Abstract
Babesia rossi infection has been reported to be associated with the high prevalence of pancreatitis in dogs. In this study, we retrospectively investigated whether pancreatitis occurs in B. gibsoni-infected dogs. The clinical manifestations, and hematological and serum biochemical examination results, including canine pancreatic-specific lipase (cPL), in 20 B. gibsoni-infected dogs were analyzed. The cPL concentration exceeded 400 µg/l in only 2 dogs, and they were suspected of having pancreatitis. Although the cPL concentration did not correlate with the degree of anemia or the level of parasitemia, it correlated with the band neutrophil count, platelet count, and blood urea nitrogen (BUN) level. Our study suggested that the prevalence of pancreatitis is lower among B. gibsoni-infected dogs than B. rossi-infected dogs.
Keywords: Babesia gibsoni, pancreatitis, retrospective study
Canine babesiosis, a tick-borne hematozoan disease, is caused by Babesia parasites. This disease is characterized by fever, lethargy, and anemia, and some cases may be severe, causing death. Babesia gibsoni, B. canis, B. vogeli, B. rossi, and B. conradae are well-known Babesia species in canine hosts [3, 6]. The degree of severity of canine babesiosis may depend on the causative Babesia species [11]. In particular, B. rossi, the dominant species found in South Africa, is highly virulent and causes acute disease, including acute kidney injury, neural symptoms, hepatic disorder, and pancreatitis [7]. These complications are considered to be the result of tissue hypoxia following anemia and concomitant systemic inflammatory response syndrome (SIRS) caused by marked cytokine release [7]. It was previously reported that B. rossi-infected dogs presenting with SIRS had a significantly higher serum pancreatic-specific lipase (Spec cPL) concentration than dogs that did not meet the criteria for SIRS, and that more than 28% of B. rossi-infected dogs had acute pancreatitis [7]. Spec cPL is one examination performed to detect canine pancreatitis and is useful for screening [10].
In Japan, B. gibsoni is an important pathogen for canine babesiosis and its infection follows a hyper-acute, acute, or chronic course [12]. The acute course is the most common, and dogs usually present increased C-reactive protein (CRP) [2] and liver enzyme levels such as alanine aminotransferase (ALT) and aspartic aminotransferase (AST) [12]. However, to our best knowledge, pancreatitis and increased Spec cPL have not been reported to be associated with B. gibsoni infection. In the present study, we retrospectively assessed whether acute pancreatitis develops in B. gibsoni-infected dogs.
