Abstract
The objectives of this study were: 1) to evaluate the effects of a fructose enriched diet (FED) on rat sperm quality, epididymal function (i.e. oxidative stress and alpha-glucosidase expression) and testosterone concentrations; 2) to determine if the administration of ghrelin (Ghrl), reverses the effects induced by FED.
After validating the protocol as an inductor of metabolic syndrome like-symptoms, adult male rats were assigned to one of the following treatments for 8 weeks: FED = 10% fructose enriched in water (v/v); FED + Ghrl = fructose enriched diet plus Ghrl (6 nmol/animal/day, s.c.) from week 6–8; or C = water without fructose (n = 5–10 animals/group).
FED significantly decreased sperm concentration and motile sperm count/ml vs C (FED: 19.0 ± 1.6 × 106sperm/ml and 834.6 ± 137.0, respectively vs C: 25.8 ± 2.8 × 106 and 1300.4 ± 202.4, respectively; p < 0.05); ghrelin injection reversed this negative effect (23.5 ± 1.6 × 106sperm/ml and 1381.7 ± 71.3 respectively). FED resulted in hypogonadism, but Ghrl could not normalize testosterone concentrations (C: 1.4 ± 0.1 ng/ml vs FED: 0.8 ± 0.2 ng/ml and FED + Ghrl: 0.6 ± 0.2 ng/ml; p < 0.05). Ghrelin did not reverse metabolic abnormalities secondary to FED. FED did not alter epididymal expression of antioxidants enzymes (superoxido-dismutase, catalase and glutathione peroxidases –Gpx-). Nevertheless, FED + Ghrl significantly increased the expression of Gpx3 (FED + Ghrl: 3.47 ± 0.48 vs FED: 0.69 ± 0.28 and C: 1.00 ± 0.14; p < 0.05). The expression of neutral alpha-glucosidase, which is a marker of epididymal function, did not differ between treatments.
In conclusion, the administration of Ghrl modulated the negative effects of FED on sperm quality, possibly by an epididymal increase in Gpx3 expression. However, Ghrl could not neither normalize the metabolism of FED animals, nor reverse hypogonadism.
Keywords: Cell biology, Developmental biology, Molecular biology, Diet, Endocrinology, Metabolic syndrome, Epididymis, Glutathione peroxidase, Oxidative stress, Neutral alpha-glucosidase
Cell biology; Developmental biology; Molecular biology; Diet; Endocrinology; Metabolic syndrome; Epididymis; Glutathione peroxidase; Oxidative stress; Neutral alpha-glucosidase
1. Introduction
Metabolic syndrome (MetS) is a cluster of abnormalities that includes overweight, dyslipemia and impaired glucose metabolism, with insulin resistance and central obesity recognized as causative factors [1, 2]. Dysregulated production of adipokines, chronic low-grade inflammation and oxidative stress are being hypothetized as the cellular/molecular bases of the metabolic abnormalities and the comorbidities associated with this pathology [reviewed in 1, 2].
Because of its systemic nature, MetS may affect several features of human physiology, including reproductive potential. In humans, MetS has been associated with hypogonadism [1, 3, 4]; in fact, some authors suggested that this trait should be added to the group of abnormalities that characterizes MetS [5]. Moreover, although without consensus [3, 6], some studies have reported a decrease in human seminal quality secondary to this pathology [4, 7, 8, 9, 10]. Specifically, some studies proposed that the oxidative stress associated to MetS and related diseases (such as obesity and type 2-diabetes) results in sperm membrane lipid peroxidation, with the consequent membrane dysfunction, motility decrease and DNA damage [reviewed in 1, 7].
Ghrelin (Ghrl) is a 28 aminoacide peptide originally described by its growth hormone stimulatory ability and its orexigenic effects [11]. Nonetheless, Ghrl has emerged as an important metabolic regulatory substance, related to glucose and lipids metabolism [12, 13]. Furthermore, Ghrl has been linked with mammals reproductive capability [14]. Particularly in males, it is known that hyperghrelinemia inhibits LH and testosterone (T) secretion and might reduce spermatogenesis, contributing to the suppression of male reproductive axis in situations of negative energy balance. Conversely, testicular Ghrl is regulated by LH secretion and GHSR-1a, the active Ghrl receptor, is expressed in rodent Leydig cells, Sertoli cells and even in germ cells. In addition, Ghrl has been shown to control the secretion of stem cell factor, suggesting that Ghrl might paracrinally regulate spermatogenesis [reviewed in 14].
It has been reported that obese patients and/or those with MetS, exhibit decreased circulating levels of Ghrl and, that progressive lower Ghrl is associated with increasing MetS severity [3, 6, 9, 15, 16, 17]. Because of its anti-inflammatory and anti-oxidant properties, Ghrl has been suggested as a positive prognostic factor in MetS [18, 19, 20, 21]. Interestingly, Catak et al. (2014) reported in a male rat MetS model that, although ghrelinemia did not vary in comparison to control animals, testicular and seminal vesicle Ghrl levels were significantly decreased [22]. In another research study developed in rabbits, Marchiani et al. (2015) demonstrated that MetS, induced by a high fatty diet for 12 weeks, reduced sperm motility, normal morphology and the capability of the acrosome to react to progesterone. Moreover, these authors proposed that the structures affected by MetS were the testis and, notably, epididymis, since the expression of some inflammatory genes was increased in these tissues [23]. Likewise in humans, we have previously linked obesity (closely related to MetS) with epididymal dysfunction [24, 25]. However, despite the essential role of epididymis in sperm function and fertilizing capability, the possible deleterious effects of MetS (or related pathologies) on this tissue have rarely been studied. Moreover, since epididymal maturation requires a fine tuned balance between reactive oxygen species (ROS) production and neutralization, systemic or local alterations in the redox state may negatively impact semen quality [7, 26]. It is important to remark that several sperm parameters may be altered by epididymal dysfunction, including motility, sperm count and even morphology [23, 27, 28].
