Life expectancy in individuals with chronic kidney disease (CKD) is unacceptably low, and cardiovascular disease remains the leading cause of death.1 Above and beyond established cardiovascular disease risk factors that are highly prevalent in CKD patients, unique risk factors such as abnormal mineral metabolism are widely hypothesized to contribute to the pathogenesis of cardiovascular disease in CKD.
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone produced mainly by osteocytes. As kidney function declines in CKD, FGF23 rises early and counteracts phosphate accumulation. Elevated FGF23 levels are independently associated with increased risk of cardiovascular disease and mortality in different populations, including among those with CKD.2 In animal models and in vitro, FGF23 has a direct pathogenic effect, causing left ventricular (LV) hypertrophy by activating fibroblast growth factor receptor 4 on cardiac myocytes. In prior human observational studies, higher FGF23 has been associated with arrhythmias, and decreased LV systolic function.3 Given animal data suggesting that FGF23 may induce LV hypertrophy, and human data demonstrating associations of elevated FGF23 with LV hypertrophy4—one potential pathologic driver of diastolic dysfunction—we hypothesized that higher FGF23 levels may be associated with diastolic dysfunction, a common complication of CKD.
Methods
Please see Supplementary Material.
Results
Baseline characteristics of the study cohort are shown in Table 1, stratified by quartiles of intact (i)FGF23. Seventy percent of the 47 participants were men, and 62% had preserved LV ejection fraction (EF). The mean (±SD) age was 61 (±20) years, mean phosphate was 5.0 (±1.4) mg/dl; mean LVEF was 51 (±13) %, mean left atrial volume was 58 (±27) ml, mean tricuspid velocity was 2.6 (±0.5) m/sec, and the mean E (transmitral early filling velocity)/A (transmitral late filling velocity) ratio was 1.4 (±0.5). A total of 94% (44 of 47) of patients had LV diastolic dysfunction, and 53% (25 of 47) had LV hypertrophy (LVH).
Table 1.
Baseline characteristics by quartiles of FGF23
| Variablea | Full cohort | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|---|
| N | 47 | 9 | 12 | 14 | 12 |
| iFGF23 (pg/ml) | 32–361 | 363–1074 | 1195–3195 | 3926–22,369 | |
| Age (yr) | 61 (±20) | 66 (±22) | 57 (±18) | 55 (±18) | 68 (±21) |
| Weight (kg) | 68 (±18) | 61 (±16) | 80 (±18) | 66 (±19) | 63 (±12) |
| Male (%) | 70 | 67 | 75 | 64 | 75 |
| EF ≥ 50%, n (%) | 29 (62) | 5 (56) | 9 (75) | 10 (71) | 5 (42) |
| LV hypertrophyb n (%) | 25 (53) | 4 (44) | 5 (42) | 6 (43) | 10 (83) |
| TR jet velocity (cm/s) | 2.6 (±0.5) | 2.5 (±0.7) | 2.3 (±0.3) | 2.7 (±0.3) | 2.9 (±0.3) |
| E to A ratioc | 1.4 (±0.5) | 0.9 (±0.4) | 1.1 (±0.6) | 1.5 (±0.6) | 2.1 (±0.4) |
| LA volume (ml) | 58 (±27) | 56 (±24) | 55 (±25) | 55 (±21) | 65 (±37) |
| Hemoglobin (g/dl) | 11.7 (±1.4) | 11.4 (±1.0) | 11.4 (±1.0) | 12.2 (±1.5) | 11.5 (±1.7) |
| Calcium (mg/dl) | 8.6 (±0.7) | 8.5 (±0.5) | 8.6 (±0.4) | 8.6 (±0.7) | 8.6 (±0.9) |
| Phosphate (mg/dl) | 5 (±1.4) | 3.9 (±0.8) | 4.7 (±1.0) | 5.4 (±1.3) | 5.8 (±1.7) |
EF, ejection fraction; iFGF, intact fibroblast growth factor; LA, left atrium; LV, left ventricle; TR, tricuspid regurgitation
Data are reported as mean (±SD) for continuous variables, and % for categorical variables.
