Abstract
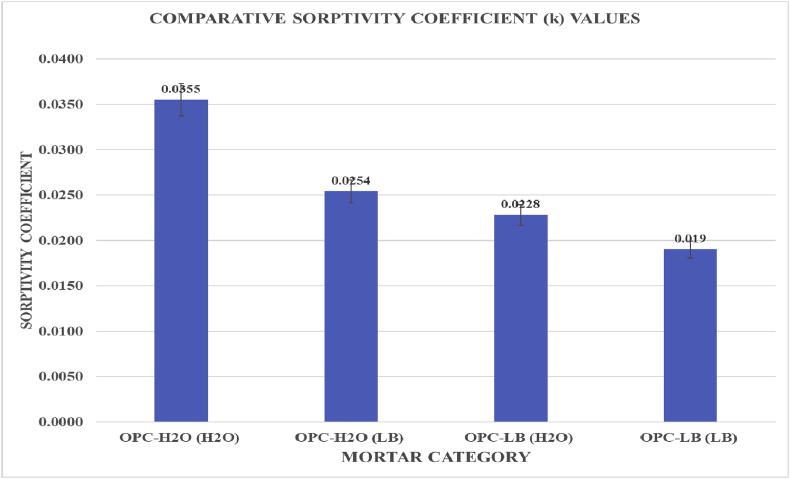
Cement structures are subject to degradation either by aggressive media or development of micro/macro cracks which create external substance ingress pathways. Microbiocementation can be employed as a self-intelligent solution to this deterioration process. This paper presents study results on the effects of Lysinibacillus sphaericus microbiocementation on Ordinary Portland cement (OPC), normal consistency, setting time, soundness, compressive strength and water sorptivity. Microbial solutions with a concentration of 1.0 × 107 cells/ml were mixed with OPC to make prisms at a water/cement ratio of 0.5. Mortar prisms of 160 mm × 40 mm x 40mm were used in this study. A maximum compressive strength gain of 17% and 19.8% was observed on the microbial prism at the 28th and 56th day of curing respectively. A minimum of 0.0190 and a maximum of 0.0355 water sorptivity coefficient was observed on the OPC microbial prism and OPC control prism, after 28th day of curing respectively. Scanning electron microscope images taken after the 28th day of curing showed formation of vast calcium silicate hydrates and more calcite deposits on microbial mortars. Statistical findings of this study indicate that Lysinibacillus sphaericus significantly retarded both the setting time and normal consistency, but has no influence on the mortar soundness.
Keywords: Chemical engineering, Physical chemistry, Lysinibacillus sphaericus, Compressive strength, Water sorptivity coefficient, Setting time, Soundness, Microbiocemntation, Ordinary Portland cement
Chemical engineering, Physical chemistry, Lysinibacillus sphaericus; Compressive strength; water sorptivity coefficient; setting time; Soundness; microbiocemntation; Ordinary Portland cement.
1. Introduction
Mortar/concrete is one of the most widely used construction material by mankind and it is the main material used for the infrastructure development of every country [1, 2]. Microorganisms in soils and waters play a major role on physico-mechanical properties and durability of building materials through the process generally referred to as microbial biocementation [3]. The process is affected by variation in temperatures, humidity, type of cement, type and concentration of bacteria and soil/water pH among others [4, 5]. Through microbial biocementation, calcium carbonate is deposited in cement mortar or concrete matrix. Such deposits have recently emerged as promising binders for protecting and consolidating various building materials [3, 6].
It is generally accepted that the durability of concrete/mortar is related to the characteristics of its pore structure [6]. Degradation mechanisms of concrete/mortar often depend on the way potentially aggressive substances can penetrate into the concrete/mortar, possibly causing damage [7]. The permeability of the concrete/mortar is dependent on the porosity and on the connectivity of the pores [8, 9]. The more open the pore structure of the concrete/mortar, the more vulnerable the material is to degradation mechanisms caused by penetrating substances [1, 10].
OPC based structures are well known of achieving high early compressive and flexural strength large content and early hydration of C3S [1, 2, 11]. Despite the various OPC concrete/mortar advantages, it has more open pore structure than blended cements structures [1, 9, 12, 13]. This is one of the basic principles of adding blends such as pozzolana in cements to decrease these void spaces and consequently improve on porosity and connectivity of the pores. Addition of any blend/pozzolanic material during cement manufacturing process has a limitation to avoid compromising other beneficial characteristics of the clinker [14]. OPC mortar/concrete structures also have a high tendency to form cracks during and after curing allowing aggressive substances to penetrate into the structure. Permeability or cracks are one of the main causes of concrete/mortar deterioration and decrease in durability. Treatment of cracks and pores in concrete/mortar are generally divided into passive and active treatments. Passive treatments can only heal the surface cracks, while active treatments can heal both interior and exterior cracks [15, 16].
