Abstract
Most women with epithelial ovarian cancer (EOC) present with late-stage disease. As a result, globally, EOC is responsible for >150,000 deaths a year. Thus, a better understanding of risk factors for developing EOC is crucial for earlier screening and detection to improve survival. To that effort, there have been suggestions that there is an association of schizophrenia and cancer, possibly because metabolic changes are a hallmark of both cancer and schizophrenia (SZ). Perturbed choline metabolism has been documented in both diseases. Our objective was to use Mendelian randomization to evaluate whether SZ increased risk for developing EOC or the converse, and, whether SZ impacted 1- or 2-glycerophosphocholine (1- or 2-GPC) metabolites. We found that SZ conferred a weak but increased risk for EOC, but not the reverse (no evidence that EOC caused SZ). SZ was also causally associated with lower levels of two 1- or 2-GPC species and with suggestively lower levels in an additional three 1- or 2-GPCs. We postulate that perturbed choline metabolism in SZ may mimic or contribute to a “cholinic” phenotype, as observed in EOC cells.
Keywords: Schizophrenia, Ovarian cancer, Mendelian randomization, Choline, Metabolism
1. Introduction
Though epithelial ovarian cancer (EOC) is rare, across the globe, over 200,000 new cases of ovarian cancer are diagnosed yearly [1]. Because the majority of diagnoses are advanced-stage disease with poor prognosis and cancer recurrence after treatment [2,3], globally, over 150,000 women die from this cancer each year [4]. Aside from family history of breast or ovarian cancer, high body mass index in adolescence, diagnosis of endometriosis, and taller height, most established risk factors are hormonal or reproductive [1]. A recent Mendelian randomization (MR) study of 13 known observational risk factors only confirmed genetic liability to lifetime smoking and endometriosis as causal [5]. Thus, identification of additional risk factors is crucial for designing prevention strategies and for earlier diagnosis of EOC.
Because EOC and breast cancer share many similar risk factors and schizophrenia (SZ) has been reported to be a causal risk factor for breast cancer in a recent Mendelian randomization (MR) study [6], we hypothesized that SZ or something about its disease pathogenesis, perhaps metabolic, may be a risk factor for EOC. One possibility is energy metabolism, as it is important in both cancer [7] and schizophrenia (SZ) [8,9]. In particular, aberrant choline metabolism has been documented for EOC [10,11] and also noted in the brains of schizophrenics [8]. We conducted an MR study of SZ and EOC and its subtypes, and, to assess whether EOC might influence SZ, we also tested the reverse: the causal impact of EOC on SZ. As proof of principle that SZ may impact on circulating metabolites that have been associated with EOC, we performed MR of SZ on 15 circulating 1- or 2-glycerophosphocholine (1- or 2-GPC) metabolites.
2. Materials and methods
2.1. Conceptual framework
MR is an instrumental-variables technique that exploits the random assortment of alleles, using, most commonly, single-nucleotide polymorphisms (SNPs) as instruments (“proxies”) [12,13]. With summary results from genome-wide association studies (GWASs), we used two-sample MR to test the causal relationships between 1) EOC and SZ and 2) SZ and 1- or 2-GPC species. We presumed the following assumptions: the chosen instrumental variables (SNPs) are strongly associated with exposures (assumption 1); the instrumental variables are not associated with confounders (assumption 2); and the instrumental variables are only associated with the outcomes through the exposures (assumption 3) [14].
2.2. Data sources
Two-sample MR uses the summary results from two separate GWASs as its data sources: a GWAS of the exposure and a GWAS of the outcome.
2.2.1. SZ on EOC and EOC subtypes
-
1)
We selected a GWAS of SZ [15]; Ripke et al. (2014) meta-analyzed 49 case-control studies of SZ along with 1235 parent affected-offspring trios (total study size = 35,476 SZ cases and 46,839 controls). From their GWAS results, we extracted the summary statistics for SNPs strongly associated with SZ (p-value <5e-8).
