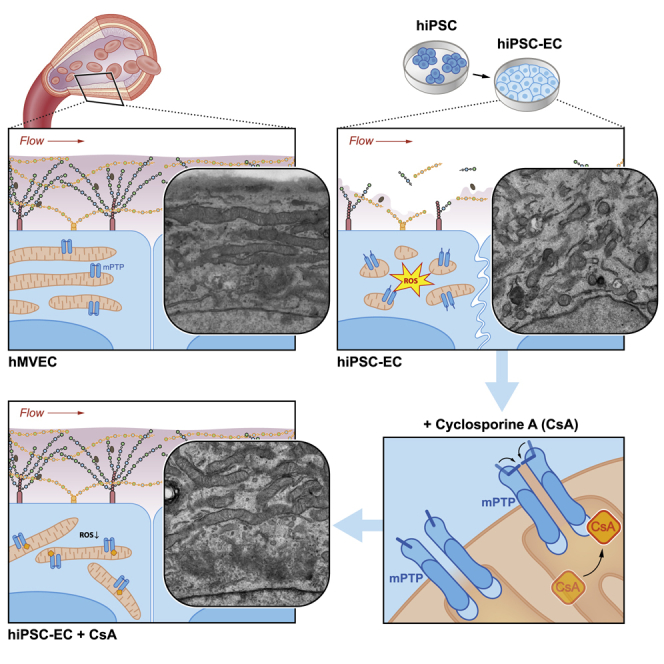
Summary
Human induced pluripotent stem cells (hiPSCs) are used to study organogenesis and model disease as well as being developed for regenerative medicine. Endothelial cells are among the many cell types differentiated from hiPSCs, but their maturation and stabilization fall short of that in adult endothelium. We examined whether shear stress alone or in combination with pericyte co-culture would induce flow alignment and maturation of hiPSC-derived endothelial cells (hiPSC-ECs) but found no effects comparable with those in primary microvascular ECs. In addition, hiPSC-ECs lacked a luminal glycocalyx, critical for vasculature homeostasis, shear stress sensing, and signaling. We noted, however, that hiPSC-ECs have dysfunctional mitochondrial permeability transition pores, resulting in reduced mitochondrial function and increased reactive oxygen species. Closure of these pores by cyclosporine A improved EC mitochondrial function but also restored the glycocalyx such that alignment to flow took place. These results indicated that mitochondrial maturation is required for proper hiPSC-EC functionality.
Keywords: hiPSC-derived endothelial cells, hiPSC-ECs, endothelial cell differentiation, mitochondrial dysfunction, mitochondrial permeability transition pore, reactive oxygen species, cyclosporine A, glycocalyx, shear stress, maturation
Graphical Abstract
Highlights
-
•
hiPSC-ECs lack a functional glycocalyx and fail to align to flow
-
•
hiPSC-ECs have reduced mitochondrial function and increased leakage of ROS
-
•
Closing the mPTP with cyclosporine A induces mitochondrial maturation
-
•
Improved mitochondrial function restores the glycocalyx and alignment to flow
Closure of the mitochondrial permeability transition pore by cyclosporine A improved mitochondrial function and maturation of hiPSC-ECs but also restored the glycocalyx such that alignment to flow took place. This functional glycocalyx, necessary for growth factor signaling and anticoagulation, is a prerequisite for future hiPSC-EC applications in tissue engineering, organoid vascularization and therapeutic use of hiPSC-ECs.
Introduction
The ability to generate human endothelial cells (ECs) from human induced pluripotent stem cells (hiPSCs) makes it possible to consider these cells for therapeutic applications, tissue engineering, and regeneration and for disease modeling (Hasan et al., 2014, Leuning et al., 2018, Novosel et al., 2011, Phelps and Garcia, 2010). In addition to appropriate marker expression, proper functionality is required to realize most applications. While multiple protocols have described hiPSC-EC differentiation (Li et al., 2011, Ong et al., 2019, Orlova et al., 2014, Park et al., 2010, Rufaihah et al., 2011, Rufaihah et al., 2013, Wimmer et al., 2019), these ECs have not yet been able to recapitulate all features of their adult (primary) counterparts (Halaidych et al., 2018, Rosa et al., 2019, Taura et al., 2009, Zhang et al., 2017).
One key aspect of endothelial function is the ability to synthesize and maintain a glycocalyx surface layer (Broekhuizen et al., 2009, Esko and Lindahl, 2001, Reitsma et al., 2007, Weinbaum et al., 2007). In the endothelium this consists of the polysaccharides heparan sulfate, hyaluronan, and chondroitin sulfate, and the proteins that interact with them; together, they mediate most of the characteristic functional aspects of the endothelium (Quarto and Amalric, 1994, Yayon et al., 1991). For example, the heparan sulfate chains provide the specific binding motifs required for protein-receptor interaction and the formation of surface gradients of chemokines and growth factors (Rabelink et al., 2017). The surface layer also functions as a barrier to control capillary fluid filtration and is critical for endothelial shear sensing and, hence, inducing endothelial quiescence (Arisaka et al., 1995, Boels et al., 2016, Boels et al., 2017, Mulivor and Lipowsky, 2004, van den Berg et al., 2006). Glycocalyx composition is regulated by the interplay of shear sensing with subsequent downstream glycolysis inhibition, which renders glycolytic intermediates available for glucobiosynthetic pathways, such as glycocalyx synthesis (Cantelmo et al., 2016).
To assess this aspect of functionality, we examined the ability of hiPSC-ECs to respond to shear stress exposure and to express a glycocalyx surface layer, and compared their ability to adopt a shear-sensitive endothelial phenotype with human microvascular ECs (hMVECs). Since several studies have demonstrated that mitochondria are linked to cell fate determination and development (Chung et al., 2007, Facucho-Oliveira et al., 2007, Folmes et al., 2012, Hom et al., 2011, Lonergan et al., 2007, Prigione and Adjaye, 2010, Vannini et al., 2016, Xu et al., 2013, Zhang et al., 2018), we investigated the effects of closing the mitochondrial permeability transition pore (mPTP) in hiPSC-ECs. While we observed no effect of shear stress as such, in contrast to hMVECs we found that mPTP closure induced mitochondrial maturation and decreased reactive oxygen species (ROS), which restored the expression of an endothelial surface layer and adaptation to shear. In this respect, hiPSC-ECs exhibited functional maturation comparable with that of hMVECs.
Results
Differentiation of hiPSCs toward ECs
hiPSC lines used were generated using episomal plasmids (Okita et al., 2011) and RNA reprogramming (Schlaeger et al., 2015, Yoshioka et al., 2013) as described previously. For hiPSC-EC differentiation, we used a defined protocol described previously (Halaidych et al., 2018, Orlova et al., 2014) (Figures S1A and S1B). Results of RNA sequencing, all metabolic assays, and key experiments were obtained and reproduced in ECs from three different hiPSC lines (hiPSC-L72, hiPSC-L99, and hiPSC-NCRM1). All other results are from NCRM1 (also used as undifferentiated control). In all experiments, we compared hiPSC-ECs with primary hMVECs. For the aspects relevant to this study, we have not found significant differences between HUVECs (human umbilical vein endothelial cells) and hMVECs (Figures S2A–S2H).
Effects of Environmental Cues on iPSC-EC Functionality
To assess the effects of shear stress, we exposed hiPSC-ECs to a 5-dyne/cm2 laminar shear stress for 4 days. Previous studies have shown that shear stress of less than 24 h provokes an inflammatory-like response and that prolonged shear stress is necessary for the assessment of response to flow, such as alignment (Dekker et al., 2002, Dekker et al., 2006, Fledderus et al., 2007). Principal component analysis (PCA) of total RNA-sequencing data revealed that hiPSC-ECs cultured under static conditions formed a distinct population compared with hMVECs and that although flow induced a change in transcriptome, the cells were still different from the mature hMVECs (Figure 1A). Even prolonged flow had little effect on alignment of hiPSC-ECs (Figures 1B and 1C) but instead resulted in unstable focal adherence junctions between the cells (Figure 1D). Co-culturing hiPSC-ECs with human kidney-derived perivascular stromal cells for 4 days also failed to improve the alignment of hiPSC-ECs with laminar flow (Figures S3A and S3B).