Serum samples and clinical data were collected at a private animal hospital located in Yamaguchi Prefecture in western Japan. A total of 20 dogs diagnosed with acute canine babesiosis in 2014–2015 were examined in this study. The study population of dogs had a mean age of 6.9 ± 3.7 years old. Canine babesiosis was diagnosed by detecting Babesia parasites using Giemsa staining of thin blood smears. As Yamaguchi Prefecture has been an endemic area of B. gibsoni, all 20 dogs were considered to be infected with B. gibsoni. At the diagnosis, ticks were detected on three dogs (dog 9, 14 and 19). Moreover, 17 dogs had taken a walk out of door every day. Serum was collected with informed owner consent after the diagnosis of canine babesiosis and stocked at −15°C at the private animal hospital until later analysis.
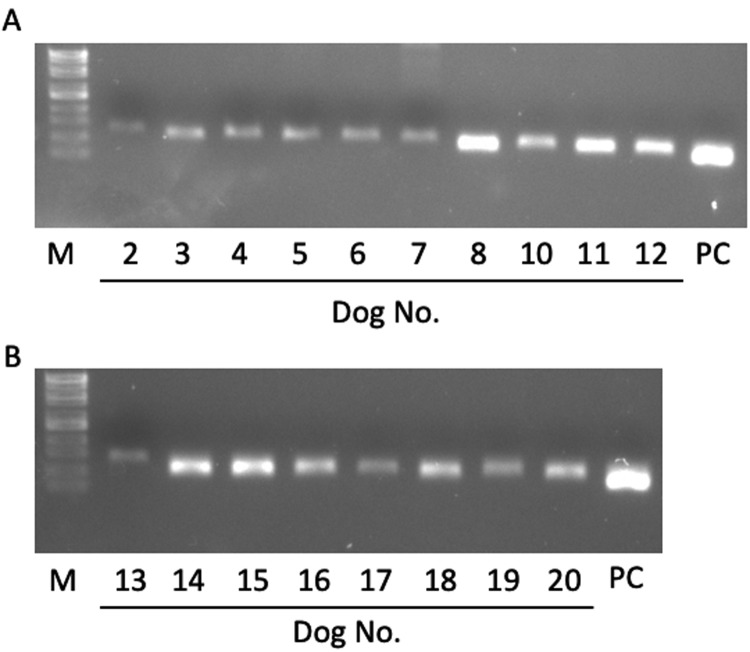
All dogs underwent a physical examination, complete blood cell count (CBC), blood biochemical analysis, and measurement of the level of parasitemia at the private animal hospital before treatment (Table 1). The range of parasitemia was 0.04−5.01%, and the hematocrit values were 9–46%. The criterion of SIRS for dog was reported previously [4]. In the present study, the heart rate and respiratory rate of many dogs were not recorded. Although we could not determine dogs with SIRS, at least seven dogs (dog 2, 4, 5, 6, 10, 11 and 12) were suspected as SIRS. No dogs presented the clinical signs of pancreatitis, such as vomiting and abdominal tenderness, at the first examination. After the stored sera were transported on ice to Iwate University, the lipase activity (reference range: 13–200 U/l) was measured using an autoanalyzer (IDEXX VetTest Chemistry Analyzer, IDEXX Laboratories, Inc., Westbrook, ME, U.S.A.). Spec cPL was measured by a commercial laboratory (IDEXX Laboratories, Tokyo, Japan). The reference interval was set at <200 µg/l and concentrations >400 µg/l were considered to indicate pancreatitis [10]. Moreover, the identification of species of Babesia parasites was performed using PCR. Genomic DNA was extracted from dog serum using NucleoSpin® Blood (Macherey-Nagel GmbH & Co, KG, Düren, Germany). Since serum of dog 1 and 9 were not enough for the DNA extraction, DNA were extracted from 18 dog serum. The primers used for the amplification of 18S rRNA of B. gibsoni were described previously [14]. Genomic DNA in reaction mixture were prepared according to the manufacture’s protocol (PlatinumTMTaq DNA polymerase, Thermo Fisher Scientific K. K., Tokyo, Japan), and then, it was amplified for 35 cycles (denaturation for 30 sec at 94°C, annealing for 30 sec at 55°C and extension for 30 sec at 72°C) followed by the final extension for 5 min at 72°C in a T100TM Thermal Cycler (BIO-RAD Laboratories, Inc., CA, U.S.A.). As a result, the fragments of 18S rRNA of B. gibsoni were detected from 18 dogs tested, suggesting that those 18 dogs were infected with B. gibsoni (Fig. 1). According to the previous studies, B. vogeli which is mainly detected