Abstract
Introduction
Soluble fms-like tyrosine kinase 1 (sFLT1) is a splice variant of the vascular endothelial growth factor (VEGF) receptor lacking the transmembrane and cytoplasmic domains and acts as a powerful antagonist of VEGF signaling. Plasma sFLT1 levels are higher in patients with chronic kidney disease (CKD) and correlate with renal dysfunction. The source of plasma sFLT1 in CKD is unclear.
Methods
Fifty-two renal biopsies were studied for sFLT1 expression using immunohistochemistry and evaluated on a 0–4 grading scale of positive cells within inflammatory infiltrates. These included drug-induced interstitial nephritis (6); allografts (12), with polyomavirus nephritis (3); diabetes mellitus (10); lupus glomerulonephritis (6); pauci-immune vasculitis (7); IgA nephropathy (6); and miscellaneous CKD (5).
Results
Forty-seven biopsies had inflammatory infiltrates of which 37 had sFLT1-positive cells: of these biopsies, 3 were grade 4, i.e., had cells that constituted more than 50% of the inflammatory infiltrate, 9 were grade 3 (25%–50%), 5 were grade 2 (10%–25%), 3 were grade 1 (10%), and 17 were grade 0.5 (<10%). There was a robust correlation (r2 = 0.89) between degree of inflammation and sFLT1-positive cells. CD68/sFLT1 co-immunostaining studies indicated that sFLT1-positive cells were histiocytes. The surrounding capillary network was reduced.
Conclusion
sFLT1-positive histiocytes are generally part of the inflammatory infiltrates noted in CKD and are particularly abundant in forms of interstitial nephritis. Their presence promotes an anti-angiogenic state locally in the tubulointerstitium that could inhibit capillary repair, contribute to peritubular capillary loss, and enhance fibrosis in CKD.
Keywords: CKD, histiocytes/macrophages, sFLT1
sFLT1 is a splice variant of the VEGF receptor lacking the cytoplasmic and transmembranous domains. By binding the VEGF ligand, signal transduction is inhibited and an anti-angiogenic state is promoted. There is ample and fairly direct evidence in humans that the latter can be both destructive/disease-producing (preeclampsia1) and beneficial/therapeutic (retinal neovascularization2; macular degeneration3). Indeed, sFLT1 presence is responsible for maintenance of the normal corneal avascular environment.4, 5 In contrast, the fenestrated vasculature, including the renal peritubular capillaries, seems, at least in part, dependent on the presence of VEGF creating a pro-angiogenic environment.6, 7 The basis for CKD is exceedingly complex, involving epithelial and capillary loss with associated inflammatory and pro-fibrotic changes. This injury is multifactorial, and progression is based on many factors, such as the ramifying effects of progressive capillary loss in the setting of continual demands on the residual nephron population. In this paper, we demonstrate that sFLT1 protein is expressed by infiltrating histiocytes in renal biopsy tissues from CKD patients and may be an important factor in the capillary, interstitial, and tubular alterations that characterize CKD.
Methods
Clinical and Molecular Studies
Renal tissue specimens used in these studies were archived kidney biopsies from patients receiving care at the Beth Israel Deaconess Medical Center. Our studies were approved by the Institutional Review Board at the Beth Israel Deaconess Medical Center. In all cases, the research was conducted according to principles having their origin in the Declaration of Helsinki and the Declaration of Istanbul. Clinical details and summary data of the biopsy specimens used in this study are provided in Table 1.
Table 1.