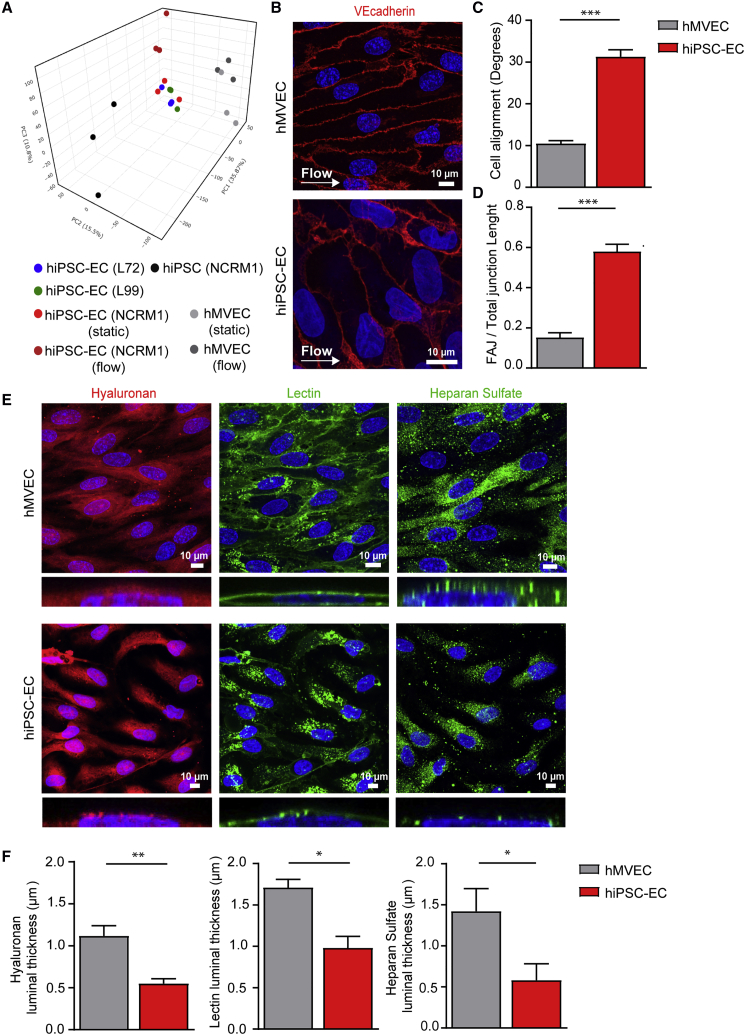
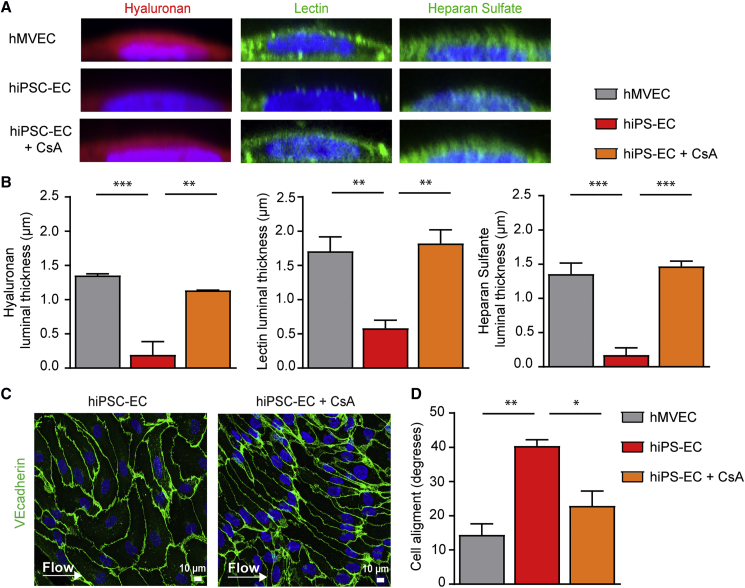
Figure 1.
hiPSC-Derived Endothelial Cells Do Not Show a Functional Response to Shear Stress Accompanied by an Insufficient Glycocalyx
(A) PCA plot of expression of all genes acquired from RNA sequencing of hiPSC NCRM1, mature ECs (hMVECs), and hiPSC-ECs NCRM1/L72/L99 in static conditions and after exposure to flow.
(B–D) Representative cross-sectional confocal images stained for VE-cadherin (red) and Hoechst (blue) after 4 days of laminar flow (5 dyne/cm2) culture of hMVECs and hiPSC-EC NCRM1 show the alignment of cells to flow (B). Quantification of cell alignment after 4 days of laminar flow culture of hMVECs and hiPSC-ECs (100 cells/group) (C). Quantification of adherence junction remodeling as the ratio of unstable focal adherence junction (FAJ) over total adherence junction length after 4 days of laminar flow culture of hMVECs and hiPSC-ECs (50 cells/group) (D).
(E and F) Representative cross-sectional and side-view confocal images stained for components of the glycocalyx (E). Hyaluronan (Neurocan, red), lectin (LEA, green) and heparan sulfate (JM403, green) after 4 days of laminar flow (5 dyne/cm2) culture of hMVECs and hiPSC-EC NCRM1 (E). Quantification of luminal thickness of hyaluronan, lectin Lycopersicon esculentum, and heparan sulfates after 4 days of laminar flow of hMVECs and hiPSC-ECs (8–16 cells/group) (F).
Values are presented as mean ± SEM of n = 3–6 independent experiments. Non-paired two-tailed Student's t test was performed; ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001.
hiPSC-ECs Lack a Functional Glycocalyx
As the endothelial glycocalyx serves a primary shear stress sensor (Arisaka et al., 1995, Mulivor and Lipowsky, 2004, van den Berg et al., 2006), we determined whether hiPSC-ECs had a functional glycocalyx. To this end, we quantified the luminal thickness of hyaluronan, lectin binding, and heparan sulfate (Figures 1E and 1F). Luminal glycocalyx expression was significantly lower in hiPSC-ECs compared with hMVECs with an overall ∼50% decrease in thickness of hyaluronan, lectin, and heparan sulfates (Figures 1E and 1F), indicating insufficient and dysfunctional surface coverage that can be important for binding of growth factors, mechanotransduction, and vascular stability. Pericyte co-culture did not increase the luminal glycocalyx thickness either (Figures S3C and S3D).
