Abstract
Left atrial enlargement (LAE) is a well-known negative prognostic factor in dogs with myxomatous mitral valve disease (MMVD). Left atrial-to-aortic root ratio (LA/Ao) is the most commonly used method to evaluate left atrial (LA) size in dogs, while the left atrial anteroposterior diameter (LAD) has been proposed as an additional measurement of LA size in different species. The aim of this study was to establish a normal reference range of LAD normalized to body weight (LADn) in dogs using allometric scales, and to evaluate the agreement between LADn and LA/Ao in the detection of LAE in dogs with MMVD. This was a retrospective, multicenter, observational study. We included 330 healthy dogs, 30 dogs with MMVD in ACVIM stage B1, 30 dogs in ACVIM stage B2, and 30 dogs in ACVIM stage C. The reference range for the LAD, depending on body weight, was between 16.91 mm and 49.68 mm. The reference range for the LADn in healthy dogs was between 10.49 and 15.72. LADn was significantly greater in dogs with MMVD compared to healthy dogs, and a significant difference in LADn was noted between different ACVIM stages (P<0.001). The most accurate cut-off value of LADn to differentiate between dogs in groups B2 and C was 20.3 (sensitivity, 83.3%; specificity, 83.3%). There was a misclassification rate of 37% between LADn and LA/Ao in the detection of LAE in group B1. This study provides a normal reference range for LAD in dogs, which can be used as an additional tool to assess LAE in dogs with MMVD.
Keywords: canine, cardiology, degenerative valvular disease, echocardiography, myxomatous mitral valve disease
Left atrial enlargement (LAE) is a common finding in dogs with hemodynamically significant myxomatous mitral valve disease (MMVD), and has a strong negative prognostic significance [5, 29]. In dogs, numerous echocardiographic methods for the assessment of LAE have been reported, including linear, cross-sectional area and volume measurements [3, 13,14,15, 19, 24, 26, 34, 36]. Linear measurements are widely used since they are less time consuming and do not require off-line analysis compared to more advanced measurements.
In humans, the most widely used linear dimension is left atrial anteroposterior diameter (LAD) obtained from the parasternal long-axis view [18]. In veterinary clinical practice, the left atrial-to-aortic root ratio (LA/Ao) in right parasternal short axis view is the most commonly used method to evaluate left atrial (LA) size in dogs [6, 8, 12, 13, 26]. This ratio provides an index of LA size that is independent of body size. Potential limitations of this method include defining the path of aortic measurement relative to valve sinuses, excluding pulmonary veins from the LA measurement and consistently timing LA measurements during the cardiac cycle [34]. Moreover, if the aortic dimension is increased/decreased or if the imagining plane is not correct, the resulting ratio can underestimate or overestimate LA size, respectively [10, 13, 26]. Finally, significant interoperator measurement variability of the LA/Ao has been reported, with possible misdiagnosis of LAE [27]. For these reasons, in cats and horses, the measurement of LAD is routinely used to evaluate LA size and LAE [1, 16, 31, 32, 37]. Given the correlation of LAD with body weight (BW), the normal reference range of LAD using allometric scales has been proposed in these two species [1, 16, 31, 37].
In dogs, the use of LAD has not been investigated extensively. To the authors’ knowledge, no previous studies have reported the allometrically-scaled normal reference range of LAD in dogs. Therefore, the aim of this study was to establish a normal reference range of LAD and LAD normalized to BW (LADn) in dogs based on allometric scales and to evaluate the agreement between LADn and LA/Ao in the detection of LAE in dogs with MMVD.
MATERIALS AND METHODS
This is a retrospective, multicenter, observational study. The clinical databases of the Istituto Veterinario di Novara and the Department of Veterinary Sciences of the University of Pisa were reviewed for echocardiographic examinations performed on healthy dogs and dogs with different stages of MMVD, from February 2014 to June 2017.
This study consisted in two phases. In the first phase, a control group of healthy dogs was generated to produce a reference range using allometric scale for LADn in dogs. In the second phase, LAD was measured in dogs with different severity of MMVD. The LADn in this group of diseased dogs was compared with the reference range derived from the healthy dogs to evaluated LAE according to LADn. In addition, the agreement between LADn and LA/Ao in the detection of LAE was evaluated.
Animals
The control group included client-owned healthy dogs referred for cardiac screening or pre-anesthesia evaluation. Health status was defined based on history, physical examination, electrocardiography, and complete echocardiographic examination.
The study group comprised client-owned dogs affected by MMVD referred for cardiologic evaluation. A diagnosis of MMVD was based on echocardiographic identification of mitral valve thickening or prolapse in combination with the presence of mitral regurgitation. Dogs with MMVD were classified into stage B1, B2 or C according to the ACVIM classification [17].
Exclusion criteria included the presence of non-sinus arrhythmia, concomitant systemic diseases that can affect the cardiovascular system (e.g. hypothyroidism, hyperadrenocorticism), congenital cardiac diseases, and the use of medication in the control group. Dogs in the study group, receiving cardiac medications as pimobendan, furosemide, torsemide, benazepril, enalapril and spironolactone were not excluded.
