Abstract
Organismal ageing is a complex process driving progressive impairment of functionality and regenerative potential of tissues. Cellular senescence is a state of stable cell cycle arrest occurring in response to damage and stress and is considered a hallmark of ageing. Senescent cells accumulate in multiple organs during ageing, contribute to tissue dysfunction and give rise to pathological manifestations. Senescence is therefore a defining feature of a variety of human age‐related disorders, including cancer, and targeted elimination of these cells has recently emerged as a promising therapeutic approach to ameliorate tissue damage and promote repair and regeneration. In addition, in vivo identification of senescent cells has significant potential for early diagnosis of multiple pathologies. Here, we review existing senolytics, small molecules and drug delivery tools used in preclinical therapeutic strategies involving cellular senescence, as well as probes to trace senescent cells. We also review the clinical research landscape in senescence and discuss how identifying and targeting cellular senescence might positively affect pathological and ageing processes.
Keywords: age‐related disorders, cellular senescence, SASP, senolytic drugs, senoprobes
Subject Categories: Ageing, Pharmacology & Drug Discovery
This article reviews the broad topic of cellular senescence, how to manipulate it ‐ novel probes and nanocarriers have potential diagnostic and therapeutic values ‐ and details a number of senotherapies that entered clinical trials.
Glossary
- Apoptosis
Controlled cell death that occurs in response to a variety of cellular stressors and as part of developmental programmes of multicellular organisms.
- Autophagy
Regulated mechanism used by cells to maintain homeostasis and normal functioning by degradation of unnecessary or dysfunctional components.
- Cellular senescence
Cellular state characterized by a stable cell cycle arrest and the implementation of a complex secretory phenotype (SASP) in response to different sources of damage and/or stress.
- Cytokines
Broad range of small secreted proteins that have specific effects on the interactions and communications between cells and the immune system.
- DDR (DNA Damage Response)
Network of cellular pathways that sense, signal and repair DNA lesions. These include a set of DNA repair mechanisms, damage tolerance processes and cell cycle checkpoint pathways.
- Metabolic reprogramming
Molecular adjustments in metabolic pathways that alter the bioenergetic profile and metabolism of the cell.
- Nanoparticles
Particles between 1 and 100 nm in size with very large surface area to volume ratios that can be functionalized for specific drug delivery targeting.
- Neutropenia
The abnormally low concentration of neutrophils in the blood. If severe, it can significantly increase the risk of infection.
- OIS (Oncogene‐Induced Senescence)
Robust and sustained antiproliferative response that can be induced by aberrant signalling resulting from an activating mutation of an oncogene, or the inactivation of a tumour suppressor gene.
- Osteoclastogenesis
The development of osteoclasts (cells responsible for bone break down and resorption) from blood cells, specifically from monocytes/macrophages.
- Oxidative stress
Imbalance caused by a higher production of free radicals that cannot be neutralized by the antioxidants produced inside the cell, resulting in damaged components.
- Progeroid (mouse) model
Genetically engineered mouse model presenting a pronounced premature ageing and typical age‐related pathologies. In particular, the text refers to Ercc1 −/Δ mice. Ercc1 is a DNA excision repair protein involved in genome maintenance.
- Proteotoxic stress
Molecular response triggered by the accumulation of misfolded proteins within the cell, which may impair cellular function.
- Renal glomerulosclerosis
Condition that refers to the scarring or hardening of the glomeruli in the kidney (the renal functional units). If left untreated, it can lead to kidney failure.
- Replicative senescence
State of cellular senescence induced by the attrition of telomeres in the cells, which triggers a specific DNA damage response.
- SAβgal (Senescence‐associated β‐galactosidase)
Lysosomal enzymatic activity increased in senescent cells that catalyses the hydrolysis of β‐galactoside into monosaccharides. It is commonly used as a marker of cellular senescence.
- SAHF (Senescence‐associated heterochromatin foci)
Regions of facultative heterochromatin within the nucleus that allow the silencing of proliferation‐related genes in the cell. It is considered a common feature of cellular senescence.
- Sarcopenia
Degenerative loss of skeletal muscle mass, quality and strength associated with ageing.
- SASP (Senescence‐associated secretory phenotype)
A robust secretion of molecules such as growth factors, chemokines, cytokines and extracellular matrix metalloproteases that occurs when a cell undergoes senescence.
- Secretome
The set of all molecules and factors secreted by a cell into the extracellular space.
- Senolytic drugs
Chemical compounds that selectively target and induce the death of senescent cells.
- Senoprobes
Molecules that have been developed and engineered to analyse or detect senescent cells for diagnostic or experimental purposes.
- Synovium
Soft connective tissue that lines the inner surface of spaces of diarthrodial joints, tendon sheaths and bursae.
- Theranostic tools
Tools aimed at simultaneously detecting and eradicating a pathological lesion or damaged area of tissue.
- Thrombocytopenia
Condition associated with low blood platelet counts.
- TIS (Therapy‐Induced Senescence)
A subtype of cellular senescence triggered by a therapeutic treatment such as chemotherapy or radiotherapy.
Introduction
Severe or irreparable cellular damage triggers a stereotyped response across vertebrates based on a stable cell cycle arrest known as cellular senescence. Senescent cells can implement a complex paracrine response, including cytokines, chemokines, growth factors, proteases and extracellular matrix remodelling factors. This senescence‐associated secretory phenotype (SASP) can play differing roles depending on the physiological context (Muñoz‐Espín & Serrano, 2014; Pérez‐Mancera et al, 2014). A major consequence of senescence appears to prevent propagation of pre‐malignant cells, providing a crucial barrier to tumorigenesis (Collado & Serrano, 2010). Cell senescence participates in other physiological processes by promoting tissue repair and regeneration (Demaria et al, 2014; Yun et al, 2015) and plays a programmed role in morphogenesis during normal embryonic development in a damage‐independent fashion (Muñoz‐Espín et al, 2013; Storer et al, 2013). Senescent cells can elicit a tissue remodelling process that includes their own elimination, recruitment of phagocytic immune cells and mobilization of nearby progenitor cells. However, upon persistent damage or during ageing, senescent cell clearance is compromised and dysfunctional cells accumulate, contributing to the generation of a chronic pro‐inflammatory microenvironment that results in a diverse range of pathological manifestations.
Cell senescence contributes to a wide variety of human age‐related pathologies, including cancer, fibrosis, cardiovascular diseases, obesity, type 2 diabetes, sarcopenia, osteoarthritis and neurological disorders (van Deursen, 2014; Muñoz‐Espín & Serrano, 2014). The genetic ablation of p16ink4a+ senescent cells in progeroid and chronic disease mouse models attenuates or even reverts tissue dysfunction, leading to an increased animal healthspan. p16ink4a+ senescent cell clearance can ameliorate adipose atrophy, sarcopenia, cataracts, cardiomyocyte hypertrophy, renal glomerulosclerosis, tumorigenesis, cancer progression, atherosclerosis, osteoarthritis and tau‐dependent disease (Baker et al, 2011, 2016; Childs et al, 2016; Demaria et al, 2017; Jeon et al, 2017; Bussian et al, 2018). Importantly, such clearance also increases mouse lifespan (Baker et al, 2016). For years, pharmacological clearance of senescent cells has proved very challenging. However, in the last few years, renewed research efforts have led to the development of senolytics, a collection of molecules capable of preferentially removing senescent cells (Childs et al, 2017; Soto‐Gamez & Demaria, 2017; Ovadya & Krizhanovsky, 2018). Taken together, emerging data suggest that cell senescence is causative of multiple human disorders (Fig 1), which can be induced by various stimuli including drugs used clinically, and that senolytics may provide exciting opportunities for therapeutic intervention.
Figure 1. Therapeutic and diagnostic opportunities in senescence‐related disorders and during ageing.
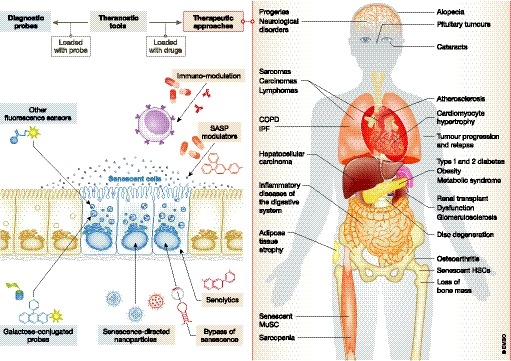
Cellular senescence is associated with multiple human disorders, offering potential interventions for targeted therapeutic and diagnostic approaches. The development of galactose‐conjugated and fluorescent probes to detect and highlight senescent cells offers an important opportunity for longitudinal monitoring of senescence in clinical trials. Pharmacologically active small compounds known as senolytics inhibit pro‐survival pathways in senescent cells leading to apoptosis, a therapeutic strategy that may additionally be enhanced by the use of immune modulators promoting natural clearance of senescent cells. Also, a variety of drugs can manipulate the SASP and its detrimental effects, thus suggesting a potential clinical use. Interventions to bypass senescence represent an interesting alternative, although this approach should be taken with caution due to the risk of uncontrolled proliferation and cancer initiation. Finally, nanoparticles encapsulating cytotoxic drugs, tracers and/or small molecules can be used as theranostic tools, both for therapeutic and diagnostic purposes. Of note, the benefits of therapeutic approaches for the prevention or elimination of senescent cells in vivo have been validated in an increasing number of conditions. Genetic manipulation to inactivate the senescence pathway or to ablate senescent cells in murine models produced (mostly) a beneficial impact irrespective of the disorder or condition investigated, including adipose atrophy, cataracts, IPF, sarcopenia, kidney dysfunction, atherosclerosis, premature ageing of the haematopoietic system, osteoarthritis, cardiomyocyte hypertrophy, loss of bone mass, type 2 diabetes, tumorigenesis, neurological disorders and natural ageing. Furthermore, clearance of senescent cells by treatment with senolytic drugs, a more clinically relevant approach, showed in vivo benefits in, among other disorders, atherosclerosis, premature ageing of the haematopoietic system, myocardial infarction, IPF, osteoarthritis, osteoporosis, type 1 diabetes, obesity‐induced metabolic syndrome and neuropsychiatric disorders, tau‐dependent pathologies, cancer and natural ageing. IPF, idiopathic pulmonary fibrosis; HSC, hematopoietic stem cells; MuSC, muscle stem cells.
Besides stable cell cycle arrest and SASP production (see Fig 2 for relevant signalling pathways), another hallmark of senescent cells is their resistance to damage‐induced apoptosis through survival pathway upregulation (Childs et al, 2014, 2017; Soto‐Gamez et al, 2019). Some senolytics exploit their capacity to stimulate cell death pathways (see below). In the absence of a single universal hallmark, senescent cells can be identified by combining a number of markers (Sharpless & Sherr, 2015), including upregulation of p16 ink4a and other cell cycle inhibitors, exclusion of proliferative markers, formation of specialized heterochromatin domains (senescence‐associated heterochromatin foci, SAHF) and persistent activation of the DNA damage response (DDR) machinery. Although imperfect, detection of increased activity of lysosomal senescence‐associated β‐galactosidase (SAβgal) remains the most widely used indicator of cellular senescence (Sharpless & Sherr, 2015), explaining why many senescence detection probes are based on detecting its enzymatic activity.
Figure 2. Regulation of the cell cycle arrest and inflammatory SASP in the induction of cellular senescence and its interconnection with apoptosis.
