Autosomal dominant polycystic kidney disease (ADPKD) is a common cause of chronic kidney disease (CKD) worldwide. It is also the most common genetic cause of CKD, resulting from mutations in the PKD1 and PKD2 genes.S1 The clinical course is characterized by progressive enlargement of renal cysts, often culminating in end-stage renal disease (ESRD).
Fibroblast growth factor 23 (FGF23) is a hormone secreted by osteocytes that induces phosphaturia and decreases renal 1α-hydroxylase expression,S2 physiologically functioning as a homeostatic regulator of phosphate and a counterregulatory hormone to 1,25-dihydroxyvitamin D (1,25D). In CKD, FGF23 levels increase very early and continue to rise as the glomerular filtration rate decreases.S3,S4 Increased FGF23 levels in CKD help to maintain normophosphatemia until late-stage CKDS4,S5; however, these elevated FGF23 levels are also associated with adverse, “off-target” effects, including CKD progression,S6–S8 cardiac hypertrophy,S9 and overall mortality.S7 Interestingly, ADPKD patients with early-stage CKD have higher circulating levels of FGF23 than do non-ADPKD patients with similar kidney function.1, 2
Regulation of FGF23 is complex and remains incompletely understood. Several mineral metabolism factors may increase FGF23 production, including phosphate,S10,S11 1,25D,S10,S12 parathyroid hormone,S13 and calcium.S14 Recently, it has also been demonstrated that non-mineral factors, such as inflammation,S15 iron deficiency,S15–S18 and erythropoietin (EPO)3, 4, 5, 6, 7, 8 can also increase FGF23 production.
There is evidence suggesting that EPO production is increased in ADPKD. In kidneys from ADPKD patients, it has been shown that stromal cells in the cyst walls express EPO mRNA, and that the cyst fluid can contain high concentrations of EPO.S19 This cystic EPO production may be secondary to regional hypoxia-induced upregulation of hypoxia-inducible factor 2α.S20 In cohorts of ESRD patients, it has been observed that ADPKD patients have higher serum EPO levels than do non-ADPKD patients.9,S21 As EPO has recently been identified as a novel determinant of FGF23 production,3, 4, 5, 6, 7, 8 we hypothesized that EPO may contribute to FGF23 production in ADPKD.
Therefore, we evaluated associations between serum EPO and circulating FGF23 levels in subjects from the Halt Progression of Polycystic Kidney Disease (HALT-PKD) study A, a cohort of ADPKD patients with estimated glomerular filtration rates (eGFRs)S22 of at least 60 ml/min per 1.73 m2. Then, in a separate, smaller cohort of ADPKD patients with an eGFR of at least 60 ml/min per 1.73 m2 in whom research protocol bone biopsies were performed, we evaluated associations among serum EPO, circulating FGF23 levels, and bone FGF23. In both cohorts, we measured concentrations of C-terminal (total) FGF23 and intact FGF23. Whereas the C-terminal (total) FGF23 assay detects both the full-length, intact hormone and its C-terminal proteolytic fragments, thus functioning as a surrogate measure of overall FGF23 production, the intact FGF23 assay detects only the full-length form of the hormone (Supplementary Figure S1).
Results
ADPKD HALT-PKD Study A Cohort
This cohort consisted of 78 ADPKD subjects, characterized in Table 1. The mean (SD) age was 37 (8) years. A total of 55% of subjects were male; 97% of subjects were white. All subjects had an estimated eGFR of at least 60 ml/min per 1.73 m2, and the mean eGFR was 91 (17) ml/min per 1.73 m2. Kidney volumes were elevated compared with published normal valuesS23; however, the average liver volume for body surface area was within the reference range.S24 The mean serum EPO concentration was 15.8 (6.9) mIU/ml, and the median (interquartile range) serum EPO concentration was 14.2 (11.5, 18.8) mIU/ml, which is elevated compared to the median EPO level observed in a cohort of 6777 subjects from the general population living at sea level (7.8 [5.9, 10.3] mIU/ml).S25 Although levels were elevated in the ADPKD cohort, the contribution of altitude-mediated effects cannot be excluded.
Table 1.
