Abstract
MPT64 is a specific protein that is secreted by Mycobacterium tuberculosis complex (MTBC). The objective of this study was to obtain optimum culture conditions for MPT64 synthetic gene expression in Escherichia coli BL21 (DE3) by response surface methodology (RSM). The RSM was undertaken to optimize the culture conditions under different cultivation conditions (medium concentration, induction time and inducer concentration), designed by the factorial Box-Bhenken using Minitab 17 statistical software. From the randomized combination, 15 treatments and three center point repetitions were obtained. Furthermore, expression methods were carried out in the flask scale fermentation in accordance with the predetermined design. Then, the MPT64 protein in the cytoplasm of E. coli cell was isolated and characterized using sodium dodecyl sulfate polyacrilamide electrophoresis (SDS-PAGE) then quantified using the ImageJ program. The optimum conditions were two-fold medium concentration (tryptone 20 mg/mL, yeast extract 10 mg/mL, and sodium chloride 20 mg/mL), 5 h of induction time and 4 mM rhamnose. The average concentration of recombinant MPT64 at optimum conditions was 0.0392 mg/mL, higher than the predicted concentration of 0.0311 mg/mL. In conclusion, the relationship between the selected optimization parameters strongly influenced the level of MPT64 gene expression in E. coli BL21 (DE3).
Keywords: Environmental science, Microbiology, MPT64, Response surface methodology, Box-Behnken design, Rhamnose, Medium, Induction
Environmental science; Microbiology; MPT64; Response surface methodology; Box-Behnken design; Rhamnose; Medium; Induction
1. Introduction
MPT64 is one of the major secreted proteins (24 kDa) from Mycobacterium tuberculosis shown to be a specific protein that distinguishes the Mycobacterium tuberculosis complex (MTBC) from mycobacteria other than M. tuberculosis (MOTT) (M. chelonae, M. avium, M. intracellularae, M. flavescens, M. pregrinum, M. vaccae, M. aurum, M. mucogenicum, and M. xenopi) that cause a similar disease (Hasegawa et al., 2002; Abe et al., 1999; Toihir et al., 2011). Both groups of mycobacteria show signs and symptoms of pulmonary infection that often resemble tuberculosis (Falkinham, 1996; Hillemann et al., 2005). Thus, the identification of both groups of mycobacteria become crucial to provide effective diagnosis and treatment of the affected individuals, and also to prevent transmission to other healthy individuals. The prevention of tuberculosis transmission has an important role in decreasing tuberculosis patients in worldwide because of the ease of tuberculosis transmission. In 98% of tuberculosis cases, M. tuberculosis is transmitted through the air when the affected person coughs. Eventhough the immune system of some individuals is reported to be able to rid the infection without treatment. But, in others, M. tuberculosis can replicate inside the macrophages for several weeks by subverting the alveolar macrophages’ efforts in its degradation (Cruz and Starke, 2014). The study of BCG-vaccinated newborns reported that only 50% have a positive tuberculin skin test (TST) result, and 80–90% lose reactivity within 5 years (Ashley and Siebenmann, 1967). Of course, it is also affected by BCG product and nutritional status (Dunn et al., 2016).
The lack of adequate detection tools, coupled with the disease severity, makes rapid diagnosis for M. tuberculosis essential. Researchers have been concerned with the development of a new diagnostic using antibodies that can quickly and accurately diagnose the causative agent of infection, such as the immunochromatography method for detection of tuberculosis, Chikungunya virus, Adenovirus, and Mycoplasma pneumoniae (Toihir et al., 2011; Jain et al., 2018; Tsutsumi et al., 1999; Namkoong et al., 2018). Thus, the MPT64 antibodies may act as a valuable tool in diagnostic screening of suspected patients. Antibodies are highly useful for diagnostic applications due to their specificity and affinity towards desired antigens. MPT64 antibody production requires large amounts of MPT64 protein as the antigen. Thus, in this study, the culture conditions of Escherichia coli BL21 (DE3) containing MPT64 synthetic gene, constructed in an expression vector that is regulated by a rhamnose-inducible promoter, were optimized to obtain large amounts of MPT64 protein.
E. coli has been studied as a highly successful host cell system for the production of a variety of heterologous proteins owing to its rapid growth on inexpensive substrates and ease of scale up. Generally, the presence of an expression vector in the host cell may cause a metabolic burden, which may lead to plasmid instability and reduce specific growth rate (DeLisa et al., 2001). Therefore, gaining optimum culture conditions to facilitate overproduction of recombinant proteins is very important. Several culture condition parameters such as growth medium concentration, induction time and inducer concentration, could affect E. coli growth. The chemical and nutritional components of the growth medium can directly affect the host cell growth during the production of target proteins (Zhang and Greasham, 1999). The carbon source type and its amount in culture medium is important for microorganism biosynthetic pathways to gain production of the recombinant protein. However, acetate and other acidic byproducts can be accumulated during batch fed cultivation. It can diminish the cell growth as well as the production of recombinant proteins (Turner et al., 1994). Medium supplementation with yeast extract and tryptone have been reported to decrease acetate accumulation (Korz et al., 1995). In addition, yeast extract is a goodsource of trace components and can relieve the responses of cellular stress such as proteases production during synthesis of recombinant proteins (Lim et al., 2000). The optical density of the host cell is related to the biomass level of the host cell, thus the optimal induction time to overexpress target protein must be determined (Shojaosadati et al., 2008). The inducer, in an appropriate concentration, is needed to regulate the transcription of the target gene (Donovan et al., 1996). Thus, those parameters must be optimized to obtain an optimum yield of MPT64. But, varying single factors at a time to achieve an apparent optimum point to optimize the overexpression conditions are labor intensive that cannot explain the interactions between the different factors involved, thus it cannot identify the true optimal conditions for protein overexpression (Morowvat et al., 2015). Several studies have been done to adopt a statistical methodology to optimize gene expression in host cells. In another study, a Response Surface Methodology (RSM) based on a Box-Behnken design (BBD) was used during protein production from E. coli BL21-SI to reach the maximum production of the protein target (Ferreira et al., 2007; Maldonado et al., 2007). Response surface methodology (RSM) is a kind of analysis process in which certain factors are selected to obtain the desired response and it has been widely used in recent years (Pournejati et al., 2014). RSM can determine the effects of various independent factors on a dependent factor (Zhang et al., 2010). Therefore, in order to achieve the highest efficiency and production of recombinant MPT64 protein in E. coli, the culture conditions of E. coli were optimized using Box–Behnken design with response surface methodology (RSM).
