Abstract
MircoRNAs (miRNAs) are a diverse family of highly-conserved small non-coding RNAs, which range from approximately 18 to 25 nucleotides in size. They regulate gene expression transcriptionally or post-transcriptionally via binding to the 3’-untranslated region (3’-UTR) of target message RNAs (mRNAs). MiRNAs have emerged as molecular regulators that participate in physiological and pathological processes of diverse malignancies. Among them, miRNA-145 (miR-145) played a profound role in tumorigenesis and progression of various neoplasms. In this review, we summarized the recent findings regarding miR-145, to elucidate its functional roles in cell invasion and migration of diverse human malignancies, and considered it a potential biomarker for cancer diagnosis, screening, and prognosis.
Keywords: MiR-145, cancers, biomarker, invasion and migration
Introduction
Global cancer statistics alarm that cancers rank as the leading cause of death, and cancer-caused mortality is soaring globally. Treatment and regular surveillance contribute to relieving the remarkably rising economic burden of cancers [1]. Cell invasion and migration are the major characteristics of metastatic tumors, which account for high mortality rate and poor prognosis of cancers. Despite the advances in surgical resection, radiotherapy and chemotherapy, it is urgent to search and develop novel and alternative therapeutic approaches of cancers. In this regard, mircoRNAs (miRNAs) have been verified to stress their roles as metastasis activators or suppressors, which may serve as diagnostic biomarkers and therapeutic targets [2].
MiRNAs, a novel class of small noncoding RNAs, regulate gene expression at the transcriptional or post-transcriptional level through binding to the 3’ untranslated regions (3’-UTR) of target message RNAs (mRNAs) [3]. A variety of miRNAs have multiple target genes involved in cell growth and signaling pathways, dysregulation of which may participate in many fundamental cellular processes of various cancers, including tumorigenesis, proliferation, apoptosis, metastasis and drug-resistance. Among them, miRNA-145 (miR-145) appears to be a vital factor for tumor aggressiveness and prognosis. MiR-145, located in a fragile region of chromosome 5q, was first reported in mouse heart muscle, and later verified in humans [4-6]. It was remarkably expressed in germ lines and mesoderm-derived tissues, including ovary, uterus, prostate, testis, spleen and heart [7]. Based on available researches, miR-145 downregulated in a wide range of cancers, including colorectal cancer (CRC), non-small-cell lung cancer (NSCLC), breast cancer (BCa), osteosarcoma (OS), cervical cancer, prostate cancer (PCa), gastric cancer (GC), ovarian cancer (OC), glioma (GBM) and bladder cancer. In this review, we summarized the roles of miR-145 in invasion and migration of various tumors, and explored its potential role as a candidate biomarker for cancer diagnosis, monitoring and prognosis in humans, shedding new light on potential treatment of cancers.
MiR-145 in tumor invasion and migration
MiR-145 in CRC
MiR-145 played an inhibitory role in CRC in that it reduced cell migration and invasion, apparently by suppressing P21-activated kinase 4 (PAK4) [8] , tumor suppressor candidate 3 (TUSC3) [9], p70S6K1 [10], sex-determining region Y-box 9 (SOX9) [11], myosin VI (MYO6) [12], insulin receptor substrate-1 (IRS-1) [13], SMAD-interacting protein 1 (SIP1) [14] and fascin-1 (FSCN1) [15]. MiR-145 targeted PAK4 to suppress cell migration through inhibition of the LIMK1-conflin signaling pathway. PAK4, a subfamily of serine/threonine kinases, modulated actin cytoskeleton reorganization by phosphorylating LIMK1 [16]. Subsequently, activated PAK4 stimulated LIMK1 to phosphorylate cofilin, which acted synergistically to facilitate cell migration [17]. Besides, miR-145 was clarified to block mitogen-activated protein kinase (MAPK) pathway by targeting TUSC3. TUSC3, located on chromosome region 8p22 [18], was reported to enhance epithelial-mesenchymal transition (EMT) and cancer progression through regulating MAPK, PI3K/Akt and Wnt/β-catenin pathways in CRC [19]. However, whether miR-145 regulated the latter two pathways warranted further investigation. EMT is a critical step of tumor cell metastasis [20]. The MAPK pathway participated in gene expression and cell growth by phosphorylating specific target protein substrates. MAPKs were composed of three well-characterized subfamilies: extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK), and p38 protein kinases [21]. MiR-145 downregulated hypoxia-inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF) by targeting p70S6K1, both were important regulators in tumor angiogenesis. P70S6K1, one downstream mammalian target of rapamycin (mTOR), acted as a key regulator in protein synthesis. Additionally, the mTOR/p70S6K1 is a vital signaling pathway in regulating cellular functions [10,22-24]. LncRNA MALAT1 functioned as an endogenous RNA (ceRNA) to sponge miR-145, and inhibited malignant progression of CRC via the lncRNA MALAT1/miR-145/SOX9 axis. LncRNA MALAT1, expressed from chromosome 11q13, is a noncoding RNA of more than 8000 nt [25]. SOX9 was determined as an oncogene, which was reported to regulate metastasis by activating Wnt/β-catenin signal [26], but it was unclear whether this pathway functioned in CRC. Similarly, lncRNA SOX21-AS1/miR-145/MYO6 axis aggravated the malignant development of CRC. LncRNA SOX21-AS1 was identified as a cancer-correlated molecular in human cancers, low expression level of which was associated with advanced stage in oral squamous cell carcinoma, while its upregulation positively correlated with tumor size and advanced stage in lung adenocarcinoma [27,28]. Of note, sponging miRNA was also a significant regulatory mechanism of circRNA functions. Circ_001569 exerted its tumor promoting function via inhibiting miR-145, and subsequently up-regulated E2F5, BAG4 and FMNL2, which were all targets of miR-145 [29] (Figure 1 and Table 1).
Figure 1.
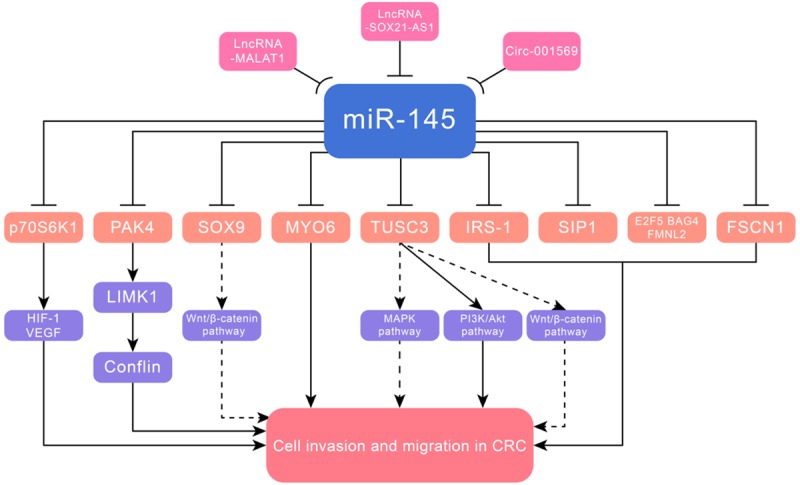
MiR-145 suppressed cell invasion and metastasis in CRC via targeting PAK4, TUSC3, p70S6K1, SOX9, MYO6, IRS-1, SIP1, FSCN1, E2F5, BAG4 and FMNL2.
Table 1.