Microbial concrete/mortar biologically produce calcium carbonate (limestone) to seal pores that appear within the concrete/mortar matrix or heal cracks that appear on the surface of concrete/mortar structures [2, 16]. These microbrial deposits could also establish nucleation sites which enhance early cement hydration process leading to improved compressive and flexural strengths. Specific types of the bacteria genus such as Bacillus species along with a calcium-based nutrient such as calcium lactate, or calcium nitrate could be added to the ingredients of the concrete when it is being mixed [14]. These self-healing agents can lie dormant within the concrete/mortar for up to 200 years [15, 16]. However, when a concrete/mortar structure develops a crack or is damaged and water starts to seep through the cracks that appear in the concrete/mortar, the spores of the bacteria germinate on contact with the water and the nutrients. Once activated, the bacteria start to feed on the calcium-containing nutrient consuming oxygen. The soluble calcium-containing nutrient is converted to insoluble calcium carbonate [9, 15, 16]. The calcium carbonate solidifies on the cracked surface, thereby sealing it up.
Oxygen is an essential element in the process of rebar corrosion and as such when the microbial activity consumes it, this improves the durability of steel reinforced concrete constructions. Thus, consumption of oxygen during the microbial conversion of calcium-containing nutrient to calcium carbonate has an additional advantage over sealing of pores/cracks [17].
Addition of bacterium in OPC mortar/concrete matrix is an innovative treatment which improve the transport/migration properties of OPC making these microbial treated concrete/mortars to act as intelligent systems that are different from the usually prepared OPC cement structures [13, 14]. These smart structures have self-sensing and self-healing properties towards external factors such as change in temperature, pH, humidity and concrete/mortar pore solution chemistry [5, 9, 16].
Microbiologically Induced Calcite Precipitation (MICP) reactions [18, 19, 20] can be summarized as:
| CO(NH2)2 + H2O → NH2COOH + NH3 | (1) |
| NH2COOH + H2O → NH3 + H2CO3 | (2) |
| 2NH3 + 2H2O → 2NH4+ + 2OH− | (3) |
| H2CO3 →HCO3− + H+ | (4) |
| HCO3− + H+ + 2OH − → CO32− + 2H2O | (5) |
| Ca2+ + Bacterial cell → Bacterial Cell-Ca2+ | (6) |
| Bacterial Cell-Ca2+ + CO32− → Cell-CaCO3 | (7) |
2. Materials and method
2.1. Materials
2.1.1. Cement chemical analysis
In this study, the Ordinary Portland Cement (OPC 42N) and standard sand manufactured according to ISO 679:1989, EN 196–1 [21] used were tested according to Kenyan Cement Standards specifications, KS EAS 18–1: 2017 [22]. 100 g sample of the test cement sample was ground to pass through a 76 μm mesh sieve. The ground sample was used for chemical analysis of cement oxides using X-ray fluorescence in the usual manner [22]. Loss on Ignition was done in accordance to ASTM D7348: 2013 [23].
2.1.2. Microbial culturing nutrients
Analytical grade (AR) chemicals were used in preparing the culture media. Calcium lactate, C6H10O6Ca, Peptone from casein and other animal proteins, Meat extract, Agar, sodium hydrogen carbonate (NaHCO3), anhydrous sodium carbonate (Na2CO3), distilled water among other nutrients were purchased from Chem-Labs Limited, Nairobi, Kenya. Lysinibacillus sphaericus bacteria (DSM 28) was purchased from Leibniz-Institut DSMZ-Deutsche Sammlung von, Germany.
2.2. Lysinibacillus sphaericus microbial culturing
The Lysinibacillus sphaericus microbial solution was cultured using nutrients as per the supplier manual. The liquid medium chosen for culturing the bacteria consisted of 5.00 g of peptone added to 3.00 g of meat extract and 3.95 g of calcium acetate per liter of distilled water was mixed to obtain liquid medium per stock culture. Initially, this mixture was sterilized for 20 min at a temperature of 121 °C by autoclaving. This mixture was then cooled to room temperature. After cooling, a 1M Na-sesquicarbonate solution (1.0 ml in 10.0 ml) prepared by mixing 4.2 g NaHCO3 with 5.3 g anhydrous Na2CO3 and made up-to 1 L using distilled water was added to the stock culture to achieve a pH of 9.7. The Lysininbacillus sphaericus spore powder sample was added to this mixture in a laminar flow chamber. These cultures were then incubated on a shaker incubator at 130 rotations per minute maintained at 30 °C for 72 h. Optical density test was conducted using spectrophotometer for determining the quantity of culture solution required to mix. This test was conducted in bacteria growing medium which was considered as blank. This solution was also taken to be the reference, for experimentation of optical density of microbial solution. Separately, 0.5 mL of blank and bacteria solution of 0.5 mL were placed in the spectrophotometer at a wavelength of 600 nm and the machine set to read. The microbial concentration was observed to be 1.0 × 107 cells/mL using the spectrophotometer. This microbial culture concentration was maintained throughout the mortar samples preparation as well as in prism curing solution.
