Abstract
In human medicine, computed tomography (CT) is the gold standard for visceral fat measurement. Research shows that the visceral fat area (VFA) of the umbilical slice is significantly correlated with the visceral fat volume (VFV). In veterinary medicine, however, few studies have evaluated visceral fat using CT. This study aimed to evaluate the visceral fat in dogs using CT images, and determine if the slice significantly correlated with VFV to simplify visceral fat measurements. This retrospective study includes data on 90 dogs that underwent whole-body CT scans for diagnostic purposes. VFV was calculated as the product of VFA and thickness in each CT slice; the correlation between VFV and VFA was analyzed at the level of each lumbar vertebra. Visceral fat percentage (VF%) was calculated as the ratio of the product of VFV and fat density to the body weight. Visceral fat area percentage (VFA%) was calculated as the ratio of VFA to the body area, and its correlation with the VF% and the body condition score (BCS) was analyzed. VFA was highly correlated with VFV at the level of each lumbar vertebra, with the highest correlation (r=0.964) at the L3 level. VFA% was significantly correlated with VF% (r=0.930) and weakly correlated with BCS (r=0.523). This study demonstrates that it is sufficient to use only the L3 slice for visceral fat evaluation and that the evaluation can be based on VFA% of the L3 level.
Keywords: computed tomography, visceral fat, visceral obesity
In human medicine, epidemiological studies have determined an association between severe obesity and mortality due to increased rates of cardiovascular disease and diabetes [15, 18, 19]. However, research shows that risk factors for cardiovascular disease are not present in 30% of individuals with obesity [7]. Therefore, many studies reemphasize the clinical observation of another earlier study that the distribution of fat tissue is the critical factor to consider when determining the relationship between obesity and metabolic abnormalities [31]. Visceral obesity, defined as the excessive accumulation of visceral fat, has been reported as a critical risk factor for diabetes mellitus, cardiovascular disease, dyslipidemia, and hypertension. [5, 22, 27] The visceral fat secretes many bioactive substances, such as free fatty acids (FFAs), tumor necrosis factor-α (TNF-α), and angiotensinogen. FFAs are reported to cause dyslipidemia by reducing the levels of high-density lipoprotein (HDL) cholesterol and increasing those of low-density-lipoprotein (LDL) cholesterol [5]. TNF-α was found to contribute to the onset of diabetes mellitus by increasing the insulin resistance with the involvement of glycogen synthase, phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, activator protein-2, acetyl-CoA carboxylase, and tyrosine aminotransferase [13]. Angiotensinogen, which is secreted in greater amounts in the visceral fat than in the subcutaneous fat, is implicated in the pathogenesis of hypertension associated with visceral obesity [10]. Hence, the regional distribution of adipose tissue is considered to be more important than the total amount of body fat [27].
In veterinary medicine, obesity has been reported as a risk factor for endocrine, orthopedic, cardiorespiratory, and urinary tract disorders [9]. Endocrine diseases, including diabetes mellitus, hypothyroidism, hyperadrenocorticism, and insulinoma, have been associated with obesity [9]. Additionally, the incidence of both traumatic and degenerative orthopedic disorders is reported to be increased in obese dogs; [6, 26] and obesity in small dogs exacerbates cardiorespiratory disorders, especially tracheal collapse [34]. Visceral fat is considered to be more strongly associated with obesity-related diseases than is subcutaneous fat, due to the differences in the metabolic and endocrine function of different adipose depots [1]. Presently, only two studies have clarified the association between visceral fat and diseases in dogs. These studies determined that excess visceral fat is associated with cardiovascular disease [28] and with hyperadrenocorticism [4].
In human medicine, body fat is measured by dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis, air displacement plethysmography, computed tomography (CT), quantitative magnetic resonance, and magnetic resonance imaging [8]. For the measurement of visceral fat, however, CT is the gold standard [33]. It has been reported that the visceral fat area of the umbilical CT slice is significantly correlated with the visceral fat mass [29], which, in human medicine, allows for visceral fat estimation through analysis of the umbilical slice alone. In some veterinary medicine studies, both DEXA and the deuterium oxide dilution method have been used as gold standards for total fat measurement [12]. The body condition score (BCS)—reported to correlate well with body fat measured by DEXA [21]—is the most widely used method, in veterinary practice, for evaluation of body fat. Notably, several prior studies have evaluated visceral fat using CT [12, 17, 23, 28]; however, slices of different levels were used in each study, since it is not clear which slice shows the greatest correlation with the visceral fat mass in veterinary medicine. The objective of this study was to evaluate visceral fat in dogs using CT images and to determine which slice is significantly correlated with the visceral fat volume (VFV) to simplify the visceral fat measurement.