Target genes of miR-145 in cancer invasion and metastasis
| MiR-145 | Disease | Target genes | Reference |
|---|---|---|---|
| Downregulated | CRC | PAK4 | [8] |
| TUSC3 | [9] | ||
| p70S6K1 | [10] | ||
| SOX9 | [11] | ||
| MYO6 | [12] | ||
| IRS-1 | [13] | ||
| SIP1 | [14] | ||
| FSCN1 | [15] | ||
| E2F5, BAG4 and FMNL2 | [29] | ||
| BCa | ARF6 | [30] | |
| fascin and JAM-A | [31] | ||
| c-Myc | [32] | ||
| ROCK1 | [33] | ||
| CCNE2 | [34] | ||
| OCT4 | [35] | ||
| MMP11 and Rab27a | [36] | ||
| RTKN | [37] | ||
| MUC1 | [38] | ||
| FSCN1 | [84] | ||
| NSCLC | MUC1 | [48] | |
| NEDD9 | [49] | ||
| FOXM1 | [50] | ||
| MTDH (AEG-1) | [51] | ||
| FSCN1 | [85,86] | ||
| GBM | ADAM17 and EGFR | [56] | |
| ROCK1 | [57] | ||
| ABCG2 | [58] | ||
| CTGF | [59] | ||
| SOX9 and ADD3 | [60] | ||
| OC | MTDH | [68] | |
| RASA1 | [69] | ||
| DNMT3A and HK2 | [70] | ||
| HMGA2 | [71] | ||
| Bladder cancer | PAK1 | [78] | |
| ZEB1/2 and FSCN1 | [79] | ||
| KLF4 | [88] | ||
| CDK6 | [91] | ||
| Sp1 | [92] | ||
| PCa | FSCN1 | [87] | |
| SWAP70 | [93] | ||
| HEF1 (NEDD9 or Cas-L) | [94] | ||
| GC | FSCN1 | [82] | |
| Ets1 | [101] | ||
| SOX9 | [104] | ||
| CTNND1 | [105] | ||
| N-cadherin and ZEB2 | [106] | ||
| Cervical cancer | SIP1 | [107] | |
| MYPT1 | [110] | ||
| OS | VEGF | [112] | |
| MMP16 | [113] | ||
| CDK6 | [114] | ||
| HCC | ADAM17 | [62] | |
| Renal cell carcinoma | ADAM17 | [63] | |
| ESCC | FSCN1 | [80] | |
| LSCC | FSCN1 | [83] | |
| MYO5A | [120] | ||
| Pancreatic cancer | MUC13 | [121] | |
| OSCC | c-Myc and CDK6 | [122] | |
| Endometrial cancer | SOX11 | [123] | |
| Melanoma | NRAS | [124] | |
| ICC | NUAK1 (ARK5) | [125] | |
| Retinoblastoma | ADAM19 | [126] | |
| Thyroid cancer | AKT3 | [130] | |
| Upregulated | esophageal cancer | SMAD5 | [131] |
MiR-145 in BCa
MiR-145 mediated targets directly related to cell invasion and migration in BCa were ADP-ribosylation factor 6 (ARF6) [30], fascin, junctional adhesion molecule-A (JAM-A) [31], c-Myc [32], Rho-associated protein kinase 1 (ROCK1) [33], cyclin E2 (CCNE2) [34], octamer-binding transcription factor 4 (OCT4) [35], matrix metalloproteinase 11 (MMP11), Rab GTPase family 27a (Rab27a) [36], RTKN [37] and metastasis gene mucin 1 (MUC1) [38]. LncRNA-RoR/miR-145/ARF6 pathway was demonstrated to inhibit invasion in BCa. The small GTPase ARF6, a novel target of miR-145, participated in membrane traffic or cytoskeleton organization, and regulated E-cadherin localization [39]. Similarly, the KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1) gene, located at 11p15.5, interacted with miR-145 to modulate its target CCNE2, forming a KCNQ1OT1/miR-145/CCNE2 axis [40]. In addition, miR-145 inhibited cell invasion and migration by targeting fascin and JAM-A, which were major contributors to the cytoskeletal phenotype. The cell-cell adhesion JAM-A influenced epithelial cell morphology and migration. Knockdown of JAM-A reduced β1-integrin expression, which was implicated in BCa via effects on growth, and whether miR-145/JAM-A/β1-integrin functioned in BCa required further exploration [41,42]. P53 indirectly inhibited the oncogene c-Myc through directly binding the promoter of miR-145 at the p53 response element [32]. Moreover, eIF4E and CDK4 were direct target genes of c-Myc [43,44]. The regulation mechanisms of miR-145 through DNA methylation and p53 gene mutation were first reported in prostate cancer [45]. MiR-145 inhibited growth and migration via targeting ROCK1, a serine/threonine protein kinase, which was a critical regulator of actin cytoskeleton reorganization [46]. MUC1, a member of extensively O-glycosylated proteins, created a physical barrier that protected epithelia from damage [47]. MiR-145/MUC1 also exerted its tumor inhibitory function in NSCLC, and the exact mechanism will be elucidated in the next segment (Figure 2 and Table 1).
Figure 2.
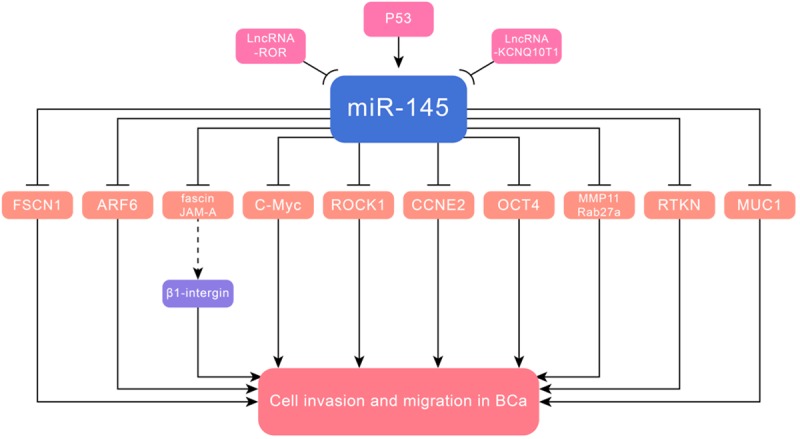
MiR-145 inhibited cell invasion and metastasis in BCa via targeting ARF6, fascin, JAM-A, c-Myc, ROCK1, CCNE2, OCT4, MMP11, Rab27a, RTKN, MUC1 and FSCN1.
MiR-145 in NSCLC
MiR-145 inhibited cell invasion and migration in NSCLC by targeting MUC1 [48], NEDD9 [49], forkhead box transcription factor M1 (FOXM1) [50] and metadherin (MTDH) [51]. MiR-145 was downregulated in NSCLC, due to its methylation, correlated with a more aggressive tumor phenotype via targeting MUC1. Abnormal promoter DNA hypermethylation, a vital epigenetic signature, was associated with transcriptional repression of various miRNAs, resulting in signaling activation that could enhance invasion and migration [52]. ERβ could promote NSCLC vasculogenic mimicry formation and cell invasion by modulating ERβ/MALAT1/miR-145/NEDD9 signaling. Moreover, ERβ upregulated expression of lncRNA-MALAT1 via binding to the estrogen response elements, which were located on the promoter of lncRNA-MALAT1. Consequently, it inhibited miR-145 and increased NEDD9 as miR-145 directly targeted NEDD9. LINC00339 acted as a ceRNA to sponge miR-145, and inhibited malignant progression of NSCLC cells via LINC00339/miR-145/FOXM1 axis. FOXM1, a transcription factor from the FOX family, was a vital component of the KRAS/ERK pathway in respiratory epithelial cells [53]. LncRNA small nucleolar RNA host gene 1 (SNHG1) modulated MTDH by acting as a miR-145 sponge. MTDH, also named astrocyte elevated gene-1 (AEG-1), was identified as an oncogene that controlled growth and aggressiveness of cancers [54]. A close association of MTDH with PI3K/AKT pathway was unveiled in numerous studies, however, it warranted further research to identify whether miR-145 affected migration and invasion of NSCLC cells via regulating this pathway [55] (Figure 3 and Table 1).
Figure 3.