2.3. Mortar prism moulding and testing
Mortar mix prisms were fabricated according to KS EAS 18–1: 2017 [22]. 450 g of OPC was placed in the mix basin of an automatic programmable mixer model number JJ-5. 225.0 ml of distilled water was then added. The mix basin and its contents were clamped onto the automatic programmable mixer and allowed to run for 3 min 1350 ± 5 g of the standard sand was placed in an automatic pour-trough little by little until all the 1350 ± 5 g sample was added while the mixer was still running at a speed of 30 revolutions per minute (rpm). The machine was let to run for 10 min. The mortar prepared had w/c ratio of 0.50 and was sufficient to prepare three mortar prisms. Once the mortar was mixed, it was poured into steel moulds of 40 mm × 40 mm x 160 mm. Using a trowel, the mortar paste was scooped from the automatic programmable mixing basin and placed in a compaction mould of a jolting compaction machine with 60 rpm vibrations. Leveling of the paste was done with a mould trowel in each of the three chambers of the mould after every jolting cycle until a good finish was achieved at the surface. The mould with the mortar paste was then placed in a humid chamber maintained at 95% humidity and 27.0 °C for 24 h. The mortar was then demoulded from the moulds after 24 h to obtain usual OPC mortar. The distilled-water prepared mortars were categorized into two categories depending on their curing regime: The first category was cured in distilled water (labelled as OPC-H2O (H2O). The second category was cured in microbial solution (labelled as OPC-H2O [LB]). The above procedure was repeated but this time using 225 ml of microbial solution as mix media instead of distilled water which resulted to two more mortar categories: The third category was the OPC mortar prepared using microbial solution and cured in distilled water (labelled as OPC-LB [H2O]), while the forth category was the OPC mortar prepared using the microbial solution and cured in microbial solution (labelled as OPC-LB [LB]). The mortars were labeled for identification and placed in requisite water or microbial solution for curing in a chamber maintained at 27 ± 1 °C for curing. The compressive strength tests were conducted at the 2nd, 7th, 14th, 28th and 56th day of curing. Compressive strength tests in this study were performed on three samples per category for obtaining average results [24].
2.4. Fresh paste tests preparation
Two categories of fresh cement paste for fresh paste tests; Normal consistency, Soundness and Setting time were separately prepared. The first category of the paste was prepared using distilled water as the mix media and was labelled OPC (H2O). The second category of fresh cement paste was prepared using microbial solution as the mix media and was labelled OPC (LB).
2.4.1. Normal consistency test
A cement paste for OPC (H2) weighing 300.0 g was prepared in accordance to IS 4031–4:1988 [25]. Standard consistency was calculated using Eq. (8). The weight of water for OPC (H2O) fresh cement paste refers to weight of distilled water used while for OPC (LB) fresh cement paste category, the weight of water refers to the weight of microbial solution used to make the microbial fresh cement paste.
| (8) |
Normal consistency tests in this study were performed on three samples per category for obtaining average results [24].
2.4.2. Soundness test
The soundness test was done in accordance to KS EAS 148–3: 2017 [26]. A lightly oiled mould was placed on a lightly oiled glass sheet and filled with mortar paste formed by gauging cement with 0.78 times the distilled water to prepare OPC (H2O) fresh cement paste. The same procedure was repeated but now with 0.78 times of the microbial solution instead of distilled water for OPC (LB) fresh cement paste to give a paste of standard consistency for each mortar category [25, 26]. Similar test was repeated on three fresh cement pastes samples per category to obtain averages [24].
2.4.3. Setting time of cement
2.4.3.1. Initial setting time
A fresh sample of cement paste for each mortar category was prepared in accordance to KS EAS 148–3: 2017 [26]. This test was done on OPC (H2O) and also on OPC (LB) cement pastes. Initial setting time tests in this study were performed on three samples per cement paste category for obtaining average results [24].
2.4.3.2 Final setting time.
A fresh sample of cement paste for each mortar category was prepared in accordance to KS EAS 148–3: 2017 [26]. This test was done on OPC (H2O) and also on OPC (LB) cement pastes. Final setting time tests in this study were performed on three samples per cement paste category for obtaining average results [24].
2.5. Sorptivity test
Sorptivity test was done on the four categories of mortar prisms OPC-H2O (H2O), OPC-H2O (LB), OPC-LB (H2O) and OPC-LB (LB) after the 28th day of curing. Sorptivity test was carried out following the method prescribed by Achal et al. (2016) [27]. To calculate the sorptivity coefficient, the 28th day cured mortar prism was dried at 100 °C in a ventilated oven. The mortar prism was then submerged into water at a height of 5 mm above the base of the mortar prism with the 40 mm × 40 mm side facing downward. At regular time intervals (15 min, 30 min; 1 h, 1.5 h, 3 h, 5 h, 8 h, 24 h, 72 h, 96 h, 120 h, 144 h, and 168 h), the mortar prism was removed from the water and its new weight determined after drying the submerged surface with a clean wet towel. Immediately after the measurement, the mortar prism was then re-submerged again into water up to the 168th hour. Three samples for each mortar category were exposed to this test simultaneously and the triplicate results obtained for averaging. The sorptivity coefficient (k), was obtained by using Eq. (9):
| (9) |
where, Q was the amount of water absorbed, A is the cross-section of the specimen that was in contact with water and t was the exposure time. On plotting a graph of Q/A against square root of time, values of k were determined graphically.
3. Results and discussion
3.1. Cement oxides
The results for the chemical analysis of cement oxides and LOI in percent by mass for the OPC test cement are given in Table 1.
Table 1.
