Abstract
The older adult population (65 years or older) with advanced or end-stage kidney disease is steadily growing, but rates of transplantation within this cohort have not increased in a similar fashion. Physical deconditioning, resulting in poor post-transplantation outcomes, is a primary concern among older renal patients. The assessment of physical function often holds more weight in the selection process for older candidates, despite evidence showing benefits of transplantation to this vulnerable population. Although several frailty assessment tools are being used increasingly to assess functional status, there is no standardized selection process for older candidates based on these assessment results. Also, it is unknown if timely targeted physical therapy interventions in older patients result in significant improvement of functioning capacity, translating to higher listing and transplantation rates, and improved post-transplantation outcomes. It is therefore of upmost importance not only to incorporate an effective objective functional status assessment process into selection and waitlist evaluation protocols, but also to have targeted interventions in place to maintain and improve physical conditioning among older renal patients. This paper reviews the commonly utilized assessment tools, and their applicability to older patients with renal disease. We also propose the need for definitive selection and waitlist management guidelines to formulate a streamlined assessment of functional capacity and transplant eligibility, as well as a process to maintain functional status, thereby increasing the access of older patients to renal transplantation.
Keywords: elderly, physical assessment, transplant, transplant candidacy
The global population is aging. More than 1.5 billion individuals are expected to be 65 years or older by the year 2050.1 Similar trends are seen in the end-stage renal disease (ESRD) population, with the number of individuals 65 years and older needing renal replacement therapy steadily on the rise.2 The merits of kidney transplantation among all ages are well known. Despite this, from 2007 to 2016, the transplantation rates per 100 dialysis years have remained between 2.4 and 2.6 for patients aged 65–75 years and between 0.3 and 0.4 for patients older than 75 years in the United States.2
Chronic kidney disease (CKD) accelerates the functional deterioration process through several pathways: protein energy wasting, oxidative stress, and chronic inflammation.3 The greatest hurdle to kidney transplantation is identifying “the suitable candidate”—an individual likely to benefit from receiving a transplant. Several factors contribute to candidate eligibility. Age alone is no longer a contraindication to transplantation.4 Yet, kidney transplantation often is not offered as an option to older renal patients. A study conducted on older first-time kidney transplant candidates reported that 76.3% of those with predicted 3-year post-transplantation survival rates of 87.6% or higher were neither listed for transplantation nor referred for living-donor transplants.5 Many transplant centers are wary of providing transplants for older candidates given the multiple coexisting comorbidities and higher risks of early post-transplantation mortality and morbidity,6 despite research suggesting that older candidates also derive benefit from receiving a transplant compared with remaining dialysis-dependent.7, 8, 9, 10, 11, 12, 13
Mounting evidence highlights an association between impaired physical functioning and poor post-transplantation outcomes. As a result, tools that assess physical functioning and functional limitations are increasingly being utilized to risk-stratify transplant candidates. However, the lack of biological understanding of true functional impairment, inability to distinguish functional impairment from the natural aging process, lack of clear cut-off values to determine transplant eligibility, as well as knowledge gaps regarding effective utilization of tools to improve candidate selection and post-transplantation outcomes result in inconsistent use of these tools. The American Society of Transplantation recently released consensus guidelines on best practices for assessing frailty risks for transplantation of various solid organs; however the ideal method to measure frailty risks has not yet been identified.14 Currently, the impact of poor assessment results on transplant eligibility and selection remains at the discretion of the transplant centers, which have varied policies on utilizing assessment tools during their candidate selection process, with no standardization of the type of tool or the method of administration.14 Additionally, functioning capacity assessments are not routinely performed on kidney transplant waitlist candidates to determine change in conditioning and thus eligibility status.
Evidence suggests that the selection process is more stringent for older candidates compared with that for their younger counterparts.6 Older renal patients, even those without significant comorbidities, are at a higher risk of early mortality and rehospitalizations resulting in poor quality of life and significant medical and financial healthcare burdens.15 Not surprisingly, significant emphasis is placed on thorough medical and functional status evaluation during transplant eligibility assessment for older transplant candidates. In clinical practice, older candidates are more likely to be perceived as frail without objective assessments of their functional status, based on their appearance or self-reports about activity levels. Some transplant centers have an arbitrary age cut-off for transplantation that unfairly restricts transplant access for the older ESRD/CKD population. These subjective disparities inadvertently impact transplantation referrals and rates among older candidates, especially women.4,6,16, 17, 18
This paper reviews the commonly used, validated, performance-capacity assessment tools, with a focus on the benefits and shortcomings of applying these tools to older renal patients. Our aim is to promote awareness regarding the lack of standardized objective selection guidelines based on functioning capacity, the need for a multidimensional model including counseling, assessment recommendations, and targeted preventative interventions for older renal candidates (including those on the deceased-donor waitlist), to ensure more referrals, objective selection, and maintenance of continued eligibility for transplantation. Ultimately, we seek to highlight the need for further research efforts and greater participation from all stakeholders to create an effective functional model for this cohort without jeopardizing post-transplantation outcomes.
Physical Function and Physical Activity Assessment Tools: Applicability and Limitation for Use in Older Transplant Candidates
Selecting an appropriate older candidate for kidney transplantation is challenging and complex. The primary reason for graft loss in an older transplant patient is death with a functioning graft.19 The natural process of aging is well known to cause loss of muscle mass, decreased cardiovascular efficiency resulting in reduced aerobic capacity, muscle strength, and functional mobility.20 The ideal functional status assessment tool is one that is multidimensional and evaluates several areas that affect overall physical functioning and subsequent poor outcomes. Assessment tools measure functioning abilities in a variety of ways: (i) physical activity (e.g., self-report, step counters); (ii) performing capacity (e.g., self-report, timed walk test, short physical performance battery); and (iii) physiological impairment (e.g., cardiorespiratory fitness, muscle strength/endurance).21
Which assessment tool best determines functional abilities in the older CKD/ESRD patient? Are the various available assessment tools interchangeable and equal in comparing functional impairment? These questions are yet to be answered. To be used consistently in the clinical setting, assessment tools need to be completely objective, easy to administer, reproducible, and without a significant monetary or time investment requirement.