Echocardiography
All echocardiographic studies were performed by a board-certified cardiologist or by residents supervised by a cardiologist using ultrasound systems (Vivid I, GE Healthcare, Chicago, IL, U.S.A.; Xario XG, Toshiba Corporation, Utsunomiya, Japan) with phased-array transducers (1.5–8 Mhz). All dogs were examined gently restrained in right and left lateral recumbency, without sedation. Standard M-mode, two-dimensional Doppler echocardiographic images and video-loops were recorded with continuous ECG monitoring as previously reported [2, 25, 35].
In all dogs, the LA/Ao was measured from a right parasternal short-axis view at the heart base as previously described [26]. In brief, we measured the internal short-axis diameter of the aorta along the commissure between the noncoronary and right coronary aortic valve cusps on the 1st frame after aortic valve closure [26]. We then measured internal short-axis diameter of the LA in the same frame in a line extending from and parallel to the commissure between the noncoronary and left coronary aortic valve cusps to the distant margin of the left atrium [26].
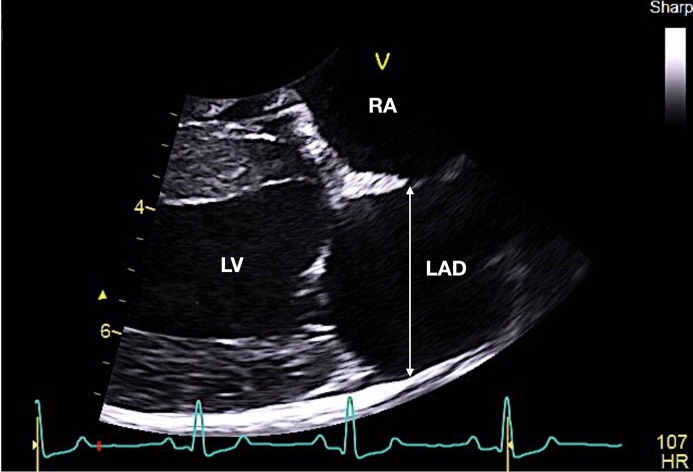
Normal LA dimension was defined as an LA/Ao ratio <1.6 [17, 26]. The LAD was measured at end-systole (a frame just before mitral valve opening), from the right parasternal long axis view [26, 34]. The measurement was done evaluating the widest distance, parallel to the mitral valve annulus, from the inner wall of the middle of interatrial septum to the inner wall of the posterior free wall. The distance from blood-tissue interface to blood-tissue interface was used (Fig. 1).
Fig. 1.
Representative measurement of left atrial anteroposterior diameter (LAD) obtained from the parasternal long-axis view in a healthy dog. The LAD was measured at end-systole (a frame just before mitral valve opening), evaluating the widest distance, parallel to the mitral valve annulus, from the inner wall of the middle of interatrial septum to the inner wall of the posterior free wall. The distance from the blood-tissue interface to blood-tissue interface was used (white arrow). LV, left ventricle; RA right atrium
All echocardiographic measurements were carried out off-line by a single operator (FM) on three consecutive cardiac cycles, and the mean values were used.
Statistical analysis
Statistical analysis was performed with a commercially available statistical software package (SAS Institute Inc., Cary, NC, U.S.A.). Descriptive statistics were generated. The normality of data distribution was tested using the Shapiro Wilks test. Non-normally distributed data were reported as median and range. A non-parametric Kruskal-Wallis test was applied to assess differences between groups (healthy vs B1 vs B2 vs C) for demographic and echocardiographic variables. Bonferroni correction was used to perform pairwise contrasts between groups. The differences for categorical variables (as sex) were tested using a chi-square test. In healthy dogs, an ANCOVA model was applied to test the significance of sex, BW, and age on LAD.
To account for differences that can be expected with the large variation in BW of dogs, BW-dependent regression-based reference ranges were determined.
A linear regression analysis of the logarithmic form of the allometric equation log (Y)=log (a) + b X log (M) was performed [9]. Y represents LAD, M is BW, a is the proportionality constant, and b is the scaling exponent. Regression yields the constant b, which is the slope of the regression line, and a, which is the antilog of the intercept. The regression can be rewritten as the allometric form: Y=a X Mb. Using the estimates obtained by the regression analysis, 95% prediction intervals of the LAD values for normal dog were calculated. The assumptions of linear models on the residuals (for ANCOVA and regression models) were graphically assessed. The LAD normalized to BW (LADn) was calculated using the results of allometric scaling. To verify the effect of the breed on LADn values, we compared this measurement between the most represented breeds (composed by at least 20 dogs) in the control group.
A receiver operating characteristic (ROC) curve analysis was adopted to calculate the sensitivity and specificity of LADn in detection of clinical stage. The Youden index was used to identify the best cut-off value of LADn to discriminate between B2 and C dogs.
The correlation between LA/Ao and LADn in dogs with MMVD was quantified using the Spearman’s rank coefficient (r).
According to the LA/Ao, LAE was defined as a LA/Ao≥1.6. According to LADn, LAE was defined as a value of LADn greater than the upper reference limit of the 95% prediction interval calculated with the allometric scales. Based on these thresholds, a 2 × 2 contingency table was calculated for all MMVD animals and for the different groups (B1, B2, and C). A misclassification rate in the definition of LAE was calculated, as percentage of cases incorrectly attributed to wrong classes of the severity of the disease.