Characteristics of the Autosomal Dominant Polycystic Kidney Disease Halt Progression of Polycystic Kidney Disease (ADPKD HALT-PKD) study A cohort (n = 78)
| Parameter | Value |
|---|---|
| Age (yr) | 37 (8) |
| Sex (% male) | 55 |
| Ethnicity (% white) | 97 |
| Weight (kg) | 83.9 (19.1) |
| Height (cm) | 174.6 (10.4) |
| Body surface area (m2) | 2.01 (0.26) |
| Body mass index (kg/m2) | 27.3 (5.5) |
| Estimated glomerular filtration rate (ml/min per 1.73 m2) | 91 (17) |
| Total kidney volume (ml) | 1207 (648) |
| Height-adjusted total kidney volume (ml/m) | 686 (348) |
| Liver volume (ml) | 2012 (433) |
| Systolic blood pressure (mm Hg) | 130 (15) |
| Diastolic blood pressure (mm Hg) | 78 (11) |
| Erythropoietin (mIU/ml) | 15.8 (6.9) |
| Hemoglobin (g/dl) | 14.6 (1.3) |
| C-terminal (total) FGF23 (RU/ml) | 22.9 (12.8) |
| Intact FGF23 (pg/ml) | 53.8 (34.3) |
| Alkaline phosphatase (IU/L) | 54.0 (15.7) |
| Phosphate (mg/dl) | 3.4 (0.6) |
| Calcium (mg/dl) | 9.3 (0.4) |
| Parathyroid hormone (pg/ml) | 28.4 (16.3) |
| 25OH vitamin D3 (ng/ml) | 32.9 (9.7) |
| 1,25(OH)2 vitamin D3 (pg/ml) | 38.0 (13.2) |
| 24,25(OH)2 vitamin D3 (pg/ml) | 3.7 (1.9) |
FGF, fibroblast growth factor.
Data presented as percentages or mean (SD).
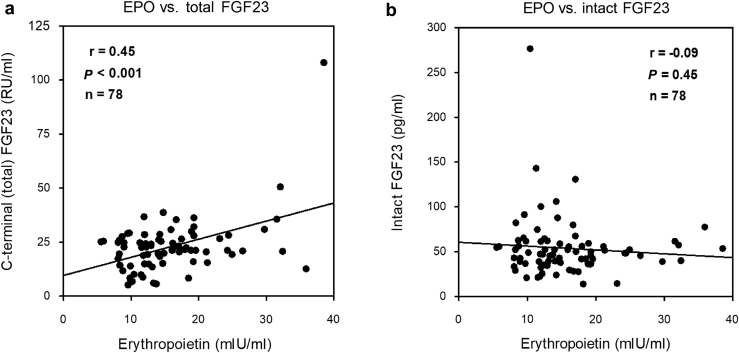
In our cohort, serum EPO levels positively correlated with circulating C-terminal (total) FGF23 concentrations (r = 0.45, P < 0.001; Figure 1a), but not with circulating intact FGF23 concentrations (r = –0.09, P = 0.45; Figure 1b). Serum EPO levels were not associated with height-adjusted total kidney volume (r = –0.05, P = 0.65) or with liver volume (r = 0.05, P = 0.67). In multiple linear regression modeling, in a model adjusted for age, sex, eGFR, height-adjusted total kidney volume, and liver volume, EPO remained positively and independently associated with total FGF23 (β = 0.45, P < 0.001; Table 2). In a model further adjusted for serum phosphate, calcium, parathyroid hormone, 1,25D, and hemoglobin, EPO remained positively and independently associated with total FGF23 (β = 0.39, P = 0.001; Table 2). In contrast to total FGF23, in no model was intact FGF23 associated with EPO (Table 3).
Figure 1.
Associations between erythropoietin and fibroblast growth factor 23 (FGF23) in the Autosomal Dominant Polycystic Kidney Disease Halt Progression of Polycystic Kidney Disease (ADPKD HALT-PKD) study A cohort. In patients with ADPKD, serum erythropoietin (EPO) levels positively correlated with circulating C-terminal (total) FGF23 concentrations (a), but not with circulating intact FGF23 concentrations (b).
Table 2.