Today, more than 75 functional status assessment tools are available, with new assessment tools being developed on an ongoing basis. Although reviewing all available tools is beyond the scope of this paper, the most frequently used tools are discussed in the following sections (Table 1).
Table 1.
Commonly used functional assessment tools in clinical practice: benefits and limitations when used in older renal transplant candidates
| Functional assessment tools | Methods | Benefits | Limitations |
|---|---|---|---|
| Physical assessment questionnaires | Self-reported ability to perform varied tasks using SF36, IADL, PASE |
|
|
| Karnofsky Performance Status Scale | Assigned score of 0%–100% based on reported functional abilities |
|
|
| Fried’s Frailty Phenotype Score | Score of 0–5 on domains, namely: (1) weight loss
0 = nonfrail 1–2 = prefrail ≥3 = frail |
|
|
| Frailty Index | Index of cumulative deficits (functional impairments, cognitive impairments, laboratory findings, disabilities); scored 0–1 Scoring interpretation: 0 = good health status 0.5 = fair health status 1 = poor health status |
|
|
| Physical performance capacity measures | Walking speed, grip strength, repeat chair stands, 6-min walk test, timed up-and-go tests |
|
|
| SPPB | Measures lower-extremity strength Score from 0–4 on: (1) standing balance
|
|
|
| Morphometric measurements |
|
|
|
| Cardiopulmonary fitness test | Tests exercise tolerance by measuring peak oxygen uptake using incremental treadmill or stationary bike |
|
|
IADL, instrumental activities of daily living; PASE, Physical Activity Scale for the Elderly; SF36, Short Form-36 Physical Function Scale; SPPB, Short Physical Performance Battery.
Physical Assessments Questionnaires
The easiest method to assess physical conditioning is through self-reported ability to perform varied tasks, through questionnaires such as the Short Form-36 physical function scale, the Katz Independence in Daily Living, the Instrumental Activities of Daily Living, or the Physical Activity Scale for the Elderly. In the older transplant population, studies utilizing self-reported assessment tools have established an association between functional status and patient survival.22, 23, 24, 25, 26 The primary concern with using questionnaires as assessment tools is their subjective nature, owing to self-reporting. The possibility of selection bias and inaccurate reporting, and the difficulty in using them longitudinally to quantify improvement with interventions, makes them less than ideal for physical functioning assessment as a means to determine transplant eligibility.
Karnofsky Performance Scale (KPS)
The KPS is one of the earliest assessment tools27 and has been in use since 1949. With the KPS, providers (users) assign individuals a score between 0% (dead) and 100% (active, no limitations), based on their ability to perform daily activities and the level of assistance they need to do them. Developed primarily to assess performance status in patients with advanced cancer, it is now a widely used metric to assess functional abilities in candidates both pre- and post-transplantation. The Organ Procurement and Transplantation Network requires reporting of KPS results at the time of transplantation for all adult recipients, as a surrogate for measuring frailty.
The KPS allows for quick, easy evaluation of a person’s functional abilities. However, assessment of ability to perform daily activities, and symptoms experienced, is based on an individual’s perception of their own abilities, which is not always reproducible. Also, classification of symptoms as mild or more significant, and decisions about which symptoms are of concern, are vaguely described and are determined primarily by the user assigning a score. This method raises concerns regarding reliability and validity given the variability in user reporting.27 The Scientific Registry of Transplant Recipients highlighted this pitfall in an analysis of mean KPS results across varied transplantation programs (kidney, liver, and lung) in different transplant centers countrywide.28 Significant variations in KPS reporting across centers, which did not attenuate when adjusted for age, gender, race, or disease cause, showcased the unreliability of the KPS, owing to user reporting differences.28 Although less user-reporting variability is seen when individuals with good performance status are scored, greater variability is observed when scoring an individual whose performance status is on the lower end of the spectrum.27 This inaccurate determination of performance status disproportionately impacts older renal patients, as they often fall into the lower end of the performance status range, thereby making the KPS an inadequate tool for this vulnerable group.
Fried’s Frailty Phenotype Score
Frailty is frequently and often wrongly used to describe a person who appears to be deconditioned. Older candidates are often mislabeled as “frail” primarily because of their advanced age and appearance. Frailty is defined in 1 of 2 ways: (i) as a syndrome of decreased reserve, resulting in decreased ability to recover from stressors, and an increased risk of poor health outcomes29, 30, 31; and (ii) as a cumulative index of deficits.32
The Fried’s frailty phenotype (FFP) score, originally characterized in community-dwelling elderly adults, assesses frailty in 5 domains: weight loss, exhaustion, physical activity, grip strength, and walking speed. Scores range from 0 to 5; the frailty spectrum ranges from non-frail (score of 0), to pre-frail (score of 1–2), and ultimately to frail (score of ≥3). Multiple factors, in addition to advancing age, contribute to frailty, including loss of muscle mass (sarcopenia), low activity, poor endurance, loss of strength, cognitive status, socioeconomic influences, and disease state.29 Patients with advanced CKD or end-stage kidney disease are known to be frail regardless of their age.33, 34, 35, 36, 37, 38 Measured frailty by FFP scoring has shown significant correlation with post-transplantation outcomes.39, 40, 41, 42, 43, 44 It is associated with increased risks of drug intolerance, post-transplantation delirium, delayed graft function, graft loss, early hospital readmission, and mortality regardless of the transplant recipient’s age.39, 40, 41, 42, 43, 44 The FFP score is the only available tool that is well validated in the CKD, older, and transplant populations.