hiPSC-ECs Have Reduced Mitochondrial Function
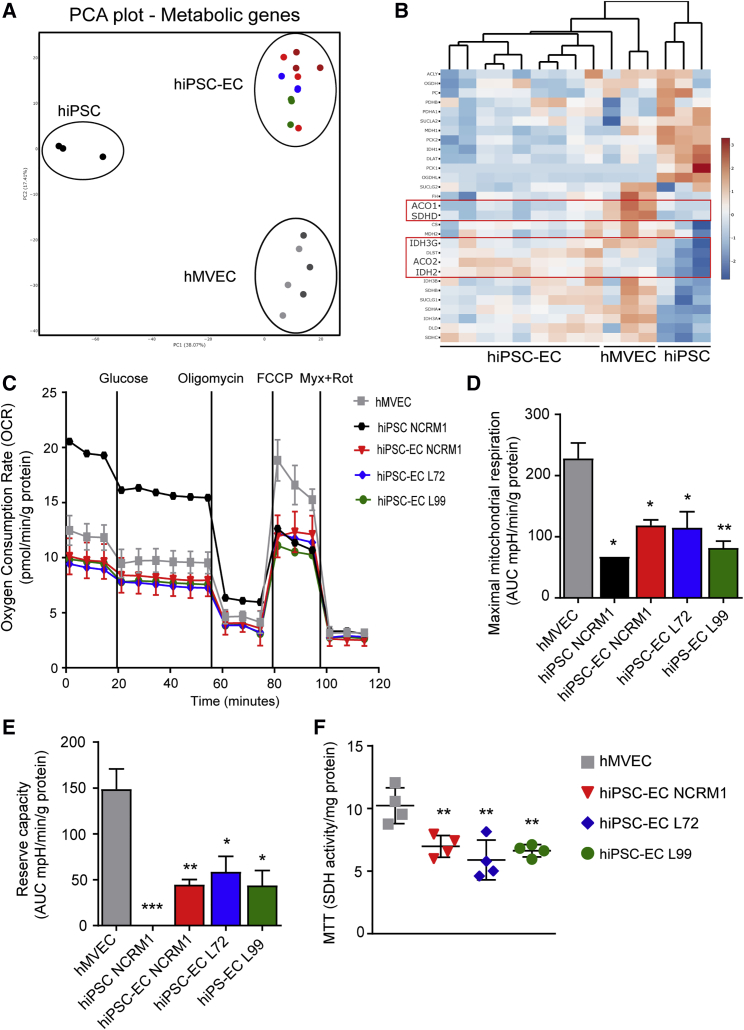
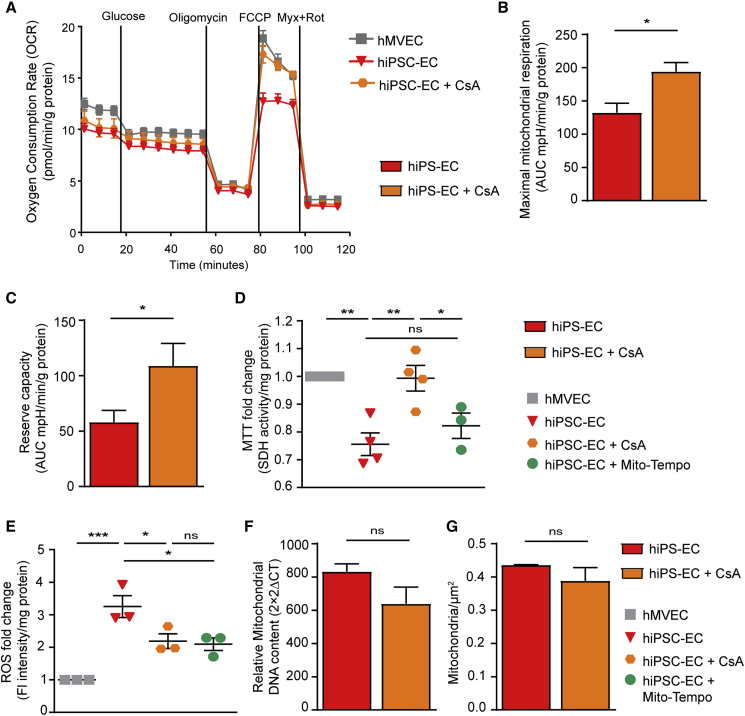
Since endothelial function is intrinsically linked to its metabolism (Bierhansl et al., 2017, Eelen et al., 2015), we investigated the expression of metabolic enzyme genes. PCA revealed separate clustering of undifferentiated hiPSCs, hiPSC-ECs, and hMVECs, indicating that hiPSC-ECs exhibited a different metabolic gene-expression profile with hMVECs (Figure 2A). Functional measurements of the extracellular acidification rate (ECAR), an indicator of lactate production, showed similar glucose-induced glycolysis and maximal glycolytic capacity in three different hiPSC-EC cell lines compared with hMVECs (Figures S4A and S4B). Furthermore, ATP production was similar (Figure S4C), dismissing the lack of energy production. RNA sequencing, however, showed a clear downregulation of several enzymes related to the tricarboxylic acid cycle, including isocitrate dehydrogenase and aconitase 1 (Figure 2B). The oxygen consumption rate (OCR), an indicator of mitochondrial function, showed dysfunction in hiPSC-ECs from all three hiPSC lines (Figure 2C) (Zhang et al., 2012) demonstrated by significantly reduced maximum mitochondrial respiration and mitochondrial reserve capacity (Figures 2D and 2E). This reduced mitochondrial activity was further confirmed by reduced NAD(P)H-dependent reduction of the tetrazolium dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Figure 2F).
Figure 2.
hiPSC-ECs Have Dysfunctional Mitochondria
(A) PCA plot of expression of all metabolic genes acquired from RNA sequencing of hiPSC NCRM1, hiPSC-ECs NCRM1/L72/L99, and mature ECs (hMVECs).
(B) Heatmap of RNA-sequencing results of metabolic genes involved in the mitochondrial metabolism. Scale bar represents Z scores: blue indicates lower gene expression and red a higher gene expression.
(C–E) Using a Seahorse XF flux analyzer, the oxygen consumption rate (OCR), an indicator of metabolic function, revealed mitochondrial dysfunction in three different hiPSC-EC cell lines (C). Both maximal mitochondrial respiration (D) and mitochondrial reserve capacity (E) were decreased (n = 4).
(F) Mitochondrial activity was also tested by MTT (n = 4).
Values are presented as mean ± SEM of n = 3–5 independent experiments. One-way ANOVA was performed; ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001.
hiPSC-ECs Have Immature Mitochondria
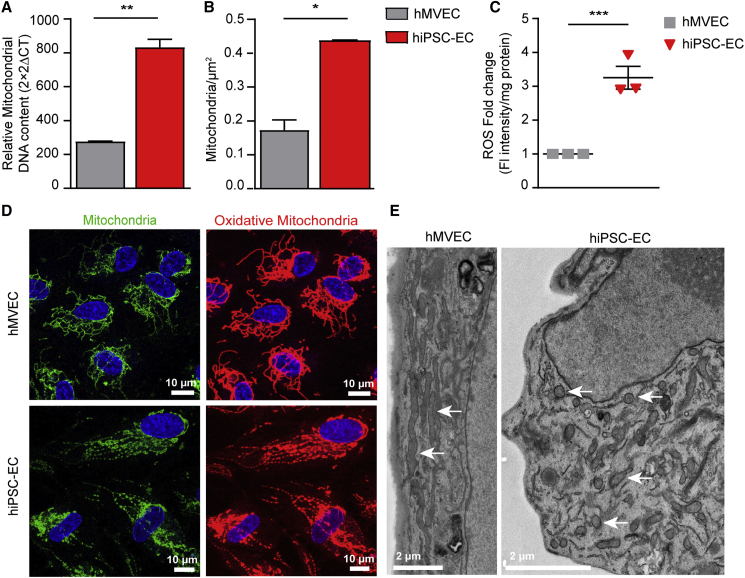
hiPSC-ECs have higher numbers of mitochondria and mitochondrial DNA compared with hMVECs (Figures 3A and 3B). However, both confocal microscopy and transmission electron microscopy (TEM) revealed a distinctly different morphology of hiPSC-EC mitochondria compared with hMVECs (Figures 3D, 3E, and S5A). TEM of the hiPSC-ECs showed round mitochondria with paucity of cristae, characteristic of immaturity. This was associated with increased cell-associated ROS (Figure 3C).
Figure 3.
hiPSC-ECs Have an Increased Amount of Immature Mitochondria
(A) Mitochondrial DNA measured by qPCR.
(B) Mitochondrial density quantified on transmission electron microscopy (TEM) stitches (21 cells/group).
(C) Fold change of fluorescent intensity/mg protein of ROS dye.
(D) Representative cross-sectional confocal images stained for MitoTracker red (oxidative mitochondria) and MitoTracker green (mitochondria).
(E) TEM images show the ultrastructure of mitochondria of hMVECs and hiPSC-EC NCRM1.
Values are presented as mean ± SEM of n = 3 independent experiments. Non-paired two-tailed Student's t test was performed; ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001.