in Okinawa Prefecture in Southern Japan was not identified in Yamaguchi Prefecture [5, 8]. It was considered that dog 1 and 9 were also infected with B. gibsoni. Data were analyzed using a commercial statistical software package (StatView5, SAS institute Inc., Cary, NC, U.S.A.). The correlation of lipase activity, cPL concentration, degree of anemia, the level of parasitemia, and the results of clinical pathological parameters were evaluated using Pearson product-moment correlation and the test of no correlation (r≥0.7 was strong correlation, 0.4≤ r<0.7 was moderate correlation, 0.2≤ r<0.4 was weak correlation, and r<0.2 was no correlation). A P-value of ≤0.05 was considered significant.
Table 1. The results of the Spec canine pancreatic-lipase (cPL) value, parasitemia, the parameters of complete blood cell count, biochemical parameters in dogs with Babesia gibsoni infection.
| Dog No. |
||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Breed | T. P. | S. T. | M. D. | Shiba | CH | M. D. | M. D. | M. D. | Shiba | PM |
| Sex | Male | Female | Male | Female | Male | Male | Female | Male | Male | Male |
| Age | 9 | 4 | 9 | 2 | 5 | 6 | 6 | 15 | 7 | 8 |
| Body temperature (°C) | 39.6 | 39.3 | 39.8 | 39.2 | 39.5 | 40.3 | 39.8 | 38.9 | 40.1 | 39.2 |
| Respiratory rate (/min) | 20 | ND | 20 | 30 | ND | ND | ND | ND | ND | 12 |
| Spec cPL (µg/l) | 73 | 104 | 104 | 66 | 49 | 60 | 44 | 234 | 186 | 124 |
| Lipase (U/l) | 349 | 505 | 407 | 1,662 | 457 | 427 | 709 | 640 | 366 | 377 |
| Parasitemia (%) | 2.85 | 0.3 | 1.5 | 0.18 | 0.41 | 0.06 | 0.3 | 1.4 | 5.01 | 1.3 |
| WBC (/µl) | 12,200 | 4,800 | 11,200 | 10,000 | 4,200 | 3,800 | 6,400 | 14,400 | 15,924 | 28,225 |
| Band neutrophil | 0 | 48 | 0 | 0 | 84 | 0 | 0 | 144 | 0 | 282 |
| Segmented neutrophil | 10,126 | 3,120 | 8,288 | 8,600 | 2,562 | 2,964 | 4,416 | 8,532 | 15,526 | 21,733 |
| Lymphocyte | 732 | 1,248 | 2,576 | 400 | 1,218 | 152 | 1,472 | 5,472 | 79 | 4,798 |
| Monocyte | 1,220 | 336 | 224 | 900 | 336 | 684 | 512 | 288 | 318 | 1,411 |
| Eosinophil | 122 | 48 | 112 | 100 | 0 | 0 | 0 | 144 | 0 | 0 |
| RBC (×106/µl) | 2.67 | 4.80 | 3.03 | 2.55 | 5.38 | 4.85 | 6.34 | 4.83 | 1.85 | 1.00 |
| Hb (g/dl) | 6.3 | 11.4 | 7.5 | 5.6 | 12.0 | 11.8 | 15.7 | 11.6 | 4.0 | 8.6 |
| Ht (%) | 19 | 30 | 21 | 15 | 38 | 35 | 46 | 35 | 11 | 9 |
| Plat (×103/µl) | 41 | 9 | 46 | 28 | 41 | 11 | 12 | 29 | 12 | 47 |
| Glucose (mg/dl) | 99 | 89 | 103 | 97 | 110 | 111 | 105 | 95 | 91 | 103 |
| Total protein (g/dl) | 6.2 | 6.0 | 6.0 | 6.8 | 6.0 | 6.8 | 7.8 | 5.6 | 6.6 | 6.2 |
| Albumin (g/dl) | 2.3 | 2.6 | 2.9 | 2.8 | 2.9 | 3.0 | 3.5 | 2.4 | 2.4 | 2.1 |
| ALT (U/l) | 38 | 60 | 57 | 21 | 61 | 69 | 51 | 48 | 29 | 499 |
| AST (U/l) | 30 | 62 | 33 | 21 | 49 | 72 | 56 | 37 | 50 | 112 |
| ALP (U/l) | 391 | 680 | 279 | 365 | 636 | 418 | 342 | 417 | 128 | >3,500 |