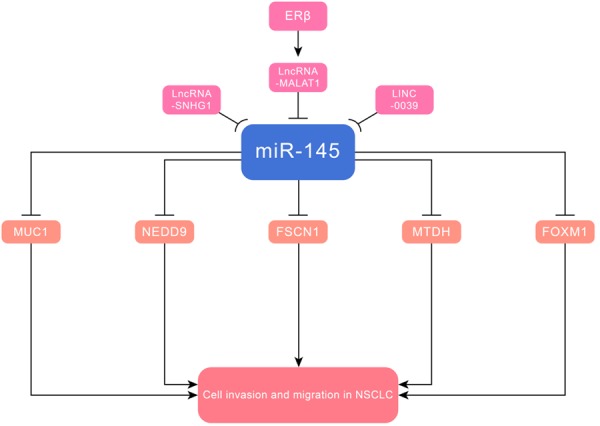
MiR-145 suppressed cell invasion and metastasis in NSCLC via targeting MUC1, NEDD9, FOXM1, MTDH and FSCN1.
MiR-145 in GBM
MiR-145 was reported to play a tumor-suppressive role in GBM, and its several gene targets were identified, including A disintegrin and metalloproteinase 17 (ADAM17) and EGFR [56], ROCK1 [57], ABCG2 [58], connective tissue growth factor (CTGF) [59], SOX9 and adducin 3 (ADD3) [60]. ADAM17 originally validated as the protease of the tumor necrosis factor-α (TNF-α) [61]. The miR-145/ADAM17 axis also played a role in hepatocellular carcinoma (HCC) [62] and renal cell carcinoma [63]. Besides, LncUCA1/miR-145/ADAM17 was shown to activate EMT and take part in the metastasis of nasopharyngeal carcinoma [64]. MiR-145 significantly inhibited cell invasion at least partially via downregulation of the RhoA/ROCK1 pathway. ABCG2 was an ATP-binding cassette transporter protein, which correlated with the phenotype of cancer stem cells [65]. MiR-145 targeted CTGF, which in turn downregulated downstream SPARC and focal adhesion kinase (FAK)/pFAK, leading to suppression of cell migration. CTGF could activate the FAK and extracellular signal-regulated kinase (ERK) pathways in chondrosarcoma cells [66]. CTGF, located on chromosome 6q23.1, is a member of the CCN family and a cell-adhesion factor [67]. Besides, miR-145 modulated proliferation, adhesion, and invasion of glioblastoma cells by targeting SOX9 and ADD3. The former was presumed to downregulate expression of cell cycle progression-related genes, including c-Myc, N-Myc and cyclin D1, resulting in inhibition of cell cycle progression. The latter inhibited the transcription of genes involved in cell migration (E-cadherin, N-cadherin), contributing to inhibition of cell invasion (Figure 4 and Table 1).
Figure 4.
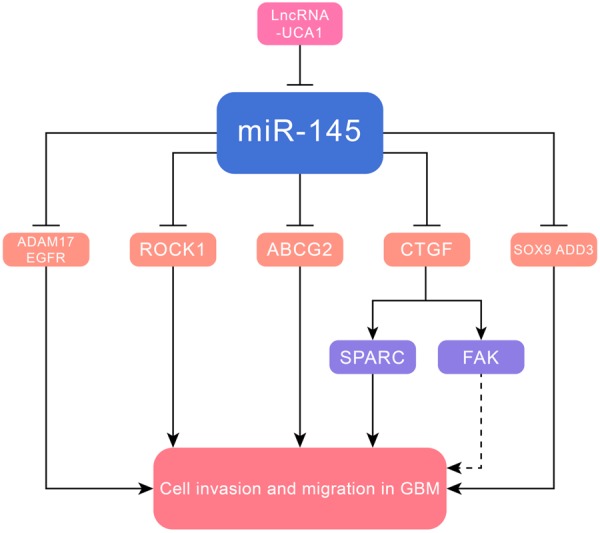
MiR-145 inhibited cell invasion and metastasis in GBM via targeting ADAM17, EGFR, ROCK1, ABCG2, CTGF, SOX9 and ADD3.
MiR-145 in OC
MiR-145 suppressed cell invasion and migration of OC, whose mediated targets were identified, including MTDH [68], RASA1 [69], DNA methyltransferases 3A (DNMT3A), hexokinase-2 (HK2) [70] and high-mobility group A2 (HMGA2) [71]. MiR-145 inhibited tumor growth and metastasis by targeting MTDH in high-grade serous OC, the most common and aggressive subtype of epithelial ovarian cancer. Circ-ITCH interacted with miR-145 to modulate its target RASA1, forming a circ-ITCH/miR-145/RASA1 axis. Circ-ITCH generated from several exons of itchy E3 ubiquitin protein ligase (ITCH), which was located on chromosome 20q11.22 on the plus strand [72]. RASA1, a member of the RAS-GAP family, could stimulate the GTPase activity of normal RASp21, resulting in aberrant intracellular signaling through RAF-MEK-ERK and PI3K-Akt pathways [73]. MiR-145 perturbed the Warburg effect by targeting DNMT3A and HK2, thereby inhibiting cell growth. The former was one of DNA methyltransferases that could promote the Warburg effect, and the latter was one of glycolytic enzymes controlled the Warburg effect. DNA methylation, which is controlled by DNMT, is a major epigenetic rule that controlled chromosomal stability and gene expression. Of note, DNMT3A did not interact with HK2, which was mediated by miR-145. MiR-145 overexpression correlated with DNMT3A downregulation, and DNMT3A also suppressed miR-145 through DNA methylation, suggesting the existence of a mutual negative feedback between miR-145 and DNMT3A [74,75]. The Warburg effect described by Warburg in the 1930s, is a well-known feature in cancer-specific metabolism and its emergence is associated with tumor progression [76,77] (Figure 5 and Table 1).
Figure 5.
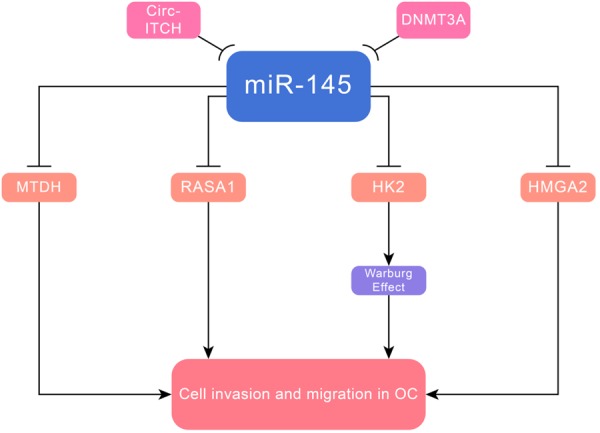
MiR-145 suppressed cell invasion and metastasis in OC via targeting MTDH, RASA1, DNMT3A, HK2 and HMGA2.
MiR-145 in bladder cancer
MiR-145 inhibited bladder cancer cell invasion via targeting PAK1, which promoted EMT through phosphorylating Snail. Both miR-145 and PAK1 modulated the activity of MMP-9, which was a critical step in tumor invasion [78]. LncRNA-UCA1, acting as an inducer of EMT, enhanced bladder cancer invasion and metastasis through the miR-145-ZEB1/2-FSCN1 pathway. Additionally, lncRNA-UCA1 and miR-145 constituted a reciprocal repression regulatory loop. Both ZEB1 and ZEB2 bound the E-BOX sequence in the E-cadherin promoter, thus repressing the initiation of EMT [79]. A recent study also reported a similar mechanism, in which up-regulation of lncRNA ROR regulated FSCN1 by serving as a molecular sponge for miR-145, contributing to inhibition of cell invasion and migration in ESCC [80]. FSCN1, an actin-binding protein, is involved in the formation of cytoplasmic microfilament bundles and actin-based cell surface protrusions [81]. MiR-145/FSCN1 also played a role in gastric cancer [82], laryngeal squamous cell carcinoma (LSCC) [83], BCa [84], NSCLC [85,86] and PCa [87]. MiR-145 targeted Kruppel-like factor 4 (KLF4), which regulated PTBP1, resulting in impairment of the Warburg effect (the PTBP1/PKMs axis) [88]. KLF4, a zinc-finger transcription factor expressed in the epithelium of various tissues, played a pivotal role in maintaining the self-renewal of embryonic stem cells [89,90]. Circ_0058063 served as a ceRNA that absorbed miR-145 to regulate its expression in bladder cancer. It regulated cell growth and migration of bladder cancer through modulating miR-145, which was mediated by CDK6 [91]. Overexpression of miR-145 obviously inhibited the transcription of Slug via down-regulating the specificity protein 1 (Sp1)/nuclear factor-κB (NF-κB) signaling pathway, which was an EMT-associated transcription factor [92] (Table 1).