Therefore, the objectives of this study were to evaluate: a) the effects of a fructose enriched diet (FED: 10% fructose v/v), commonly used as a MetS model, on rat sperm quality and plasma T concentrations; b) if the detrimental effects of the FED on sperm quality are associated with an increase in epididymal oxidative stress or an alteration in neutral alpha-glucosidase expression (enzyme involved in epididymal maturation that is experimentally and clinically used as an epididymal functional marker [28, 29]) and, c) if Ghrl administration may prevent/reverse the possible negative effects induced by the FED.
2. Material and methods
2.1. Animals
Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the Medicine School of the Cordoba National University (UNC-RHCS 674/09). This animal ethics committee approved the protocol used in this study (Ref. 73/18; October 25, 2018). The study complies with the ARRIVE guidelines.
We used adult (70–90 days) male inbreed Wistar rats maintained on a 12:12 h light:dark basis at 22 ± 2 °C with water and food (Grupo Pilar-Gepsa, Cordoba, Argentina) provision ad libitum.
2.2. Protocol validation (5 weeks of treatment)
An initial group of male rats were randomly assigned to one of the following treatments, in order to validate our experimental protocol as an inductor of MetS-related symptoms:
-
-
FED = fructose enriched diet: free access to food and water with 10% fructose (v/v), for 5 weeks.
-
-
C = control: free access to food and water, for 5 weeks.
It is important to remark that this is a commonly used protocol inductor of MetS, applied in general for 4–6 weeks [30, 31].
Body weight was evaluated before treatment, during (once a week) and at the end of the experimental period. Other morphometric parameters evaluated were: waist circumference, weight of visceral fat, weight of epididymal fat and total abdominal fat weight. Metabolic profile was evaluated by the quantification of: plasma glucose, cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerides.
2.3. Experimental groups (8 weeks of treatment)
After validation of the protocol, a new group of male rats were randomly assigned to one of the following experimental groups:
-
-
FED = fructose enriched diet: (v/v), for 8 weeks.
-
-
FED + Ghrl = fructose enriched diet plus ghrelin: a subgroup of FED animals, from week 6–8, were also injected s.c. with 6 nmol/animal/day of Ghrl.
-
-
C = control: free access to food and water, for 8 weeks.
Treatments were applied for 8 weeks, in order to assure that the evaluated spermatozoa were formed and matured under the effects of the diet (i.e. to cover the whole spermatogenic cycle and epididymal transit). Ghrelin was dissolved in isotonic solution and administered by subcutaneous injections twice a day (with 3 nmol/animal in each injection, at 9 a.m. and 5 p.m.), for the last two weeks of treatment. Control and FED animals received the vehicle in the same regimen. The doses of Ghrl employed in this study have been previously used by several authors [32, 33].
As for protocol validation, morphometric and metabolic parameters were quantified. Sperm functional activity, plasma T and epididymal expression of antioxidants enzymes and neutral alpha-glucosidase (Ganc) were also assayed.
2.4. Plasma metabolic profile
After 12 h fasting, rats were anesthetized by isofluorane and blood collected from abdominal aorta. Serum glucose, cholesterol, triglycerides, HDL and LDL were evaluated with kits from GT Laboratories (Rosario, Argentine).
2.5. Sperm functional activity
After euthanasia, caudal epididymal spermatozoa were obtained and incubated for 15 min in 5 ml of modified Tyrode's medium at 37 °C (with 95% air: 5% CO2) [34]. After incubation, sperm concentration and motility were quantified in a Makler counting chamber (Sefi-Medical Instruments, Israel) [35]. Motility was expressed as percentage of motile cells (progressive plus non-progressive gametes). Sperm viability was assessed using the supravital stain Hoechst 33258 as previously described [36] and evaluated with epifluorescence microscope. The percentage of immature gametes was evaluated, quantifying the percentage of spermatozoa showing a persistent cytoplasmic drop [29]. Acrosomal reaction was assayed by the coomassie blue technique [37] and the results were expressed as percentage of spermatozoa with intact acrosome.
2.6. Plasma testosterone concentration
T concentration was determined by an in-house enzyme immunoassay using a polyclonal anti-T antibody, T standard and their corresponding horseradish peroxidase conjugate (T R156/7, Department of Population Health and Reproduction, C. Munro, UC Davis, CA, USA). The assay sensitivity was 0.047 ng/ml. Intra-assay and inter-assay coefficient of variation were less than 10% and 15%, respectively.
2.7. Antioxidants enzymes and Ganc epididymal expression
After euthanasia, the proximal portion of the epididymis was sampled, snap frozen in liquid nitrogen and conserved at -80 °C until mRNA extraction. Messenger RNA was extracted using Trizol reagent (Thermo Scientific) according manufacturer protocol, its concentration determined with Nanodrop, and stored at -80 °C until cDNA conversion. An aliquot of the extracted RNA was converted to cDNA using M-MLV Reverse Transcriptase (Promega), following the manufacturer protocol, and stored at -20 °C until its use.
The mRNA expressions of Ganc, catalase (Cat), glutathione peroxidase 3 and 5 (Gpx3 and Gpx5), superoxide dismutase 1 (Sod1) and Actb (β-actin), as housekeeping gene, were measured by qRT-PCR, using primers (Invitrogen) and SYBR Green PCR Master Mix (Applied Biosystems). Primers were designed with Primer Express software (Applied Biosystems) from the corresponding Rattus novergicus mRNA. Table 1 reports primers sequences and accession numbers.
