Abstract
Purpose
As an alleviative treatment measured in patients with unresectable advanced pancreatic cancer, radiofrequency ablation (RFA) needed more clinical data to prove its advantages and to explore limitations in its utilization. This study was determined to observe the efficacy of RFA, and to explore its impact on perioperative periphery carcinoma as well as the normal pancreatic tissues.
Methods
Clinical data of 32 patients with pancreatic cancer accepted RFA surgery were collected. Followed up patients’ pain degree and the changes in serum tumor markers CA19-9 and CA 242 before and after surgery. Ex vivo, gave human pancreatic cancer cell line PANC-1 heat treatment to simulate the heat exposure condition periphery carcinoma was experienced during RFA surgery, and to observe the proliferation rate and HSP70 expression change compared with control group.
Results
Of the 32 patients, 1 died of upper gastrointestinal hemorrhage, and 29 survived for more than 5 months, 2 of which for more than 16 months. The average CA19-9 and CA 242 levels of the patients were significantly decreased in 3 months after surgery (t = 9.873, 5.978, P < 0.001). During in vitro experiments, the proliferation rate of PANC-1 cells after heating was significantly increased, accompanied with the increased HSP70 expression. The addition of HSP70 inhibitor can inhibit the rise of proliferation after heat therapy.
Conclusion
Utilizing RFA treat patients with unresectable advanced pancreatic cancer, could effectively relieve the pain, decline jaundice, and deduce tumor marker levels significantly. However, it failed to extend the long-term survival rate of the patients significantly. This study found that a higher proliferative rate accompanied with a higher HSP70 expression level were observed on in vitro cultured pancreatic carcinoma cells after heat treatment, which could be altered by HSP70 inhibitor. And these findings indicated that the heat exposure might impact periphery carcinoma during RFA surgery and HSP70 might play an important role in patients’ prognosis.
Keywords: Pancreatic cancer, Radiofrequency ablation, Proliferation, HSP70
Highlights
-
•
RFA alleviated the symptoms in pancreatic cancer.
-
•
Higher Hsp70 expression induced proliferation in PANC-1.
-
•
Heat expose impact pancreatic cells.
1. Introduction
Pancreatic cancer is highly malignant, and radical resection is the only treatment method [1,2], but currently, only 15–20% of patients are eligible for surgical resection, and 30–40% of patients are diagnosed as non-metastatic and unresectable advanced pancreatic cancer [3,4]. For patients who have lost their opportunity to accept a surgery, even with chemotherapy, the survival time rarely exceeds 500 days [5,6]. For such patients, palliative treatment is an option that can extend life and improve living quality, such as Radiofrequency Ablation (RFA).
Radiofrequency ablation is a thermotherapy technique that uses high-frequency alternating current to cause tissue coagulation and necrosis, thereby killing tumor cells. It can be used percutaneously or in combination with endoscopic ultrasonography [7]. Comparing with traditional radical resection, it is easier to recover after RFA surgery [8]. Although RFA has been widely used to treat solid tumors such as liver cancer [9,10] and colorectal cancer [11], since pancreatic tissue is sensitive to heat, rich in blood vessels, and the anatomical position is close to arteries and bile ducts, there's a high risk in applying thermotherapy technique. As RFA application becomes increasingly mature, the incidence of postoperative complications has decreased significantly [7,[12], [13], [14]]. A clinical survey in Italy pointed out that RFA can significantly eliminate pancreatic tumors, resulting in an average survival rate of 185 days [6].
However, this data suggests that RFA still cannot improve the patient's five-year survival rate. Considering the absence of membrane on pancreatic tissue, cancerous tissue tends to invade nearby blood vessels and bile ducts, increasing the difficulty of completely eliminating tumor tissue [15]. Although studies have shown that residual cancerous tissue after general resection does not affect the prognosis [16], the residual tumor tissue underwent heat exposure after RAF may have different manifestation, because studies have indicated that heat exposure increased the heat tolerance and viability of residual tumor tissue [[17], [18], [19]]. Studies have shown that heat stress can induce synthetic Heat Shock Protein 70 (HSP70) in a variety of cells [20], increasing the survival rate of cells under high temperature stress [21,22]. However, there are currently no related studies on pancreatic cancer. Also, as a key marker of tumors, increased expression of HSP70 itself reveals an increase in tumor proliferation and migration [23,24], and whether or not heat stress also increases the invasiveness of pancreatic cancer by inducing an increase in HSP70 expression still needs to be studied.
Recently, a study showed that combined using with anti-HSP micellar quercetin in RFA could significantly reduce the subsequent systemic tumor growth [25], which suggested that expression change of HSP caused by RFA might be an important risk factor. And also, this study proposal a question is that how local thermal injury pass the signal away to the distant tumor.