In clinical practice, however, frailty typically isn’t measured, but perceived frailty is used as a surrogate, despite existing knowledge that measured and perceived frailty do not necessarily correlate with one another.16 Results from the American Society of Transplantation frailty assessment survey across different solid organ transplants, in the kidney transplant cohort: 31.8% of the survey takers reported never performing any standardized frailty assessment when evaluating for transplant candidacy, and the FFP score was utilized by only 3.6% of the survey takers who reported assessing frailty for candidacy evaluation.14 Additionally, 2 of the 5 components of the FFP score—unintentional weight loss and exhaustion—are subjective and self-reported, and therefore, not quantifiable. For example, one individual might be more active and report greater exhaustion, whereas another who has an extremely sedentary lifestyle may deny feeling exhausted. Measuring unintentional weight loss in patients with ESRD is also challenging due to the fluid-weight fluctuations seen in this population.45,46
Using perceived frailty as an assessment tool negatively impacts evaluation of an older transplant candidate. Despite knowledge of how to calculate a frailty score, nephrologists are more likely to perceive and label older candidates and those with more comorbidities as frail..16 Nephrologists, nurse practitioners, and candidates themselves often perceive older individuals with ESRD as frail when they are not frail as measured by the FFP score.16 Although the FFP is a great tool, its subjective components, the inability to accurately assess certain components in the older renal cohort, and discrepancies between perceived and objective FFP measurements, make it less suitable for use in the clinical setting for older kidney transplant candidacy selection.
Frailty Index
The frailty index (FI), developed as part of the Canadian Study of Health and Aging in older Canadians, measures frailty as an index of cumulative deficits.31,32 The FI takes into consideration factors (such as socioeconomic and cognitive impairments) contributing to frailty that are not measured by FFP. Approximately 30–70 deficits (such as functional impairments, cognitive impairments, laboratory findings, disabilities) can be measured with this process, and a ratio of the number of deficits to the total number of items considered can be calculated to determine the FI score.21 A score of 0 = good health, 0.5 = fair health, and 1 = poor health.
Since the FI incorporates a wider range of factors affecting frailty than the FFP score, it is more sensitive and precise. The FI also exhibits greater ability to identify individuals with moderate to severe frailty and to better assess mortality risk compared with the FFP score.47
One of the primary limitations of utilizing the FI is its inability to distinguish frailty from disability or functional dependence.48 The FI is also time-consuming to administer, making it less appropriate for use in the clinical setting. Although the FI is well validated in the older and surgical populations, it still needs research and validation among the transplant population.49,50
Muscle Function Measurements
Loss of muscle mass and muscle strength is part of the natural aging process. Starting as early as the fourth decade of life, and by the eighth decade, individuals lose almost 50% of their muscle mass.51 Reduced muscle mass results in reduced muscle strength, power, and endurance, all of which are essential for physical fitness.21 Reduced muscle mass and function is frequently observed in patients with advanced CKD, especially older adults, who are at the greatest risk.52 However, poor physical performance does not always equate to loss of muscle mass or strength and often is reversible with early initiation of strength/muscle-building activities. Therefore, it is crucial to recognize muscle loss and functioning impairments early, to identify at-risk older renal patients in whom performance capabilities can be improved with interventions. Physical performance abilities can be measured in several ways, as detailed below in this section.
Physical Performance Capacity Measures
Performance capacity in specific muscle groups can be assessed using tools such as walking speed, grip strength, repeat chair stands, the 6-minute walk test, or timed up-and-go tests. There are several advantages to using these tools: ease of use in the clinical setting, low or no cost, and time efficiency. However, each of these tools has been developed to answer a specific question about particular muscle groups, and impairment in performing one particular physical function does not always imply overall physical dysfunction. Variability in the choice of tool used makes standardizing and interpreting research results difficult. For example, walking speed, although it is a well validated clinical marker of survival and functional status in older community dwellers, has not been well validated amongst cohorts with comorbid conditions such as CKD.53 Assessment of grip strength in the older advanced-CKD person also poses challenges. Grip strength is significantly worse in an arm that has an arteriovenous dialysis access.47,54 Furthermore, older ESRD patients, frail or not, are known to have low grip strength.47,54 These factors imply that grip strength does not accurately represent functional abilities in older renal patients if used as a surrogate for performance capacity.
Applicability of walking speed and grip strength as stand-alone measures, especially when assessing functional status in the older transplant candidate, is questionable. Of all the performance capacity tools, the 6-minute walk test (6MWT) is the one most frequently used as a stand-alone test in clinical practice. In small ESRD cohort studies, better performance on the 6MWT correlated with improved quality of life.55 However, using the 6MWT in the dialysis population can be unreliable due to variability resulting from changes in fluid volume status (slow walking speed if volume overloaded) and the timing of test administration (before or after dialysis)..21 In the pretransplantation selection process, the 6MWT is often used as both a surrogate marker of cardiopulmonary fitness and a predictor of mortality and morbidity.55 However, the 6MWT does not estimate maximal exercise capacity and cannot be used in lieu of cardiopulmonary fitness tests.21 Its testing parameter allows patients to walk at their own pace and to stop and rest when necessary. Thus, maximal blood pressure, heart rate, or exercise capacity that represents an individual’s functional abilities may never be achieved. Additionally, studies assessing the 6MWT in the older kidney transplant cohort are currently lacking.
Several challenges exist to using any one particular physical capacity measuring tool, as noted earlier, and concurrent use of additional assessment tools is needed for more-reliable results.
