Abstract
Aquaporin-2 (AQP2), a vasopressin-regulated water channel, plays an important role in renal water homeostasis. It has been reported that the level of AQP2 in human urine is altered during pregnancy. However, little is known about the level of urinary AQP2 in pregnant cattle. In this study, we examined the level of AQP2-bearing extracellular vesicles (uEV-AQP2), which account for most urinary AQP2, in both heifers and cows during the gestational and postpartum periods. The level of uEV-AQP2 was significantly decreased during gestation in comparison with the other cattle examined. Similarly, the levels of EV marker proteins in uEVs, including tumor susceptibility gene 101 (TSG101) protein and apoptosis-linked gene 2-interacting protein X (ALIX), were significantly decreased during gestation. There were significant correlations between the levels of uEV-AQP2 and uEV-TSG101, or uEV-ALIX. Immunohistochemistry data from pregnant and non-pregnant cattle supported the notion that the level of uEV-AQP2 was decreased during gestation. These data indicate that the level of uEV-AQP2 is decreased in pregnant cattle, possibly through a decrease in both the number of EVs released into the urine and renal AQP2 expression.
Keywords: aquaporin-2 (AQP2), exosomes, Japanese Black cattle, pregnancy, urinary extracellular vesicles
Urine is a non-invasive biomarker source for many diseases, and includes components useful for this purpose, such as electrolytes, glucose, proteins, amino acids, erythrocytes, and ketones [5]. In addition to these traditional components, renal functional proteins, such as aquaporin-1 (AQP1), AQP2, sodium-potassium-chloride co-transporter 2, and sodium-chloride cotransporter, have been found in urinary extracellular vesicles (uEVs) [25], which are now receiving considerable attention as a possible source for discovery of novel biomarkers that might be used as indicators of cellular pathological change [24].
In 1992, AQP1 was discovered as a water channel protein by Agre’s group [26], and since then PCR-based homologous cloning has identified 12 other AQP isoforms in mammals [9, 13, 22]. Among these AQPs, AQP2 has been shown to be the most important protein for the water homeostasis regulated by vasopressin [13, 22]. AQP2 is expressed in principal cells of kidney collecting ducts. Vasopressin regulates AQP2 in two ways: short-term and long-term. Short-term regulation occurs within one hr after activation of the V2 receptor by vasopressin in the principal cells. This leads to accumulation of AQP2 on the apical membrane through the vesicular trafficking, and this apical expression of AQP2 provides a portal for water reabsorption. In long-term regulation, the renal abundance of AQP2 is increased through transcriptional regulation upon exposure to vasopressin. Increased renal abundance of AQP2 enhances water reabsorption, thereby increasing the body’s extracellular fluid volume.
AQP2 in the uEVs (uEV-AQP2) was first discovered by Kanno et al. [14] and a subsequent proteomic analysis performed by Pistukun et al. confirmed this observation [25]. Later, using differential centrifugation, Miyazawa et al. [20] found that most AQP2 (more than 80%) in the urine is localized to uEVs.
In pregnant humans, it is known that extracellular fluid volume is increased by 30–50% [27, 28]. Buemi et al. [4] have reported that the level of urinary AQP2 in humans is increased during the gestational period. Furthermore, Ohara et al. [23] have reported that upregulation of renal AQP2 contributes to the water retention in pregnant rats. These data suggest that the state of body fluid in pregnancy is regulated by renal expression of AQP2, which is reflected by its urinary excretion. However, in pregnant cattle, the level of urinary AQP2 has yet to be clarified.
In the present study, we examined the level of uEV-AQP2 from 5 heifers (H), 19 cows during gestation (G), and 10 cows during the postpartum period (P).
MATERIALS AND METHODS
Animals
All studies were performed with approval from the University of Miyazaki in accordance with the Guidelines for the Care and Use of Laboratory Animals in the University of Miyazaki. Spot urine samples (10–40 ml) were obtained from Japanese Black cattle in the morning by urethral catheterization. Urine samples were obtained from pregnant cows, including 6 cows in the first trimester (G1), 7 cows in the second trimester (G2), and 6 cows in the last trimester of pregnancy (G3).