Therefore, this study aimed to carry out prognostic analysis for patients receiving RFA treatment to observe the efficacy of RFA in advanced pancreatic cancer; and by means of heat exposure treatment for human pancreatic cancer cell line PANC-1 cultured in vitro, to observe the impact of heat stress on the proliferation of tumor cells; and after that, the medium of PANC-1 cells after heat treatment was collected to culture human pancreas cell strain HPC-Y5 to observe the affect. And thus, to provide a fundamental research basis for improving the safety of RFA application.
2. Materials and methods
2.1. Participants
From January 2015 through June 2016, of the patients treated advanced pancreatic cancer with RFA at the Third Affiliated Hospital of Qiqihar Medical University, 32 met the following inclusion criteria:
-
1.
Age above 18 years old. Be able to sign their own Informed Consent Form.
-
2.
Meet typical clinical symptoms such as abdominal pain, back pain, jaundice, weight loss, etc.
-
3.
Diagnosed as advanced pancreatic cancer according to WHO's diagnostic criterion 2003 Digestive System Neoplasm.
-
4.
Free from disturbance of consciousness, nor failure of major organs such as heart, liver and kidney. Free from symptoms of infection, nor coagulation disorders.
-
5.
Be able to cooperate with pain rating and willing to participate in follow-up.
Among which, 20 male patients and 12 female patients with an average age of 43 years old. Tumor dia. 7.5 cm∼10.4 cm. Serum CA19-9 and CA 242 levels were measured 1 day before and 3 months after surgery, and they were followed up by telephone every 3 months to understand the prognosis.
Our study is in compliance with the survey of Declaration of Helsinki on human subjects. The study protocol was ethically approved by the Institutional Review Board of the Qiqihar Medical University. All the participants provided written Informed Consent Form. And every effort was exerted to reduce the pain of patients.
2.2. Pain rating
Pain levels before and after surgery were assessed by Numerical Rating Scale (NRS) and Verbal Rating Scale (VRS). The specific implementation standard refers to: [25]. And, observe whether there are sigh and groan, and determine vital signs such as heart rate and blood pressure of the patients to assist in the assessment of pain levels.
2.3. Surgical methods
The tumor location and RFA puncture point and the direction of needle insertion were determined by computerized tomography performed in 32 patients. After performing general anesthesia on the patient, the plate electrode of the radiofrequency ablation instrument (Beijing Wei'er Fu Electronics Co., model WE7568) was attached closely to the patient's lower extremities. Under the guidance of B-ultrasound, an umbrella shaped microelectrode was implanted inside the tumor, with EVB electrode connected, and the heating was controlled by a computer program. Repeat times and each performing time was set according to the location and size of the tumor. Patients' vital signs were carefully monitored during the surgery. rest in supine position was taken after surgery, and nutritional support, anti-inflammatory drugs and antacid were administered.
2.4. Determination of serum CA199 and CA242
Take 3 ml fasting venous blood in the early morning and place it in a biochemical tube. Separate out serum by centrifugal process. Serum CA199 and CA242 levels were detected by chemiluminescence immunoassay (BPCL ultra-weak chemiluminescence analyzer, Institute of Biophysics, Chinese Academy of Sciences, Beijing).
2.5. Cell culture and treatment
Human pancreatic cancer cell line PANC-1 (ATCC, CRL-1469) and human pancreas cell strain HPC-Y5 (ScienCell, KL) was cultured in a medium prepared by 90% Dulbecco modified medium (Catalog No.: 30–2002), 10% heat-inactivated fetal calf serum (10099, Australia) and 1% penicillin and streptomycin. At the time of cell passage, remove the medium solution, flush for 3 times with PBS, and after it is digested with 0.25% trypsin plus 0.02% EDTA, remove the pancreatin, add a fresh medium, mix well, then dispense it into new flasks. At heat treatment, implant PANC-1 into 75 cm2 culture flasks (density 2 × 105 cells/mL). Heat in water baths at 37 °C, 42 °C and 50 °C for 5, 10, 30 min respectively, and then put them back into the incubator. Replace the solution every other day and passage the adherent cells into a six-pore plate, and the cells would be collected for testing after they grow up to an 80% confluence.
2.6. Western-blotting
After PANC-1 cells were cultured to an 80% confluence, they were flushed for 3 times with pre-cooled PBS, then a protein lysate (solarbio, r 0020) was added. React for 30 min on ice, then collect the upper protein solution by centrifugation after cell debris was collected. The concentration of the samples was determined by BCA method. The protein was separated by SDS-PAGE electrophoresis and transferred onto a PVDF membrane. Block with 5% w/v skimmed milk powder (1 × TBST), and then continue to incubate the membrane with the following primary antibodies: Cyclin D1 Antibody (CST#2922) (1:1000), HSP70 Antibody (CST#4873) (1:1000), GADPH (CST#51332) (1:1000).