Short Performance Physical Battery
Developed by the National Institute of Aging, the short performance physical battery (SPPB) objectively assesses physical abilities by measuring lower-extremity strength. To overcome shortcomings of using one particular performance metric, it combines use of 3 physical performance measures: standing balance, walking speed, and chair stand tests. Each area is scored from 0 to 4, for a composite score of 0–12, with higher scores indicating better functional status. A composite score of <10 is considered to indicate impairment and is associated with loss of mobility, poor quality of life, recurrent hospitalizations, longer hospitalization lengths, nursing home admissions, and mortality.19,56, 57, 58, 59
The SPPB is not impacted by user variation and has been extensively researched and validated in older population groups, making it a great tool for identifying mobility impairments and risk-stratifying older transplant candidates.19 It also is not laborious, requiring 5–10 minutes to administer.56 In a prospective study including 700 kidney transplant patients, the SPPB was found to be non-inferior to FFP scoring with regard to strength of association and prediction of mortality.60 A lower SPPB score was also associated with a 2.3-fold higher risk of post-transplantation mortality, independent of FFP scores.60 The SPPB is slowly emerging as a tool that is completely objective, is not time consuming, requires no personnel training for testing, and has high reliability. In addition, it is a perfect alternative for candidates with fistulas or grafts who have impaired grip strength and thus ideal for assessing older transplant candidates. Given that the SPPB measures lower-extremity impairment, alternative measurement tools are necessary for those with lower-extremity amputations or lower-extremity deficits due to vascular disease or neurologic issues.
Morphometric Measurements
Morphometric measurements, such as muscle mass, muscle density, and visceral fat, are known predictors of cardiovascular and postoperative mortality risks, including in the transplant population.61,62 Such measurements are completely objective and successfully predict risk of poor outcomes among kidney transplant waitlist candidates.62 Imaging studies are necessary to determine morphometric measurements. In transplant candidates, abdominal computed tomography (CT)/magnetic resonance imaging (MRI) is regularly performed as a part of candidacy evaluation; therefore, taking these measurements may not be associated with additional cost. Morphometric assessments are commonly used to measure sarcopenia and morphometric age.
Sarcopenia
Sarcopenia, the involuntary loss of muscle mass and therefore muscle strength, was originally thought to be a result of aging alone. It has now been determined to be multifactorial and can result from ongoing disease processes and malnutrition. Sarcopenia prevalence increases with worsening renal function and is associated with poor outcomes, such as physical disability, poor quality of life, and death.63, 64, 65 Therefore, even a small reduction in sarcopenia prevalence can significantly reduce healthcare costs.66
For diagnosing sarcopenia, muscle mass can be measured by anthropometry, bioelectrical impedance analysis, dual energy X-ray absorptiometry, or CT/MRI.67 CT and MRI are well validated for diagnosing sarcopenia but costly, therefore not the first choice to diagnose sarcopenia in the general population.67 Per current US guidelines, in the older population, sarcopenia should be suspected in older adults who are nonambulatory, have low walking speed, or need assistance getting up from a chair, and diagnosis of sarcopenia should be made using a dual energy X-ray absorptiometry scan.67 These vague criteria for performing objective imaging testing have resulted in underdiagnosis of sarcopenia in the general population.67 In the kidney transplant population, abdominal CT or MRI is regularly performed as part of the evaluation testing. However, sarcopenia assessment via psoas muscle measurement is not routinely calculated, resulting in infrequent diagnosis of sarcopenia in the transplant population as well.
Morphometric Age Analysis
It has been long known that chronological age is a poor proxy for a person’s functional status and physiological reserve. Morphometric age is objective, precise, and reproducible, and it better correlates with outcomes in the transplant population.68,69 It is calculated by software that computes psoas muscle area, psoas muscle density, and percentage of aortic wall calcification measured on abdominal CT imaging. Data show that chronologically older patients with a low morphologic age have increased survival compared with those who have a higher morphologic age.69 Determining morphologic age, although it is more precise and reliable compared with taking sarcopenia measurements, is still in the process of being researched and validated in the transplant population. Conducting the necessary measurements also requires experienced personnel and specialized software.
Cardiopulmonary Fitness Test
The association between cardiopulmonary fitness and overall health is well established.70 Cardiopulmonary fitness effectively predicts overall mortality and cardiovascular risks in any population group.71 Limited data in the kidney transplant cohort also show a correlation between lower peak oxygen uptake and increased post–kidney transplant hospital readmissions and mortality.72,73 Exercise tolerance testing, using an incremental treadmill or stationary bike, allows measurement of peak oxygen uptake, thus assessing maximal endurance and performance capacity. Advanced CKD and ESRD candidates have other comorbid conditions, and a tendency to be fluid overloaded, which negatively impact their aerobic capacity.21 Therefore, cardiopulmonary testing in this population can be limited due to early discontinuation of testing and inability to achieve maximal exercise capacity, owing to symptom development. Also, cardiopulmonary tests need to be adjusted for age, gender, and weight, which is challenging in this cohort, given the significant fluctuations in weight that occur based on fluid volume status. Furthermore, the need for trained personnel as well as significant monetary resources limits their popularity for use in the kidney transplant population.