Summary of morphological data and results of sFLT1 immunohistochemistry in human kidney biopsies
| Patient no. | Clinical diagnosis | IFTA (0–4) | Creatinine (mg %) | Inflammation (0–4) | sFLT1+ histiocytes (0–4) |
|---|---|---|---|---|---|
| 1 | Polyoma | 1 | 0.8 | 4 | 4 |
| 2 | Polyoma | 1 | 0.7 | 3 | 3 |
| 3 | Polyoma | 1 | 3 | 0.5 | 0.5 |
| 4 | Interstitial nephritis | 4 | 2.8 | 4 | 3 |
| 5 | Intrestitial nephritis | 4 | 6.3 | 4 | 3 |
| 6 | Interstitial nephritis | 4 | 3.4 | 3 | 3 |
| 7 | Interstitial nephritis | 4 | 3.6 | 3 | 3 |
| 8 | Interstitial nephritis | 2 | 7.6 | 1 | 0.5 |
| 9 | Interstitial nephritis | 1 | 2.6 | 4 | 4 |
| 10 | SLE | 4 | 2 | 0.5 | 1 |
| 11 | SLE | 1 | 0.6 | 0.5 | 0.5 |
| 12 | SLE | 1 | 0.5 | 0 | 0 |
| 13 | SLE | 1 | 1.4 | 0 | 0 |
| 14 | SLE | 3 | 4.3 | 1 | 0 |
| 15 | SLE | 1 | 0.8 | 0.5 | 0.5 |
| 16 | DM | 2 | 3.7 | 4 | 4 |
| 17 | DM | 3 | 2.1 | 2 | 3 |
| 18 | DM | 3 | 1.4 | 2 | 0.5 |
| 19 | DM | 4 | 3.7 | 2 | 2.5 |
| 20 | DM | 3 | 5.9 | 0.5 | 0 |
| 21 | DM | 2 | 2.1 | 0 | 0 |
| 22 | DM | 2 | 2 | 0.5 | 0 |
| 23 | DM | 3 | 6.4 | 0.5 | 0.5 |
| 24 | DM | 3 | 1.5 | 1 | 0 |
| 25 | DM | 3 | 1.5 | 1 | 0.5 |
| 26 | Transplant | 4 | 1.5 | 0.5 | 0.5 |
| 27 | Transplant | 2 | 13 | 0.5 | 0.5 |
| 28 | Transplant | 1 | 3.1 | 0 | 0 |
| 29 | Transplant | 3 | 2.2 | 1 | 0.5 |
| 30 | Transplant | 2 | 2 | 0.5 | 0 |
| 31 | Transplant | 1 | 2.3 | 1 | 0 |
| 32 | Transplant | 2 | 1.3 | 2 | 2 |
| 33 | Transplant | 3 | 2.8 | 1 | 1 |
| 34 | Transplant | 1 | 1 | 0.5 | 0.5 |
| 35 | ANCA | 3 | 2.2 | 2 | 0.5 |
| 36 | ANCA | 3 | 5.4 | 3 | 3 |
| 37 | ANCA | 1 | 4 | 0.5 | 0.5 |
| 38 | ANCA | 2 | 6.6 | 1.5 | 2 |
| 39 | ANCA | 1 | 0.8 | 0.5 | 0.5 |
| 40 | ANCA | 1 | 5.5 | 0 | 0 |
| 41 | ANCA | 1 | 7 | 1 | 0 |
| 42 | Hypertension | 2 | 1.4 | 1 | 1 |
| 43 | Hypertension | 2 | 0.8 | 0.5 | 0.5 |
| 44 | Hypertension | 2 | 1.9 | 1 | 0 |
| 45 | CKD GN | 4 | 4.7 | 1.5 | 1.5 |
| 46 | DM + IgA | 2 | 6 | 1.5 | 2 |
| 47 | IgA | 3 | 1.3 | 1.5 | 3 |
| 48 | IgA | 3 | 3.8 | 2.5 | 2 |
| 49 | IgA | 3 | 1.2 | 0.5 | 0 |
| 50 | IgA | 2 | 1.9 | 0.5 | 0.5 |
| 51 | IgA | 1 | 1.1 | 0.5 | 0 |
| 52 | TMA | 3 | 2 | 0.5 | 0.5 |
| Median | — | 2 | 2.15 | 1 | 0.5 |
| Lower quartile | — | 1 | 1.4 | 0.5 | 0 |
| Upper quartile | — | 3 | 3.85 | 2 | 2 |
ANCA, anti-neutrophilic cytoplasmic antibody disease; CKD, chronic kidney disease; DM, diabetes mellitus; GN, glomerulonephritis; IFTA, interstitial fibrosis and tubular atrophy; sFLT1+, soluble fms-like tyrosine kinase 1; SLE, systemic lupus erythematosus; TMA, thrombotic microangiopathy.