Multiple linear regression modeling, dependent variable: C-terminal (total) fibroblast growth factor 23 (n = 78)
| Model | Independent variables | Standardized coefficient | P value | Variance inflation factor | Adjusted R2 |
|---|---|---|---|---|---|
| 1 | EPO | 0.45 | <0.001 | 1.00 | 0.19 |
| 2 | EPO | 0.45 | <0.001 | 1.06 | 0.18 |
| Age | 0.12 | 0.33 | 1.39 | ||
| Sex | –0.12 | 0.32 | 1.27 | ||
| eGFR | 0.07 | 0.57 | 1.50 | ||
| Height-adjusted TKV | 0.12 | 0.32 | 1.23 | ||
| Liver volume | 0.03 | 0.78 | 1.17 | ||
| 3 | EPO | 0.39 | 0.001 | 1.36 | 0.25 |
| Age | 0.08 | 0.53 | 1.59 | ||
| Sex | –0.17 | 0.26 | 2.21 | ||
| eGFR | 0.13 | 0.30 | 1.54 | ||
| Height-adjusted TKV | 0.13 | 0.27 | 1.42 | ||
| Liver volume | 0.00 | 0.97 | 1.25 | ||
| Phosphate | 0.02 | 0.86 | 1.14 | ||
| Calcium | –0.30 | 0.011 | 1.33 | ||
| PTH | –0.05 | 0.63 | 1.25 | ||
| 1,25D | –0.28 | 0.019 | 1.40 | ||
| Hemoglobin | –0.05 | 0.72 | 2.16 |
1,25D, 1,25-dihydroxyvitamin D; eGFR, estimated glomerular filtration rate; EPO, erythropoietin; PTH, parathyroid hormone; TKV, total kidney volume.
Table 3.
Multiple linear regression modeling, dependent variable: intact fibroblast growth factor 23 (n = 78)
| Model | Independent variables | Standardized coefficient | P value | Variance inflation factor | Adjusted R2 |
|---|---|---|---|---|---|
| 1 | EPO | –0.09 | 0.45 | 1.00 | 0.00 |
| 2 | EPO | –0.07 | 0.54 | 1.06 | 0.03 |
| Age | 0.04 | 0.79 | 1.39 | ||
| Sex | –0.18 | 0.16 | 1.27 | ||
| eGFR | –0.02 | 0.90 | 1.50 | ||
| Height-adjusted TKV | 0.05 | 0.66 | 1.23 | ||
| Liver volume | 0.18 | 0.14 | 1.17 | ||
| 3 | EPO | –0.07 | 0.58 | 1.36 | 0.00 |
| Age | 0.09 | 0.54 | 1.59 | ||
| Sex | –0.13 | 0.46 | 2.21 | ||
| eGFR | –0.04 | 0.79 | 1.54 | ||
| Height-adjusted TKV | 0.05 | 0.70 | 1.42 | ||
| Liver volume | 0.19 | 0.14 | 1.25 | ||
| Phosphate | 0.08 | 0.49 | 1.14 | ||
| Calcium | 0.06 | 0.64 | 1.33 | ||
| PTH | –0.11 | 0.39 | 1.25 | ||
| 1,25D | 0.03 | 0.82 | 1.40 | ||
| Hemoglobin | 0.03 | 0.84 | 2.16 |
1,25D, 1,25-dihydroxyvitamin D; eGFR, estimated glomerular filtration rate; EPO, erythropoietin; PTH, parathyroid hormone; TKV, total kidney volume.
In a sensitivity analysis, FGF23 values were log-transformed prior to analysis. As in the original analysis, serum EPO levels positively correlated with log-transformed circulating total FGF23 concentrations (r = 0.35, P = 0.002; Supplementary Figure S2A), but not with log-transformed circulating intact FGF23 concentrations (r = –0.04, P = 0.73; Supplementary Figure S2B). In multiple linear regression modeling, in the partially adjusted and fully adjusted models, EPO remained positively and independently associated with log-transformed total FGF23 (β = 0.37, P = 0.002, and β = 0.32, P = 0.009, respectively; Supplementary Table S1). As in the original analysis, in no model was log-transformed intact FGF23 associated with EPO (Supplementary Table S2).