-
2)
Next, we identified the largest GWAS of EOC and its subtypes [16]; Phelan et al. (2017) meta-analyzed GWAS from six Ovarian Cancer Association Consortium (OCAC) and two Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) projects (total study size = 25,509 cases and 40,941 controls of European ancestry) for women who had been genotyped using the Illumina Custom Infinium array (OncoArray). We extracted summary data for the SZ-associated SNPs from within the GWAS of overall EOC (n = 25,509), high-grade serous carcinoma (n = 13,037), mucinous carcinoma (n = 2566), endometrioid carcinoma (n = 2810), and clear-cell carcinoma (n = 1366).
2.2.2. EOC on SZ (reverse direction)
For the MR of EOC on SZ, we selected SNPs strongly associated with EOC (p-value <5e-8) from the Phelan et al. [16] GWAS of EOC.
We extracted the summary data for the EOC-associated SNPs from the Ripke et al. [15] GWAS of SZ.
2.2.3. SZ on 15 1- or 2-GPC species
For the MR of SZ on 1- or 2-GPC species, we extracted SZ-associated SNPs (p-value <5e-8) from Ripke et al. [15].
We then selected GWASs of circulating metabolites in, depending on the metabolite, up to 7824 adult individuals from two European population studies (TwinsUK and the Cooperative Health Research in the Region of Augsburg (KORA) study) performed by Shin et al. (2014). We extracted summary statistics for SZ-associated SNPs for 15 1- or 2-GPC metabolites: 1-arachidonoylglycerophosphocholine, 1-palmitoleoylglycerophosphocholine, 1-eicosatrienoylglycerophosphocholine, 1-docosahexaenoylglycerophosphocholine, 1-eicosadienoylglycerophosphocholine, 1-palmitoylglycerophosphocholine, 1-heptadecanoylglycerophosphocholine, 1-oleoylglycerophosphocholine, 1-stearoylglycerophosphocholine, 1-linoleoylglycerophosphocholine, 2-palmitoylglycerophosphocholine, 2-oleoylglycerophosphocholine, 2-stearoylglycerophosphocholine, 2-linoleoylglycerophosphocholine, 1-myristoylglycerophosphocholine [17].
2.3. Statistical procedure
2.3.1. SZ on EOC and EOC subtypes
To implement MR for the tests of SZ on overall EOC and SZ on EOC subtypes, we used the summary statistics for SZ-associated SNPs identified in and extracted from Ripke et al. [15] and the summary statistics for the Ripke-SZ-associated SNPs extracted from Phelan et al. [16] to calculate the odds ratio (OR) for overall EOC and for each subtype per unit log odds higher liability to SZ for each SNP instrument (Wald ratio) and conducted a meta-analysis of the Wald ratios in a combined instrument with the inverse-weighted variance (IVW) MR method [18].
2.3.2. EOC on SZ
To implement MR for the test of EOC on SZ, we used the summary statistics for EOC-associated SNPs identified in and extracted from Phelan et al. [16] and the summary statistics for the Phelan-EOC-associated SNPs extracted from Ripke et al. [15] to calculate the odds ratio (OR) for SZ per unit log odds higher liability to EOC for each SNP instrument (Wald ratio) and meta-analyzed the results with the IVW method. The MR of SZ on EOC and the reverse MR of EOC on SZ comprise a bidirectional analysis. Because the validity of bidirectional MR methods depends on the independence of the two instruments [12], we checked that there was no overlap or linkage disequilibrium (LD) between the genetic variants included in the instruments for SZ and EOC. We used LDassoc, a free and publicly available web tool, to assess whether instruments were in LD [19].