Histology and Immunohistochemistry
Archived paraffin sections (4 μm) on polysine-coated slides (Fisher, Atlanta, GA) were deparaffinized and rehydrated. Optimal staining was achieved with an antigen retrieval method that was performed in 10 mmol/l citric acid, pH 6.00, for 30 minutes. Endogenous peroxidase was quenched with 3% H2O2 in ddH2O for 15 minutes. Sections were blocked with 2.5% normal horse serum at room temperature for 40 minutes and incubated 40 minutes with a 1:200 dilution of primary sFLT1 that recognizes the N-terminal region of FLT1/sFLT1 (catalog no. AF321; R&D Systems, Framingham, MA), and CD34 or CD68 antibodies (Agilent, Santa Clara, CA). Specific labeling was detected with an ImmPRESS HRP Anti-Goat or Anti-Mouse IgG (Peroxidase) Polymer Detection Kit (Vector Laboratories, Burlingame, CA). All primary antibodies used in this study are listed in Table 2. The enzymatic reaction product was achieved by using DAB substrate to give a brown precipitate, and the sections were counterstained with hematoxylin, dehydrated, and mounted in Permount. Sections with no primary antibody were used as negative control slides. For positive controls, tissues previously shown to express the antigen of interest by immunohistochemistry (IHC) were used.8, 9 We also used, as a negative control for sFLT1 IHC, a Goat IgG Isotype Control (catalog no. 02-6202; Thermo Fisher Scientific, Waltham, MA)9 and for CD34 and CD68, a mouse Universal Negative Control for Mouse Primary Antibodies (catalog no. IS75061-2; Agilent).
Table 2.
Antibody list
| Antibody | Source | Vendor |
|---|---|---|
| VEGF R1/FLT1 | Polyclonal Goat IgG | R&D Systems (Framingham, MA) #AF321 |
| VEGF R1 (soluble) | Polyclonal Rabbit IgG | Thermo Fisher Scientific (Waltham, MA) #36-1100 |
| CD68 | Monoclonal Mouse Clone #KP1 | Agilent (Santa Clara, CA) #IR60961-2 |
| CD34 | Monoclonal Mouse Clone #QBEnd 10 | Agilent #IR63261-2 |
| Goat IgG | Isotype control | Thermo Fisher Scientific #02-6202 |
| Mouse IgG | Isotype control | Agilent #IS75061-2 |
| Rabbit IgG | Isotype control | Agilent #IR60066-2 |
FLT1, fms-like tyrosine kinase 1; VEGF, vascular endothelial growth factor.
Immunofluorescence
Snap-frozen, nonfixed kidney biopsies were double immunofluorescence stained with sFLT1/CD68 antibodies or sFLT-1/CD34 antibodies. Five-micrometer cryosections of kidney biopsies were cut and equilibrated in phosphate-buffered saline for 10 minutes at 37 °C, followed by incubation with sFlt-1 (1:200; AF231; R&D Systems), CD68 (FLEX Monoclonal Mouse Anti-Human CD68, Clone KP1, (Agilent), or FLEX Monoclonal Mouse Anti-Human CD34 Class II, Clone QBEnd 10, (Agilent) for 30 minutes at 37 °C. Slides were then rinsed with phosphate-buffered saline and incubated for 30 minutes with VectaFluor R.T.U. DyLight 594 Anti-Goat IgG or VectaFluor R.T.U. DyLight 488 Anti-Mouse IgG (Vector Laboratories), respectively. Slides were rinsed twice in phosphate-buffered saline and mounted.