MATERIALS AND METHODS
Study design
This is a retrospective study that includes dogs, referred to the Veterinary Medical Center of the University of Tokyo from May through July 2018, that underwent whole-body CT scans for diagnostic purposes. The animals were included in the study, regardless of the type of disease. We obtained informed client consent from the owners of all animals. Since there was an insufficient number of dogs of BCS 1 and BCS 5 categories, we additionally included dogs meeting those requirements; these animals were referred to the medical center from August 2017 through March 2018. The following characteristics were analyzed: breed, sex, age, body weight, and BCS (Scale from 1 to 5).
Computed tomography
An 80-row multi-slice CT scanner (Aquilion Prime; Canon Medical Systems Corp., Tochigi, Japan) was used for imaging. The dogs were fasted for more than 12 hr before the CT scan. Scanning was performed with the dogs positioned in a prone position on the CT table, while under general anesthesia. Scanning settings were 120 kV, 50–300 mA, 0.5 sec tube rotation time, 65.0 helical pitch, 240.0–500.0 mm field of view, and a 512 × 512 matrix.
Body fat assessment
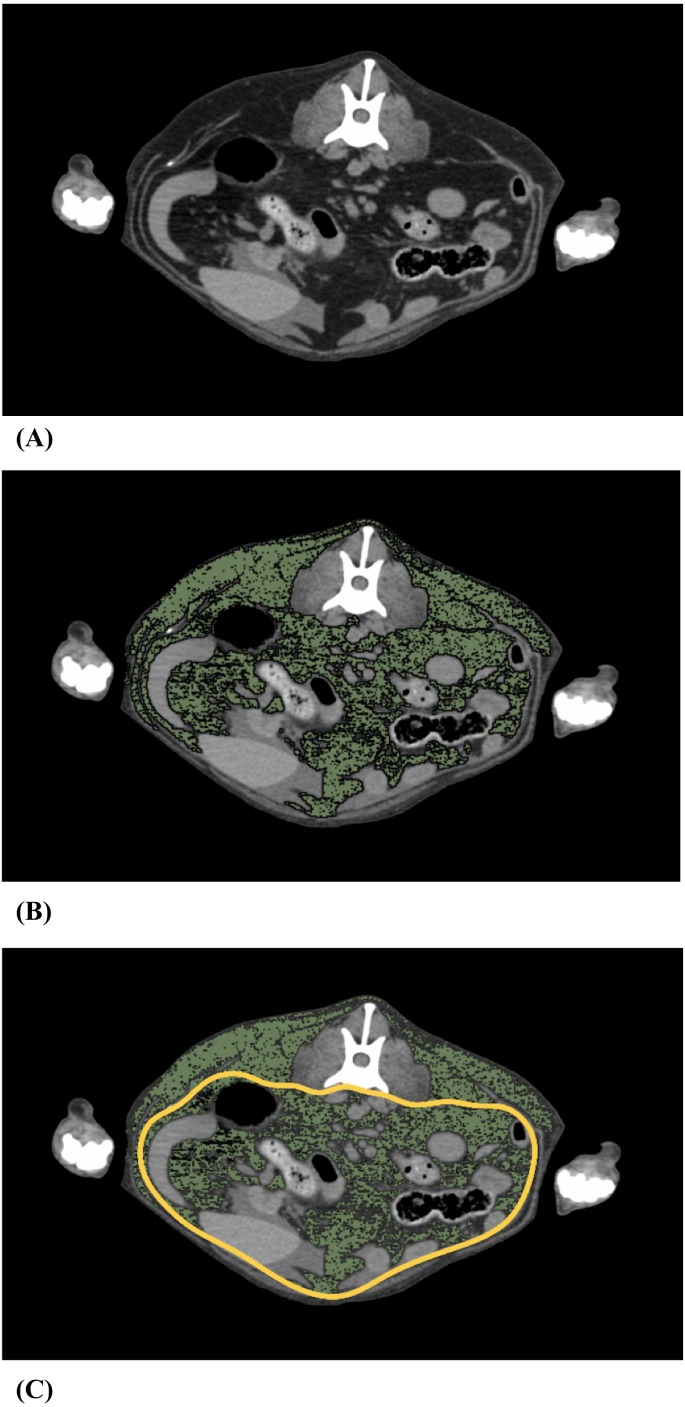
OsiriX DICOM Viewer (Pixmeo SARL Inc., Bernex, Switzerland) was used for body fat analysis. The analysis was performed from the top of the diaphragm to the anus. On each CT transverse image, (Fig. 1A) fat was identified based on the attenuation range of −135 to −105 Hounsfield units (HU) of fat [12]. The pixels with an attenuation range of −135 to −105 HU were colored green (Fig. 1B). The region of interest (ROI) was marked by surrounding the visceral cavity based on the abdominal wall musculature [12], and then, we measured the visceral fat area (VFA) and the subcutaneous fat area (SFA) (Fig. 1C). Hence, VFA was defined as the fat area inside the abdominal musculature, and SFA was defined as the fat outside the abdominal wall musculature. We performed fat separation in each 6 mm slice, and calculated VFV as the product of the VFA and thickness, and calculated subcutaneous fat volume (SFV) as the product of the SFA and thickness [36]. In summary, the definition of VFV is the fat volume in the abdominal cavity, and the definition of SFV is the fat volume outside the abdominal cavity from the top of the diaphragm to the anus.
Fig. 1.
Measurement of visceral fat. (A) Representative L3 level computed tomography (CT) image of a dog with body condition score (BCS) 3. (B) Fat tissue was colored green using an attenuation range of −135 to −105 Hounsfield units (HU). (C) Fat tissue was differentiated into visceral fat and subcutaneous fat based on the abdominal wall musculature. The yellow line represents the abdominal wall musculature.