To verify the suitability of the LAD in a clinical setting, intra- and interobserver measurement variability were determined with the coefficient of variation (CV). For intraobserver variability, one operator (FM) calculated LAD and LA/Ao from 12 randomly selected echocardiographic studies (3 healthy dogs, 3 from the B1 group, 3 from the B2 group, and 3 from the C group) on two different occasions at 30 days apart. Interobserver variability was assessed by two operators (FM, OD) who measured the LAD and LA/Ao from the same 12 randomly selected echocardiographic studies.
A value of P<0.05 was considered statistically significant.
RESULTS
Study population
A total of 420 dogs of different breeds were included, 330 healthy control dogs and 90 dogs with MMVD. In the control group 44 dogs were Labrador Retriever, 37 were mixed-breed dogs, 30 were Golden Retrievers, 27 Boxers, 22 English Bulldogs, 20 Doberman Pinschers, 11 French Bulldogs, 11 German Shepherds, 9 Pugs, 8 Yorkshire Terriers, 8 Jack Russel Terriers, 6 Beagles, 6 Cavalier King Charles Spaniels, 6 English Cocker Spaniels, 5 Border Collies, 5 Australian Shepherds, 4 Chihuahuas and the remaining 71 dogs were of other 48 different breeds. In the MMVD group, 37 were mixed-breed dogs, 7 were Cavalier King Charles Spaniels, 5 Chihuahuas, 4 Yorkshire Terriers and the remaining 37 dogs were of other 20 different breeds.
Baseline clinical and echocardiographic characteristics of all dogs are shown in Table 1. Dogs with MMVD were significantly older (11.7 years, range: 3–16 years) than control dogs (3.6 years; range, 1–15.7 years; P<0.001). In addition, dogs with MMVD had a lower BW (9.3 kg; range, 1.5–43 kg) in comparison to healthy control dogs (25.7 kg; range, 1.8–65 kg; P<0.001).
Table 1. Demographic data and echocardiographic variables of control dogs and dogs with myxomatous mitral valve disease (MMVD) included in the study.
| Control | MMVD (stage B1) | MMVD (stage B2) | MMVD (stage C) | |
|---|---|---|---|---|
| No. cases | 330 | 30 | 30 | 30 |
| Age (years) | 3.6 (1–15.7) | 10.9 (3–13.2)a) | 13.6 (5.6–15)a) | 12 (6–16)a) |
| BW (kg) | 25.7 (1.8–65) | 10 (1.5–43)a) | 10.7 (3.5–39)a) | 8.2 (1.8–32.5)a) |
| Sex (female/male) | 185/145 | 14/16a) | 13/17a) | 8/22b) |
| LAD (mm) | 36.3 (14.9–58.4) | 32.5 (18.6–49) | 49.2 (27–63)c) | 48.4 (22.3–69.9)c) |
| LADn | 12.9 (9.7–17.2) | 15.4 (11.4–18.13)a) | 18.8 (16.3–28.9)c) | 23.3 (16.38–33.5)b) |
| LA/Ao | 1.4 (0.9–1.59) | 1.4 (1.1–1.5) | 1.8 (1.7–3)c) | 2.3 (1.8–3.6)b) |
BW, body weight; LAD, left atrial anteroposterior diameter; LADn, left atrial anteroposterior diameter normalized to body weight; LA/Ao, left atrial-to-aortic root ratio. a) P<0.001 vs. control group, b) P<0.001 vs. control group, B1 and B2, c) P<0.001 vs. control group and B1. Data represent median (min-max) or number of cases.