2.7. Real-time PCR
Take PANC-1 cells with 80% confluence, extract and purify RNA with Trizol (Invitrogen™), and then reversely transcribed it into cDNA. The target sequence was amplified by the primers list as follows: GADPH, forward primer (5’→3′): TCTGGCACCACACCTTCTAC, reverse primer (5’→3′): ATGTCACGCACGATTTCC; Cyclin-D1, forward primer (5’→3′): TGGCTGAAGTCACCTCTTG; reverse primer (5’→3′): GTGCTTGGAAATGGAATGG; HSP7, forward primer (5’→3′): TGACTGTGTTGTTTCGGTTC; reverse primer (5’→3’): TACCTCCCAATGTCGTGTC.
2.8. Cell survival rate
To avoid false positive result caused by combine using of VER-155008 in colony formation experiment, CCK-8 assay was measured to find a suitable point. Human pancreatic cancer PANC-1 cells were digested with 0.25% trypsin to make 5 × 104/ml single cell suspensions, inoculated in 96 well plate with 200 μL cell suspension per well and cultured in 37 °c, 5% CO2 incubator. After 12 h change the culture medium to set experimental group, negative control group, and blank group. The experimental group was treated with VER-155008 at concentration of 25μ mol/L, for 12 h, 24 h, 36 h, and 48 h respectively. Five parallel compound holes were set up for each hole. The IOD was measured by enzyme labeling instrument in 450 nm. Calculated cell relative proliferation rate = (experimental group IOD value-blank group IOD value)/(negative control group IOD value-blank group IOD value) × 100%.
2.9. Colony formation experiment
PANC-1 cells after heated treatment or combined with VER-155008 treatment were collected and inoculated in 6-well plate and 12-well plate at 1000/well and 500/well respectively, incubated for two weeks. After culture, stained with 4% methyl alcohol for 10 min, dyed by 0.1% crystal violet.
2.10. Statistical analysis
Statistical analysis was performed using Microsoft EXCEL (Microsoft, Redmond, WA), SPSS and Prism Rev. 5.00 (GraphPad Software, San Diego, CA). It will be considered a significant difference where p is less than 0.05.
3. Results
3.1. Follow-up results
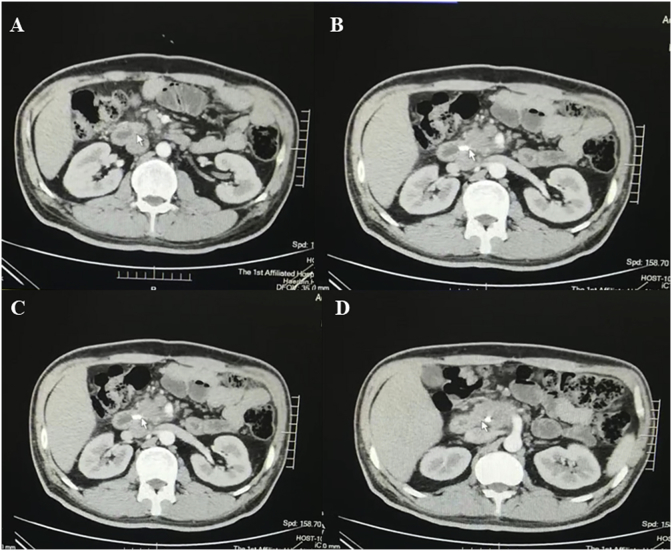
Of the 32 patients, there were 20 male patients and 12 female patients, with an average age of 43 years old. The tumor dia. in the preoperative assessment is 7.5 cm∼10.4 cm (Fig. 1). 1 patient died of gastrointestinal hemorrhage in the postoperative follow-up. Within 6 months after surgery, 1 patient died of multiple organ failure caused by distant tumor metastasis. 2 patients survived for more than 16 months.
Fig. 1.
Manifestation of CT image of a typical pancreatic cancer patient, from A to D the results of layer-by-layer scanning are shown from the patient's head downwards, with tumor location indicated by arrow.
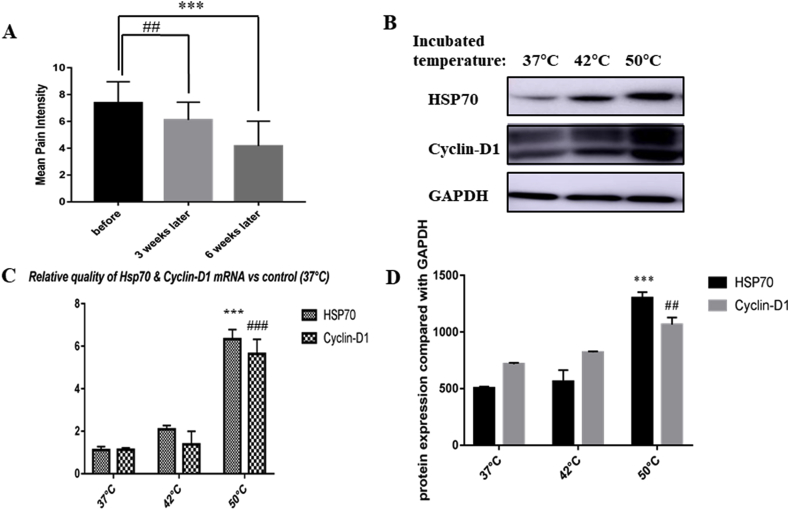
There were 29 cases of jaundice and 19 cases of abdominal pain with jaundice before surgery. All patients had pain, of which 21 cases of abdominal pain and 11 cases of back pain (Table 1). The patients' pain degree was assessed by NRS/VRS before surgery, with an average pain level of around 7.3 (Fig. 2A). The patients were asked about the pain 3 weeks after surgery. Except for 4 cases of abdominal pain without obvious relief, the other 28 cases were all improved. 20 patients had pain relief 6 weeks after surgery. The overall pain relief rate is up to 62.5% (P < 0.05). In addition, of the 14 patients requiring a painkiller, 6 no longer require a painkiller, which drops the usage rate of painkiller to 42.8% (Table 1).