We examined the correlation between VFV and VFA, and between SFV and SFA at the levels of each lumbar vertebra (L1 to L7), in the slice covering the middle part of the vertebra.
Visceral fat analysis
Visceral fat weight in whole-body (VFW) was calculated using equation (1), to account for body size differences, where the density of fat tissue (d) was 0.923 g/cm3 [11].
VFW (kg)=VFV (cm3) × d/1,000 (1)
Then, VFW was converted into VF% using equation (2).
VF% was defined as the ratio of VFW to body weight in the whole body (BW).
VF%=VFW (kg)/BW (kg) × 100 (2)
To easily evaluate the visceral fat mass, we used the visceral fat area percentage (VFA%), which was calculated by dividing VFA by body area (BA) in each transverse image, as in equation (3) [16]. VFA% was defined as the ratio of VFA to BA in each transverse image.
VFA%=VFA (cm2)/BA (cm2) × 100 (3)
Statistical analysis
Statistical analysis was performed using the JMP Pro statistical software program (SAS Institute Inc., Cary, NC, U.S.A.). Pearson’s correlation coefficient was used to determine the correlations between VFV and VFA; SFV and SFA; VF% and VFA%. Spearman’s rank correlation coefficient was used to determine the correlation between VFA% and BCS. A P-value of less than 0.05 was considered statistically significant.
RESULTS
Animals
Ninety dogs were included in this study. Of these, 36 were neutered males, 30 were spayed females, 14 were intact males, and 10 were intact females. There were 10 Miniature Dachshunds, 8 Chihuahuas, 8 mix-breed dogs, 5 Pomeranians, 5 Yorkshire Terriers, 3 Golden Retrievers, 3 Miniature Schnauzers, 3 Jack Russell Terriers, and 45 other breeds. The median age was 10 years (range, 5 months to 15 years). The median BCS was 3 (range, 1 to 5; mean, 3.16), with 3 dogs assigned a BCS of 1, 8 dogs assigned a BCS of 2, 56 dogs assigned a BCS of 3, 17 dogs assigned a BCS of 4, and 6 dogs assigned a BCS of 5. The mean body weight was 9.06 kg (range, 1.2 to 41 kg).
Relationship between VFV, SFV and cross-sectional area
The mean total fat area was highest in the L3 slice (L1: 31.67 cm2, L2: 39.15 cm2, L3: 40.54 cm2, L4: 39.15 cm2, L5: 37.65 cm2, L6: 35.37 cm2, L7: 30.39 cm2). The mean visceral fat area was also highest in the L3 slice (L1: 15.22 cm2, L2: 19.96 cm2, L3: 21.43 cm2, L4: 19.62 cm2, L5: 15.91 cm2, L6: 11.24 cm2, L7: 7.36 cm2), whereas the mean subcutaneous fat area was highest in the L6 slice (L1: 16.45 cm2, L2: 19.19 cm2, L3: 19.11 cm2, L4: 19.53 cm2, L5: 21.74 cm2, L6: 24.13 cm2, L7: 23.03 cm2).
