Abstract
Introduction
This study investigated the association of urinary transforming growth factor-β1 (uTGF-β1) with prevalent chronic kidney disease (CKD) in the HIV-infected population.
Methods
HIV-positive patients without CKD (HIV+CKD−, n = 194) and 114 with CKD (HIV+CKD+) who did not have hypertension, diabetes mellitus, or hepatitis B or C, had their urinary protein-creatinine ratio (uPCR), serum transforming growth factor (TGF)–β1, and uTGF-β1 measured. uTGF-β1-creatinine ratios (uTGF-β1Cr) were calculated. Spearman correlation was used to determine the association between uTGF-β1Cr and various attributes, and the Cuzick trend test was used to assess the presence of a linear trend in median uTGF-β1Cr levels across the stages of CKD. Multivariable robust linear regression models were used to assess independent association with variability in uTGF-β1Cr and estimated glomerular filtration rate (eGFR) levels.
Results
The age of the participants was 38.3 ± 10.3 years with 73.4% women. The median uTGF-β1Cr was higher among HIV+CKD+ (4.85 ng/mmol [25th–75th percentile 1.96–12.35] vs. 2.95 [1.02–5.84]; P = 0.001]). There was significant correlation between uTGF-β1Cr and age (P = 0.02), eGFR (P = 0.001), and uPCR (P < 0.001) in the HIV+CKD+ group. Among the HIV+CKD+ patients, there was gradual reduction in the median level of uTGF-β1Cr with CKD severity (P = 0.04). HIV+CKD+ patients had significantly higher levels of uTGF-β1Cr after controlling for potential confounders. Using eGFR as dependent variable, proteinuria explained the changes associated with uTGF-β1Cr levels.
Conclusion
HIV+CKD+ patients express higher levels of uTGF-β1 especially in the early stages of CKD apparently related to proteinuria levels.
Keywords: CKD, HIV, Nigeria, urinary TGF-β1
The early diagnosis and treatment of CKD presents a definite opportunity for reducing the incidence of end-stage renal disease. This is especially important in regions of high HIV prevalence, which, unfortunately, tend to have dysfunctional health care systems. TGF-β1 is a 112-amino acid, 25-KDa ubiquitous protein believed to play a central role in renal fibrosis,1 both as a proliferative, and in some conditions, anti-proliferative factor.2
In diabetic nephropathy, overexpression of TGF-β1 has been found to occur in the kidneys, and in vivo studies have documented increased expression of TGF-β1 in proximal tubular cells and mesangial cells cultured in high glucose concentrations.3 Also, in glomerulosclerosis, TGF-β1 has been observed to be centrally involved in extracellular matrix expansion.4 Indeed, renal disease associated with expansion of extracellular matrix (diabetic nephropathy, lupus nephritis, focal and segmental glomerulonephritis, and IgA nephropathy) have all been associated with increased expression of TGF-β1, whereas renal conditions that do not have extracellular matrix accumulation (thin basement membrane disease, minimal change disease) seem not to have increased expression of TGF-β1.5 The former may be the case with HIV-related nephropathy in which expansion of the extracellular matrix occurs. Studies have shown that the administration of anti-TGF-β1 substances attenuate extracellular matrix production6, 7 and may lead to retardation of CKD progression in renal diseases associated with increased extracellular matrix production.
Some studies in patients with CKD who are HIV-positive have demonstrated elevated levels of TGF-β1 in renal tissue,8 serum,9 and in the urine10 of patients with HIV-associated nephropathy. Our study aim was to investigate the association of uTGF-β1 with prevalent CKD in HIV-positive patients in Nigeria.
Methods
Study Participants
This was a cross-sectional study involving 194 HIV-positive patients without CKD (HIV+CKD−) and 114 HIV-positive patients with CKD (HIV+CKD+) seen at the HIV clinic and the renal unit of the University of Uyo Teaching Hospital, Nigeria (Ethical review number: UUTH/AD/S/96/VOL.XIX/15). The facility’s HIV clinic offers care to approximately 50 ambulant HIV-positive patients daily with approximately 3500 patients on active enrollment. Screening for renal disease is not routinely done because patients pay out-of-pocket. Ambulant HIV-positive patients on antiretroviral (ARV) treatment at the HIV clinic who gave informed consent were recruited into the study. CKD was defined as eGFR of <60 ml/min per 1.73 m2 persisting in 2 measurements at least 3 months apart and/or urine protein-creatinine ratio (uPCR) of ≥ 0.05 g/mmol creatinine.11 The patients’ sociodemographic (age, sex, ethnicity) and clinical characteristics (e.g., body mass index and blood pressures) were collected using standard techniques. Data regarding the patients’ CD4 count at initiation of care and current CD4 count levels, viral load at initiation of care, and current levels and ARV regimen also were collected. All patients were on ARVs. The ARV regimen used in this program included zidovudine-based regimens (AZT-3TC-EFV or AZT/3TC/NVP), tenofovir-based regimen (TDF-3TC-EFV or TDF-FTC-EFV), abacavir-based regimen (ABC-3TC-EFV), and less frequently, protease-inhibitor regimen. Patients with hypertension, diabetes mellitus, or hepatitis B or C coinfection were excluded from the study.