Table 1.
Characteristics of the studied population.
| Variables | All patients (before surgery, n = 32) | All patients (6 months after, n = 31) |
|---|---|---|
| Gender | ||
| Male | 20 | 19 |
| Female | 12 | 12 |
| Average Age (years) | 43 ± 5.64 | |
| Tumor Size (centimeters) | 7.5–10.4 | |
| Symptoms | ||
| Jaundice | 29 | 0 |
| Abdominal pain | 21 | 12 |
| Back pain | 11 | 4 |
| Oral analgesic medication | 14 | 8 |
Fig. 2.
A: The statistical results of pain scores of 32 patients before and 3 weeks and 6 weeks after surgery. Pain of the patients was assessed by applying verbal rating scale and numerical rating scale respectively; the rating data of the patients before and 3 weeks and 6 weeks after surgery were collected; and the data of single variance analysis showed that pain improvement was statistically significant (compared with pre operation, pain improved 3 weeks after surgery, ##p < 0.01; and compared with pre operation, pain improved significantly 6 weeks after surgery ***p < 0.001). B, D: Western-blotting results of heat-treated cells at 42 °C and 50 °C and control cells at 37 °C. The results of three independent replicates after single-variance analysis showed that the proliferation rate was significantly increased in the 50 °C group compared with the control group (##p < 0.01), and the HSP70 expression was significantly increased (***p < 0.001). C: Real-time PCR results of heat-treated cells at 42 °C and 50 °C and control cells at 37 °C. The results of three independent replicates after single-variance analysis showed that the proliferation rate was significantly increased in the 50 °C group compared with the control group (###p < 0.001), and the HSP70 expression was significantly increased (***p < 0.001).
3.2. Changes in tumor marker serum level
Carbohydrate antigens 19-9 (CA19-9) and CA242 are glycoproteins which are present in small amounts in the pancreas and gallbladder of adults. When the body's pancreatic tumor tissue proliferates, the sharp increase in the level of these glycoproteins can faithfully and reliably reflect the growth of the tumor. Notably, although CA19-9 is not sensitive enough for screening early lesions, but it's the most frequently used biomarker for Pancreatic ductal adenocarcinoma (PDAC). And also, CA242 is identified as a relatively late biomarker for the diagnosis, prognosis and surveillance of PDAC [26,27]. So, we chose these two biomarkers as surveillances to predict prognosis for our patients. From the changes in CA19-9 and CA242 levels before and 3 months after treatment, it can be seen that the levels of both serum tumor markers decreased 3 months after treatment with radiofrequency ablation, and the difference was remarkable by comparison (Table 2).
Table 2.
Serum CA19-9 and CA242 levels before and 3 months after of treatment (U/mL).
| Pre-RFA | 90-Day Post-RFA | Delta | t | p | |
|---|---|---|---|---|---|
| CA19-9 | 247.19 ± 112.41 | 77.34 ± 64.07 | 163.21 ± 97.87 | 9.873 | <0.01 |
| CA242 | 65.42 ± 44.60 | 39.31 ± 32.03 | 22.74 ± 26.60 | 5.978 | <0.01 |
3.3. Pancreatic cancer cells after heat treatment had a higher proliferation rate
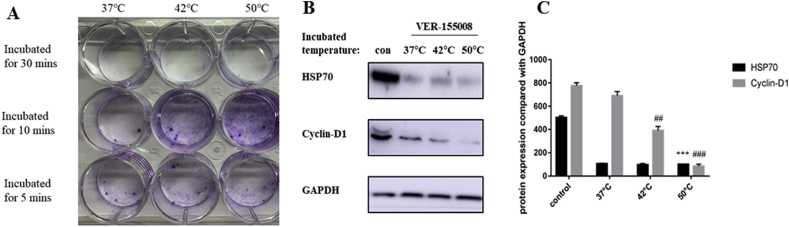
In order to observe the impact of heat stress on pancreatic tumor tissue, human pancreatic cancer cell line PANC-1 was cultured and heated in a water bath at 37 °C, 42 °C and 50 °C for 5 min, 10 min, and 30 min respectively to simulate heat exposure process of residual tumor tissue at the marginal zone during RFA treatment. Colony formation test showed that when incubated at 50 °C for 10 min, the proliferation rate of PANC-1 cells was significantly increased (Fig. 3A). And also, cells after heat treatment were collected for detection of Cyclin-D1 and HSP70 expressions, which were then compared with the control group (water bath group at 37 °C) to observe the changes in cell proliferation rate and HSP70 level (Fig. 2B–D). Because cyclin D1 expression was proved to be positive correlated with cell proliferation, especially in carcinoma cells [28,29].