| Total cholesterol (mg/dl) | 173 | 225 | 114 | 175 | 265 | 181 | 289 | 209 | 162 | 248 |
| Total bilirubin (mg/dl) | 0.7 | 0.5 | 0.9 | 0.4 | 0.3 | 0.7 | 1.7 | 0.4 | 1.1 | 1.4 |
| BUN (mg/dl) | 21.7 | 14.9 | 10.8 | 27.9 | 15.5 | 11.2 | 19.7 | 22.8 | 16.0 | 27.4 |
| Creatinine (mg/dl) | 0.3 | 0.4 | 0.3 | 0.7 | 0.4 | 0.3 | 0.5 | 0.5 | 0.5 | 0.3 |
| Na (mEq/l) | 148 | ND | 145 | 149 | 143 | 144 | 148 | 148 | 147 | 151 |
| K (mEq/l) | 3.4 | ND | 3.7 | 4.0 | 3.8 | 4.1 | 3.5 | 4.1 | 3.6 | 4.1 |
| Cl (mEq/l) | 119 | ND | 114 | 117 | 113 | 110 | 121 | 112 | 119 | 115 |
| Ca (mg/dl) | 8.1 | 8.5 | 8.8 | 9.5 | 8.6 | 9.3 | 9.6 | 8.7 | 8.3 | 8.9 |
| CRP (mg/dl) | >20 | >20 | 15 | 13 | >20 | >20 | 16 | 18 | 15 | 13 |
|
Dog No. |
||||||||||
| 11 | 12* | 13 | 14* | 15 | 16 | 17 | 18 | 19 | 20 | |
| Breed | PL | M. D. | mix | S. T. | BG | M. D. | CH | S. T. | mix | CKCS |
| Sex | Male | Female | Male | Female | Female | Female | Female | Male | Female | Male |
| Age | 7 | 8 | 10 | UK | 3 | 1 | 10 | 13 | 0 | 7 |
| Body temperature (°C) | 39.7 | 40.3 | 40.7 | 38.0 | 39.9 | 39.0 | 38.3 | 38.7 | 40.5 | 38.8 |
| Respiratory rate (/min) | 30 | 40 | ND | 20 | ND | 20 | ND | ND | ND | ND |
| Spec cPL (µg/l) | 48 | 661 | 92 | 655 | 52 | 78 | 58 | 198 | 88 | 112 |
| Lipase (U/l) | 309 | 2,111 | 236 | 1,343 | 295 | 333 | 224 | 675 | 293 | 553 |
| Parasitemia (%) | 0.4 | 0.27 | 0.16 | 0.62 | 2.0 | 0.11 | 0.04 | 0.8 | 0.06 | 0.94 |
| WBC (/µl) | 9,300 | 7,129 | 6,100 | 29,000 | 8,727 | 16,900 | 16,200 | 4,074 | 8,800 | 18,300 |
| Band neutrophil | 0 | 0 | 0 | 676 | 0 | 338 | 0 | 0 | 0 | 296 |
| Segmented neutrophil | 7,174 | 5,775 | 4,036 | 25,520 | 6,894 | 13,182 | 14,742 | 1,140 | 5,368 | 8,436 |
| Lymphocyte | 1,594 | 1,069 | 610 | 1,740 | 1,309 | 2,873 | 972 | 2,077 | 3,344 | 4,958 |
| Monocyte | 88 | 142 | 854 | 966 | 523 | 507 | 486 | 855 | 88 | 888 |
| Eosinophil | 0 | 142 | 0 | 96 | 0 | 0 | 0 | 0 | 0 | 222 |
| RBC (×106/µl) | 3.59 | 3.96 | 6.80 | 1.88 | 2.02 | 0.79 | 1.93 | 3.37 | 3.45 | 1.83 |
| Hb (g/dl) | 9.0 | 9.4 | 15.7 | 4.6 | 5.0 | 2.3 | 4.5 | 7.6 | 7.1 | 4.6 |
| Ht (%) | 27 | 26 | 44 | 14 | 16 | 9 | 12 | 22 | 25 | 14 |
| Plat (×103/µl) | 23 | 52 | 24 | 99 | 17 | 71 | 89 | 81 | 24 | 13 |
| Glucose (mg/dl) | 96 | 79 | 91 | 88 | 94 | 89 | 110 | 101 | 89 | 90 |
| Total protein (g/dl) | 7.6 | 6.8 | 8.4 | 6.8 | 6.8 | 7.2 | 8.0 | 7.4 | 7.0 | 6.6 |
| Albumin (g/dl) | 2.7 | 2.2 | 3.0 | 2.4 | 2.2 | 2.1 | 2.7 | 3.0 | 2.5 | 2.7 |
| ALT (U/l) | 26 | 15 | 44 | 33 | 27 | >1,000 | 63 | 66 | 12 | 28 |
| AST (U/l) | 59 | 56 | 52 | 35 | 49 | 623 | 21 | 25 | 91 | 25 |
| ALP (U/l) | 573 | 353 | 979 | 287 | 192 | 432 | 1,310 | 382 | 847 | 279 |
| Total cholesterol (mg/dl) | 201 | 112 | 411 | 124 | 129 | 114 | 175 | 298 | 372 | 176 |
| Total bilirubin (mg/dl) | 1.1 | 0.5 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 | 0.5 | 0.4 | 0.9 |