Spot urine samples for uTGF-β1 assay were collected, processed, and stored using the manufacturer’s instructions. All samples were centrifuged with the supernatant stored in 2 aliquots at −20 °C within 2 hours of collection. Blood samples (4 ml) were collected, serum separated, and stored in 2 aliquots (for serum creatinine and TGF-β1 assay). Serum and urine creatinine were measured with RANDOX creatinine kits (RANDOX, Crumlin, UK) using the modified Jaffe reaction. Serum and urinary TGF-β1 were measured using Biovision (San Francisco, CA) TGF-β1 (human) enzyme-linked immunosorbent assay kit (catalog #K4342–100) in duplicates. The intra-assay and inter-assay coefficient of variation was 8.6% and 7.0%, respectively. The sensitivity of the assay was <1 pg/ml. To ameliorate the effect of varying urine concentrations, uTGF-β1Cr ratio (pg/mmol) was derived by dividing urinary TGF-β1 (in pg/l) by urinary creatinine (in mmol/l) and later converted to ng/mmol. We estimated the glomerular filtration rate using the 4-variable Modification of Diet in Renal Disease12 and the CKD-Epidemiology Collaboration13 equations. Participants’ kidney function was staged using the Kidney Disease Outcome Quality Initiative classification.11
All data analysis was performed with Stata 15.1 (StataCorp, College Station, TX). Numerical variables were reported as means (±SD) or median (25th–75th percentile). Categorical variables are reported as frequencies (percentages). Comparison of quantitative variables was performed using the Student t test (or its nonparametric equivalent, the Mann-Whitney U test). Comparison of categorical variables was performed using the χ2 test. The Spearman rank correlation test was used to investigate the continuous association between uTGF-β1Cr and certain demographic and clinical variables (age, serum creatinine, serum TGF-β1, uPCR, and CD4 count) and a formal comparison of the correlation coefficients undertaken using the Stata module for comparison of correlation coefficients based on the Steiger test.14 The Cuzick trend test was used to assess the presence of a linear trend in median uTGF-β1Cr levels across the stages (1–5) of CKD. Univariable and multivariable robust linear regression models were built to determine factors independently associated with variability in uTGF-β1Cr and eGFR levels. The Akaike information criteria was used to determine the best multivariable model that explains the variability in uTGF-β1Cr levels. P value less than 0.05 was deemed statistically significant.
Results
Demographic and Clinical Characteristics
A total of 194 HIV+CKD− and 114 HIV+CKD+ were recruited into the study. The mean age of the participants was 38.3 ± 10.3 years with statistically significant difference between the groups (P = 0.02). The sex distribution showed a female preponderance (73% vs. 27%). Table 1 summarizes the demographic and clinical characteristics of the study participants by CKD status. The level of serum TGF-β1 and urinary TGF-β1 was higher among the HIV+CKD+ patients than in HIV+CKD− patients, although this difference did not achieve statistical significance. However, when urinary TGF-β1 level was standardized by using the uTGF-β1Cr ratio, those with HIV+CKD+ had significantly higher levels (Table 1).
Table 1.