Fig. 3.
A: PANC-1 cells after heat treatment at 37 °C, 42 °C, 50 °C for 5 min, 10 min and 30 min respectively were collected to plant into 6-well plate at 1000 cells/well for 7 days. Then use crystal violet to show colon formation. B–C: The cells were subjecting to a heat treatment after being pretreated with HSP70 inhibitor VER-155008 25 μM for an hour, and cell protein was collected to observe the change in HSP70 expression and the variation of proliferation rate by means of Western-blotting. The experimental results of three independent replicates after single-variance analysis showed that VER-155008 can significantly inhibit the expression of HSP70 in cells (***p < 0.001), and the proliferation rate of cells applied with an inhibitor and subjected to a heat radiation was significantly decreased (##p < 0.01, ###p < 0.001).
3.4. HSP70 inhibitor altered the proliferation increase in pancreatic cancer cells after heat treatment
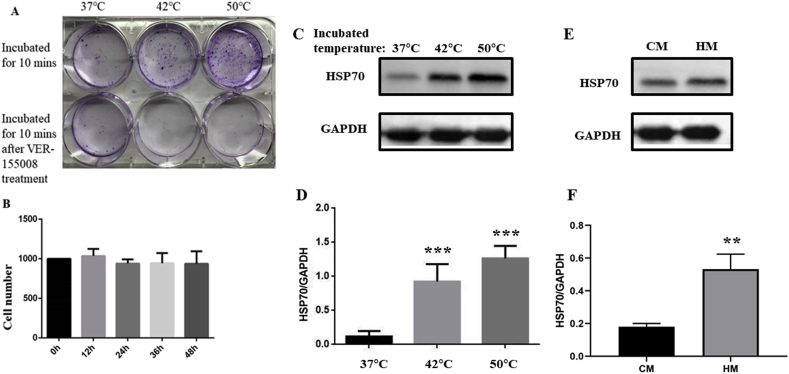
HSP70 inhibitor ver-155008 was applied to PANC-1 cells after heat treatment to identify if HSP70 change is the cause of increase in proliferation. The results showed that the viability of PANC-1 cells subjected to a heat treatment after pretreatment with HSP70 inhibitor ver-155008 decreased significantly (Fig. 4A, Fig. 3B-C). The concentration of ver-155008 was 25 μM according to Ref. [30]. And also, by using CCK-8 assay, the possibility that ver-155008 treatment itself caused the death of PANC-1 was excluded (Fig. 4B).
Fig. 4.
A: Crystal violet to show colony formation in cells treated with/without VER-155008 during heat treatment. B: Using 25 μM VER-155008 to treat PANC-1 for different times, CCK-8 showed that VER-155008 did not cause PANC-1 death. C, D: HSP70 expression change in human pancreas cell line HPC-Y5 after heat treatment (***p < 0.001). E, F: Expression of HSP70 of HPC-Y5 cells in two kinds of medium, CM: control medium, HM: medium came from heat treated PANC-1(**p < 0.01).
3.5. Carcinoma cells after heat injury might pass the signal to normal cells around
To further investigate the affect caused by heat injury on normal pancreas cells, we applied heat treatment on human pancreas cells strain HPC-Y5 in the same way. And then, to examine the expression change of HSP70 (Fig. 4. C, D). And also, to verify the indirect influence from heat exposure pancreatic carcinoma cells to the normal cells around, we collected medium from heat treated PANC-1 to replace the medium for HPC-Y5, exchange the medium synchronously. After 7 days culture, to examine the expression of HSP70 again (Fig. 4. E, F). From that we can see, even though the signals contented in heat treated PANC-1's medium didn't have the strong activation effect as heat treatment on HPC-Y5, but it still raised expression of HSP70 in HPC-Y5.
4. Discussion
30–40% of patients with pancreatic cancer are not eligible for radical resection [4], while radiofrequency ablation (RFA) is a treatment means that can be applied to advanced unresectable pancreatic cancer [31]. RFA causes protein denaturation and coagulative necrosis by heating, and blocks the blood supply to the tumor, further causing tumor cell ischemia and delaying tumor growth [32,33]. At the same time, RFA can also enhance the immunity of patients by promoting the immune function of T cells, natural killer cells and macrophages, thereby limiting tumor cell proliferation [34]. However, clinical data indicated that RFA cannot significantly prolong the patients' five-year survival rate [6]. In this study, based on a retrospective investigation on 32 patients treated with RFA, by detecting the changes in the level of serum tumor markers and observing the improvement of the patients' symptoms, the same conclusion was reached: although RFA can indeed effectively eliminate pancreatic tumors, relieve pain, subside jaundice and improve symptoms, it still cannot effectively prolong the patients' survival time.