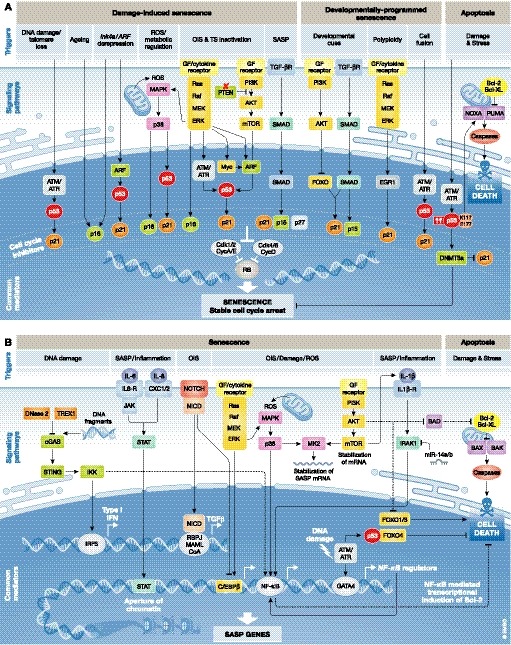
(A) Most senescence‐inducing triggers converge in the activation of the cell cycle inhibitor pathways p53/p21 and/or p16INK 4a. These result in the inhibition of cyclin‐dependent kinase 1 (CDK1), CDK2, CDK4 and CDK6, which prevents the phosphorylation of the retinoblastoma protein (RB), leading to the suppression of S‐phase genes and an ensuing stable cell cycle arrest. DNA‐damaging triggers activate the DNA damage response (DDR) pathway resulting in the activation of p53 and p21. Ageing and epigenetic derepression of the Ink4a/ARF locus also lead to the activation of cell cycle inhibitors p16 and p21. ROS lead to the activation of the MAPK signalling pathway and its downstream effector p38. The aberrant expression of oncogenes or the loss of tumour suppressors leads to p53 activation through the Ras‐Raf‐MEK‐ERK or AKT signalling pathways, and TGFβ, and important factor of the SASP, leads to p15, p21 and p27 upregulation via SMAD signalling. Other triggers such as developmental cues and polyploidy activate the AKT, SMAD and/or Ras‐Raf‐MEK‐ERK pathway for p21 upregulation, while processes such as cell fusion signal through the DDR for p53 activation. In response to damage and different types of stress high levels of p53 with specific post‐translational modifications (such as acetylated K117 and E177) target DNMT3a, a suppressor of p21 and senescence, and trigger the apoptotic programme by upregulating PUMA and NOXA, which in turn activate the caspase cascade leading to cell death. (B) SASP implementation is orchestrated by the activation of the transcription factors NF‐κB and C/EBPβ through upstream signalling pathways. DNA‐damaging agents, ROS and OIS, generally activate the expression of SASP TFs via the AKT and/or the Ras‐Raf‐MEK‐ERK axis. In addition, DNA fragments are also known to trigger the activation of the cGAS/STING signalling, resulting in the activation of the IRF3 TF and subsequent transcription of Type 1 IFN. OIS‐derived SASP is dynamic and can also be orchestrated by NOTCH signalling, a process that restrains the inflammatory secretion by inhibiting C/EBPβ at initial stages, and allows the activation of SASP‐related super enhancers through NF‐κB later on. Accumulating increased levels of TFs reinforce the senescent phenotype through autocrine and paracrine signalling. SASP‐derived inflammatory chemokines such as IL‐6 and IL‐8 promote epigenetic modifications reinforcing the cell cycle arrest through the JAK/STAT cascade, while IL‐1α stimulates the activity of NF‐κB and C/EBPβ promoting a positive feedback loop on the secretion of other cytokines. Finally, senescence promotes survival networks by the regulation anti‐apoptotic pathways. This includes PI3K‐AKT signalling, which can inhibit pro‐apoptotic BAD and FOXO1/3, and phosphorylate caspase‐9; anti‐apoptotic FOXO4, that is present in senescent cells and interacts with p53; and NF‐κB, that may also promote survival responses by transcriptional induction of anti‐apoptotic proteins of the Bcl‐2 family. ATM/ATR, ataxia‐telangiectasia mutated and Rad3‐related homologue; IFN, interferon; OIS, oncogene‐induced senescence; ROS, reactive oxygen species; SASP, senescence‐associated secretory phenotype; TFs, transcription factors; TS, tumour suppressor.
Here, we review approaches to identify and therapeutically target senescent cells (Fig 1). These strategies comprise senolytics and novel drug delivery tools to target senescence. Additional innovative interventions to manipulate cell senescence include the modulation of the SASP and associated signalling pathways, immunotherapy and promoting the artificial reactivation of proliferation. We discuss novel probes for senescent cell visualization, and their potential utility in medical diagnosis as well as for monitoring accumulation or elimination of senescent cells. We comment on the clinical development of senescence‐targeted strategies and future translational considerations, highlighting novel opportunities as well as current challenges.
Therapeutic approaches
Senescent cells as target of therapy
Senescence and apoptosis have been proposed as alternative cell fates in the context of damage and stress. Pro‐apoptotic cellular changes are often actively anti‐senescent (Fig 2A), while senescent cells are highly resistant to apoptosis. Several molecular mechanisms of the senescent phenotype directly contribute to increased survival (Fig 2B) (Childs et al, 2014; Soto‐Gamez et al, 2019). Accordingly, targeting pro‐survival pathways, i.e. those involving the BCL‐2 family of proteins, the p53 or PI3K/AKT pathways (Table EV1, Figs 2B and 3), is currently the most common strategy to promote senescent cell elimination in damaged or aged tissues.
Figure 3. Therapeutic approaches targeting cellular senescence.
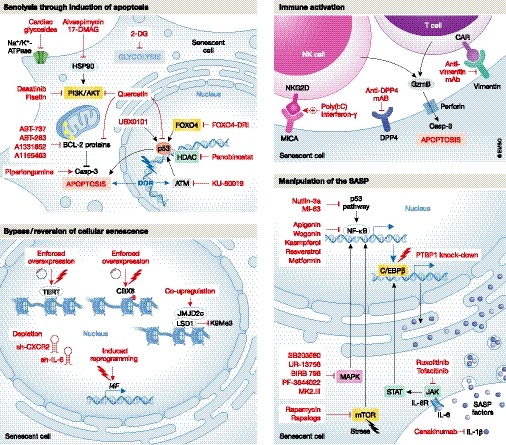
To prevent the deleterious effects of cellular senescence, four different strategies can be potentially implemented. The inhibition of pro‐survival pathways by the use of apoptosis‐inducing drugs is a leading approach. First and second generation of inhibitors of the BCL‐2 cell death regulator family of proteins can induce selective apoptosis of senescent cells. Targeting senescence metabolism through glycolysis blockade and attenuation of ATM, HDAC, FOXO4 activities as well as the PI3K cascade have also been reported as effective approaches. A second strategy is the activation of the immune system against senescent cells to stimulate their clearance. Enhancing the cytotoxic activity of NK against senescent cells, and manipulating the humoral innate immunity with the use of antibodies against receptors, such as DPP4 and vimentin, are proposed attractive strategies. Thirdly, manipulation of the SASP without compromising the cell cycle arrest of senescent cells has also proven beneficial in particular settings. A large number of molecules can interfere with NF‐κB and C/EBPβ transcriptional activities or their upstream regulators, dampening the expression of SASP factors, such as IL‐1, IL‐6 and IL‐8, and thus reducing the senescence‐derived inflammatory milieu. Lastly, genetic and epigenetic manipulation of cells, including the induction of reprogramming, have been proposed as a means of bypassing or reverting the state of cellular senescence, although these approaches should be taken with caution given the potential risk of cancer initiation. BCL‐2, B‐cell lymphoma 2; CAR, chimeric antigen receptor; GzmB, granzyme B; HDAC, histone deacetylase; HSP90, heat shock protein 90; i4F, inducible four Yamanaka factors; LSD1, lysine‐specific histone demethylase 1A; MICA, MHC class I polypeptide‐related sequence A; NK, natural killer; TERT, telomerase reverse transcriptase.
Inhibition of pro‐survival pathways by single senolytics
Deregulation of the BCL‐2 family of proteins, major regulators of programmed cell death, is commonly observed in multiple haematological, autoimmune, degenerative disorders and cancer (Singh et al, 2019). Navitoclax (ABT‐263), a specific inhibitor of BCL‐2, BCL‐xL and BCL‐W, induced selective apoptosis of a variety of cells undergoing senescence induced by ionizing radiation, oncogene expression or replicative exhaustion, including human umbilical vein epithelial cells (HUVECs), human lung fibroblasts and MEFs, but not in senescent human primary preadipocytes (Zhu et al, 2016). Administration of ABT‐263 also depleted senescent cells in vivo, mitigating premature ageing of the haematopoietic system in sublethally irradiated mice, thereby participating in bone marrow and muscle haematopoietic stem cells rejuvenation in normally aged mice (Chang et al, 2016). In addition, ABT‐263 treatment reduced the expression levels of several SASP factors in aged murine lungs, including Cdkn2a (encoding for p16ink4a), Tnfa (encoding for TNF‐α) and Ccl5 (encoding for CCL5). In a model of articular joint injury, the selective elimination of senescent cells in the articular cartilage and synovium by ABT‐263 reduced features of post‐traumatic osteoarthritis (Jeon et al, 2017); age‐related symptoms were ameliorated by targeting senescent osteoblast progenitors obtained from aged mice (Kim et al, 2017a). ABT‐263 reduced the levels of several SASP components by eliminating the senescent progenitor cells, resulting in attenuation of osteoclastogenesis in bone marrow stromal cell cultures from aged mice (Kim et al, 2017a).
ABT‐737 is an analogue of ABT‐263, validated in IMR90 human fibroblasts and in mouse embryonic fibroblasts (MEFs) where the anti‐apoptotic proteins BCL‐2, BCL‐W and BCL‐xL are highly induced upon different senescence‐inducing stimuli (Yosef et al, 2016). ABT‐737 eliminated senescent cells in the lungs and epidermis of irradiated mice, resulting in increased hair‐follicle stem cell proliferation. However, both ABT‐263 and ABT‐737 are toxic for neutrophils and platelets (Cang et al, 2015), which may limit their clinical development. Second‐generation inhibitors of the BCL‐2 family of proteins include the BCL‐xL inhibitors A1331852 and A1155463, which induced selective apoptosis of senescent HUVEC and IMR90 cells, but not of senescent human preadipocytes (Zhu et al, 2017). Preadipocytes appear resistant to BCL2 family member blockade, suggesting heterogeneity in cell‐intrinsic senescence pathways (Zhu et al, 2016). These BCL‐xL selective inhibitors enhance the efficacy of standard chemotherapy in mouse models of breast cancer, non‐small‐cell lung cancer (NSCLC) and ovarian cancer, while avoiding the exacerbation of cytotoxic chemotherapy‐induced neutropenia observed with ABT‐263 (Leverson et al, 2015). While more BCL‐2 family inhibitors are currently in development, targeting the BH4 domain is promising for enhancing senolytic sensitivity; BH4 domain is present in all the pro‐survival members of the family (BCL‐2, BCL‐xL, BCL‐W, MCL‐1 and BFL‐1) and necessary for their anti‐apoptotic activity (Liu et al, 2016).
Piperlongumine, isolated from trees of the genus Piper, is a natural senolytic agent. Initially found to inhibit tumour growth in a xenograft mouse model of NSCLC, it was later shown to trigger apoptosis and preferentially kill senescent cells induced by oncogene expression, ionizing radiation or replicative exhaustion (Wang et al, 2016). Panobinostat (an FDA‐approved histone deacetylase inhibitor) has senolytic activity in NSCLC, and head and neck squamous cell carcinoma (HNSCC) cell lines previously treated with clinically relevant cytotoxic drugs (cisplatin and paclitaxel) (Samaraweera et al, 2017). Panobinostat increased caspase 3/7 activity and decreased Bcl‐xL expression in chemotherapy‐induced senescent cells. Recently, cardiac glycosides (CGs) have been identified as a family of compounds with potent senolytic activity (Guerrero et al, 2019; Triana‐Martínez et al, 2019). CGs target the cell membrane Na+/K+‐ATPase pumps causing a disbalanced electrochemical gradient that makes senescent cells more vulnerable. These compounds have been validated ex vivo in senescent preneoplastic cells and in vivo in models of lung fibrosis, therapy‐induced senescence, and aged mice.
Combinations of drugs with senolytic activity
The tyrosine kinase inhibitor dasatinib (which inhibits SRC, c‐KIT, ephrin receptors and other kinases) and the flavonoid quercetin (which has multiple targets including kinases and receptors, and inhibits the PI3K‐AKT pathway) are effective in combination at eliminating senescent cells in vitro and in vivo, selectively targeting a wide range of senescent cell types (Zhu et al, 2015; Roos et al, 2016). A single administration of dasatinib and quercetin in aged mice was sufficient to improve cardiovascular function and also reduced the expression of p16ink4a and prevalence of SAβGal‐positive cells after localized limb irradiation (Zhu et al, 2015). Periodic drug administration extended healthspan in progeroid mice, delaying age‐related symptoms and pathologies. The combination of dasatinib and quercetin (D + Q) in mice resulted in increased survival and improved health. D + Q treatment prevented and alleviated physical dysfunction in naturally aged mice and mice transplanted with senescent preadipocytes, and reduced senescent cell prevalence and pro‐inflammatory cytokine secretion in explants of human adipose tissue obtained from obese individuals (Xu et al, 2018).