MiR-145 in PCa
MiR-145 mediated targets directly related to cell invasion and migration in PCa were FSCN1 [87], SWAP70 [93] and human enhancer of filamentation 1 (HEF1) [94]. The PCAT-1/miR-145/FSCN1 regulatory axis inhibited cell invasion and migration in prostate cancer. Prostate cancer associated lncRNA transcript 1 (PCAT-1), a highly specific lncRNA, was a prostate-specific regulator located in the chromosome 8q30 gene [95,96]. SWAP70 was a 70-kDa nuclear protein originally isolated from activated B lymphocytes, whose overexpression altered the actin organization and lamellipodial morphology [97,98]. HEF1, also known as NEDD9 or Cas-L, is a cytoplasmic scaffolding protein that dramatically decreased E-cadherin and promoted EMT in prostate cancer, which were directly targeted by miR-145. HEF1 was also implicated to the TGF-β signaling pathway, which was important in EMT [94,99]. However, it was unclear whether miR-145/HEF1/TGF-β functioned in PCa (Table 1).
MiR-145 in GC
LncRNA taurine-upregulated gene 1 (TUG1) enhanced cell invasion in GC via negatively regulating miR-145 [100]. MiR-145 repressed the V-ets erythroblastosis virus E26 oncogene homolog 1 (Ets1) expression at the posttranscriptional level through directly targeting its 3’-UTR in GC cells [101]. The cellular homolog of retroviral V-ets oncogene, was a member of the Ets transcription factor family that participated in the migration, invasion, and angiogenesis of cancer cells [102,103]. LncRNA SNHG14 was remarkedly up-regulated in GC and could sponge miR-145, thus upregulating SOX9 and involving in PI3K/AKT/mTOR pathway [104]. Catenin-δ1 (CTNND1) was a member of the cadherin-catenin complex, and its cytoplasmic expression was inhibited by miR-145. Additionally, the miR-145 mediated decrease of N-cadherin rescued the membranous localization of E-cadherin and CTNND1, thus inhibiting cell invasion [105]. ZEB2, bound the E-BOX sequence in the E-cadherin promoter and suppressed EMT, leading to GC invasiveness [106] (Table 1).
MiR-145 in cervical cancer
MiR-145 inhibited migration and invasion of cervical cancer cells through targeting SIP1, a key activator of EMT. It also modulated the expression of Snail (SNAI1), up-regulation of which correlated with up-regulation of vimentin (VIM) and down-regulation of E-cadherin (CDH1), both of which were EMT-associated markers [107]. The zinc finger protein SIP1, also known as ZEB2, functioned as a transcriptional repressor of CDH1 [108]. MiR-145 expression in cervical cancer cells was wild-type p53-dependent, which enhanced the effects of p53 by suppressing the p53 inhibitors, thus inhibiting cell invasion [109]. MiR-145 negatively regulated cell invasion through targeting MYPT1 by directly binding to its 3’-UTR, thus increasing phosphorylation of myosin light chain (pMLC) and inhibiting cervical cell viability, migration and invasion. Besides, pMLC was involved in migration and invasion through modulating actomyosin contractile activity and cytoskeletal reorganization post-translationally [110]. MiR-145 could induce cancer stem cell (CSC) differentiation, whose overexpression down-regulated core stem cell transcription factors, such as OCT4, SOX2 and KLF4, which were essential for CSC maintenance, thereby decreasing tumor invasion and colony formation in cervical cancer [111] (Table 1).
MiR-145 in OS
MiR-145 was reported to play a tumor-suppressive role in OS, and its several gene targets were identified so far, including VEGF [112], MMP16 [113], CDK6 [114]. VEGF, an apparently endothelial cell-specific mitogen, was a signal protein that played a significant role in tumor development and metastasis [115,116]. MMP16 was one of the most significant MMPs in cell migration and tissue remodeling, which could directly degrade a few matrix molecules and triggered pro-MMP2 [117,118]. MMPs were the family of zinc- and calcium-dependent endopeptidases that played a crucial role in tumor metastasis and angiogenesis [119]. Futhermore, MMPs shared a number of features with another family of ADAMs, as mentioned above (Table 1).
MiR-145 in other cancers
MiR-145 could inhibit cell invasion and migration by targeting MYO5A in LSCC [120], mucin 13 (MUC13) in pancreatic cancer [121], c-Myc and CDK6 in oral squamous cell carcinoma (OSCC) [122], SOX11 in endometrial cancer [123], NRAS in melanoma [124], Novel (nua) kinase family 1 (NUAK1) in intrahepatic cholangiocarcinoma (ICC) [125] and ADAM19 in retinoblastoma [126]. Among these target genes, NRAS, belonging to the RAS superfamily of GTPases, were upstream factors of MAPK (RAS/RAF/MEK/ERK) signaling pathway and played an essential role in the progression of various cancers [127]. In addition, overexpression of miR-145 suppressed growth and invasion of ICC by targeting NUAK1/Akt/FOXO1 signaling, which was also associated with downregulation of MMP. NUAK1, an AMP-activated protein kinase also known as ARK5, could be phosphorylated and activated by Akt [128,129]. Futhermore, miR-145 inhibited growth and metastasis of thyroid cancer cells mediated by the PI3K/Akt pathway, as it directly targeted AKT3 and thus reducing Akt phosphorylation [130]. MiR-145 was a potential protective miRNA of most cancers, but intriguingly, its upregulation stimulated both migration and invasion by targeting SMAD5 in esophageal cancer, serving as a positive regulator of SMAD5 expression an independent prognostic factor for overall survival of esophageal cancer [131] (Table 1).
MiR-145 in potential clinical application
Accumulating evidence suggested that miR-145 played profound roles in tumor migration and invasion via targeting key transcription factors or critical pathways, and miR-145 expression was of promising clinical utility. Low miR-145 expression was associated with advanced stage disease and tumor aggressiveness, indicating it might serve as a powerful predictor of outcomes and a potential biomarker of poor prognosis in cancer patients. A number of studies reported that dysregulated miRNAs showed alterations at the early stages of tumorigenesis. Moreover, miRNAs can be circulated in body fluid, suggesting their values as non-invasive biomarkers. Circulating miRNAs were uniquely useful for stratification and of intriguing quality, which made them potential biomarkers for cancers [132]. The biomarkers found in patient biofluids (i.e. blood and urine) were likely to be more representative of the whole tumor’s genomic landscape compared to tumor sampling [133,134]. With a better understanding of miR-145 and its targets, along with its associated pathways, we could disclose a brand new mechanism in malignancy therapy. Taken together, miR-145 might serve a useful biomarker of poor prognosis, monitor cancer progression and treatment response, and optimize personalized treatment regimens.