Analysis of urine parameters
Initially, we tested urine with Multistix®PRO 10 LS Urinalysis Strips (Siemen Healthcare Diagnostics, Inc., Tokyo, Japan) for examination of health status. Samples yielding normal results including subclinical proteinuria were used, and these samples were subjected to determination of urinary creatinine and electrolyte concentrations (Na, K, Cl, and Ca) using a autoanalyzer (Fuji Film Medical, Tokyo, Japan), and urine osmolality using a osmometer (Osmostation OM-6060; Arkray, Kyoto, Japan).
Isolation of uEVs
uEVs were isolated from urine within 24 hr after collection using an ultracentrifugation technique that is considered to be a gold standard for isolation of EVs [2, 12, 16]. The urine was centrifuged at 1,000 g for 15 min for removal of cell debris. The volume of the 1,000 g supernatant was adjusted to include a certain amount of creatinine. Thereafter, we added protease inhibitor mixture (3.75% pAPMSF, 5% of 0.5 mM EDTA solution, 0.5% leupeptin in PBS) to the samples at 4% and centrifuged them again at 17,000 g for 15 min. The 17,000 g supernatant was retained. The pellet was dissolved in 50 µl of isolation solution (10 mM trimethanolamine, 250 mM sucrose, 50 mg/ml dithiothreitol (DTT), and 8 mM HEPES, pH 7.6) and the pellet solution was incubated at 37°C with vortexing for 1 min. Thereafter, the volume of the pellet solution was adjusted to the original volume by addition of isolation solution without DTT and the solution was centrifuged again at 17,000 g for 15 min. The first and second supernatants after centrifugation at 17,000 g were mixed, and the mixture was spun at 200,000 g (Beckman Coulter, Fullerton, CA, U.S.A.), at 25°C for 1 hr. The resulting pellet was dissolved in a solution containing a protease inhibitor cocktail (Complete®, Roche Diagnostics, Tokyo, Japan) and the pellet solution was mixed with 4 × sample buffer (8% SDS, 50% glycerol, 250 mM Tris-HCl, 0.05% bromophenol blue, 200 mM DTT), followed by an incubation at 37°C for 30 min. The final samples were stored at −80°C until immunoblotting analysis.
Immunoblotting
After separation by SDS-PAGE, the protein was transferred on to a polyvinylidene difluoride (PVDF) membranes. After a blocking with a 5% skim milk in a tris-buffered solution containing 0.05% Tween-20 (TTBS), the PVDF membrane was incubated in TTBS containing 1.7% skim milk and primary antibody for 1 hr at 30°C. Thereafter, the PVDF membrane was washed with TTBS and was then incubated with TTBS containing 1.7% skim milk and anti-rabbit secondary antibody for 45 min at 30°C. After washing the PVDF membrane with TTBS, proteins on the membrane associated with antibodies were visualized by a Super Signal chemiluminescence detection system (Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) and the visualized bands were quantified using the ImageQuant TL software (GE Healthcare, Uppsala, Sweden). Antibodies used in this study were anti-AQP2 (catalog no. AQP-002, Alomone Labs, Jerusalem, Israel), anti-tumor susceptibility gene 101 (TSG101) (catalog no. ab-125011, Abcam, Cambridge, U.K.), anti- apoptosis-linked gene 2-interacting protein X (ALIX) (catalog no. sc-49268, Santa Cruz Biotechnology, Dallas, TX, U.S.A.), and peroxidase-conjugated anti-rabbit IgG secondary antibody (catalog no. 7074, Cell Signaling Technology, Danvers, MA, U.S.A.).
One constant control sample, comprising a mixture of the samples from three healthy humans, was loaded on the same gel and the control band intensity was used to quantify and normalize the band intensity of each bovine sample. These human samples were obtained with informed consent, and the study protocols were approved by the Faculty of Agriculture, University of Miyazaki Institutional Review Board, in accordance with the Ethical Guidelines for Clinical Studies in Japan.