Creating a Better Paradigm for Older Transplant Candidates
The older renal cohort is significantly different from other populations. Research suggests that older adults with advanced CKD/ESRD have more disabilities compared with older chronic obstructive pulmonary disease or heart failure patients.74 Prehabilitation with pretransplant physical activity regimens is not the usual practice, despite strong evidence showing exercise training benefits to ESRD patients and kidney transplant recipients.75, 76, 77, 78, 79 Prehabilitation data in the transplant population are limited, but evidence in surgical populations shows significant improvement in short- and long-term outcomes with prehabilitation.80, 81, 82, 83
In the older population, it is of utmost importance to carefully consider the ethical, financial, and medical implications of offering kidney transplantation. Although selection decisions for candidates on either end of the spectrum (not impaired or with severely impaired functional abilities) are relatively straightforward, eligibility decisions for candidates who are moderately impaired at the time of initial evaluation, or who become severely deconditioned while on the waitlist, are more challenging. The upper age limit and extent of functional impairment at which transplantation ceases to offer a benefit are still unknown. Therefore, denying transplantation to an older individual solely on the basis of poor functioning capacity raises ethical concerns of bias. Additionally, no consensus or guidelines have been established on how to manage candidates with poor functional reserve. At what point does the risk of transplantation outweigh the benefits? How would transplantation affect overall quality of life? Can targeted exercise paradigms slow, halt, or reverse functional deconditioning sufficiently to minimize peritransplantation risk without affecting outcomes? When does functional impairment become significantly advanced or irreversible? These are crucial questions to address during the transplant decision-making process for older adults.
There is need for a multidimensional paradigm that incorporates counseling, objective functional assessment, and prehabilitation into the transplantation selection process of the older CKD and ESRD cohort from the time of referral from a nephrologist until post-transplantation. This paradigm is important for (i) risk stratification, (ii) identification of candidates likely to benefit from preventative interventions, and (iii) monitoring response to targeted interventions. Approaches and tools that can be used include those described in the following 2 sections.
At the Nephrologist’s Office
Current evidence identifies poor counseling by nephrologists and other medical staff, as well as insufficient encouragement to engage in physical activities, as barriers to patient participation in exercise programs.84, 85, 86, 87 Training of general nephrologists and dialysis staff is crucial for honing their skills to counsel older renal patients regarding kidney transplantation, provide timely transplant referrals, and educate patients early regarding the need for physical activity to maintain good functional abilities.
Self-report questionnaires are great tools for counseling older candidates about their transplant eligibility and the probability of poor post-transplantation outcomes.88 Simple objective clinical tools, such as the SPPB, chair stands, and the 6MWT, can be used to identify the highest-risk older renal patients who are unlikely to benefit from transplantation and also to measure performance improvement with physical activity interventions. These tools are easy to administer and can rule out the sickest candidates while allowing other older candidates an opportunity to undergo a more thorough evaluation at a transplant center. Albeit that training of personnel is pivotal for accurately administering tests such as the SPPB, free training materials and other helpful tools are easily accessible at no cost, from the National Institute of Aging’s website, and these can assist immensely with the personnel training process.
At the Transplant Center: Initial Candidacy Evaluation and Waitlist Management
Defined thresholds can help identify older candidates in whom the transplant risks outweigh the benefits, and those who should be eligible to receive only living-donor transplants to minimize time to transplantation, as well as the rates of further deconditioning and death while on the waiting list. These cutoff criteria need to be defined using objective, well validated, and reliable measures. Also, ongoing physical training interventions need to be incorporated once patients are approved for transplantation, to maintain and improve functionality.
Of all the above-mentioned functional-capacity assessment tools, the SPPB and morphometric measurements are the most objective and validated tests currently available. The SPPB is a great tool for use during the evaluation process and can also be used to monitor progress following targeted physical therapy interventions. Abdominal CT imaging and/or MRI are routinely obtained during the transplant candidacy evaluation, and they are often repeated during waitlist re-evaluations. Therefore, diagnosing sarcopenia by psoas muscle measurements and determining morphometric age in this cohort are not associated with additional cost and can assist in early diagnosis of muscle mass loss.
Based on the currently available clinical data, utilizing both the SPPB and imaging studies for the detection of sarcopenia appears to be the preferred option for the initial evaluation and waitlist management of the physical functional status of older patients with CKD and ESRD.
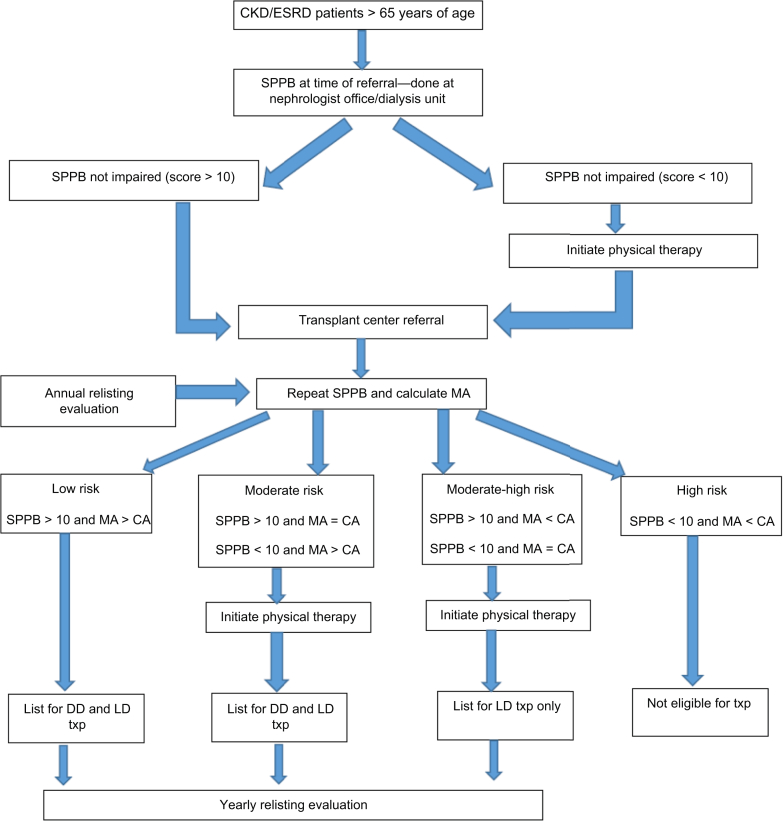
To simulate a potential objective assessment strategy, we propose a model utilizing these 2 tools to stratify older candidates into 4 risk groups (low, moderate, moderate-high, high) based on their physical functional capacity. Determination of transplant eligibility and exercise interventions can be made based on this stratification (Figure 1). We selected morphologic age rather than sarcopenia because it is more precise and has no variability in its definition. We acknowledge that the proposed model is a suggestion that requires testing and that other alternate integrative models using objective measurements also need to be researched and compared in order to eventually create a customized toolset for functional assessment, progress tracking, and provision of recommendations for exercise interventions.