Left atrial size
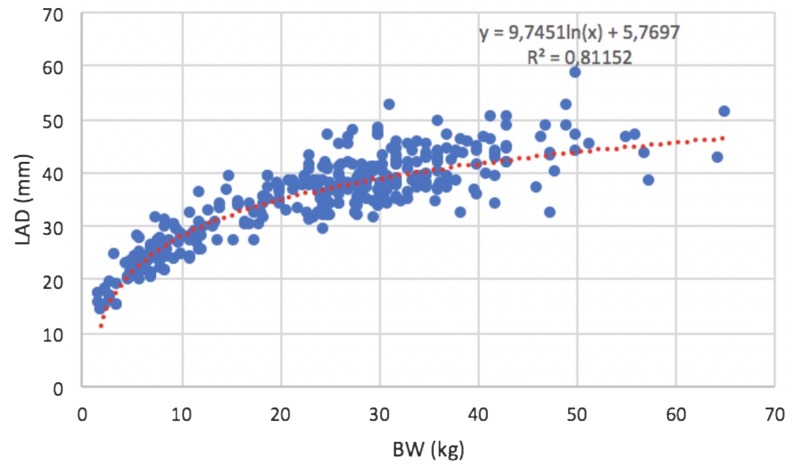
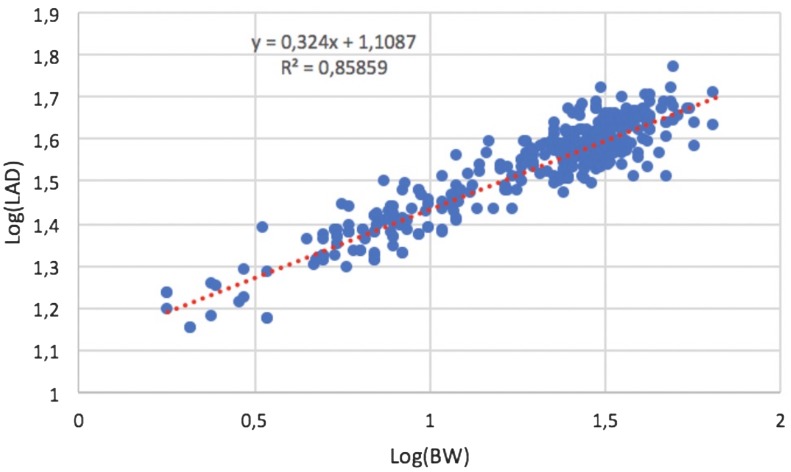
In the control group, the median value of LA/Ao was 1.4 (0.9–1.59), while the median value of LAD was 36.3 mm (range, 14.9–58.4 mm). No significant effect of sex or age on LAD was found (P=0.078 and P=0.409, respectively). A curvilinear positive correlation was found between LAD and BW in control dogs (R2=0.81; P<0.001) (Fig. 2). After logarithmic transformation, LAD and BW showed a strong positive linear correlation (R2=0.86; P<0.001) (Fig. 3). Results of linear regression analysis of logarithmically transformed echocardiographic variables, including the proportionality constant (a) and the allometric scaling exponent (b), are reported in Table 2. The reference range for the LAD was between 16.91 and 49.68 mm. The 95% prediction intervals of LAD according to different BW in heathy dogs are reported in Table 3.
Fig. 2.
Left atrial anteroposterior diameter (LAD) versus body weight (BW), the curvilinear regression line and R2 of the model.
Fig. 3.
Left atrial anteroposterior diameter (LAD) versus body weight (BW) after logarithmic transformation. The equation of regression line and R2 of the model. Note that the relationship between the log of BW and the log of LAD is linear.
Table 2. Results of linear regression analysis of logarithmically transformed left atrial anteroposterior diameter (LAD) and body weight including the proportionality constants (a) and allometric scaling exponents (b) from 330 healthy control dogs.
| Log (a) | a | 95% Prediction Interval for a | b | R2 | SE of Y est | ||
|---|---|---|---|---|---|---|---|
| LAD | 0.045 | 1.11 | 10.49 | 15.72 | 0.324 | 0.86 | 0.045 |
a: Antilog (log−1) of the y-intercept of the regression line; b: scaling exponent, slope of the regression; R2: coefficient of determination of the model; SE of Y est: standard error of the Y estimate.
Table 3. Body weight (BW)-dependent reference interval (95% prediction interval) of left atrial anteroposterior diameter (LAD) in 330 heathy dogs.
| BW (kg) | LAD (mm)a) |
|---|---|
| 2 | 13.1–19.7 |
| 3 | 15.0–22.4 |
| 4 | 16.4–24.6 |
| 5 | 17.7–26.5 |
| 6 | 18.7–28.1 |
| 7 | 19.7–29.5 |
| 8 | 20.6–30.8 |
| 9 | 21.4–32.0 |
| 10 | 22.1–33.1 |
| 15 | 25.2–37.8 |
| 20 | 27.7–41.5 |
| 25 | 29.8–44.6 |
| 30 | 31.6–47.3 |
| 35 | 33.2–49.7 |
| 40 | 34.7–51.9 |
| 50 | 37.3–55.8 |
| 60 | 39.5–59.2 |
| 70 | 41.6–62.3 |
a) Allometric equation with 95% prediction intervals: Y=10.49 to 15.72 × M0.324.
According to the allometric scales, the formula to calculate the LADn was: LAD (mm)/BW (kg)0.324. In the control group, the median LADn was 12.92 and the 95% reference range for the LADn was between 10.49 and 15.72.
Considering the most represented breeds in the control group (Labrador Retriever, Golden Retriever, Boxer, English Bulldog, Doberman Pinscher) no differences in the LADn values were found among these breeds as well as in comparison with mixed-breed dogs (P=0.08).
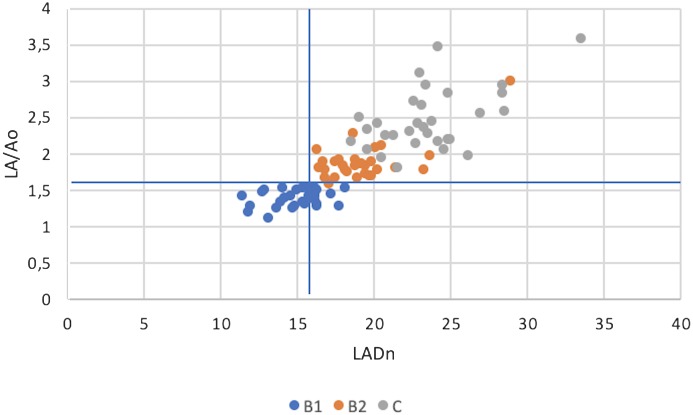
In dogs with MMVD, LADn was significantly greater (P<0.001) compared to healthy dogs and a significant difference in LADn was also noted among different clinical stages (P<0.001) (Table 1). In dogs with MMVD, a positive correlation was found between LADn and LA/Ao (r=0.87; P<0.001) (Fig. 4). LADn identified LAE in 11 dogs of the group B1, resulting in a misclassification rate of 37% vs. the LA/Ao method. In groups B2 and C, LADn and LA/Ao were perfectly concordant in identifying LAE (100% of cases).