Table 1.
Sequence of primers and accession number for each gene for qRT-PCR analysis.
| Gene | Forward primer | Reverse primer | Accession number |
|---|---|---|---|
| Sod1 | CCACTGCAGGACCTCATTTTAAT | TCTCCAACATGCCTCTCTTCATC | NM_017050 |
| Cat | TCAGCGACCGAGGGATTC | GGTGTGTGAGCCATAGCCATT | NM_012520 |
| Gpx3 | AAGAAGAACTTGGCCCATTCG | GGCTCCTGTTTGCCAAATTG | NM_022525 |
| Gpx5 | CAGCTAAGAGTCTTCTATCTCGTTCCA | GTAGCAGTCCATCTTCATCTTTTCC | NM_001105738 |
| Ganc | GACTGTGGCAAGATTGCATTCTA | AGTGAAGCCCGGTAGGTGGTA | NM_001145840 |
| Actb | TCTGTGTGGATTGGTGGCTCTA | CCTGCTTGCTGATCCACATCT | NM_031144 |
All samples were run in triplicate for each gene and for β-actin. There were no differences in β-actin expression among the groups. The change in threshold cycle (ΔCt) between the mean Ct for each gene and the mean Ct for β-actin mRNA from the same animal were used to calculate the relative mRNA expression for each gene. The effect of FED (at 5 and 8 weeks) and FED + Ghrl on each gene was analyzed by ANOVA using the ΔCt values. Data are shown as the mean fold change in expression in the FED (5 and 8 weeks) and FED + Ghrl groups relative to the C group; fold change in each sample was calculated as 2-ΔΔCt, where ΔΔCt is the difference between ΔCt in each sample and the mean ΔCt in the C group.
2.8. Statistical analysis
Results were expressed as Mean ± SEM and were analyzed by one-way ANOVA with LSD Fisher as post-hoc comparison analysis. In all cases, p values under 0.05 were considered of statistical significance. Statistical analyses were performed with Infostat 2016p (Group Infostat, Facultad de Ciencias Agropecuarias – Universidad Nacional de Córdoba, Argentina).
3. Results
Morphometric and metabolic results from male rats treated with the FED or C protocol for 5 weeks are shown in Table 2. As can be seen, FED increased all the morphometric parameters evaluated (particularly epididymal fat, that increased 33% compared to C), although only visceral fat and total abdominal fat reached statistical significance, increasing in 96% and 63% respectively compared to C. In addition, FED significantly increased the plasma concentrations of cholesterol (in 27%), LDL (in 82%) and triglycerides (in 49%). Glucemia and HDL concentration did not differ between groups.
Table 2.
Morphometric and metabolic parameters of male rats treated for 5 weeks with a fructose enriched diet (FED).
| Paremeters | C (n = 5) | FED (n = 5) |
|---|---|---|
| Body weight (g) | 352.5 ± 8.8 | 409.8 ± 9.8 |
| Body weight gain (g) | 30.0 ± 4.1 | 32.5 ± 4.4 |
| Waist circumference (cm) | 16.6 ± 1.5 | 19.5 ± 1.4 |
| Visceral fat (g) | 4.8 ± 0.4 a | 9.4 ± 1.3 a |
| Epididymal fat (g) | 5.4 ± 0.4 | 7.2 ± 1.0 |
| Total abdominal fat (g) | 10.2 ± 0.7 b | 16.6 ± 2.1 b |
| Plasma glucose (mg/dl) | 92.4 ± 3.5 | 85.2 ± 3.8 |
| Cholesterol (mg/dl) | 49.0 ± 2.4 c | 63.2 ± 3.3 c |
| HDL (mg/dl) | 24.4 ± 1.4 | 22.4 ± 0.9 |
| LDL (mg/dl) | 12.2 ± 1.9 d | 22.2 ± 3.1 d |
| Triglycerides (mg/dl) | 62.2 ± 7.3 e | 92.8 ± 9.8 e |
FED animals had free access to food and water enriched with fructose (10% fructose v/v) for 5 weeks. Control animals (C) received water without fructose for the same period. Body weight gain = final body weight – initial body weight. HDL = high-density lipoproteins. LDL = low-density lipoproteins. Identical letters indicate significant differences (p < 0.05).
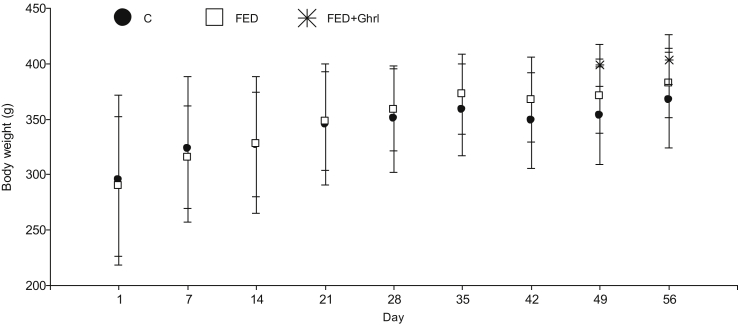
Once validated the protocol, treatments were applied for 8 weeks in order to cover spermatogenesis and epididymal transit (Fig. 1 and Tables 3, 4, and 5). Fig. 1 shows body weight evolution of male rats from groups FED, FED + Ghrl and C. No significant differences were observed in this parameter between groups. An increase in body weight independent of treatment was observed in male rats (days 1 vs 7 vs 14 vs 21 and 28 vs 35 vs 42, 49 and 56; p < 0.05). Food intake of FED + Ghrl animals was similar than that of FED or C males (results not shown).