Mitochondrial Maturation by Permeability Transition Pore Closure
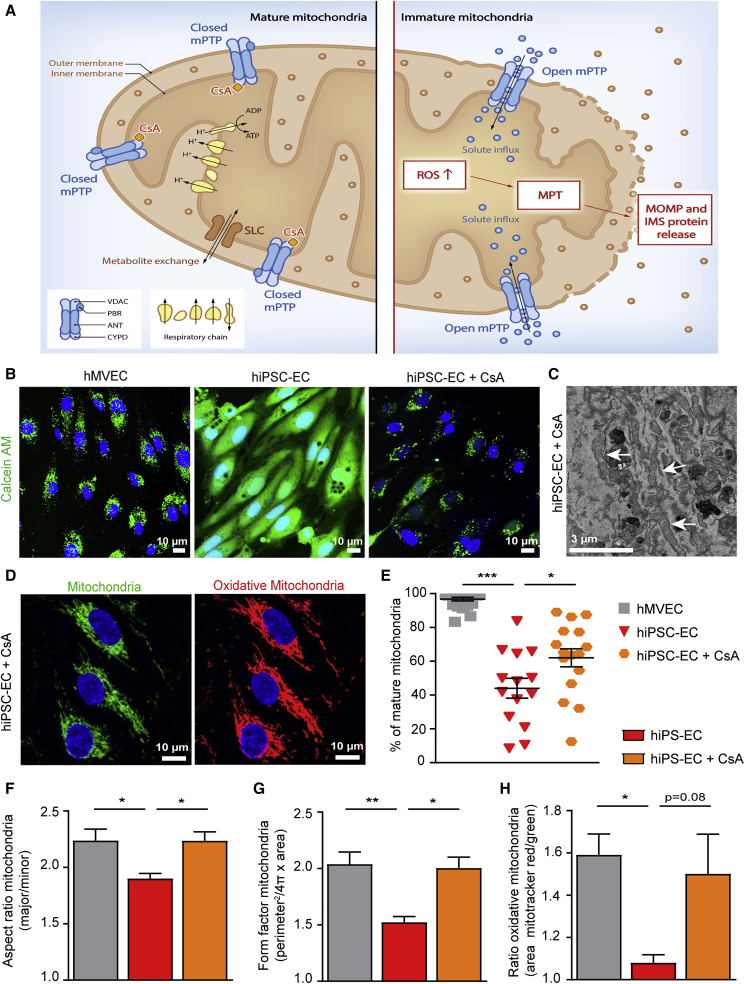
The high number of immature mitochondria and increased intracellular ROS in hiPSC-ECs suggested that the mitochondrial membrane permeability transition pore (mPTP) in hiPSC-ECs might be constitutively open (Halestrap, 2009, Hom et al., 2011). During differentiation of hiPSC to hiPSC-ECs, this mPTP transporter should close in order to allow maturation of the mitochondria (Hom et al., 2011). Cyclosporine A (CsA), an immunosuppressant, binds to mitochondrial cyclophilin D (CYPD) to block the calcium ion-induced permeability transition pore mPTP (Figure 4A), and treatment with CsA has been shown to induce mitochondrial maturation in myocytes (Brookes et al., 2004, Crompton et al., 1999, Halestrap, 2009, Hom et al., 2011). To determine whether the mPTP was open in hiPSC-ECs, we used the cobalt/calcein quenching method (Petronilli et al., 1999). In untreated hiPSC-ECs, calcein fluorescence leaked from the mitochondria due to an open mPTP, and calcein AM fluorescence was observed throughout the cell. However, when hiPSC-ECs were treated with 1.5 mM CsA for 30 min, only mitochondrial calcein fluorescence was observed, indicating that CsA closed the mPTP, preventing calcein leakage from the mitochondria. In untreated hMVECs, calcein fluorescence was only observed in the mitochondria, confirming the closed mPTP in mature ECs (Figures 4B and S5B).
Figure 4.
Treatment with Cyclosporine A Results in Closure of the mPTP and Subsequent Maturation of the Mitochondria
(A) Schematic overview of mature and immature mitochondria. Cyclosporine A (CsA) binds to cyclophilin D (CYPD) and thereby closes the mitochondrial permeability transition pore (mPTP). This prevents leakage of ROS and intermembrane space (IMS) proteins due to mitochondrial outer membrane permeabilization during the opening of mPTP.
(B) To determine the state of the mPTP in hiPSC-ECs, the cobalt/calcein AM (green) quenching method was used. hiPSC-EC NCRM1 treated with CsA for 30 min prevented calcein leakage, indicating that CsA closed the mPTP.
(C) TEM image shows the ultrastructure of mitochondria of hiPSC-ECs treated with 500 nM CsA during differentiation.
(D) Representative cross-sectional confocal images stained for MitoTracker red (oxidative mitochondria) and MitoTracker green (mitochondria) of hiPSC-ECs NCRM1 treated with CsA. (E) Quantification of percentage of mature mitochondria on TEM stitches (21 cells/group). (F and G) Quantitative analysis of mitochondrial morphology by analyzing MitoTracker confocal images. Individual particles (mitochondria) were analyzed for circularity and lengths of major and minor axes. From these values, aspect ratio (AR; major/minor) (F) and form factor (FF; perimeter2/4π × area) (G) were calculated. Both FF and AR have a minimal value of 1 when a particle is a small perfect circle, and the values increase as the shape becomes elongated. AR is a measure of mitochondrial length, and an increase of FF represents increase in length and branching (10 cells/group).
(H) Quantitative analysis of the area stained by MitoTracker red (oxidative mitochondria) divided by the area stained for MitoTracker green (mitochondria) (10 cells/group).
Values are presented as mean ± SEM of n = 3–5 independent experiments. One-way ANOVA was performed; ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001.
Treatment of hiPSC-ECs with CsA for a week during differentiation starting on day 5 revealed high numbers of mature, elongated cristae-rich mitochondria similar to those observed in hMVECs on TEM (Figures 4C and 4E). The same morphology was observed with confocal microscopy using MitoTracker dyes (Figures 4D and S5A). Analysis of the mitochondrial morphology further supported the maturation of the mitochondria, with a higher aspect ratio and form factor in hiPSC-ECs treated with CsA, indicating increased elongation and reduced circularity of the mitochondria (Figures 4F and 4G). A trend toward more oxidative mitochondria was also observed (Figure 4H).
Improved Mitochondrial Function after Treatment with CsA
CsA treatment during the differentiation of hiPSC-ECs restored mitochondrial function by increasing maximal mitochondrial respiration and reserve capacity and increasing mitochondrial activity as detected by the MTT to levels similar to those in hMVECs (Figures 5A–5D). Concomitantly, CsA led to significantly reduced levels of ROS (Figure 4E). This reduction was also observed after treating the cells with MitoTEMPO (Figure 5E), a mitochondria-specific antioxidant, which further suggested that ROS is produced by the dysfunctional mitochondria (Figure 5E). However, treatment with MitoTEMPO during differentiation did not lead to mitochondrial maturation (Figure 5D). The reduction in ROS after treatment with CsA is not due to changes in mitochondrial content, since the amount of mitochondria is not significantly different (Figures 5F and 5G) Together, these findings indicated that maturation of mitochondria could be induced by closure of the mPTP with CsA.
Figure 5.
Treatment with Cyclosporine A Results in Improved Mitochondrial Function in hiPSC-ECs
(A–C) The OCR revealed increased mitochondrial function in hiPSC-EC NCRM1 treated with 500nM CsA during differentiation (A).
Both maximal mitochondrial respiration (B) and mitochondrial reserve capacity (C) were increased in hiPSC-ECs treated with CsA (n = 3).
(D) Mitochondrial activity was also tested by MTT after addition of 500 nM CsA or 10 mM MitoTEMPO during differentiation. CsA results in an increased mitochondrial activity, whereas MitoTEMPO did not increase mitochondrial activity.
(E) Fold change of fluorescent intensity per mg of protein of ROS staining shows reduced ROS of CsA-treated cells. The same effect was obtained by addition of 10 mM MitoTEMPO.
(F and G) Mitochondrial DNA measured with qPCR (F) and mitochondrial density quantified on TEM stitches (21 cells/group) (G) after treatment of CsA during differentiation.