Pathology Evaluation
As shown in Table 1, interstitial fibrosis and tubular atrophy were evaluated using a 4-tier scale: mild, approximately 5% of renal parenchyma involved (grade 1); moderate, 10%–25% (grade 2); 25%–50% (grade 3); and severe, greater than 50% (grade 4). Inflammation was quantified similarly on a 4-tier scale.10
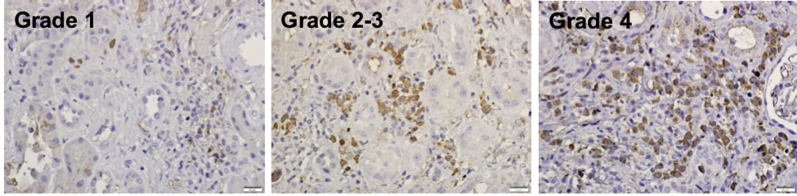
sFLT1 immunostaining was quantified (Figure 1) on a 5-tier scale: sFLT1-positive cells that constituted more that 50% of the inflammatory infiltrate (grade 4); 25%–50% (grade 3); 10%–25% (grade 2); 10% (grade 1); and <10% (grade 0.5).
Figure 1.
Grading scale for immunohistochemical staining of soluble fms-like tyrosine kinase 1 (sFLT1) in kidney biopsies: sFLT1-positive cells that constituted more than 50% of the inflammatory infiltrate (grade 4), 25%–50% (grade 3), 10%–25% (grade 2), 10% (grade 1), and <10% (grade 0.5). Bars = 20 μm.
Statistics
Pearson’s correlation coefficients were calculated to determine the association between sFlt1-positive cells and inflammatory cell numbers. Median, lower, and upper quartiles were calculated for interstitial fibrosis and tubular atrophy, creatinine, inflammation, and sFLT-1 immunostaining in Table 1 with Microsoft Excel version 16.27 (Microsoft, Redmond, WA).
Results
A total of 52 renal biopsies were studied using immunohistochemistry and immunofluorescence staining for sFLT1, CD34, and CD68, to identify the location, cell type expression, and proximity to capillaries for sFLT1 expression in various kidney diseases. Table 1 includes a summary of all the data, including clinical diagnosis, inflammation, interstitial fibrosis and tubular atrophy score, and results of sFLT1 immunohistochemistry staining as described in the Methods. The criteria for selection of the cases were random, but an effort was made to find varying degrees of kidney injury. The biopsies were immunostained for sFLT1 and evaluated on a 0–4 grading scale of positive cells within inflammatory infiltrates (Figure 1). Cases studied included: drug-induced interstitial nephritis (6); allografts (12), 3 with polyomavirus nephritis; diabetes mellitus (10); lupus glomerulonephritis (6); anti-neutrophilic cytoplasmic antibody disease (7); IgA/ Henoch-Schönlein purpura nephropathy (6); and miscellaneous chronic kidney diseases (5; Table 1). A total of 47 biopsies had inflammatory infiltrates, of which 37 had sFLT1-positive cells: 3 biopsies had cells that constituted more than 50% of the inflammatory infiltrate (grade 4); 9 were grade 3 (25%–50%); 5 were grade 2 (10%–25%); 3 were grade 1 (10%); and 17 were grade 0.5 (<10%). The positive cells were generally single but formed clusters that became diffuse. Cases of polyomavirus nephritis (2), drug-associated interstitial nephritis (5), advanced diabetic kidneys (3), anti-neutrophilic cytoplasmic antibody (1), and Henoch-Schönlein purpura (1) were the most positive (12 of 37), but significant (≥10%) positivity was seen in many biopsies (25 of 37).
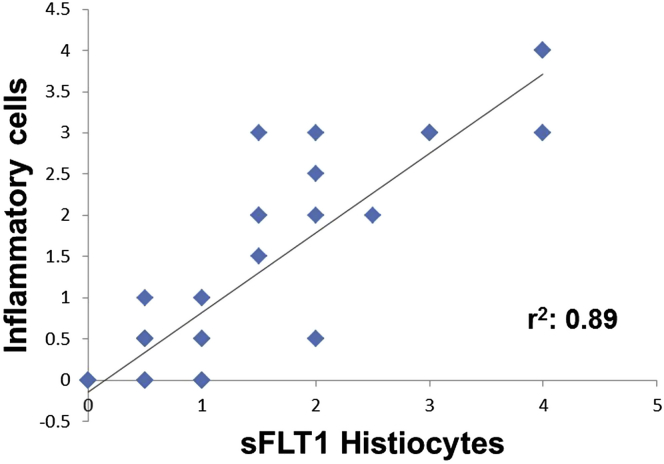
In Figure 2, we show that there is a significant correlation between sFLT1-positive cells and degree of inflammation (r2 = 0.9), but some inflammatory foci were negative. No correlation was seen between inflammation and fibrotic changes (perhaps indicating that the fibrotic changes might be an ongoing/evolving process).