Fig. 1.
Body weight evolution of male rats treated for 8 weeks with a fructose enriched diet (FED; 10% fructose v/v in drinking water). A subgroup of FED animals received ghrelin (s.c., 6 nmol/animal/day) for the last two weeks of treatment (FED + Ghrl, i.e. from day 42–56). FED and C (control animals) were injected s.c., during this last two weeks of treatment, with isotonic solution (ghrelin vehicle).Values are expressed as Mean±SDM. Number of animals/treatment: C = 7; FED = 10; FED + Ghrl = 5. An increase in food intake independently of treatment was seen in male rats: days 1 vs 7 vs 14 vs 21 and 28 vs 35 vs 42, 49 and 56 (p < 0.05).
Table 3.
Morphometric and metabolic parameters of male rats treated for 8 weeks with a fructose enriched diet (FED), with or without the administration of ghrelin for the last two weeks of treatment.
| Paremeters | C (n = 9) | FED (n = 10) | FED + Ghrl (n = 5) |
|---|---|---|---|
| Body weight (g) | 367.9 ± 16.2 | 384.7 ± 8.5 | 403.6 ± 10.0 |
| Body weight gain (g) | 125.3 ± 9.0 | 132.9 ± 9.4 | 114.2 ± 20.7 |
| Waist circumference (cm) | 16.7 ± 1.6 | 15.8 ± 0.7 | 17.2 ± 0.4 |
| Visceral fat (g) | 8.1 ± 0.9 | 11.1 ± 1.2 | 10.5 ± 1.4 |
| Plasma glucose (mg/dl) | 97.4 ± 2.7 | 98.6 ± 4.0 | 92.8 ± 5.3 |
| Cholesterol (mg/dl) | 54.8 ± 3.0 a-b | 72.8 ± 4.7 a | 76.6 ± 7.2 b |
| HDL (mg/dl) | 22.3 ± 1.7 c | 28.8 ± 2.1 | 30.8 ± 3.9 c |
| LDL (mg/dl) | 20.0 ± 3.4 | 26.4 ± 4.5 | 28.6 ± 4.7 |
| Triglycerides (mg/dl) | 63.2 ± 5.3 d-e | 88.2 ± 9.6 d | 86.2 ± 7.1 e |
FED + Ghrl animals had free access to food and water enriched with fructose (10% fructose v/v) for 8 weeks and, from week 6 onward, received a daily subcutaneous injection of ghrelin (6 nmol/animal/day). FED animals had free access to food and water enriched with fructose (10% fructose v/v) for 8 weeks. Control animals (C) received water without fructose for the same period. These two groups were injected, during the last two weeks of treatment, with isotonic solution (ghrelin vehicle). Body weight gain = final body weight – initial body weight. HDL = high-density lipoproteins. LDL = low-density lipoproteins. Identical letters indicate significant differences (p < 0.05).
Table 4.
Epididymal sperm functional activity and plasma testosterone of male rats treated for 8 weeks with a fructose enriched diet (FED), with or without the administration of ghrelin for the last two weeks of treatment.
| Parameter | C (n = 9) | FED (n = 10) | FED + Ghrl (n = 5) |
|---|---|---|---|
| Testicular weight (g) | 2.9 ± 0.1 | 3.0 ± 0.1 | 2.9 ± 0.1 |
| Concentration (x106/ml) | 25.8 ± 2.8 a | 19.0 ± 1.6 a | 23.5 ± 1.6 |
| Motility (%) | 50.9 ± 4.8 | 48.9 ± 5.3 | 59.2 ± 2.5 |
| Motile sperm count/ml | 1300.4 ± 202.4 b | 834.6 ± 137.0 b-c | 1381.7 ± 71.3 c |
| Cytoplasmic drop (%) | 0.1 ± 0.1 | 0.4 ± 0.2 | 0.0 ± 0.0 |
| Viability (%) | 89.4 ± 3.0 | 88.7 ± 1.2 | 89.2 ± 2.1 |
| Acrosomal reaction (%) | 92.7 ± 1.9 | 91.5 ± 1.9 | 90.0 ± 2.2 |
| Testosterone (ng/ml) | 1.4 ± 0.1 d-e | 0.8 ± 0.2 d | 0.6 ± 0.2 e |
FED + Ghrl animals had free access to food and water enriched with fructose (10% fructose v/v) for 8 weeks and, from week 6 onward, received a daily subcutaneous injection of ghrelin (6 nmol/animal/day). FED animals had free access to food and water enriched with fructose (10% fructose v/v) for 8 weeks. Control animals (C) received water without fructose for the same period. These two groups were injected, during the last two weeks of treatment, with isotonic solution (ghrelin vehicle). Motile sperm count/ml = concentration x motility. Identical letters indicate significant differences (p < 0.05).
Table 5.