Fig. 4.
Scatterplot showing the association between the left atrial anteroposterior diameter normalized to body weight (LADn) and left atrial-to-aortic root ratio (LA/Ao) in dogs with myxomatous mitral valve disease. A linear positive correlation was found between LADn and LA/Ao (r=0.87; P<0.001). The lines represent the threshold for identification of left atrial enlargement based on LA/Ao (1.6) and LADn (15.72).
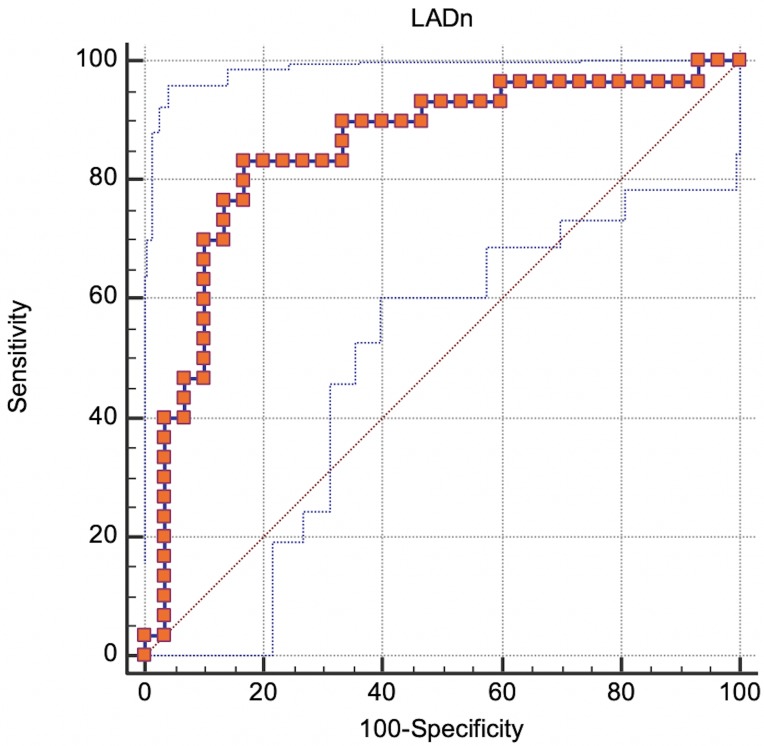
According to the ROC curve analysis, a LADn of 20.3 was the best cut-off to differentiate between dogs in groups B2 and C (sensitivity=83.3%; specificity=83.3%; AUC=0.851; P<0.001) (Fig. 5).
Fig. 5.
ROC curve analysis: true positive rate (Sensitivity) plotted in function of the false positive rate (100-Specificity) for different cut-off points. 95% confidence bounds were also reported (blue dot lines). Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a particular decision threshold. A test with perfect discrimination (no overlap in the two distributions) has a ROC curve that passes through the upper left corner (100% sensitivity, 100% specificity). Therefore, the closer the ROC curve is to the upper left corner, the higher the overall accuracy of the test.
Intraobserver and interobserver measurement variability for LAD yielded average CVs of 1.8% (95% Confidence Interval: 1.0–2.7%) and 2.2% (95% Confidence Interval: 1.0–3.4%), respectively. Intraobserver and interobserver measurement variability for LA/Ao was 4% (95% Confidence Interval: 1.1–7.0%) and 6.9% (95% Confidence Interval: 3.4–10.3%), respectively.
DISCUSSION
Our study provides the normal reference ranges for LAD and LADn in a large multiple-breed sample of healthy dogs, over a relatively wide range of BWs. This study revealed that LAD strongly correlates with BW in healthy dogs. A similar result was found in humans, cats, and horses where normalization of LAD by an allometric model successfully removes the effect of body size [7, 9, 16, 22, 28, 30, 31, 36]. Differently, the LAD did not correlate with age or sex in the present study. This is surprising, since most human studies note an age-related increase in LA size [23]. However, controversy exists regarding whether this LA enlargement is part of the normal ageing process or related to subclinical heart disease [20]. The truncated age range in our study (median 3.6 years) might have reduced our ability to observe a relationship between age and LAD.
We also found no significant differences in LAD between sex. Contrary to humans, sex differences in LA size have been reported, with men having larger LA sizes than women. However, these differences are nearly completely accounted for by variation in body size [33].
In our study, LADn increased with the severity of MMVD and had a good sensitivity and specificity in the differentiation of dogs with subclinical MMVD from dogs with clinical MMVD. In the natural history of MMVD, LA size has been shown to increase with increasing class of heart failure [4]. Thus, using the normal reference range found in the present study, LADn might be useful as an additional parameter in clinical management of dogs with MMVD.