Figure 2.
The expression of soluble fms-like tyrosine kinase 1 (sFLT1) in kidney biopsies is positively correlated with kidney inflammation (n = 52 biopsies). sFLT1 protein expression localized in kidney biopsies by immunohistochemistry. A 4-tier grading system was used to semiquantitatively measure sFLT1 and inflammation in the kidney biopsies.
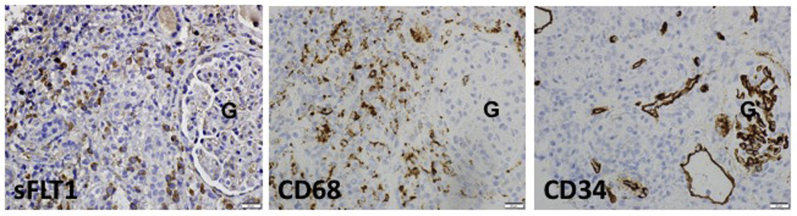
Immunohistochemical assays for sFLT1, CD68 (macrophage marker), and CD34 (endothelial marker) were done in a subset of kidney biopsies from our cases. In Figure 3 (interstitial nephritis case), we find that many interstitial cells are sFLT1-positive (Figure 3a). In the same tissue area, CD68 immunostaining demonstrated, that histiocytes were also abundant (Figure 3b). CD34 immunostaining in this tissue shows a loss of capillary networks (Figure 3c).
Figure 3.
Representative images of immunohistochemical assay for soluble fms-like tyrosine kinase 1 (sFLT1), CD68 (macrophage marker), and CD34 (endothelial marker) immunoreactivity (brown precipitate) in renal biopsies. Bars = 20 μm. G, glomerulus.
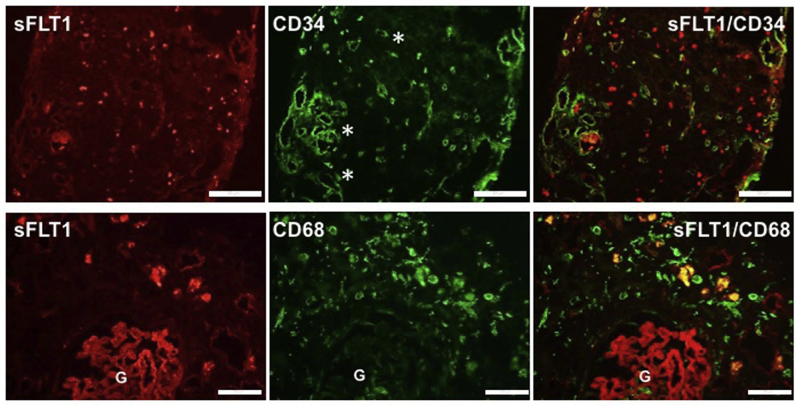
A colocalization assay for sFLT1 (red), and CD34 (green immunofluorescence) demonstrates that in areas where sFLT1 staining is high, there is loss of CD34 stain in the renal biopsy (Figure 4; upper panels show the interstitial nephritis case). This supports data from Figure 3, suggesting that the presence of sFLT1 initiates an anti-angiogenic state that causes the loss of capillary networks. Also, a double immunofluorescence assay for sFLT1 (red) and CD68 (green) shows colocalization in several cells, proving that macrophages express sFlt-1 in kidney interstitium (Figure 4; lower panels show the anti-neutrophilic cytoplasmic antibody case).
Figure 4.
