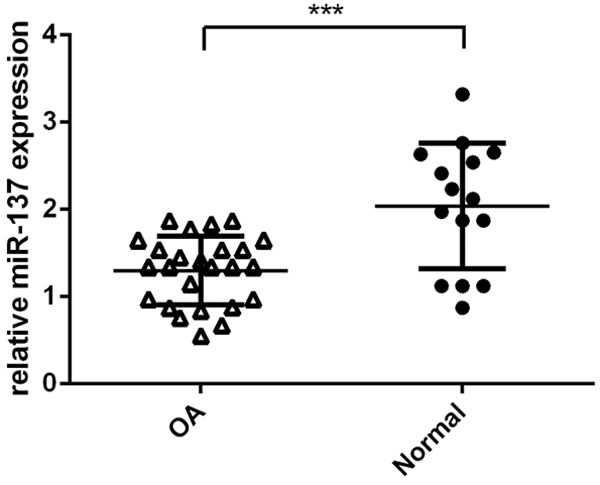
miR-137 expression was downregulated in the OA issues. The expression level of miR-137 in the OA cases and normal control patients was measured by qRT-PCR assay. ***P < 0.001.
ADAMTS-5 was a direct target gene of miR-137 in the chondrocytes
To further study the mechanism of miR-137 in OA, the software targetscan (http://www.targetscan.org/vert_72/) was utilized to find the target gene interacted with miR-137. As shown in the Figure 3A, we demonstrated that ADAMTS-5 contained potential binding sites with miR-137. Moreover, we showed that the miR-137 expression was upregulated in the chondrocytes after treatment with miR-137 mimic (Figure 3B). Dual-luciferase reporter analysis was done to confirm whether ADAMTS-5 was a direct target gene of miR-137. Our results proved that ectopic expression of miR-137 significantly suppressed luciferase activity of ADAMTS-5-WT vector but not the ADAMTS-5-MUT (Figure 3C). Furthermore, we indicated that elevated expression of miR-137 decreased the protein expression of ADAMTS-5 in the chondrocytes (Figure 3D).
Figure 3.
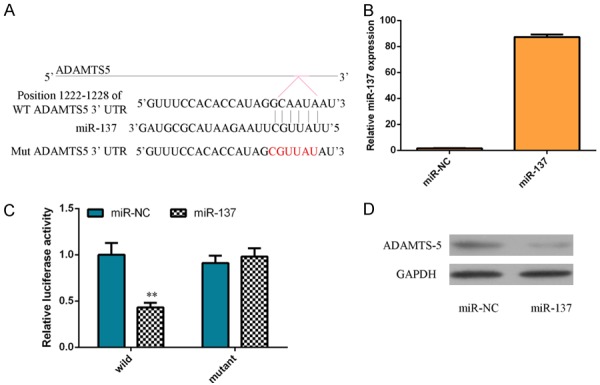
ADAMTS-5 was a direct target gene of miR-137 in the chondrocytes. A. ADAMTS-5 contained potential binding sites with miR-137 by using the software targetscan (http://www.targetscan.org/vert_72/). B. miR-137 expression was upregulated in the chondrocytes after treatment with miR-137 mimic. C. Ectopic expression of miR-137 significantly suppressed luciferase activity of ADAMTS-5-WT vector but not the ADAMTS-5-MUT. D. Elevated expression of miR-137 decreased the protein expression of ADAMTS-5 in the chondrocytes.
IL-1β induces the ADAMTS-5 expression in the chondrocytes
Then, we study the expression of ADAMTS-5 in the chondrocytes after treatment of inflammatory mediator IL-1β. Our result showed that IL-1β (10 ng/ml) enhanced the ADAMTS-5 expression in the chondrocytes at the time-dependently (Figure 4A). In additional, we demonstrated that the ADAMTS-5 expression level was downregulated after treatment with 0 (as a control), 5, 10 and 20 ng/ml IL-1β (Figure 4B).