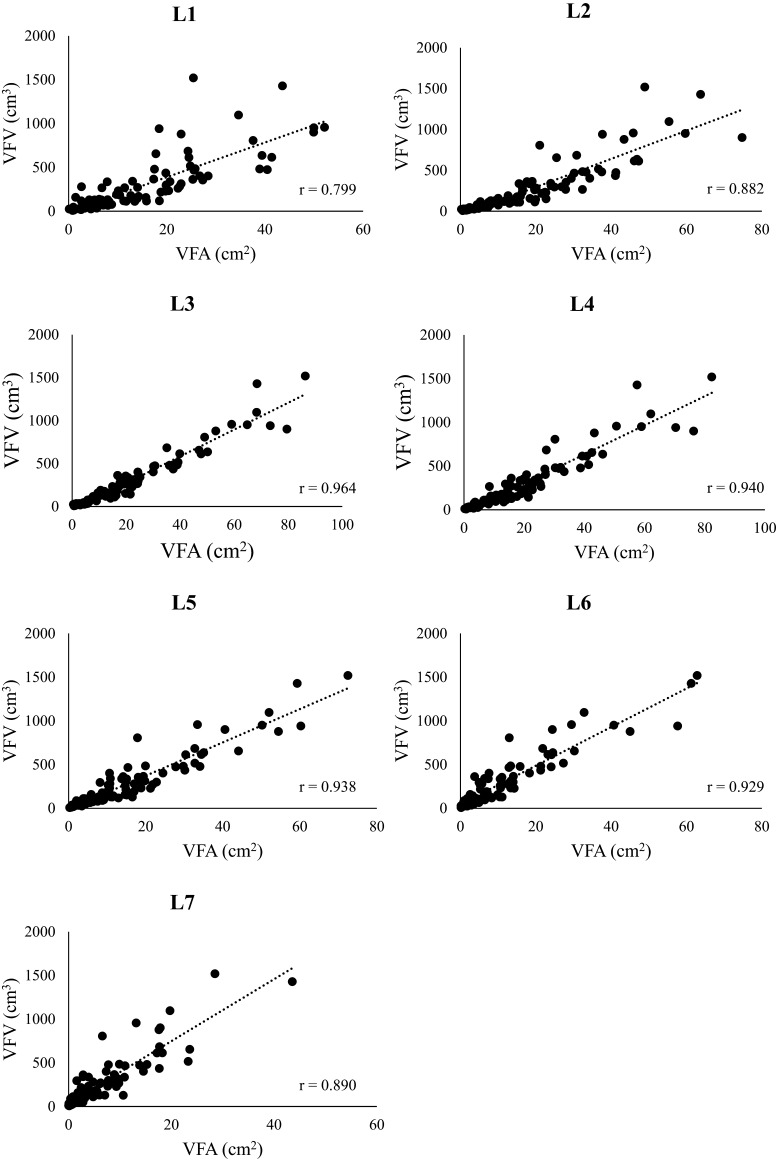
The correlation between VFV and VFA at the level of each lumbar vertebra is presented in Fig. 2. The VFA was significantly correlated with the VFV at all vertebral levels (P<0.05; L1: r=0.799, L2: r=0.882, L3: r=0.964, L4: r=0.940, L5: r=0.938, L6: r=0.929, L7: r=0.890), with the highest correlation observed at the L3 level (r=0.964).
Fig. 2.
Relationship between the visceral fat area and the visceral fat volume. This figure shows the correlation between visceral fat area (VFA) and visceral fat volume (VFV) at the level of each lumbar vertebra (L1 to L7), measured by using an attenuation range of −135 to −105 Hounsfield units (HU) in computed tomography (CT) images. The statistical analysis used Pearson’s correlation coefficient.
SFA was significantly correlated with SFV (P<0.05; L1: r=0.971, L2: r=0.966, L3: r=0.958, L4: r=0.953, L5: r=0.954, L6: r=0.930, L7: r=0.901), with the highest correlation observed at the L1 level (r=0.971).