In another sensitivity analysis, the outlying total FGF23 and intact FGF23 data points (see Figure 1) were removed from the data set prior to analysis. As in the original analysis, serum EPO levels were positively correlated with circulating total FGF23 concentrations (r = 0.27, P = 0.016; Supplementary Figure S3A), but not with circulating intact FGF23 concentrations (r = –0.03, P = 0.79; Supplementary Figure S3B). In multiple linear regression modeling, in the partially adjusted and fully adjusted models, EPO remained positively and independently associated with total FGF23 (β = 0.29, P = 0.017, and β = 0.29, P = 0.014, respectively; Supplementary Table S3). As in the original analysis, in no model was intact FGF23 associated with EPO (Supplementary Table S4).
ADPKD Bone Biopsy Cohort
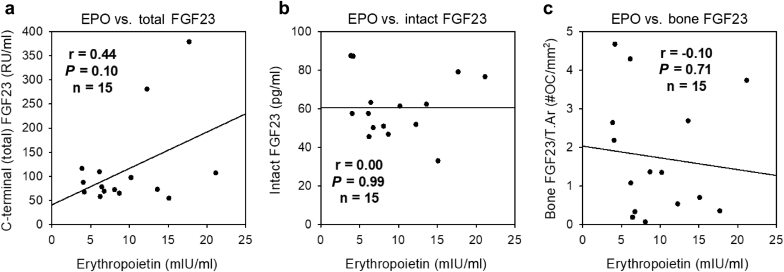
This cohort consisted of 15 ADPKD subjects who underwent bone biopsy as part of a research protocol, characterized in Table 4. The mean (SD) age was 29 (10) years, and 40% of subjects were male. All subjects had an estimated eGFR of at least 60 ml/min per 1.73 m2, and the mean eGFR was 106 (20) ml/min per 1.73 m2. The mean serum EPO concentration was 9.7 (5.3) mIU/ml. In this cohort, serum EPO levels tended to positively correlate with circulating total FGF23 concentrations (r = 0.44, P = 0.10; Figure 2a), but not with circulating intact FGF23 concentrations (r = 0.00, P = 0.99; Figure 2b) or with bone FGF23 levels (r = –0.10, P = 0.71; Figure 2c).
Table 4.
Characteristics of the autosomal dominant polycystic kidney disease bone biopsy cohort (n = 15)
| Parameter | Value |
|---|---|
| Age (yr) | 29 (10) |
| Sex (% male) | 40 |
| Weight (kg) | 78.5 (13.6) |
| Height (cm) | 174.9 (8.3) |
| Body surface area (m2) | 1.95 (0.19) |
| Body mass index (kg/m2) | 25.7 (4.0) |
| Estimated glomerular filtration rate (ml/min per 1.73 m2) | 106 (20) |
| Erythropoietin (mIU/ml) | 9.7 (5.3) |
| Erythroferrone (ng/ml) | 16.3 (7.3) |
| Hepcidin (ng/mL) | 30.0 (22.4) |
| Iron (mcg/dl) | 96.6 (39.2) |
| Ferritin (ng/ml) | 292.3 (174.3) |
| Hemoglobin (g/dl) | 14.4 (1.8) |
| C-terminal (total) FGF23 (RU/ml) | 113.9 (91.4) |
| Intact FGF23 (pg/ml) | 60.6 (15.8) |
| Bone FGF23/tissue areaa | 1.7 (1.5) |
| Phosphate (mg/dl) | 4.0 (0.5) |
| Calcium (mg/dl) | 9.3 (0.3) |
| Parathyroid hormone (pg/ml) | 27.1 (8.8) |
| 25OH vitamin D3 (ng/ml) | 29.7 (9.3) |
| 1,25(OH)2 vitamin D3 (pg/ml) | 46.0 (13.3) |
| 24,25(OH)2 vitamin D3 (pg/ml) | 2.6 (0.8) |
FGF, fibroblast growth factor.
Data presented as percentages or mean (SD).
Bone FGF23/tissue area is defined as the number of osteocytes with positive staining divided by the total tissue area analyzed (mm2).
Figure 2.