Expression of antioxidants enzymes and neutral alpha-glucosidase in epididymis of male rats treated for 5 or 8 weeks with a fructose enriched diet (FED), with or without ghrelin administration from week 6–8 inclusive.
| Parameter | C (n = 11) | FED 5 weeks (n = 6) | FED 8 weeks (n = 5) | FED + Ghrl (n = 5) |
|---|---|---|---|---|
| Superoxido dismutase (Sod1) | 1.00 ± 0.16 | 0.89 ± 0.18 | 0.82 ± 0.42 | 0.68 ± 0.16 |
| Catalase (Cat) | 1.00 ± 0.12 | 0.86 ± 0.15 | 0.96 ± 0.19 | 1.57 ± 0.54 |
| Glutation peroxidase 3 (Gpx3) | 1.00 ± 0.14 a | 0.76 ± 0.11 | 0.69 ± 0.28 b | 3.47 ± 0.48 a-b |
| Glutation peroxidase 5 (Gpx5) | 1.00 ± 0.24 | 0.60 ± 0.08 | 0.98 ± 0.30 | 1.31 ± 0.16 |
| Neutral alpha-glucosidase (Ganc) | 1.00 ± 0.10 | 0.84 ± 0.10 | 1.36 ± 0.27 | 1.09 ± 0.62 |
Epididymal enzymes expression of each gene was quantified by qRT-PCR. Data are shown as the mean fold change in expression (±SEM) in the FED (5 and 8 weeks) and FED + Ghrl groups relative to the control group.
As depicted in Table 3, FED significantly increased cholesterol and triglycerides concentrations. The injection of Ghrl did not reverse dyslipidemia. Nevertheless, Ghrl administration significantly increased HDL concentrations when compared to C.
Regarding the reproductive parameters (Table 4), FED significantly reduced sperm concentration and motile sperm count/ml compared to C. This reduction in motile sperm count was prevented/reversed by the injection of Ghrl during the last two weeks. Furthermore, FED animals were hypogonadic, evidenced by lower T levels in comparison to C. Decreased plasma T levels were also detected in FED + Ghrl (vs C).
Table 5 shows the relative expression of antioxidants enzymes (Sod1, Cat, Gpx3 and Gpx5) and Ganc in the epididymis of C, FED (for 5 and 8 weeks) and FED + Ghrl groups. FED modified, neither at 5 nor at 8 weeks, the expression of antioxidants enzymes or that of neutral alpha-glucosidase. The co-administration of Ghrl to the FED animals from weeks 6–8, significantly increased the expression of GPx3 compared to FED and C.
4. Discussion
The objectives of this study were to evaluate in male rats, if a FED that provokes MetS-related symptoms, exerts deleterious effects on plasma T and semen quality and if the co-administration of Ghrl reverse/prevent these effects. We aimed to evaluate also, if these deleterious effects are associated with increased oxidative stress in epididymis (structure involved in sperm maturation) and if Ghrl may ameliorate the oxidative stress in this tissue.
After validating the model of FED, as an inductor of MetS-related symptoms, we applied the diet for 8 weeks in order to cover spermatogenesis and epididymal transit. We found that FED significantly decreased sperm concentration, while Ghrl co-administration increased this parameter to values comparable to C (although not reaching statistical significance vs FED group). Because of the ability of Ghrl of reversing/preventing the decrease in sperm concentration, the motile sperm count/ml increased significantly in FED + Ghrl vs FED. Although this variable is the product of other two (concentration and motility), we consider it has an important biological meaning. In order to fertilize an egg, it is necessary for the sperm to move.
It would be necessary to determine in our experimental conditions, which mechanisms are implicated in sperm concentration reduction; which is actually the most important variable of sperm quality that changed in our study. Classically, a decrease in sperm concentration has been attributed to alterations in spermatogenesis [38]. Nevertheless, Marchiani et al (2015) and Mallidis et al (2011) described, in rabbits with MetS, a decrease in sperm quality without detecting histological alterations in the testis [23, 39]. Besides, since Ghrl was administered only during the last two weeks of treatment, its effects would only cover the last part of spermatogenesis and all the epididymal transit. So, it is feasible that the beneficial effects of Ghrl are associated to epididymal physiology; for example increasing sperm survival and/or concentrating gametes [28]. Concordantly, Marchiani et al. (2015) found in a rabbit MetS model, an altered epididymal expression of Aqp1 (aquaporin 1), and suggested that this may underlie defective fluid resorption in the organ [23].
Although not all [3, 6, 39], several other authors found alterations in sperm quality secondary to MetS or MetS-related diets [4, 7, 8, 9, 10, 23, 40]. The sperm variables most affected are sperm concentration and motility. Ferramosca et al. (2017), fed male rats with a high-fat diet (enriched with 35% of fat and 15% sucrose) during 4 weeks, and found a significant decrease (of more than 40%) in sperm concentration and in motility; the authors attributed motility reduction to an increase in sperm oxidative stress [40]. This oxidative stress might affect, for example, epididymal sperm survival.
As reviewed by Noblanc et al (2011), spermatozoa are particularly susceptible to oxidative damage for three major reasons: first, post-testicular spermatozoa are silent cells, with a compacted DNA, which are not able to participate in any transcriptional activation when challenged by oxidative stress. Second, spermatozoa are also silent in terms of protein synthesis, because of the loss of most of their cytoplasm and sub-cellular organelles. Third, spermatozoa are highly reactive to oxidative injury because their membranes have high concentrations of PUFAs, lipids prone to oxidation [26]. Furthermore, since sperm maturation processes and specifically, epididymal maturation, require the action of H2O2 for sperm proteins sulfoxidation, oxidative-antioxidative balance must be strictly controlled [26]. For this reason, mammalian epididymis expresses, among other antioxidants, several glutathione peroxidases (Gpxs). To date, is the organ in which one can find expressed, in different levels and sub-territories, most of the known Gpxs (from Gpx1 to Gpx8) [41,42]. Gpx3 and Gpx5 are quantitatively the most abundant Gpxs, representing more than 95% of the epididymal Gpxs [26]. Besides, Gpx5 is specific from this tissue [26, 41].