2.3.3. SZ on 15 1- or 2-GPC species
To implement MR for the tests of SZ on 15 1- OR 2-GPC metabolites, we used the summary statistics for the SZ-associated SNPs identified in and extracted from Ripke et al. [15] and Ripke-SZ-associated SNPs extracted from the Shin et al. [17] GWAS of circulating 1- or 2-GPC metabolites. We calculated the log10 increase in 1- or 2-GPC metabolite per unit log odds higher liability to SZ for each SNP proxy and meta-analyzed the Wald ratios with the IVW method. We performed 15 meta-analyses, one for each of the 15 1- or 2-GPC metabolites. To account for multiple testing, we set the P-value threshold for the analyses of SZ on 1- or 2-GPCs to the Bonferroni-corrected value of 0.0033 (P = .05/15).
2.4. Two-sample MR
For all instruments, where the exposure SNPs were not available in the outcome data, we identified LD proxy SNPs in linkage disequilibrium (R2 > 0.8 and within 250 kb of the target SNP). We implemented all two-sample MR tests in R version 3.5.1 using the “TwoSampleMR” package [20]. All data are publicly available and accessible through the MR-Base website, a curated database of complete GWASs and platform for performing two-sample MR [21].
2.5. Sensitivity analyses
To detect possible violations of the third MR assumption (assumption 3 above), we performed various sensitivity analyses. We applied IVW radial regression for each MR test. IVW radial regression uses the P-value from Cochran's Q-statistic (P[het]) to identify possible heterogeneity among an instrument's ratio estimates [22]. Heterogeneity can indicate pleiotropy. For all analyses, we removed heterogenous SNPs and report the results of the MR analysis with them removed, along with IVW radial regression performed again after their removal to demonstrate either absence of evidence for heterogeneity or continued evidence for it. The tables of results include the number of SNPs included in each analysis. We also implemented MR-Egger regression, weighted median, and weighted mode estimators, which make different assumptions about the underlying nature of potential pleiotropy. Briefly, if the MR-Egger, weighted median, and weighted mode estimators have the same direction and comparable magnitude of effects as the IVW estimate, this strengthens confidence in the validity of the instruments.
3. Results
3.1. SZ and EOC
SZ was associated with overall EOC (IVW OR per log odds higher liability to SZ: 1.09, 95% CI: 1.04, 1.14; P = .0004) and high-grade serous ovarian carcinoma (IVW OR 1.07, 95% CI: 1.01, 1.13; P = .033). The comparisons of the MR estimators for both reveal little evidence for pleiotropy: the direction of the effect was consistent across methods and the effects were of relatively the same magnitude for the IVW, weighted median, and weighted mode estimators (Table 1; Fig. 1). IVW radial regression indicated no evidence of heterogeneity (overall EOC: P[het] = 0.5238; high-grade serous: P[het] = 0.4254).