Associations between erythropoietin (EPO) and fibroblast growth factor 23 (FGF23) in the autosomal dominant polycystic kidney disease (ADPKD) bone biopsy cohort. In patients with ADPKD, serum EPO levels tended to positively correlate with circulating C-terminal (total) FGF23 concentrations (a), but not with circulating intact FGF23 concentrations (b) or with bone FGF23 levels (c).
In this cohort, we also characterized iron parameters. Serum ferritin levels tended to inversely correlate with circulating total FGF23 concentrations (r = –0.48, P = 0.07; Supplementary Figure S4A), but not with circulating intact FGF23 concentrations (r = –0.17, P = 0.54; Supplementary Figure S4B) or with bone FGF23 levels (r = –0.01, P = 0.97; Supplementary Figure S4C). Serum iron concentrations were not associated with total FGF23 (r = –0.17, P = 0.54), intact FGF23 (r = 0.02, P = 0.94), or bone FGF23 (r = –0.30, P = 0.28).
Also, serum EPO positively correlated with the hepcidin-regulatory hormone erythroferrone (ERFE) (r = 0.69, P = 0.004; Supplementary Figure S5A), but ERFE was not correlated with serum hepcidin (r = –0.05, P = 0.87; Supplementary Figure S5B). Ferritin was positively correlated with hepcidin (r = 0.66, P = 0.007; Supplementary Figure S5C).
Discussion
Previous studies comparing cohorts of ADPKD versus non-ADPKD patients with similar kidney function revealed higher serum EPO levels9,S21 and higher circulating FGF23 concentrations1, 2 in the ADPKD groups. As EPO can induce FGF23 production,3, 4, 5, 6, 7, 8 we hypothesized that EPO and FGF23 levels would positively correlate in our ADPKD cohorts. We observed that serum EPO concentrations positively and independently correlated with circulating total FGF23 levels, but not with circulating intact FGF23 levels, suggesting complex EPO-induced effects on FGF23 regulatory mechanisms. Also, we observed that serum EPO concentrations were more strongly associated with circulating total FGF23 levels than with bone FGF23 levels, which may be consistent with EPO effects on extra-osseous FGF23 production. These findings are significant in that they are consistent with what has been observed in preclinical studies regarding the effects of EPO on FGF23,3, 4, 5, 6, 7, 8 and they suggest that EPO may play a role in FGF23 metabolism in ADPKD patients.
Molecular regulation of FGF23 is complex, as FGF23 is regulated by many factors at both the transcriptional and posttranslational stages. Intracellularly, some translated FGF23 protein may be cleaved into N-terminal and C-terminal fragments, such that what is secreted from the cell is a mix of full-length, intact, bioactive FGF23 protein and cleaved FGF23 protein fragments, the biological relevance of which is unclear. Regulation of FGF23 posttranslational proteolytic cleavage is important, as this regulatory step determines the ratio of intact FGF23 to fragmented FGF23 in the circulation. Multiple enzymes are involved in the regulation of FGF23 cleavage. Proprotein convertases furinS26 and PC5/6S27 can cleave intact FGF23 at a 176RHTR179 cleavage site. O-glycosylation of the cleavage site by GALNT3 protects intact FGF23 from cleavage.S28 Conversely, Fam20C phosphorylates intact FGF23 adjacent to the cleavage motif, inhibiting O-glycosylation, allowing cleavage.S29 The relative effects of these enzymes determine how much translated FGF23 protein is cleaved prior to secretion.
There are two different types of enzyme-linked immunosorbent assays (ELISAs) used to measure circulating FGF23 concentrations. The first type of FGF23 ELISA is the “C-terminal” ELISA. This assay uses capture and detection antibodies that both recognize epitopes within the C-terminal tail of FGF23, distal to the 176RHTR179 cleavage site. Therefore, this assay can detect both full-length, uncleaved FGF23 protein and the cleaved C-terminal FGF23 proteolytic fragment, thus measuring the total amount of translated FGF23 protein (intact FGF23 + cleaved FGF23 = total FGF23; Supplementary Figure S1). Conversely, the second type of FGF23 ELISA—the intact FGF23 ELISA—uses a capture antibody that recognizes an epitope within the N-terminal region, proximal to the cleavage site, and a detection antibody that recognizes an epitope within the C-terminal region, distal to the cleavage site. Therefore, this assay detects only full-length, intact FGF23 (Supplementary Figure S1). Using both ELISAs can provide a sense of cleavage activity. For instance, if FGF23 concentrations are increased using the total FGF23 ELISA but not the intact FGF23 ELISA, then this suggests that increased FGF23 translation has been offset by increased posttranslational cleavage, resulting in increased circulating concentrations of FGF23 fragments (as reflected by the total FGF23 ELISA), but not intact FGF23 protein (as reflected by the intact FGF23 ELISA).