It is thought that cell senescence contributes to idiopathic pulmonary fibrosis (IPF), a progressive and debilitating chronic disease with limited therapeutic options (Naikawadi et al, 2016). Ex vivo treatment of mouse primary alveolar epithelial type II cells from fibrotic lungs with D + Q reduced senescence and fibrosis markers (Lehmann et al, 2017). The combination mitigated fibrotic lung disease in a bleomycin‐injury mouse model, clearing senescent cells and improving pulmonary and physical health of treated animals (Schafer et al, 2017). D + Q also cleared senescent cells in the medial segments of blood vessels, improving the vascular phenotype associated with age‐related vascular pathology (Roos et al, 2016). Likewise, chronic treatment reduced intimal aortic plaque calcification in a hypercholesterolaemic mouse model of atherosclerosis. D + Q are well‐tolerated drugs and are now being trialled in human patients in a variety of senescence‐associated conditions (see Targeting senescence clinically in age‐related disorders).
Of note, a panel of flavonoid polyphenols distinct from quercetin has been screened for senolytic activity. Fisetin reduced senescence markers in multiple tissues in progeroid and naturally aged mice (Yousefzadeh et al, 2018), and administration of fisetin in normally aged mice restored tissue homeostasis, reduced age‐related dysfunction and extended lifespan. Future therapeutic outcomes will be required for initial proof of principle of combination therapies.
Targeting pathways involved in senescence
The p53 axis, another key controller of apoptosis and senescence, is a promising target for novel senolytic strategies. FOXO transcription factors can interact with p53, inhibiting p53‐mediated apoptosis and favouring cell cycle arrest and senescence (Wang et al, 2008). A D‐retro inverso (DRI)‐isoform of FOXO4 was developed which causes p53 nuclear exclusion and resultant death of senescent cells (Baar et al, 2017). FOXO4‐DRI selectively eliminated human senescent fibroblasts in vitro through p53‐mediated apoptosis. In naturally and experimentally aged mice, FOXO‐DRI counteracted doxorubicin‐induced senescence and chemotoxicity, minimizing hepatotoxicity and loss of body weight, and reducing features of frailty and loss of renal function (Baar et al, 2017).
High‐throughput experimental approaches are capable of identifying novel senolytic drugs and targets. A screening platform based on SAβgal activity identified HSP90, a ubiquitously expressed chaperone with a role in protein stabilization, as a potential target (Fuhrmann‐Stroissnigg et al, 2017). HSP90 inhibitors downregulate the anti‐apoptotic PI3K/AKT pathway and reduce senescence markers in a variety of human and mouse cell lines. In a progeroid mouse model, the HSP90 inhibitor 17‐DMAG [alvespimycin, which has been tested in clinical trials in different solid tumours and lymphomas (Trepel et al, 2010)] reduced p16ink4a expression levels, extended healthspan and delayed the onset of various age‐related clinical markers (Fuhrmann‐Stroissnigg et al, 2017). A similar high‐throughput screening approach identified KU‐60019, an inhibitor of the DDR protein ataxia‐telangiectasia mutated (ATM), as an effective anti‐senescent agent (Kang et al, 2017). ATM can mediate mechanisms that control senescence through regulating lysosomal acidification. Treatment with KU‐60019 decreased SAβgal activity in senescent fibroblasts, removed dysfunctional mitochondria and resulted in metabolic reprogramming. KU‐60019 therapy accelerated cutaneous wound healing in aged mice, and inhibition of ATM activity also attenuated senescence (Kang et al, 2017); KU‐60019 is therefore a promising candidate target for treatment of age‐related diseases.
Senescent cells are “hypermetabolic”, and this may potentially be therapeutically targetable (Dörr et al, 2013). Metabolic reprogramming is required for senescent cells to cope with the high energetic demands of the senescent programme, including SASP‐coupled proteotoxic stress (featured by high production of SASP factors, increased oxidative stress resulting in misfolded or toxic proteins, and increased endoplasmic reticulum stress/unfolded protein response/ubiquitination/autophagy cascade). Accordingly, senescent cells are more sensitive to treatment with 2‐DG, a decoy substrate for glycolytic metabolism or specific inhibitors of lysosomal V‐ATPases (Dörr et al, 2013). How metabolically targeted drugs can achieve sufficient specificity for senescent over non‐senescent cells in vivo to allow successful translation remains an open question.
Manipulation of the SASP
Although inducing the selective apoptosis of senescent cells using senolytic drugs could be therapeutically beneficial, dampening the detrimental effects of the SASP without compromising senescent cell cycle arrest may prove more advantageous in particular settings. Seminal work showing that the SASP and cell cycle arrest are independently regulated (Coppé et al, 2011) opened the door to differentially targeted therapeutic approaches. Senescent cells implement programmed secretion of growth factors, matrix metalloproteases, chemokines and cytokines that can trigger a wide range of autocrine and paracrine effects. Some of these [such as immune activation and reinforcement of growth arrest and differentiation (Hong et al, 2007; Anestakis et al, 2015)] are crucial for the resolution of tissue damage, but others (such as cell growth, migration and invasion) can be disadvantageous in certain contexts including cancer (Pérez‐Mancera et al, 2014; Gonzalez‐Meljem et al, 2018; Lee & Schmitt, 2019). Activation of various signalling pathways, including key drivers such as mammalian target of rapamycin (mTOR), mitogen‐activated protein kinase (MAPK) signalling, phosphoinositide 3 kinase (PI3K) signalling and GATA4/p62‐mediated autophagy, orchestrates this complex secretome (reviewed in Faget et al, 2019) (Fig 2B). These cascades converge in the activation of the NF‐κB and the CCAAT/enhancer binding protein beta (C/EBPβ) pathways. The great diversity of potential targets able to modulate the cascades driving the expression of the SASP prompted the development of a number of molecules and antibodies to interfere with NF‐κB and C/EBPβ transcriptional activities at different levels (Table EV2 and Fig 3).
Modulation of the upstream regulators of NF‐κB activity
mTOR is a serine/threonine kinase implicated in a wide variety of cellular processes. It is thought to interact with the MAPK pathway by increasing the translation of MAPKAPK2 (Herranz et al, 2015), ultimately resulting in NF‐κB activation and nuclear translocation. mTOR can also regulate membrane‐bound IL‐1α expression (Laberge et al, 2015), rendering it an attractive target for selective inhibitors. mTOR inhibition by rapamycin in normal human fibroblasts and non‐tumorigenic human breast cells suppresses the secretion of inflammatory cytokines including IL‐6, and selectively decreases IL‐1α translation, thereby diminishing NF‐κB transcriptional activity (Laberge et al, 2015). Rapamycin also suppresses the ability of senescent fibroblasts to stimulate prostate tumour growth in mice (Imrali et al, 2016) and blocks the translation of MAPKAPK2, leading to degradation of several SASP components transcripts, including IL‐8 and IL‐1α (Herranz et al, 2015). Treatment of murine lung WI‐38 fibroblasts with rapamycin resulted in a significant decrease in IL‐6, IL‐1β and Vcam‐1 transcription and decreased Stat3 pathway activation (Wang et al, 2017). Newer mTOR inhibitors with comparatively advantageous pharmacological properties have been developed and may also target detrimental effects of the SASP (Lamming et al, 2013; Leontieva et al, 2015). The effects of such “rapalogs”, including everolimus, temsirolimus and deforolimus on senescence remain however unclear.
Inhibitors of members of the MAPK pathway have also been investigated as SASP modulators. The p38MAPK inhibitor SB203580 reduces mRNA levels and secretion of several SASP components reducing NF‐κB transcriptional activation and paracrine effects of the SASP in human senescent cells (Freund et al, 2011). The next‐generation p38MAPK inhibitors UR‐13756 and BIRB 796 also suppress IL‐6 expression in human senescent fibroblasts, and treatment of cells with the MAPKAPK2 inhibitors PF‐3644022 and MK2.III attenuates the SASP (Alimbetov et al, 2016). In addition, treatment of astrocytes with ginsenoside F1, an enzymatically modified derivative of ginsenoside Rg1 that targets p38MAPK, robustly decreased IL‐6 and IL‐8 secretion (Hou et al, 2018). Conditioned media from senescent astrocytes treated with ginsenoside F1 were less able than controls to induce paracrine‐activated cell migration of glioblastoma cells.
Nutlin‐3a results in p53 stabilization by inhibiting Mdm2 and was shown to inhibit the activity of NF‐κB in a p53‐dependent manner (Dey et al, 2008). Nutlin‐3a is therefore an attractive anti‐cancer therapy since it could simultaneously activate p53 and suppress NF‐κB. Indeed, the cytokine response to DDR requires ATM, NBS1 and CHK2, but not the cell cycle arrest enforcers p53 and pRb (Rodier et al, 2009). Nutlin‐3a treatment of human fibroblasts not only decreased the secretion of certain SASP interleukins, but the collected conditioned medium was able to suppress breast cancer cell invasiveness (Wiley et al, 2018). MI‐63, a next‐generation Mdm2 inhibitor, showed similar results (Wiley et al, 2018), confirming the potential of these drugs to attenuate detrimental paracrine effects of the SASP. Many Mdm2‐MdmX inhibitors have entered clinical trials with the hope of restoring p53 function in a variety of malignancies; thus far, no drugs in this class have been approved.
Direct modulation of NF‐κB binding and activity
Metformin is a well‐tolerated drug used extensively in treatment of type 2 diabetes. It reduces NF‐κB nuclear translocation, preventing the activation of the NF‐κB pathway, and has been extensively investigated as a possible SASP modulator (Moiseeva et al, 2013). Treatment of Ras‐mutant IRM‐90 fibroblasts with metformin significantly inhibited the secretion of several SASP components, including CXCL‐5, IL‐6, IL‐8 and IL‐1β, by interfering with IKK/NF‐κB activation (Moiseeva et al, 2013). In addition, metformin activates AMPKα, resulting in mTOR signalling pathway inhibition (Sinnett‐Smith et al, 2013). Resveratrol (Pitozzi et al, 2013) and the flavonoids wogonin, kaempferol and apigenin (Lim et al, 2015; Perrott et al, 2017) are natural compounds thought to interfere with NF‐κB through their interaction with IκB kinases and effectively attenuate the SASP in specific contexts. Glucocorticoids, steroid hormones with potent anti‐inflammatory activity, can also suppress the SASP by modulating NF‐κB transcriptional activity. Treatment with cortisol and corticosterone suppressed the secretion of several SASP components, including IL‐6 and IL‐1α, impairing the ability of the SASP to stimulate breast cancer cell invasion in vitro (Laberge et al, 2012).
Modulation of the upstream regulators of C/EBPβ activity
SASP‐derived pro‐inflammatory effects are linked to JAK/STAT pathway activation, which may sustain cytokine production by activation of the transcription factor C/EBPβ (Faget et al, 2019). Treatment of irradiation‐induced senescent adipocytes with the JAK1/2 inhibitor ruxolitinib significantly reduced SASP factor secretion (Xu et al, 2015). Ruxolitinib treatment of aged mice notably diminished systemic and adipose tissue inflammation and improved mice fitness, demonstrating anti‐inflammatory potential for treatment of chronic disorders. Additionally, oncogene‐induced senescence is accompanied by dynamic fluctuation of NOTCH1 ligand activity, driving a TGF‐β‐rich secretome while suppressing the pro‐inflammatory SASP through C/EBPβ repression (Hoare et al, 2016). NOTCH1 is upregulated within NRAS‐senescent hepatocytes and negatively controls senescence immunosurveillance promoting tumorigenesis. Modulation of this pathway should be considered in potential cancer therapeutic translation.
Other ways of modulating the SASP
Other potential SASP‐associated targets have been reported, including the alternative splicing modulator polypyrimidine tract binding protein 1 (PTBP1), which regulates a pro‐inflammatory secretome. Inhibition of PTBP1 attenuated SASP‐induced tumour promotion in a mouse model of hepatocellular carcinoma (Georgilis et al, 2018). PTBP1 may therefore be a potential therapeutic target.
Targeting of specific components of the SASP known to be deleterious in certain conditions could provide more precise, potentially biomarker‐directed, therapeutic strategies, including through neutralization of well‐defined cytokines (such as IL‐1, IL‐6 or IL‐8 or their receptors) by monoclonal antibodies. Although such antibodies have been developed (van Rhee et al, 2014), their effects on senescence‐associated phenotypes remain uncertain. Intriguingly, canakinumab, a monoclonal antibody directed against IL‐1β and approved for the treatment of cryopyrin‐associated periodic syndrome and other rare autoinflammatory disorders, has been investigated in a number of more common inflammatory disorders—these disorders are linked with senescence and their true mechanism of action may lie in the SASP modulation. Of note, in the phase 3 CANTOS clinical trial for ischaemic heart disease (NCT01327846), patients treated with canakinumab had a very significantly reduced risk of being subsequently diagnosed with lung cancer (Ridker et al, 2017). Since pre‐malignant lung adenomas are characterized by accumulation of senescent cells (Collado et al, 2005), it is tempting to speculate that senescent cells are targeted specifically by canakinumab.