Figure 4.
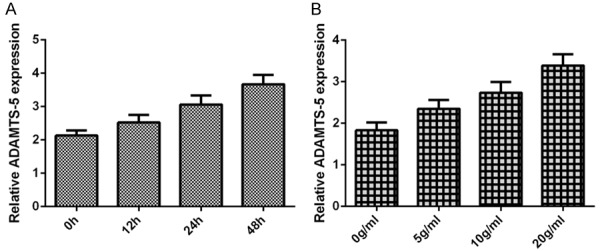
IL-1β induces the ADAMTS-5 expression in the chondrocytes. A. The expression of ADAMTS-5 was measured by qRT-PCR assay and GAPDH was used as the internal control. B. The miR-137 expression level was downregulated after treatment with 0 (as a control), 5, 10 and 20 ng/ml IL-1β.
ADAMTS-5 expression was upregulated in the OA issues and was negatively correlated with miR-137 in OA
Next, we investigated the expression level of ADAMTS-5 in the OA cases and normal control patients. Our data suggested that the ADAMTS-5 expression level was higher in the OA cases than in the control patients (Figure 5A). Moreover, we showed that the expression of ADAMTS-5 was negatively correlated with the miR-137 expression level in OA tissues (Figure 5B).
Figure 5.
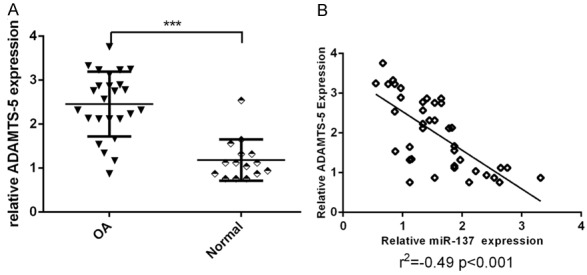
ADAMTS-5 expression was upregulated in the OA issues and was negatively correlated with miR-137 in OA. A. The ADAMTS-5 expression level was higher in the OA cases than in the control patients. B. The expression of ADAMTS-5 was negatively correlated with the miR-137 expression level in OA tissues. ***P < 0.001.
Overexpression of miR-137 suppressed cell growth, ECM degradation and inflammation in chondrocytes
We demonstrated that ectopic expression of miR-137 suppressed cell growth in the chondrocytes (Figure 6A). Then, we showed that overexpression of miR-137 inhibited the IL-1 expression in the chondrocytes (Figure 6B). In addition, miR-137 overexpression promoted the aggrecan expression in the chondrocytes (Figure 6C). Ectopic expression of miR-137 enhanced type X collagen expression in the chondrocytes (Figure 6D). Moreover, we demonstrated that overexpression of miR-137 suppressed the ADAMTS-4 (Figure 6E) and MMP-13 (Figure 6F) expression in chondrocytes.
Figure 6.
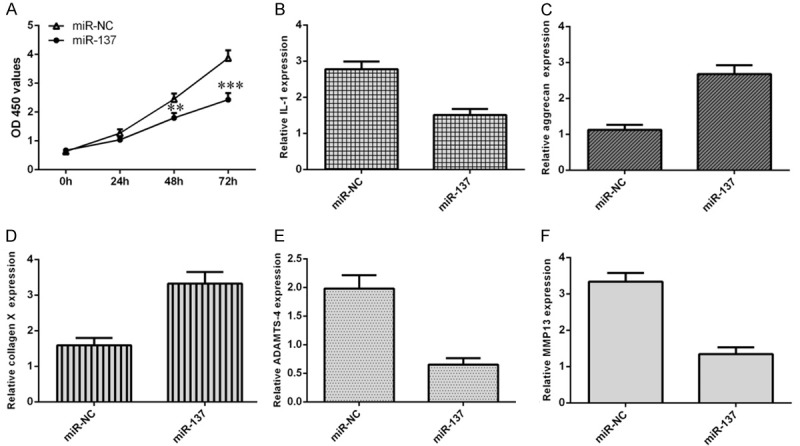
Overexpression of miR-137 suppressed cell growth, ECM degradation and inflammation in chondrocytes. A. CCK-8 assay was performed to detect the cell growth. B. Overexpression of miR-137 inhibited the IL-1 expression in the chondrocytes. C. miR-137 overexpression promoted the aggrecan expression in the chondrocytes. D. Ectopic expression of miR-137 enhanced type X collagen expression in the chondrocytes. E. Overexpression of miR-137 suppressed the ADAMTS-4 expression in chondrocytes. F. The expression of MMP-13 was detected by qRT-PCR assay and GAPDH was used as the internal control. **P < 0.01 and ***P < 0.001.