Interestingly, EPO stimulates both FGF23 mRNA transcription and FGF23 posttranslational cleavage.3, 4, 5, 6, 7, 8 The mechanisms by which EPO may induce FGF23 mRNA transcription are unknown. Regarding cleavage, the mechanisms are also unknown; however, some murine data suggest that EPO may be associated with decreased GALNT3 mRNA expression, which could allow for increased FGF23 proteolytic cleavage.4, 8 The coupling of increased transcription with increased posttranslational cleavage results in cellular secretion of large amounts of FGF23 fragments, markedly out of proportion to intact FGF23, resulting in much greater increases in circulating C-terminal (total) FGF23 than intact FGF23. For instance, mice with chronically high endogenous EPO levels8 or acutely increased endogenous EPO levels4, 5 have large increases in circulating FGF23 fragments, but attenuated increases in circulating intact FGF23 concentrations. Similarly, in mice3, 4, 5, 6, 7, 8 and humans,3, 7 a single dose of exogenous EPO acutely increases FGF23 fragment concentrations out of proportion to intact FGF23. Consistent with EPO-induced coupling of increased FGF23 transcription with increased posttranslational cleavage, in human CKD cohorts, serum EPO levels or exogenous EPO dose are positively and independently associated with total FGF23 levels, but not intact FGF23.8 In the ADPKD cohorts presented here, we observed similar patterns, as serum EPO was associated with total FGF23 but not intact FGF23. As the biological roles of FGF23 fragments remain incompletely defined, the implications of EPO-induced increased fragment production are unclear and require further study.
In our ADPKD cohort that underwent bone biopsy, we measured both circulating and bone FGF23 levels. Serum EPO concentrations correlated more strongly with circulating total FGF23 than with bone FGF23, which may be consistent with observations that EPO affects extra-osseous FGF23 production. However, the absence of an association between EPO and bone FGF23 may also be due to a lack of power, given the small sample size, and/or limitations related to immunohistochemistry. Also, our assessment of this cohort was limited by the absence of bone Fgf23 mRNA expression data.
Previous studies have presented data regarding EPO effects on extra-osseous FGF23 production. Murine studies have demonstrated that the bone marrow is a novel site of EPO-induced FGF23 production.3, 4, 5, 6, 7, 8 Although it is unknown which exact marrow cell subpopulation can produce FGF23 in response to EPO, murine studies have demonstrated EPO-induced FGF23 expression in erythroid lineage cells.3, 4, 7 However, other non-skeletal tissues could potentially produce FGF23 in ADPKD. In murine PKD models, Spichtig et al. demonstrated that the cells lining renal cysts express FGF23 mRNA and protein.S30 More recently, in human ADPKD patients with hepatic cysts, Bienaime et al. found that the hepatic cysts express FGF23 mRNA and protein.2 In this cohort, plasma total FGF23 levels independently correlated with liver volume, and surgical reduction of the cystic liver mass was associated with decreased plasma total FGF23 levels.2 In our ADPKD bone biopsy group, one subject had numerous liver cysts; this subject also had the highest circulating total FGF23 concentration. However, in our larger ADPKD cohort, serum EPO levels were not associated with kidney volume or liver volume. Although the stimuli of potential renal and hepatic cystic FGF23 production are unknown, it is possible that local hypoxia may increase FGF23 expression, as hypoxia stimulates hypoxia-inducible factor 1α (HIF1α) expression, and HIF1α can bind to a site in the proximal FGF23 promoter to induce transcription.S31