Table 1.
MR of SZ on overall EOC and four ovarian cancer subtypes.
| Method | N |
Lower |
Upper |
P-Value | ||
|---|---|---|---|---|---|---|
| SNPs | OR | 95% CI | 95% CI | |||
| Overall EOC | Inverse variance weighted | 66 | 1.09 | 1.04 | 1.14 | 0.0004 |
| MR Egger | 66 | 1.18 | 1.00 | 1.39 | 0.0600 | |
| MR Egger intercept | 66 | 0.99 | 0.98 | 1.01 | 0.3345 | |
| Weighted median | 66 | 1.10 | 1.03 | 1.18 | 0.0077 | |
| Weighted mode | 66 | 1.08 | 0.91 | 1.27 | 0.3778 | |
| High grade serous | Inverse variance weighted | 62 | 1.07 | 1.01 | 1.13 | 0.0333 |
| MR Egger | 62 | 1.08 | 0.85 | 1.35 | 0.5386 | |
| MR Egger intercept | 62 | 1.00 | 0.98 | 1.02 | 0.9344 | |
| Weighted median | 62 | 1.05 | 0.97 | 1.14 | 0.2171 | |
| Weighted mode | 62 | 1.06 | 0.86 | 1.30 | 0.5857 | |
| Mucinous | Inverse variance weighted | 65 | 1.09 | 0.98 | 1.21 | 0.1258 |
| MR Egger regression | 65 | 1.22 | 0.84 | 1.77 | 0.3027 | |
| MR Egger intercept | 65 | 0.99 | 0.96 | 1.02 | 0.5282 | |
| Weighted median | 65 | 1.17 | 1.00 | 1.36 | 0.0510 | |
| Weighted mode | 65 | 1.36 | 0.97 | 1.91 | 0.0797 | |
| Endometriod | Inverse variance weighted | 64 | 1.10 | 0.99 | 1.22 | 0.0804 |
| MR Egger regression | 64 | 1.33 | 0.88 | 1.99 | 0.1778 | |
| MR Egger intercept | 64 | 0.98 | 0.95 | 1.02 | 0.3478 | |
| Weighted median | 64 | 1.14 | 0.99 | 1.32 | 0.0753 | |
| Weighted mode | 64 | 1.23 | 0.87 | 1.74 | 0.2503 | |
| Clear cell | Inverse variance weighted | 65 | 1.06 | 0.92 | 1.22 | 0.4227 |
| MR Egger | 65 | 1.18 | 0.67 | 2.07 | 0.5622 | |
| MR Egger intercept | 65 | 0.99 | 0.95 | 1.04 | 0.6968 | |
| Weighted median | 65 | 1.15 | 0.94 | 1.40 | 0.1780 | |
| Weighted mode | 65 | 1.21 | 0.77 | 1.91 | 0.4083 | |
Fig. 1.
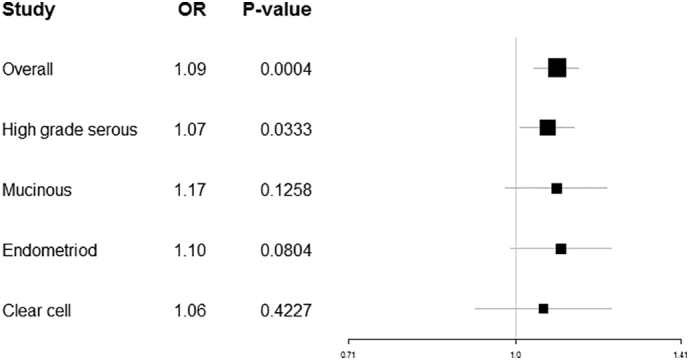
MR results of genetic liability to SZ on overall EOC and four EOC subtypes.
Null, increased risks were observed for the remaining subtypes, and IVW radial regression revealed no evidence for heterogeneity: mucinous carcinoma (IVW OR 1.09; 95% CI 0.98, 1.21; P = .1258; P[het] = 0.7236); endometrioid carcinoma (OR 1.10; 95% CI 0.99, 1.22; P = .0804; P[het] = 0.9568); and clear-cell carcinoma (OR 1.06; 95% CI 0.92, 1.22; P = .4227; P[het] = 0.8990). The sensitivity estimators were relatively consistent for all tests (same direction of effect and comparable magnitudes of effect).
3.2. EOC on SZ
Genetic liability to EOC did not causally contribute to risk for SZ (Table 2).
Table 2.
Results of MR of EOC on SZ.