Upper panels (interstitial nephritis case): representative images of immunofluorescence assay for soluble fms-like tyrosine kinase 1 (sFLT1; red) and CD34 (endothelial marker; green) in a renal biopsy. The areas to the right of the asterisks show reduced or absent CD34 staining. Bars = 100 μm. Lower panels (anti-neutrophilic cytoplasmic antibody case): representative images of immunofluorescence assay for sFLT1 (red), and CD68 (macrophage marker; green) in a renal biopsy. Yellow immunofluorescence demonstrates colocalization of the 2 antigens, which demonstrates that macrophages express sFLT1 in kidney interstitium. Bars = 20 μm.
Since the antibody used to study sFLT1 expression also detects FLT1, we performed additional staining with an antibody that has been reported to be specific to the unique C-terminal region of sFLT1. As noted in Supplementary Figure S1, these studies showed similar expression staining patterns as seen in Figure 1; however, the staining with the sFLT1-specific antibody was less intense. These data suggest that both sFLT1 and FLT1 may be upregulated in the infiltrating histiocytes in CKD. To further characterize the specificity of sFLT1/VEGFR1 antibody (Thermo Fisher catalog no. 36-1100), human monocyte cells were stimulated with phorbol myristate acetate11 and evaluated for sFLT1 expression in the lysates by Western blot (Supplementary Figure S2). We detected the 130-kDa sFLT1 isoform, as the predominant band in monocytes stimulated with phorbol myristate acetate compared to nonstimulated monocytes using this antibody. These findings suggest that the Thermofisher antibody used in the immunohistochemistry predominantly detects the sFLT1 isoform.
Discussion
Here, we report that sFLT1-expressing histiocytes are present in a variety of tubulo-interstitial diseases and correlate with the degree of inflammation noted in CKD. An interesting point is that these sFLT1-expressing histiocytes were adjacent to areas of peritubular capillary loss. We speculate that the reduction of capillaries in the tubulointerstitum noted in CKD biopsies may be secondary to recruitment of macrophages expressing sFLT1 to sites of renal injury by creation of localized anti-angiogenic milieu. sFLT1 is known to be produced by the placenta during pregnancy, and the clinical significance of circulating sFLT1 in kidney diseases was first reported in association with preeclampsia, a major renal complication of pregnancy.1 Circulating soluble FLT1 levels are increased in patients with preeclampsia and are responsible for systemic endothelial injury, including the glomerular capillary damage typical of that condition.12 Overexpression of sFLT1 in pregnant rats induced hypertension, proteinuria, and glomerular endotheliosis.12 Multiple studies have demonstrated that circulating sFLT1 is a marker for diagnosis of preeclampsia.13 In preeclampsia, the major source of circulating sFLT1 is the placenta, as evidenced by the dramatic fall in sFLT1 levels following delivery. Elevated sFLT1 levels have also been noted in other nonpregnancy diseases; however, systemic levels were significantly lower than those that have been reported during preeclampsia, suggesting that local levels may be important.14, 15, 16 Our data suggest that inflammatory mononuclear cells such as histiocytes or macrophages may be a source for local production of sFLT1 at sites of injury, such as the tubulointerstitium in the context of CKD.
Di Marco et al.15, 17 documented that plasma levels of soluble VEGF receptor sFLT1 were important to the increased cardiovascular risk that accompanies CKD, and after multivariate regression analysis were found to be exclusively associated with renal function. They identified circulating monocytes as a possible source. In a more recent study, Yuan et al.18 (2013) showed that circulating levels of VEGF and sFLT1 in CKD were associated with biomarkers of inflammation and that a high sFLT1 with concomitant high IL-6 correlated with increased mortality, which remained significant after adjustment for age and gender, body mass index, and comorbidities. Also, increased levels of sFLT1 were detected during acute anti-neutrophilic cytoplasmic antibody–-associated vasculitis, leading to an antiangiogenic state that hinders endothelial repair.19
Hammadah et al.20 (2016) evaluated 791 heart failure patients undergoing elective coronary angiogram. High levels of sFLT1 were associated with adverse cardiovascular outcomes. These increased sFLT1 levels were also associated with worse renal function, although they still independently predicted long-term survival.20 Chapal et al.21 (2013) found that increased plasma sFLT1 correlated with delayed graft function and early loss of peritubular capillaries in the kidney graft (136 consecutive renal transplant patients assessed for delayed graft function and peritubular capillary loss).