Histology
Two formalin-fixed kidney samples from female cattle were analyzed. These samples had been kept at the University of Tokyo after pathological examination. The causes of death included a pregnancy-related disease and decubitus caused by arthritis. The kidney samples were cut into sections 2 µm thick, and then subjected to immunostaining. For immunohistostaining, the sections were deparaffinized and rehydrated, and the antigens were retrieved by incubating each specimen in distilled water at 121°C for 5 min. Each slide was then reacted with the primary antibody against AQP2 at 37°C for 1 hr, and was subsequently incubated with Envision System Labelled Polymer Reagent (Dako, Tokyo, Japan) at 37°C for 45 min. The region of AQP2 labeling was visualized by treatment with 3, 3′-diaminobenzidine tetrahydrochloride, and the specimen was counterstained with hematoxylin. Each specimen was scanned and its image was acquired using a NanoZoomer 2.0 RS virtual slide scanner (C10730-13, Hamamatsu Photonics K.K., Shizuoka, Japan) with the NDP. view2 software package (U12388-01, Hamamatsu Photonics K.K.).
Statistical analysis
The BoxPlotR (http://boxplot.tyerslab.com) was used for generation of box plots [29]. Differences between the groups were analyzed by the analysis of variance with Kruskal-Wallis test and subsequent Steel-Dwass test. Spearman rank correlation and least-squares regression were used to analyze the linear relationship between two variables. Programs of the tests available at https://www.R-project.org/.
RESULTS
Alterations in the urine parameters of cattle in the present study are summarized in Table 1. Since spot urine samples were used, all electrolyte values were normalized by urinary creatinine concentration [30, 32]. Among these parameters, a significant difference was evident only in the K:creatinine ratio between the G and the P groups.
Table 1. Urinary parameters.
| Parameters | Heifer (H Group) |
Gestation (G Group) |
Postpartum (P Group) |
|---|---|---|---|
| n | 5 | 19 | 10 |
| Age (years) | 1.44 ± 0.38 | 6.80 ± 0.74a) | 9.02 ± 0.98a) |
| Osmolality (mOsm/kg H2O) | 687.8 ± 127.6 | 799.8 ± 64.5 | 814.8 ± 29.9 |
| pH | 8.2 ± 0.1 | 8.0 ± 0.3 | 8.6 ± 0.2 |
| Na: creatinine ratio | 1.99 ± 0.48 | 1.41 ± 0.19 | 1.66 ± 0.21 |
| K: creatinine ratio | 4.06 ± 1.19 | 6.33 ± 0.86 | 9.27 ± 1.32a) |
| Ca: creatinine ratio | 0.05 ± 0.00 | 0.10 ± 0.03 | 0.10 ± 0.02 |
| Cl: creatinine ratio | 3.11 ± 0.53 | 4.33 ± 0.47 | 3.26 ± 0.39 |
a) P<0.05 vs. the H group. There were no statistical significant differences between the G and P groups.
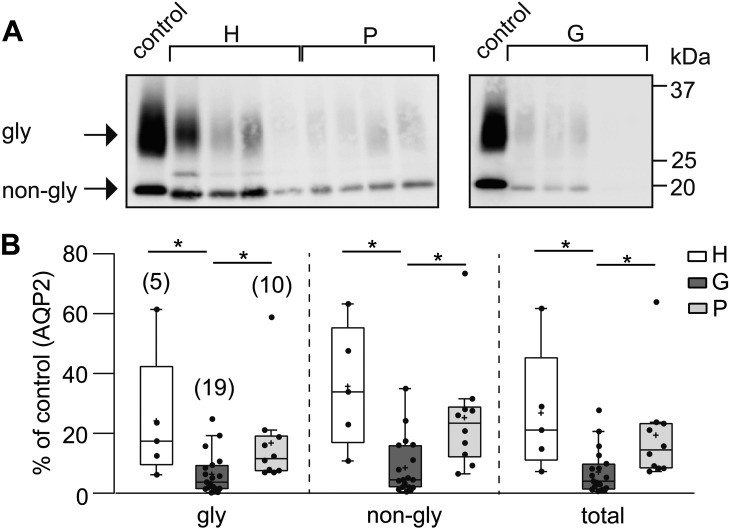
Figure 1 shows the results of immunoblot analysis for uEV-AQP2. As reported previously for AQP2 in bovine samples [17], both glycosylated and non-glycosylated AQP2 were detected in the uEVs of cattle. Quantitative analysis showed that the levels of glycosylated, non-glycosylated and glycosylated + non-glycosylated (total) AQP2 were significantly decreased in the G group relative to the other two groups. When we compared total AQP2 between the three trimester groups (G1, G2, and G3) after normalization with the mean value in the P groups, the values decreased as pregnancy progressed (the mean ± SE; G1, 50.0 ± 24.2%, n=6; G2, 33.5 ± 7.1%, n=7; G3, 26.2 ± 13.3%, n=6), but these values did not differ significantly.