Values are presented as mean ± SEM of n = 3–4 independent experiments. One-way ANOVA or non-paired two-tailed Student's t test was performed; ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001; ns, not significant.
Closure of mPTP Restores Glycocalyx Formation and Improves Functionality of hiPSC-ECs
After treating the hiPSC-ECs with CsA during differentiation, we exposed them to 4 days of laminar flow to assess the glycocalyx and morphological responses. The mPTP was still closed after 4 days of flow, assessed by the cobalt/calcein method as described earlier, even without addition of CsA during the flow (Figure S5C). CsA significantly increased luminal thickness of hyaluronan, lectin, and heparan sulfates compared with untreated hiPSC-ECs (Figures 6A, 6B, and S6A). Given the role of glycocalyx as a shear stress mechanotransducer, we hypothesized that normalization of the glycocalyx might lead to restoration of alignment to shear. Indeed, treatment of hiPSC-ECs with CsA resulted in improved alignment to the shear stress (Figures 6C, 6D, and S6B).
Figure 6.
Treatment with Cyclosporine A Restores the Glycocalyx and Improves Alignment to Flow
(A) Representative side-view confocal images stained for hyaluronan (Neurocan, red), lectin (LEA, green), and heparan sulfate (JM403, green) after 4 days of laminar flow culture of hMVECs, hiPSC-ECs control, and hiPSC-EC NCRM1 treated with 500 nM CsA during differentiation.
(B) Quantification of luminal thickness of hyaluronan, lectin Lycopersicon esculentum, and heparan sulfates after 4 days of laminar flow of hMVECs, hiPSC-ECs, and hiPSC-ECs treated with CsA (8–16 cells/group).
(C) Representative cross-sectional confocal images of VE-cadherin (green) and Hoechst (blue) show the alignment of cells to flow.
(D) Quantification of cell alignment after 4 days of laminar flow culture of hMVECs, hiPSC-ECs, and hiPSC-ECs treated with CsA (100 cells/group).
Values are presented as mean ± SEM of n = 3 independent experiments. One-way ANOVA was performed; ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001.
Discussion
ECs exposed to laminar shear stress express a thick glycocalyx on their surface that plays an important role in reducing vascular permeability and endothelial anti-inflammatory, antithrombotic, and antiangiogenic properties. Our results show that prolonged exposure to shear stress initially failed to induce endothelial quiescence or maturation in hiPSC-ECs. We showed that hiPSC-ECs essentially lack a functional glycocalyx and do not align to the shear stress as primary ECs. Several studies have also demonstrated a role for pericytes in inducing EC quiescence and vessel stability (Lennon and Singleton, 2011, Sweeney and Foldes, 2018). Our data, however, showed that additional co-culture of hiPSC-ECs with pericytes under shear also failed to induce endothelial quiescence and the synthesis of a glycocalyx layer. Nonetheless, we recently demonstrated that pericyte-EC signaling actually requires an intact glycocalyx layer, implicating the dysfunctional glycocalyx as the potential cause for failure of hiPSC-ECs and pericyte interaction (van den Berg et al., 2019).
It has been well documented that in general, when hiPSCs commit to differentiation, they undergo metabolic remodeling so that they become less reliant on glycolysis and more dependent upon mitochondrial respiration (Folmes et al., 2012, Xu et al., 2013, Zhang et al., 2018). This has also been described for mesodermal differentiation (Cliff et al., 2017), even though ECs preserve relatively high glycolysis (Cantelmo et al., 2016, Eelen et al., 2018, Wong et al., 2017). One hypothesis is that failure to establish an accurate metabolic switch may have negatively affected the maturation and functionality of iPSC-ECs. It was therefore noteworthy that glycolysis was not different between hiPSC-ECs and hMVECs.
The Seahorse data did show, however, reduced mitochondrial capacity for oxidative phosphorylation, despite higher overall mitochondrial content in the hiPSC-ECs. Furthermore, ultrastructural analysis showed immature, round mitochondria that lacked mature cristae development and were associated with increased ROS leakage. Together, these data indicated an open mPTP, which is characteristic of mitochondrial immaturity. Previous studies have shown the importance of mitochondria and mPTP activity in differentiation and stem cell fate (Cho et al., 2014, Hom et al., 2011, Hou et al., 2013, Wanet et al., 2015, Yan et al., 2009). Chemical closure of the mPTP has been shown to increase mitochondrial maturation, regulate redox signaling, and enhance differentiation of cardiomyocytes by 10- to 20-fold (Hom et al., 2011, Yan et al., 2009). Our data showed that prolonged closure of mPTP with CsA in hiPSC-ECs resulted in more functional mature mitochondria and prevention of ROS leakage. Furthermore, we found that maturation of the mitochondria led to functional improvements that included restoration of the glycocalyx and subsequent restoration of the mechanotransduction in hiPSC-ECs. Moreover, reduction of ROS may also be directly involved in restoration of glycocalyx, since ROS can lead to direct heparan sulfate and hyaluronan fragmentation by oxidative reductive depolymerization (Moseley et al., 1997, Rubio-Gayosso et al., 2006, Uchiyama et al., 1990).
As ECs generate ATP mostly through glycolysis, the mitochondria act as signaling organelles via generation of ROS in addition to producing the metabolic intermediates necessary for cell growth. This activates transcriptional networks and, via metabolic intermediates, controls epigenetic regulation (Bekkering et al., 2018, Daiber et al., 2017, Kalucka et al., 2018, Schell and Rutter, 2017). The impact of mitochondrial maturation on these regulatory functions requires further study. The elongated mature mitochondria of hiPSC-ECs treated with CsA illustrate the importance of efficient biosynthetic organelles to support sufficiently the proliferation required for angiogenesis (Bruning et al., 2018, Diebold et al., 2019). This angiogenic capacity together with a functional glycocalyx, necessary for growth factor signaling and anticoagulation, is a prerequisite for future hiPSC-EC applications in tissue engineering (Leuning et al., 2018) and organoid vascularization (van den Berg et al., 2018), and in future therapeutic applications of hiPSC-ECs, such as wound healing (Clayton et al., 2018). This study demonstrated that mitochondrial maturation can be pharmacologically regulated in hiPSC-derived cells to generate more mature and representative differentiation products.
Experimental Procedures
hiPSC Culture and EC Differentiation
NCRM1 was obtained from RUCDR (reprogramming of CD34+ cord blood using episomal vectors). LUMC0072iCTRL01 (L72) and LUMC0099iCTRL04 (L99) were generated by the LUMC iPSC core facility from fibroblasts on mouse embryonic fibroblasts using a Simplicon RNA Reprogramming Kit (Millipore-Merck, Amsterdam, the Netherlands) and ReproRNA (STEMCELL Technologies) respectively, as described previously (Yoshioka et al., 2013) and further cultured in TeSR-E8 medium (STEMCELL). The hiPSC-ECs were generated from these lines according to Orlova et al. (2014). In short, hiPSCs maintained on Matrigel-coated (Corning) plates in TeSR-E8 medium were routinely enzymatically passaged weekly. Differentiation was induced 4 days after passaging (day 0). Mesoderm specification was induced by adding bone morphogenetic protein 4 (R&D Systems), activin A (Miltenyi Biotec), small-molecule inhibitor of glycogen synthase kinase 3β (Tocris Bioscience), and vascular endothelial growth factor (VEGF; Miltenyi Biotec). Mesoderm-inducing factors were removed on day 3 of differentiation and replaced by vascular specification medium containing VEGF and the transforming growth factor β (TGF-β) pathway small-molecule inhibitor SB431542 (Tocris Bioscience). SB431542 allows expansion of ECs by inhibiting the antiproliferative activities of TGF-β also present in the culture. Vascular specification medium was refreshed on days 7 and 9 of differentiation and hiPSC-ECs were isolated on day 10 using anti-CD31 antibody-coupled magnetic beads (Dynabeads; Miltenyi Biotech). hiPSC-ECs were then transferred to EC serum-free medium (EC-SFM; Gibco, Thermo Fisher Scientific) to which platelet-poor plasma (1% v/v) (Biomedical Technologies), 50 μg/mL VEGF-165 (R&D Systems), and 100 μg/mL basic fibroblast growth factor (Miltenyi Biotech) had been added (EC-SFM full medium) at 37°C with 5% CO2 and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin; Life Technologies Gibco).