Evaluation of visceral fat
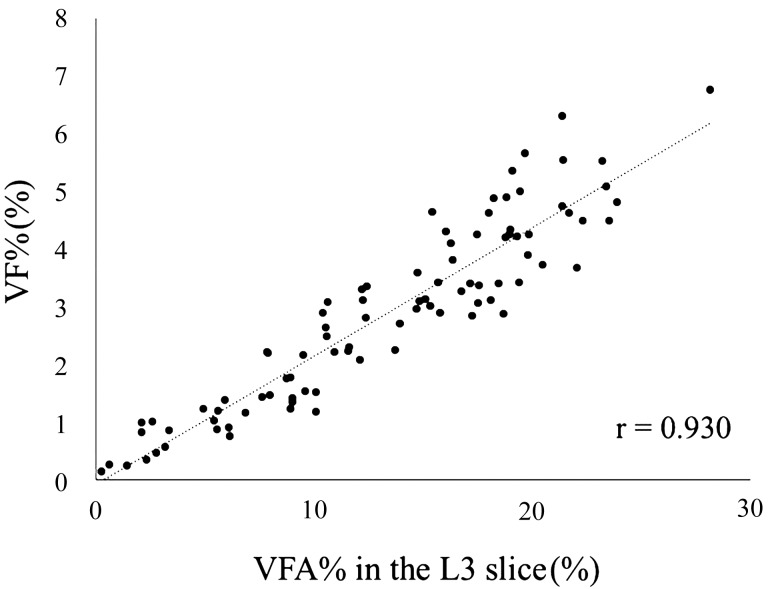
Since the highest correlation between the VFA and VFV was observed at the L3 level, we calculated the VFA% in the L3 slice and analyzed its correlation with the VF%. We found that the VFA% in the L3 slice was significantly correlated with the VF% (P<0.05; r=0.930) (Fig. 3).
Fig. 3.
Relationship between visceral fat area percentage and visceral fat percentage. Visceral fat area percentage (VFA%), the ratio of visceral fat area to body fat area in the L3 slice, was compared to visceral fat percentage (VF%), the ratio of visceral fat weight to body weight. The statistical analysis used the Pearson’s correlation coefficient.
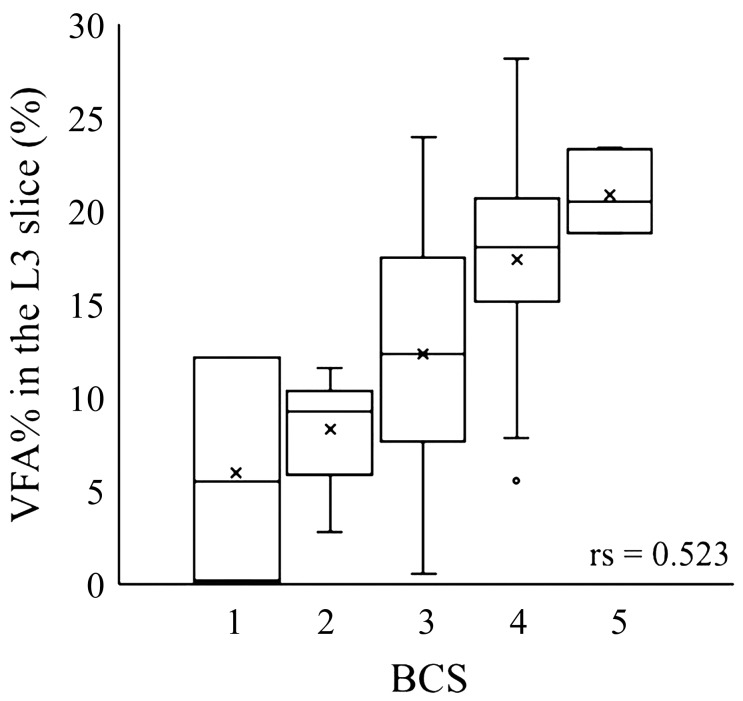
We also analyzed the correlation between the VFA% in the L3 slice and BCS (Fig. 4). The BCS demonstrated a positive correlation with VFA%, but the correlation was not strong (P<0.05; rs=0.523).
Fig. 4.
Relationship between body condition score and visceral fat area percentage in the L3 slice. Visceral fat area percentage (VFA%) in the L3 slice, the ratio of visceral fat area to body area, was compared to body condition score (BCS). Statistical analysis used the Spearman’s rank correlation coefficient.