Demographic and clinical characteristics
| Variables | Total (N = 308) | HIV+CKD+ (n = 114) | HIV+CKD− (n = 194) | P |
|---|---|---|---|---|
| Age (yr) | 38.3 ± 10.3 | 39.9 ± 10.4 | 37.1 ± 10.1 | 0.02 |
| Female sex, n (%) | 226 (73.4) | 85 (74.6) | 141 (72.7) | 0.72 |
| Duration on ARV (25th–75th percentile) | 5 (2–8) | 5 (2–8) | 5 (2–8) | 0.27 |
| Systolic blood pressure (mm Hg) | 129.0 ± 26.3 | 137.0 ± 26.9 | 124.5 ± 24.8 | <0.001 |
| Diastolic blood pressure (mm Hg) | 77.7 ± 14.5 | 80.8 ± 14.5 | 75.2 ± 14.1 | 0.001 |
| Mean arterial pressure (mm Hg) | 91.2 ± 13.9 | 93.0 ± 13.5 | 90.1 ± 14.1 | 0.07 |
| Weight (kg) | 61.4 ± 11.9 | 59.1 ± 11.5 | 62.7 ± 12.0 | 0.01 |
| Height (m) | 1.63 ± 0.09 | 1.63 ± 0.08 | 1.63 ± 1.0 | 0.38 |
| Body mass index (kg/m2) | 23.2 ± 4.4 | 22.5 ± 4.4 | 23.6 ± 4.4 | 0.03 |
| Waist circumference (cm) | 80.1 ± 9.5 | 79.3 ± 9.2 | 81.3 ± 9.6 | 0.07 |
| Serum creatinine (μmol/l) | 85.3 (67.6–108.8) | 110.8 (90–144) | 74 (61–88) | <0.001 |
| eGFRCKD-EPI (ml/min per 1.73 m2) | 80.3 (58.6–105.1) | 56.1 (41.9–75.1) | 97.6 (78.3–113.3) | <0.001 |
| eGFRMDRD (ml/min per 1.73 m2) | 74.7 (55.8–97.9) | 53.9 (39.9–69.5) | 90.2 (72.5–111.2) | <0.001 |
| uPCR (g/mmol creatinine) | 0.04 (0.02–0.09) | 0.11 (0.03–0.20) | 0.03 (0.02–0.04) | <0.001 |
| CD4 count at enrollment (cells/μl) | 207 (80–380) | 226 (69–384.5) | 197 (89–372) | 0.84 |
| Current CD4 count (cells/μl) | 499 (308–693) | 472.5 (274–675) | 500 (329–714) | 0.32 |
| Current Log viral load (copies/ml) | 2.9 ± 1.5 | 2.8 ± 1.4 | 3.0 ± 1.6 | 0.49 |
| CKD stage, n (%) | ||||
| 1 | 18 (15.8) | |||
| 2 | 30 (26.3) | |||
| 3 | 48 (42.1) | |||
| 4 | 8 (7.0) | |||
| 5 | 10 (8.8) | |||
| Serum TGF-β1 (ng/l) | 13.7 (3.7–42.5) | 20.1 (3.7–47.8) | 13.0 (3.7–37.1) | 0.22 |
| uTGF-β1 (ng/l) | 32.5 (19.2–46.6) | 33.2 (21.4–45.5) | 32.5 (18.2–47.9) | 0.64 |
| uTGF-β1Cr (ng/mmol) | 3.7 (1.7–7.5) | 4.8 (2.0–11.5) | 2.9 (1.2–5.7) | <0.001 |
| Antiretroviral regimens, n (%) | ||||
| • Zidovudine-based | 205 (66.5) | 83 (72.8) | 122 (62.9) | |
| • Tenofovir-based | 88 (28.6) | 26 (22.8) | 62 (31.9) | 0.09 |
| • Abacavir-based | 11 (3.6) | 4 (3.5) | 7 (3.6) | |
| • Protease-inhibitor-based | 4 (1.3) | 1 (0.9) | 3 (1.6) |
CKD, chronic kidney disease; eGFRCKD-EPI, estimated glomerular filtration rate using CKD-EPI equation; eGFRMDRD, estimated glomerular filtration rate using the 4-variable Modification of Diet in Renal Disease formula; HIV+CKD+, HIV-positive patients with CKD; HIV+CKD−, HIV-positive patients without CKD; TDF, tenofovir disoproxil fumarate; TGF- β1, transforming growth factor-beta1; uPCR, urine protein-creatinine ratio; uTGF-β1, urinary transforming growth factor-beta 1; uTGF-β1Cr, urinary transforming growth factor-beta-1–Creatinine ratio.