OPC chemical analysis results.
| Sample | Cement Composition % w/w ±S.D |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | SO3 | Na2O | K2O | CaO | MgO | Fe2O3 | MnO | LOI | |
| Avg | 3.643 ± 0.010 | 22.182 ± 0.010 | 2.695 ± 0.021 | 0.410 ± 0.001 | 0.975 ± 0.006 | 64.627 ± 0.042 | 2.084 ± 0.025 | 3.403 ± 0.012 | 0.173 ± 0.001 | 1.519 ± 0.001 |
The chemical analysis results shows that the test OPC met the minimum chemical composition requirements [22, 23]. Using Bogues formula [28], the average phase composition for the test OPC is 65.115 ± 0.854%, 14.485 ± 0.913%, 3.899 ± 0.013% and 10.355 ± 0.018% for C3S, C2S, C3A and C4AF respectively. These study results confirm that the test cement has the major cement phases that meets the Kenya Bureau of standards acceptable cement phases range [26].
3.2. Normal consistency, setting time and Soundness for Control and Microbial OPC
Table 2 gives the results for normal consistency, Setting time and Soundness for Control and Microbial OPC.
Table 2.
Normal consistency, Setting time and Soundness for Control and Microbial OPC.
| Test Cement mortar | Setting Time (min) |
Normal consistency (%) | Soundness (mm) | |
|---|---|---|---|---|
| Initial | Final | |||
| OPC (H2O) | 98.0 ± 5.0 | 178.0 ± 5.0 | 28.0 ± 0.05 | 1.0 ± 0.05 |
| OPC (LB) | 78.0 ± 5.0 | 167.0 ± 5.0 | 26.4 ± 0.05 | 1.0 ± 0.05 |
The OPC (LB) cement paste showed a significant difference (tcalc = 0.00004729, p = 0.05) in normal consistency with OPC (LB) being statistically lower than the OPC (H2) cement paste as shown in Table 2. This was in agreement with the findings of Mutitu and co-authors [5, 29, 30]. The soundness of both OPC (LB) and OPC (H2) cement pastes exhibited no significant difference (tcalc = 0.5, p = 0.05) and hence they were similar. The initial and final setting time for OPC (LB) cement paste were statistically lower than for OPC (H2) cement paste. The initial and final setting time for both OPC (H2) and OPC (LB) were significantly different with tcalc = 0.0005288 and tcalc = 0.003881, p = 0.05 respectively. Thus, the addition of Lysinibacillus sphaericus microbial solution as mortar making mix media in the preparation of mortars significantly retards both the initial and final setting time as well the normal consistence, but has no significant influence on the mortar soundness.
3.3. Scanning electron microscope (SEM) analysis
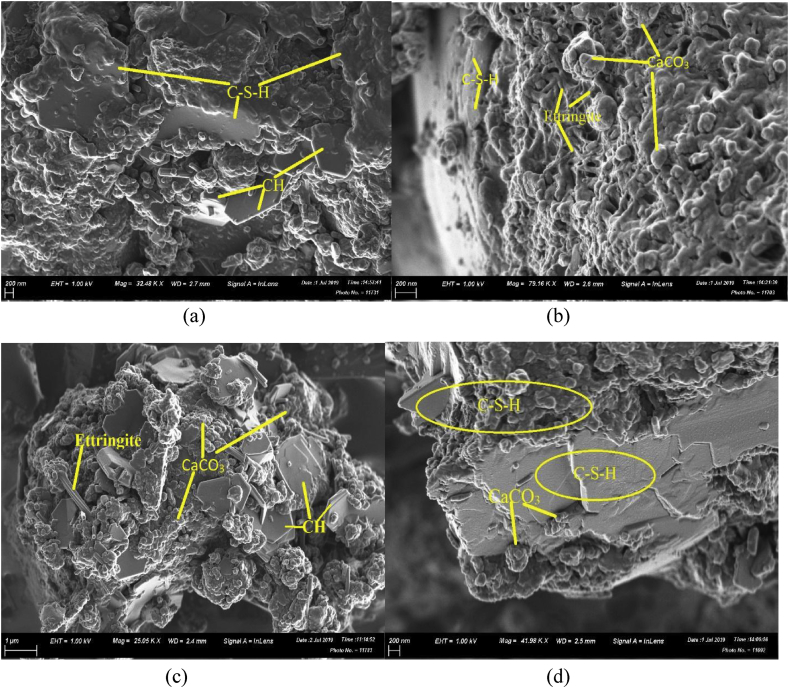
Fig. 1(a) to (d) shows SEM analysis for both control and microbial mortar prisms after 28th day of curing. The SEM images display the formation of calcium-silicate-hydrate, C–S–H, calcium carbonate precipitation, CaCO3, needle type ettringite, Ettr., and presence of portlandite/calcium hydroxide, CH.
Fig. 1.
SEM analysis for (a) OPC-H2O (H2O), (b) OPC-H2O (LB), (c) OPC-LB (H2O) and (d) OPC-LB (LB).
As SEM images in Fig. 1 illustrate, the OPC-H2O (H2O) mortar had no visible calcium carbonate deposits. However, the microbial mortars OPC-H2O (LB), OPC-LB (H2O) and OPC-LB (LB) showed significant calcium carbonate precipitates. This could be attributed to the MICP deposits from the Lysinibacillus sphaericus either present in mix media or present in the cultured curing solution [5, 31, 32]. There a lot of observable differences between the SEMs including morphology of CSH which densifies from b to c to d.