DISCUSSION
We analyzed the correlation between VFA and VFV to determine which slice showed the greatest correlation with the VFV, in order to simplify visceral fat measurement. The VFA of the L3 slice was found to have the highest correlation with the VFV. In dogs, the position of the umbilicus varies by breed; however, it is generally considered to be located around the L3 cross-section [17]. Hence, our findings are similar to those obtained in human medicine. In humans, the fat area of the umbilical slice is the greatest, and it best allows differentiation of subcutaneous fat from visceral fat [3]. In the present study, the mean total fat area was highest in the L3 slice, indicating that this slice would best allow for separation of the fat area into the subcutaneous and visceral fat area. In previous studies, slices of various vertebral levels (L2, L3, L4, and L5) were used to evaluate visceral fat, [23, 28] and there was not a uniform method for evaluation. This study demonstrates that it is sufficient to use only the L3 slice to evaluate visceral fat. However, it should be noted that our study has the following limitation: the number of large-sized dogs included in the study was fewer than small sized dogs.
In our study, the VFA% in the L3 slice was significantly correlated with VF%. Body fat percentage, that is, the fat tissue weight to body weight ratio is considered to be a useful clinical and research measurement used to account for body size differences in different breeds [30]. Correspondingly, we calculated the VF% as the visceral fat weight to body weight ratio to account for body size differences. Although VF% is the ratio of visceral fat to the whole body weight, it requires that all slices of the abdominal area be evaluated; therefore, it is not clinically useful. Consequently, we calculated the VFA% in order to evaluate the visceral fat more easily. As noted, VFA% was significantly correlated with VF%. Hence, we suspect that the VFA% is the index which reflects the visceral fat distribution in the whole body.
In the present study, the BCS did not strongly correlate with the VFA% in the L3 slice, indicating that the visceral fat mass differs among dogs of the same BCS. Therefore, the possibility exists that dogs have visceral obesity too. However, VFA% has previously been reported to significantly correlate with the BCS (r=0.812) [16]. The discrepancy in the findings may be caused by the small sample size and the limited number of breeds (only one breed) that were included in the previous study. In human medicine, the International Diabetes Federation defines metabolic syndrome as visceral obesity with more than two of the following findings: elevated triglyceride levels, reduced levels of high-density lipoprotein cholesterol, elevated blood pressure, and elevated fasting plasma glucose levels [2]. In dogs, hyperlipidemia is reportedly associated with obesity [35]. However, the use of the term “metabolic syndrome” in dogs is probably not appropriate, because there is no scientific evidence that a high BCS is associated with clinical outcomes such as elevated blood pressure and elevated plasma glucose [32]. In the present study, the BCS did not significantly correlate with VFA%. Thus, further research is needed in order to analyze the association between visceral obesity and clinical outcomes in dogs.
In humans, the easiest way to measure visceral fat is by measuring the abdominal circumference at the level of the umbilicus. The waist circumference is reported to be significantly correlated with the VFA at the umbilical level (r=0.76) [14]; however, it does not allow for accurate quantification of the abdominal adipose tissue depot and may be unrelated to the amount of visceral fat [20]. Bioelectrical impedance analysis (BIA) and ultrasonography have been reported as useful methods substitutes to the waist circumference [24, 25]. Nonetheless, further research is needed to assess the use of non-anesthetic methods, such as BIA and ultrasonography, for visceral fat evaluation in dogs.
In conclusion, we established that the VFA of the L3 CT slice could be used to evaluate canine visceral fat, and VFA% of L3 could reflect the visceral fat distribution in the whole body. Further study is needed to evaluate simpler methods for measuring visceral fat without anesthesia and clarifying its clinical importance in dogs.