Immune activation to target senescent cells
The immune system plays a fundamental role in senescent cell clearance and it is thought that the age‐dependent decline of the immune system is partially responsible for the accumulation of senescent cells in tissues over time. Clearance of senescent cells is driven by CD4(+) T cells and monocytes/macrophages through a process known as senescence surveillance (reviewed in Burton & Stolzing, 2018). Natural killer (NK) cells can mediate elimination of senescent hepatic stellate cells in a model of damage‐induced liver fibrosis, resulting in less fibrotic scarring and facilitating fibrosis resolution (Krizhanovsky et al, 2008). Accordingly, administration of polyinosinic–polycytidylic acid (polyI:C; a NK Toll‐like receptor 3 [TLR3] ligand) and interferon‐γ enhances the cytotoxic activity of NK cells against activated hepatic stellate cells and ameliorates liver fibrosis (Radaeva et al, 2006). This process appears to be mediated by activation of the NK cell receptor NKG2D, which recognizes ligands on the surface of infected, damaged or stressed cells. Ligands of NKG2D are elevated in senescent cells, and NKG2D was required for NK cell‐mediated senescent cell clearance protecting against liver fibrosis (Sagiv et al, 2016).
Activation of antibody‐dependent cell‐mediated cytotoxicity (ADCC) may also preferentially eliminate senescent cells. The surface peptidase DPP4 is enriched on senescent human diploid fibroblasts versus normal cells. An anti‐DPP4 antibody triggered ADCC via NK cells, which eliminated senescent cells in vitro (Kim et al, 2017b). An oxidized form of membrane‐bound vimentin was recently described as a novel marker for senescent murine lung fibroblasts (Frescas et al, 2017). The resultant hypothesis that humoral innate immunity may recognize and target the oxidized form of vimentin in senescent fibroblasts is yet to be tested in vivo.
A variety of new interventions focused on the activation of the immune system have emerged as effective treatments for a wide variety of diseases. Since senescent cells are immunogenic, reactivation of immune cell effector mechanisms against these cells, or senescent cell manipulation to increase their immunogenicity, are attractive clinical development strategies.
Bypassing and reverting senescence
Although cell senescence was originally described as an irreversible cell cycle arrest, in vitro reactivation of cell proliferation after induction of senescence can be achieved. For example, enforced telomerase activity induces the bypass of replicative senescence without inactivation of the p16ink4a/Rb pathway or abrogation of p53 expression (Pellegrini et al, 2004). In an OIS model, IL‐6 exerts an antiproliferative effect, and its depletion allows cells to bypass OIS. This phenomenon was associated with the suppression of p15INK4B (Kuilman et al, 2008). Inhibition of the chemokine receptor CXCR2 also alleviates replicative senescence and OIS (Acosta et al, 2008). Ectopic overexpression of the polycomb group (PcG) protein CBX8, required for proliferation of diploid human and mouse fibroblasts, also allows senescence bypass and immortalizes primary MEFs through direct repression of the Ink4a‐Arf locus (Dietrich et al, 2007). In a seminal study, in vivo reprogramming of somatic cells was shown to restore their proliferative potential (Abad et al, 2013), a relevant approach for future applications in tissue repair and regenerative medicine. Interestingly, pools of cells enriched in senescent cells from centenarians were reprogrammed in vitro to pluripotent stem cells (Lapasset et al, 2011).
These studies open up the possibility of bypassing senescence therapeutically. However, such approaches should be considered with caution. A recent study showed that therapy‐induced senescent (TIS) cells can acquire both functional and phenotypic stemness features in in vivo models of lymphoma and leukaemia, developing stronger and more aggressive tumour growth potentials (Milanovic et al, 2018). In this pioneering work, lymphoma cells that escaped a previously senescent state presented a higher tumour‐initiating capacity than cells that were never senescent, and this feature was driven by the activation of the canonical Wnt signalling pathway in TIS. This experimental approach used mice carrying loss‐of‐function alleles at the Suv39h1 locus, and it remains to be seen if the required epigenetic changes associated with reversion of cellular senescence occur in nature.
Probes for diagnosis
Senescent cells have increased lysosomal content, with high levels of lysosomal β‐galactosidase, a feature used for decades to highlight senescent cells in vitro and in vivo (Sharpless & Sherr, 2015). Multiple fluorescent probes for tracking β‐galactosidase activity have been developed over the last years. Although having the potential to target senescent cells, many probes have been validated only in human cells transfected with plasmids harbouring the Escherichia coli lacZ gene, resulting in cytoplasmic overexpression of bacterial β‐galactosidase. However, this approach does not recapitulate cellular senescence, as it does not correspond with the endogenous lysosomal β‐galactosidase activity associated with senescence. Also, some probes have been tested in particular human cancer cell lines naturally expressing high levels of lysosomal β‐galactosidase (Table EV3). Here, we focus on fluorescent probes validated in bona fide senescent cells/tissues. We believe these sets of probes to be the most promising for translation to in vivo models of senescence‐related disorders and/or the accumulation of senescent cells during ageing (see Table EV3 and Fig 4).
Figure 4. Novel diagnostic and therapeutic approaches for targeting senescent cells: probes and nanoparticles.
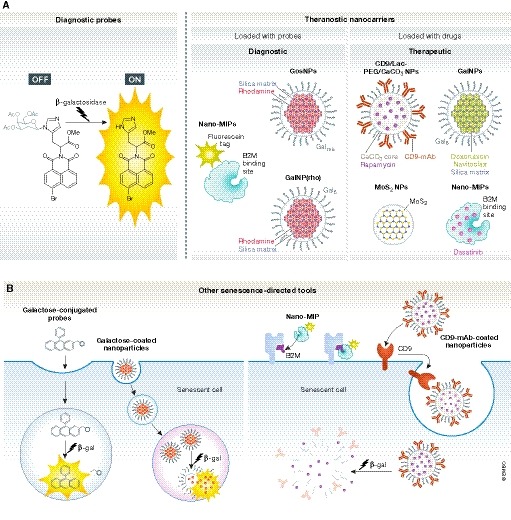
(A) Representative structural images of some of the novel tools developed for the detection and targeting of senescent cells. Diagnostic probes are either fluorescent or chromogenic and can be detected upon β‐galactosidase catalytic reaction. Nanocarriers are loaded or tagged with either fluorescent particles (such as rhodamine) or drugs/senolytics (doxorubicin, navitoclax, rapamycin) for different clinical interventions. Most of the senescence‐directed nanoparticles are coated or conjugated to galactose‐derived residues or have been designed to bind to specific receptors. (B) Tracking the β‐galactosidase activity of senescent cells is one of the commonest strategies for the development of probes and nanoparticles. The enzymatic activity cleaves galactose residues conjugated to endocytosed probes or nanoparticles and allows the release of carriers or the emission of colour/fluorescence within the lysosomal compartment. Other developed tools can bind to receptors present on the membrane to either allow the detection of senescent cells (Nano‐MIPs) or subsequently become endocytosed and processed by β‐galactosidase activity (CD9‐mAb‐coated nanoparticles). B2M, beta‐2 microglobulin; β‐gal, β‐galactosidase; GalNPs, 6‐mer galacto‐oligosaccharides‐conjugated nanoparticles; GosNPs, galacto‐oligosaccharides‐conjugated nanoparticles; MIPs, molecularly imprinted particles; NPs, nanoparticles; PEG, polyethylene glycol.
Fluorescent probes validated in vitro
The first reported ratiometric two‐photon fluorescent probe to track senescent cells in vitro, initially tested in human diploid fibroblasts (HDFs) undergoing replicative senescence, consists of a naphthalene‐based fluorescent moiety (SG1) containing a β‐D‐galactopyranoside‐derived benzyl carbamate at the β‐gal hydrolytic site and a solubilizing group (Lee et al, 2014). This probe produces a blue‐to‐yellow emission response to β‐gal and has high insensitivity to pH and reactive oxygen species, high photostability and low cytotoxicity. Gal‐Pro, another senescence‐specific fluorescent probe, was recently validated in vitro using HDFs undergoing oxidative stress‐induced senescence (Zhang et al, 2017). Gal‐Pro is based on a hemicyanine skeleton conjugated with a D‐galactose residue via a glycosidic bond displaying near‐infrared emission. The probe exhibited a rapid and sensitive turn‐on response to SAβgal in living cells, with high photostability and low background fluorescence.
Fluorescent probes validated in vivo
The fluorescent probe AHGa can trace senescent cells in vivo in mice bearing human melanoma SK‐MEL‐103 tumour xenografts treated with senescence‐inducing chemotherapy (Lozano‐Torres et al, 2017). AGHa is an OFF‐ON two‐photon fluorescent probe comprising a naphthalimide fluorophore, an L‐histidine methyl ester linker and an acetylated galactose bonded to one of the aromatic nitrogen atoms of the L‐histidine through an N‐glycosidic bond that can be hydrolysed by β‐galactosidase activity. Whereas tumours of mice treated with palbociclib (a potent senescence‐inducing CDK4/6 inhibitor) and injected intravenously with AHGa displayed a clear fluorescent signal, tumours of untreated mice showed negligible fluorescence, and the probe was not activated significantly in endogenous tissues. The combination of selectivity, sensitivity and straightforward synthesis makes AHGa an efficient and attractive OFF‐ON two‐photon probe for the in vitro and in vivo imaging of senescence. NIR‐BG is an activatable molecular probe synthesized by glycosylation of a hemicyanine dye and a protected galactosyl bromide, with far‐red excitation, near‐infrared emission and high turn‐on ratio upon SAβgal activation (Wang et al, 2019). This probe was validated in a variety of human cancer cells (HeLa and MCF7) undergoing camptothecin and/or radiation‐induced senescence, and also in mice bearing HeLa xenografted tumours undergoing chemotherapy‐induced senescence. HMRef‐βGal is a highly membrane permeable and β‐gal‐sensitive fluorescence probe, which utility was demonstrated for in vivo visualization of small tumours initiated by intraperitoneal injection of different ovarian cancer cells expressing endogenous high levels of lysosomal β‐gal (Asanuma et al, 2015). HMRef‐βGal remains untested in senescence itself. HMRef‐βGal is based on a spirocyclization strategy that uses a hydroxymethyl rhodol derivative bearing a β‐gal moiety.
Development of fluorescent probes to track β‐gal activity thus represents an innovative approach to monitor senescent cells in vivo for potential bioimaging applications. However, clinical applications of fluorescent probes are still challenged by a limited tissue penetration depth (~1 cm), which makes them more suitable for specific tissues, such as the skin. We await next‐generation probes for senescence monitoring, which may include activatable contrast agents (for MRI detection) and radiotracers (for PET detection). Galacto‐conjugation of PET radionuclides could then be an interesting approach to track senescent tissues in vivo.
Nanoparticles as diagnostic and therapeutic tools
Nanomedicine is an innovative approach for cell type‐/biomarker‐/phenotype‐specific cargo delivery. Although the technological success achieved in this field is considerable, incomplete knowledge of nano‐bio interactions and nanoparticle biodistribution in mammals, potential toxic effects, clearance pathways and challenges with scaling up the manufacturing process have impeded the translational applications and commercial development of various promising formulations. Nevertheless, their specificity in cell targeting and versatility in cargo encapsulation makes them ideally suited for modulation/elimination of senescent cells (Table EV4 and Fig 4).
The first targeted cargo delivery system for senescent cells was based on functionalized mesoporous silica nanoparticles (NPs) (Agostini et al, 2012). Here, spherical particles (~100 nm size) encapsulating rhodamine were coated with galacto‐oligosaccharides of different lengths (Gos), preventing the release of the cargo out of a silica matrix known as MCM‐41. Cellular uptake of GosNPs occurs via endocytosis, and, after fusion with lysosomal vesicles, the beads are eventually released by exocytosis. Preferential release of rhodamine in SAβgal‐positive human senescent fibroblasts from dyskeratosis congenita patients was observed, but not in (control) proliferative human NSCLC cells.