| BUN (mg/dl) | 12.8 | 45.3 | 10.6 | 45.9 | 13.5 | 37.3 | 30.5 | 60.1 | 9.0 | 54.6 |
| Creatinine (mg/dl) | 0.4 | 0.7 | 0.7 | 0.3 | 0.3 | 0.2 | 0.3 | 1.0 | 0.9 | 0.2 |
| Na (mEq/l) | 147 | 152 | 148 | 142 | 147 | 148 | 146 | 150 | 130 | 147 |
| K (mEq/l) | 3.9 | 4.1 | 3.5 | 3.3 | 3.3 | 3.7 | 3.3 | 4.1 | 3.3 | 3.8 |
| Cl (mEq/l) | 116 | 121 | 113 | 107 | 112 | 121 | 109 | 109 | 110 | 112 |
| Ca (mg/dl) | 9.0 | 9.0 | 9.2 | 7.8 | 9.4 | 8.6 | 8.5 | 10.7 | 8.9 | 9.0 |
| CRP (mg/dl) | >20 | 12 | >20 | >20 | >20 | 9.1 | >20 | 4.6 | >20 | >20 |
T. P.=Toy Poodle, S. T.=Shih Tzu, M. D.=Miniature Dachshund, CH=Chihuahua, PM=Pomeranian, PL=Papillon, BG=Beagle, CKCS=Cavalier King Charles Spaniel, mix=mix breed. ND=not determined. UK=unknown. *Dogs having high Spec cPL concentration.
Fig. 1.
The detection of the partial 18S rRNA of Babesia gibsoni in 18 dogs. Gel electrophoresis analysis of PCR products from 18 dog serum. Lane number showed the dog number. Lane PC, positive control (genomic DNA from cultured B. gibsoni isolate). Lane M, molecular marker.
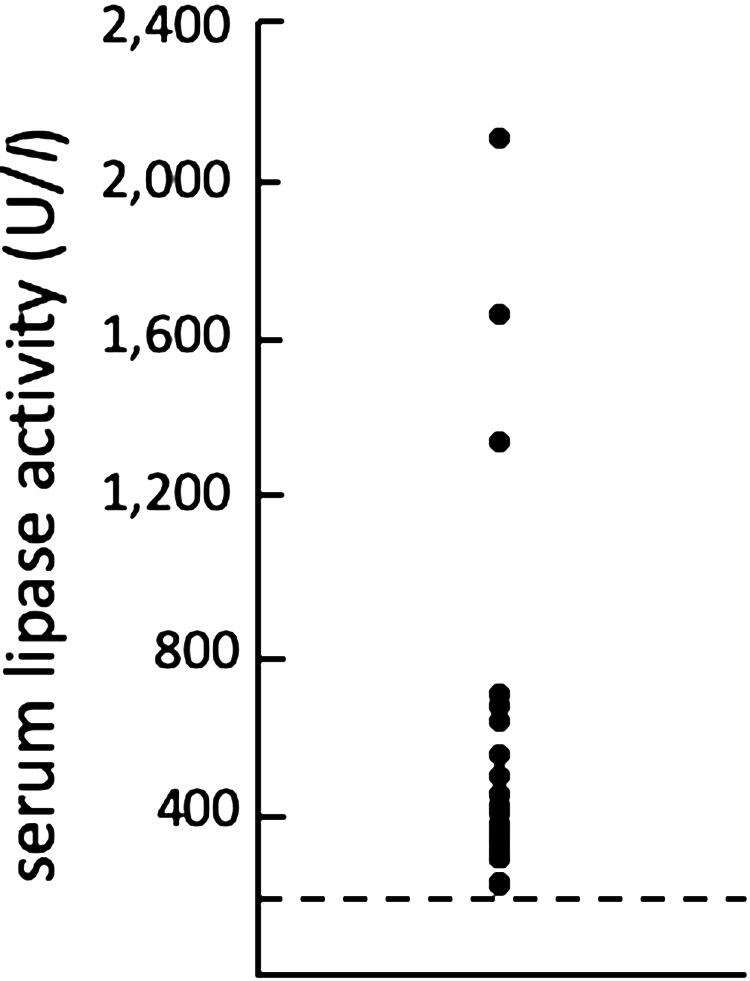
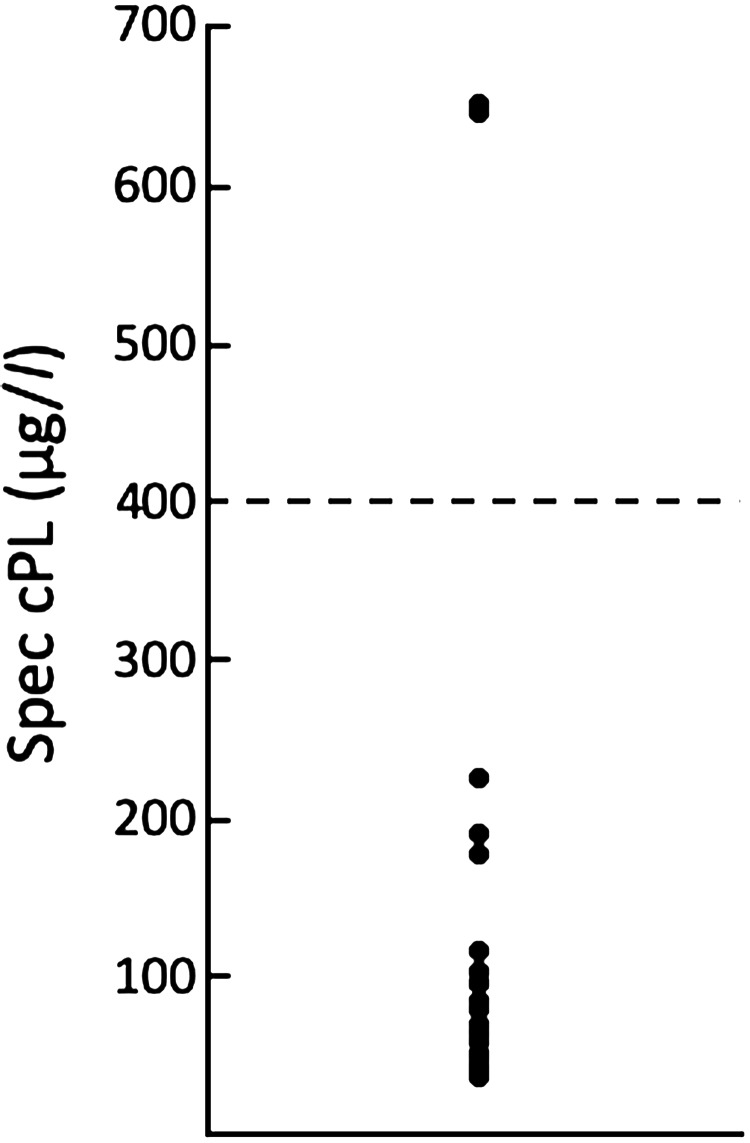
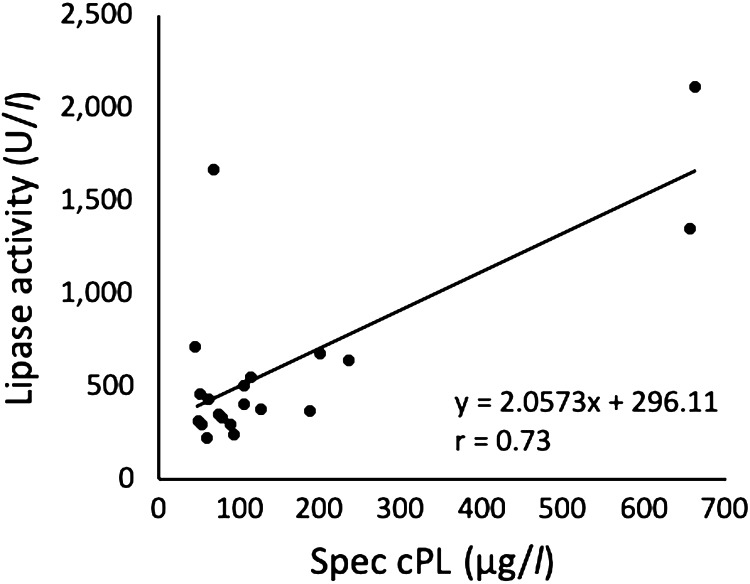
To investigate the prevalence of pancreatitis, the lipase activity and the Spec cPL concentration were examined. As shown in Fig. 2, all dogs had lipase activity greater than 200 U/l. The range of the Spec cPL concentration for all tested dogs was 44–655 µg/l (Fig. 3). Although one dog (dog 4) with high lipase activity had a low cPL concentration, the cPL concentrations were strongly positively correlated with the lipase activity (r=0.73; P=0.0002) (Fig. 4). Two of 20 dogs (dog 12 and 14) had a cPL concentration above 400 µg/l and they were suspected of having pancreatitis based on the reference range of Spec cPL. In our study, the prevalence of pancreatitis among B. gibsoni-infected dogs was 10% (2/20). Although dog 12 was suspected as SIRS, dog 14 was not suspected as SIRS. The CBC and serum biochemical parameter measurements of all dogs are shown in Table 1. In 2 dogs (dog 12 and 14), the concentrations of CRP and alkaline phosphatase (ALP), which are supportive parameters for pancreatitis, were higher than the normal ranges. In the field of medicine, hypotension, ischemia, and circulation insufficiency are known causes of acute pancreatitis [1]. Therefore, we suspected that anemia induced pancreatitis in the B. gibsoni-infected dogs. However, the degree of anemia and the level of parasitemia in the two dogs were moderate among the 20 dogs (Table 1). Therefore, the degree of anemia and the level of parasitemia may not be related to the pathogenesis of pancreatitis. Additionally, dogs with hyperadrenocorticism or glucocorticoid administration were reported to have a high cPL concentration [1, 9]. Thus, the increased cPL concentration in the two dogs was due to other causes.