This could be attributed to the calcium carbonate precipitation by Lysinibacillus sphaericus which is largely attributed to increase in compressive strength as well decrease in sorptivity coefficient. Image (c) from Fig. 1, clearly shows biodeposition over ettringite needles resulting to formation of biofilms on their surface and plugging of the pores on the mortar structure. This could explain why MICP technique has been used to remove sulphate and clean crusts from marble monuments [33, 34, 35].
3.4. Compressive strength
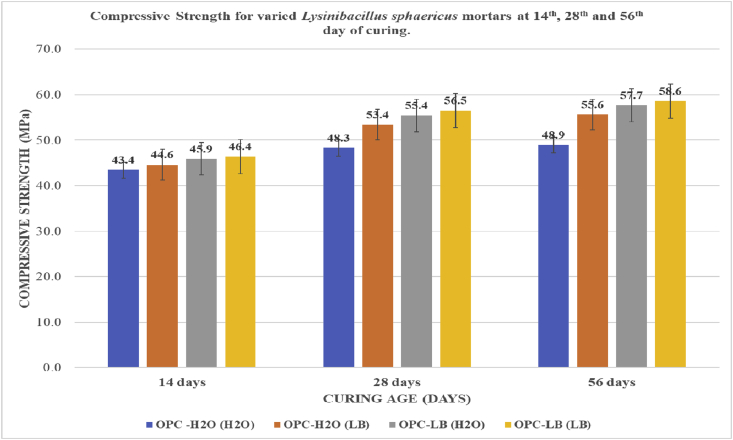
The compressive strength results obtained at 14th, 28th and 56th day of curing are summarized in Fig. 2.
Fig. 2.
Results for Compressive Strength of test mortars at 14th, 28th and 56th day of Curing.
Compressive strength for OPC-H2O (H2O), OPC-H2O (LB), OPC-LB (H2O) and OPC-LB (LB) mortar prisms were determined using a compressive strength machine at the 2nd, 7th, 14th, 28th and 56th day of curing. Across all mortar categories, there was no significant difference in their compressive strengths at 2nd and 7th day of curing and hence they have not been reported in this study. Perhaps the bacteria had not precipitated significant quantities of calcium carbonate or the cement material had not reacted with the precipitation to form a strength beneficial material. A summary of significance difference in compressive strength among different mortar categories have been presented in Table 3.
Table 3.
TCalc. values summary for mortar categories compressive strength across 2nd, 7th, 14th, 28th and 56th day of curing. (TCrit. = 0.5, p = 0.05).
| MORTAR CATEGORIES | TCalc. Values (x10−3) |
||||
|---|---|---|---|---|---|
| 2nd day | 7th day | 14th day | 28th day | 56th day | |
| OPC-H2O (H2O) vs. OPC-H2O (LB) | 500 | 500 | 1.4271 | 0.0012 | 0.0270 |
| OPC-H2O (H2O) vs. OPC-LB (H2O) | 500 | 500 | 0.5483 | 0.0001 | 0.0002 |
| OPC-H2O (H2O) vs. OPC-LB (LB) | 500 | 500 | 0.0254 | 0.0332 | 0.2756 |
| OPC-H2O (LB) vs. OPC-LB (H2O) | 500 | 500 | 0.1061 | 0.0262 | 0.1222 |
| OPC-H2O (LB) vs. OPC-LB (LB) | 500 | 500 | 0.0833 | 0.0905 | 1.4317 |
| OPC-LB (H2O) vs. OPC-LB (LB) | 500 | 500 | 6.2184 | 3.2996 | 31.7364 |
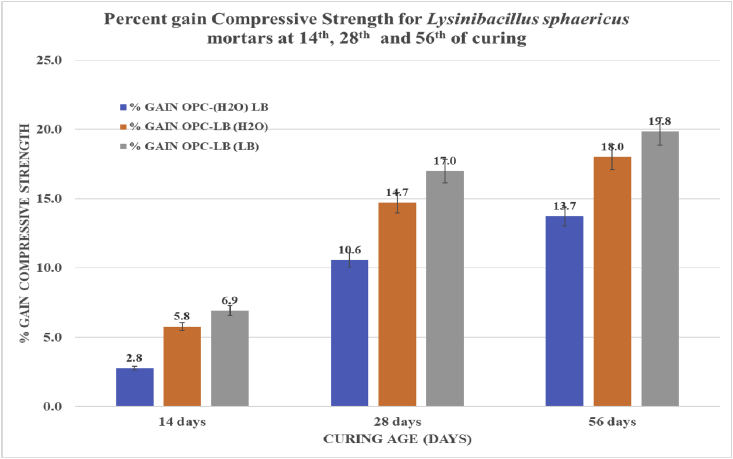
The compressive strength for all mortar categories increased with increase in curing age as depicted on Fig. 3. Across all curing ages, OPC-LB (LB) exhibited the highest compressive strength as well the highest percent gain in compressive strength than the other mortar categories. The highest compressive strength and percent compressive strength gain was observed at the 56th day of curing at 58.6MPa and 19.8% as shown on Fig. 2 and Fig. 3 respectively. There was observed a statistically significant difference in compressive strength and percent compressive strength gain both from one curing age to another as well from one microbial mortar category to another for all microbial mortar categories. as shown in Table 2. The increase in compressive strength could be attributed to the materials precipitated by the Lysinibacillus sphaericus being involved in the hydration process forming calcium silicate hydrate responsible for strength development. The added Ca2+ together with calcium acetate, in presence of the microbial cell-wall nucleation site readily combine with the precipitated CO32- and crystallizes out as calcium silicate hydrate (C–S–H) [5, 16, 27, 30].