Discussion and prospects
There was growing body of evidences on the functions of miRNAs in regulating cell migration and invasion of diverse malignancies, which attracted much attention and research interest. MiR-145 functioned via regulating its downstream molecules, either directed or indirectly through its upstream RNA molecules, such as lncRNA and circRNAs, which both served as the ceRNAs to sequester miRNAs away from target mRNAs, or lncRNAs derepressed target mRNAs expression by competitively binding to miRNAs [135]. MiRNAs were characterized as critical components of cancer biological processes, including tumorigenesis, proliferation, differentiation, apoptosis, metastasis, angiogenesis, drug-resistance and EMT regulation. Notably, EMT and angiogenesis could trigger tumor invasion and metastasis. For instance, miR-145 played a profound role in angiogenesis and vascular development via targeting the friend leukemia virus integration 1 (Fli1), an early marker of hemangioblast transcription and differentiation, thus inhibiting migration [136,137]. Tumor migration and invasion, the commonly known causes of cancer-related deaths, were involved in advanced stages of tumor progression. In this review, we summarized the recent findings regarding miR-145 and focused on its mechanistic involvement in cell migration and invasion of multiple cancers. In conclusion, miR-145 could be a good candidate for the targeted therapy of cancers, especially the invasive cancers. Above all, miRNA-based therapeutics is promising, and elevation or inhibition of miR-145 has been proposed as a possible therapeutic strategy of cancers, but further investigation is needed prior to clinical application.
Acknowledgements
We thank Dan-Dan Wang for useful discussions and help in revision of the present paper. This work was supported by the National Key Research and Development Program of China (2016YFC0905900) and National Natural Science Foundation of China (81872365).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs--the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 3.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. a comprehensive review. EMBO Mol Med. 2017;9:852. doi: 10.15252/emmm.201707779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaksman O, Stavnes HT, Kaern J, Trope CG, Davidson B, Reich R. miRNA profiling along tumour progression in ovarian carcinoma. J Cell Mol Med. 2011;15:1593–1602. doi: 10.1111/j.1582-4934.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 6.Michael MZ, O’ Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 7.Lakshmipathy U, Love B, Adams C, Thyagarajan B, Chesnut JD. Micro RNA profiling: an easy and rapid method to screen and characterize stem cell populations. Methods Mol Biol. 2007;407:97–114. doi: 10.1007/978-1-59745-536-7_8. [DOI] [PubMed] [Google Scholar]
- 8.Sheng N, Tan G, You W, Chen H, Gong J, Chen D, Zhang H, Wang Z. MiR-145 inhibits human colorectal cancer cell migration and invasion via PAK4-dependent pathway. Cancer Med. 2017;6:1331–1340. doi: 10.1002/cam4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang H, Li K, Zheng J, Dou X, Zhao Y, Wang L. microRNA-145 regulates tumor suppressor candidate 3 and mitogen-activated protein kinase pathway to inhibit the progression of colorectal cancer. J Cell Biochem. 2018 doi: 10.1002/jcb.28122. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang BH. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Zhang X, Hu X, Zhou W, Zhang P, Zhang J, Yang S, Liu Y. The effects of lncRNA MALAT1 on proliferation, invasion and migration in colorectal cancer through regulating SOX9. Mol Med. 2018;24:52. doi: 10.1186/s10020-018-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei AW, Li LF. Long non-coding RNA SOX21-AS1 sponges miR-145 to promote the tumorigenesis of colorectal cancer by targeting MYO6. Biomed Pharmacother. 2017;96:953–959. doi: 10.1016/j.biopha.2017.11.145. [DOI] [PubMed] [Google Scholar]
- 13.Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem. 2007;282:32582–32590. doi: 10.1074/jbc.M702806200. [DOI] [PubMed] [Google Scholar]
- 14.Sathyanarayanan A, Chandrasekaran KS, Karunagaran D. microRNA-145 downregulates SIP1-expression but differentially regulates proliferation, migration, invasion and Wnt signaling in SW480 and SW620 cells. J Cell Biochem. 2018;119:2022–2035. doi: 10.1002/jcb.26365. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Zhu J, Ou C, Deng Z, Chen M, Huang W, Li L. MicroRNA-145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin-1. Br J Cancer. 2014;110:2300–2309. doi: 10.1038/bjc.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Li X, Ke Q, Li Y, Liu F, Zhu G, Li F. DGCR6L, a novel PAK4 interaction protein, regulates PAK4-mediated migration of human gastric cancer cell via LIMK1. Int J Biochem Cell Biol. 2010;42:70–79. doi: 10.1016/j.biocel.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed T, Shea K, Masters JR, Jones GE, Wells CM. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal. 2008;20:1320–1328. doi: 10.1016/j.cellsig.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 18.MacGrogan D, Levy A, Bova GS, Isaacs WB, Bookstein R. Structure and methylation-associated silencing of a gene within a homozygously deleted region of human chromosome band 8p22. Genomics. 1996;35:55–65. doi: 10.1006/geno.1996.0322. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang S, Ding Y, Lin J. TUSC3 promotes colorectal cancer progression and epithelial-mesenchymal transition (EMT) through WNT/beta-catenin and MAPK signalling. J Pathol. 2016;239:60–71. doi: 10.1002/path.4697. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 22.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 23.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Liu Z, Jiang B, Peng R, Ma Z, Lu J. SOX9 overexpression promotes glioma metastasis via wnt/β-catenin signaling. Cell Biochem Biophys. 2015;73:205–212. doi: 10.1007/s12013-015-0647-z. [DOI] [PubMed] [Google Scholar]
- 27.Yang CM, Wang TH, Chen HC, Li SC, Lee MC, Liou HH, Liu PF, Tseng YK, Shiue YL, Ger LP, Tsai KW. Aberrant DNA hypermethylation-silenced SOX21-AS1 gene expression and its clinical importance in oral cancer. Clin Epigenetics. 2016;8:129. doi: 10.1186/s13148-016-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X, Huang C, He X, Liu X, Ji J, Zhang E, Wang W, Guo R. A novel long non-coding RNA, SOX21-AS1, indicates a poor prognosis and promotes lung adenocarcinoma proliferation. Cell Physiol Biochem. 2017;42:1857–1869. doi: 10.1159/000479543. [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y, Yang S, Zeng Z, Liao W, Ding YQ, Liang L. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7:26680–26691. doi: 10.18632/oncotarget.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eades G, Wolfson B, Zhang Y, Li Q, Yao Y, Zhou Q. lincRNA-RoR and miR-145 regulate invasion in triple-negative breast cancer via targeting ARF6. Mol Cancer Res. 2015;13:330–338. doi: 10.1158/1541-7786.MCR-14-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M, Ibrahim SA, Peddibhotla S, Teng YH, Low JY, Ebnet K, Kiesel L, Yip GW. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569–6580. doi: 10.1038/onc.2010.386. [DOI] [PubMed] [Google Scholar]
- 32.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng M, Sun X, Li Y, Zuo W. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol. 2016;37:8189–8196. doi: 10.1007/s13277-015-4722-2. [DOI] [PubMed] [Google Scholar]