Factors Associated With uTGF-β1Cr
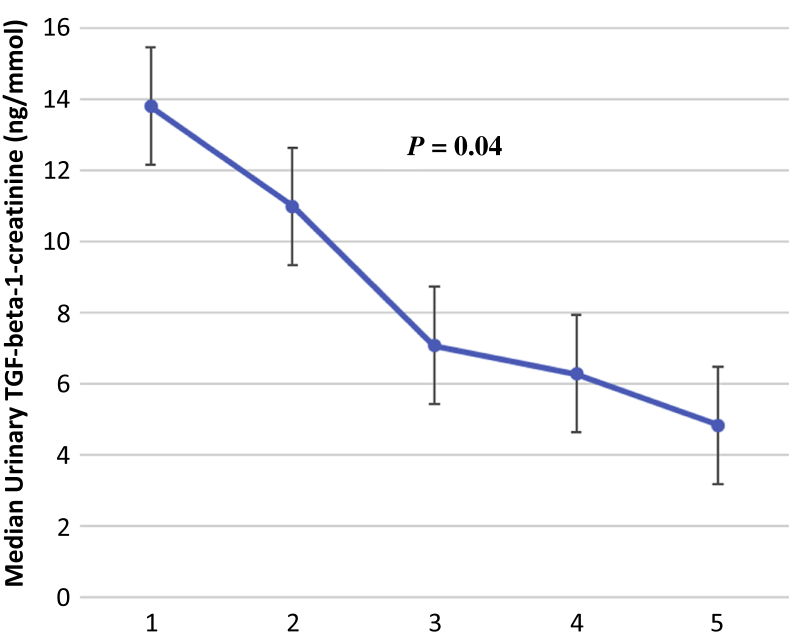
Age and uPCR had significant correlation with uTGF-β1Cr, whereas no significant correlation was found with mean arterial blood pressure, body mass index, CD4 count, and serum TGF-β1 for the total study population (Table 2). However, in the HIV+CKD+ group there was significant correlation with age, serum creatinine, uPCR, and eGFR. There was significant positive correlation between uPCR and uTGF-β1Cr (rho = 0.32; P < 0.001). A significantly higher correlation was found between uTGF-β1Cr and eGFR in the HIV+CKD+ group than the HIV+CKD− group. For the HIV+CKD+ group, median uTGF-β1Cr significantly decreased with severity of CKD (Figure 1). There was no correlation between serum TGF-β1 and eGFR (rho = −0.04; P = 0.52) or between serum TGF-β1 and serum creatinine (rho = 0.09; P = 0.19).
Table 2.
Correlations of uTGF-β1Cr with demographic and biochemical parameters
| HIV+CKD+ group (n = 114) |
HIV+CKD− group (n = 194) |
P-for difference | Total (N = 308) |
||||
|---|---|---|---|---|---|---|---|
| Rho | P | Rho | P | Rho | P | ||
| Age (yr) | 0.20 | 0.02 | 0.09 | 0.22 | 0.35 | 0.19 | 0.001 |
| Serum creatinine (μmol/l) | −0.35 | <0.001 | −0.01 | 0.85 | 0.003 | 0.01 | 0.98 |
| Serum TGF-β1 (pg/ml) | −0.17 | 0.11 | 0.03 | 0.75 | 0.09 | −0.03 | 0.66 |
| mABP (mm Hg) | 0.04 | 0.62 | 0.04 | 0.64 | 0.99 | 0.07 | 0.22 |
| BMI (kg/m2) | 0.02 | 0.85 | −0.14 | 0.07 | 0.18 | −0.11 | 0.06 |
| Waist circumference (cm) | −0.03 | 0.73 | −0.07 | 0.32 | 0.74 | −0.06 | 0.26 |
| eGFRMDRD (ml/min per 1.73 m2) | 0.35 | <0.001 | 0.03 | 0.70 | 0.01 | 0.003 | 0.95 |
| eGFRCKD-EPI (ml/min per 1.73 m2) | 0.35 | <0.001 | −0.005 | 0.99 | 0.003 | −0.02 | 0.81 |
| CD4 count (cells/μl) | −0.05 | 0.68 | −0.15 | 0.13 | 0.40 | −0.09 | 0.20 |
| uPCR (g/mmol) | 0.31 | <0.001 | 0.14 | 0.05 | 0.13 | 0.32 | <0.001 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; HIV+CKD+, HIV-positive patients with CKD; HIV+CKD−, HIV-positive patients without CKD; mABP, mean arterial blood pressure; TGF-β1, transforming growth factor-beta-1; uPCR, urine protein-creatinine ratio.
Figure 1.
Median urinary transforming growth factor (TGF) levels across chronic kidney disease stages.