Fig. 3.
Percent gain Compressive strength of Lysinibacillus sphaericus mortars at 14th, 28th and 56th day of curing.
In this study, it has been found that the Lysinibacillus sphaericus biomineralization process enhance the compressive strength hence improving durability properties of OPC cement structures. The enhanced MICP process in this study, could also be attributed to the metabolic conversion of the organic acetate added as a microbial feed in form of calcium acetate which was aerobically oxidized under improved alkaline conditions by this ureolytic alkaliphilic Bacillus spp. according to the following equation [5, 16]:
| CH3COO1- + 2O2 → 2CO2 + OH1- + H2O | (10) |
| CO2 + OH1- → HCO31- | (11) |
| HCO31- + OH1- → CO32- + H2O | (12) |
| Bacterial Cell-Ca2+ + CO32− → Cell-CaCO3 | (13) |
3.5. Water sorptivity
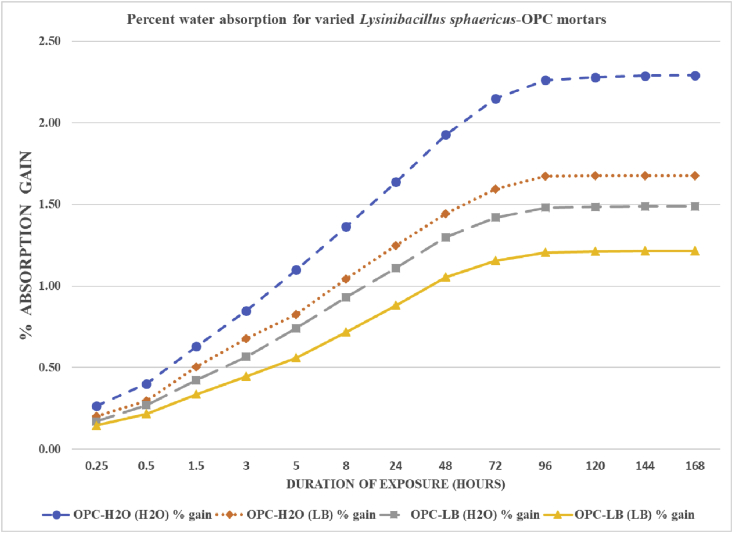
As shown in Fig. 4, throughout the water exposure duration, OPC-H2O (H2O), exhibited the highest percent water absorption while the OPC-LB (LB), had the lowest. All the test mortars exhibited a gradual rise in water absorption but after the 120th hour of water exposure the water sorption almost became constant. Perhaps the mortars became water saturated such that they could not absorb more water.
Fig. 4.
Percent water absorption for varied Lysinibacillus sphaericus OPC mortars after 28th day of curing.
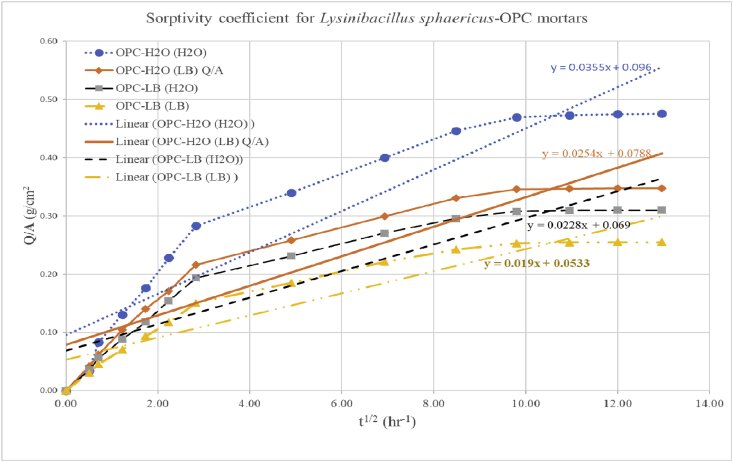
As shown on Fig. 5 and summarized in Fig. 6, it is evident that all the microbial mortar prisms OPC-H2O (LB), OPC-LB (H2O) and OPC-LB (LB) had a higher water absorption resistance than the non-microbial OPC-H2O (H2O) mortar prism. The decrease in water sorption could be attributed to the calcium carbonate precipitation which is also evident in the SEM images as shown in Fig. 1 (a) as compared to (b), (c) and (d). Other researchers though using different bacteria such as Mutitu and his co-authors [5], Achal and his co-workers [27] using Sporosarcina pasteurii and Dhamia and his co-workers [36] using Bacillus megaterium bacteria also observed water absorption reduction in the microbial mortars as compared with mortars prepared without microbial media. All these studies attributed the decrease in water permeability and porosity to the calcium carbonate precipitation which seals the pores in the mortar matrix inhibiting water migration [5, 27, 37].