In our smaller ADPKD cohort, we also characterized circulating iron parameters. Like EPO, iron deficiency also couples increased FGF23 mRNA transcription with increased FGF23 posttranslational cleavage.S15–S18 In human cohorts, it has been observed that iron deficiency is associated with increased circulating total FGF23 levels, but not intact FGF23, consistent with elevated concentrations of FGF23 proteolytic fragments.S32,S33 Similarly, in our ADPKD cohort, we observed that serum ferritin levels tended to inversely correlate with circulating C-terminal (total) FGF23 but not intact FGF23. Also, we observed that EPO strongly and positively correlated with ERFE, a hormone secreted by erythroid precursor cells that acts on the liver to suppress hepcidin.S34 Decreased hepcidin allows for increased activity of the cellular iron exporter ferroportin, resulting in increased dietary iron absorption from enterocytes and increased mobilization of body iron stores.S35 This EPO-ERFE-hepcidin axis couples erythropoietic stimuli with enhanced iron availability for erythropoiesis. In our subjects, ERFE did not correlate with serum hepcidin levels, but ferritin did, suggesting a possibly stronger effect of iron and/or inflammation on hepcidin than ERFE. In humans, we have previously observed that both phlebotomy-induced blood loss (which causes compensatory increases in serum EPO concentrations) and exogenous EPO administration results in acutely increased ERFE levels and decreased hepcidin levels.S36 However, in cross-sectional analysis of other small human cohorts, we also have observed that EPO correlates with ERFE, but ERFE does not correlate with hepcidin, suggesting multifactorial regulation of hepcidin production.S37
Although both EPO and iron status may affect FGF23 production and metabolism, other factors may also be contributory. Specifically in ADPKD, alterations in klotho and sclerostin may be important. Compared to early-stage CKD patients without ADPKD, early-stage CKD patients with ADPKD have significantly lower soluble klotho levels.S38 Compared to ESRD patients without ADPKD, ESRD patients with ADPKD have significantly higher circulating sclerostin levels.S39 Therefore, specific ADPKD-associated differences in mineral metabolism parameters may also contribute to the FGF23 profile in this condition.
Although our study is novel in that it is the first to assess associations between EPO and FGF23 in ADPKD patients, it has several limitations, including small sample sizes, the lack of non-ADPKD control groups, incomplete characterization (for instance, lack of iron studies in the first cohort), and the associative nature of our results. Additionally, altitude-mediated effects may have influenced serum EPO levels; however, this would not be expected to affect EPO-FGF23 correlations. Lastly, we observed that circulating total FGF23 levels were lower in the first cohort than in the second cohort. Although the reasons for this difference are unclear, we hypothesize that it could be related to differences observed when measuring total FGF23 in serum versus plasma with the Immutopics/Quidel ELISA (San Diego, CA).S40 Regardless, the pattern of EPO-FGF23 associations (a much stronger association between EPO and total FGF23 than between EPO and intact FGF23) was similar in both cohorts, suggesting similar potential effects of EPO on FGF23. Indeed, consistent with previous murine and human studies, our data suggest EPO-induced coupling of increased FGF23 transcription with increased posttranslational cleavage. Larger studies of ADPKD patients are needed to more robustly assess the possible EPO-FGF23 relationship in ADPKD and its potential clinical consequences.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The work in this letter was performed with the support of the National Institute of Diabetes, Digestive, and Kidney Disease of the National Institutes of Health research grants R01-DK094796 (to BYG and MBC) and K08-DK111980 (to MRH).
Author Contributions
Research idea and study design: MRH, IBS, BYG, MBC; data acquisition: MRH, RCP, BYG; data analysis/interpretation: MRH, IBS, BYG, MBC; manuscript drafting: MRH; manuscript review and revision: MRH, IBS, RCP, WW, ZY, KLN, GMB, VET, ABC, RDP, TIS, KTB, BYG, MBC.
Footnotes
Figure S1. The C-terminal (total) fibroblast growth factor 23 (FGF23) and intact FGF23 enzyme-linked immunosorbent assays (ELISAs). Whereas the C-terminal (total) FGF23 ELISA detects both the full-length, intact protein and its C-terminal proteolytic fragments, the intact FGF23 assay detects only the full-length form of the hormone.