Univariable and Multivariable Linear Regression Models
After adjusting for the effect of age at the time of data collection, sex differences, mean arterial blood pressure, waist circumference, eGFR, ARV regimen, and CD4 count at enrollment, patients with HIV+CKD+ persistently had higher levels of uTGF-β1Cr compared with HIV+CKD− (Table 3). The magnitude of the effect of uPCR on uTGF-β1Cr levels was noted to be much higher than that of eGFR. The type of ARV regimen administered, differences in sex, body mass index, CD4 count, and serum TGF-β1 were not significantly associated with uTGF-β1Cr at the multivariable level (Table 3). Older age was significantly associated with higher uTGF-β1Cr levels. A little more than a quarter (88 [28.6%]) of the sample population were on tenofovir disoproxil fumarate. Sensitivity analysis done (without patients using tenofovir disoproxil fumarate) did not show any significant qualitative difference. All independent associations seen in the initial model were maintained.
Table 3.
Linear regression models for uTGF-β1Cr prediction
| Univariable β (95% CI) P value | Multivariable β (95% CI) P value | |
|---|---|---|
| Study groups | ||
| HIV+CKD− | 1 | 1 |
| HIV+CKD+ | 2.66 (1.85–3.47) <0.001 | 3.97 (2.49–5.45) <0.001 |
| Age (yr) | 0.04 (0.004–0.08) 0.03 | 0.07 (0.02–0.12) 0.01 |
| Sex | ||
| Female | 1 | 1 |
| Male | −0.98 (−1.13 to 0.94) 0.85 | −0.51 (−1.78 to 0.76) 0.43 |
| mABP (mm Hg) | 0.04 (0.01–0.06) 0.01 | 0.01 (−0.02 to 0.05) 0.44 |
| BMI (kg/m2) | −0.13 (−0.23 to −0.03) 0.02 | 0.03 (−0.19 to 0.25) 0.83 |
| Waist circumference (cm) | −0.03 (−0.74 to 0.02) 0.32 | −0.05 (−0.15 to 0.06) 0.39 |
| ARV regimen | ||
| Non–TDF-based | 1 | 1 |
| TDF-based | −0.76 (−1.81 to 0.29) 0.15 | −2.57 (−6.07 to 0.93) 0.15 |
| CD4 count (cells/μl) | −0.01 (−0.01 to 0.006) 0.81 | −0.0006 (−0.007 to 0.006) 0.91 |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | −0.10 (−0.02 to 0.01) 0.24 | 0.03 (0.01–0.05) 0.01 |
| uPCR (g/mmol) | 12.10 (10.58–13.62) <0.001 | 6.62 (2.04–11.20) 0.01 |
| Serum TGF-β1 (pg/ml) | 0.002 (−0.001 to 0.002) 0.99 | 0.0001 (−0.001 to 0.003) 0.93 |
ARV, antiretrovirals; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; eGFR (CKD-EPI), estimated glomerular filtration rate using the chronic kidney disease epidemiology collaboration equation; HIV+CKD+, HIV-positive patients with CKD; HIV+CKD−, HIV-positive patients without CKD; mABP, mean arterial blood pressure; TDF, tenofovir disoproxil fumarate; TGF-β1, transforming growth factor-beta-1; uPCR, urine protein-creatinine ratio.
Table 4 shows univariable and multivariable robust linear regression models with eGFRCKD-EPI as dependent variable. The relationship between uTGF-β1Cr and renal function seen in previous analysis appears to be dependent on proteinuria levels. In both multivariable models, serum TGF-β was not independently associated with uTGF-β1Cr or eGFRCKD-EPI (Tables 3 and 4).
Table 4.