2. Materials and methods
2.1. Materials
The materials used in this study were agar bacto (Oxoid), agarose (Sigma-Aldrich), ammonium persulfate (Bio Basic INC), aquades, glacial acetic acid, bis-acrylamide, Coomassie brilliant blue R-250, ethanol, glycerol, kanamycin Sulfate (Sigma-Aldrich), Unstained Protein MW Marker, L-rhamnosa (Sigma-Aldrich), sodium chloride (Brand), sodium dodesyl sulphate (Brand), tris (hydroxymethyl) aminomethane (Brand), TEMED, glycine, tryptone (1'st Base), urea (Brand), and yeast extract (1'st Base).
2.2. Bacterial strain and vector system
Escherichia coli BL21 (DE3) (Invitrogen, CA, USA) harboring a recombinant plasmid was used as host cell for production of the MPT64 protein. A synthetic construct encoding MPT64 was inserted into the EcoRI and SapI cloning sites (ATUM, California, USA). The plasmid contains the strong inducible T7 promoter under the control of rhaBAD promotor ((PrhaBAD) sequence and a kanamycin resistance cassette. The recombinant plasmid was transformed into E. coli BL21 (DE3) using the electophoration method. The transformant was selected using Luria Bertani (LB) agar plates containing 25 μg/mL of kanamycin and the selected transformed bacteria were cultivated in LB broth medium for 18 h. The bacterial suspension was then stored in 20% (v/v) glycerol at −80 °C for long-term usage.
2.3. Cell growth of E. coli BL21 (DE3) under different medium concentration
The rate of transformant cell growth was monitored using a spectrophotometer at 600 nm. The purpose of this method was to determine the growth phase of E. coli BL21 (DE3) transformant which correlated with the determination of effective induction time. Optical density measurement (OD) is one of the most commonly used techniques in microbiology to determine microbial growth in the culture media at certain times. The cell density was measured based on the assumption that the obtained OD values were proportional to the cell number (N) or cell concentration (C), according to the law of Lambert Beer (Stevenson et al., 2016). In this study, the OD measurements of bacterial culture were carried out using the eppendorf biophotometer plus. A cloudy cell suspension will absorb and scatter light. The higher the cell concentration exhibited the higher the turbidity level. The experiment was carried out by inoculating the tranformant from the glycerol stocks into a volume of 5 mL LB broth medium containing 30 μL kanamycin (25 μg/mL) under different medium concentrations of 1, 1.5 and 2- fold. The medium was then incubated overnight at 37 °C with shaking at 180 rpm. To start the cultivation, 1 mL of transformant preculture was pipetted and suspended in 99 ml of LB broth medium containing 494 μL kanamycin (25 μg/mL), followed by incubation at 37 °C with shaking at 180 rpm. Samples of tranformant cultures were taken in situ every 1 h to monitor cell growth by measuring the optical density (OD) at 600 nm (OD600).
2.4. Determination of optimum culture conditions
Determination of optimum culture conditions for the expression of recombinant MPT64 protein was carried out using the factorial RSM Box-Bhenken design level -1, 0, +1 using Minitab 17 statistical software. Each experiment was performed under different conditions of medium concentration, induction time after inoculating the preculture and inducer concentration as described in experimental design (Table 1). From the randomized combination, 15 treatments and three center point repetitions were obtained. Furthermore, expression methods were carried out in the flask scale fermentation in accordance with the predetermined design. The experimental design is shown in Table 2.
Table 1.
Experimental range of the three variables studied using box-behnken design in terms of actual and coded factors.
| Component | Level |
||
|---|---|---|---|
| -1 | 0 | +1 | |
| Rhamnosa concentration (mM) | 0.025 | 2.0125 | 4 |
| Induction time (h) | 2 | 3.5 | 5 |
| Medium concentration (-fold) | 2 | 1.5 | 1 |
Table 2.
Box-Behnken design with the independent variable values.