Primary Human Microvascular EC Culture
hMVECs were isolated from human kidney cortical tissue and were purchased from Cell Systems (ACBRI-128, Kirkland, WA). They were available at passage 3 (<12 cumulative population doublings) cryopreserved in CSC Cell Freezing Medium (4Z0-705). hMVECs were also cultured in EC-SFM full medium at 37°C with 5% CO2 and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin).
Flow Experiments
Shear experiments were performed using an Ibidi flow system (Ibidi). Cells were cultured for 4 days at a constant laminar shear stress of 5 dyne/cm2, at moderated physiological flow (Buchanan et al., 2014, Gautam et al., 2006) in EC-SFM full medium as previously described (Boels et al., 2016, van den Berg et al., 2019). Cells were seeded into closed perfusion chambers (ibiTreat 0.4 μ-Slide I or VI; Luer) at a concentration of 1.4 × 106 cells/mL and allowed to adhere for 3 h. Thereafter, the chamber was connected to a computer-controlled air-pressure pump and a fluidic unit with a two-way switching valve. The pump setup allowed pumping of 16 mL of cell-culture medium from two reservoirs in a unidirectional way through the flow channel over the monolayer of ECs at a constant shear stress of 5 dyne/cm2. Medium was refreshed after 1 day of culture. The chamber and the reservoirs containing the medium were kept in an incubator at 37°C and 5% CO2. RNA was isolated from cells subjected to shear stress in a 0.4 μ-Slide I Luer flow chamber, while the six lanes of a 0.4 μ-Slide VI Luer were used for immunofluorescent staining.
MitoTracker Staining and Quantification
MitoTracker probes (Invitrogen) were used to visualize mitochondria. Live cells were loaded with MitoTracker Green FM, MitoTracker Red CM-H2XRos (100 nM, Thermo Fisher, M7513) and Hoechst 33258 (Life Technologies, H3569) for 30 min at 37°C and imaged using a Leica SP8 white-light laser confocal immunofluorescence microscope. Analysis of mitochondrial morphology was quantified as described previously (Koopman et al., 2005, Yu et al., 2006). After filtering for median of two pixels, making the picture binary and thresholding, the individual particles were analyzed for circularity (4π × area/perimeter2) and length of the minor and minor axes.
The ratio between MitoTracker red and green was also quantified by thresholding and selection of single cells, followed by measuring the stained area of MitoTracker red and MitoTracker green. For each independent experiment (n = 5), 10 cells per group were analyzed with up to 250 particles per cell.
Fluorescent Quantification
Surface hyaluronan expression (Ncan-dsRed), lectin binding (LEA; 10 μg/mL, Sigma-Aldrich, L2895) and heparan sulfate (JM403; 1.1 mg/mL, gift from Dr. van der Vlag and Dr. Kuppevelt [Nijmegen Center for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, the Netherlands]) were quantified on 4 cells per field of view essentially as described earlier (Boels et al., 2016, van den Berg et al., 2019). From a side view, resliced from a line (10 pixels wide) over the nucleus, three additional lines were drawn at the nuclear position and the mean fluorescence was calculated between the distance from half-maximum signal of the nuclear staining to half-maximum signal at the luminal end. Eight to 20 cells per group were analyzed from each independent experiment (n = 4). The difference between stable adherence junctions and focal adherence junctions (FAJ) upon junction remodeling (Huveneers et al., 2012) was quantified as the ratio of FAJ length over total junction length (Timmerman et al., 2015). One hundred cells were analyzed from each independent experiment (n = 4).
Metabolic Assays
To measure endothelial glycolytic flux, we seeded hMVECs and hiPSC-ECs overnight at 4 × 104 cells per well on fibronectin-coated (Sigma-Aldrich) Seahorse XF96 polystyrene tissue culture plates (Seahorse Bioscience). The plate was incubated in unbuffered DMEM assay medium (Sigma-Aldrich) for 1 h in a non-CO2 incubator at 37°C before measuring in an XFe 96 extracellular flux analyzer (Seahorse Bioscience). OCR (data not shown) and ECAR were measured over 4-min periods with 2-min mixing in each cycle, with five cycles in total. Inhibitors and activators were used at the following concentrations: glucose (10 mM), oligomycin (3 μM), 2-deoxyglucose (100 mM), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP; 1 μM), antimycin A (1.5 μM), and rotenone (3 μM) (all Sigma-Aldrich). Cellular protein content was determined with a BCA-protein kit from Pierce (Thermo Fischer Scientific), and the data are presented as ECAR normalized to protein. Each measurement was averaged from triplicate wells. To calculate these parameters, we considered basal OCR as the last value prior to injection of the first additive (oligomycin). Likewise, we considered OCR on oligomycin as the last value prior to FCCP injection, OCR on FCCP as the last value prior to antimycin plus rotenone injection, and non-mitochondrial OCR as the last value recorded after antimycin plus rotenone.
As described previously (Dranka et al., 2010, Fink et al., 2012), we then calculated the following parameters. Basal respiration was determined as OCR in the basal state minus non-mitochondrial OCR. Maximal respiration was calculated as OCR on FCCP minus non-mitochondrial OCR. The reserve capacity was calculated as maximal respiration minus basal respiration.
To measure intracellular reactive oxygen species, we used the Cellular ROS/Superoxide detection assay kit (Abcam, ab139476). ECs plated in a black 96-well plate (20,000 cells per well) were incubated with ROS/Superoxide detection mix (2 μM) for 30 min at 37°C in darkness. As positive and negative controls, the ROS inducer pyocyanin (200 μM) and ROS inhibitor N-acetyl-L-cysteine (5 mM) were used 1 h or 30 min in advance, respectively. Cells were then washed twice with washing buffer and immediately observed for fluorescein and rhodamine in a Molecular Devices Spectramax i3x. Cellular protein content was determined with a BCA-protein kit from Pierce (Thermo Fisher Scientific), and the data are presented as ROS normalized to protein. Each measurement was averaged from quadruplicate wells.
CsA (Sigma, #30024) was added at a concentration of 500 nM to the vascular medium during differentiation, starting on day 5 of vascular medium, and continued for 1 week.
Statistical Analysis
Results are presented as mean ± SD or mean ± SEM, where n is the number of biological independent experimental replicates. Differences between groups were assessed by non-paired two-tailed Student's t test, paired two-tailed Student's t test, or, when not normally distributed, by two-tailed F test. Differences between more than two groups were assessed by ANOVA. p values of <0.05 were considered statistically significant.
Author Contributions
G.L.T. and B.M.v.d.B. designed the research study, conducted experiments, acquired data, and wrote the manuscript. G.W., S.J.D. conducted experiments, acquired data, and provided helpful comments. M.C.A., T.K. and W.M.P.J.S conducted experiments and acquired data. C.L.M., V.V.O., and C.W.v.d.B. read the manuscript and provided helpful comments. P.C. read the manuscript, provided helpful comments, and acquired funding. T.J.R. designed the research study, wrote the manuscript, and acquired funding.
Acknowledgments
We acknowledge the support of Ellen Lievers, Rozemarijn de Koning, Loes Wiersma, and Manon Zuurmond (LUMC, Leiden, the Netherlands). We thank hiPSC core facility, LUMC, Leiden, the Netherlands, for providing two hiPSC lines (LUMC0072iCTRL01 and LUMC0099iCTRL04). This work is supported by the partners of ‘Regenerative Medicine Crossing Borders’ (RegMed XB) and Powered by Health∼Holland, Top Sector Life Sciences & Health. G.L.T. is funded by the LUMC MD/PhD track and C.W.v.d.B. is supported by the Wiyadharma fellowship (Bontius Stichting, LUMC). V.V.O. and C.L.M. received support from H2020 TECHNOBEAT.