Fig. 2.
A plot depicting the serum lipase activity in 20 dogs diagnosed with acute Babesia gibsoni infection. The dashed line indicates the upper limit of the reference range (200 U/l).
Fig. 3.
A plot depicting the Spec canine pancreatic-lipase (cPL) concentration in 20 dogs diagnosed with acute Babesia gibsoni infection. The dashed line indicates the threshold for the diagnosis of canine pancreatitis (400 µg/dl).
Fig. 4.
Relationship between the Spec canine pancreatic-lipase (cPL) concentration and the lipase activity in 20 dogs infected with Babesia gibsoni.
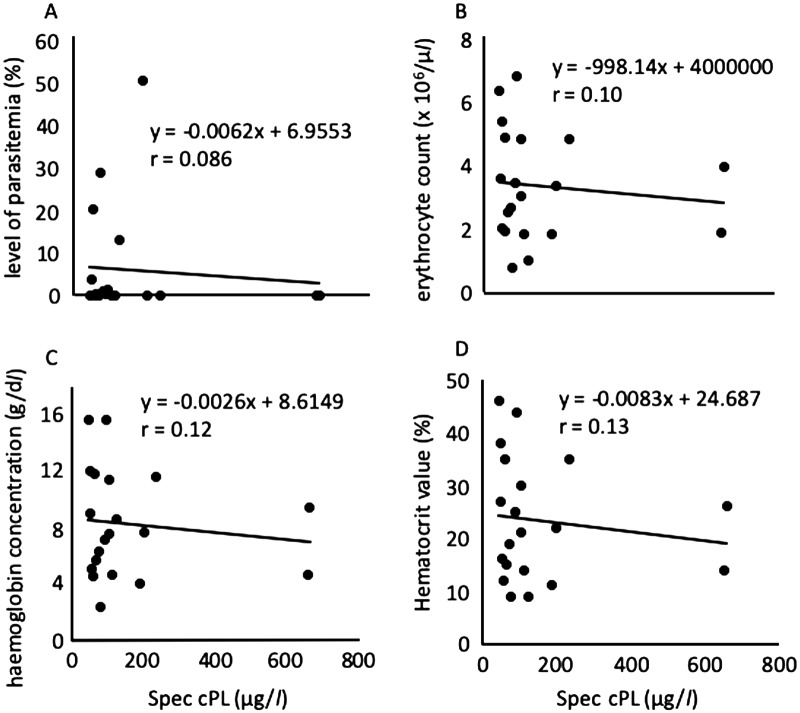
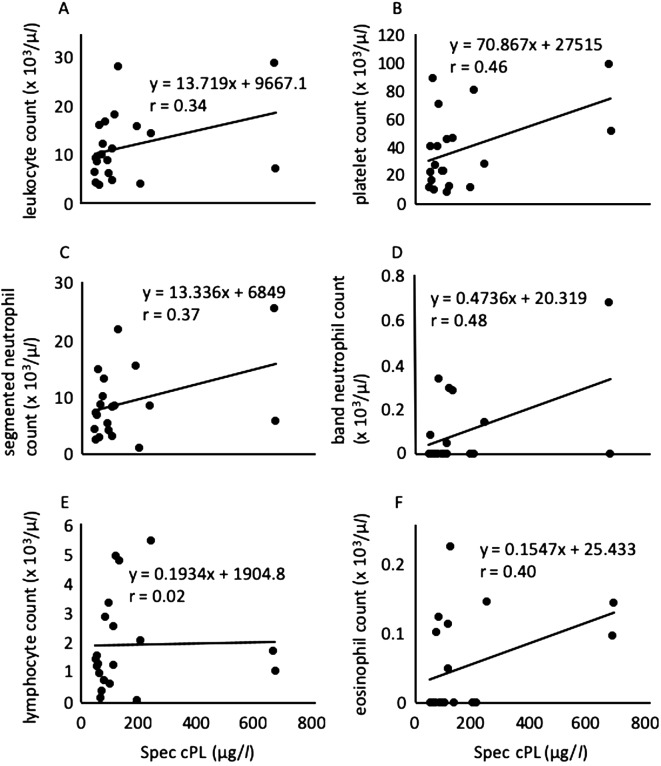
We next analyzed the correlation between the cPL concentration and the level of parasitemia, the degree of anemia (erythrocyte count, and hemoglobin [Hb] and hematocrit [Ht] levels), and leukocyte and platelet counts in all tested dogs (Figs. 5 and 6). Consistent with the above supposition, the cPL concentration was not correlated with the level of parasitemia (r=0.086; P=0.72), erythrocyte count (r=0.10; P=0.66), Hb level (r=0.12; P=0.61), or Ht level (r=0.13; P=0.58) (Fig. 5). The cPL concentration was previously reported not to correlate with the level of parasitemia or the degree of anemia in B. rossi-infected dogs [7]. Similarly, the lipase activity was not correlated with the level of parasitemia or the degree of anemia (data not shown). These results suggested that B. gibsoni does not directly damage the pancreas and that the severity of anemia in B. gibsoni infection is not related to the pancreatitis. On the other hand, the cPL concentration was moderately positively correlated with the band neutrophil count (r=0.48; P=0.031) (Fig. 6D) and platelet count (r=0.46; P=0.040) (Fig. 6B). However, it was not correlated with the leukocyte count (r=0.34; P=0.15), segmented neutrophil count (r=0.37; P=0.10), lymphocyte count (r=0.02; P=0.93), or eosinophil count (r=0.40; P=0.08) (Fig. 6A, 6C, 6E and 6F). The cPL concentration in B. rossi-infected dogs was significantly positively correlated with the immature neutrophil count [7]. This previous finding is consistent with the result of this study. The inflammation with pancreatitis may be related to the increase in the band neutrophil and platelet counts.