Discussion
Identification of pivotal miRNAs correlated with pathogenesis of OA may assist to OA prognosis and diagnosis. In our study, we firstly showed that IL-1β decreased the expression of miR-137 in the chondrocytes and we demonstrated that the miR-37 expression level was lower in the OA cases than in the control patients. Dual-luciferase reporter analysis was performed to confirm that ADAMTS-5 was a direct target gene of miR-137. Furthermore, we indicated that elevated expression of miR-137 decreased the protein expression of ADAMTS-5 in the chondrocytes. In additional, we showed that IL-1β induces the ADAMTS-5 expression in the chondrocytes. The ADAMTS-5 expression level was higher in the OA cases than in the control patients. We showed that the expression of ADAMTS-5 was negatively correlated with the miR-137 expression level in OA tissues. Overexpression of miR-137 suppressed cell growth, ECM degradation and inflammation in chondrocytes. These preliminary data elucidated that miR-137 suppressed OA progression via inhibiting cell growth, inflammation and ECM degradation.
A series of researches indicated that miR-137 played critical functional roles in the development of several tumors [35-37]. For instances, Qi et al. [33] reported that the expression of miR-137 was downregulated in melanoma cell lines and tissues and miR-137 knockdown suppressed melanoma cell invasion and migration partly through regulating PIK3R3 expression. Huang et al. [38] indicated that miR-137 expression was decreased in hepatocellular carcinoma cell lines and overexpression of miR-137 suppressed hepatocellular carcinoma cell migration and invasion via inhibiting EZH2 expression. Bi et al. [39] showed that miR-137 expression level was downregulated in colon cancer cells and ectopic expression of miR-137 decreased the colon cancer cell invasion, proliferation and migration by modulating TCF4 expression. However, the involvement and underlying mechanism of miR-137 in development of OA remains unknown. In the present study, we investigated the expression of miR-137 in the OA cases and normal control patients. Our results suggested that the miR-137 expression level was lower in the OA cases than in the control patients. Moreover, we showed that IL-1β decreased the expression of miR-137 in the chondrocytes. Ectopic expression of miR-137 suppressed cell growth in the chondrocytes. Furthermore, we indicated that overexpression of miR-137 suppressed the ADAMTS-4 and MMP-13 expression. These results suggested that that miR-137 suppressed OA progression via inhibiting cell growth, inflammation and ECM degradation.
Previous studies indicated that miRNAs involved in cell physiological and pathological processes by modulating several target genes expression and their signal pathways [40,41]. ADAMTS-5, one member of ADAMTS family, played pivotal roles in the development of OA [42-44]. Glasson et al. [45] indicated that erosion of cartilage and aggrecan early loss was decreased by depletion of ADAMTS-5 in one murine model of the OA. This result suggested that ADAMTS-5 played important roles in the progression of OA. However, the modulation of ADAMTS-5 functioning in the progression of OA remains unclear. In our study, we used software targetscan (http://www.targetscan.org/vert_72/) to predict the target gene interacted with miR-137. We found that ADAMTS-5 contained potential binding sites with miR-137. Dual-luciferase reporter analysis was performed to confirm that ADAMTS-5 was a direct target gene of miR-137. Ectopic expression of miR-137 significantly suppressed luciferase activity of ADAMTS-5-WT vector but not the ADAMTS-5-MUT. Furthermore, we indicated that elevated expression of miR-137 decreased the protein expression of ADAMTS-5 in the chondrocytes.