Fig. 1.
Comparison of aquaporin-2 (AQP2) levels in urinary extracellular vesicles (uEVs) between heifers (H), cows during gestation (G), and cows in the postpartum period (P). (A) Typical immunoblots of AQP2 in uEVs (uEV-AQP2) in the H, G, and P groups are shown. The upper and lower bands correspond to the glycosylated (gly) and non-glycosylated (non-gly) forms of AQP2, respectively. Control indicates the band for one constant control (see the MATERIALS AND METHODS section). (B) Quantitative summary of immunoblotting results. After normalization of the intensity of each band against the band intensity of the control, the data are shown as dot and box plots. Total indicates gly + non-gly AQP2. Numbers in parentheses indicate the number of animals examined. *P<0.05.
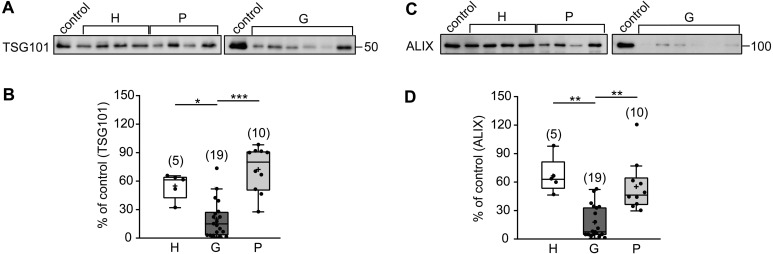
It has been reported that TSG101 and ALIX are marker proteins for uEVs [7, 24]. Therefore, we next examined the levels of uEV-TSG101 and -ALIX in the three groups. As shown in Fig. 2, when we compared the level of uEV-TSG101 in the G group was significantly lower than in the other two groups. On the other hand, there was not significant difference in the levels between the H and P groups. Similarly, when we compared the levels of uEV-ALIX in the three groups, that in the G group was significantly lower than those in the other two groups.
Fig. 2.
Comparison of levels of uEV-tumor susceptibility gene 101 protein (TSG101) and -apoptosis-linked gene 2-interacting protein X (ALIX) between the H, G, and P groups. (A) Typical immunoblots of uEV-TSG101 in the H, G, and P groups are shown. (B) Quantitative summary of the immunoblotting results in (A). *P<0.05 and ***P<0.001, respectively. (C) Typical immunoblots of uEV-ALIX in the three groups are shown. (D) Quantitative summary of immunoblotting results in (C). **P<0.01.
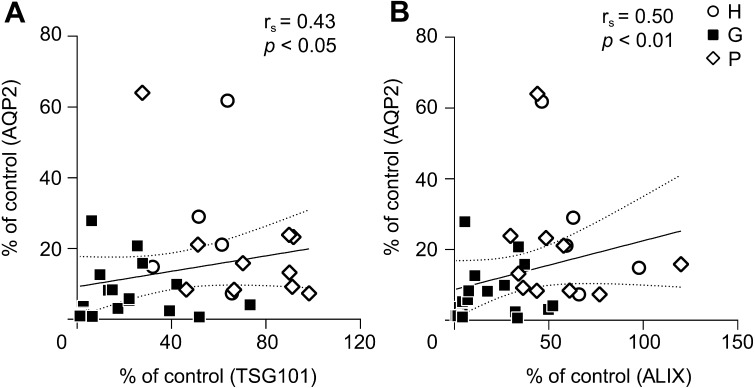
Figures 1 and 2 show that the level of uEV-AQP2 was associated with the levels of marker proteins for uEVs. In order to clarify this relationship, we performed correlation analyses. As shown in Fig. 3, there were significant correlations between the level of uEV-AQP2 and those of uEV-TSG101, or uEV-ALIX.
Fig. 3.