- 34.Feng W, Wang C, Liang C, Yang H, Chen D, Yu X, Zhao W, Geng D, Li S, Chen Z, Sun M. The dysregulated expression of KCNQ1OT1 and its interaction with downstream factors miR-145/CCNE2 in breast cancer cells. Cell Physiol Biochem. 2018;49:432–446. doi: 10.1159/000492978. [DOI] [PubMed] [Google Scholar]
- 35.Kuang WB, -C Deng Q, Deng CT, Li WS, Zhang YG, Shu SW, Zhou MR. MiRNA regulates OCT4 expression in breast cancer cells. Eur Rev Med Pharmacol Sci. 2018;22:1351–1357. doi: 10.26355/eurrev_201803_14478. [DOI] [PubMed] [Google Scholar]
- 36.Tang L, Wei D, Yan F. MicroRNA-145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple-negative breast cancer. Cancer Gene Ther. 2016;23:258–265. doi: 10.1038/cgt.2016.27. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, Zhou H, Zhao RC. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34:1461–1466. [PubMed] [Google Scholar]
- 38.Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 40.Chiesa N, De Crescenzo A, Mishra K, Perone L, Carella M, Palumbo O, Mussa A, Sparago A, Cerrato F, Russo S, Lapi E, Cubellis MV, Kanduri C, Cirillo Silengo M, Riccio A, Ferrero GB. The KCNQ1OT1 imprinting control region and non-coding RNA: new properties derived from the study of Beckwith-Wiedemann syndrome and Silver-Russell syndrome cases. Hum Mol Genet. 2012;21:10–25. doi: 10.1093/hmg/ddr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McSherry EA, McGee SF, Jirstrom K, Doyle EM, Brennan DJ, Landberg G, Dervan PA, Hopkins AM, Gallagher WM. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int J Cancer. 2009;125:1343–1351. doi: 10.1002/ijc.24498. [DOI] [PubMed] [Google Scholar]
- 42.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 43.Jones RM, Branda J, Johnston KA, Polymenis M, Gadd M, Rustgi A, Callanan L, Schmidt EV. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol Cell Biol. 1996;16:4754–4764. doi: 10.1128/mcb.16.9.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, O’Connell BC, Mateyak MK, Tam W, Kohlhuber F, Dang CV, Sedivy JM, Eick D, Vogelstein B, Kinzler KW. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci U S A. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32:772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7:657–671. doi: 10.2217/fca.11.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khodarev NN, Pitroda SP, Beckett MA, MacDermed DM, Huang L, Kufe DW, Weichselbaum RR. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye Z, Shen N, Weng Y, Li K, Hu L, Liao H, An J, Liu L, Lao S, Cai S. Low miR-145 silenced by DNA methylation promotes NSCLC cell proliferation, migration and invasion by targeting mucin 1. Cancer Biol Ther. 2015;16:1071–1079. doi: 10.1080/15384047.2015.1046024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu W, Ding J, He M, Chen Y, Wang R, Han Z, Xing EZ, Zhang C, Yeh S. Estrogen receptor beta promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene. 2019;38:1225–1238. doi: 10.1038/s41388-018-0463-1. [DOI] [PubMed] [Google Scholar]
- 50.Yuan Y, Haiying G, Zhuo L, Ying L, Xin H. Long non-coding RNA LINC00339 facilitates the tumorigenesis of non-small cell lung cancer by sponging miR-145 through targeting FOXM1. Biomed Pharmacother. 2018;105:707–713. doi: 10.1016/j.biopha.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Lu Q, Shan S, Li Y, Zhu D, Jin W, Ren T. Long noncoding RNA SNHG1 promotes non-small cell lung cancer progression by up-regulating MTDH via sponging miR-145-5p. FASEB J. 2018;32:3957–3967. doi: 10.1096/fj.201701237RR. [DOI] [PubMed] [Google Scholar]
- 52.Duraisamy S, Kufe T, Ramasamy S, Kufe D. Evolution of the human MUC1 oncoprotein. Int J Oncol. 2007;31:671–677. [PubMed] [Google Scholar]
- 53.Kalinichenko VV, Kalin TV. Is there potential to target FOXM1 for ‘undruggable’ lung cancers? Expert Opin Ther Targets. 2015;19:865–867. doi: 10.1517/14728222.2015.1042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y, Zhong DW. AEG-1 is associated with hypoxia-induced hepatocellular carcinoma chemoresistance via regulating PI3K/AKT/HIF-1alpha/MDR-1 pathway. EXCLI J. 2016;15:745–757. doi: 10.17179/excli2016-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng X, Thiel KW, Leslie KK. Drug resistance mediated by AEG-1/MTDH/LYRIC. Adv Cancer Res. 2013;120:135–157. doi: 10.1016/B978-0-12-401676-7.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, Chopp M, Zheng X, Katakowski M, Buller B, Jiang F. MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol Rep. 2013;29:67–72. doi: 10.3892/or.2012.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan X, Cheng Q, Peng R, Ma Z, Chen Z, Cao Y, Jiang B. ROCK1, a novel target of miR-145, promotes glioma cell invasion. Mol Med Rep. 2014;9:1877–1882. doi: 10.3892/mmr.2014.1982. [DOI] [PubMed] [Google Scholar]
- 58.Shi L, Wang Z, Sun G, Wan Y, Guo J, Fu X. miR-145 inhibits migration and invasion of glioma stem cells by targeting ABCG2. Neuromolecular Med. 2014;16:517–528. doi: 10.1007/s12017-014-8305-y. [DOI] [PubMed] [Google Scholar]
- 59.Lee HK, Bier A, Cazacu S, Finniss S, Xiang C, Twito H, Poisson LM, Mikkelsen T, Slavin S, Jacoby E, Yalon M, Toren A, Rempel SA, Brodie C. MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PLoS One. 2013;8:e54652. doi: 10.1371/journal.pone.0054652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rani SB, Rathod SS, Karthik S, Kaur N, Muzumdar D, Shiras AS. MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro Oncol. 2013;15:1302–1316. doi: 10.1093/neuonc/not090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bell JH, Herrera AH, Li Y, Walcheck B. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J Leukoc Biol. 2007;82:173–176. doi: 10.1189/jlb.0307193. [DOI] [PubMed] [Google Scholar]
- 62.Yang XW, Zhang LJ, Huang XH, Chen LZ, Su Q, Zeng WT, Li W, Wang Q. miR-145 suppresses cell invasion in hepatocellular carcinoma cells: miR-145 targets ADAM17. Hepatol Res. 2014;44:551–559. doi: 10.1111/hepr.12152. [DOI] [PubMed] [Google Scholar]
- 63.Doberstein K, Steinmeyer N, Hartmetz AK, Eberhardt W, Mittelbronn M, Harter PN, Juengel E, Blaheta R, Pfeilschifter J, Gutwein P. MicroRNA-145 targets the metalloprotease ADAM17 and is suppressed in renal cell carcinoma patients. Neoplasia. 2013;15:218–230. doi: 10.1593/neo.121222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu J, Du M, Zhang Q, Zhang W, Fan Y, Yin L, Fei Q, Jiang X, Chen W, Zhu H, Yan P, He X, Bian X. Long noncoding RNA UCA1 promotes the proliferation, invasion, and migration of nasopharyngeal carcinoma cells via modulation of miR-145. Onco Targets Ther. 2018;11:7483–7492. doi: 10.2147/OTT.S182290. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Wang F, Xue X, Wei J, An Y, Yao J, Cai H, Wu J, Dai C, Qian Z, Xu Z, Miao Y. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Tan TW, Lai CH, Huang CY, Yang WH, Chen HT, Hsu HC, Fong YC, Tang CH. CTGF enhances migration and MMP-13 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Biochem. 2009;107:345–356. doi: 10.1002/jcb.22132. [DOI] [PubMed] [Google Scholar]
- 67.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 68.Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, Li Y, Yang Q, Liu J, Wei JJ, Shao C, Liu Z, Kong B. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5:10816–10829. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu J, Wang L, Chen J, Gao H, Zhao W, Huang Y, Jiang T, Zhou J, Chen Y. The circular RNA circ-ITCH suppresses ovarian carcinoma progression through targeting miR-145/RASA1 signaling. Biochem Biophys Res Commun. 2018;505:222–228. doi: 10.1016/j.bbrc.2018.09.060. [DOI] [PubMed] [Google Scholar]