In our experimental conditions, the expression of the epididymal antioxidant enzymes Cat, Sod1, Gpx3 and Gpx5, did not differ by FED (at week 5 or 8).These results seem to indicate that FED did not increase oxidative stress at epididymal level. Regretfully, we did not evaluate enzymes content and/or activity, in order to reassure this assumption. In any way, the co-administration of Ghrl significantly increased Gpx3 expression, even in comparison with the C group. It is important to remark that Gpxs mediate, as Cat, the recycling of H2O2 to water. Nevertheless, Cat is activated when H2O2 concentrations are far above physiological levels, being an acute stress-response scavenger. Nonetheless, Gpxs deal with small physiological adjustments of H2O2 concentrations, and are more versatile than Cat in the substrates they can metabolize. Gpxs can recycle organic peroxidized molecules, including those in free PUFAs and in complex membranes, acting therefore not only as scavenger but also, as repairing enzymes [reviewed in 26]. Further studies are necessary in order to elucidate if the increase in epididymal Gpx3, induced by Ghrl, functions as a “protective” mechanism naturally provoked by this substance; and/or an amplified reaction by Ghrl to some degree of systemic/local oxidative stress not evidenced by the other antioxidant enzymes and/or the detection methods used in this study.
There are few studies investigating the effects of Ghrl on Gpxs activity and/or expression; none of them in epididymis. Nevertheless, the majority of these studies have detected an antioxidant effect of Ghrl which is even more potent than in basal conditions. That means that Ghrl significantly increases Sod, Cat and/or Gpxs expression and/or activity, compared to control groups [32, 43, 44, 45, 46]. Asadi et al (2018), demonstrated that Ghrl administration to rats with varicocele significantly increased Sod and Gpxs testicular levels and reduced malondialdehyde, not only vs varicocele rats but also vs control ones. These authors informed an improvement in sperm count and viability in Ghrl injected rats vs those with varicocele [43]. Similar results were observed in another study in rats, in which testicular toxicity was induced by cadmium [46]. Furthermore in humans, failure in spermatozoa Gpxs expression has been correlated with infertility [47, 48]. Finally, studies conducted in knockout models for Gpxs have demonstrated that Gpxs play important roles in mammalian sperm physiology [reviewed in 26]. Therefore, it might be possible in our study that the reversion in sperm abnormalities caused by Ghrl would be attributable to this increase in Gpx3 expression.
Additionally to sperm quality, FED significantly reduced plasma T concentrations. This feature has been found by several authors, not only in animal models but also in humans [3, 4, 23, 39]. Nonetheless, some authors actually found an increase in this androgen in MetS models [22]. Hypogonadism in MetS and MetS-related models has been attributed to an increase in T aromatization to estradiol, which occurs in adipose tissue [reviewed in 49]. This may explain why Ghrl co-administration did not prevent/reverse T decrease, since morphometric parameters were similar in FED and FED + Ghrl groups.
Although epididymal function and specifically Ganc expression is T-dependent [27], we did not find any alteration in the expression of this enzyme. Since epididymal intraluminal T levels are much higher than plasma concentrations [50, 51], it is possible that plasma androgen levels are not as important for epididymal function as local androgen concentrations.
Regarding metabolism and in concordance with previous publications [22, 30], FED provoked MetS-related abnormalities. Specifically, after 5 weeks of treatment, FED significantly increased visceral and total abdominal fat, and resulted in dyslipidemia (by enhancing total cholesterol, LDL and triglycerides levels). The effects of FED at 8 weeks were not as noticeable as that at 5 weeks, since only cholesterol and triglycerides levels varied significantly. It seems that male rats achieved some kind of “compensation”. This compensation should not be attributed to a reduction in food intake, since this parameter was similar in the three experimental groups. Although it might be related to a decreased in fructose enriched water intake, this parameter was not measured. In any case, the administration of Ghrl did not reverse dyslipidemia. Nevertheless, Ghrl significantly increased HDL levels, which is a good prognostic lipoprotein for cardiovascular risk [52]. Besides, it has been reported that during fasting, Ghrl interacts with a species of HDL associated with clusterin, paraoxonase and apolipoprotein A-I, linking hunger with growth hormone release [53]. An increase in plasma Ghrl due to the exogenous administration of the peptide, might rise the levels of this particular HDL.
In summary, the application of a FED to male rats for 8 weeks significantly decreased sperm concentration and plasma T levels. The co-administration of Ghrl during the last two weeks of treatment prevented/reversed sperm quality impairment, without reversing hypogonadism. It is possible that an epididymal increase in the expression of Gpx3 might be responsible, at least in part, for sperm quality recovery.
Declarations
Author contribution statement
Nicolás David Ramírez, Eugenia Mercedes Luque, Xavier Michael Jones, Pedro Javier Torres, María José Moreira Espinoza, Verónica Cantarelli: Performed the experiments; Analyzed and interpreted the data.
Marina Flavia Ponzio: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ana Arja, María Belén Rabaglino: Conceived and designed the experiments; Analyzed and interpreted the data.
Ana Carolina Martini: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by grants from the Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba (SECyT-UNC).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Clarisa Lagares for the care of our experimental animals and technical support.