Relationship between uEV-aquaporin-2 and -TSG101, or -ALIX. (A) Analysis of the correlation between uEV-TSG101 and -AQP2. The line is the least-squares regression line. Total (glycosylated + non-glycosylated forms) uEV-AQP2 was used for this analysis. (B) Analysis of the correlation between uEV-ALIX and -AQP2.
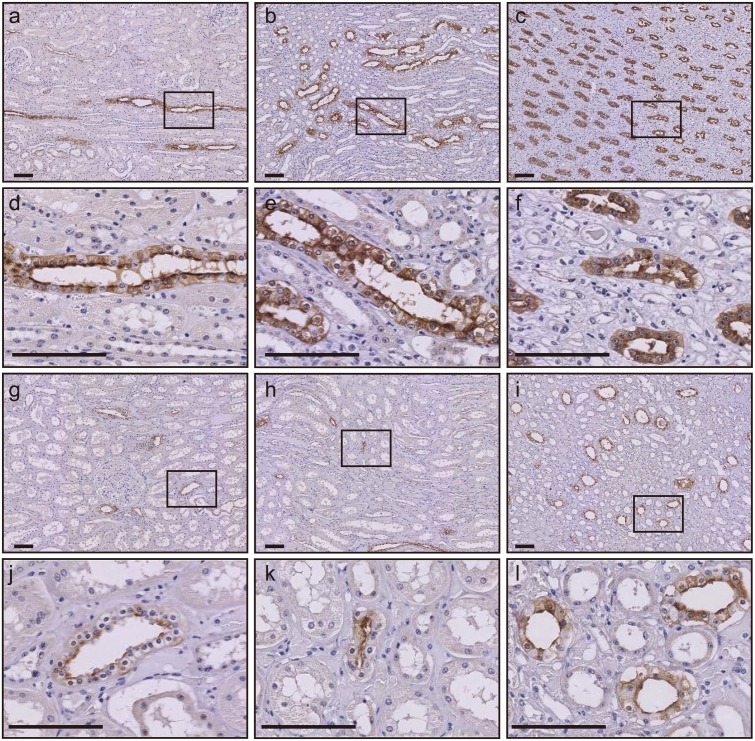
Several previous studies have suggested that the level of uEV-AQP2 is related to the degree of its renal expression [1, 2, 19]. Although the number of samples we examined was limited, we were able to immunohistochemically analyze renal AQP2 expression in two kidney samples from pregnant and non-pregnant cows (Fig. 4). In the non-pregnant case, AQP2 was especially expressed at the apical membrane, and to a degree in the cytosol, of renal collecting duct cells in the medulla. This expression pattern was in good agreement with a previous report [18]. On the other hand, in the kidney from the pregnant cow, the number of AQP2-positive cells was decreased and its expression level was weaker than that in the kidney from the non-pregnant case. When we quantitatively analyzed the whole kidney area using immunohistochemistry imaging data [19], the AQP2-positive area and the intensity of AQP2 expression in the pregnant cow were 50.4 and 86.9%, respectively, relative to those in the non-pregnant cow.
Fig. 4.
Immunohistochemistry of renal aquaporin-2 in a pregnant and a non-pregnant cattle. Kidney sections from a non-pregnant (a−f) and a pregnant cow (g−l) were stained with anti-AQP2 antibody. Representative images of the cortex (a, d, g, and j), outer medulla (b, e, h, and k) and inner medulla (c, f, i, and l) are shown. The smaller black box in a, b, c, g, h, and i corresponds to the d, e, f, j, k, and l, respectively. Brown staining indicates the presence of AQP2. Bars=100 µm.
DISCUSSION
In the present work, we have shown for the first time that the level of uEV-AQP2 in pregnant cows is decreased in comparison with that in heifers or in the postpartum period. This decrease was correlated with the levels of uEV marker proteins such as TSG101 and ALIX. Since the levels of uEV marker proteins have been reported to reflect the number of EVs released into urine [7, 24], the decrease in the level of uEV-AQP2 might result from the decreased number of EVs released into urine in pregnant cattle. Furthermore, when we performed immunohistochemical analysis, although the number examined was limited, reduced renal expression of AQP2 was observed in pregnant cattle. Therefore, in addition to a lower number of EVs, the decreased renal expression of AQP2 was also thought to contribute to the reduced level of uEV-AQP2 in pregnant cattle.