| Experiment | Box Bhenken Design | Rhamnosa concentration (mM) | Induction time (h) | Medium concentration (-fold) | ||
|---|---|---|---|---|---|---|
| 1 | 0 | -1 | -1 | 2.0125 | 2.0 | 1.00 |
| 2 | 0 | 0 | 0 | 2.0125 | 3.5 | 1.50 |
| 3 | 0 | +1 | -1 | 2.0125 | 5.0 | 1.00 |
| 4 | 0 | 0 | 0 | 2.0125 | 3.5 | 1.50 |
| 5 | -1 | +1 | 0 | 0.0250 | 5.0 | 1.50 |
| 6 | +1 | +1 | 0 | 4.0000 | 5.0 | 1.50 |
| 7 | +1 | -1 | 0 | 4.0000 | 2.0 | 1.50 |
| 8 | +1 | 0 | +1 | 4.0000 | 3.5 | 2.00 |
| 9 | +1 | 0 | -1 | 4.0000 | 3.5 | 1.00 |
| 10 | 0 | -1 | +1 | 2.0125 | 2.0 | 2.00 |
| 11 | -1 | 0 | +1 | 0.0250 | 3.5 | 2.00 |
| 12 | 0 | 0 | 0 | 2.0125 | 3.5 | 1.50 |
| 13 | -1 | 0 | -1 | 0.0250 | 3.5 | 1.00 |
| 14 | 0 | +1 | +1 | 2.0125 | 5.0 | 2.00 |
| 15 | -1 | -1 | 0 | 0.0250 | 2.0 | 1.50 |
2.5. Overexpression and isolation of MPT64 protein
Recombinant cells were precultured by inoculating a loopfull of the positive transformant into 5 mL of LB broth medium containing 30 μL kanamycin (25 μg/mL) and incubated at 37 °C for 18 h with 180 rpm shaking. After that, 1000 μL of the incubated cells were inoculated in a flask containing 99 mL of LB broth medium and kanamycin (25 μg/mL), followed by culture incubation at 37 °C until an OD600 of 0,6-1 was reached (approximately 3–4 h). Before induction by L-Rhamnose, a volume of 1 mL of culture was separated as t0, then culture was centrifuged at 6.000 g at 4 °C for 20 min to isolate the cell pellet from the supernatant. The cytoplasm fractions were isolated by suspending the cell pellet in 500 μL Tris-Cl EDTA buffer, resuspended and followed by cell lysis using a sonicator for 7 min with 15 s on and off. The supernatant was then separated from the cell debris by centrifugation at 6.000 g at 4 °C for 30 min. The supernatant obtained was labeled as (S) fraction. The expressed recombinant MPT64 was analyzed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
2.6. Immunochromatography method
In addition to the SDS PAGE results, the presence of MPT64 protein in the cytoplasm fraction was confirmed using immunochromatographic kit SD Bioline TB Ag MPT64 (Standard Diagnostics, Inc., Korea). The MPT64 protein in the SDS PAGE gel band was isolated and suspended in kit extraction buffer, then 100 μL of the suspension was dropped in the sample area. The results were be obtained in approximately 15 min. The validity of the tool kit was determined by the appearance of the band in the control line. The positive result of the sample was determined by the appearance of two bands on the test and control line.
2.7. Quantification of recombinant MPT64 protein using ImageJ program
The concentration of MPT64 protein in SDS-PAGE gel bands was carried out using the ImageJ program. MPT64 protein concentration was calculated by the linear regression equation of y = a + bx using Bovine Serum Albumin (BSA) as a standard. Quantification reflected the relative amounts as a ratio of each MPT64 protein band relative to the lane's loading control. The BSA standard concentrations were 0; 0.01; 0.05; 0.10; 0.15; and 0.2 mg/mL as a range for the standard curve. After running and destaining the gel, the picture was captured or scanned and saved as a tif and as a jpg. Recombinant MPT64 protein levels were calculated based on the area of the band using a linear regression equation y = a + bx from the standard BSA. The dependent variable (Y) was the area of the band and the independent variable (X) was the level of protein.
2.8. Data analysis
The combined effect of three independent variables, including rhamnosa concentration variables, induction time, and concentration of medium components, were analyzed using Minitab software 17. The analysis of variance (ANOVA) was generated to determine the significance of the equation model using the lack-of-fit test. The lack of fit test provided the details to determine the suitability of the model compared to the data by comparing the probability value (P-value) with the level of significance. The significance of each coefficient (linear or quadratic) was determined using the Student's t-test with 0.05 probability level. A value of α = 0.05 indicated that the maximum possibility of error was 5%. The hypotheses used in this test were as follows: H0 (The model was appropriate (there was no lack of fit) if p > 0.05) and H1 (Model was not appropriate (there was a lack of fit) if p < 0.05). The optimum condition for the expression of recombinant MPT64 protein was determined by contour plot, 3D surface, and optimizer curve.