Published: October 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.10.005.
Accession Numbers
RNA-sequencing data are available under accession number ArrayExpress: E-MTAB-8392.
Supplemental Information
References
- Arisaka T., Mitsumata M., Kawasumi M., Tohjima T., Hirose S., Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann. N. Y Acad. Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- Bekkering S., Arts R.J.W., Novakovic B., Kourtzelis I., van der Heijden C., Li Y., Popa C.D., Ter Horst R., van Tuijl J., Netea-Maier R.T. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172:135–146.e9. doi: 10.1016/j.cell.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Bierhansl L., Conradi L.C., Treps L., Dewerchin M., Carmeliet P. Central role of metabolism in endothelial cell function and vascular disease. Physiology. 2017;32:126–140. doi: 10.1152/physiol.00031.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boels M.G., Avramut M.C., Koudijs A., Dane M.J., Lee D.H., van der Vlag J., Koster A.J., van Zonneveld A.J., van Faassen E., Grone H.J. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65:2429–2439. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
- Boels M.G.S., Koudijs A., Avramut M.C., Sol W., Wang G., van Oeveren-Rietdijk A.M., van Zonneveld A.J., de Boer H.C., van der Vlag J., van Kooten C. Systemic monocyte chemotactic protein-1 inhibition modifies renal macrophages and restores glomerular endothelial glycocalyx and barrier function in diabetic nephropathy. Am. J. Pathol. 2017;187:2430–2440. doi: 10.1016/j.ajpath.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Broekhuizen L.N., Mooij H.L., Kastelein J.J., Stroes E.S., Vink H., Nieuwdorp M. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr. Opin. Lipidol. 2009;20:57–62. doi: 10.1097/MOL.0b013e328321b587. [DOI] [PubMed] [Google Scholar]
- Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Bruning U., Morales-Rodriguez F., Kalucka J., Goveia J., Taverna F., Queiroz K.C.S., Dubois C., Cantelmo A.R., Chen R., Loroch S. Impairment of angiogenesis by fatty acid synthase inhibition involves mTOR malonylation. Cell Metab. 2018;28:866–880.e15. doi: 10.1016/j.cmet.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C.F., Verbridge S.S., Vlachos P.P., Rylander M.N. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adh. Migr. 2014;8:517–524. doi: 10.4161/19336918.2014.970001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantelmo A.R., Conradi L.-C., Brajic A., Goveia J., Kalucka J., Pircher A., Chaturvedi P., Hol J., Thienpont B., Teuwen L.-A. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30:968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.W., Park J.-S., Heo H.J., Park S.-W., Song S., Kim I., Han Y.-M., Yamashita J.K., Youm J.B., Han J. Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells. J. Am. Heart Assoc. 2014;3:e000693. doi: 10.1161/JAHA.113.000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Dzeja P.P., Faustino R.S., Perez-Terzic C., Behfar A., Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007;4(Suppl 1):S60–S67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton Z.E., Tan R.P., Miravet M.M., Lennartsson K., Cooke J.P., Bursill C.A., Wise S.G., Patel S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff T.S., Wu T., Boward B.R., Yin A., Yin H., Glushka J.N., Prestegaard J.H., Dalton S. MYC controls human pluripotent stem cell fate decisions through regulation of metabolic flux. Cell Stem Cell. 2017;21:502–516.e9. doi: 10.1016/j.stem.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Virji S., Doyle V., Johnson N., Ward J.M. The mitochondrial permeability transition pore. Biochem. Soc. Symp. 1999;66:167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- Daiber A., Di Lisa F., Oelze M., Kroller-Schon S., Steven S., Schulz E., Munzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017;174:1670–1689. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R., van Soest S., Fontijn R.D., Salamanca S., de Groot P.G., Vanbavel E., Pannekoek H., Horrevoets A. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- Dekker R.J., Boon R.A., Rondaij M.G., Kragt A., Volger O.L., Elderkamp Y.W., Meijers J.C., Voorberg J., Pannekoek H., Horrevoets A.J. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- Diebold L.P., Gil H.J., Gao P., Martinez C.A., Weinberg S.E., Chandel N.S. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat. Metab. 2019;1:158–171. doi: 10.1038/s42255-018-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranka B.P., Hill B.G., Darley-Usmar V.M. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G., de Zeeuw P., Simons M., Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116:1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G., de Zeeuw P., Treps L., Harjes U., Wong B.W., Carmeliet P. Endothelial cell metabolism. Physiol. Rev. 2018;98:3–58. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J.D., Lindahl U. Molecular diversity of heparan sulfate. J. Clin. Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facucho-Oliveira J.M., Alderson J., Spikings E.C., Egginton S., St John J.C. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J. Cell Sci. 2007;120:4025–4034. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- Fink B.D., Herlein J.A., O'Malley Y., Sivitz W.I. Endothelial cell and platelet bioenergetics: effect of glucose and nutrient composition. PLoS One. 2012;7:e39430. doi: 10.1371/journal.pone.0039430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledderus J.O., van Thienen J.V., Boon R.A., Dekker R.J., Rohlena J., Volger O.L., Bijnens A.-P.J.J., Daemen M.J.A.P., Kuiper J., van Berkel T.J.C. Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood. 2007;109:4249. doi: 10.1182/blood-2006-07-036020. [DOI] [PubMed] [Google Scholar]
- Folmes C.D., Dzeja P.P., Nelson T.J., Terzic A. Mitochondria in control of cell fate. Circ. Res. 2012;110:526–529. doi: 10.1161/RES.0b013e31824ae5c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M., Shen Y., Thirkill T.L., Douglas G.C., Barakat A.I. Flow-activated chloride channels in vascular endothelium: shear stress sensitivity, desensitization dynamics, and physiological implications. J. Biol. Chem. 2006;281:36492–36500. doi: 10.1074/jbc.M605866200. [DOI] [PubMed] [Google Scholar]
- Halaidych O.V., Freund C., van den Hil F., Salvatori D.C.F., Riminucci M., Mummery C.L., Orlova V.V. Inflammatory responses and barrier function of endothelial cells derived from human induced pluripotent stem cells. Stem Cell Rep. 2018;10:1642–1656. doi: 10.1016/j.stemcr.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hasan A., Paul A., Vrana N.E., Zhao X., Memic A., Hwang Y.S., Dokmeci M.R., Khademhosseini A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials. 2014;35:7308–7325. doi: 10.1016/j.biomaterials.2014.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom J.R., Quintanilla R.A., Hoffman D.L., de Mesy Bentley K.L., Molkentin J.D., Sheu S.S., Porter G.A., Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev. Cell. 2011;21:469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Mattson M.P., Cheng A. Permeability transition pore-mediated mitochondrial superoxide flashes regulate cortical neural progenitor differentiation. PLoS One. 2013;8:e76721. doi: 10.1371/journal.pone.0076721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S., Oldenburg J., Spanjaard E., van der Krogt G., Grigoriev I., Akhmanova A., Rehmann H., de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalucka J., Bierhansl L., Conchinha N.V., Missiaen R., Elia I., Bruning U., Scheinok S., Treps L., Cantelmo A.R., Dubois C. Quiescent endothelial cells upregulate fatty acid beta-oxidation for vasculoprotection via redox homeostasis. Cell Metab. 2018;28:881–894.e13. doi: 10.1016/j.cmet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Koopman W.J., Visch H.J., Verkaart S., van den Heuvel L.W., Smeitink J.A., Willems P.H. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am. J. Physiol. Cell Physiol. 2005;289:C881–C890. doi: 10.1152/ajpcell.00104.2005. [DOI] [PubMed] [Google Scholar]
- Lennon F.E., Singleton P.A. Hyaluronan regulation of vascular integrity. Am. J. Cardiovasc. Dis. 2011;1:200–213. [PMC free article] [PubMed] [Google Scholar]