Fig. 5.
Relationship between the Spec canine pancreatic-lipase (cPL) concentration and the level of parasitemia (A), erythrocyte count (B), haemoglobin concentration (C), and Hematocrit value (D) in 20 dogs infected with Babesia gibsoni.
Fig. 6.
Relationship between the Spec canine pancreatic-lipase (cPL) concentration and leukocyte count (A), platelet count (B), segmented neutrophil count (C), band neutrophil count (D), lymphocyte count (E), and eosinophil count (F) in 20 dogs infected with Babesia gibsoni.
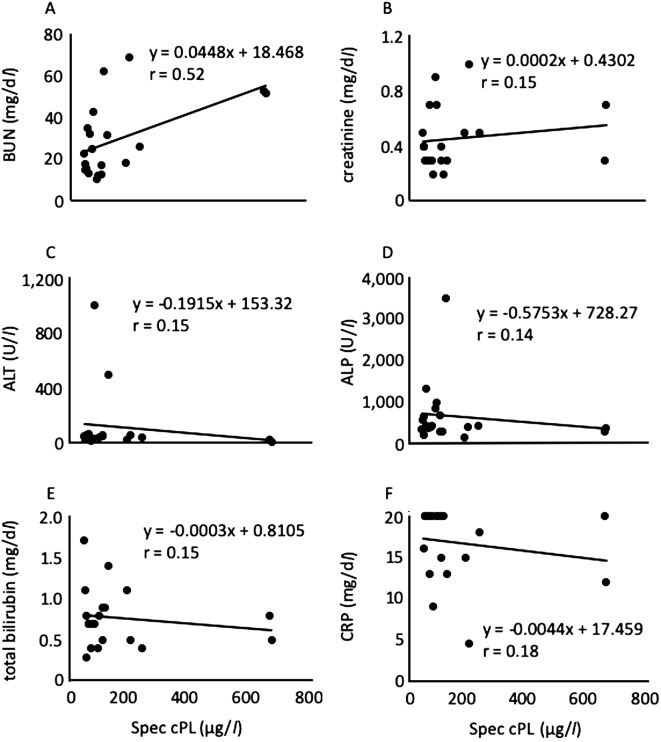
In addition, we examined the correlation between the cPL concentration and the other biochemical parameters. In the present study, the values of ALT (dog 16), ALP (dog 10) and CRP (dog 1, 2, 5, 6, 11, 13, 14, 15, 17, 19 and 20) were greater than the measurement limit. Therefore, the values of measurement limit were used for the analysis. As a result, the blood urea nitrogen (BUN) level was moderately positively correlated (r=0.52; P=0.019) with the cPL concentration (Fig. 7A), whereas CRP (r=0.18; P=0.46), ALT (r=0.15; P=0.53), ALP (r=0.14; P=0.55), total bilirubin (r=0.17; P=0.47), and creatinine (r=0.15; P=0.52) levels were not correlated (Fig. 7B–F). Additionally, the cPL concentrations of dogs having high concentration of CRP were less than 200 (µg/l) except for dog 14. Moreover, the BUN level was moderately positively correlated (r=0.48; P=0.033) with the lipase activity. The BUN level was previously reported to be a prognosis factor for pancreatitis [13]. The BUN levels of the 2 dogs suspected of having pancreatitis in this study (dog 12 and 14) were higher than the normal range (Table 1). Accordingly, it is possible that the BUN level is related to the severity of pancreatitis in canine B. gibsoni infection, although the reason remains unknown.