A number of researches have been investigating the mechanisms by which EVs are released [11]. EV release comprises several steps, including EV genesis, the mechanism of cargo sorting into EVs, and the release steps. Many molecules involved in this process have been identified and studied, including hepatocyte growth factor-related tyrosine kinase substrate, TSG101, charged multivesicular body protein 4C (CHMP4C), signal transducing adaptor molecule 1, vacuolar protein sorting 4 homolog B, vesicular trafficking 1, ALIX, syntenin, syndecan, CD9, Rab family proteins, V-ATPase, and ISG15 ubiquitin-like modifier (ISG15). Among them, ISG15, a ubiquitin-like protein, is noteworthy, because the level of ISG15 mRNA in blood has been reported to increase after artificial insemination in cattle [10]. ISG15 is induced by interferon (IFN)-τ derived from the conceptus in cattle. One of the characteristics of ISG15 includes its conjugation to target proteins (ISGylation). Recently, using ISG15-knock out mice and mice expressing an inactive form of the ISG15 deconjugating enzyme, USP18C61A, Villarroya-Beltri et al. [31] have suggested that the ISGylation is related to regulation of the EV release. Although the level of ISG15 in renal cells, the major cells producing uEVs, has not been examined in cattle, activation of the mechanism involving ISG15 might decrease the number of EVs released into the urine of pregnant cattle. Future studies will need to identify the molecules responsible for the release of uEVs, and their function in cattle.
In a human study, Buemi et al. [4] examined urine from 45 pregnant primiparas, and observed that the excretion of urinary AQP2 during all three trimesters was increased. However, in a study of 17 healthy pregnant women, Nielsen et al. [21] found no significant difference from non-pregnant women in the level of uEV-AQP2. Despite the difference between these two reports, uEV-AQP2 in humans does not decrease during gestation. However, the present study clearly showed that uEV-AQP2 was decreased in pregnant cattle. The reason for this difference is currently unclear. As ISG15 is also known to be induced by pregnancy in humans [3], the different degree of renal activation of the ISGylation pathway between humans and cattle might be partly responsible. Furthermore, it is known that humans and cattle differ in both renal anatomy and physiology [8, 18]. For example, the bovine kidney is distinctively lobulated, whereas the human kidney is not. The maximal urine concentration ability of cattle is 2,000–2,200 mOsm/kg H2O, whereas that of humans is 1,400 mOsm/kg H2O [6]. Therefore, a further study is needed to clarify the reason for this difference.
It has been reported that renal AQP2 is up-regulated in pregnant rats, and that this might contribute to the water retention [23]. In this study, although the number of samples was limited, we showed that the level of renal AQP2 expression was decreased in a pregnant cow. Although it is currently unclear whether water retention occurs in cattle, the decreased expression of renal AQP2 might protect cattle from pregnancy-induced water retention through inhibition of water reabsorprtion. A future study examining the relationship between body fluid volume and renal AQP2 expression in cattle may clarify the reason for this difference.
Urinary excretion of potassium was higher in the P group than in the H group. Intake of potassium has been reported to correlate with urinary potassium excretion in lactating cows [15]. Although potassium intake was not measured in this study, potassium intake in the P group appeared to be high than that in the H group.
Our present study has demonstrated that pregnant cows show a lower level of uEV-AQP2 and this decrease tends to be lowered as pregnancy progressed. A reduction in the number of EVs released into urine and reduced renal AQP2 expression during gestation in cattle might contribute to this. These data suggest that uEV-AQP2 might be applicable as a marker for detection of pregnancy and/or pregnancy-related diseases.
CONFLICT OF INTEREST STATEMENT
None declared.
Acknowledgments
The work is supported by JSPS KAKENHI, 16K15047 (M.I.), 18H02348 (M.I.), 15K18784 (H.S.), and 18K05996 (H.S.).