This study also compared LADn and LA/Ao in the evaluation of LAE in dogs with MMVD.
When LADn was used to differentiate between dogs in stage B1 versus stage B2, more dogs were classified as having LAE using LADn in comparison to LA/Ao, with an overall misclassification of 37%. This discrepancy could be explained as follows. Firstly, the time points of measurement are slightly different in the two methods, with the LA/Ao method using early diastole and the LAD method using end systole. However, since these time points are very close to each other, theoretically only minimal variation in LA size is expected. Secondly, the LA is a three-dimensional structure and its enlargement may occur in an asymmetrical way. As LAD and LA/Ao do not evaluate LAE in the same plane, different LA geometries could lead to different assessment of LAE according to which method is used. For this reason, volume-based methods have been proposed to be more accurate in the detection of LAE in comparison to linear measurements [17]. A recent study in dogs showed that LAD-based estimation of LA volume has good correlation with LA volume obtained by real-time three-dimensional echocardiography [36]. However, this issue was not addressed in the present study, and critical comparisons would require a gold standard for the assessment of LAE, such as magnetic resonance imaging or validated three-dimensional echocardiography.
Measurement variability was considered good for all measurements in this study. The LAD method showed a less measurement variability in comparison with LA/Ao, especially the interobserver measurement variability was significantly lower based on the 95% confidence interval of the CV. This result is in line with a previous study in which LAD showed a very low within-day variability (4.2%) compared to LA/Ao (10.9%) [36]. A possible explanation for this discrepancy is that the LA/Ao depends on two different echocardiographic variables, each one with its own variability. Based on our results and a previous study [36], LAD has less measurement variability compared to conventional LA/Ao. This might be the consequence by defining a clear and precise far-field LA border while maintaining an appropriate view through the aortic root during image acquisition, as well as due to the difficulty to get a consistent timing of the aortic valve closure which it has been shown to affect the LA/Ao measurement [11]. Furthermore LAD measurement is not influenced by the presence of the pulmonary veins, as may occur for LA/Ao, since it is measured from the interatrial septum to the posterior free wall of the left atrium. For this reason, LAD could be more suitable for novice examiners. LA dimension is a key parameter for both diagnostic and treatment decision making process, thus we believe that LAD should be added in the standard echocardiographic evaluation of dogs, and that LAD and LA/Ao are two complementary, simple and rapid measurement that should be integrated in the overall assessment of LA size.
The present study has some limitations. First, the study group was significantly older in comparison to the control group. Given the acquired and highly prevalent nature of MMVD in dogs, an age-matched control group could not be obtained. However, a recent study in dogs found no evidence for correlation between LA size and age [14]. Second, we only included 5 breeds in the evaluation of breed influence on LAD values. However, based on this preliminary analysis, it is reasonable to believe that breed does not significantly affect LADn. Further studies may be necessary to better establish if breed-specific reference values are necessary considering differences in type of breed, size, and conformation of the thorax [15]. Third, systemic blood pressure was not measured in all dogs. Systemic hypertension could have possibly led to geometrical modification of the heart. The most common cardiac change associated with hypertensive cardiomyopathy in dogs is cardiomegaly associated with left ventricular concentric hypertrophy and the presence of aortic insufficiency [21]. However, none of the dogs in the present study had clinical or echocardiographic signs of systemic hypertension. Finally, heart rate was not considered, and this could have influenced evaluation of LA size [14]. However, no dogs showed severe tachycardia or bradycardia according to medical records.
In conclusion, this study provides the normal reference range of LAD and LADn in dogs that can be used as a complementary tool in the assessment of LAE, especially in dogs affected by MMVD. Future studies are needed to compare the accuracy of LADn and LA/Ao in the definition of LAE using a gold standard technique for evaluation of chamber size.
