See Clinical Research on Page 1717
IgA vasculitis (IgA-V), a multisystem disorder affecting predominantly the skin, joints, and gastrointestinal tract, and primary IgA nephropathy (IgAN) are 2 frequent diseases sharing the role of IgA as marker and player in their physiopathology. In this issue of Kidney International Reports, Suzuki and his collaborators1 evaluated new biomarkers shared by IgA-V and primary IgAN in their pediatric forms. They identified an increase in serum levels and production of circulating hypogalactosylated IgA1 (Gd-IgA1) and Gd-IgA1–specific IgG autoantibodies in all patients with IgA-V with kidney involvement and patients with IgAN compared with the controls.
Isolated increased IgA levels are insufficient to cause glomerular IgA1 deposition and necessitate posttranslational modification of the O-glycan moieties in its hinge region with reduced terminal galactose in O-linked oligosaccharides. The consequence is an increased fraction of polymeric IgA with reduced galactosylation. This is the common characteristic between idiopathic and secondary forms of IgAN and IgA-V with renal involvement.2 Hypogalactosylation of the hinge region of IgA1 (Gd-IgA1) represents the first pathological “hit” for kidney IgA1 deposition. Interestingly, using peripheral blood mononuclear cells from patients, the authors found reduced expression of core β1,3 galactosyltransferase (C1β3GalT1) that promotes addition of galactose to GalNac residues, and its cofactor C1β3GalT1-specific molecule (COSMC), in both IgAN and IgA-V compared with healthy patients and patients with IgA-V without nephritis. Furthermore, activity of N-acetyl galactosaminide α-2,6 sialyltransferase (ST6GalNacII), which prevents addition of galactose to GalNac by attached N-acetylneuraminic acid (NeuNAc) residues to GalNac, was also increased in both IgAN and IgA-V compared with healthy patients and patients with IgA-V without nephritis. All these findings corroborate that IgA-V and IgAN share pathogenic features. Mesangial IgA deposition is not necessarily associated with kidney disease, with a frequency ranging from 2% to 16%.3,4 Mesangial IgA deposition may remain inert. They need another “hit” to promote inflammation and finally glomerulonephritis. Gd-IgA1 may represent an interesting biomarker used in screening, diagnosis, and monitoring of progression of IgA-V and IgAN, but is not sufficient alone.
Gd-IgA1–specific IgG autoantibodies could be the second “hit” of the glomerulonephritis.5 The incomplete galactosylation exposing GalNac generates neo-epitopes that are recognized by naturally occurring IgG and IgA antibodies specific for GalNac, leading to immune complex formation based on antigen (IgA1)–antibody (IgG or IgA) recognition.6 The authors found increased Gd-IgA1 IgG antibodies in both IgA-V and IgAN compared with controls using the in-house sandwich enzyme-linked immunosorbent assay (ELISA) method.
Biomarkers are the holy grail of IgAN. Biomarker discovery relies on intimate knowledge of the disease pathophysiology and is based on the hypothesis-driven method of research. A biomarker is defined as an indicator that measures and evaluates normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.7
Is Gd-IgA1 a good biomarker? If the biomarker is available in only specialized laboratories, its value is poor. Gd-IgA1 could be tested by 2 approaches, none validated or easily accessible. The authors measured serum Gd-IgA1 with a snail helix aspersa agglutinin (HAA) lectin-based ELISA assay. HAA use is hindered by certain limitations, such as bioactivity and stability that vary depending on the batch of the product, the absence of a validated measurement platform, and consensus criteria in techniques. Therefore, Gd-IgA1 is not easily measured and restricted to specialized laboratories, which are not available in current practices. Noteworthy, a lectin independent ELISA assay using a unique anti-Gd-IgA1 monoclonal antibody KM55, commercially available, has been developed by a Japanese research group and will possibly overcome this major problem of reproducibility and accessibility.8 Second, a good biomarker should clearly distinguish pathologic and healthy patients. In this study, the distribution of the Gd-IgA1 values show an overlap between the patient and control populations, making this biomarker unlikely to substitute the kidney biopsy for diagnosis or to predict prognosis. In the literature, Gd-IgA1–specific IgG autoantibodies have a better relationship with diagnosis, disease severity, and progression and seems to be a better powerful biomarker in this study. The authors were not able to carry out a receiver operating characteristics (ROC) curve analysis given the small sample size. Biomarker classification performance should be quantified with appropriate metrics, such as true-positive rate, false-positive rate, and ROC curves. A clear research methodology and goals will improve efficiency for successful biomarkers and prevent wastage of resources and effort on failed biomarkers. Third, a biomarker could be used in all the populations affected by the disease, irrespective of age, sex, and geographical origins.