REFERENCES
- 1.Adolphe J. L., Silver T. I., Childs H., Drew M. D., Weber L. P.2014. Short-term obesity results in detrimental metabolic and cardiovascular changes that may not be reversed with weight loss in an obese dog model. Br. J. Nutr. 112: 647–656. doi: 10.1017/S0007114514001214 [DOI] [PubMed] [Google Scholar]
- 2.Alberti K. G., Zimmet P., Shaw J.2006. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 23: 469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 3.Borkan G. A., Gerzof S. G., Robbins A. H., Hults D. E., Silbert C. K., Silbert J. E.1982. Assessment of abdominal fat content by computed tomography. Am. J. Clin. Nutr. 36: 172–177. doi: 10.1093/ajcn/36.1.172 [DOI] [PubMed] [Google Scholar]
- 4.Cho K. D., Paek J., Kang J. H., Chang D., Na K. J., Yang M. P.2014. Serum adipokine concentrations in dogs with naturally occurring pituitary-dependent hyperadrenocorticism. J. Vet. Intern. Med. 28: 429–436. doi: 10.1111/jvim.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebbert J. O., Jensen M. D.2013. Fat depots, free fatty acids, and dyslipidemia. Nutrients 5: 498–508. doi: 10.3390/nu5020498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edney A. T., Smith P. M.1986. Study of obesity in dogs visiting veterinary practices in the United Kingdom. Vet. Rec. 118: 391–396. doi: 10.1136/vr.118.14.391 [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E., Haffner S. M., Mitchell B. D., Stern M. P.1991. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia 34: 416–422. doi: 10.1007/BF00403180 [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D., Shaheen I., Zafar K.2008. State-of-the-art measurements in human body composition: A moving frontier of clinical importance. Int. J. Body Compos. Res. 6: 141–148. [PMC free article] [PubMed] [Google Scholar]
- 9.German A. J.2006. The growing problem of obesity in dogs and cats. J. Nutr. 136 Suppl: 1940S–1946S. doi: 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- 10.Giacchetti G., Faloia E., Sardu C., Camilloni M. A., Mariniello B., Gatti C., Garrapa G. G., Guerrieri M., Mantero F.2000. Gene expression of angiotensinogen in adipose tissue of obese patients. Int. J. Obes. Relat. Metab. Disord. 24 Suppl 2: S142–S143. doi: 10.1038/sj.ijo.0801305 [DOI] [PubMed] [Google Scholar]
- 11.Gifford A., Kullberg J., Berglund J., Malmberg F., Coate K. C., Williams P. E., Cherrington A. D., Avison M. J., Welch E. B.2014. Canine body composition quantification using 3 tesla fat-water MRI. J. Magn. Reson. Imaging 39: 485–491. doi: 10.1002/jmri.24156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishioka K., Okumura M., Sagawa M., Nakadomo F., Kimura K., Saito M.2005. Computed tomographic assessment of body fat in beagles. Vet. Radiol. Ultrasound 46: 49–53. doi: 10.1111/j.1740-8261.2005.00009.x [DOI] [PubMed] [Google Scholar]
- 13.Jaganathan R., Ravindran R., Dhanasekaran S.2018. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can. J. Diabetes 42: 446–456.e1. doi: 10.1016/j.jcjd.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 14.Janssen I., Heymsfield S. B., Allison D. B., Kotler D. P., Ross R.2002. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am. J. Clin. Nutr. 75: 683–688. doi: 10.1093/ajcn/75.4.683 [DOI] [PubMed] [Google Scholar]
- 15.Kannel W. B.1985. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am. Heart J. 110: 1100–1107. doi: 10.1016/0002-8703(85)90224-8 [DOI] [PubMed] [Google Scholar]
- 16.Kim D., Noh D., Oh T., Lee K.2018. Body fat assessment by computed tomography and radiography in normal Beagle dogs. J. Vet. Med. Sci. 80: 1380–1384. doi: 10.1292/jvms.18-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T., Koie H., Kusumi A., Kitagawa M., Kanayama K., Otsuji K.2014. Comparative investigation of body composition in male dogs using CT and body fat analysis software. J. Vet. Med. Sci. 76: 439–446. doi: 10.1292/jvms.13-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann G. V.1974. The influence of obesity on health (first of two parts). N. Engl. J. Med. 291: 178–185. doi: 10.1056/NEJM197407252910405 [DOI] [PubMed] [Google Scholar]