3. Results
3.1. Growth and production curve of E. coli BL21 (DE3)
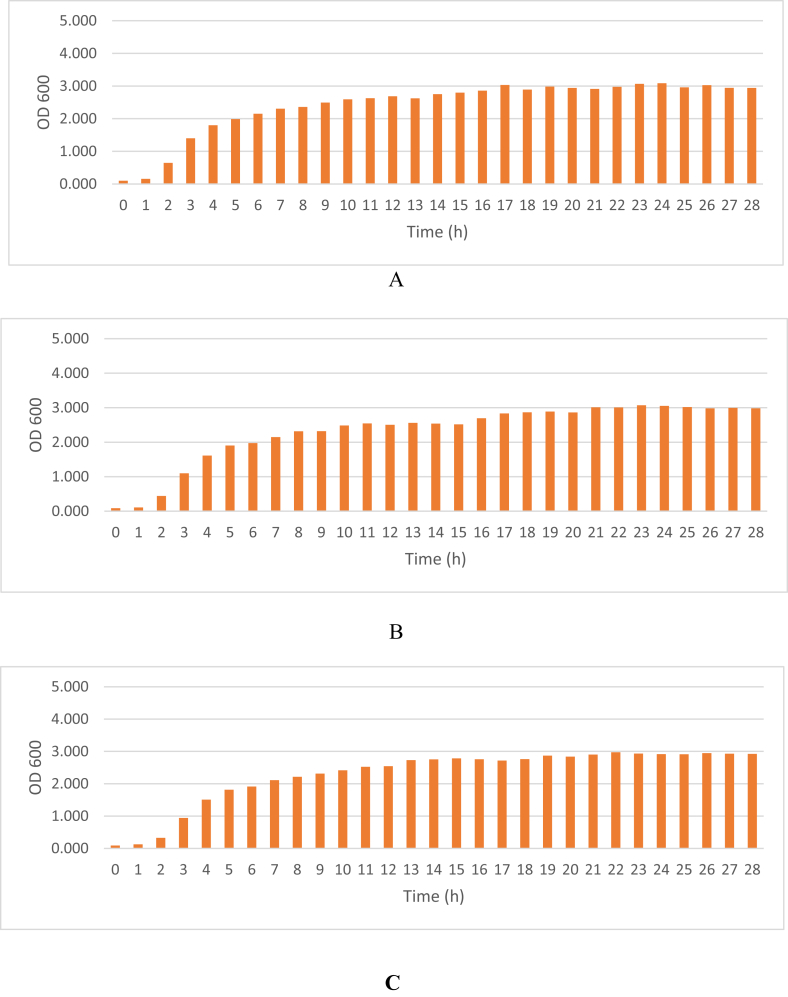
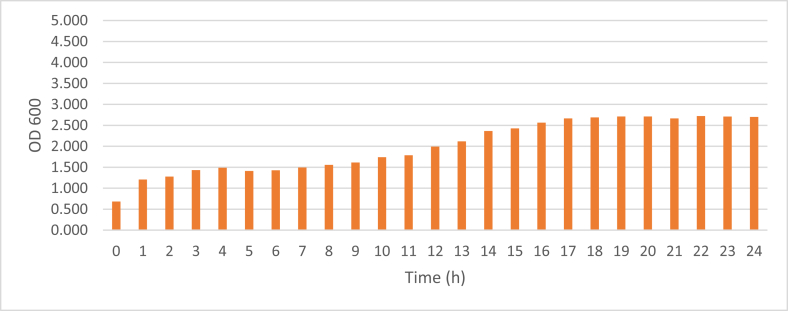
The growth curve of the transformant can be seen in Fig. 1. The lag phase occurred at the 1st h, the exponential phase was in the 2nd to 16th h, and the stationary phase started at the 17th h. Meanwhile, the curve of MPT64 production was performed in Fig. 2.
Fig. 1.
Growth curve of E. coli BL21 (DE3) harboring the recombinant expression vector under different medium concentrations. Notes: A (1-fold medium concentration); B (1.5-fold medium concentration); C ((2.0-fold medium concentration).
Fig. 2.
Production curve of E. coli BL21 (DE3) harboring the expression vector recombinant with L-rhamnosa induction.
3.2. Optimization of culture condition
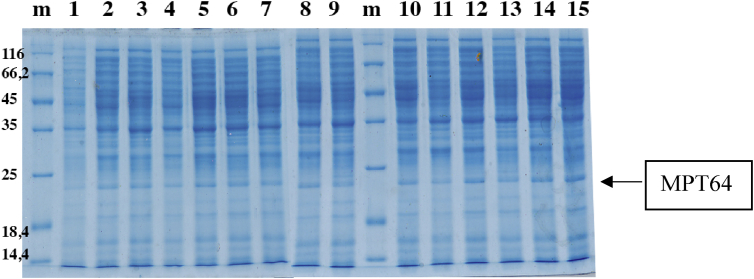
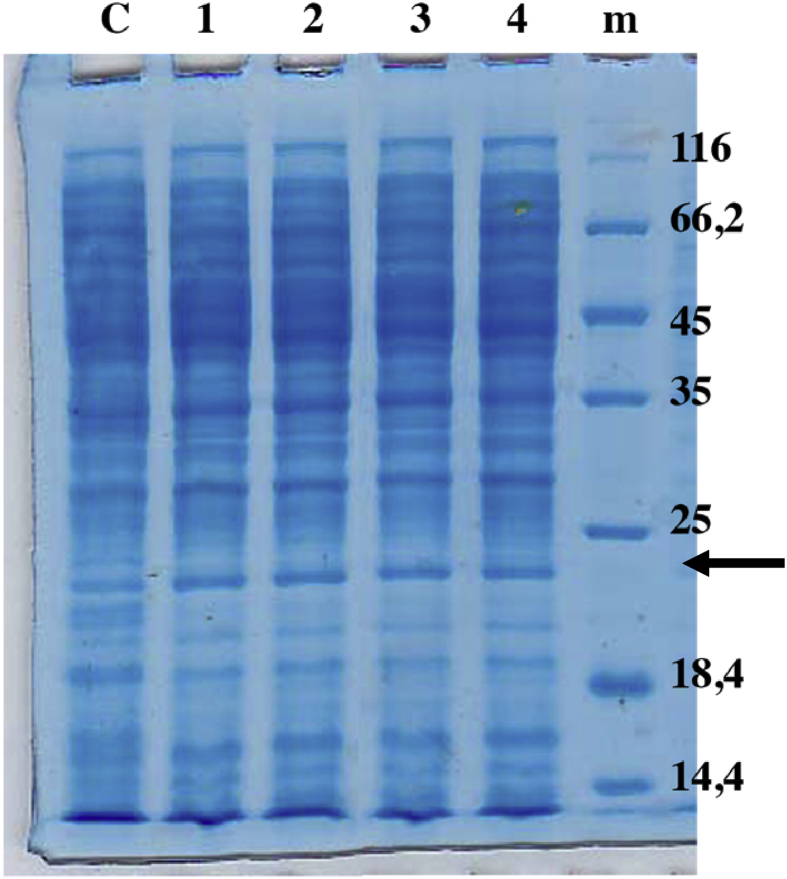
The expression of recombinant MPT64 protein in the cytoplasmic fraction was carried out in flask scale fermentation based on the experimental design; PAGE analysis is shown in Fig. 3.