Left ventricular wall thickness >11 mm is defined as LV hypertrophy.
E (transmitral early filling velocity) / A (transmitral late filling velocity) ratio.
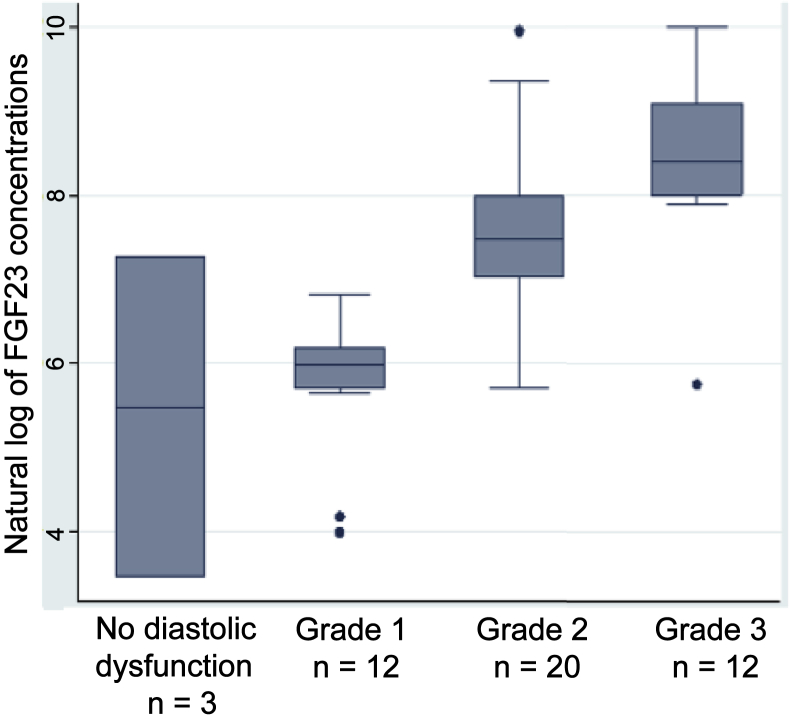
Median iFGF23 was elevated at 1135 (interquartile range: 361, 3195) pg/ml, which is comparable to values observed in end-stage kidney disease cohorts.5 The strongest association was between higher levels of iFGF23 and grades of diastolic dysfunction (rs = 0.75; P < 0.001), followed by serum phosphate and iFGF23 (rs = 0.51; P < 0.001). In the univariate model, elevated levels of natural log–transformed iFGF23 were significantly associated with a higher grade of LV diastolic dysfunction (R2 = 0.51; 95% confidence interval for slope, 1.7–3.4; P < 0.001). In a multivariate model, this relationship remained significant and was essentially unaltered after adjusting for age, phosphate, and LVEF (R2 = 0.5; 95% confidence interval for slope, 1.01–1.5; P < 0.001; Figure 1).
Figure 1.
Box plots showing the relationship between the grading of diastolic dysfunction and the natural logarithm of intact fibroblast growth factor 23 (FGF23) in 47 hemodialysis patients. Elevated levels of natural log–transformed intact FGF23 were significantly associated with a higher grade of left ventricular diastolic dysfunction (R2 = 0.51; 95% confidence interval for slope, 1.7–3.4; P < 0.001).
Discussion
In this study of 47 patients with end-stage kidney disease treated with hemodialysis, we found that higher levels of iFGF23 were associated with LV diastolic dysfunction. The association appeared to have a step-wise linear relationship with grades of severity of diastolic dysfunction, independently of LVEF. Although observational data reported here cannot prove causality, these data parallel observations made in vitro and in experimental animals demonstrating that FGF23 induces LVH. This suggests that a similar biology may be ongoing in hemodialysis patients, promoting diastolic dysfunction. Because diastolic dysfunction is strongly associated with mortality and morbidity, it is important to identify its potential drivers. Future studies are needed to determine if FGF23 lowering may lead to less diastolic dysfunction in hemodialysis patients.