In summary, our data identified that IL-1β decreased the expression of miR-137 in the chondrocytes and miR-137 expression level was lower in the OA cases than in the control patients. Overexpression of miR-137 suppressed cell growth, ECM degradation and inflammation in chondrocytes. ADAMTS-5 was a direct target gene of miR-137. These preliminary data elucidated that miR-137 suppressed OA progression via inhibiting cell growth, inflammation and ECM degradation.
Acknowledgements
Our study was supported by Key R&D Program of Shandong Province (Public Welfare Project) (2017 GSF218065).
Disclosure of conflict of interest
None.
References
- 1.Li YF, Li SH, Luo YT, Liu Y, Yu NH. LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol. 2017;36:571–580. doi: 10.1089/dna.2017.3678. [DOI] [PubMed] [Google Scholar]
- 2.Li YF, Li SH, Liu Y, Luo YT. Long noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for mir-27b. Cell Physiol Biochem. 2017;43:602–610. doi: 10.1159/000480532. [DOI] [PubMed] [Google Scholar]
- 3.Hu JL, Wang Z, Shan YJ, Pan Y, Ma J, Jia L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/beta-catenin axis. Cell Death Dis. 2018;9:711. doi: 10.1038/s41419-018-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park S, Lee M, Chun CH, Jin EJ. The lncRNA, nespas, is associated with osteoarthritis progression and serves as a potential new prognostic biomarker. Cartilage. 2019;10:148–156. doi: 10.1177/1947603517725566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang QY, Wang WC, Zhang F, Deng YW, Long ZL. NEAT1/miR-181c regulates osteopontin (OPN)-mediated synoviocyte proliferation in osteoarthritis. J Cell Biochem. 2017;118:3775–3784. doi: 10.1002/jcb.26025. [DOI] [PubMed] [Google Scholar]
- 6.Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL, Qian LH, Qian DW, Duan JA. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem. 2011;18:977–1001. doi: 10.2174/092986711794940905. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, Wu YD, Xu D, Yan XF. Long noncoding RNA UFC1 promotes proliferation of chondrocyte in osteoarthritis by acting as a Sponge for miR-34a. DNA Cell Biol. 2016;35:691–695. doi: 10.1089/dna.2016.3397. [DOI] [PubMed] [Google Scholar]
- 8.Iliopoulos D, Malizos KN, Tsezou A. Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis. 2007;66:1616–1621. doi: 10.1136/ard.2007.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallu S, Francin PJ, Guillaume C, Gegout-Pottie P, Netter P, Mainard D, Terlain B, Presle N. Obesity affects the chondrocyte responsiveness to leptin in patients with osteoarthritis. Arthritis Res Ther. 2010;12:R112. doi: 10.1186/ar3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36:1149–1160. doi: 10.1177/147323000803600601. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Shen JX, Chan MT, Wu WK. MicroRNA-379 suppresses osteosarcoma progression by targeting PDK1. J Cell Mol Med. 2017;21:315–323. doi: 10.1111/jcmm.12966. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.She K, Yan H, Huang J, Zhou H, He J. miR-193b availability is antagonized by LncRNA-SNHG7 for FAIM2-induced tumour progression in non-small cell lung cancer. Cell Prolif. 2018;51 doi: 10.1111/cpr.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JJ, Ren JC, Hao SJ, Ma F, Xin Y, Jia WX, Sun YD, Liu ZF, Yu H, Jia JH, Li WJ. MiRNA-491-5p inhibits cell proliferation, invasion and migration via targeting JMJD2B and serves as a potential biomarker in gastric cancer. Am J Transl Res. 2018;10:525–534. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Fan Y, Zhang XL, Gao LL, Ma JF. Role of miRNA-21/PTEN on the high glucose-induced EMT in human mesothelial peritoneal cells. Am J Transl Res. 2018;10:2590–2599. [PMC free article] [PubMed] [Google Scholar]