- Leuning D.G., Witjas F.M.R., Maanaoui M., de Graaf A.M.A., Lievers E., Geuens T., Avramut C.M., Wiersma L.E., van den Berg C.W., Sol W. Vascular bioengineering of scaffolds derived from human discarded transplant kidneys using human pluripotent stem cell-derived endothelium. Am. J. Transplant. 2018;19:1328–1343. doi: 10.1111/ajt.15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Hu S., Ghosh Z., Han Z., Wu J.C. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev. 2011;20:1701–1710. doi: 10.1089/scd.2010.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T., Bavister B., Brenner C. Mitochondria in stem cells. Mitochondrion. 2007;7:289–296. doi: 10.1016/j.mito.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley R., Waddington R.J., Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim. Biophys. Acta. 1997;1362:221–231. doi: 10.1016/s0925-4439(97)00083-5. [DOI] [PubMed] [Google Scholar]
- Mulivor A.W., Lipowsky H.H. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- Novosel E.C., Kleinhans C., Kluger P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Ong S.B., Lee W.H., Shao N.Y., Ismail N.I., Katwadi K., Lim M.M., Kwek X.Y., Michel N.A., Li J., Newson J. Calpain inhibition restores autophagy and prevents mitochondrial fragmentation in a human iPSC model of diabetic endotheliopathy. Stem Cell Reports. 2019;12:597–610. doi: 10.1016/j.stemcr.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova V.V., van den Hil F.E., Petrus-Reurer S., Drabsch Y., Ten Dijke P., Mummery C.L. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 2014;9:1514–1531. doi: 10.1038/nprot.2014.102. [DOI] [PubMed] [Google Scholar]
- Park S.W., Jun Koh Y., Jeon J., Cho Y.H., Jang M.J., Kang Y., Kim M.J., Choi C., Sook Cho Y., Chung H.M. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762–5772. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- Petronilli V., Miotto G., Canton M., Brini M., Colonna R., Bernardi P., Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophysical J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., Garcia A.J. Engineering more than a cell: vascularization strategies in tissue engineering. Curr. Opin. Biotechnol. 2010;21:704–709. doi: 10.1016/j.copbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione A., Adjaye J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int. J. Dev. Biol. 2010;54:1729–1741. doi: 10.1387/ijdb.103198ap. [DOI] [PubMed] [Google Scholar]
- Quarto N., Amalric F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J. Cell Sci. 1994;107(Pt 11):3201–3212. doi: 10.1242/jcs.107.11.3201. [DOI] [PubMed] [Google Scholar]
- Rabelink T.J., van den Berg B.M., Garsen M., Wang G., Elkin M., van der Vlag J. Heparanase: roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat. Rev. Nephrol. 2017;13:201–212. doi: 10.1038/nrneph.2017.6. [DOI] [PubMed] [Google Scholar]
- Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A.M.J., Oude Egbrink M.G.A. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa S., Praça C., Pitrez P.R., Gouveia P.J., Aranguren X.L., Ricotti L., Ferreira L.S. Functional characterization of iPSC-derived arterial- and venous-like endothelial cells. Sci. Rep. 2019;9:3826. doi: 10.1038/s41598-019-40417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Gayosso I., Platts S.H., Duling B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- Rufaihah A.J., Huang N.F., Jame S., Lee J.C., Nguyen H.N., Byers B., De A., Okogbaa J., Rollins M., Reijo-Pera R. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2011;31 doi: 10.1161/ATVBAHA.111.230938. e72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufaihah A.J., Huang N.F., Kim J., Herold J., Volz K.S., Park T.S., Lee J.C., Zambidis E.T., Reijo-Pera R., Cooke J.P. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am. J. Translational Res. 2013;5:21–35. [PMC free article] [PubMed] [Google Scholar]
- Schell J.C., Rutter J. Mitochondria link metabolism and epigenetics in haematopoiesis. Nat. Cell Biol. 2017;19:589–591. doi: 10.1038/ncb3540. [DOI] [PubMed] [Google Scholar]
- Schlaeger T.M., Daheron L., Brickler T.R., Entwisle S., Chan K., Cianci A., DeVine A., Ettenger A., Fitzgerald K., Godfrey M. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 2015;33:58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M., Foldes G. It takes two: endothelial-perivascular cell cross-talk in vascular development and disease. Front. Cardiovasc. Med. 2018;5:154. doi: 10.3389/fcvm.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura D., Sone M., Homma K., Oyamada N., Takahashi K., Tamura N., Yamanaka S., Nakao K. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler. Thromb. Vasc. Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- Timmerman I., Heemskerk N., Kroon J., Schaefer A., van Rijssel J., Hoogenboezem M., van Unen J., Goedhart J., Gadella T.W., Jr., Yin T. A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1. J. Cell Sci. 2015;128:3041–3054. doi: 10.1242/jcs.168674. [DOI] [PubMed] [Google Scholar]
- Uchiyama H., Dobashi Y., Ohkouchi K., Nagasawa K. Chemical change involved in the oxidative reductive depolymerization of hyaluronic acid. J. Biol. Chem. 1990;265:7753–7759. [PubMed] [Google Scholar]
- van den Berg B.M., Spaan J.A., Rolf T.M., Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H915–H920. doi: 10.1152/ajpheart.00051.2005. [DOI] [PubMed] [Google Scholar]
- van den Berg C.W., Ritsma L., Avramut M.C., Wiersma L.E., van den Berg B.M., Leuning D.G., Lievers E., Koning M., Vanslambrouck J.M., Koster A.J. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 2018;10:751–765. doi: 10.1016/j.stemcr.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B.M., Wang G., Boels M.G.S., Avramut M.C., Jansen E., Sol W.M.P.J., Lebrin F., van Zonneveld A.J., de Koning E.J.P. Glomerular function and structural integrity depend on hyaluronan synthesis by glomerular endothelium. J. Am. Soc. Nephrol. 2019;30:1886–1897. doi: 10.1681/ASN.2019020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini N., Girotra M., Naveiras O., Nikitin G., Campos V., Giger S., Roch A., Auwerx J., Lutolf M.P. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat. Commun. 2016;7:13125. doi: 10.1038/ncomms13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A., Arnould T., Najimi M., Renard P. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 2015;24:1957–1971. doi: 10.1089/scd.2015.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S., Tarbell J.M., Damiano E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Wimmer R.A., Leopoldi A., Aichinger M., Wick N., Hantusch B., Novatchkova M., Taubenschmid J., Hammerle M., Esk C., Bagley J.A. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565:505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.W., Marsch E., Treps L., Baes M., Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36:2187–2203. doi: 10.15252/embj.201696150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Duan S., Yi F., Ocampo A., Liu G.-H., Izpisua Belmonte J.C. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Yan P., Nagasawa A., Uosaki H., Sugimoto A., Yamamizu K., Teranishi M., Matsuda H., Matsuoka S., Ikeda T., Komeda M. Cyclosporin-A potently induces highly cardiogenic progenitors from embryonic stem cells. Biochem. Biophysical Res. Commun. 2009;379:115–120. doi: 10.1016/j.bbrc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J.D., Leder P., Ornitz D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yoshioka N., Gros E., Li H.R., Kumar S., Deacon D.C., Maron C., Muotri A.R., Chi N.C., Fu X.D., Yu B.D. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell. 2013;13:246–254. doi: 10.1016/j.stem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Robotham J.L., Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Nuebel E., Wisidagama D.R., Setoguchi K., Hong J.S., Van Horn C.M., Imam S.S., Vergnes L., Malone C.S., Koehler C.M. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 2012;7:1068–1085. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chu L.F., Hou Z., Schwartz M.P., Hacker T., Vickerman V., Swanson S., Leng N., Nguyen B.K., Elwell A. Functional characterization of human pluripotent stem cell-derived arterial endothelial cells. Proc. Natl. Acad. Sci. U S A. 2017;114:e6072–e6078. doi: 10.1073/pnas.1702295114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Menzies K.J., Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145 doi: 10.1242/dev.143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.