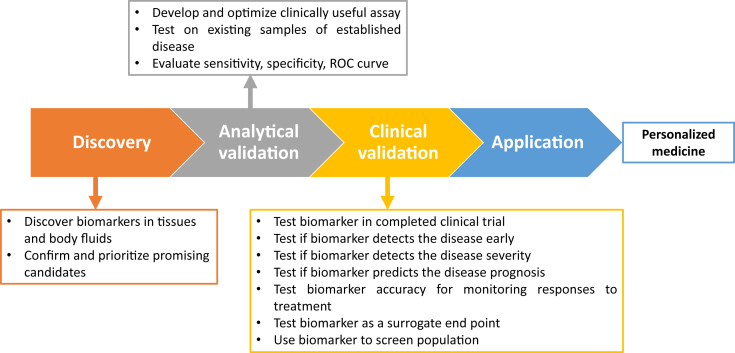
Gd-IgA and Gd-IgA1–specific IgG autoantibodies seem to be specific biomarkers in both IgAN and IgA-V, but are not validated in different populations and applicable in clinical practice. Although a large number of studies have been devoted to identifying highly sensitive and specific biomarker(s) for IgA-V and IgAN, the attempts around the so-called liquid biopsy are still in progress. The discovery of IgA-V and IgAN diagnostic, prognostic, and therapeutic biomarkers is a milestone of translational research to validate the concept of “bench to the bedside.” We propose a flow for identification of biomarkers in nephrology (Figure 1). In current clinical practice, the histopathological evaluation by kidney biopsy remains the gold standard for the most common glomerulonephritis worldwide.
Figure 1.
Biomarker step-by-step method.
In summary, it seems that the best approach for IgA-V and IgAN may be to find multiple biomarkers that can be combined as part of a panel. An overall collaborative strategy of development, calibration, and evaluation will help to achieve higher specificity and sensibility. The molecular profiling approaches by the multi-omics concept may be useful.
Disclosure
The author declared no competing interests.
References
- 1.Suzuki H., Moldoveanu Z., Julian B.A. Autoantibodies specific for galactose-deficient IgA1 in IgA vasculitis with nephritis. Kidney Int Rep. 2019;4:1717–1724. doi: 10.1016/j.ekir.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert T., Berthelot L., Cambier A. Molecular insights into the pathogenesis of IgA nephropathy. Trends Mol Med. 2015;21:762–775. doi: 10.1016/j.molmed.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Varis J., Rantala I., Pasternack A. Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol. 1993;46:607–610. doi: 10.1136/jcp.46.7.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki K., Honda K., Tanabe K. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286–2294. doi: 10.1046/j.1523-1755.63.6s.2.x. [DOI] [PubMed] [Google Scholar]
- 5.Novak J., Tomana M., Matousovic K. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 6.Tomana M., Novak J., Julian B.A. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 8.Yasutake J., Suzuki Y., Suzuki H. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant. 2015;30:1315–1321. doi: 10.1093/ndt/gfv221. [DOI] [PMC free article] [PubMed] [Google Scholar]