Fig. 5.
Sorptivity coefficients for varied Lysinibacillus sphaericus-OPC mortars after 28th day of curing.
Fig. 6.
Comparative Sorptivity coefficients for varied Lysinibacillus sphaericus mortars after 28th day of curing.
4. Conclusion
-
1.
Lysinibacillus sphaericus has the ability to precipitation-controlled amount of calcium carbonate, enough to improve both compressive strengths as well the pore structure lowering water ingress.
-
2.
Compressive strength of the mortar increased with increase in the population/concentration of Lysinibacillus sphaericus bacteria. This was exhibited by the higher strengths in microbial mortars cured in cultured solutions showing higher strengths than those cured in distilled water.
-
3.
Incorporation of Lysinibacillus sphaericus into OPC mortar enhanced the nucleation sites for calcite precipitation leading to improved overall microstructure of the mortar. The calcite precipitation filled the pores of the mortar reducing water permeation.
-
4.
Incorporation of calcium acetate as a feed in the microbial culturing introduced more Ca2+ in the mortar matrix. This enhances formation of the cement-compatible calcium carbonate which plugs the pores as shown by the SEM images improving both the compressive strength as well lowering water sorptivity.
Declarations
Author contribution statement
Daniel Karanja Mutitu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jackson Muthengia Wachira, Romano Mwirichia, Joseph Karanja Thiong'o, Onesmus Mulwa Munyao, Genson Muriithi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Data availability statement
The raw or any additional data used to support the findings of this study are restricted by the University of Embu, Board of Postgraduate in order to avoid plagiarism since this study findings are intended to form part of a PhD thesis yet to be published.
Acknowledgements
Access of Scholarly repository and library materials from University of Embu and Kenyatta University, Kenya, is gratefully acknowledged. My sincere gratitude goes to the technical staff of Savannah Cement Ltd. and in particular Mr. Mulwa as well as the University of Pretoria, South Africa for allowing me to use their cement laboratory facilities for my laboratory work operations and related tests. Special thanks also go to my three great supervisors herewith acknowledged as my co-authors for their continued support, insights, guidance and critique throughout this study.
References
- 1.Wachira J.M., Thiong’o J.K., Marangu J.M., Muriithi L.G. Physicochemical performance of Portland – rice husk ash-calcined clay-dried acetylene lime sludge cement in sulphate and chloride media. Adv. Mater. Sci. Eng. 2019;2019 [Google Scholar]
- 2.Mutitu D.K. Chemistry Department, Kenyatta University; Nairobi: 2013. Diffusivity of Chloride and Sulphate Ions into Mortar Cubes Made Using Ordinary Portland and Portland Pozzolana Cements; pp. 69–83. [Google Scholar]
- 3.Azadi M., Ghayoomi M., Shamskia N. Physical and mechanical properties of reconstructed bio-cemented sand. Soils Found. 2017;57:698–706. [Google Scholar]
- 4.Chahal N., Siddique R., Rajor A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of fly ash concrete. Constr. Build. Mater. 2012;4:98–105. [Google Scholar]
- 5.Mutitu D.K., Mulwa O.M., Wachira J.M., Mwirichia R., Thiong’o J.K., Marangu J.M. Effects of biocementation on some properties of cement-based materials incorporating Bacillus species bacteria – a review. J. Sustain. cement-based Mater. 2019:5–12. [Google Scholar]
- 6.Luo M., Qjan C. Influences of bacteria based self-healing agents on cementitious materials hydration kinetics and compressive strength. Constr. Build. Mater. 2016;4:1132–1141. [Google Scholar]
- 7.Nosouhian F., Mostofinejad D., Hasheminejad H. 2016. Concrete Durability Improvement in a Sulphate Environment Using Bacteria; pp. 1–12. [Google Scholar]
- 8.Qian C.X., Yu X.N., Wang X. A study on the cementation interface of bio-cement. Mater. Char. 2018;59:1186–1193. [Google Scholar]
- 9.Verma R.K., Chaurasia L., Bisht V. Bio-mineralization and bacterial carbonate precipitation in mortar and concrete. Biosci. Bioeng. 2015;1:5–11. [Google Scholar]
- 10.Maes M., De Belie N. Procedure of concrete Solutions. 6th International on concrete Repair; 6: 20–22nd June, 2016. 2016. Service life estimation of cracked and healed concrete in marine environment. Thessaloniki, Greece. [Google Scholar]
- 11.Jeffrey J.W., Mondal P., Mideley C.M. Mechanisms of cement hydration. Cement Concr. Res. 2012;41:1208–1223. [Google Scholar]
- 12.Alizadeh R., Beaudoin J.J., Raki L. Mechanical properties of calcium silicate hydrates. Mater. Struct. 2011;44:3–8. [Google Scholar]