| Method | N |
Lower |
Upper |
P-Value | ||
|---|---|---|---|---|---|---|
| SNPs | OR | 95% CI | 95% CI | |||
| Overall EOC | Inverse variance weighted | 9 | 0.95 | 0.87 | 1.04 | 0.2586 |
| MR Egger | 9 | 0.97 | 0.77 | 1.22 | 0.8220 | |
| MR Egger intercept | 9 | 0.99 | 0.98 | 1.01 | 0.3345 | |
| Weighted median | 9 | 0.94 | 0.86 | 1.03 | 0.2075 | |
| Weighted mode | 9 | 0.95 | 0.84 | 1.06 | 0.3791 | |
3.3. SZ on 1- or 2-GPC species
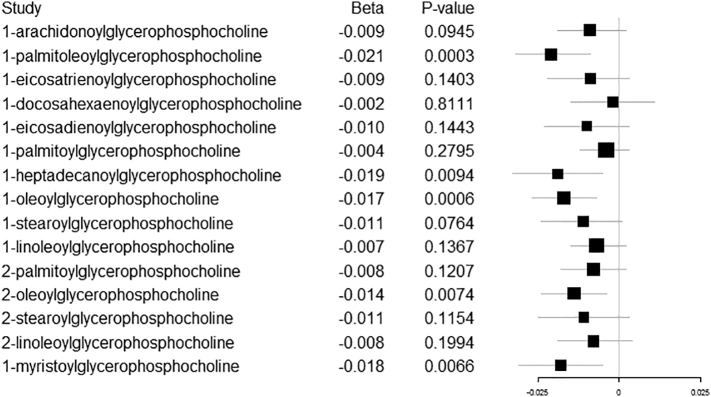
Two of the 15 tests of SZ on circulating 1- or 2-GPC species showed evidence for causal association and 3 had suggestive associations (Table 3). SZ was associated with 1-palmitoleoylglycerophosphocholine (IVW beta log10 increase per log odds higher liability to SZ: -0.021, 95% CI: −0.032, −0.009; P = .0003; P[het] = 0.7337). Similarly, SZ was associated with 1-oleoylglycerophosphocholine (IVW beta: -0.017, 95% CI: −0.027, −0.007; P = .0006; P[het] = 0.8507). Suggestive associations were observed for 1-heptadecanoylglycerophosphocholine (IVW beta: -0.019, 95% CI: −0.033, −0.005; P = .0066; P[het] = 0.8664); 2-oleoylglycerophosphocholine (IVW beta: -0.014, 95% CI: −0.024, −0.004; P = .0074; P[het] = 0.8664); and1-myristoylglycerophosphocholine (IVW beta: -0.018, 95% CI: −0.031, −0.005; P = .0066; P[het] = 0.9419). The comparison of the MR estimators showed little evidence for pleiotropy for the 1- or 2-GPC metabolites after Bonferroni correction. The suggestively associated 1- or 2-GPC metabolites were also robust to the MR sensitivity analyses, except for 1-heptadecanoylglycerophosphocholine for which there was some discrepancy in the direction of effects for the MR estimators, indicating potential pleiotropy. For all the 1- or 2-GPCs, including the 10 that had null associations, SZ (Fig. 2) lowered the 1- or 2-GPCs.
Table 3.
Results of MR of SZ on circulating glycerophosphocholines (1- or 2-GPCs).
| Method | N |
Lower |
Upper |
P-Value | |||
|---|---|---|---|---|---|---|---|
| SNPs | Beta | Std. Error | 95% CI | 95% CI | |||
| 1-palmitoleoylglycerophosphocholine | Inverse variance weighted | 60 | -0.021 | 0.006 | −0.032 | −0.009 | 0.0003 |
| MR Egger regression | 60 | −0.032 | 0.026 | −0.082 | 0.019 | 0.2275 | |
| MR Egger intercept | 60 | 0.001 | 0.002 | −0.003 | 0.005 | 0.6696 | |
| Weighted mode | 60 | −0.049 | 0.020 | −0.088 | −0.009 | 0.0188 | |
| Weighted median | 60 | −0.028 | 0.009 | −0.045 | −0.011 | 0.0014 | |
| 1-heptadecanoylglycerophosphocholine | Inverse variance weighted | 58 | −0.019 | 0.007 | −0.033 | −0.005 | 0.0094 |
| MR Egger regression | 58 | 0.030 | 0.035 | −0.037 | 0.098 | 0.3836 | |
| MR Egger intercept | 58 | −0.004 | 0.003 | −0.009 | 0.001 | 0.1516 | |
| Weighted mode | 58 | 0.001 | 0.026 | −0.050 | 0.052 | 0.9675 | |
| Weighted median | 58 | −0.012 | 0.010 | −0.033 | 0.008 | 0.2298 | |
| 1-oleoylglycerophosphocholine | Inverse variance weighted | 56 | −0.017 | 0.005 | −0.027 | −0.007 | 0.0006 |
| MR Egger regression | 56 | −0.030 | 0.022 | −0.073 | 0.012 | 0.1694 | |
| MR Egger intercept | 56 | 0.001 | 0.002 | −0.002 | 0.004 | 0.5295 | |
| Weighted mode | 56 | −0.023 | 0.015 | −0.052 | 0.006 | 0.1314 | |