REFERENCES
- 1.Abbott J. A., MacLean H. N.2006. Two-dimensional echocardiographic assessment of the feline left atrium. J. Vet. Intern. Med. 20: 111–119. doi: 10.1111/j.1939-1676.2006.tb02830.x [DOI] [PubMed] [Google Scholar]
- 2.Bonagura J. D., O’Grady M. R., Herring D. S.1985. Echocardiography. Principles of interpretation. Vet. Clin. North Am. Small Anim. Pract. 15: 1177–1194. doi: 10.1016/S0195-5616(85)50364-2 [DOI] [PubMed] [Google Scholar]
- 3.Boon J., Wingfield W. E., Miller C. W.1983. Echocardiographic indices in the normal dog. Vet. Radiol. 24: 214–221. doi: 10.1111/j.1740-8261.1983.tb00718.x [DOI] [Google Scholar]
- 4.Borgarelli M., Haggstrom J.2010. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet. Clin. North Am. Small Anim. Pract. 40: 651–663. doi: 10.1016/j.cvsm.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Borgarelli M., Savarino P., Crosara S., Santilli R. A., Chiavegato D., Poggi M., Bellino C., La Rosa G., Zanatta R., Haggstrom J., Tarducci A.2008. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J. Vet. Intern. Med. 22: 120–128. doi: 10.1111/j.1939-1676.2007.0008.x [DOI] [PubMed] [Google Scholar]
- 6.Boswood A., Häggström J., Gordon S. G., Wess G., Stepien R. L., Oyama M. A., Keene B. W., Bonagura J., MacDonald K. A., Patteson M., Smith S., Fox P. R., Sanderson K., Woolley R., Szatmári V., Menaut P., Church W. M., O’Sullivan M. L., Jaudon J. P., Kresken J. G., Rush J., Barrett K. A., Rosenthal S. L., Saunders A. B., Ljungvall I., Deinert M., Bomassi E., Estrada A. H., Fernandez Del Palacio M. J., Moise N. S., Abbott J. A., Fujii Y., Spier A., Luethy M. W., Santilli R. A., Uechi M., Tidholm A., Watson P.2016. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial. J. Vet. Intern. Med. 30: 1765–1779. doi: 10.1111/jvim.14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown D. J., Rush J. E., MacGregor J., Ross J. N., Jr., Brewer B., Rand W. M.2003. M-mode echocardiographic ratio indices in normal dogs, cats, and horses: a novel quantitative method. J. Vet. Intern. Med. 17: 653–662. doi: 10.1111/j.1939-1676.2003.tb02496.x [DOI] [PubMed] [Google Scholar]
- 8.Chetboul V., Tissier R.2012. Echocardiographic assessment of canine degenerative mitral valve disease. J. Vet. Cardiol. 14: 127–148. doi: 10.1016/j.jvc.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Cornell C. C., Kittleson M. D., Della Torre P., Häggström J., Lombard C. W., Pedersen H. D., Vollmar A., Wey A.2004. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 18: 311–321. doi: 10.1111/j.1939-1676.2004.tb02551.x [DOI] [PubMed] [Google Scholar]
- 10.Cunningham S. M., Rush J. E., Freeman L. M., Brown D. J., Smith C. E.2008. Echocardiographic ratio indices in overtly healthy Boxer dogs screened for heart disease. J. Vet. Intern. Med. 22: 924–930. doi: 10.1111/j.1939-1676.2008.0121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson D., Caivano D., Patteson M., Rishniw M.2016. The times they are a-changin’: Two-dimensional aortic valve measurements differ throughout diastole. J. Vet. Cardiol. 18: 15–25. doi: 10.1016/j.jvc.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Häggström J., Boswood A., O’Grady M., Jöns O., Smith S., Swift S., Borgarelli M., Gavaghan B., Kresken J. G., Patteson M., Ablad B., Bussadori C. M., Glaus T., Kovacević A., Rapp M., Santilli R. A., Tidholm A., Eriksson A., Belanger M. C., Deinert M., Little C. J., Kvart C., French A., Rønn-Landbo M., Wess G., Eggertsdottir A. V., O’Sullivan M. L., Schneider M., Lombard C. W., Dukes-McEwan J., Willis R., Louvet A., DiFruscia R.2008. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J. Vet. Intern. Med. 22: 1124–1135. doi: 10.1111/j.1939-1676.2008.0150.x [DOI] [PubMed] [Google Scholar]
- 13.Hansson K., Häggström J., Kvart C., Lord P.2002. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 43: 568–575. doi: 10.1111/j.1740-8261.2002.tb01051.x [DOI] [PubMed] [Google Scholar]
- 14.Höllmer M., Willesen J. L., Tolver A., Koch J.2013. Left atrial volume and phasic function in clinically healthy dogs of 12 different breeds. Vet. J. 197: 639–645. doi: 10.1016/j.tvjl.2013.05.045 [DOI] [PubMed] [Google Scholar]
- 15.Höllmer M., Willesen J. L., Tolver A., Koch J.2016. Comparison of four echocardiographic methods to determine left atrial size in dogs. J. Vet. Cardiol. 18: 137–145. doi: 10.1016/j.jvc.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Huesler I. M., Mitchell K. J., Schwarzwald C. C.2016. Echocardiographic assessment of left atrial size and function in Warmblood Horses: reference intervals, allometric scaling, and agreement of different echocardiographic variables. J. Vet. Intern. Med. 30: 1241–1252. doi: 10.1111/jvim.14368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keene B. W., Atkins C. E., Bonagura J. D., Fox P. R., Häggström J., Fuentes V. L., Oyama M. A., Rush J. E., Stepien R., Uechi M.2019. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 33: 1127–1140. doi: 10.1111/jvim.15488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang R. M., Badano L. P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F. A., Foster E., Goldstein S. A., Kuznetsova T., Lancellotti P., Muraru D., Picard M. H., Rietzschel E. R., Rudski L., Spencer K. T., Tsang W., Voigt J. U.2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16: 233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc N., Scollan K., Sisson D.2016. Quantitative evaluation of left atrial volume and function by one-dimensional, two-dimensional, and three-dimensional echocardiography in a population of normal dogs. J. Vet. Cardiol. 18: 336–349. doi: 10.1016/j.jvc.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 20.Leung D. Y., Boyd A., Ng A. A., Chi C., Thomas L.2008. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am. Heart J. 156: 1056–1064. doi: 10.1016/j.ahj.2008.07.021 [DOI] [PubMed] [Google Scholar]