Fig. 7.
Relationship between the Spec canine pancreatic-lipase (cPL) concentration and blood urea nitrogen (BUN) (A), creatinine (B), alanine aminotransferase (ALT) (C), Alkaline phosphatase (ALP) (D), total bilirubin (E), and C-reactive protein (CRP) (F) in 20 dogs infected with Babesia gibsoni.
In conclusion, the prevalence of pancreatitis may be low among B. gibsoni-infected dogs. In previous reports, the prevalence of pancreatitis among B. rossi-infected dogs was greater than 28% [7]. The prevalence of pancreatitis among B. gibsoni-infected dogs in this study (10%) was less than that among B. rossi-infected dogs. In B. rossi infection, the different clinical signs are considered to be the result of tissue hypoxia following anemia and concomitant SIRS caused by marked cytokine release [7]. As anemia is also main symptom in B. gibsoni infection, there should be some difference between B. gibsoni infection and B. rossi infection in dogs. Therefore, further studies using more samples from B. gibsoni-infected dogs with severe anemia are necessary to elucidate the relationship between B. gibsoni infection in dogs and pancreatitis.
REFERENCES
- 1.Binker M. G., Cosen-Binker L. I.2014. Acute pancreatitis: the stress factor. World J. Gastroenterol. 20: 5801–5807. doi: 10.3748/wjg.v20.i19.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown A. L., Shiel R. E., Irwin P. J.2015. Clinical, haematological, cytokine and acute phase protein changes during experimental Babesia gibsoni infection of beagle puppies. Exp. Parasitol. 157: 185–196. doi: 10.1016/j.exppara.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 3.Gad Baneth. 2018. Babesia in domestic dogs. pp. 241–258. In: Parasitic Protozoa of Farm Animals and Pets, 1st ed. (Florin-Jacobsen, M. and Schnittger, L. eds.), Springer Nature, Berlin. [Google Scholar]
- 4.Hauptman J. G., Walshaw R., Olivier N. B.1997. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet. Surg. 26: 393–397. doi: 10.1111/j.1532-950X.1997.tb01699.x [DOI] [PubMed] [Google Scholar]
- 5.Inokuma H., Yoshizaki Y., Shimada Y., Sakata Y., Okuda M., Onishi T.2003. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 41: 3494–3498. doi: 10.1128/JCM.41.8.3494-3498.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjemtrup A. M., Wainwright K., Miller M., Penzhorn B. L., Carreno R. A.2006. Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet. Parasitol. 138: 103–111. doi: 10.1016/j.vetpar.2006.01.044 [DOI] [PubMed] [Google Scholar]
- 7.Köster L. S., Steiner J. M., Suchodolski J. S., Schoeman J. P.2015. Serum canine pancreatic-specific lipase concentrations in dogs with naturally occurring Babesia rossi infection. J. S. Afr. Vet. Assoc. 86: E1–E7. doi: 10.4102/jsava.v86i1.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubo S., Tateno M., Ichikawa Y., Endo Y.2015. A molecular epidemiological survey of Babesia, Hepatozoon, Ehrlichia and Anaplasma infections of dogs in Japan. J. Vet. Med. Sci. 77: 1275–1279. doi: 10.1292/jvms.15-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mawby D. I., Whittemore J. C., Fecteau K. A.2014. Canine pancreatic-specific lipase concentrations in clinically healthy dogs and dogs with naturally occurring hyperadrenocorticism. J. Vet. Intern. Med. 28: 1244–1250. doi: 10.1111/jvim.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohta H., Morita T., Yokoyama N., Osuga T., Sasaki N., Morishita K., Nakamura K., Takiguchi M.2017. Serial measurement of pancreatic lipase immunoreactivity concentration in dogs with immune-mediated disease treated with prednisolone. J. Small Anim. Pract. 58: 342–347. doi: 10.1111/jsap.12652 [DOI] [PubMed] [Google Scholar]
- 11.Schoeman J. P.2009. Canine babesiosis. Onderstepoort J. Vet. Res. 76: 59–66. doi: 10.4102/ojvr.v76i1.66 [DOI] [PubMed] [Google Scholar]
- 12.Taboada J., Lobetti R.2006. Babesiosis. pp. 722–736. In: Infectious Disease of the Dog and Cat, 3rd ed. (Greene, C. E. eds.), Saunders Elsevier, St. Louis. [Google Scholar]
- 13.Vitale D. S., Hornung L., Lin T. K., Nathan J. D., Prasad S., Thompson T., Abu-El-Haija M.2019. Blood urea nitrogen elevation is a marker for pediatric severe acute pancreatitis. Pancreas 48: 363–366. doi: 10.1097/MPA.0000000000001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamasaki M., Tajima M., Yamato O., Hwang S. J., Ohta H., Maede Y.2008. Heat shock response of Babesia gibsoni heat shock protein 70. J. Parasitol. 94: 119–124. doi: 10.1645/GE-1279.1 [DOI] [PubMed] [Google Scholar]