- 16.Hosseini V, Mohammadi-Yeganeh S, Ghanbarian H, Hashemi SM, Khojasteh A. The power of precise bioinformatics prediction of miRNA:mRNA interactions:miR-4699 as a potential inducer of Wnt signaling pathway. J Cell Biochem. 2018;119:5960–5969. doi: 10.1002/jcb.26791. [DOI] [PubMed] [Google Scholar]
- 17.Zheng H, Zhang F, Lin X, Huang C, Zhang Y, Li Y, Lin J, Chen W. MicroRNA-1225-5p inhibits proliferation and metastasis of gastric carcinoma through repressing insulin receptor substrate-1 and activation of beta-catenin signaling. Oncotarget. 2016;7:4647–4663. doi: 10.18632/oncotarget.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Peng Z, Zhao Y, Chen L. microRNA-25 inhibits cell apoptosis of human gastric adenocarcinoma cell line AGS via regulating CCNE1 and MYC. Med Sci Monit. 2016;22:1415–1420. doi: 10.12659/MSM.896118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin K, Liu M, Zhang M, Wang F, Fen M, Liu Z, Yuan Y, Gao S, Yang L, Zhang W, Zhang J, Guo B, Xu J, Liang H, Chen X, Guan W. miR-208a-3p suppresses cell apoptosis by targeting PDCD4 in gastric cancer. Oncotarget. 2016;7:67321–67332. doi: 10.18632/oncotarget.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin G, Zhou H, Xue Y, Yao B, Zhao W. MicroRNA-340 promotes the tumor growth of human gastric cancer by inhibiting cyclin G2. Oncol Rep. 2016;36:1111–1118. doi: 10.3892/or.2016.4876. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Wang KX, Xu H, Hong Y. Integrative miRNA analysis identifies hsa-miR-3154, hsa-miR-7-3, and hsa-miR-600 as potential prognostic biomarker for cervical cancer. J Cell Biochem. 2018;119:1558–1566. doi: 10.1002/jcb.26315. [DOI] [PubMed] [Google Scholar]
- 22.Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. 2010;1:e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, Jeyaseelan K. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One. 2011;6:e22839. doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai WF, Liu GS, Lam CK, Florea S, Qian J, Zhao W, Pritchard T, Haghighi K, Lebeche D, Lu LJ, Deng J, Fan GC, Hajjar RJ, Kranias EG. Up-regulation of micro-RNA765 in human failing hearts is associated with post-transcriptional regulation of protein phosphatase inhibitor-1 and depressed contractility. Eur J Heart Fail. 2015;17:782–793. doi: 10.1002/ejhf.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Gupta S, Du WW, Yang BB. MicroRNA-17 inhibits tumor growth by stimulating T-cell mediated host immune response. Oncoscience. 2014;1:531–539. doi: 10.18632/oncoscience.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, Qiu G. MicroRNA-10b promotes nucleus pulposus cell proliferation through RhoC-Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS One. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Chen LQ, Li Q, Wang J, Jin S, Zheng HM, Lin J, He F, Zhang H, Ma S, Mei J, Yu J. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J Cell Mol Med. 2017;21:3347–3359. doi: 10.1111/jcmm.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Zhang HY, Sun QY, Yang J, Zeng C, Ding CH, Cai DZ, Liu AL, Bai XC. Chondrocyte mTORC1 activation stimulates miR-483-5p via HDAC4 in osteoarthritis progression. J Cell Physiol. 2019;234:2730–2740. doi: 10.1002/jcp.27088. [DOI] [PubMed] [Google Scholar]