Figure 1.
Model for selection of older transplant candidates incorporating targeted intervention and allocation to a type of transplant. CA, chronological age; CKD, chronic kidney disease; DD, deceased donor; ESRD, end-stage renal disease; LD, living donor; MA, morphologic age; SPPB, Short Performance Physical Battery; Txp, transplant.
In the current setting, there exists a great need for tailored selection criteria among the older advanced CKD and ESRD populations, for referral to transplantation and beyond. Development of such criteria ultimately will lead to increased transplant access and improvements in outcomes for this cohort.
Future Directions
Throughout this review, the lack of definite selection strategies and the utilization of arbitrary methods to assess functional status have been highlighted as limitations when assessing kidney transplant candidacy in older adults. Undoubtedly, a multidimensional approach is needed to create an effective strategy for improved transplant candidacy determination. Such an approach has not yet been developed, but the Frailty Committee of the American Society of Transplantation is actively working to develop recommendations for better assessment of functional status, and to propose necessary interventions, in different solid organ groups. Provider education is crucial to minimize perception biases, increase early transplant center referrals, and ensure timely counseling on exercise interventions. Involvement of dialysis unit and transplant center non-physician staff for exercise counseling, and incorporation of physical therapy and nutritional experts into the selection paradigm, is vital to an effective and sustainable model. Significant prospective research is necessary to assess whether early identification of functional impairment and timely, targeted physical therapy would lead to more transplantation and improved outcomes in this population. A clinical trial is currently underway to determine if exercise interventions would improve functional status in frail or pre-frail kidney transplant candidates.89 The role of barriers (such as having multiple comorbidities, fatigue, depression) to participation in exercise regimens also needs further investigation so that sustainable therapy plans can be developed. Exercise programs in varied settings (at dialysis, in-center on non-dialysis days, or home-based regimens) need to be developed, compared, and stratified to provide maximum benefit to this particular group.
Access to physical therapy, duration of therapy approval, and costs covered for staff as well as facility use, are in large part dictated by insurance companies and policymakers. Elimination by the US Congress of a yearly payment maximum for physical therapy for Medicare clients in 2018 is a step toward achieving better physical functioning, especially in the older advanced CKD cohort.90 More policies are necessary to better provide for sustainable prehabilitation programs for all older advanced CKD and ESRD candidates.
Ultimately, standardized assessment techniques, guidelines at a national level, targeted interventions, research, and buy-in from all stakeholders are vital for developing a selection process model specifically designed for older potential transplant candidates.
Disclosure
All the authors declared no competing interests.
References
- 1.United Nations, Population Division World population prospects 2019. Available at: http://esa.un.org/unpd/wpp
- 2.United States Renal Data System website Available at: https://www.usrds.org/2018/view/v2_06.aspx
- 3.Kasiske B.L., Cangro C.B., Hariharan S. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1(suppl 2):S3–S95. [PubMed] [Google Scholar]
- 4.Thamer M., Hwang W., Fink N.E. U.S. nephrologists' attitudes towards renal transplantation: results from a national survey. Transplantation. 2001;71:281–288. doi: 10.1097/00007890-200101270-00020. [DOI] [PubMed] [Google Scholar]
- 5.Grams M.E., Kucirka L.M., Hanrahan C.F. Candidacy for kidney transplantation of older adults. J Am Geriatr Soc. 2012;60:1–7. doi: 10.1111/j.1532-5415.2011.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucirka L.M., Grams M.E., Segev D.L. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12:351–357. doi: 10.1111/j.1600-6143.2011.03865.x. [DOI] [PubMed] [Google Scholar]
- 7.Rao P.S., Merion R.M., Ashby V.B. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 8.Pinter J., Hanson C.S., Tong A. ‘I feel stronger and younger all the time’—perspectives of elderly kidney transplant recipients: thematic synthesis of qualitative research. Nephrol Dial Transplant. 2016;31:1531–1540. doi: 10.1093/ndt/gfv463. [DOI] [PubMed] [Google Scholar]
- 9.Laupacis A., Keown P., Pus N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 11.Heldal K., Leivestad T., Hartmann A. Kidney transplantation in the elderly—the Norwegian experience. Nephrol Dial Transplant. 2008;23:1026. doi: 10.1093/ndt/gfm719. [DOI] [PubMed] [Google Scholar]
- 12.Oniscu G.C., Brown H., Forsythe J.L. How old is old for transplantation? Am J Transplant. 2004;4:2067. doi: 10.1111/j.1600-6143.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 13.Rebollo P., Ortega F., Baltar J.M. Health-related quality of life (HRQOL) of kidney transplanted patients: variables that influence it. Clin Transplant. 2000;14:199. doi: 10.1034/j.1399-0012.2000.140304.x. [DOI] [PubMed] [Google Scholar]
- 14.Kobashigawa J., Dadhania D., Bhorade S. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19:984–994. doi: 10.1111/ajt.15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swidler M. Considerations in starting a patient with advanced frailty on dialysis: Complex biology meets challenging ethics. Clin J Am Soc Nephrol. 2013;8:1421–1428. doi: 10.2215/CJN.12121112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salter M.L., Gupta N., Massie A.B. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52. doi: 10.1186/s12877-015-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segev D.L., Kucirka L.M., Oberai P.C. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. JAm Soc Nephrol. 2009;20:621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolzmann K.L., Bautista L.E., Gangnon R.E. Trends in kidney transplantation rates and disparities. J Natl Med Assoc. 2007;99:923–932. [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik J.M., Ferrucci L., Pieper C.F. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 20.Gault M.L., Willems M.E. Aging, functional capacity and eccentric exercise training. Aging Dis. 2013;4:351–363. doi: 10.14336/AD.2013.0400351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painter P., Marcus R.L. Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol. 2013;8:861–872. doi: 10.2215/CJN.06590712. [DOI] [PubMed] [Google Scholar]