- 19.Mann G. V.1974. The influence of obesity on health (second of two parts). N. Engl. J. Med. 291: 226–232. doi: 10.1056/NEJM197408012910504 [DOI] [PubMed] [Google Scholar]
- 20.Maurovich-Horvat P., Massaro J., Fox C. S., Moselewski F., O’Donnell C. J., Hoffmann U.2007. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int. J. Obes. 31: 500–506. doi: 10.1038/sj.ijo.0803454 [DOI] [PubMed] [Google Scholar]
- 21.Mawby D. I., Bartges J. W., d’Avignon A., Laflamme D. P., Moyers T. D., Cottrell T.2004. Comparison of various methods for estimating body fat in dogs. J. Am. Anim. Hosp. Assoc. 40: 109–114. doi: 10.5326/0400109 [DOI] [PubMed] [Google Scholar]
- 22.Moller D. E., Kaufman K. D.2005. Metabolic syndrome: a clinical and molecular perspective. Annu. Rev. Med. 56: 45–62. doi: 10.1146/annurev.med.56.082103.104751 [DOI] [PubMed] [Google Scholar]
- 23.Müller L., Kollár E., Balogh L., Pöstényi Z., Márián T., Garai I., Balkay L., Trencsényi G., Thuróczy J.2014. Body fat distribution and metabolic consequences - Examination opportunities in dogs. Acta Vet. Hung. 62: 169–179. doi: 10.1556/AVet.2013.057 [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro-Filho F. F., Faria A. N., Azjen S., Zanella M. T., Ferreira S. R.2003. Methods of estimation of visceral fat: advantages of ultrasonography. Obes. Res. 11: 1488–1494. doi: 10.1038/oby.2003.199 [DOI] [PubMed] [Google Scholar]
- 25.Ryo M., Maeda K., Onda T., Katashima M., Okumiya A., Nishida M., Yamaguchi T., Funahashi T., Matsuzawa Y., Nakamura T., Shimomura I.2005. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 28: 451–453. doi: 10.2337/diacare.28.2.451 [DOI] [PubMed] [Google Scholar]
- 26.Smith G. K., Mayhew P. D., Kapatkin A. S., McKelvie P. J., Shofer F. S., Gregor T. P.2001. Evaluation of risk factors for degenerative joint disease associated with hip dysplasia in German Shepherd Dogs, Golden Retrievers, Labrador Retrievers, and Rottweilers. J. Am. Vet. Med. Assoc. 219: 1719–1724. doi: 10.2460/javma.2001.219.1719 [DOI] [PubMed] [Google Scholar]
- 27.Tchernof A., Després J. P.2013. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93: 359–404. doi: 10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]
- 28.Thengchaisri N., Theerapun W., Kaewmokul S., Sastravaha A.2014. Abdominal obesity is associated with heart disease in dogs. BMC Vet. Res. 10: 131. doi: 10.1186/1746-6148-10-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokunaga K., Matsuzawa Y., Ishikawa K., Tarui S.1983. A novel technique for the determination of body fat by computed tomography. Int. J. Obes. 7: 437–445. [PubMed] [Google Scholar]
- 30.Turner R. B. S., Hepworth G., Wilson K., Tyrrell D., Dunshea F. R., Mansfield C. S.2019. Abdominal volume computed tomography assessment of body composition in dogs. BMC Vet. Res. 15: 21. doi: 10.1186/s12917-018-1768-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vague J.1996. Sexual differentiation. A determinant factor of the forms of obesity. 1947. Obes. Res. 4: 201–203. doi: 10.1002/j.1550-8528.1996.tb00535.x [DOI] [PubMed] [Google Scholar]
- 32.Verkest K. R.2014. Is the metabolic syndrome a useful clinical concept in dogs? A review of the evidence. Vet. J. 199: 24–30. doi: 10.1016/j.tvjl.2013.09.057 [DOI] [PubMed] [Google Scholar]
- 33.Wajchenberg B. L.2000. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21: 697–738. doi: 10.1210/edrv.21.6.0415 [DOI] [PubMed] [Google Scholar]
- 34.White R. A. S., Williams J. M.1994. Tracheal collapse in the dog—is there really a role for surgery? A survey of 100 cases. J. Small Anim. Pract. 35: 191–196. doi: 10.1111/j.1748-5827.1994.tb01685.x [DOI] [Google Scholar]
- 35.Xenoulis P. G., Steiner J. M.2015. Canine hyperlipidaemia. J. Small Anim. Pract. 56: 595–605. doi: 10.1111/jsap.12396 [DOI] [PubMed] [Google Scholar]
- 36.Zhao B., Colville J., Kalaigian J., Curran S., Jiang L., Kijewski P., Schwartz L. H.2006. Automated quantification of body fat distribution on volumetric computed tomography. J. Comput. Assist. Tomogr. 30: 777–783. doi: 10.1097/01.rct.0000228164.08968.e8 [DOI] [PubMed] [Google Scholar]