References
- 1.Kasturi S.S., Tannir J., Brannigan R.E. The metabolic syndrome and male infertility. J. Androl. 2008;29:251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 2.Srikanthan K., Feyh A., Visweshwar H. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginianpopulation. Int. J. Med. Sci. 2016;13:25–38. doi: 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventimiglia E., Capogrosso P., Colicchia M. Metabolic síndrome in white European men presenting for primary couple’s infertility: investigation of the clinical and reproductive burden. Andrology. 2016;4:944–951. doi: 10.1111/andr.12232. [DOI] [PubMed] [Google Scholar]
- 4.Lotti F., Corona G., Degli Innocenti S. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013;1:229–239. doi: 10.1111/j.2047-2927.2012.00031.x. [DOI] [PubMed] [Google Scholar]
- 5.Makhside N. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J. Urol. 2005;3:827–834. doi: 10.1097/01.ju.0000169490.78443.59. [DOI] [PubMed] [Google Scholar]
- 6.Pilatz A., Hudemann C., Wolf J. Metabolic syndrome and the seminal cytokine network in morbidly obese males. Andrology. 2017;5:23–30. doi: 10.1111/andr.12296. [DOI] [PubMed] [Google Scholar]
- 7.Rosety I., Elosegui S., Pery M.T. Asociación entre obesidad abdominal y daño oxidative seminal en pacientes con síndrome metabólico. Rev. Med. Chile. 2014;142:732–737. doi: 10.4067/S0034-98872014000600007. [DOI] [PubMed] [Google Scholar]
- 8.Montanino Oliva M., Minutolo E., Lippa A. Effect of myoinositol and antioxidants on sperm quality in men with metabolic syndrome. Int. J. Endocrinol. 2016;2016:1674950. doi: 10.1155/2016/1674950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leisegang K., Bouic P.J., Henkel R.R. Metabolic syndrome is associated with increased seminal inflammatory cytokines and reproductive dysfunction in a case-controlled male cohort. Am. J. Reprod. Immunol. 2016;76:155–163. doi: 10.1111/aji.12529. [DOI] [PubMed] [Google Scholar]
- 10.Leisegang K., Udodong A., Bouic P.J. Effect of the metabolic syndrome on male reproductive function: a case-controlled pilot study. Andrologia. 2014;46:167–176. doi: 10.1111/and.12060. [DOI] [PubMed] [Google Scholar]
- 11.Kojima M., Hosoda H., Date Y. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 12.Kojima M., Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.Y., Asakawa A., Fujimiya M. Ghrelin gene products and the regulation of food intakeand gut motility. Pharmacol. Res. 2009;61:430–481. doi: 10.1124/pr.109.001958. [DOI] [PubMed] [Google Scholar]
- 14.García M.C., López M., Álvarez C.V. Role of ghrelin in reproduction. Reproduction. 2007;133:531–540. doi: 10.1530/REP-06-0249. [DOI] [PubMed] [Google Scholar]
- 15.Pulkkinen L., Ukkola O., Kolehmainen M. Ghrelin in diabetes and metabolic syndrome. Int. J. Pept. 2010;2010:248948. doi: 10.1155/2010/248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ukkola O., Poykko S.M., Antero Kesaniemi Y. Low plasma ghrelin concentration is an indicator of metabolic syndrome. Ann. Med. 2006;38:274–279. doi: 10.1080/07853890600622192. [DOI] [PubMed] [Google Scholar]
- 17.Ukkola O. Ghrelin and metabolic disorders. Curr. Protein Pept. Sci. 2009;10:2–7. doi: 10.2174/138920309787315220. [DOI] [PubMed] [Google Scholar]
- 18.Barazzoni R., Zanetti M., Semolic A. High-fat diet with acyl-ghrelin treatment leads to weight gain with low inflammation, high oxidative capacity and normal triglycerids in rat muscle. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixit V.D., Schaffer E.M., Pyle R.S. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kheradmand A., Alirezaei M., Birjandi M. Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul. Pept. 2010;162:84–89. doi: 10.1016/j.regpep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 21.El Eter E., Al Tuwaijiri A., Hagar H. In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. J. Gastroenterol. Hepatol. 2007;22:1791–1799. doi: 10.1111/j.1440-1746.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 22.Catak Z., Aydin S., Sahin I. Regulatory neuropeptides (ghrelin, obestatin and nesfatin-1) levels in serum and reproductive tissues of female and male rats with fructose-induced metabolic syndrome. Neuropeptides. 2014;48:167–177. doi: 10.1016/j.npep.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Marchiani S., Vignozzi L., Filippi S. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol. Cell. Endocrinol. 2015;401:12–24. doi: 10.1016/j.mce.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Martini A.C., Tissera A., Estofán D. Overweight and seminal quality: a study in 794 patients. Fertil. Steril. 2010;94:1739–1743. doi: 10.1016/j.fertnstert.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Luque E.M., Tissera A., Gaggino M.P. Body mass index and human sperm quality: neither one extreme nor the other. Reprod. Fertil. Dev. 2017;29:731–739. doi: 10.1071/RD15351. [DOI] [PubMed] [Google Scholar]
- 26.Noblanc A., Kocer A., Chabory E. Glutathione peroxidases at work in epididymal spermatozoa: an example of the dual effect of reactive oxygen species on mammalina male fertilizing ability. J. Androl. 2011;32:641–650. doi: 10.2164/jandrol.110.012823. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud A.M., Geslevich J., Kint J. Seminal plasma α-glucosidase activity and male infertility. Hum. Reprod. 1998;13:591–595. doi: 10.1093/humrep/13.3.591. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan R., Mieusset R. The human epididymis: its function in sperm maturation. Hum. Reprod. 2016;22:574–587. doi: 10.1093/humupd/dmw015. [DOI] [PubMed] [Google Scholar]