Linear regression models for eGFR prediction
| Univariate β (95% CI) P value | Multivariate β (95% CI) P value | |
|---|---|---|
| Age (yr) | −0.87 (−1.16 to −0.57) <0.001 | −0.86 (−1.15 to −0.56) <0.001 |
| Sex | ||
| Female | 1 | 1 |
| Male | −0.58 (−8.28 to 7.13) 0.88 | 4.00 (−3.41 to 11.41) 0.29 |
| uPCR (g/mmol creatinine) | −24.27 (−36.18 to −12.36) <0.001 | −29.57 (−42.68 to −16.46) <0.001 |
| uTGF-β1Cr (ng/mmol) | −0.12 (−0.36 to 0.11) 0.31 | 0.18 (−0.09 to 0.44) 0.19 |
| Serum TGF-β1 (pg/ml) | −0.001 (−0.01 to 0.01) 0.89 | −0.001 (−0.01 to 0.01) 0.91 |
| BMI (kg/m2) | 0.24 (−0.54 to 1.02) 0.55 | 0.09 (−0.64 to 0.82) 0.81 |
| CD4 count (cells/μl) | −0.01 (−0.03 to 0.01) 0.24 | −0.01 (−0.03 to 0.01) 0.28 |
| ARV regimen | ||
| Non-TDF regimen | 1 | 1 |
| TDF regimen | 8.88 (1.57–16.19) 0.02 | 6.10 (−1.22 to 13.42) 0.10 |
ARV, antiretrovirals; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; TDF, tenofovir disoproxil fumarate; TGF-β1, transforming growth factor-beta1; uPCR, urine protein-creatinine ratio; uTGF-β1Cr, transforming growth factor-beta-1–creatinine.
Discussion
This study found significantly higher levels of uTGF-β1Cr in HIV+CKD+ patients compared with HIV+CKD− patients even after controlling for other potential confounders. This difference also occurred despite both groups having similar levels of serum TGF-β1. There was significant positive correlation between uTGF-β1Cr and uPCR. Older age and eGFR were also independently associated with differences in uTGF-β1Cr levels. Among the HIV+CKD+ individuals, the levels of uTGF-β1Cr progressively decreased across CKD stages 1 to 5. Ultimately, uTGF-β1Cr levels were fully dependent on uPCR levels.
TGF-β1 is known to induce renal fibrosis through multiple pathways, including direct action on fibroblasts and other cells that cause extracellular matrix synthesis; inhibition of antifibrotic pathways, and induction of cell loss through apoptosis.15 In the “canonical” pathway of TGF-β1–induced renal fibrosis, the binding of TGF-β1 to its twin transmembrane receptors leads to the activation (phosphorylation) of smad2 and smad3, which is then translocated to the nucleus with the help of smad4 protein. The activation of smad2 and smad3 is usually associated with the inhibition of smad7, which is known to have antifibrotic activity. The binding of smad3 to gene promoters leads to transcription of profibrotic molecules. This leads to increased laying down of extracellular matrix in the kidneys and subsequent fibrosis.15 In the kidneys, TGF-β1 is expressed on the renal tubular epithelial cells and glomerular basement membrane16 and also in the myofibroblasts occurring in the interstitium during chronic kidney injury.5 The increased expression of TGF-β1 in persistent renal injury17 manifests as increased urinary excretion of TGF-β1.18 This has been documented in renal diseases associated with increase in extracellular matrix production,6, 7 including HIV-related nephropathy.10 The finding of increased urinary excretion of TGF-β1 in patients with HIV and CKD was corroborated by our study. Others also have found a positive correlation between urinary albumin excretion and uTGF-β1,19 which was also corroborated by our study. Indeed, we observed a stronger effect of uPCR (compared with eGFR) on uTGF-β1Cr levels in this study. This is probably due to the activation of proximal tubular cells by persistent proteinuria leading to increased production of TGF-β1.20 In the multivariable model with eGFR as the dependent variable, uPCR was shown to clearly explain the changes associated with uTGF-β1Cr.
We also noted a stepwise reduction in urinary TGF-β1 levels in individuals with more severe CKD (stages 4 and 5). In advanced CKD, where tubular atrophy and severe tubulointerstitial fibrosis has set in, there are reduced numbers of myofibroblasts and functional proximal tubular epithelial cells (and thus TGF-β expression) in the tubulointerstitium, which may lead to reduced excretion of urinary TGF-β1. This explanation suggests a type of “burn-out” of TGF-β1 activity as CKD progresses. Some investigators have reported lower interstitial density measurements for TGF-β1 in advanced CKD from patients with HIV-associated nephropathy and diabetic nephropathy compared with HIV-positive and HIV-negative controls.21 Similarly, some studies have shown a reduction in circulating TGF-β1 and TGF-β1 mRNA in patients with end-stage kidney disease.22, 23, 24 A 12-month follow-up study25 among patients with CKD with a wide range of etiology (not including HIV), found significantly higher levels of tubulointerstitial TGF-β1 mRNA in patients with renal disease who did not have progressive CKD compared with those who had progressive CKD. This suggested a protective effect of TGF-β1, but a 12-month follow-up period may not be adequate to assess long-term renal outcomes, and serial TGF-β mRNA measures were not available in this study. Studies with contrary findings26, 27 (higher TGF-β1 immunohistochemical and mRNA expression in the renal tubulo-interstitium with increased fibrosis) were noted to have been done in patients with stages 1 to 3 CKD and not very advanced disease. Indeed, one study26 used only patients with serum creatinine less than 2.0 mg/dl (approximately 177 μmol/l), understandably because of the risk involved in the biopsy of fibrotic kidneys. It appears that serial measurements of urinary TGF-β1 in progressive HIV+CKD+ patients, being a noninvasive procedure, may more succinctly document the temporal profile of uTGF-β1 in progressive CKD.