- 21.Misbach C., Gouni V., Tissier R., Trehiou-Sechi E., Petit A. M., Carlos Sampedrano C., Pouchelon J. L., Chetboul V.2011. Echocardiographic and tissue Doppler imaging alterations associated with spontaneous canine systemic hypertension. J. Vet. Intern. Med. 25: 1025–1035. doi: 10.1111/j.1939-1676.2011.0771.x [DOI] [PubMed] [Google Scholar]
- 22.Neilan T. G., Pradhan A. D., Weyman A. E.2008. Derivation of a size-independent variable for scaling of cardiac dimensions in a normal adult population. J. Am. Soc. Echocardiogr. 21: 779–785. doi: 10.1016/j.echo.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 23.Nikitin N. P., Witte K. K. A., Thackray S. D. R., Goodge L. J., Clark A. L., Cleland J. G. F.2003. Effect of age and sex on left atrial morphology and function. Eur. J. Echocardiogr. 4: 36–42. doi: 10.1053/euje.4.1.36 [DOI] [PubMed] [Google Scholar]
- 24.O’Grady M. R., Bonagura J. D., Powers J. D., Herring D. S.1986. Quantitative cross-sectional echocardiography in the normal dog. Vet. Radiol. 27: 34–49. doi: 10.1111/j.1740-8261.1986.tb00001.x [DOI] [Google Scholar]
- 25.Quiñones M. A., Otto C. M., Stoddard M., Waggoner A., Zoghbi W. A., Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. 2002. Recommendations for quantification of Doppler echocardiography: a report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 15: 167–184. doi: 10.1067/mje.2002.120202 [DOI] [PubMed] [Google Scholar]
- 26.Rishniw M., Erb H. N.2000. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J. Vet. Intern. Med. 14: 429–435. doi: 10.1111/j.1939-1676.2000.tb02252.x [DOI] [PubMed] [Google Scholar]
- 27.Rishniw M.2016. Interobserver variability in two-dimensional echocardiographic left atrial measurements is complex. Research Communication, 25th ECVIM-CA Congress, Lisbon, Portugal.
- 28.Rovira S., Muñoz A., Rodilla V.2009. Allometric scaling of echocardiographic measurements in healthy Spanish foals with different body weight. Res. Vet. Sci. 86: 325–331. doi: 10.1016/j.rvsc.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 29.Sargent J., Muzzi R., Mukherjee R., Somarathne S., Schranz K., Stephenson H., Connolly D., Brodbelt D., Fuentes V. L.2015. Echocardiographic predictors of survival in dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 17: 1–12. doi: 10.1016/j.jvc.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 30.Scansen B. A., Morgan K. L.2015. Reference intervals and allometric scaling of echocardiographic measurements in Bengal cats. J. Vet. Cardiol. 17 Suppl 1: S282–S295. doi: 10.1016/j.jvc.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 31.Schober K., Stephanie S., Vedat Y.2017. Reference intervals and allometric scaling of two-dimensional echocardiographic measurements in 150 healthy cats. J. Vet. Med. Sci. 79: 1764–1771. doi: 10.1292/jvms.17-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith S., Dukes-McEwan J.2012. Clinical signs and left atrial size in cats with cardiovascular disease in general practice. J. Small Anim. Pract. 53: 27–33. doi: 10.1111/j.1748-5827.2011.01143.x [DOI] [PubMed] [Google Scholar]
- 33.Spencer K. T., Mor-Avi V., Gorcsan J., 3rd., DeMaria A. N., Kimball T. R., Monaghan M. J., Perez J. E., Weinert L., Bednarz J., Edelman K., Kwan O. L., Glascock B., Hancock J., Baumann C., Lang R. M.2001. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart 85: 272–277. doi: 10.1136/heart.85.3.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strohm L. E., Visser L. C., Chapel E. H., Drost W. T., Bonagura J. D.2018. Two-dimensional, long-axis echocardiographic ratios for assessment of left atrial and ventricular size in dogs. J. Vet. Cardiol. 20: 330–342. doi: 10.1016/j.jvc.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 35.Thomas W. P., Gaber C. E., Jacobs G. J., Kaplan P. M., Lombard C. W., Moise N. S., Moses B. L.1993. Recommendations for standards in transthoracic two-dimensional echocardiography in dogs and cats. J. Vet. Intern. Med. 7: 247–252. doi: 10.1111/j.1939-1676.1993.tb01015.x [DOI] [PubMed] [Google Scholar]
- 36.Tidholm A., Bodegård-Westling A., Höglund K., Ljungvall I., Häggström J.2011. Comparisons of 2- and 3-dimensional echocardiographic methods for estimation of left atrial size in dogs with and without myxomatous mitral valve disease. J. Vet. Intern. Med. 25: 1320–1327. doi: 10.1111/j.1939-1676.2011.00812.x [DOI] [PubMed] [Google Scholar]
- 37.Vezzosi T., Schober K. E.2019. Doppler-derived echocardiographic evidence of pulmonary hypertension in cats with left-sided congestive heart failure. J. Vet. Cardiol. 23: 58–68. doi: 10.1016/j.jvc.2019.01.007 [DOI] [PubMed] [Google Scholar]