This silica bead‐based nanotechnology was recently refined by using a homogenous coating consisting of a 6‐mer galacto‐oligosaccharide (Gal) and validated in models of damage‐induced and chemotherapy‐induced senescence (Muñoz‐Espín et al, 2018). It was reported that gal‐encapsulated rhodamine, GalNP(rho), is preferentially released in a variety of human cancer cell lines (including melanoma, head and neck squamous cell carcinoma and NSCLC cells) undergoing palbociclib‐induced senescence. GalNP(rho) was selectively activated in senescent lesions in vivo, using palbociclib‐treated tumour xenografts and fibrotic lungs damaged by bleomycin. In the latter case, lung epithelial cells and fibroblasts exhibiting enriched signatures of senescence label with rhodamine preferentially upon GalNP(rho) administration when compared with control mice.
In addition to cell labels, nanoparticles can encapsulate small molecules capable of killing senescent cells, potentially widening the therapeutic window of these agents (Table EV4 and Fig 4). Gal‐encapsulated doxorubicin, GalNP(dox), induced apoptosis in palbociclib‐induced senescent cells, but not in control (proliferative) cells, thus validating its therapeutic senescence‐targeting potential (Muñoz‐Espín et al, 2018). Of note, GalNP(dox) treatment restored lung function in mice with bleomycin‐induced pulmonary fibrosis and reduced the lung fibrotic scar. In addition, GalNP(dox) promoted tumour regression in combination with palbociclib in mice bearing tumour xenografts of human squamous cell carcinoma or melanoma cells. Besides chemotherapy drugs, encapsulation of senolytics should increase their therapeutic specificity. A combination of palbociclib and galacto‐encapsulated navitoclax also reduced tumour xenograft growth, reinforcing the concept that this nanotechnology is an efficient therapeutic tool to specifically deliver drugs into senescent cells. Reassuringly, galacto‐encapsulation diminished undesirable toxicities of doxorubicin and navitoclax (cardiotoxicity and thrombocytopenia, respectively). Collectively, studies with GalNP nanocarriers provide an important and versatile advance in the ability to deliver small compounds to multiple types of senescent lesions in vivo, providing proof of concept of potential therapeutic and diagnostic applications in the clinic.
Porous calcium carbonate nanoparticles (CaCO3, ~130 nm size) loaded with rapamycin have also been used to target senescent cells (Thapa et al, 2017). CaCO3(Rapa) NPs were wrapped with a conjugate of lactose (Lac; to facilitate cargo release by lysosomal β‐galactosidase activity), and polyethylene glycol (to stabilize carriers in blood and prevent opsonization). Lac/CaCO3(Rapa) NPs were functionalized with a monoclonal antibody against CD9, a cell surface glycoprotein receptor overexpressed in senescent cells, to further promote senescent HDF targeting. Treatment of HDFs with CD9‐Lac/CaCO3(Rapa) resulted in anti‐senescence effects as defined by decreased β‐gal and p53/p21/CD9/cyclin D1 expression, reduced population doubling time, enhanced cell proliferation/migration, reduced expression the SASP components and prevention of cell cycle arrest. Calcium carbonate nanoparticles loaded with a coumarin fluorescent dye, CD9‐Lac/CaCO3(C9H6O2), exhibited high cellular uptake by senescent HDFs, indicating that this nanotechnology is also suitable for imaging senescence. In another study, pretreatment of human aortic endothelial cells with molybdenum disulphide nanoparticles (MoS2 NPs) inhibited H2O2‐induced senescence by preventing lysosomal and mitochondrial dysfunction (Ke et al, 2018). Exposure to MoS2 NPs promoted autophagy in these cells, resulting in improved endothelial cell functionality. Interestingly, another recent study shows that molecularly imprinted nanoparticles (nanoMIPs) can be designed to target epitopes of surface proteins in senescent cells, such as β2 microglobulin (B2M) in senescent EJ bladder cells (Ekpenyong‐Akiba et al, 2019). NanoMIPs were internalized after B2M‐binding and had a cytotoxic effect when loaded with the senolytic dasatinib. Fluorescently tagged nanoMIPs detected senescent cells in the abdominal cavity of naturally aged mice, while no signs of toxicity were found at a single dose.
Nanoparticles therefore offer a versatile and novel strategy for in vitro and in vivo targeting of senescent cells with potential diagnostic and therapeutic applications, including as theranostic tools, aimed at simultaneously detecting and eradicating senescent lesions associated with age and numerous human pathologies.
Targeting senescence clinically in age‐related disorders
Elimination of senescent cells can ameliorate and even reverse a variety of age‐related disorders in preclinical studies (Muñoz‐Espín & Serrano, 2014; Childs et al, 2017; Soto‐Gamez & Demaria, 2017; Ovadya & Krizhanovsky, 2018), holding exciting promises for the development of novel therapeutic strategies against these important pathologies (Fig 1). To this end, clinical trials are in progress where senescent cells are the therapeutic targets of systemically delivered small molecules (Table EV5). Encouraged by successful preclinical results, the combination of D + Q is being tested in some age‐related disorders, including chronic kidney disease, which has multiple systemic consequences (NCT02848131), idiopathic pulmonary fibrosis (NCT02874989) and in haematopoietic stem cell transplant survivors who are at increased risk of premature ageing (NCT02652052).
Most approved cancer therapeutics are designed to eliminate tumour cells by inducing apoptosis. The critical regulator of apoptosis, Bcl‐2 (target of navitoclax), is overexpressed in the majority of small cell lung cancer (SCLC) tumours (Ikegaki et al, 1994). Although correlative analyses suggested several putative biomarkers of clinical benefit, navitoclax showed limited efficacy as a single agent in advanced and recurrent SCLC (Rudin et al, 2012), suggesting these tumours are not sensitive at steady state and do not have high senescent cell burden, or that sufficient senolytic concentrations were not achieved. Besides promoting apoptosis, many of the standard chemotherapies and targeted therapies used in the clinic can also induce senescence in tumour cells (Ewald et al, 2010; Petrova et al, 2016), which may be important for at least a portion of therapeutic resistance. TIS translates into slower proliferation rates, but the SASP produced by senescent cells can potentially promote an invasive phenotype and an increased growth in neighbouring non‐senescent tumour cells (Pérez‐Mancera et al, 2014; Gonzalez‐Meljem et al, 2018). Combining standard senescence‐inducing chemotherapies with senolytic agents therefore represents an attractive approach for treating solid tumours. Several phase 1 and phase 1/2 clinical trials combining senescence‐inducing chemotherapies or targeted therapies with navitoclax are ongoing or completed, including with cisplatin, etoposide and navitoclax in SCLC patients (NCT00878449), dabrafenib, trametinib and navitoclax treating patients with BRAF mutant metastatic melanoma (NCT01989585), and osimertinib and navitoclax in patients with EGFR‐positive advanced NSCLC (NCT02520778). Navitoclax is also combined with gemcitabine (NCT00887757), paclitaxel (NCT00891605), docetaxel (NCT00888108), irinotecan (NCT01009073), erlotinib (NCT01009073), sorafenib (NCT02143401) or trametinib (NCT02079740) in advanced solid tumours. Two trials from the last group have already published results in small subsets of patients with varying solid tumours. A total of forty‐six patients were treated with gemcitabine and a dose escalation of navitoclax (NCT00887757), demonstrating good tolerability and safety for the combination, but no objective responses were observed (Cleary et al, 2014). A total of eleven patients received the combination of navitoclax and the tyrosine kinase inhibitor erlotinib (NCT01009073), which was also well tolerated but did not result in any objective responses (Tolcher et al, 2015). Another phase 1 study combining navitoclax with carboplatin/paclitaxel in nineteen patients with solid tumours showed modest efficacy (Vlahovic et al, 2014).
In these cases, based on heterogeneous cancer‐type patient cohorts, analyses were exploratory, and hence, conclusions on the efficacy should be taken with caution. It is not clear if objective responses were not observed because (i) the tested chemotherapies did not induce senescence in these patients; (ii) senolytics did not achieve sufficient intratumoural concentrations; (iii) senolytics were ineffective at killing senescent cells; (iv) cancer‐type specificities were not representative of preclinical models; or (v) a combination of these/other factors. It is, however, imperative to persist in the approach: future studies should explore promising targeting agents where senescence induction is known to be robust in human patients with specific cancer types well represented by appropriate preclinical models. Embedding translational science into these trial protocols, with the analysis of senolytic effects on pre‐ and post‐treatment biopsies, or the use of senescent cell imaging approaches, should help refine strategies and direct them to patients most likely to benefit.
Age‐related immune senescent remodelling is likely to contribute to the decline of the immune system, chronic inflammatory state, risk for frailty, chronic disease and functional decline in older individuals (Akbar et al, 2016). Patients with activated PI3K Delta syndrome present a dominant mutation in the PI3K catalytic subunit p110δ, resulting in T‐cell senescence and immunodeficiency (Lucas et al, 2014). Administration of a selective PI3Kδ inhibitor leniolisib (CDZ173) showed a reduction of senescent T cells and a decrease in inflammatory markers in a trial involving six patients (NCT02435173), and an extension study is ongoing (NCT02859727).
Treatment of mice with metformin is associated with a reduction in oxidative stress and inflammation, resulting in extension of lifespan and healthspan (Barzilai et al, 2016). Three clinical trials in older patients are evaluating the premise that metformin may be an effective “anti‐ageing” drug (NCT02325245, NCT02570672 and NCT03451006), with changes in frailty indices as their primary outcome measures. A fourth clinical trial (NCT02432287) tests the hypothesis that metformin will result in changes in the transcriptome, reverting the expression profiles of older adults with impaired glucose intolerance towards those seen in younger patients, in muscle and adipose tissue. A phase 3 clinical trial (NCT03309007) aims to investigate the effects of a short treatment with metformin on cellular senescence and autophagy in older adults with pre‐diabetes. Confirming initial hypotheses of improvements seen in autophagy and senescence would justify starting further clinical trials with metformin as an anti‐ageing therapy, but these should ideally be in disease‐specific populations with objective, clinically relevant endpoints.
A phase 2 clinical trial (NCT02874924) is measuring effects of rapamycin (sirolimus) in patients over 70 years old on general parameters of immune health, including levels of inflammatory serum cytokines and polyclonal T‐cell activation. Secondary outcomes include improvement in physical, cognitive and cardiovascular functions. A phase 1 clinical trial (NCT01649960) involving low‐dose rapamycin in older adults with coronary artery disease has already been completed, with the assessment of frailty by physical performance as a primary outcome, and the analysis of the SASP and quantification of the levels of senescent preadipocytes as secondary outcomes. Other approaches are in development, including a further trial (NCT03353597) evaluating the effects of monthly plasma transfusions of young healthy male donors to older subjects (> 40) in order to reverse epigenetic and other markers of senescence. The outcomes of the study are the assessment of cell DNA methylation levels to calculate an epigenetic age, as well as detection of changes in cognitive, renal and pulmonary function, muscle strength, telomere length and expression levels of IGF‐1 and p16INK4a in blood and skin biopsies.
Despite the increasing prevalence and societal burden of age‐related diseases, trials targeting senescence are still relatively few in number, and we await an objective initial proof of principle. It is important that we reassess the best way to translate promising preclinical observations into well‐designed translational approaches and effective therapeutic strategies. Importantly, a number of Biotechnology Companies are now developing translational activities in the field of senescence including, among others: Unity Biotechnology (Bcl‐2 inhibitors), Senolytic Therapeutics (NPs to target SAβgal‐positive cells), Oisin biotechnologies (NPs to target p16‐positive cells), Antoxerene (FOXO4 peptides), CellAge (multigenic senescence signatures), and Everon Biosciences and Siewa Therapeutics (senescence immunotherapy strategies).
Future perspectives
This review recapitulates a collection of innovative approaches to manipulate and trace cellular senescence, including some already tested and validated in animal models of human pathologies and a number of ongoing studies in humans. Successful clinical translation of strategies targeting senescent cells may have a significant impact on the treatment of multiple human age‐related disorders, as well as increase lifespan and healthspan by delaying the chronological ageing of damaged tissues and organs. Nevertheless, several important challenges must be considered to optimize translation of senescence‐targeted therapeutic strategies to the clinic.
Human cell cultures have provided considerable data on senotherapies (see Tables EV1 and EV4) and senoprobes (see Table EV3). Approaches have employed a variety of senescence‐inducing stressors, different cell types and different regulatory mechanisms. However, most approaches have excluded the complex 3D microenvironment of diseased tissues in living organisms, and their relevance for translation to human diseases remains therefore unclear. The method used to induce senescence (replicative stress, oncogene activation, damaging compounds, irradiation, carcinogens, etc.) strongly determines the key drivers and the signalling pathways involved, a process that is also critically dependent on the cellular type and tissue of origin (Salama et al, 2014). Senescent cells in culture are a highly heterogeneous population, and these subpopulations consequently may present different vulnerabilities to senotherapies and senoprobes. It is therefore important that we ask the right clinical question (e.g. Does drug X enhance the efficacy of drug Y in condition Z?) in the right model that is as accurate a representation of the human condition as possible.