- 70.Zhang S, Pei M, Li Z, Li H, Liu Y, Li J. Double-negative feedback interaction between DNA methyltransferase 3A and microRNA-145 in the Warburg effect of ovarian cancer cells. Cancer Sci. 2018;109:2734–2745. doi: 10.1111/cas.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim TH, Song JY, Park H, Jeong JY, Kwon AY, Heo JH, Kang H, Kim G, An HJ. miR-145, targeting high-mobility group A2, is a powerful predictor of patient outcome in ovarian carcinoma. Cancer Lett. 2015;356:937–945. doi: 10.1016/j.canlet.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Lee JY, Kim S, Kim YT, Lim MC, Lee B, Jung KW, Kim JW, Park SY, Won YJ. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer. 2018;18:601. doi: 10.1186/s12885-018-4498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wittinghofer A. Felix hoppe-seyler lecture 1998: signal transduction via ras. Biological Chemistry. 1998;379:933–938. [Google Scholar]
- 74.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 75.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 76.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 77.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 78.Kou B, Gao Y, Du C, Shi Q, Xu S, Wang CQ, Wang X, He D, Guo P. miR-145 inhibits invasion of bladder cancer cells by targeting PAK1. Urol Oncol. 2014;32:846–854. doi: 10.1016/j.urolonc.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Xue M, Pang H, Li X, Li H, Pan J, Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107:18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shang M, Wang X, Zhang Y, Gao Z, Wang T, Liu R. LincRNA-ROR promotes metastasis and invasion of esophageal squamous cell carcinoma by regulating miR-145/FSCN1. Onco Targets Ther. 2018;11:639–649. doi: 10.2147/OTT.S157638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol. 2005;37:1787–1804. doi: 10.1016/j.biocel.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Chen JJ, Cai WY, Liu XW, Luo QC, Chen G, Huang WF, Li N, Cai JC. Reverse correlation between microRNA-145 and FSCN1 affecting gastric cancer migration and invasion. PLoS One. 2015;10:e0126890. doi: 10.1371/journal.pone.0126890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao W, Zhang C, Li W, Li H, Sang J, Zhao Q, Bo Y, Luo H, Zheng X, Lu Y, Shi Y, Yang D, Zhang R, Li Z, Cui J, Zhang Y, Niu M, Li J, Wu Z, Guo H, Xiang C, Wang J, Hou J, Zhang L, Thorne RF, Cui Y, Wu Y, Wen S, Wang B. Promoter methylation-regulated miR-145-5p inhibits laryngeal squamous cell carcinoma progression by targeting FSCN1. Mol Ther. 2019;27:365–379. doi: 10.1016/j.ymthe.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao H, Kang X, Xia X, Wo L, Gu X, Hu Y, Xie X, Chang H, Lou L, Shen X. miR-145 suppresses breast cancer cell migration by targeting FSCN-1 and inhibiting epithelial-mesenchymal transition. Am J Transl Res. 2016;8:3106–3114. [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Lin Q. MicroRNA-145 inhibits migration and invasion by down-regulating FSCN1 in lung cancer. Int J Clin Exp Med. 2015;8:8794–8802. [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Yang X, Wu H, Zhou W, Liu Z. MicroRNA-145 inhibits migration and invasion via inhibition of fascin 1 protein expression in non-small-cell lung cancer cells. Mol Med Rep. 2015;12:6193–6198. doi: 10.3892/mmr.2015.4163. [DOI] [PubMed] [Google Scholar]
- 87.Xu W, Chang J, Du X, Hou J. Long non-coding RNA PCAT-1 contributes to tumorigenesis by regulating FSCN1 via miR-145-5p in prostate cancer. Biomed Pharmacother. 2017;95:1112–1118. doi: 10.1016/j.biopha.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 88.Minami K, Taniguchi K, Sugito N, Kuranaga Y, Inamoto T, Takahara K, Takai T, Yoshikawa Y, Kiyama S, Akao Y, Azuma H. MiR-145 negatively regulates Warburg effect by silencing KLF4 and PTBP1 in bladder cancer cells. Oncotarget. 2017;8:33064–33077. doi: 10.18632/oncotarget.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, McClintick J, Zhong L, Edenberg HJ, Yoder MC, Chan RJ. Murine embryonic stem cell differentiation is promoted by SOCS-3 and inhibited by the zinc finger transcription factor Klf4. Blood. 2005;105:635–637. doi: 10.1182/blood-2004-07-2681. [DOI] [PubMed] [Google Scholar]
- 91.Sun M, Zhao W, Chen Z, Li M, Li S, Wu B, Bu R. Circ_0058063 regulates CDK6 to promote bladder cancer progression by sponging miR-145-5p. J Cell Physiol. 2019;234:4812–4824. doi: 10.1002/jcp.27280. [DOI] [PubMed] [Google Scholar]
- 92.Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J, Shi ZZ. miR-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the Sp1/NF-kappaB signaling pathway in esophageal squamous cell carcinoma. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18091833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiyomaru T, Tatarano S, Kawakami K, Enokida H, Yoshino H, Nohata N, Fuse M, Seki N, Nakagawa M. SWAP70, actin-binding protein, function as an oncogene targeting tumor-suppressive miR-145 in prostate cancer. Prostate. 2011;71:1559–1567. doi: 10.1002/pros.21372. [DOI] [PubMed] [Google Scholar]
- 94.Guo W, Ren D, Chen X, Tu X, Huang S, Wang M, Song L, Zou X, Peng X. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem. 2013;114:1606–1615. doi: 10.1002/jcb.24502. [DOI] [PubMed] [Google Scholar]
- 95.Walsh AL, Tuzova AV, Bolton EM, Lynch TH, Perry AS. Long noncoding RNAs and prostate carcinogenesis: the missing ‘linc’? Trends Mol Med. 2014;20:428–436. doi: 10.1016/j.molmed.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 96.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X, Jing X, Wang X, Siddiqui J, Wei JT, Robinson D, Iyer HK, Palanisamy N, Maher CA, Chinnaiyan AM. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borggrefe T, Wabl M, Akhmedov AT, Jessberger R. A B-cell-specific DNA recombination complex. J Biol Chem. 1998;273:17025–17035. doi: 10.1074/jbc.273.27.17025. [DOI] [PubMed] [Google Scholar]
- 98.Hilpela P, Oberbanscheidt P, Hahne P, Hund M, Kalhammer G, Small JV, Bahler M. SWAP-70 identifies a transitional subset of actin filaments in motile cells. Mol Biol Cell. 2003;14:3242–3253. doi: 10.1091/mbc.E03-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ren K, Li Z, Li Y, Zhang W, Han X. Long noncoding rna taurine-upregulated gene 1 promotes cell proliferation and invasion in gastric cancer via negatively modulating miRNA-145-5p. Oncol Res. 2017;25:789–798. doi: 10.3727/096504016X14783677992682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng L, Pu J, Qi T, Qi M, Li D, Xiang X, Huang K, Tong Q. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol Cancer Res. 2013;11:182–193. doi: 10.1158/1541-7786.MCR-12-0534. [DOI] [PubMed] [Google Scholar]
- 102.Furlan A, Vercamer C, Desbiens X, Pourtier A. Ets-1 triggers and orchestrates the malignant phenotype of mammary cancer cells within their matrix environment. J Cell Physiol. 2008;215:782–793. doi: 10.1002/jcp.21360. [DOI] [PubMed] [Google Scholar]
- 103.Seth A, Papas TS. The c-ets-1 proto-oncogene has oncogenic activity and is positively autoregulated. Oncogene. 1990;5:1761–1767. [PubMed] [Google Scholar]
- 104.Liu Z, Yan Y, Cao S, Chen Y. Long non-coding RNA SNHG14 contributes to gastric cancer development through targeting miR-145/SOX9 axis. J Cell Biochem. 2018;119:6905–6913. doi: 10.1002/jcb.26889. [DOI] [PubMed] [Google Scholar]