REFERENCES
- 1.Abdeen A., Sonoda H., El-Shawarby R., Takahashi S., Ikeda M.2014. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am. J. Physiol. Renal Physiol. 307: F1227–F1237. doi: 10.1152/ajprenal.00140.2014 [DOI] [PubMed] [Google Scholar]
- 2.Asvapromtada S., Sonoda H., Kinouchi M., Oshikawa S., Takahashi S., Hoshino Y., Sinlapadeelerdkul T., Yokota-Ikeda N., Matsuzaki T., Ikeda M.2018. Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am. J. Physiol. Renal Physiol. 314: F584–F601. doi: 10.1152/ajprenal.00184.2017 [DOI] [PubMed] [Google Scholar]
- 3.Bebington C., Bell S. C., Doherty F. J., Fazleabas A. T., Fleming S. D.1999. Localization of ubiquitin and ubiquitin cross-reactive protein in human and baboon endometrium and decidua during the menstrual cycle and early pregnancy. Biol. Reprod. 60: 920–928. doi: 10.1095/biolreprod60.4.920 [DOI] [PubMed] [Google Scholar]
- 4.Buemi M., D’Anna R., Di Pasquale G., Floccari F., Ruello A., Aloisi C., Leonardi I., Frisina N., Corica F.2001. Urinary excretion of aquaporin-2 water channel during pregnancy. Cell. Physiol. Biochem. 11: 203–208. doi: 10.1159/000047807 [DOI] [PubMed] [Google Scholar]
- 5.Chau K., Hutton H., Levin A.2016. Laboratory assessment of kidney disease: Glomerular filtration rate, urinalysis, and proteinuria. pp. 780–803. In: Brenner and Rector’s The Kidney, 10th ed. (Skorecki, K., Chertow, G. M., Marsden, P. A., Taal, M. W. and Yu, A. S. L. eds.), Elsevier, Philadelphia. [Google Scholar]
- 6.Epstein F. H., Kleeman C. R., Hendrikx A.1957. The influence of bodily hydration on the renal concentrating process. J. Clin. Invest. 36: 629–634. doi: 10.1172/JCI103462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdbrügger U., Le T. H.2016. Extracellular vesicles in renal diseases: more than novel biomarkers? J. Am. Soc. Nephrol. 27: 12–26. doi: 10.1681/ASN.2015010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frandson R. D., Wilke W. L., Fails S. D.2009. The urinary system. pp. 383–398. In: Anatomy and Physiology of Farm Animals, 7th ed., Wiley-Blackwell, Ames. [Google Scholar]
- 9.Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S.1993. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552. doi: 10.1038/361549a0 [DOI] [PubMed] [Google Scholar]
- 10.Han H., Austin K. J., Rempel L. A., Hansen T. R.2006. Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows. J. Endocrinol. 191: 505–512. doi: 10.1677/joe.1.07015 [DOI] [PubMed] [Google Scholar]
- 11.Hessvik N. P., Llorente A.2018. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 75: 193–208. doi: 10.1007/s00018-017-2595-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huebner A. R., Cheng L., Somparn P., Knepper M. A., Fenton R. A., Pisitkun T.2016. Deubiquitylation of protein cargo is not an essential step in exosome formation. Mol. Cell. Proteomics 15: 1556–1571. doi: 10.1074/mcp.M115.054965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda M., Matsuzaki T.2015. Regulation of aquaporins by vasopressin in the kidney. Vitam. Horm. 98: 307–337. doi: 10.1016/bs.vh.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 14.Kanno K., Sasaki S., Hirata Y., Ishikawa S., Fushimi K., Nakanishi S., Bichet D. G., Marumo F.1995. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 332: 1540–1545. doi: 10.1056/NEJM199506083322303 [DOI] [PubMed] [Google Scholar]
- 15.Kume S., Nonaka K., Oshita T., Kozakai T., Kojima H.2004. Potassium excretion of dry, pregnant and lactating cows fed forage. Nihon Chikusan Gakkaiho 75: 179–184. doi: 10.2508/chikusan.75.179 [DOI] [Google Scholar]
- 16.Livshits M. A., Khomyakova E., Evtushenko E. G., Lazarev V. N., Kulemin N. A., Semina S. E., Generozov E. V., Govorun V. M.2015. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 5: 17319. doi: 10.1038/srep17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michałek K., Dratwa-Chałupnik A., Ciechanowicz A. K., Malinowski E.2014. Aquaporin 2: Identification and analysis of expression in calves’ urine during their first month of life. Can. J. Anim. Sci. 94: 653–659. doi: 10.4141/cjas-2014-023 [DOI] [Google Scholar]