Fig. 3.
SDS PAGE analysis of MPT64 expression under different optimized culture conditions. Notes: m: Marker, 1–15: experiment condition, (arrow): recombinant MPT64 protein [∼ 24 kDa].
Recombinant MPT64 protein levels were calculated based on the area of the protein band in the SDS-PAGE gel. The BSA standard linear regression equation was y = 188757x + 311.79 with 0.9988 as a coefficient of determination. The values of recombinant MPT64 concentrations obtained from 15 experimental units can be seen in Table 3. Furthermore, the optimum conditions were determined based on the results of statistical data analysis so that the obtained equation can be used to determine the predictions of recombinant MPT64 protein levels. The predicted and experimental responses were presented in Table 3.
Table 3.
The experimental results for the production of MPT64 recombinant.
| Experiment | Rhamnosa concentration (mM) | Induction time (h) | Medium concentration (-fold) | Protein Concentration (mg/mL) |
|
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | 2.0125 | 2.0 | 1.00 | 0.0079 | 0.0133 |
| 2 | 2.0125 | 3.5 | 1.50 | 0.0211 | 0.0197 |
| 3 | 2.0125 | 5.0 | 1.00 | 0.0204 | 0.0185 |
| 4 | 2.0125 | 3.5 | 1,50 | 0.0122 | 0.0197 |
| 5 | 0.0250 | 5.0 | 1,50 | 0.0202 | 0.0216 |
| 6 | 4.0000 | 5.0 | 1.50 | 0.0226 | 0.0284 |
| 7 | 4.0000 | 2.0 | 1.50 | 0.0190 | 0.0176 |
| 8 | 4.0000 | 3.5 | 2.00 | 0.0245 | 0.0240 |
| 9 | 4.0000 | 3.5 | 1.00 | 0.0154 | 0.0113 |
| 10 | 2.0125 | 2.0 | 2.00 | 0.0184 | 0.0202 |
| 11 | 0.0250 | 3.5 | 2.00 | 0.0128 | 0.0169 |
| 12 | 2.0125 | 3.5 | 1.50 | 0.0258 | 0.0197 |
| 13 | 0.0250 | 3.5 | 1.00 | 0.0179 | 0.0184 |
| 14 | 2.0125 | 5.0 | 2.00 | 0.0284 | 0.0229 |
| 15 | 0.0250 | 2.0 | 1.50 | 0.0304 | 0.0245 |
The second-order regression equation explained the levels of MPT64 production as the interactions of medium concentration, induction time and inducer concentration, which can be presented in the following equation:
| Responses = 0.0059 − 0.01051x − 0.0067y + 0.0394 z + 0.000282x2 − 0.00099y2 + 0.0127z2 + 0.00115x.y + 0.00356x.z − 0,00081y.z | (1) |
Notes: X (inducer concentration); Y (induction time); Z (medium concentration).
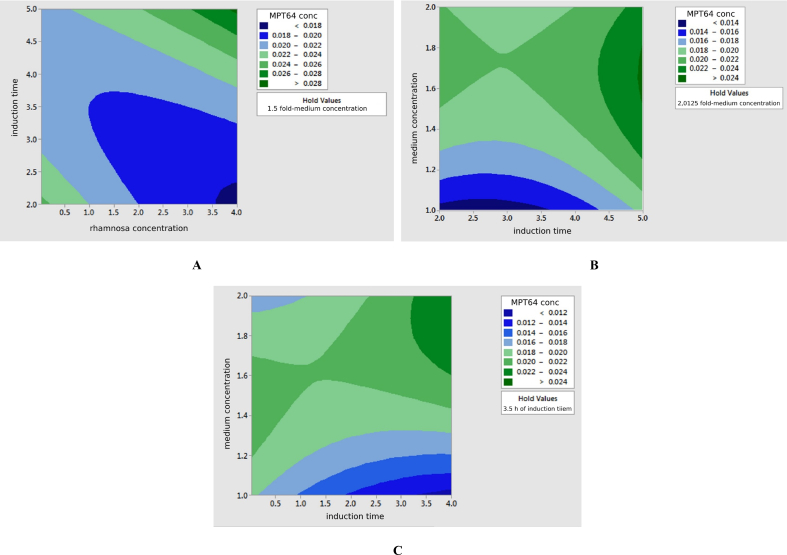
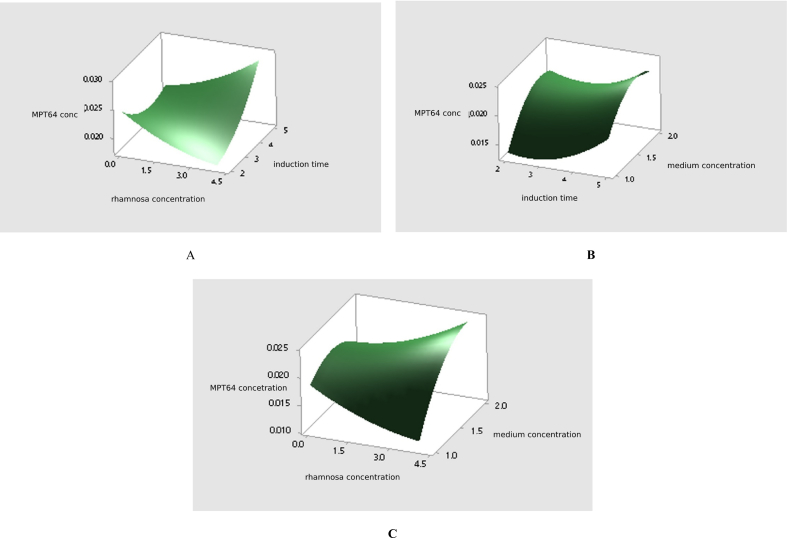
Interactions between RSM Box-Behnken design expression variables in the form of contour plots are shown in Fig. 4 and its 3D surface forms were presented in Fig. 5.