The markedly elevated uTGF-β1Cr found in patients in stages 1 and 2 CKD present a window of opportunity to reverse or slow down progression if early diagnosis is made and available intervention, like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, are used. Studies have shown reduction in uTGFβ1 levels attributable to administration of angiotensin-converting enzyme inhibitors28 or prednisolone29 in IgA nephropathy. The actions of these medications may not be unconnected with the fact that persistent proteinuria is known to activate complements in the renal tubular epithelial cells leading to a cascade of events that eventuate in renal fibrosis.30, 31 Reducing proteinuria using angiotensin receptor blockers or angiotensin-converting enzyme inhibitors may indirectly reduce TGF-β1 profibrotic activity in the kidneys and slow down CKD progression; however, it is yet to be shown, in a properly conducted clinical trial, that this will be the case among patients with HIV with early CKD. The type of ARV regimen used did not appear to have any effect on variability in uTGF-β1Cr levels in this study. This includes tenofovir use, which has been known to be associated with proximal tubular dysfunction. We are not aware of any direct link between tenofovir-related renal fibrosis and TGF-β1 levels. It may also have been more informative to compare uTGF-β1Cr levels between ARV-naïve and ARV-exposed patients. Unfortunately, all patients in this study had been commenced on ARV.
A major limitation of this study is the lack of renal histopathology among the patients with CKD. This could have determined if the degree of fibrosis (glomerular, tubular, and interstitial) correlated with uTGFB1 in the CKD population. Another limitation of our study is the cross-sectional design as this design does not allow for serial assessment of this marker as a measure of progressive deterioration of kidney function. Therefore, it is difficult to ascertain from this work if uTGF-β1Cr increase predates the occurrence of persistent proteinuria. Again, because the levels of uTGF-β1Cr appear to wane with advanced CKD, it is possible to miss cases of advanced CKD if this test is used as a stand-alone. Also, the lack of HIV-negative controls limited comparison of uTGF-β1Cr levels in the patients with HIV and the general population. Despite the limitations, the result of our study is an important addition to the current body of literature and suggests that uTGF-β1Cr is an important marker of CKD in patients who are HIV-positive. Whether this biomarker can be useful for monitoring kidney response to treatment still needs to be studied.
Conclusion
Patients with HIV and CKD express higher levels of TGF-β1 activity in urine, especially in the early stages of CKD, explained by proteinuria levels. Persistent proteinuria remains a veritable tool for early CKD detection in the HIV population.
Disclosure
All the authors declared no competing interests.