| Weighted median | 56 | −0.019 | 0.007 | −0.032 | −0.005 | 0.0076 | |
| 2-oleoylglycerophosphocholine | Inverse variance weighted | 57 | −0.014 | 0.005 | −0.024 | −0.004 | 0.0074 |
| MR Egger regression | 57 | −0.016 | 0.024 | −0.063 | 0.031 | 0.5086 | |
| MR Egger intercept | 57 | 0.000 | 0.002 | −0.003 | 0.004 | 0.9233 | |
| Weighted mode | 57 | −0.006 | 0.015 | −0.036 | 0.024 | 0.7033 | |
| Weighted median | 57 | −0.011 | 0.007 | −0.026 | 0.003 | 0.1157 | |
| 1-myristoylglycerophosphocholine | Inverse variance weighted | 59 | −0.018 | 0.007 | −0.031 | −0.005 | 0.0066 |
| MR Egger regression | 59 | −0.012 | 0.030 | −0.071 | 0.047 | 0.6953 | |
| MR Egger intercept | 59 | 0.000 | 0.002 | −0.005 | 0.004 | 0.8347 | |
| Weighted mode | 59 | −0.024 | 0.021 | −0.066 | 0.018 | 0.2648 | |
| Weighted median | 59 | −0.020 | 0.009 | −0.039 | −0.002 | 0.0332 | |
Fig. 2.
MR results of genetic liability to SZ on 15 1- or 2-GPCs.
The results for the IVW estimates of individual SNPs for all models and the table with the complete list of findings for the MR of SZ on the 15 1- or 2-GPC metabolites, including the null associations, are available in the Supplement.
4. Discussion
As the prevalence of SZ ranges from 4 to 7 per 1000 persons worldwide [23], both it and EOC are rare enough that there are few epidemiologic studies examining SZ as a risk factor for EOC. Those that have examined the influence of SZ on EOC have observed mixed findings. Comparing the incidence rate ratio (IRR) of EOC among those with and without SZ, a null but decreased risk for EOC has been reported (IRR 0.98; 95% confidence interval (CI) not specifically provided, but included 1) [24]. A large retrospective study in Sweden of 59,233 patients with SZ reported a null but increased risk for EOC (standardized incidence ratio, SIR, 1.13; 95% CI 0.95, −1.34) among schizophrenics after diagnosis, but a decreased risk for EOC prior to diagnosis (SIR 0.48; 95% CI 0.35, −0.65) [25]. These previous reported associations between SZ and EOC could reflect residual confounding, reverse causation, or measurement error.
A major strength of our investigation is that we leveraged large GWAS to examine these two rare diseases. Our approach optimized power to find associations. A weakness of our study is that we were not able to assess the strength of our MR instruments (F-statistics), due to missing effect-allele frequencies in the Ripke et al. [15] GWAS. However, this is balanced out by the advantage of the two-sample MR design: weak instruments bias causal associations towards the null in two-sample MR. Thus, if our SZ instruments were weak, we likely would not have observed the causal associations we did, as our findings would have been attenuated to the null. Although the MR of SZ on overall EOC was statistically well powered, our analysis of SZ on the EOC subtypes may have been unpowered due to the small number of cases. Thus, we cannot rule out the possibility that SZ is associated with mucinous, clear cell, and/or endometrioid carcinomas, as it was with overall EOC and high-grade serous carcinoma, the most common of the subtypes.