Fig. 4.
The contour plot of the RSM design showing the influence of interaction between gene expression variable. A: interaction of induction time and rhamnose concentration; B: induction time interaction and medium concentration; C: interaction of medium concentration and rhamnose concentration.
Fig. 5.
The 3D surface interaction of variable on the expression response using the Box-Behnken RSM design. A: interaction of induction time and rhamnose concentration; B: induction time interaction and medium concentration; C: interaction of medium concentration and rhamnose concentration.
After the optimum conditions were determined, the recombinant MPT64 protein expression was carried out by four repetitions and the results were characterized by SDS-PAGE, presented in Fig. 6. This was done to determine the suitability of the model. The results of running were then compared with data center points and levels of the predictive protein. Data on the results of recombinant MPT64 protein expression under optimum conditions are shown in Table 4.
Fig. 6.
Characterization of recombinant MPT64 protein at optimum expression conditions using the SDS-PAGE method. C: center point, 1–5: repeat sequence of experiments under optimum conditions, m: Marker, (arrow): recombinant MPT64 protein [∼ 24 kDa].
Table 4.
Validation of optimization results.
| Component Experiment |
Response |
|||
|---|---|---|---|---|
| Condition | Rhamnose concentration (mM) | Induction Time (h) | Medium Concentration (-fold) | MPT64 Protein Concentration (mg/mL) |
| Center point | 2.0125 | 3.5 | 1.5 | 0.0212 |
| Optimum | 4.0 | 5 | 1.9495 | 0.0311 |
| Repetitions of the optimum condition (4 times) | 4.0 | 5 | 1.9495 | 0.0441 |
| 4.0 | 5 | 1.9495 | 0.0406 | |
| 4.0 | 5 | 1.9495 | 0.0386 | |
| 4.0 | 5 | 1.9495 | 0.0336 | |
Model Eq. (1) was a function of three variables: rhamnosa concentration, induction time, and medium concentration which were used to find the optimum point of recombinant MPT64 protein production.
3.3. MPT64 recombinant protein characterization using the immunochromatography method
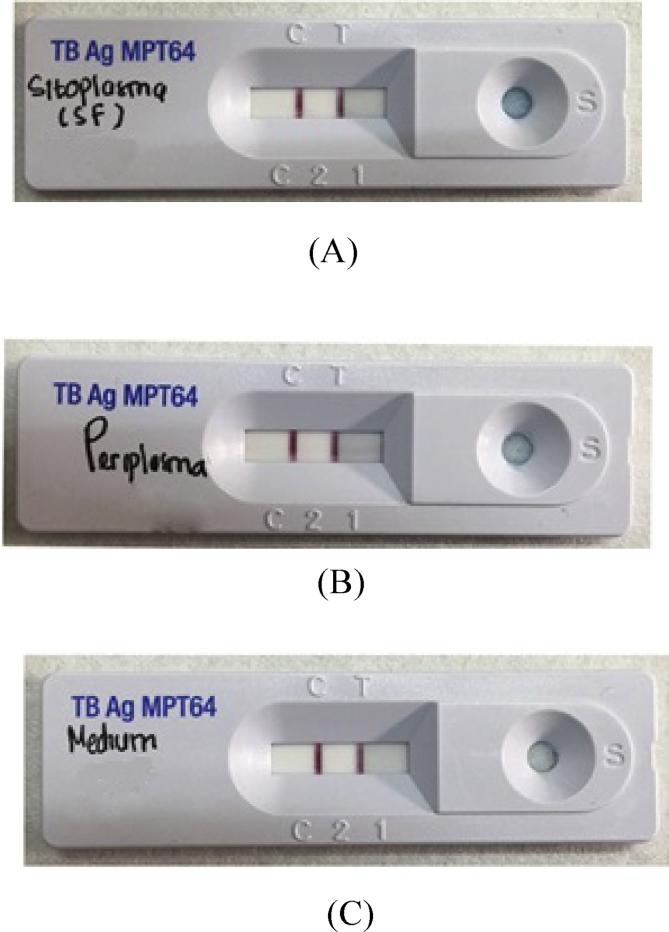
After expression validation using the optimum conditions, the expression results were characterized using the SDS-PAGE method. Then the protein was identified by an immunochromatography test kit. The commercial immunochromatography kit used in this study was SD Bioline Ag MPT64. A 24 kDa recombinant MPT64 protein was isolated from SDS-PAGE gel (figure 9 in the supplementary material), then it was suspended in an extraction buffer and dropped on the sample site. The sample was allowed to flow on the nitrocellulose membrane for approximately 15 min. The results of identification using the immunochromatography method are shown in Fig. 7.
Fig. 7.
The identification results of the recombinant MPT64 proteins using immunochromatographic methods. (A) dissolved fraction (cytoplasm), (B) periplasma fraction, (C) medium fraction.