- 22.Kutner N.G., Zhang R., Bowles T., Painter P. Pretransplant physical functioning and kidney patients' risk for posttransplantation hospitalization/death: evidence from a national cohort. Clin J Am Soc Nephrol. 2006;1:837–843. doi: 10.2215/CJN.01341005. [DOI] [PubMed] [Google Scholar]
- 23.Reese P.P., Bloom R.D., Shults J. Functional status and survival after kidney transplantation. Transplantation. 2014;97:189–195. doi: 10.1097/TP.0b013e3182a89338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese P.P., Shults J., Bloom R.D. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis. 2015;66:837–845. doi: 10.1053/j.ajkd.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas S.E., Reese P.P., Huan Y. Pretransplant physical activity predicts all-cause mortality in kidney transplant recipients. Am J Nephrol. 2012;35:17–23. doi: 10.1159/000334732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prihodova L., Nagyova I., Rosenberger J. Health-related quality of life 3 months after kidney transplantation as a predictor of survival over 10 years: a longitudinal study. Transplantation. 2014;97:1139–1145. doi: 10.1097/01.TP.0000441092.24593.1e. [DOI] [PubMed] [Google Scholar]
- 27.Kelly C.M., Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:6186543. doi: 10.1155/2016/6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder JP, Salowski NJ, Lamb KE, et al., Karnofsky assessment score and its use in risk adjustment of transplant outcomes in the United States. Paper presented at: American Transplant Congress. June 2–6, 2012; Boston, MA.
- 29.Xue Q.L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X.S., Lentine K.L., Koraishy F.M. Implications of frailty for peritransplant outcomes in kidney transplant recipients. CurrTransplant Rep. 2019;6:16–25. doi: 10.1007/s40472-019-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockwood K., Song X., MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 33.Bao Y., Dalrymple L., Chertow G.M. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansen K.L., Chertow G.M., Jin C., Kutner N.G. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 35.McAdams-DeMarco M.A., Law A., Salter M.L. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco M.A., Suresh S., Law A. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reese P.P., Cappola A.R., Shults J. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38:307–315. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzpatrick J., Sozio S.M., Jaar B.G. Frailty, body composition and the risk of mortality in incident hemodialysis patients: the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease study. Nephrol Dial Transplant. 2019;34:346–354. doi: 10.1093/ndt/gfy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAdams-DeMarco M.A., Law A., King E. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2014;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAdams-DeMarco M.A., Law A., Salter M.L. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAdams-DeMarco M.A., Law A., Tan J. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99:805. doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAdams-DeMarco M.A., Ying H., Olorundare I. Frailty and health-related quality of life in end stage renal disease patients of all ages. J Frailty Aging. 2016;5:174–179. [PMC free article] [PubMed] [Google Scholar]
- 43.Haugen C.E., Mountford A., Warsame F. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol. 2018;29:1752–1759. doi: 10.1681/ASN.2018010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAdams-DeMarco M.A., King E.A., Luo X. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective Cohort study. Ann Surg. 2016;266:1084–1090. doi: 10.1097/SLA.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Pilsum Rasmussen S., Konel J., Warsame F. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol. 2018;19:8. doi: 10.1186/s12882-017-0806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehfuss J.P., Berceli S.A., Barbey S.M. The spectrum of hand dysfunction after hemodialysis fistula placement. Kidney Int Rep. 2016;2:332–341. doi: 10.1016/j.ekir.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uppal S., Igwe E., Rice L.W. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol. 2015;137:98–101. doi: 10.1016/j.ygyno.2015.01.532. [DOI] [PubMed] [Google Scholar]
- 48.Koller K., Rockwood K. Frailty in older adults: implications for end-of-life care. Cleve Clin J Med. 2013;80:168–174. doi: 10.3949/ccjm.80a.12100. [DOI] [PubMed] [Google Scholar]
- 49.Farhat J.S., Velanovich V., Rubinfeld I.S. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72:1526–1530. doi: 10.1097/TA.0b013e3182542fab. discussion 1530–1531. [DOI] [PubMed] [Google Scholar]