References
- 1.Sporn M.B., Roberts A.B., Wakefield L.M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105:1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lan H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma K., Ziyadeh F.N. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995;44:1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 4.Isaka Y., Fujiwara Y., Ueda N. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993;92:2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto T., Noble N.A., Cohen A.H. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 6.Border W.A., Okuda S., Languino L.R. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature. 1990;346:371. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 7.Border W.A., Noble N.A., Yamamoto T. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T., Noble N.A., Miller D.E. Increased levels of transforming growth factor-beta in HIV-associated nephropathy. Kidney Int. 1999;55:579–592. doi: 10.1046/j.1523-1755.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 9.Yushchuk N.D., Gadzhikulieva M.M., Balmasova I.P. [The role of immune factors in the progression of chronic kidney diseases in HIV infection] Terapevticheskii arkhiv. 2016;88:56–61. doi: 10.17116/terarkh201688356-61. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell B.I., Byron M.M., Ng R.C. Elevation of non-classical (CD14+/lowCD16++) monocytes is associated with increased albuminuria and urine TGF-beta1 in HIV-infected individuals on stable antiretroviral therapy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey A.S., Coresh J., Bolton K. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–S266. [PubMed] [Google Scholar]
- 12.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caci H. Boston College Department of Economics; Boston, MA: 2000. CORTESTI: Stata Module to Test Equality of Two Correlation Coefficients. Statistical Software Components S407302. [Google Scholar]
- 15.Meng X.-M., Nikolic-Paterson D.J., Lan H.Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 16.Yoshioka K., Takemura T., Murakami K. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993;68:154–163. [PubMed] [Google Scholar]
- 17.Bruijn J.A., Roos A.J. Transforming growth factor-beta and the glomerular extracellular matrix in renal pathology. J Lab Clin Med. 1994;123:34–47. [PubMed] [Google Scholar]
- 18.Tsakas S., Goumenos D.S. Accurate measurement and clinical significance of urinary transforming growth factor-beta1. Am J Nephrol. 2006;26:186–193. doi: 10.1159/000093178. [DOI] [PubMed] [Google Scholar]
- 19.Honkanen E., Teppo A.-M., Törnroth T. Urinary transforming growth factor-beta 1 in membranous glomerulonephritis. Nephrol Dial Transplant. 1997;12:2562–2568. doi: 10.1093/ndt/12.12.2562. [DOI] [PubMed] [Google Scholar]
- 20.Goumenos D.S., Tsakas S., El Nahas A.M. Transforming growth factor-β1 in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant. 2002;17:2145–2152. doi: 10.1093/ndt/17.12.2145. [DOI] [PubMed] [Google Scholar]
- 21.Nel M., Buys J.-M., Botha F. The functionality of African-specific variants in the TGFB1 regulatory region and their potential role in HIVAN. Clin Exp Nephrol. 2018;22:764–772. doi: 10.1007/s10157-017-1516-4. [DOI] [PubMed] [Google Scholar]
- 22.Füth R., Herder C., Förster S. Evaluation of diagnostic relevance of mRNA levels in peripheral blood: predictive value for mortality in hemodialysis patients. Cytokine. 2004;27:166–172. doi: 10.1016/j.cyto.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Knerr K., Füth R., Hemsen P. Chronic inflammation and hemodialysis reduce immune competence of peripheral blood leukocytes in end-stage renal failure patients. Cytokine. 2005;30:132–138. doi: 10.1016/j.cyto.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A., Gupta V., Chaudhary M. Depleted TGF-β1 levels in end stage renal disease patients from North India. Gene. 2014;534:440–443. doi: 10.1016/j.gene.2013.09.116. [DOI] [PubMed] [Google Scholar]
- 25.Eikmans M., Baelde H.J., Hagen E.C. Renal mRNA levels as prognostic tools in kidney diseases. J Am Soc Nephrol. 2003;14:899–907. doi: 10.1097/01.asn.0000056611.92730.7b. [DOI] [PubMed] [Google Scholar]
- 26.Iwano M., Akai Y., Fujii Y. Intraglomerular expression of transforming growth factor-beta 1 (TGF-β1) mRNA in patients with glomerulonephritis: quantitative analysis by competitive polymerase chain reaction. Clin Exp Immunol. 1994;97:309–314. doi: 10.1111/j.1365-2249.1994.tb06086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bódi I., Kimmel P.L., Abraham A.A. Renal TGF-β in HIV-associated kidney diseases. Kidney Int. 1997;51:1568–1577. doi: 10.1038/ki.1997.215. [DOI] [PubMed] [Google Scholar]
- 28.Park H.C., Xu Z.G., Choi S. Effect of losartan and amlodipine on proteinuria and transforming growth factor-β1 in patients with IgA nephropathy. Nephrol Dial Transplant. 2003;18:1115–1121. doi: 10.1093/ndt/gfg090. [DOI] [PubMed] [Google Scholar]
- 29.Haramaki R., Tamaki K., Fujisawa M. Steroid therapy and urinary transforming growth factor-β1 in IgA nephropathy. Am J Kidney Dis. 2001;38:1191–1198. doi: 10.1053/ajkd.2001.29209. [DOI] [PubMed] [Google Scholar]
- 30.Sheerin N., Sacks S.J. Leaked protein and interstitial damage in the kidney: is complement the missing link? Clin Exp Immunol. 2002;130:1. doi: 10.1046/j.1365-2249.2002.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangan G.K., Pippin J.W., Coombes J.D. C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney Int. 2005;67:492–503. doi: 10.1111/j.1523-1755.2005.67106.x. [DOI] [PubMed] [Google Scholar]