The importance of VEGF-A in maintaining peritubular vasculature was addressed by Dimke et al.,7 who found that this factor was expressed in tubular epithelial cells and that its receptor was largely restricted to adjacent peritubular capillaries. Embryonic deletion of tubular VEGF-A markedly reduced renal VEGF-A and resulted in the formation of a smaller kidney, with a striking reduction of density of peritubular capillaries. Serum VEGF-A was unaltered, even though there was a striking decrease in the renal parenchyma. Thus, any interference with VEGF-A function by sFLT1 has the potential to compromise peritubular vasculature as a consequence of both local production and serum elevation.
Masuda et al.22 demonstrated that angiogenic capillary repair plays an important role in recovery from glomerular damage in rats with experimentally induced glomerulonephritis. In their model, they systemically administered VEGF165, which successfully induced glomerular repair by stimulation of angiogenesis, and vascular remodeling. In contrast, an antagonist of VEGF165 increased glomerular injury in rats in an experimental model of mesangioproliferative nephritis.23 Thus, it seems that the angiogenic and anti-angiogenic balance/imbalance may play a general role in influencing the outcome of established kidney diseases.
Our study has some implications for future studies. The antibody used to detect sFLT1 also identifies FLT1, which is membrane bound and acts as a negative regulator of VEGF signaling.24 Although we were able to validate our findings with a second antibody that predominantly detects sFLT1, follow-up studies using quantitative polymerase chain reaction or in situ hybridization in fresh tissue to differentiate sFLT1 from FLT1 are needed to determine whether the paracrine effects of sFLT1 or the juxtracrine effects of FLT1 are responsible for the loss of capillaries in areas of renal inflammation. We also do not know whether the renal inflammation is the cause or the consequence of the renal disease. Studies of renal failure models in animals should shed light on the role of infiltrating monocytes during CKD. It is also important to peform studies with additional markers, such as arginase-1, to understand whether these sFLT1-expressing histiocytes are of the M1 or M2 pattern.25
In summary, our studies using archived renal biopsies from a variety of CKDs implicate a population of sFLT1-positive histiocytes that could also be responsible for elevated plasma and urine levels and putatively create a local anti-angiogenic state as well. We speculate that sFLT1-positive cells are a part of proinflammatory infiltrates in CKD and may be extremely abundant in forms of tubulointerstitial nephritis. Their presence may promote a localized anti-angiogenic state that could inhibit capillary repair, contribute to peritubular capillary loss, and enhance fibrosis in CKD.
Disclosure
ZKZ is a part-time employee at Radikal Therapeutics Inc., a biotech company developing therapies for kidney diseases. SAK is a co-inventor on patents related to angiogenic biomarkers that are held by Beth Israel Deaconess Medical Center. SAK has financial interest in Aggamin LLC, which is developing therapies for preeclampsia. All the other authors declared no competing interests.
Acknowledgments
Institutional support was provided by the Department of Medicine at Beth Israel Deaconess Medical Center, Boston, MA.
Author Contributions
ZKZ designed the experiments, performed double immunofluorescence stainings, did statistical analysis, and wrote the article. SR interpreted histology results and wrote the article. AL performed cell culture experiments and Western blotting. EP performed immunohistochemistry and reviewed the article. SAK and MT interpreted results and reviewed the article.
Footnotes
Supplementary Methods.
Figure S1. Immunohistochemical staining with VEGFR1 antibody (R&D Systems, Framingham, MA; A, C, and E) and soluble VEGFR1 antibody (Thermo Fisher Scientific, Waltham, MA; B, D, and F) of the same kidney biopsies. Bars = 100 μm.