Despite major advances, translation of senescence targeting to the clinic should be approached with caution, since senescence plays both beneficial and detrimental roles depending on the pathological context (Muñoz‐Espín & Serrano, 2014) and it is crucial that we examine this thoroughly in appropriate preclinical models. It is well known that cell senescence prevents the expansion of damaged and pre‐malignant cells in a cell autonomous manner, and hence, it is an important barrier against tumorigenesis (Collado & Serrano, 2010). This correlates with the observation that, among other senescence‐related genes, the vast majority of human cancer cells accumulate mutations in the p53‐p21 and/or the p16‐Rb axis (Vergel & Carnero, 2010). A relevant concern is that bypassing or reversing senescence could indeed promote tumorigenesis (Krimpenfort et al, 2001). Accordingly, there is evidence that particular populations of therapy‐induced senescent cancer cells can acquire phenotypic and functional stemness features, resulting in cell cycle re‐entry, self‐renewal capacity and a more aggressive tumour phenotype (Milanovic et al, 2018). This is less critically important in the context of attempts to improve treatments for patients with advanced cancer where more risk is often ethically acceptable, but nonetheless this requires close interrogation in preclinical models.
Besides cell autonomous roles, the SASP is crucially implicated in the recruitment of T cells and macrophages facilitating immunosurveillance in liver precancerous lesions (Burton & Stolzing, 2018) although the senescent secretome may also be endowed with an intrinsic potential to promote chronic inflammation and tumour progression (Gonzalez‐Meljem et al, 2018). It is known that the SASP facilitates tissue repair in disorders caused by severe damage and injury. This is the case for senescent activated stellate cells, capable of limiting liver fibrosis by a reduced secretion of extracellular matrix components, enhanced secretion of extracellular matrix degrading enzymes and increased immunosurveillance (Krizhanovsky et al, 2008). In addition, senescent fibroblasts accumulate in granulation tissues of healing cutaneous wounds and express antifibrotic genes (Jun & Lau, 2010). Senescence, however, intriguingly mediates fibrotic pulmonary disease, and removal of senescent cells using senolytics, therapeutic nanoparticles and anti‐inflammatory compounds reverts fibrosis and improves lung function in mice (Hecker et al, 2014; Lehmann et al, 2017; Schafer et al, 2017; Muñoz‐Espín et al, 2018). The antagonistic roles of cell senescence in a number of mouse models of human fibrotic diseases highlight the importance of a more detailed understanding of the triggers, intrinsic mechanisms and pathways driving the stable cell cycle arrest and distinct factors secreted by senescent cells before widespread clinical trials, particularly in otherwise healthy populations. Therefore, the development of appropriate and more accurate animal disease models capable of ensuring a causative role of senescence and specific beneficial effects of its manipulation is still a priority before definitively moving into clinical development, with judicious assessment of predicted beneficial/toxic effects of investigational strategies. Moreover, due to significant differences between humans and mice, preclinical studies will require a more detailed characterization of the peculiarities and mechanisms of action of senescence in patients, along with a more extensive validation of senotherapies and senoprobes by using specimens of human tissue ex vivo and xenografts in mice (with fully humanized immune systems), to prioritize the most promising disease‐specific candidates for clinical application.
The need to develop specific biomarkers of senescent cells remains a limiting factor for efficient translation of strategies targeting senescence. SASP components and the associated immunoregulation could provide tools for the selection of patients (in a biomarker‐directed manner) and for assessing sensitivity or resistance to senotherapies (and to help drive further clinical development of on‐target, pharmacodynamically active therapeutics). These include screening, diagnostic, monitoring, prognosis and pharmacodynamic tools. In the case of senotherapies, pharmacodynamic biomarkers are keys in measuring the on‐target responses and are crucial for dose optimization studies. Unfortunately, there is currently no universal marker for cellular senescence available (Sharpless & Sherr, 2015), and quantitative methods in basic research have been limited to cytochemical protocols and flow cytometry analyses (Debacq‐Chainiaux et al, 2009; Biran et al, 2017). One of the first methodologies to image and monitor senescence noninvasively in vivo was based on a sequential reporter‐enzyme bioluminescence technology to track β‐gal activity by using Lugal, a caged galactoside‐luciferin conjugate (Wehrman et al, 2006). Recent studies validated the use of nanoparticles and fluorescent probes to target senescent cells in mouse models (Lozano‐Torres et al, 2017; Muñoz‐Espín et al, 2018; Wang et al, 2019). Nanoparticles open up the possibility of encapsulating tracers and contrast agents for the imaging of senescence location and burden, and to be used as imaging biomarkers to direct promising therapies to populations most likely to benefit from them. This will however require the adaptation of current preclinical tools to deep tissue penetration bioimaging techniques. For example, diagnostic GalNPs could release gadolinium or positron‐emitting radioisotopes in senescent lesions to allow detection by MRI or PET, respectively. Gal‐encapsulation methods and senoprobes could also serve to monitor the response of solid tumours to the administration of senescence‐inducing chemotherapies, or the senescence burden in patients with senescence‐associated disorders, before and after senotherapy to provide a pharmacodynamic biomarker of response. In addition to cancer and other chronic pathologies, cellular senescence is a defining feature of a wide variety of human pre‐malignant lesions (Collado & Serrano, 2010). Based on this, it is tempting to speculate that GalNPs and senoprobes could be utilized in the early diagnosis of pre‐malignant tumours. Senescence‐specific NPs could also potentially be employed as theranostic tools, aimed at the simultaneous detection and eradication of senescent lesions associated with numerous pathologies, or during ageing. The latter would likely require regular, intermittent interventions to eliminate senescent cells or to manipulate a damaging SASP, while minimizing off‐target effects in normal cells and on‐target manipulation of beneficial senescent cells.
Beneficial senescent cells are plentiful, but biomarkers and drug sensitivities that might distinguish these from pathological senescent cells are lacking. Cellular senescence contributes centrally to the physiological processes of (i) repair, facilitating wound healing through secretion of platelet‐derived growth factor AA (Demaria et al, 2014); (ii) regeneration, promoting tissue reprogramming of nearby cells in the context of injury and ageing (Mosteiro et al, 2016; Chiche et al, 2017; Ritschka et al, 2017) including limb regrowth in salamanders (Yun et al, 2015); and (iii) embryonic development, playing an active role in tissue remodelling and morphogenesis (Muñoz‐Espín et al, 2013; Storer et al, 2013). The potential targeting of beneficial senescent cells remains unexplored in the context of senotherapies and senoprobes, not helped by the short lifespans of the widely used animal models. Manipulation of cell senescence during these processes may compromise patient health. In this regard, there are several possible strategies to overcome this problem and optimize selectivity. An important approach will be the development of second generation versions of pharmacologically active compounds and probes already tested in preclinical studies but which target pathological senescent cells specifically, exploiting synthetic lethal vulnerabilities. Another emerging possibility to increase specificity is to modify the therapeutic or diagnostic agent to be activated by an external stimulus or enzymatic reaction. This is the case for galactose‐based nanoparticles, encapsulation methods and probes (Agostini et al, 2012; Lozano‐Torres et al, 2017; Thapa et al, 2017; Muñoz‐Espín et al, 2018), which are preferentially activated by the increased lysosomal β‐gal function of senescent cells. Alternatively, accumulation of SASP components and enzymes in the senescent intercellular space might also be employed to stimulate drug delivery systems and inactive pro‐senolytics, or activatable probes, for imaging. Ideally, synthetically lethal compounds would be loaded into nanocarriers targeting senescent (or even pathologically senescent) cells, thereby extensively widening their therapeutic windows. This will require a more detailed information of the diseased cell‐specific signalling pathways and vulnerabilities at the mechanistic level.
In the absence of such data, we should still move forward with the knowledge we have. More refined BCL‐2 family inhibitors capable of preventing dose‐limiting toxicities, such as thrombocytopenia and neutropenia (Cang et al, 2015) should be tested. In the case of nanomedicine, translational applications potentially have significant long‐term safety concerns, and it is important to understand the cellular uptake and intracellular trafficking of specific NPs, biocompatibility and biodistribution properties, PK/PD analyses and routes of elimination of the core materials, which heavily rely on their physicochemical properties. For instance, opsonin binding can trigger recognition and clearance by the mononuclear phagocyte system and accumulation of NPs in liver and spleen (Mahmoudi et al, 2011), with the potential for sequestration and long‐term toxicity.
Other alternatives might include strategies based on the administration of combinations of senotherapies (such as mTOR inhibitors with other senescence modulating drugs (Han et al, 2015), potentially reducing required doses of each agent and off‐target effects), or the use of direct routes of administration to directly target the senescent tissue or organ and reduce exposure of non‐targeted tissues. Examples include inhalation of aerosols for pulmonary drug delivery, or preparations of injectable formulations of senotherapies for in situ treatments. Current studies are focusing on the identification of specific epitopes, proteins and surface receptors in senescent cells, which would facilitate the design of antibody‐based therapeutic or diagnostic formulations with increased selectivity and reduced side effects. Some of the identified potential markers, such as DEP1 and B2MG (Althubiti et al, 2014), or DCR2 (Collado et al, 2005), although overexpressed in senescent cells are also present in other cells and damaged tissues. A possible target is surface DPP4, which is preferentially expressed in senescent but not proliferating human diploid fibroblasts (Kim et al, 2017b). A recent in vitro study employs CD9 receptors in combination with increased SAβgal activity, for dual NP targeting and drug delivery in senescent cells (Thapa et al, 2017), but further studies in vivo are required to demonstrate the translational applications of these approaches. Short or intermittent exposures to senotherapies may also decrease their potential of driving adverse effects while maintaining their therapeutic benefits. This might be particularly relevant in treatments aimed at maintaining tissue homeostasis and function, and to delay ageing. However, it remains unclear how to justify preventative senotherapies outside the context of disease. Extensive preclinical testing will have to be completed before moving clinical trials towards otherwise healthy populations.
Besides the aforementioned strategies to manipulate cell senescence, other emerging features of senescent cells may be used to develop novel therapeutics, biomarkers and/or diagnostic tools in the near future. Senescent cells and SASP factors are accompanied by a significantly increased release of extracellular vesicles and exosomes (Lehmann et al, 2008) containing proteins, lipids and microRNAs, affecting nearby tissue and potentially having relevant roles in immune regulation (Xu & Tahara, 2013; Urbanelli et al, 2016; Borghesan et al, 2019). Small exosome‐like extracellular vesicles can be important mediators of the pro‐tumorigenic functions of the senescent secretome. This process involves extracellular vesicle‐associated EphA2 secreted from senescent cells, which binds to ephrin‐A1 that is highly expressed in several cancer cell types and promotes proliferation (Takasugi et al, 2017). Exosomes released by senescent prostate cancer cells subjected to radiation therapy were enriched in B7‐H3 protein, an immune checkpoint ligand. It is tempting to speculate that manipulation and identification of extracellular vesicles secreted by senescent cells might be used in (immuno)therapeutic approaches. Another interesting alternative to modulate the SASP and the immune response could be through modulation of the cGAS‐cGAMP‐STING signalling pathway. This pathway detects cytoplasmic chromatin fragments in response to DNA damage in senescent cells, activates type I interferons and other cytokines, and mediates autoinflammatory diseases (Li & Chen, 2018). Cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthase (cGAS) and the adaptor protein STING are key drivers of the senescent secretome in primary human cells and in mice (Dou et al, 2017; Glück et al, 2017; Yang et al, 2017). Mice deficient in cGAS and STING show impaired immunosurveillance of oncogenic RAS and reduced tissue inflammation after ionizing radiation. Furthermore, this pathway is activated in cancer cells and correlates with pro‐inflammatory gene expression in human cancers (Li & Chen, 2018). It is therefore reasonable to explore novel therapeutic and/or diagnostic strategies based on the manipulation of the cGAS‐cGAMP‐STING signalling pathway. Several small‐molecule antagonists of STING with efficacy in the treatment of autoinflammatory disease in mice have already been characterized (Haag et al, 2018).
The wealth of potential therapeutic targets that senescence presents in disease are myriad. However, our study of the roles and pathways of senescence in specific pathologies is much less advanced. We advocate translational studies to elucidate these in the human disease of interest, with appropriate preclinical validation of putative therapeutic strategies in appropriate models before the strategies predicted to be most safe and efficacious are considered for clinical trials.