- 30.Steponaitiene R, Kupcinskas J, Langner C, Balaguer F, Venclauskas L, Pauzas H, Tamelis A, Skieceviciene J, Kupcinskas L, Malfertheiner P, Link A. Epigenetic silencing of miR-137 is a frequent event in gastric carcinogenesis. Mol Carcinog. 2016;55:376–386. doi: 10.1002/mc.22287. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Li Y, Shen H, Li H, Long L, Hui L, Xu W. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett. 2013;587:73–81. doi: 10.1016/j.febslet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek B, Schadendorf D, Diederichs S, Eichmuller SB. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–775. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 33.Qi J, Wang WW, Chen W, Lu WY, Shang AQ. Mechanism of miR-137 regulating migration and invasion of melanoma cells by targeting PIK3R3 gene. J Cell Biochem. 2018 doi: 10.1002/jcb.28124. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Fan XC, Yuan JS, Xie J, Pan ZP, Yao X, Sun XY, Zhang P, Zhang L. Long non-protein coding RNA DANCR functions as a competing endogenous RNA to regulate osteoarthritis progression via miR-577/SphK2 axis. Biochem Biophys Res Commun. 2018;500:658–664. doi: 10.1016/j.bbrc.2018.04.130. [DOI] [PubMed] [Google Scholar]
- 35.Li KK, Yang L, Pang JC, Chan AK, Zhou L, Mao Y, Wang Y, Lau KM, Poon WS, Shi Z, Ng HK. MIR-137 suppresses growth and invasion, is downregulated in oligodendroglial tumors and targets CSE1L. Brain Pathol. 2013;23:426–439. doi: 10.1111/bpa.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Xia B, Meng F, Lou G. miR-137 suppresses cell growth in ovarian cancer by targeting AEG-1. Biochem Biophys Res Commun. 2013;441:357–363. doi: 10.1016/j.bbrc.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 37.Althoff K, Beckers A, Odersky A, Mestdagh P, Koster J, Bray IM, Bryan K, Vandesompele J, Speleman F, Stallings RL, Schramm A, Eggert A, Sprussel A, Schulte JH. MiR-137 functions as a tumor suppressor in neuroblastoma by downregulating KDM1A. Int J Cancer. 2013;133:1064–1073. doi: 10.1002/ijc.28091. [DOI] [PubMed] [Google Scholar]
- 38.Huang B, Huang MP, Li Q. MiR-137 suppresses migration and invasion by targeting EZH2-STAT3 signaling in human hepatocellular carcinoma. Pathol Res Pract. 2018;214:1980–1986. doi: 10.1016/j.prp.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Bi WP, Xia M, Wang XJ. miR-137 suppresses proliferation, migration and invasion of colon cancer cell lines by targeting TCF4. Oncol Lett. 2018;15:8744–8748. doi: 10.3892/ol.2018.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji F, Zhang H, Wang Y, Li M, Xu W, Kang Y, Wang Z, Cheng P, Tong D, Li C, Tang H. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone. 2013;56:220–226. doi: 10.1016/j.bone.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Jin Y, Peng D, Shen Y, Xu M, Liang Y, Xiao B, Lu J. MicroRNA-376c inhibits cell proliferation and invasion in osteosarcoma by targeting to transforming growth factor-alpha. DNA Cell Biol. 2013;32:302–309. doi: 10.1089/dna.2013.1977. [DOI] [PubMed] [Google Scholar]
- 42.Saito T, Nishida K, Furumatsu T, Yoshida A, Ozawa M, Ozaki T. Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes. Osteoarthritis Cartilage. 2013;21:165–174. doi: 10.1016/j.joca.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Wu M, Jiang S, Ding W, Luo Q, Shi J. Expression of ADAMTs-5 and TIMP-3 in the condylar cartilage of rats induced by experimentally created osteoarthritis. Arch Oral Biol. 2014;59:524–529. doi: 10.1016/j.archoralbio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]