- 18.Michałek K., Laszczyńska M., Ciechanowicz A. K., Herosimczyk A., Rotter I., Oganowska M., Lepczyński A., Dratwa-Chałupnik A.2014. Immunohistochemical identification of aquaporin 2 in the kidneys of young beef cattle. Biotech. Histochem. 89: 342–347. doi: 10.3109/10520295.2013.858828 [DOI] [PubMed] [Google Scholar]
- 19.Mikoda N., Sonoda H., Oshikawa S., Hoshino Y., Matsuzaki T., Ikeda M.2019. A bell-shaped pattern of urinary aquaporin-2-bearing extracellular vesicle release in an experimental model of nephronophthisis. Physiol. Rep. 7: e14092. doi: 10.14814/phy2.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazawa Y., Mikami S., Yamamoto K., Sakai M., Saito T., Yamamoto T., Ishibashi K., Sasaki S.2018. AQP2 in human urine is predominantly localized to exosomes with preserved water channel activities. Clin. Exp. Nephrol. 22: 782–788. doi: 10.1007/s10157-018-1538-6 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen M. R., Frederiksen-Møller B., Zachar R., Jørgensen J. S., Hansen M. R., Ydegaard R., Svenningsen P., Buhl K., Jensen B. L.2017. Urine exosomes from healthy and hypertensive pregnancies display elevated level of α-subunit and cleaved α- and γ-subunits of the epithelial sodium channel-ENaC. Pflugers Arch. 469: 1107–1119. doi: 10.1007/s00424-017-1977-z [DOI] [PubMed] [Google Scholar]
- 22.Nielsen S., Frøkiaer J., Marples D., Kwon T. H., Agre P., Knepper M. A.2002. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 82: 205–244. doi: 10.1152/physrev.00024.2001 [DOI] [PubMed] [Google Scholar]
- 23.Ohara M., Martin P. Y., Xu D. L., St John J., Pattison T. A., Kim J. K., Schrier R. W.1998. Upregulation of aquaporin 2 water channel expression in pregnant rats. J. Clin. Invest. 101: 1076–1083. doi: 10.1172/JCI649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oshikawa S., Sonoda H., Ikeda M.2016. Aquaporins in urinary extracellular vesicles (exosomes). Int. J. Mol. Sci. 17: E957. doi: 10.3390/ijms17060957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pisitkun T., Shen R. F., Knepper M. A.2004. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U.S.A. 101: 13368–13373. doi: 10.1073/pnas.0403453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston G. M., Carroll T. P., Guggino W. B., Agre P.1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–387. doi: 10.1126/science.256.5055.385 [DOI] [PubMed] [Google Scholar]
- 27.Salas S. P., Marshall G., Gutiérrez B. L., Rosso P.2006. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208. doi: 10.1161/01.HYP.0000200042.64517.19 [DOI] [PubMed] [Google Scholar]
- 28.Schrier R. W., Briner V. A.1991. Peripheral arterial vasodilation hypothesis of sodium and water retention in pregnancy: implications for pathogenesis of preeclampsia-eclampsia. Obstet. Gynecol. 77: 632–639. [PubMed] [Google Scholar]
- 29.Spitzer M., Wildenhain J., Rappsilber J., Tyers M.2014. BoxPlotR: a web tool for generation of box plots. Nat. Methods 11: 121–122. doi: 10.1038/nmeth.2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang K. W., Toh Q. C., Teo B. W.2015. Normalisation of urinary biomarkers to creatinine for clinical practice and research—when and why. Singapore Med. J. 56: 7–10. doi: 10.11622/smedj.2015003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarroya-Beltri C., Baixauli F., Mittelbrunn M., Fernández-Delgado I., Torralba D., Moreno-Gonzalo O., Baldanta S., Enrich C., Guerra S., Sánchez-Madrid F.2016. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 7: 13588. doi: 10.1038/ncomms13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H., Yuen P. S., Pisitkun T., Gonzales P. A., Yasuda H., Dear J. W., Gross P., Knepper M. A., Star R. A.2006. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 69: 1471–1476. doi: 10.1038/sj.ki.5000273 [DOI] [PMC free article] [PubMed] [Google Scholar]