4. Discussion
Recently, Escherichia coli has become a widely used host cell for recombinant gene expression that offers many advantages, such as ease of growth in inexpensive media, rapid biomass accumulation, and can live with high cell density in the fermentation process, thus, E. coli need a simple process to scale-up (Mergulhao et al., 2005). Bacterial growth is a complex process involving the synthesis (anabolic) and breakdown (catabolic) of numerous cell constituents and metabolites (Maier, 2009). In this study, the growth behaviour of recombinant E. coli BL21 (DE3) cells containing MPT64 overproducing plasmid pD861-SR:319895 were investigated at 37 °C. MPT64 is a specific protein that is secreted by Mycobacterium tuberculosis complex (MTBC), which distinguishes it from mycobacteria other than M. tuberculosis (MOTT). Therefore, anti-MPT64 antibodies can be used as a main component of a diagnositic tool to detect the presence of M. tuberculosis in clinical samples. To produce the antibodies, the optimization of the MPT64 gene expression was needed to obtain the MPT64 protein in a large amounts.
The growth curves with different concentration medium showed similar results. Thus, the medium concentration as a single factor did not influence the growth of E. coli BL21 (DE3) significantly. A lag phase of transformant E. coli BL21 (DE3) was found in the first hour in the cell growth of E. coli. The exponential phase of cell growth was completed within 16 h after inoculation, after which the E. coli cells entered their stationary phase. The charts on the growth curve indicated the OD600 values of the different phases of transformant cell growth. This information was essential to determine time ranges of cell induction using rhamnose. Lag phase begins after an inoculum is inoculated into fresh medium. This phase is a transition phase to the exponential phase after the initial population has doubled (Yates and Smotzer, 2007). In the lag phase, E. coli cells are thought to be physiologically adaptating to the culture conditions. The phase may provide a time requirement for specific messenger RNA (mRNA) induction and protein synthesis to meet new culture needs. The lag phase usually occurs within minutes to several hours and the length of it can be controlled by the type of medium as as well as on the initial inoculum size. The next phase of bacterial growth is the exponential phase. The characteristic of the exponential phase is the most rapid growth of bacteria under the conditions present in the growth system. During the exponential phase, the rate of cells number increase in the culture is proportional to the number of cells present at any particular time. Thus, after that, the bacterial culture may reach stationary phase, at which point carbon and essential nutrient/energy sources are depleted. When a carbon source is depleted, it does not mean that bacterial growth stops, because cell lysis can provide a source of nutrients (Maier, 2009). Therefore, expression of the MPT64 gene was induced between 2-5 h after the preculture inoculation in the exponential stage of cell growth. After induction by rhamnose, the production curves were developed to determine the time of cell harvesting so that the optimal amount was obtained. From the curve, it was found that the optimum time to harvest the cell was at 22 h after inoculation. The most increased numbers of cells indicated an increased number of gene expression results.
The statistically based experimental design can be used as a tool in optimizing the induction conditions that may lead to a several fold increment in recombinant MPT64 production. RSM has been widely used in recent years and it can determine the optimum effects of various independent factors on a dependent factor with less experimental requirements (Pournejati et al., 2014; Zhang et al., 2010; Salihu et al., 2011). The design studied was shown to be effective in determining the vital induction conditions to improve the yield of recombinant protein in E. coli (Setiawan et al., 2018; Indriyani et al., 2019). The expression of recombinant proteins in the host E. coli was influenced by several factors such as medium components, temperature, pH, induction time, and concentration inducer. According to Zhang et al.(2009), the strength of induction is an important parameter for optimizing recombinant protein expression (Zhang et al., 2009). In this study, induction concentration and the time of induction used varied to determine the level of metabolic load that can be given to cells. In addition, the concentration of the medium was as important as those factors because the flux of metabolic pathways, cell density, and recombinant protein production can be affected. As LB medium containing tryptone concentration and yeast extract was higher, often resulting in higher cell culture density. LB medium contains abundant amino acids to support protein expression in E. coli. Complex nitrogen sources such as tryptone can cause higher plasmid stability (Korz et al., 1995). In this study, the lower limit used was normal medium concentration, while the upper limit used was twofold medium concentration. The selected medium concentration to be used was not too high considering the growth of bacteria that will increase to a certain degree and remain constant with the addition of nutrients. In addition, it may influence the effective cost of large scale production in the future.
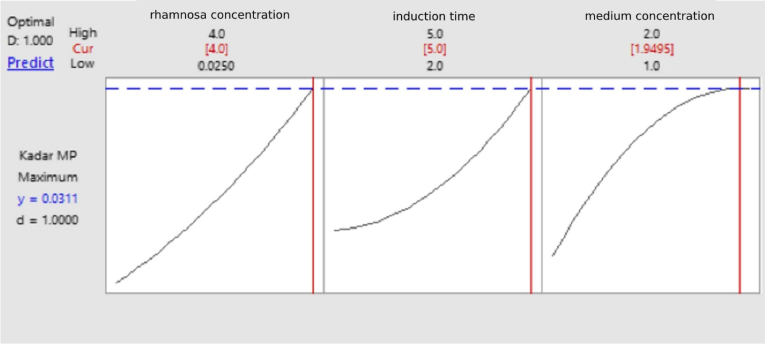
The value of coefficient of determination showed that 99.9% of the band area was determined by the protein concentration. Meanwhile, the 0.1% of the band area was influenced by other factors. Interactions between expression variables in this test were studied using contour and surface plots. In the contour plot and 3D surface (A) it was seen that the recombinant MPT64 protein levels reached the optimal amount of greater than 0.028 mg/mL at 5 h induction time and 4 mM rhamnose concentration. On contour plots and 3D surfaces (B) it is shown that the optimal point of protein content was greater than 0.024 mg/mL at 5 h induction time and the medium component concentration was 1.5–1.8 times greater. Furthermore, in the contour plot and 3D surface (C), the interaction between the 4 mM rhamnose concentration and the concentration of the medium component yielded 2 times the optimal protein content which was greater than 0.024 mg/mL. This suggested that the rhamnose concentration as inducer in the medium had a very significant effect on the MPT64 production. Based on Table 4, the calculation of recombinant MPT64 protein levels at the center point was 0.0202 mg/mL at rhamnose concentration of 2.0125 mM, 3.5 h of induction time, and 1.5-fold times the medium concentration. The optimum condition for the expression of recombinant MPT64 protein is based on the optimizer curve in Fig. 8 which is at a medium concentration of 2 times (tryptone 20 mg/mL, yeast extract 10 mg/mL, sodium chloride 20 mg/mL), induction time of 5 h, and 4 mM rhamnose concentration. The table also presented that the average recombinant MPT64 level produced under optimum conditions was 0.0392 mg/mL compared to its predicted level of 0.0311 mg/mL. The recombinant MPT64 protein content produced was 26.05% greater than the results of the recombinant MPT64 protein prediction model. The value that is not significantly different between the predicted and experimental results verified the validity of the model and the existence of the optimal point. From the results of the characterization of the model validation showed an increase in the amount of protein expressed in the cytoplasm. In addition, protein was secreted extracellularly in small amounts. The result of the commercial MPT64 immunochromatography kit showed two bands in red color at the position of the control and the test line. This indicated that the MPT64 protein was expressed intracellular and secreted extracellularly.