Figure S2. Associations between erythropoietin (EPO) and log-transformed fibroblast growth factor 23 (FGF23) in the Autosomal Dominant Polycystic Kidney Disease Halt Progression of Polycystic Kidney Disease (ADPKD HALT-PKD) study A cohort. In patients with ADPKD, serum EPO levels positively correlated with log-transformed circulating C-terminal (total) FGF23 concentrations (A), but not with log-transformed circulating intact FGF23 concentrations (B).
Figure S3. Associations between erythropoietin (EPO) and fibroblast growth factor 23 (FGF23) in the Autosomal Dominant Polycystic Kidney Disease Halt Progression of Polycystic Kidney Disease (ADPKD HALT-PKD) study A cohort, excluding outlying points. In patients with ADPKD, serum EPO levels positively correlated with circulating C-terminal (total) FGF23 concentrations (A), but not with circulating intact FGF23 concentrations (B).
Figure S4. Associations between ferritin and fibroblast growth factor 23 (FGF23) in the autosomal dominant polycystic kidney disease (ADPKD) bone biopsy cohort. In patients with ADPKD, serum ferritin levels tended to inversely correlate with circulating C-terminal (total) FGF23 concentrations (A), but not with circulating intact FGF23 concentrations (B) or with bone FGF23 levels (C).
Figure S5. Associations among iron-related parameters in autosomal dominant polycystic kidney disease (ADPKD) subjects. In patients with ADPKD, serum erythropoietin (EPO) levels positively correlated with erythroferrone (ERFE) (A), but ERFE did not correlate with hepcidin (B). Serum ferritin positively correlated with hepcidin (C).
Table S1. Multiple linear regression modeling, dependent variable: log-transformed C-terminal (total) fibroblast growth factor 23 (FGF23) (n = 78).
Table S2. Multiple linear regression modeling, dependent variable: log-transformed intact fibroblast growth factor 23 (FGF23) (n = 78).
Table S3. Multiple linear regression modeling, dependent variable: C-terminal (total) fibroblast growth factor (FGF)23, excluding outlying point (n = 77).
Table S4. Multiple linear regression modeling, dependent variable: intact fibroblast growth factor (FGF)23, excluding outlying point (n = 77).
Supplementary References.
Supplementary Methods.
Supplementary Material
References
- 1.Pavik I., Jaeger P., Kistler A.D. Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int. 2011;79:234–240. doi: 10.1038/ki.2010.375. [DOI] [PubMed] [Google Scholar]
- 2.Bienaime F., Ambolet A., Aussilhou B. Hepatic production of fibroblast growth factor 23 in autosomal dominant polycystic kidney disease. J Clin Endocrinol Metab. 2018;103:2319–2328. doi: 10.1210/jc.2018-00123. [DOI] [PubMed] [Google Scholar]
- 3.Clinkenbeard E.L., Hanudel M.R., Stayrook K.R. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica. 2017;102:e427–e430. doi: 10.3324/haematol.2017.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabadi S., Udo I., Leaf D.E. Acute blood loss stimulates fibroblast growth factor 23 production. Am J Physiol Renal Physiol. 2018;314:F132–F139. doi: 10.1152/ajprenal.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flamme I., Ellinghaus P., Urrego D., Kruger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PloS One. 2017;12 doi: 10.1371/journal.pone.0186979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toro L., Barrientos V., Leon P. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 2018;93:1131–1141. doi: 10.1016/j.kint.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Daryadel A., Bettoni C., Haider T. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Archiv. 2018;470:1569–1582. doi: 10.1007/s00424-018-2171-7. [DOI] [PubMed] [Google Scholar]
- 8.Hanudel MR, Eisenga MF, Rappaport M, et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans [e-pub ahead of print]. Nephrol Dial Transplant. Available at: 10.1093/ndt/gfy189. Accessed September 8, 2019. [DOI] [PMC free article] [PubMed]
- 9.Chandra M., Miller M.E., Garcia J.F. Serum immunoreactive erythropoietin levels in patients with polycystic kidney disease as compared with other hemodialysis patients. Nephron. 1985;39:26–29. doi: 10.1159/000183332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.