Figure S2. Representative Western blot analysis in U937 cells stimulated with phorbol myristate acetate. Using sFLT1/VEGFR1 antibody (Thermo Fisher Scientific, Waltham, MA, catalog no. 36-1100), the 130-kDa band represents the soluble form of FLT1, and the 180-kDa band is the full-length/membrane-bound FLT1 protein. The experiment was done twice, independently, with similar results.
Supplementary Material
References
- 1.Levine R.J., Maynard S.E., Qian C. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 2.Saint-Geniez M., Maharaj A.S., Walshe T.E. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford K.M., Saint-Geniez M., Walshe T. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:9478–9487. doi: 10.1167/iovs.11-8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambati B.K., Nozaki M., Singh N. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambati B.K., Patterson E., Jani P. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br J Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamba T., Tam B.Y., Hashizume H. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 7.Dimke H., Sparks M.A., Thomson B.R. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol. 2015;26:1027–1038. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zsengellér Z.K., Rajakumar A., Hunter J.T. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016;6:313–319. doi: 10.1016/j.preghy.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Covarrubias A.E., Lecarpentier E., Lo A. AP39, a modulator of mitochondrial bioenergetics, reduces antiangiogenic response and oxidative stress in hypoxia-exposed trophoblasts: relevance for preeclampsia pathogenesis. Am J Pathol. 2019;189:104–114. doi: 10.1016/j.ajpath.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zsengellér Z.K., Rosen S. The use of cytochrome C oxidase enzyme activity and immunohistochemistry in defining mitochondrial injury in kidney disease. J Histochem Cytochem. 2016;64:546–555. doi: 10.1369/0022155416660291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daigneault M., Preston J.A., Marriott H.M. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maynard S.E., Min J.Y., Merchan J. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal S., Cerdeira A.S., Redman C. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE Study. Hypertension. 2018;71:306–316. doi: 10.1161/HYPERTENSIONAHA.117.10182. [DOI] [PubMed] [Google Scholar]
- 14.Rolfo A., Attini R., Nuzzo A.M. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83:177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 15.Di Marco G.S., Reuter S., Hillebrand U. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009;20:2235–2245. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wewers T.M., Mayer A.B., Pfleiderer A. Increased soluble fms-like tyrosine kinase 1 after ischemia reperfusion contributes to adverse clinical outcomes following kidney transplantation. Kidney Int. 2019;95:1091–1102. doi: 10.1016/j.kint.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Di Marco G.S., Kentrup D., Reuter S. Soluble Flt-1 links microvascular disease with heart failure in CKD. Basic Res Cardiol. 2015;110:30. doi: 10.1007/s00395-015-0487-4. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J., Guo Q., Qureshi A.R. Circulating vascular endothelial growth factor (VEGF) and its soluble receptor 1 (sVEGFR-1) are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant. 2013;28:2356–2363. doi: 10.1093/ndt/gft256. [DOI] [PubMed] [Google Scholar]
- 19.Le Roux S., Pepper R.J., Dufay A. Elevated soluble Flt1 inhibits endothelial repair in PR3-ANCA-associated vasculitis. J Am Soc Nephrol. 2012;23:155–164. doi: 10.1681/ASN.2010080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammadah M., Georgiopoulou V.V., Kalogeropoulos A.P. Elevated soluble Fms-like tyrosine kinase-1 and placental-like growth factor levels are associated with development and mortality risk in heart failure. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapal M., Néel M., Le Borgne F. Increased soluble Flt-1 correlates with delayed graft function and early loss of peritubular capillaries in the kidney graft. Transplantation. 2013;96:739–744. doi: 10.1097/TP.0b013e31829f4772. [DOI] [PubMed] [Google Scholar]
- 22.Masuda Y., Shimizu A., Mori T. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostendorf T., Kunter U., Eitner F. VEGF(165) mediates glomerular endothelial repair. J Clin Invest. 1999;104:913–923. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibuya M. VEGF-VEGFR signals in health and disease. Biomol Ther (Seoul) 2014;22:1–9. doi: 10.4062/biomolther.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z., Ming X.F. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol. 2014;5:533. doi: 10.3389/fimmu.2014.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.