In order to explore the factors that affect the survival time of patients treated with RFA, we observed the impact of heat radiation on tumor cell proliferation. By giving heat treatment for human pancreatic cancer cell line PANC-1, a tumor tissue model after heat stress was established, its proliferation rate was determined, and was compared with the control group. It was observed that the expression of Cyclin-D1 in the heat stress model increased, which indicates that the proliferation rate of pancreatic cancer cells subjecting to a heat radiation was increased, because some studies had testified that Cyclin-D1 related to the cell proliferation [28,29]. Since radiation causes an increase in the synthesis of heat shock proteins, thereby enhancing the endurance capacity of cells to heat radiation and increasing cell viability [35], we detected the changes in HSP70 expression after heating, and by pretreating the cells with HSP70 inhibitor VER-155008, it was determined that HSP70 plays an important role in the regulation of proliferation of pancreatic cancer cells subjected to a heat stress. Although this conclusion needs to be verified in in-vivo experiments, we can still see the important effect of heat radiation generated by RFA on surrounding residual cancerous tissue and the key role of HSP70 in this in-vitro experiment.
Moreover, studies have indicated that when RFA is applied to treat liver cancer, the expression of HSP70 in tumor tissue away from ablation center would also increase, thereby inducing the growth of systemic tumors [23]. Another study found that incomplete RFA can induce cancerization in operation-surrounding tissues of mice with liver cancer [17]. Therefore, it is of great significance to study on how to apply HSP70 inhibitor to RFA surgery or to inhibit the impact of heat stress [36] so as to reduce subsequent risks. But since the members of heat shock protein family are important for normal function, this need more related experiments to testify the safety.
Also, since RFA is a treatment using high-frequency alternating current to create frictional heating of the tissue surrounding the electrode, to cause desiccation and coagulative necrosis, some studies focused to research the way to deduce the located heat. One study showed that regional flow influenced heat accumulation [37.].
Furthermore, some studies move the sight to improve the surgery technical, such as using computational model to set special plan for individual [38] or different organs [39,40]. And also, some researchers considered the influence caused by transferred energy [41]. Moreover, a study elaborated the electromagnetic wave frequency dependence of lung's electrical conductivity effects on heat generation of RFA [42].
This remind us to explore the relationship between heated carcinoma cells and normal tissue. And we found that the heat injured cells would pass the injury signal to normal pancreas cells in some way, and increased their HSP70 expression. This data suggested there might be another way to block this signal pass way to prevent heat injury caused subsequent cancerization.
These existing studies and our experimental results have verified that although there are many factors affecting the prognosis of RFA surgery, RFA surgery is effective in the treatment of pancreatic cancer, worthen further studies to increase its efficacy and safety.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgements
This study was supported by Scientific Technology Program of Qiqihar (SFGG-201643).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100700.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Perinel J., Adham M. Palliative therapy in pancreatic cancer-palliative surgery. Transl. Gastroenterol. Hepatol. 2019;4:28. doi: 10.21037/tgh.2019.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torphy R.J., Chapman B.C., Friedman C., Nguyen C., Bartsch C.G., Meguid C., Ahrendt S.A., McCarter M.D., Del Chiaro M., Schulick R.D., Edil B.H., Gleisner A. Ann Surg Oncol; 2019. Quality of Life Following Major Laparoscopic or Open Pancreatic Resection. [DOI] [PubMed] [Google Scholar]
- 3.Seufferlein T., Hammel P., Delpero J.R., Macarulla T., Pfeiffer P., Prager G.W., Reni M., Falconi M., Philip P.A., Van Cutsem E. Optimizing the management of locally advanced pancreatic cancer with a focus on induction chemotherapy: expert opinion based on a review of current evidence. Cancer Treat Rev. 2019;77:1–10. doi: 10.1016/j.ctrv.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Lekka K., Tzitzi E., Giakoustidis A., Papadopoulos V., Giakoustidis D. Contemporary management of borderline resectable pancreatic ductal adenocarcinoma. Ann. Hepatobiliary Pancreat. Surg. 2019;23:97–108. doi: 10.14701/ahbps.2019.23.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaum N., Hubner R.A., Valle J.W., Amir E., McNamara M.G. Adjuvant chemotherapy and outcomes in patients with nodal and resection margin-negative pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. J. Surg. Oncol. 2019;119:932–940. doi: 10.1002/jso.25440. [DOI] [PubMed] [Google Scholar]