Concluding remarks
Global populations continue to age, increasing the prevalence of chronic age‐related pathologies, and producing an associated public health epidemic. Senescent cells accumulate during ageing, causing tissue dysfunction, and are associated with a wide variety of age‐related disorders, both in humans and animal models. Preclinical studies have convincingly concluded that the elimination of senescent cells can ameliorate and even revert the pathological manifestations of multiple disorders in mice (Fig 1). Many challenges must be overcome to ensure successful translational application of strategies. These include the need for: (i) a better understanding of the triggers and signalling pathways driving the senescent arrest and the different secretomes in the context of a particular disease; (ii) the development of more advanced and clinically relevant animal models capable of distinguishing on‐target from off‐target effects for each condition (Kirkland & Tchkonia, 2015); (iii) an increase in the selectivity of senotherapies or senoprobes, to reduce known and potential toxicities; (iv) solid experimental data concerning the biodistribution properties, PK/PD modelling and routes of elimination of nanocarriers; and (v) the optimization of the therapeutic/diagnostic window of free/encapsulated senolytic agents/combinations. Owing to the heterogeneity of patients and the complexity and multifactorial nature of ageing and age‐related disorders, it is likely that future interventions against cellular senescence will be biomarker‐ and context‐dependent (personalized), where the risk–benefit ratio can be more clearly understood. While surprisingly few early‐phase clinical studies on senotherapies have been initiated, we expect this to balloon in coming years and hope that these are designed prudently. We are entering an exciting era, where we will be able to move anti‐senescent therapies towards medical applications, a strategy that may have important impacts on precision medicine, healing, tissue repair, regeneration and, ultimately, on human longevity.
Pending issues.
-
(i)
A more detailed knowledge of the cell‐specific triggers and signalling pathways driving different senescent programmes and secretomes and how they correlate with distinct age‐related disorders. In particular, by analysing human samples and not only by the use of animal tissues.
-
(ii)
Identification of specific cell membrane/intracellular/extracellular markers/targets of senescent cells, in order to develop next‐generation senolytics, senoprobes or nanoparticles with increased selectivity and reduced off‐target effects.
-
(iii)
Development of more clinically relevant animal models recapitulating age‐related human diseases.
-
(iv)
Optimization of therapeutic/diagnostic doses of agents targeting senescence, with preclinical examination of biodistribution, PK/PD, toxicity and safety aspects in animal models.
-
(v)
Evaluation of the first human early‐phase clinical trials to provide proof of principle of the pharmaceutical impact of senotherapies on age‐related disorders and ageing.
Conflict of interest
The authors declare that they have no conflict of interest.
For more information
Supporting information
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Acknowledgements
We thank Dr Manuel Serrano (Institute for Research in Biomedicine, Barcelona), Dr Masashi Narita (CRUK Cambridge Institute), Dr Zhenguang Zhang (Department of Oncology, University of Cambridge) and Andrew Baker (Department of Chemical Engineering and Biotechnology, University of Cambridge) for critical reading of the manuscript. We also thank Helena Ariño Bassols (BioIllustra Scientific Illustration) for the initial design of the accompanying figures. The Munoz‐Espin's laboratory is supported by the Cancer Research UK (CRUK) Cambridge Centre Early Detection Programme, by a CRUK Early Detection OHSU Project Award, by a Medical Research Council (MRC) New Investigator Research Grant (NIRG) and by a Royal Society Research Grant. E.G.‐G. is holder of a “La Caixa” Foundation Scholarship for postgraduate studies at European Universities.
EMBO Mol Med (2019) 11: e10234
See the Glossary for abbreviations used in this article.
References
- Abad M, Mosteiro L, Pantoja C, Cañamero M, Rayon T, Ors I, Graña O, Megías D, Domínguez O, Martínez D et al (2013) Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502: 340–345 [DOI] [PubMed] [Google Scholar]
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Agostini A, Mondragõn L, Bernardos A, Martínez‐Máñez R, Dolores Marcos M, Sancenõn F, Soto J, Costero A, Manguan‐García C, Perona R et al (2012) Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew Chem Int Ed 51: 10556–10560 [DOI] [PubMed] [Google Scholar]
- Akbar AN, Henson SM, Lanna A (2016) Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol 37: 866–876 [DOI] [PubMed] [Google Scholar]
- Alimbetov D, Davis T, Brook AJC, Cox LS, Faragher RGA, Nurgozhin T, Zhumadilov Z, Kipling D (2016) Suppression of the senescence‐associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 17: 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althubiti M, Lezina L, Carrera S, Jukes‐Jones R, Giblett SM, Antonov A, Barlev N, Saldanha GS, Pritchard CA, Cain K et al (2014) Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis 5: e1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anestakis D, Petanidis S, Kalyvas S, Nday CM, Tsave O, Kioseoglou E, Salifoglou A (2015) Mechanisms and applications of interleukins in cancer immunotherapy. Int J Mol Sci 16: 1691–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma D, Sakabe M, Kamiya M, Yamamoto K, Hiratake J, Ogawa M, Kosaka N, Choyke PL, Nagano T, Kobayashi H et al (2015) Sensitive β‐galactosidase‐targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo . Nat Commun 6: 6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA et al (2017) Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169: 132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, Lebrasseur NK, Childs BG, Van De Sluis B, Kirkland JL, Van Deursen JM (2011) Clearance of p16 Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature 479: 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong JA, Saltness R, Jeganathan KB, Verzosa GC, Pezeshki A et al (2016) Naturally occurring p16 Ink4a‐positive cells shorten healthy lifespan. Nature 530: 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016) Metformin as a tool to target aging. Cell Metab 23: 1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V (2017) Quantitative identification of senescent cells in aging and disease. Aging Cell 16: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesan M, Fafián‐Labora J, Eleftheriadou O, Carpintero‐Fernández P, Paez‐Ribes M, Vizcay‐Barrena G, Swisa A, Kolodkin‐Gal D, Ximénez‐Embún P, Lowe R et al (2019) Small extracellular vesicles are key regulators of non‐cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep 27: 3956–3971.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DGA, Stolzing A (2018) Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev 43: 17–25 [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau‐dependent pathology and cognitive decline. Nature 562: 578–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang S, Iragavarapu C, Savooji J, Song Y, Liu D (2015) ABT‐199 (venetoclax) and BCL‐2 inhibitors in clinical development. J Hematol Oncol 8: 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge R‐M, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W et al (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche A, Le Roux I, von Joest M, Sakai H, Aguín SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P et al (2017) Injury‐induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20: 407–414 [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15: 1139–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, Van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354: 472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, Van Deursen JM (2017) Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16: 718–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JM, Lima CMSR, Hurwitz HI, Montero AJ, Franklin C, Yang J, Graham A, Busman T, Mabry M, Holen K et al (2014) A phase I clinical trial of navitoclax, a targeted high‐affinity Bcl‐2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Invest New Drugs 32: 937–945 [DOI] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M et al (2005) Tumour biology: senescence in premalignant tumours. Nature 436: 642 [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M (2010) Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 10: 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J‐P, Rodier F, Patil CK, Freund A, Desprez P‐Y, Campisi J (2011) Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem 286: 36396–36403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq‐Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence‐associated beta‐galactosidase (SA‐betagal) activity, a biomarker of senescent cells in culture and in vivo . Nat Protoc 4: 1798–1806 [DOI] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, VanSteeg H, Dollé MET et al (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF‐AA. Dev Cell 31: 722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, O'Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM et al (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7: 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen JM (2014) The role of senescent cells in ageing. Nature 509: 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Tergaonkar V, Lane DP (2008) Double‐edged swords as cancer therapeutics: simultaneously targeting p53 and NF‐kappaB pathways. Nat Rev Drug Discov 7: 1031–1040 [DOI] [PubMed] [Google Scholar]
- Dietrich N, Bracken AP, Trinh E, Schjerling CK, Koseki H, Rappsilber J, Helin K, Hansen KH (2007) Bypass of senescence by the polycomb group protein CBX8 through direct binding to the INK4A‐ARF locus. EMBO J 26: 1637–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Däbritz JHM, Lisec J, Lenze D, Gerhardt A, Schleicher K et al (2013) Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501: 421–425 [DOI] [PubMed] [Google Scholar]
- Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z et al (2017) Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550: 402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpenyong‐Akiba AE, Canfarotta F, Abd HB, Poblocka M, Casulleras M, Castilla‐Vallmanya L, Kocsis‐Fodor G, Kelly ME, Janus J, Althubiti M et al (2019) Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horizons 4: 757–768 [Google Scholar]
- Ewald JA, Desotelle JA, Wilding G, Jarrard DF (2010) Therapy‐induced senescence in cancer. J Natl Cancer Inst 102: 1536–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget DV, Ren Q, Stewart SA (2019) Unmasking senescence: context‐dependent effects of SASP in cancer. Nat Rev Cancer 19: 439–453 [DOI] [PubMed] [Google Scholar]
- Frescas D, Roux CM, Aygun‐Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, Strom E, Virtuoso LP, Wrobel M, Osterman AL et al (2017) Senescent cells expose and secrete an oxidized form of membrane‐bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci USA 114: E1668–E1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil CK, Campisi J (2011) p38MAPK is a novel DNA damage response‐independent regulator of the senescence‐associated secretory phenotype. EMBO J 30: 1536–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann‐Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A et al (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8: 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgilis A, Klotz S, Hanley CJ, Herranz N, Weirich B, Morancho B, Leote AC, D'Artista L, Gallage S, Seehawer M et al (2018) PTBP1‐mediated alternative splicing regulates the inflammatory secretome and the pro‐tumorigenic effects of senescent cells. Cancer Cell 34: 85–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glück S, Guey B, Gulen MF, Wolter K, Kang T‐W, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A (2017) Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Meljem JM, Apps JR, Fraser HC, Martinez‐Barbera JP (2018) Paracrine roles of cellular senescence in promoting tumourigenesis. Br J Cancer 118: 1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, Wolter K, Pombo J, Irvine EE, Innes AJ et al (2019) Cardiac glycosides are broad‐spectrum senolytics. Nat Metab 1: 1074–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, Heymann M, van der Goot FG, Turcatti G, Behrendt R et al (2018) Targeting STING with covalent small‐molecule inhibitors. Nature 559: 269–273 [DOI] [PubMed] [Google Scholar]
- Han B, Park D, Li R, Xie M, Owonikoko TK, Zhang G, Sica GL, Ding C, Zhou J, Magis AT et al (2015) Small‐molecule Bcl2 BH4 antagonist for lung cancer therapy. Cancer Cell 27: 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ (2014) Reversal of persistent fibrosis in aging by targeting Nox4‐Nrf2 redox imbalance. Sci Transl Med 6: 231ra47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A et al (2015) mTOR regulates MAPKAPK2 translation to control the senescence‐associated secretory phenotype. Nat Cell Biol 17: 1205–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R et al (2016) NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18: 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DS, Angelo LS, Kurzrock R (2007) Interleukin‐6 and its receptor in cancer: implications for translational therapeutics. Cancer 110: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Hou J, Cui C, Kim S, Sung C, Choi C (2018) Ginsenoside F1 suppresses astrocytic senescence‐associated secretory phenotype. Chem Biol Interact 283: 75–83 [DOI] [PubMed] [Google Scholar]
- Ikegaki N, Katsumata M, Minna J, Tsujimoto Y (1994) Expression of bcl‐2 in small cell lung carcinoma cells. Cancer Res 54: 6–8 [PubMed] [Google Scholar]
- Imrali A, Mao X, Yeste‐Velasco M, Shamash J, Lu Y (2016) Rapamycin inhibits prostate cancer cell growth through cyclin D1 and enhances the cytotoxic efficacy of cisplatin. Am J Cancer Res 6: 1772–1784 [PMC free article] [PubMed] [Google Scholar]
- Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N et al (2017) Local clearance of senescent cells attenuates the development of post‐traumatic osteoarthritis and creates a pro‐regenerative environment. Nat Med 23: 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J‐I, Lau LF (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HT, Park JT, Choi K, Kim Y, Choi HJC, Jung CW, Lee Y‐S, Park SC (2017) Chemical screening identifies ATM as a target for alleviating senescence. Nat Chem Biol 13: 616–623 [DOI] [PubMed] [Google Scholar]