Among its myriad effects, FGF23 mediates cardiac fibrosis through the activation of pro-fibrotic factors in in vitro models,6 as well as in CKD populations,7 thereby pointing to a plausible biological link to cardiac diastolic dysfunction. Our data expand on findings in pediatric nondialysis CKD patients where high FGF23 and low Klotho levels were strongly and longitudinally associated with LV diastolic function.8 In contrast, in a retrospective study of subjects with LVEF >50% and relatively preserved renal function (mean estimated glomerular filtration rate of 52 ml/min per 1.73 m2), FGF23 was not associated with diastolic dysfunction.9 In comparison to those in the later study, our study population had much more severe kidney failure, and much higher FGF23 concentrations.
Our study also confirms that FGF23 correlates with hyperphosphatemia, a known risk factor for LVH and mortality in end-stage renal disease. LVH is known to be strongly associated with mortality and also associated with diastolic dysfunction by increasing LV filling pressure, leading to left atrial enlargement. Although others have shown an association between FGF23 and LVH, 47% of our patients did not have LVH. This latter finding suggests that other mechanisms independent of LVH may contribute to myocardial remodeling associated with impairment of diastolic function.
Our study has limitations. Our sample size is modest and applicable to adult end-stage renal disease patients supported by hemodialysis. We cannot demonstrate causality or temporality, but building on previous evidence that FGF23 causes cardiac fibrosis in vitro and in animal models, our findings support the hypothesis that FGF23 is a risk factor for LV diastolic dysfunction in end-stage renal disease patients. Repeated measurements of FGF23 and other covariates, such as blood pressure at the time of echocardiographic examination, duration between last dialysis session and echocardiogram, and other cardiovascular risk factors known to affect diastolic function, were not available. Given that FGF23 is not effectively removed by dialysis,S1 we expect levels to be stable over time. Aside from CKD and hemodialysis status, the presence of comorbid conditions that could potentially confound the severity of diastolic function (diabetes, hypertension, prior myocardial infarction, dysrhythmias) was not known due to the retrospective nature of this study.
In conclusion, this study is the first to our knowledge to report an association between higher iFGF23 levels and severity of LV diastolic dysfunction in end-stage renal disease patients receiving hemodialysis. FGF23 has multiple adverse effects on the cardiovascular system, and understanding of the complex interplay of these effects in CKD patients is evolving. Future larger studies examining the relationship between FGF23 and the progression of diastolic dysfunction are warranted in patients with CKD, and ultimately, studies that target FGF23 lowering should be conducted to evaluate the effects on cardiac function.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work is supported in part by the American Heart Association (AHA18TPA3420049 to KLN), the Veterans Health Administration (VA-MERIT I01CX001901 to KLN), and the National Institute of Diabetes, Digestive, and Kidney Diseases (K08DK111980 to MRH, K24DK110427 to JHI).
Footnotes
Supplementary Material
References
- 1.Cozzolino M., Mangano M., Stucchi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl 3):iii28–iii34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakova T., Xie H., Yang W. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabner A., Amaral A.P., Schramm K. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovanovich A., Ix J.H., Gottdiener J. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231:114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jovanovich A., You Z., Isakova T. Fibroblast growth factor 23 trajectories in chronic hemodialysis patients: lessons from the HEMO study. Am J Nephrol. 2019;49:263–270. doi: 10.1159/000497445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao H., Li X., Li Q. FGF23 promotes myocardial fibrosis in mice through activation of beta-catenin. Oncotarget. 2016;7:64649–64664. doi: 10.18632/oncotarget.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leifheit-Nestler M., Kirchhoff F., Nespor J. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant. 2018;33:1722–1734. doi: 10.1093/ndt/gfy006. [DOI] [PubMed] [Google Scholar]
- 8.Tranaeus Lindblad Y., Olauson H., Vavilis G. The FGF23-Klotho axis and cardiac tissue Doppler imaging in pediatric chronic kidney disease-a prospective cohort study. Pediatr Nephrol. 2018;33:147–157. doi: 10.1007/s00467-017-3766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto Y., Fujita S., Morita H. Association between circulating FGF23, alpha-Klotho, and left ventricular diastolic dysfunction among patients with preserved ejection fraction. Heart Vessels. 2016;31:66–73. doi: 10.1007/s00380-014-0581-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.