Aberrant choline metabolism is a feature of malignant transformation in breast and ovarian cancer cell lines, where it is marked by a change from low phosphocholine (PCho) and high 1- or 2-GPC levels to high PCho and low 1- or 2-GPC levels [10,26,27]. Aberrant choline metabolism has also been noted clinically for breast cancer patients, with higher 1- or 2-GPC levels being implicated in better prognosis [28]. In studies of schizophrenics, high choline concentrations have been observed in patients prior to antipsychotic treatment [29], pointing to perturbation of choline metabolism being independent from antipsychotic treatment and not a result of it. Moreover, GPCs, specifically, have been observed to be statistically lower in patients compared to controls in certain brain regions (antipsychotic use did not appear to be the cause) [30]. Here, we observed that SZ was associated with lower levels of several circulating 1- or 2-GPC metabolites and that SZ conferred a weak but notable increased risk for EOC.
Regarding SZ conferring an increased risk for EOC, we considered the possibility that our findings were influenced by psychoses secondary to anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis, which is associated with underlying ovarian teratomas [31]. In order for this to have induced bias into the present study, the original GWAS of SZ would need to have suffered from substantial misclassification bias; i.e., mistaking cases of anti-NMDAR psychosis for SZ. Since ovarian teratomas are rare and affect only women, it is unlikely the Ripke et al. [15] GWAS contained enough instances of this error for their associations to have been remarkably influenced. Thus, the results of the present study are unlikely to be explained by this phenomenon.
Ideally, we would have tested whether these SZ-associated 1- or 2-GPC metabolites caused EOC, but we did not have appropriate genetic instruments for this purpose. Instead, we postulate that disturbed choline metabolism may explain the causal association between SZ and EOC. It is also possible that something else may underlie the connection between the two conditions. For instance, Brown (2016) proposed that SZ may involve cell transformation, similar to that of early stage cancer initiation or attenuated tumorigenesis [32] – but without taking on a complete tumor phenotype. This is a provocative hypothesis that fits with our observations but may also, if true, reflect other early features of cancer, perhaps immune rather than metabolic. Another possibility is that changes in choline metabolism in SZ perturb the one‑carbon cycle. While free choline is primarily used in phospholipid synthesis, excess choline serves as a source of one‑carbon units for methylation; choline can directly re-methylate homocysteine to contribute to methionine formation and homeostasis [33]. Thus, perhaps SZ-induced aberrant choline metabolism mimics the “cholinic phenotype” observed in EOC and/or contributes to it, maybe via perturbation of the one‑carbon cycle.
Minimally, our results highlight the possibility that something about SZ imparts risk for developing EOC. Understanding this risk has implications for the biology of both diseases and could aid efforts at earlier detection of EOC, especially if the biological process perturbed in the two conditions also occurs in the absence of SZ. Investigating alterations in choline metabolism is a starting point to focus future investigation.
Author contributions
Dr. Adams conducted the analyses and wrote the manuscript. Dr. Neuhausen edited the manuscript. Both authors interpreted the results and approved the final manuscript.
Declaration of Competing interest
The authors declare no conflicts of interest.
Acknowledgements
We have no acknowledgements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2019.100539.