- 13.Dousti A., Shekarchi M., Alizadeh R., Taheri-Motlagh A. ―Binding of externally supplied chlorides in micro silica concrete under field exposure conditions. Cement Concr. Compos. 2011;33:1071–1079. [Google Scholar]
- 14.Theodore C., Karen S. Alkali fixation of CSH in blended cement pastes and its relation to alkali silica reaction. Laboratory of construction materials. 2012;42:1049–1054. [Google Scholar]
- 15.De Belie N., Wang J. Bacteria based repair and self-healing of concrete. J. Sustain. Cement-Based Mater. 2016;5:35–56. [Google Scholar]
- 16.Ersan Y.C., Hernandez-Sanabria N., De Belie N. Enhanced crack closure performance of microbial mortar through nitrate reduction. Cement Concr. Res. 2015;70:159–170. [Google Scholar]
- 17.Stocks-Fischer S., Galinat J.K., Takemoto K., Uchikawa H. Proceedings of the 7th International Congress on the Chemistry of Cements. John Wileys; Paris: 1980. Hydration of pozzolanic cements. 26 – 28. [Google Scholar]
- 18.Mobley H.L., Hausinger R.P. Microbial ureases: significance, regulation and molecular characterization. Microbiol. Rev. 1989;9:451–480. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L., Cord-Ruwisch R. Selective enrichment and production of highly urease active bacteria by non-sterile chemostat culture. J. Ind. Microbiol. Biotechnol. 2013;40:1095–1104. doi: 10.1007/s10295-013-1310-6. [DOI] [PubMed] [Google Scholar]
- 20.Rao V.N., Meena T. A review on carbonation study in concrete. IOP Conf. Ser. Mater. Sci. Eng. 2017;263:1–10. [Google Scholar]
- 21.EN 196-1 . European Union Standards; Beckum, Germany: 2011. Cement Part 1: Composition, Specifications and Conformity Criteria for Common Cements. [Google Scholar]
- 22.KS EAS 18-1 . KEBS; Nairobi: 2017. Kenya Standard Test Method for Oxides Specification of Hydraulic Cement; pp. 59–61. [Google Scholar]
- 23.ASTM D7348 . ASTM International; West Conshohoken, PA: 2013. Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues.www.astm.org [Google Scholar]
- 24.Elvira R. Saunders, College Publishers; FortWorth: 2012. Advanced Statistical Methods of Analysis of Large Data-Sets; pp. 1–13. [Google Scholar]
- 25.IS 4031-4 . Bureau of Indian Standards; New Delhi, India: 1988. Methods of Physical Tests for Hydraulic Cement, Part 4: Determination of Consistency of Standard Cement Paste. [Google Scholar]
- 26.KS EAS 148-3 . Kenya Bureau of Standards; Nairobi: 2017. pp. 10–15. (Cement-Test Methods Part 3: Determination of Setting Times and Soundness). [Google Scholar]
- 27.Achal V., Mukherjee A., Zhang Q.Z. Unearthing ecological wisdom from natural habitats and its ramifications on development of biocement and sustainable cities. Landsc. Urban Plan. 2016;155:61–68. [Google Scholar]
- 28.Bogue, R. H. (1977). Chemistry of Portland Cement, British Patent No. 5022, October 21; p. 1927 - 1940.
- 29.Sahoo K.K., Arakha M., Sarkar P. Enhancement of properties of recycled coarse aggregate concrete using bacteria. Int J. Smart Nano Mater. 2016;9:903–910. [Google Scholar]
- 30.Thiyagarajan H., Maheswaran S., Mapa M. Investigation of Bacterial activity on Compressive Strength of cement mortar in different curing Media. ACT. 2016;14:125–133. [Google Scholar]
- 31.Vekariya M.S., Pitroda J. Bacterial Concrete. New era for construction industry. Int. J. Eng. Trends Technol. 2013;4:9–16. [Google Scholar]
- 32.Tziviloglou E., Wiktor V., Jonkers H.M. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 2016;3:122–129. [Google Scholar]
- 33.Vijay K., Murmu M. Effect of Calcium Lactate on compressive strength and self-healing of cracks in microbial concrete. Front. Struct. Civ. Eng. 2018;5:1–11. [Google Scholar]
- 34.Seifan M., Samani A.K., Berenjian A. Bioconcrete: next generation of self healing concrete. Appl. Microbiol. Biotechnol. 2016;100 doi: 10.1007/s00253-016-7316-z. 2591–2511. [DOI] [PubMed] [Google Scholar]
- 35.Boualleg S., Bencheikh M., Belagraa L., Daoudi A. The combined effect of the initial cure and the type of cement on the natural carbonation, the portlandite content and the nonevaporable water in blended cement. Ann. Mater. Sci. Eng. 2017;6:24–34. [Google Scholar]
- 36.Dhamia N.K., Reddy S.M., Mukherjee A. Improvement in strength properties of ash bricks by bacterial calcite. Ecol. Eng. 2012:39–43. [Google Scholar]
- 37.Al-Salloum Y., Abbas H., Sheikh Q.I. Effect of some biotic factors on microbially-induced calcite precipitation in cement mortar. Saudi J. Biol. Sci. 2017;24:286–294. doi: 10.1016/j.sjbs.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw or any additional data used to support the findings of this study are restricted by the University of Embu, Board of Postgraduate in order to avoid plagiarism since this study findings are intended to form part of a PhD thesis yet to be published.