- 105.Xing AY, Wang YW, Su ZX, Shi DB, Wang B, Gao P. Catenin-delta1, negatively regulated by miR-145, promotes tumour aggressiveness in gastric cancer. J Pathol. 2015;236:53–64. doi: 10.1002/path.4495. [DOI] [PubMed] [Google Scholar]
- 106.Jiang SB, He XJ, Xia YJ, Hu WJ, Luo JG, Zhang J, Tao HQ. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2305–2315. doi: 10.2147/OTT.S101853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Sathyanarayanan A, Chandrasekaran KS, Karunagaran D. microRNA-145 modulates epithelial-mesenchymal transition and suppresses proliferation, migration and invasion by targeting SIP1 in human cervical cancer cells. Cell Oncol (Dordr) 2017;40:119–131. doi: 10.1007/s13402-016-0307-3. [DOI] [PubMed] [Google Scholar]
- 108.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 109.Shi M, Du L, Liu D, Qian L, Hu M, Yu M, Yang Z, Zhao M, Chen C, Guo L, Wang L, Song L, Ma Y, Guo N. Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J Pathol. 2012;228:148–157. doi: 10.1002/path.3997. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez-Torres A, Banuelos-Villegas EG, Martinez-Acuna N, Sulpice E, Gidrol X, Alvarez-Salas LM. MYPT1 is targeted by miR-145 inhibiting viability, migration and invasion in 2D and 3D HeLa cultures. Biochem Biophys Res Commun. 2018;507:348–354. doi: 10.1016/j.bbrc.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 111.Zhou X, Yue Y, Wang R, Gong B, Duan Z. MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer stem cells. Int J Oncol. 2017;50:853–862. doi: 10.3892/ijo.2017.3857. [DOI] [PubMed] [Google Scholar]
- 112.Fan L, Wu Q, Xing X, Wei Y, Shao Z. MicroRNA-145 targets vascular endothelial growth factor and inhibits invasion and metastasis of osteosarcoma cells. Acta Biochim Biophys Sin (Shanghai) 2012;44:407–414. doi: 10.1093/abbs/gms019. [DOI] [PubMed] [Google Scholar]
- 113.Chen B, Huang Z, Zhang Y, Chen Y, Li Z. MicroRNA-145 suppresses osteosarcoma metastasis via targeting MMP16. Cell Physiol Biochem. 2015;37:2183–2193. doi: 10.1159/000438575. [DOI] [PubMed] [Google Scholar]
- 114.Li Y, Liu J, Liu ZZ, Wei WB. MicroRNA-145 inhibits tumour growth and metastasis in osteosarcoma by targeting cyclin-dependent kinase, CDK6. Eur Rev Med Pharmacol Sci. 2016;20:5117–5125. [PubMed] [Google Scholar]
- 115.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 116.Jain L, Vargo CA, Danesi R, Sissung TM, Price DK, Venzon D, Venitz J, Figg WD. The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol Cancer Ther. 2009;8:2496–2508. doi: 10.1158/1535-7163.MCT-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Al-Raawi D, Abu-El-Zahab H, El-Shinawi M, Mohamed MM. Membrane type-1 matrix metalloproteinase (MT1-MMP) correlates with the expression and activation of matrix metalloproteinase-2 (MMP-2) in inflammatory breast cancer. Int J Clin Exp Med. 2011;4:265–275. [PMC free article] [PubMed] [Google Scholar]
- 118.Azzam HS, Arand G, Lippman ME, Thompson EW. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Natl Cancer Inst. 1993;85:1758–1764. doi: 10.1093/jnci/85.21.1758. [DOI] [PubMed] [Google Scholar]
- 119.Cavdar Z, Canda AE, Terzi C, Sarioglu S, Fuzun M, Oktay G. Role of gelatinases (matrix metalloproteinases 2 and 9), vascular endothelial growth factor and endostatin on clinicopathological behaviour of rectal cancer. Colorectal Dis. 2011;13:154–160. doi: 10.1111/j.1463-1318.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhao X, Zhang W, Ji W. MYO5A inhibition by miR-145 acts as a predictive marker of occult neck lymph node metastasis in human laryngeal squamous cell carcinoma. Onco Targets Ther. 2018;11:3619–3635. doi: 10.2147/OTT.S164597. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Khan S, Ebeling MC, Zaman MS, Sikander M, Yallapu MM, Chauhan N, Yacoubian AM, Behrman SW, Zafar N, Kumar D, Thompson PA, Jaggi M, Chauhan SC. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget. 2014;5:7599–7609. doi: 10.18632/oncotarget.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shao Y, Qu Y, Dang S, Yao B, Ji M. MiR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by targeting c-Myc and Cdk6. Cancer Cell Int. 2013;13:51. doi: 10.1186/1475-2867-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang L, Yuan Z, Shi H, Bian Y, Guo R. miR-145 targets the SOX11 3’UTR to suppress endometrial cancer growth. Am J Cancer Res. 2017;7:2305–2317. [PMC free article] [PubMed] [Google Scholar]
- 124.Liu S, Gao G, Yan D, Chen X, Yao X, Guo S, Li G, Zhao Y. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K/AKT pathways. Cancer Med. 2017;6:819–833. doi: 10.1002/cam4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Xiong X, Sun D, Chai H, Shan W, Yu Y, Pu L, Cheng F. MiR-145 functions as a tumor suppressor targeting NUAK1 in human intrahepatic cholangiocarcinoma. Biochem Biophys Res Commun. 2015;465:262–269. doi: 10.1016/j.bbrc.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 126.Sun Z, Zhang A, Jiang T, Du Z, Che C, Wang F. MiR-145 suppressed human retinoblastoma cell proliferation and invasion by targeting ADAM19. Int J Clin Exp Pathol. 2015;8:14521–14527. [PMC free article] [PubMed] [Google Scholar]
- 127.Liu SM, Lu J, Lee HC, Chung FH, Ma N. miR-524-5p suppresses the growth of oncogenic BRAF melanoma by targeting BRAF and ERK2. Oncotarget. 2014;5:9444–9459. doi: 10.18632/oncotarget.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suzuki A, Lu J, Kusakai G, Kishimoto A, Ogura T, Esumi H. ARK5 is a tumor invasion-associated factor downstream of Akt signaling. Mol Cell Biol. 2004;24:3526–3535. doi: 10.1128/MCB.24.8.3526-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki A, Kusakai G, Kishimoto A, Lu J, Ogura T, Lavin MF, Esumi H. Identification of a novel protein kinase mediating Akt survival signaling to the ATM protein. J Biol Chem. 2003;278:48–53. doi: 10.1074/jbc.M206025200. [DOI] [PubMed] [Google Scholar]
- 130.Boufraqech M, Zhang L, Jain M, Patel D, Ellis R, Xiong Y, He M, Nilubol N, Merino MJ, Kebebew E. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21:517–531. doi: 10.1530/ERC-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Q, Gan H, Song W, Chai D, Wu S. MicroRNA-145 promotes esophageal cancer cells proliferation and metastasis by targeting SMAD5. Scand J Gastroenterol. 2018;53:769–776. doi: 10.1080/00365521.2018.1476913. [DOI] [PubMed] [Google Scholar]
- 132.Hoey C, Ahmed M, Fotouhi Ghiam A, Vesprini D, Huang X, Commisso K, Commisso A, Ray J, Fokas E, Loblaw DA, He HH, Liu SK. Circulating miRNAs as non-invasive biomarkers to predict aggressive prostate cancer after radical prostatectomy. J Transl Med. 2019;17:173. doi: 10.1186/s12967-019-1920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hocking J, Mithraprabhu S, Kalff A, Spencer A. Liquid biopsies for liquid tumors: emerging potential of circulating free nucleic acid evaluation for the management of hematologic malignancies. Cancer Biol Med. 2016;13:215–225. doi: 10.20892/j.issn.2095-3941.2016.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krishnamurthy N, Spencer E, Torkamani A, Nicholson L. Liquid biopsies for cancer: coming to a patient near you. J Clin Med. 2017;6 doi: 10.3390/jcm6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larsson E, Fredlund Fuchs P, Heldin J, Barkefors I, Bondjers C, Genove G, Arrondel C, Gerwins P, Kurschat C, Schermer B, Benzing T, Harvey SJ, Kreuger J, Lindahl P. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1:108. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