Appendix A. Supplementary data
Supplementary material
References
- 1.Mallen A.R., Townsend M.K., Tworoger S.S. Risk factors for ovarian carcinoma. Hematol. Oncol. Clin. N. Am. 2018;32:891–902. doi: 10.1016/j.hoc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Parkin D.M., Bray F., Ferlay J., Pisani P. Global Cancer statistics, 2002. CA Cancer J. Clin. 2002;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Bristow R.E., Puri I., Chi D.S. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol. Oncol. 2009;112(1):265–274. doi: 10.1016/j.ygyno.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global Cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Yarmolinsky J. Evaluating causal associations between previously reported risk factors and epithelial ovarian cancer: a Mendelian randomization analysis. bioRxiv. 2018 [Google Scholar]
- 6.Byrne E.M. Is schizophrenia a risk factor for breast cancer?—evidence from genetic data. Schizophr. Bull. 2018:6–11. doi: 10.1093/schbul/sby162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Khaitovich P. Metabolic changes in schizophrenia and human brain evolution. Genome Biol. 2008;9(8):1–11. doi: 10.1186/gb-2008-9-8-r124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuccoli G.S., Saia-cereda V.M., Nascimento J.M. The energy metabolism dysfunction in psychiatric disorders postmortem brains: focus on proteomic evidence. Front. Neurosci. 2017;11(September):1–14. doi: 10.3389/fnins.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iorio E. Alterations of choline phospholipid metabolism in ovarian tumor progression. Cancer Res. 2005;65(20):9369–9376. doi: 10.1158/0008-5472.CAN-05-1146. [DOI] [PubMed] [Google Scholar]
- 11.Bagnoli M. Choline metabolism alteration: A focus on ovarian cancer. Front. Oncol. 2016;6(June):4–10. doi: 10.3389/fonc.2016.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey Smith G., Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 14.Didelez V., Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 2007;16(4):309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- 15.Ripke S. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelan C., Kuchenbaecker K., Tyrer J., Kar S., Lawrenson K., Winham S. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017;49(5) doi: 10.1038/ng.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S.Y. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014;46(6):543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden J., Smith G.D., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int. J. Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machiela M.J., Chanock S.J. LDassoc: an online tool for interactively exploring genome-wide association study results and prioritizing variants for functional investigation. Bioinformatics. 2018;34(5):887–889. doi: 10.1093/bioinformatics/btx561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemani G. MR-base: A platform for systematic causal inference across the phenome using billions of genetic associations. bioRxiv. 2016:1–31. [Google Scholar]
- 21.Hemani G. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:1–29. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowden J. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int. J. Epidemiol. 2018;(August):1–15. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha S., Chant D., Welham J., Mcgrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5) doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton S.O. Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994-2003: summary of findings. Eur. J. Cancer. 2008;44(14):2074–2085. doi: 10.1016/j.ejca.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Ji J., Sundquist K., Ning Y., Kendler K.S., Sundquist J., Chen X. Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophr. Bull. 2013;39(3):527–536. doi: 10.1093/schbul/sbs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aboagye E.O., Bhujwalla Z.M. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–84. [PubMed] [Google Scholar]
- 27.Ackerstaff E., Glunde K., Bhujwalla Z.M. Choline phospholipid metabolism: a target in cancer cells? J. Cell. Biochem. 2003;90:525–533. doi: 10.1002/jcb.10659. [DOI] [PubMed] [Google Scholar]
- 28.Moestue S.A., Giskeodegard G.F., Cao M.D., Bathen T.F., Gribbestad I.S. Glycerophosphocholine (GPC) is a poorly understood biomarker in breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2012;109(38) doi: 10.1073/pnas.1208226109. E2506–E2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowland L.M. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am. J. Psychiatry. 2002;(January):130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- 30.Weber-fahr W. Altered phospholipid metabolism in schizophrenia: a phosphorus 31 nuclear magnetic resonance spectroscopy study. Psychiatry Res. 2013;214(3):365–373. doi: 10.1016/j.pscychresns.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Clark R.M., Lynch M.P., Kolp R., Zukerberg L.R., Growdon W.B., Rueda B.R. The N-methyl-D-aspartate receptor, a precursor to N-methyl-D-aspartate receptor encephalitis, is found in the squamous tissue of ovarian teratomas. Int. J. Gynecol. Pathol. 2014;33(6):598–606. doi: 10.1097/PGP.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 32.Brown J.S. Cancer immune equilibrium and schizophrenia have similar interferon-γ, tumor necrosis factor-α, and interleukin expression: a tumor model of schizophrenia. Schizophr. Bull. 2016;42(6):1407–1417. doi: 10.1093/schbul/sbw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzo A., Napoli A., Roggiani F., Tomassetti A., Bagnoli M., Mezzanzanica D. One-carbon metabolism: biological players in epithelial ovarian cancer. Int. J. Mol. Sci. 2018;19(7):2092. doi: 10.3390/ijms19072092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material