- 6.D'Onofrio M., Crosara S., De Robertis R., Butturini G., Salvia R., Paiella S., Bassi C., Mucelli R.P. Percutaneous radiofrequency ablation of unresectable locally advanced pancreatic cancer: preliminary results. Technol. Cancer Res. Treat. 2017;16:285–294. doi: 10.1177/1533034616649292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rustagi T., Chhoda A. Endoscopic radiofrequency ablation of the pancreas. Dig. Dis. Sci. 2017;62:843–850. doi: 10.1007/s10620-017-4452-y. [DOI] [PubMed] [Google Scholar]
- 8.Si M.B., Yan P.J., Hao X.Y., Du Z.Y., Tian H.W., Yang J., Han C.W., Yang K.H., Guo T.K. Surg Endosc; 2019. Efficacy and Safety of Radiofrequency Ablation versus Minimally Invasive Liver Surgery for Small Hepatocellular Carcinoma: a Systematic Review and Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- 9.Wang L.J., Zhang Z.Y., Yan X.L., Yang W., Yan K., Xing B.C. Radiofrequency ablation versus resection for technically resectable colorectal liver metastasis: a propensity score analysis. World J. Surg. Oncol. 2018;16:207. doi: 10.1186/s12957-018-1494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y., Feng M. Radiotherapy for hepatocellular carcinoma. Semin. Radiat. Oncol. 2018;28:277–287. doi: 10.1016/j.semradonc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Lemdani K., Mignet N., Boudy V., Seguin J., Oujagir E., Bawa O., Peschaud F., Emile J.F., Capron C., Malafosse R. Local immunomodulation combined to radiofrequency ablation results in a complete cure of local and distant colorectal carcinoma. OncoImmunology. 2019;8:1550342. doi: 10.1080/2162402X.2018.1550342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scopelliti F., Pea A., Conigliaro R., Butturini G., Frigerio I., Regi P., Giardino A., Bertani H., Paini M., Pederzoli P., Girelli R. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg. Endosc. 2018;32:4022–4028. doi: 10.1007/s00464-018-6217-x. [DOI] [PubMed] [Google Scholar]
- 13.Andaluz A., Ewertowska E., Moll X., Aguilar A., Garcia F., Fondevila D., Quesada R., Berjano E., Grande L., Burdio F. Endoluminal radiofrequency ablation of the main pancreatic duct is a secure and effective method to produce pancreatic atrophy and to achieve stump closure. Sci. Rep. 2019;9:5928. doi: 10.1038/s41598-019-42411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Date R.S., Siriwardena A.K. Radiofrequency ablation of the pancreas.I: definition of optimal thermal kinetic parameters and the effect of simulated portal venous circulation in an Ex-vivo porcine model. JOP. J. Pancreas (Online) 2005;6:581–587. [PubMed] [Google Scholar]
- 15.K J., Schueller G., Sedivy R. Heat shock protein expression induced by percutaneous radiofrequency ablation of hepatocellular carcinoma in vivo. Int. J. Oncol. 2004;24(3):609–613. [PubMed] [Google Scholar]
- 16.Ratnayake C.B.B., Shah N., Loveday B., Windsor J.A., Pandanaboyana S. The impact of the depth of venous invasion on survival following pancreatoduodenectomy for pancreatic cancer: a meta-analysis of available evidence. J. Gastrointest. Cancer. 2019 doi: 10.1007/s12029-019-00248-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Yoshida S., Kornek M., Ikenaga N., Schmelzle M., Masuzaki R., Csizmadia E., Wu Y., Robson S.C., Schuppan D. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58:1667–1680. doi: 10.1002/hep.26526. [DOI] [PubMed] [Google Scholar]
- 18.elez E G.S., Kumar G. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281(3):782–792. doi: 10.1148/radiol.2016152241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Wijk R O.J., de Koning E. Mild step-down heating causes increased levels of HSP68 and of HSP84 mRNA and enhances thermotolerance. Int. J. Hyperth.: Off. J. Eur. Soc. Hyperthermic Oncol., North Am. Hyperth. Group. 1994;10(1):115–125. doi: 10.3109/02656739409009337. [DOI] [PubMed] [Google Scholar]
- 20.Calderwood S.K., Khaleque M.A., Sawyer D.B., Ciocca D.R. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Beckham J.T., Wilmink G.J., Mackanos M.A., Takahashi K., Contag C.H., Takahashi T., Jansen E.D. Role of HSP70 in cellular thermotolerance. Lasers Surg. Med. 2008;40:704–715. doi: 10.1002/lsm.20713. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi K.E., Tsuji K. Modification of thermosensitivity and chemosensitivity induced by combined treatments with hyperthermia and adriamycin. Int. J. Mol. Med. 2001;8:417–422. doi: 10.3892/ijmm.8.4.417. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M., Kumar G., Gourevitch S., Levchenko T., Galun E., Torchilin V., Goldberg S.N. Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int. J. Hyperth. 