- 50.Karam J., Tsiouris A., Shepard A. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27:904–908. doi: 10.1016/j.avsg.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Metter E.J., Conwit R., Tobin J., Fozard J.L. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 52.Noori N., Kopple J.D., Kovesdy C.P. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Studenski S., Perera S., Patel K. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartmann E.L., Kitzman D., Rocco M. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4:588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohl L.M., LU Signori, Ribeiro R.A. Prognostic value of the six-minute walk test in end-stage renal disease life expectancy: a prospective cohort study. Clinics (Sao Paulo) 2012;67:581–586. doi: 10.6061/clinics/2012(06)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marsh A.P., Wrights A.P., Rejeski W.J. The virtual short physical performance battery. J Gerontol Ser A. 2015;7010:1233–1241. doi: 10.1093/gerona/glv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guralnik J.M., Ferrucci L., Simonsick E.M. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guralnik J.M., Simonsick E.M., Ferrucci L. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 59.Pavasini R., Guralnik J., Brown J.C. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14:215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nastasi A.J., McAdams-DeMarco M.A., Schrack J. Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant. 2017;18:189–196. doi: 10.1111/ajt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Englesbe M.J., Lee J.S., He K. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 62.Locke J.E., Carr J.J., Nair S. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transplant. 2017;31 doi: 10.1111/ctr.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foley R.N., Wang C., Ishani A. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 64.Delmonico M.J., Harris T.B., Lee J.S. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 65.Goodpaster B.H., Park S.W., Harris T.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 66.Janssen I., Shepard D.S., Katzmarzyk P.T., Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 67.Rubbieri G., Mossello E., Di Bari M. Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:181–184. [PMC free article] [PubMed] [Google Scholar]
- 68.Waits S.A., Kim E.K., Terjimanian M.N. Morphometric age and mortality after liver transplant. JAMA Surg. 2014;149:335–340. doi: 10.1001/jamasurg.2013.4823. [DOI] [PubMed] [Google Scholar]
- 69.Terjimanian M.N., Underwood P.W., Cron D.C. Morphometric age and survival following kidney transplantation. Clin Transplant. 2017;31 doi: 10.1111/ctr.13066. [DOI] [PubMed] [Google Scholar]
- 70.Loprinzi PD. Estimated cardiorespiratory fitness assessment as a patient vital sign. Mayo Clin Proc. 93:821–823. [DOI] [PubMed]
- 71.Greenwood S.A., Koufaki P., Mercer T.H. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis. 2015;65:425–434. doi: 10.1053/j.ajkd.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Ting S.M.S., Iqbal H., Kanji H. Functional cardiovascular reserve predicts survival pre- kidney and post- kidney transplantation. J Am Soc Nephrol. 2014;25:187–195. doi: 10.1681/ASN.2013040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ting S.M.S., Iqbal H., Hamborg T. Reduced functional measure of cardiovascular reserve predicts admission to critical care unit following kidney transplantation. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0064335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manfredini F., Mallamaci F., D’Arrigo G. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28:1259–1268. doi: 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon E.J., Prohaska T., Siminoff L.A. Needed: tailored exercise regimens for kidney transplant recipients. Am J Kidney Dis. 2005;45:769–774. doi: 10.1053/j.ajkd.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Painter P.L., Hector L., Ray K. Effects of exercise training on coronary heart disease risk factors in kidney transplant recipients. Am J Kidney Dis. 2003;42:362–369. doi: 10.1016/s0272-6386(03)00673-5. [DOI] [PubMed] [Google Scholar]
- 77.Painter P.L., Hector L., Karen R. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74:42–48. doi: 10.1097/00007890-200207150-00008. [DOI] [PubMed] [Google Scholar]
- 78.Cheng X.S., Myers J.N., Chertow G.M. Prehabilitation for kidney transplant candidates: Is it time? Clin Transplant. 2017;31 doi: 10.1111/ctr.13020. [DOI] [PubMed] [Google Scholar]
- 79.McAdams-DeMarco M.A., Ying H., Segev D.L. Prehabilitation prior to kidney transplantation: results from a pilot study. Clin Transplant. 2019;33 doi: 10.1111/ctr.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carli F., Charlebois P., Stein B. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg. 2010;97:1187–1197. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 81.Arthur H.M., Daniels C., McKelvie R. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med. 2000;133:253–262. doi: 10.7326/0003-4819-133-4-200008150-00007. [DOI] [PubMed] [Google Scholar]
- 82.Gillis C., Li C., Lee L. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 83.Beaupre L.A., Lier D., Davies D.M., Johnston D.B. The effect of a preoperative exercise and education program on functional recovery, health related quality of life, and health service utilization following primary total knee arthroplasty. J Rheumatol. 2004;31:1166–1173. [PubMed] [Google Scholar]
- 84.Delgado C., Johansen K.L. Deficient counseling on physical activity among nephrologists. Nephron Clin Pract. 2010;116:c330–c336. doi: 10.1159/000319593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kontos P.C., Miller K.L., Brooks D. Factors influencing exercise participation by older adults requiring chronic hemodialysis: a qualitative study. Int Urol Nephrol. 2007;39:1303–1311. doi: 10.1007/s11255-007-9265-z. [DOI] [PubMed] [Google Scholar]
- 86.Painter P., Carlson L., Carey S. Determinants of exercise encouragement practices in hemodialysis staff. Nephrol Nurs J. 2004;31:67–74. [PubMed] [Google Scholar]
- 87.Bennett P.N., Breugelmans L., Barnard R. Sustaining a hemodialysis exercise program: a review. Semin Dial. 2010;23:62–73. doi: 10.1111/j.1525-139X.2009.00652.x. [DOI] [PubMed] [Google Scholar]
- 88.Concepcion B.P., Forbes R.C., Schaefer H.M. Older candidates for kidney transplantation: Who to refer and what to expect? World J Transplant. 2016;6:650–657. doi: 10.5500/wjt.v6.i4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.U.S. National Library of Medicine Exercise to treat frailty in kidney transplant candidates. Available at: https://clinicaltrials.gov/ct2/show/NCT03535584?term=frailty&cond=kidney transplant&rank=1
- 90.Valenzano M. Uncapped: Medicare's Therapy Cap and the Bipartisan Budget Act of 2018. Available at: https://www.foxrehab.org/bipartisan-budget-act-2018-impacts-medicare-therapy-cap-for-therapy-professionals/