- Ke S, Lai Y, Zhou T, Li L, Wang Y, Ren L, Ye S (2018) Molybdenum disulfide nanoparticles resist oxidative stress‐mediated impairment of autophagic flux and mitigate endothelial cell senescence and angiogenic dysfunctions. ACS Biomater Sci Eng 4: 663–674 [DOI] [PubMed] [Google Scholar]
- Kim H‐N, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O'Brien CA, Jilka RL, Zhou D, Almeida M (2017a) DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell 16: 693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE, Basu SK, Ohnuma K, Morimoto C, Johnson PF et al (2017b) Identification of senescent cell surface targetable protein DPP4. Genes Dev 31: 1529–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T (2015) Clinical strategies and animal models for developing senolytic agents. Exp Gerontol 68: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A (2001) Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413: 83–86 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS (2008) Oncogene‐induced senescence relayed by an interleukin‐dependent inflammatory network. Cell 133: 1019–1031 [DOI] [PubMed] [Google Scholar]
- Laberge R‐M, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PLJ, Liu S, Demaria M, Cong Y‐S, Kapahi P et al (2012) Glucocorticoids suppress selected components of the senescence‐associated secretory phenotype. Aging Cell 11: 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge R‐M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson‐Edell KA, Liu S et al (2015) MTOR regulates the pro‐tumorigenic senescence‐associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17: 1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA (2013) Rapalogs and mTOR inhibitors as anti‐aging therapeutics. J Clin Invest 123: 980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït‐Hamou N, Leschik J, Pellestor F, Ramirez J‐M, De Vos J et al (2011) Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 25: 2248–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Heo CH, Sen D, Byun HO, Kwak IH, Yoon G, Kim HM (2014) Ratiometric two‐photon fluorescent probe for quantitative detection of β‐galactosidase activity in senescent cells. Anal Chem 86: 10001–10005 [DOI] [PubMed] [Google Scholar]
- Lee S, Schmitt CA (2019) The dynamic nature of senescence in cancer. Nat Cell Biol 21: 94–101 [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM (2008) Senescence‐associated exosome release from human prostate cancer cells. Cancer Res 68: 7864–7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Korfei M, Mutze K, Klee S, Skronska‐Wasek W, Alsafadi HN, Ota C, Costa R, Schiller HB, Lindner M et al (2017) Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo . Eur Respir J 50: 1602367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Demidenko ZN, Blagosklonny MV (2015) Dual mTORC1/C2 inhibitors suppress cellular geroconversion (a senescence program). Oncotarget 6: 23238–23248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, Belmont LD, Nimmer P, Xiao Y, Ma XM et al (2015) Exploiting selective BCL‐2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 7: 279ra40 [DOI] [PubMed] [Google Scholar]
- Li T, Chen ZJ (2018) The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 215: 1287–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Park H, Kim HP (2015) Effects of flavonoids on senescence‐associated secretory phenotype formation from bleomycin‐induced senescence in BJ fibroblasts. Biochem Pharmacol 96: 337–348 [DOI] [PubMed] [Google Scholar]
- Liu Z, Wild C, Ding Y, Ye N, Chen H, Wold EA, Zhou J (2016) BH4 domain of Bcl‐2 as a novel target for cancer therapy. Drug Discov Today 21: 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano‐Torres B, Galiana I, Rovira M, Garrido E, Chaib S, Bernardos A, Muñoz‐Espín D, Serrano M, Martínez‐Máñez R, Sancenón F (2017) An OFF‐ON two‐photon fluorescent probe for tracking cell senescence in vivo . J Am Chem Soc 139: 8808–8811 [DOI] [PubMed] [Google Scholar]
- Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M et al (2014) Dominant‐activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol 15: 88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S (2011) Protein‐nanoparticle interactions: opportunities and challenges. Chem Rev 111: 5610–5637 [DOI] [PubMed] [Google Scholar]
- Milanovic M, Fan DNY, Belenki D, Däbritz JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA et al (2018) Senescence‐associated reprogramming promotes cancer stemness. Nature 553: 96–100 [DOI] [PubMed] [Google Scholar]
- Moiseeva O, Deschênes‐Simard X, St‐Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN, Ferbeyre G (2013) Metformin inhibits the senescence‐associated secretory phenotype by interfering with IKK/NF‐κB activation. Aging Cell 12: 489–498 [DOI] [PubMed] [Google Scholar]
- Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, Fernandez‐Marcos PJ, Muñoz‐Martin M, Blanco‐Aparicio C, Pastor J et al (2016) Tissue damage and senescence provide critical signals for cellular reprogramming in vivo . Science 354: aaf445 [DOI] [PubMed] [Google Scholar]
- Muñoz‐Espín D, Cañamero M, Maraver A, Gómez‐López G, Contreras J, Murillo‐Cuesta S, Rodríguez‐Baeza A, Varela‐Nieto I, Ruberte J, Collado M et al (2013) Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118 [DOI] [PubMed] [Google Scholar]
- Muñoz‐Espín D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15: 482–496 [DOI] [PubMed] [Google Scholar]
- Muñoz‐Espín D, Rovira M, Galiana I, Giménez C, Lozano‐Torres B, Paez‐Ribes M, Llanos S, Chaib S, Muñoz‐Martín M, Ucero AC et al (2018) A versatile drug delivery system targeting senescent cells. EMBO Mol Med 10: e9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, Rock JR, Looney MR, Wolters PJ (2016) Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1: 2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, Krizhanovsky V (2018) Strategies targeting cellular senescence. J Clin Invest 128: 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Paterna P, Golisano O, Traverso CE, Rama P, Lacal P, De Luca M (2004) Telomerase activity is sufficient to bypass replicative senescence in human limbal and conjunctival but not corneal keratinocytes. Eur J Cell Biol 83: 691–700 [DOI] [PubMed] [Google Scholar]
- Pérez‐Mancera PA, Young ARJ, Narita M (2014) Inside and out: the activities of senescence in cancer. Nat Rev Cancer 14: 547–558 [DOI] [PubMed] [Google Scholar]
- Perrott KM, Wiley CD, Desprez P‐Y, Campisi J (2017) Apigenin suppresses the senescence‐associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 39: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova NV, Velichko AK, Razin SV, Kantidze OL (2016) Small molecule compounds that induce cellular senescence. Aging Cell 15: 999–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitozzi V, Mocali A, Laurenzana A, Giannoni E, Cifola I, Battaglia C, Chiarugi P, Dolara P, Giovannelli L (2013) Chronic resveratrol treatment ameliorates cell adhesion and mitigates the inflammatory phenotype in senescent human fibroblasts. J Gerontol Ser A 68: 371–381 [DOI] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B (2006) Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D‐dependent and tumor necrosis factor‐related apoptosis‐inducing ligand‐dependent manners. Gastroenterology 130: 435–452 [DOI] [PubMed] [Google Scholar]
- van Rhee F, Wong RS, Munshi N, Rossi J‐F, Ke X‐Y, Fosså A, Simpson D, Capra M, Liu T, Hsieh RK et al (2014) Siltuximab for multicentric Castleman's disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Oncol 15: 966–974 [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Ridker P, Lorenzatti A, Krum H, Varigos J et al (2017) Effect of interleukin‐1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet 390: 1833–1842 [DOI] [PubMed] [Google Scholar]
- Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, Sansom OJ, Zender L, Keyes WM (2017) The senescence‐associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev 31: 172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppé J‐P, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J (2009) Persistent DNA damage signalling triggers senescence‐associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang‐Verzosa G et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15: 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, Chu Q, Giaccone G, Khaira D, Ramalingam SS et al (2012) Phase II study of single‐agent navitoclax (ABT‐263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 18: 3163–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv A, Burton DGA, Moshayev Z, Vadai E, Wensveen F, Ben‐Dor S, Golani O, Polic B, Krizhanovsky V (2016) NKG2D ligands mediate immunosurveillance of senescent cells. Aging 8: 328–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama R, Sadaie M, Hoare M, Narita M (2014) Cellular senescence and its effector programs. Genes Dev 28: 99–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaraweera L, Adomako A, Rodriguez‐Gabin A, McDaid HM (2017) A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep 7: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y et al (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, Sherr CJ (2015) Forging a signature of in vivo senescence. Nat Rev Cancer 15: 397–408 [DOI] [PubMed] [Google Scholar]
- Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL‐2 family proteins. Nat Rev Mol Cell Biol 20: 175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett‐Smith J, Kisfalvi K, Kui R, Rozengurt E (2013) Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun 430: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto‐Gamez A, Demaria M (2017) Therapeutic interventions for aging: the case of cellular senescence. Drug Discov Today 22: 786–795 [DOI] [PubMed] [Google Scholar]
- Soto‐Gamez A, Quax WJ, Demaria M (2019) Regulation of survival networks in senescent cells: from mechanisms to interventions. J Mol Biol 431: 2629–2643 [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert‐Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J et al (2013) Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130 [DOI] [PubMed] [Google Scholar]
- Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E (2017) Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun 8: 15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa RK, Nguyen HT, Jeong JH, Kim JR, Choi HG, Yong CS, Kim JO (2017) Progressive slowdown/prevention of cellular senescence by CD9‐targeted delivery of rapamycin using lactose‐wrapped calcium carbonate nanoparticles. Sci Rep 7: 43299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher AW, LoRusso P, Arzt J, Busman TA, Lian G, Rudersdorf NS, Vanderwal CA, Kirschbrown W, Holen KD, Rosen LS (2015) Safety, efficacy, and pharmacokinetics of navitoclax (ABT‐263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother Pharmacol 76: 1025–1032 [DOI] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10: 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana‐Martínez F, Picallos‐Rabina P, Da Silva‐Álvarez S, Pietrocola F, Llanos S, Rodilla V, Soprano E, Pedrosa P, Ferreirós A, Barradas M et al (2019) Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat Commun 10: 4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanelli L, Buratta S, Sagini K, Tancini B, Emiliani C (2016) Extracellular vesicles as new players in cellular senescence. Int J Mol Sci 17: e1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergel M, Carnero A (2010) Bypassing cellular senescence by genetic screening tools. Clin Transl Oncol 12: 410–417 [DOI] [PubMed] [Google Scholar]
- Vlahovic G, Karantza V, Wang D, Cosgrove D, Rudersdorf N, Yang J, Xiong H, Busman T, Mabry M (2014) A phase I safety and pharmacokinetic study of ABT‐263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Invest New Drugs 32: 976–984 [DOI] [PubMed] [Google Scholar]
- Wang F, Marshall CB, Yamamoto K, Li G‐Y, Plevin MJ, You H, Mak TW, Ikura M (2008) Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J Mol Biol 384: 590–603 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, Zhou D, Zheng G (2016) Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging 8: 2915–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Yu Z, Sunchu B, Shoaf J, Dang I, Zhao S, Caples K, Bradley L, Beaver LM, Ho E et al (2017) Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2‐independent mechanism. Aging Cell 16: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu J, Ma X, Cui C, Deenik PR, Henderson PKP, Sigler AL, Cui L (2019) Real‐time imaging of senescence in tumors with DNA damage. Sci Rep 9: 2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrman TS, von Degenfeld G, Krutzik PO, Nolan GP, Blau HM (2006) Luminescent imaging of beta‐galactosidase activity in living subjects using sequential reporter‐enzyme luminescence. Nat Methods 3: 295–301 [DOI] [PubMed] [Google Scholar]
- Wiley CD, Schaum N, Alimirah F, Lopez‐Dominguez JA, Orjalo AV, Scott G, Desprez PY, Benz C, Davalos AR, Campisi J (2018) Small‐molecule MDM2 antagonists attenuate the senescence‐associated secretory phenotype. Sci Rep 8: 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Tahara H (2013) The role of exosomes and microRNAs in senescence and aging. Adv Drug Deliv Rev 65: 368–375 [DOI] [PubMed] [Google Scholar]
- Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V et al (2015) JAK inhibition alleviates the cellular senescence‐associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 112: E6301–E6310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24: 1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Ren J, Chen Q, Chen ZJ (2017) cGAS is essential for cellular senescence. Proc Natl Acad Sci USA 114: E4612–E4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky‐Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R et al (2016) Directed elimination of senescent cells by inhibition of BCL‐W and BCL‐XL. Nat Commun 7: 11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann‐Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL et al (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36: 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH, Davaapil H, Brockes JP (2015) Recurrent turnover of senescent cells during regeneration of a complex structure. Elife 4: e05505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li C, Dutta C, Fang M, Zhang S, Tiwari A, Werner T, Luo F‐T, Liu H (2017) A novel near‐infrared fluorescent probe for sensitive detection of β‐galactosidase in living cells. Anal Chim Acta 968: 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann‐Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB et al (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors. Aging Cell 15: 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann‐Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL‐XL inhibitors, A1331852 and A1155463. Aging 9: 955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5