2018;34:934–942. doi: 10.1080/02656736.2018.1462535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monma H., Harashima N., Inao T., Okano S., Tajima Y., Harada M. The HSP70 and autophagy inhibitor pifithrin- enhances the antitumor effects of TRAIL on human pancreatic cancer. Mol. Cancer Ther. 2013;12:341–351. doi: 10.1158/1535-7163.MCT-12-0954. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed M., Kumar G., Gourevitch S., Levchenko T., Galun E., Torchilin V., Goldberg S.N. Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int. J. Hyperth. 2018;34:934–942. doi: 10.1080/02656736.2018.1462535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira-Valente M.A., Pais-Ribeiro J.L., Jensen M.P. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Ge L., Pan B., Song F., Ma J., Zeraatkar D., Zhou J., Tian J. Comparing the diagnostic accuracy of five common tumour biomarkers and CA19-9 for pancreatic cancer: a protocol for a network meta-analysis of diagnostic test accuracy. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong D., Jia L., Zhang L., Ma N., Zhang A., Zhou Y., Ren L. Periostin and CA242 as potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer. Cancer Sci. 2018;109:2841–2851. doi: 10.1111/cas.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M., Zhou W., Zhao S., Li S., Yan D., Wang J. Eckol inhibits Reg3A-induced proliferation of human SW1990 pancreatic cancer cells. Exp. Ther. Med. 2019;18:2825–2832. doi: 10.3892/etm.2019.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kita K., Shiota M., Tanaka M., Otsuka A., Matsumoto M., Kato M., Tamada S., Iwao H., Miura K., Nakatani T., Tomita S. Heat shock protein 70 inhibitors suppress androgen receptor expression in LNCaP95 prostate cancer cells. Cancer Sci. 2017;108:1820–1827. doi: 10.1111/cas.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paiella S., De Pastena M., Romeo F., D'Onofrio M., Fontana M., Pea A., De Marchi G., Crino S.F., Bassi C., Salvia R. Ablation treatments in unresectable pancreatic cancer. Minerva Chir. 2019;74:263–269. doi: 10.23736/S0026-4733.18.07881-1. [DOI] [PubMed] [Google Scholar]
- 32.Atar M., Kadayifci A., Daglilar E., Hagen C., Fernandez-Del Castillo C., Brugge W.R. Ex vivo human bile duct radiofrequency ablation with a bipolar catheter. Surg. Endosc. 2018;32:2808–2813. doi: 10.1007/s00464-017-5984-0. [DOI] [PubMed] [Google Scholar]
- 33.Lee D P.S., Ang M.J.C., Park J.G., Yoon S., Kim C., Lee S.K., Cho K.O., Choi J. Evaluation of liver lesions by use of shear wave elastography and computed tomography perfusion imaging after radiofrequency ablation in clinically normal dogs. Am. J. Vet. Res. 2008;79:1140–1149. doi: 10.2460/ajvr.79.11.1140. [DOI] [PubMed] [Google Scholar]
- 34.Mo Z., Lu H., Mo S., Fu X., Chang S., Yue J. Ultrasound-guided radiofrequency ablation enhances natural killer-mediated antitumor immunity against liver cancer. Oncol. Lett. 2018;15:7014–7020. doi: 10.3892/ol.2018.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hundt W., O'Connell-Rodwell C.E., Bednarski M.D., Steinbach S., Guccione S. In vitro effect of focused ultrasound or thermal stress on HSP70 expression and cell viability in three tumor cell lines. Acad. Radiol. 2007;14:859–870. doi: 10.1016/j.acra.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Geranios A P.E., Papalois A. Radiofrequency ablation of the pancreas: protective effect of local cooling techniques. Am. Surg. 2015;81:483–491. [PubMed] [Google Scholar]
- 37.Smith M.K., Mutter D., Forbes L.E., Mulier S., Marescaux J. The physiologic effect of the pneumoperitoneum on radiofrequency ablation. Surg. Endosc. 2004;18:35–38. doi: 10.1007/s00464-001-8235-2. [DOI] [PubMed] [Google Scholar]
- 38.Audigier C., Mansi T., Delingette H. Lattice Boltzmann method for fast patient-specific simulation of liver tumor ablation from CT images. Med. Image Comput. Comput. Assist. Interv. 2013;16:323–330. doi: 10.1007/978-3-642-40760-4_41. [DOI] [PubMed] [Google Scholar]
- 39.Zorbas G. Simulation of radiofrequency ablation in real human anatomy. Int. J. Hyperth. 2014;30:570–578. doi: 10.3109/02656736.2014.968639. [DOI] [PubMed] [Google Scholar]
- 40.Audigier C., Mansi T., Delingette H. Efficient lattice Boltzmann Solver for patient-specific radiofrequency ablation of hepatic tumors. IEEE Trans. Med. Imaging. 2015;34:1576–1589. doi: 10.1109/TMI.2015.2406575. [DOI] [PubMed] [Google Scholar]
- 41.Thakur S., Lavito S., Grobner E. Radiofrequency thermal ablation heat energy transfer in an model. Am. Surg. 2017;83:1373–1380. [PubMed] [Google Scholar]
- 42.Yamazaki N., Watanabe H., Lu X. The relation between temperature distribution for lung RFA and electromagnetic wave frequency dependence of electrical conductivity with changing a lung's internal air volumes. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013:386–391. doi: 10.1109/EMBC.2013.6609518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.