- 29.Cooper T.G. In: Epididymis. Encyclopedia of Reproduction. Knobil E., Neill J.D., editors. Academic Press; San Diego-USA: 1998. pp. 1–17. [Google Scholar]
- 30.Rodríguez V., Rivoire M., Guizzardi S. Naringin prevents the inhibition of intestinal Ca2+ absorption induced by fructose rich diet. Arch. Biochem. Biophys. 2017;636:1–10. doi: 10.1016/j.abb.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Tarán M., Báez M., de la Paz Scribano M. Experimental model of oxidative stress markers in subclinical atherogenesis associated with metabolic syndrome. SM. J. Cardiol. Cardiov. Dis. 2018;4:1021. [Google Scholar]
- 32.Omrani H., Alipour M.R., Farajdokht F. Effects of chronic ghrelin treatment on hypoxia- induced brain oxidative stress and inflammation in a rat normobaric chronic hypoxia model. High Alt. Med. Biol. 2017;18:145–151. doi: 10.1089/ham.2016.0132. [DOI] [PubMed] [Google Scholar]
- 33.Oztas B., Sahin D., Kir H. The effect of leptin, ghrelin, and neuropeptide-Y on serum Tnf-Α, Il-1β, Il-6, Fgf-2, galanin levels and oxidative stress in an experimental generalized convulsive seizure model. Neuropeptides. 2017;61:31–37. doi: 10.1016/j.npep.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Fiol de Cuneo M., Ruiz R., Ponce A. Time-related changes in functional activity of mouse spermatozoa during in vitro or in vivo incubation. J. Exp. Anim. Sci. 1994;36:189–200. [PubMed] [Google Scholar]
- 35.Makler A. The improved ten-micrometer chamber for rapid sperm count and motility evaluation. Fertil. Steril. 1980;33:337–338. doi: 10.1016/s0015-0282(16)44606-6. [DOI] [PubMed] [Google Scholar]
- 36.Kovács A., Foote R. Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotech. Histochem. 1992;67:119–124. doi: 10.3109/10520299209110020. [DOI] [PubMed] [Google Scholar]
- 37.Larson J.L., Miller D.J. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999;52:445–449. doi: 10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Hirsh A. ABC of subfertility. BMJ. 2003;327:669–672. doi: 10.1136/bmj.327.7416.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallidis C., Agnieska C., Filippi S. Spermatogenic and sperm quality differences in an experimental model of metabolic syndrome and hypogonadal hypogonadism. Reproduction. 2011;142:63–71. doi: 10.1530/REP-10-0472. [DOI] [PubMed] [Google Scholar]
- 40.Ferramosca A., Moscatelli N., Di Giacomo M. Dietary fatty acids influence sperm quality and function. Andrology. 2017;5:423–430. doi: 10.1111/andr.12348. [DOI] [PubMed] [Google Scholar]
- 41.Devret J.R. Glutathione peroxidases expression in the mammalian epididymis and vas deferens. Andrology. In: Francavilla F., Francavilla S., Forti G., editors. Vol. 2000. 2000. pp. 427–461. (L’Aquila-Italy, Collana di Study Abruzzesi). [Google Scholar]
- 42.Devret J.R. The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol. Cell. Endocrinol. 2006;250:70–79. doi: 10.1016/j.mce.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Asadi N., Kheradmand A., Gholami M. Effect of ghrelin on the biochemical and histopathology parameters and spermatogenesis cycle following experimental varicocele in rat. Andrologia. 2018;50 doi: 10.1111/and.13106. [DOI] [PubMed] [Google Scholar]
- 44.Omrani H., Alipour M.R., Mohaddes G. Ghrelin improves antioxidant defense in blood and brain in normobaric hypoxia in adult male rats. Adv. Pharmaceut. Bull. 2015;5:283–288. doi: 10.15171/apb.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobutovic B., Sudar E., Tepacvcevic S. Effects of ghrelin on protein expression of antioxidative enzymes and iNOS in the rat liver. Arch. Med. Sci. 2014;29:806–816. doi: 10.5114/aoms.2014.44872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kheradmand A., Alirezaei M., Dezfoulian O. Biochemical and histopathological evaluations of ghrelin effects following cadmium toxicicty in the rat testis. Andrologia. 2015;47:634–643. doi: 10.1111/and.12311. [DOI] [PubMed] [Google Scholar]
- 47.Imai H.N., Hakkaku R., Iwamoto J. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 2009;284:32522–32532. doi: 10.1074/jbc.M109.016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foresta C., Flohé L., Garolla A. Male infertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002;67:967–971. doi: 10.1095/biolreprod.102.003822. [DOI] [PubMed] [Google Scholar]
- 49.Michalakis K., Mintziori G., Kaprara A. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism. 2013;62:457–478. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Robaire B., Hamzeh M. Androgen action in the epididymis. J. Androl. 2011;32:592–599. doi: 10.2164/jandrol.111.014266. [DOI] [PubMed] [Google Scholar]
- 51.Sipila P., Krutskikh A., Pujianto D.A. Regional expression of androgen receptor coregulators and androgen action in the mouse epididymis. J. Androl. 2011;32:711–717. doi: 10.2164/jandrol.110.012914. [DOI] [PubMed] [Google Scholar]
- 52.Xiang A.S., Kingwell B.A. Rethinking good cholesterol: a clinicians’ guide to understanding HDL. Lancet Diabetes Endocrinol. 2019;7:575–582. doi: 10.1016/S2213-8587(19)30003-8. [DOI] [PubMed] [Google Scholar]
- 53.Beaumont N.J., Skinner V.O., Tricia M. Ghrelin can bind to a species of high density lipoprotein associated with paraoxonase. J. Biol. Chem. 2003;278:8877–8880. doi: 10.1074/jbc.C200575200. [DOI] [PubMed] [Google Scholar]