Fig. 8.
The prediction of optimum value of recombinant MPT64 protein expression. Cur: variable of the optimum condition, y: prediction of maximum recombinant MPT64 protein levels.
5. Conclusion
The relationship between the selected optimization parameters strongly influenced the level of MPT64 gene expression in E. coli BL21 (DE3) and the optimized culture conditions for improving the protein MPT64 yield can be successfully selected by RSM using Box-Behnken design The optimum conditions were two-fold medium concentration (tryptone 20 mg/mL, yeast extract 10 mg/mL, and sodium chloride 20 mg/mL), 5 h of induction time and 4 mM rhamnose.
Declarations
Author contribution statement
Sri Agung Fitri Kusuma: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ida Parwati, Yaya Rukayadi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Tina Rostinawati, Muhammad Yusuf: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Muhammad Fadhlillah: Performed the experiments; Analyzed and interpreted the data.
Risa R. Ahyudanari: Performed the experiment.
Toto Subroto: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by DIKTI (Directorate General of Higher Education of Indonesia) and Universitas Padjadjaran through ALG (Academic Leadership Grant).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors acknowledge the supports from DIKTI (Directorate General of Higher Education of Indonesia), Universitas Padjadjaran and Pokhara University.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abe C., Hirano K., Tomiyama T. Simple and rapid identification of the Mycobacterium tuberculosis complex by immunochromatographic assay using anti-MPB64 monoclonal antibodies. J. Clin. Microbiol. 1999;37(11):3693–3697. doi: 10.1128/jcm.37.11.3693-3697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley M., Siebenmann C. Tuberculin skin sensitivity following BCG vaccination with vaccines of high and low viable counts. Can. Med. Assoc. J. 1967;97(5):1335–1339. [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Starke J. Textbook of Pediatric Infectious Diseases. Elsevier: Saunders; Philadelphia, PA: 2014. Tuberculosis; pp. 1335–1380. [Google Scholar]
- DeLisa M.P., Valdes J.J., Bentley W.E. Quorum signaling via AI-2 communicates the metabolic burden associated with heterologous protein production in Escherichia coli. Biotechnol. Bioeng. 2001;75(4):439–450. doi: 10.1002/bit.10034. [DOI] [PubMed] [Google Scholar]
- Donovan R.S., Robinson C.W., Glick B.R. Review: optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J. Ind. Microbiol. 1996;16(3):145–154. doi: 10.1007/BF01569997. [DOI] [PubMed] [Google Scholar]
- Dunn J.J., Starke J.R., Revell P.A. Laboratory diagnosis of Mycobacterium tuberculosis infection and disease in children. J. Clin. Microbiol. 2016;54(6):1434–1441. doi: 10.1128/JCM.03043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham J.O. 3rd epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996;9(2):177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S.L., Bruns R.E., Ferreira H.S., Matos G.D., David J.M., Brandao G.C. Box-behnken design: an alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007;597(2):179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hasegawa N., Miura T., Ishii K., Yamaguchi K., Lindner L.H., Merritt S. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J. Clin. Microbiol. 2002;40(3):908–912. doi: 10.1128/JCM.40.3.908-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillemann D., Rüsch-Gerdes S., Richter E. Application of the capilia TB assay for culture confirmation of Mycobacterium tuberculosis complex isolates. Int. J. Tuberc. Lung Dis. 2005;9(12):1409–1411. [PubMed] [Google Scholar]
- Indriyani A., Anggraeni N.I., Sriwidodo S., Maksum I.P. Optimization extracellular secretion of recombinant human epidermal growth factor (hegf) in Escherichia coli BL21 (DE3) Pd881-ompa-hegf by using response surface method (RSM) Int. J. Res. Pharm. Sci. 2019;10(3):1824–1831. [Google Scholar]
- Jain J., Okabayashi T., Kaur N., Nakayama E., Shioda T., Gaind R. Evaluation of an immunochromatography rapid diagnosis kit for detection of Chikungunya virus antigen in India, a dengue-endemic country. Virol. J. 2018;15(84):1–6. doi: 10.1186/s12985-018-1000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korz D.J., Rinas U., Hellmuth K., Sanders E.A., Deckwer W.D. Simple fed-batch technique for high cell density cultivation of Escherichia coli. J. Biotechnol. 1995;39(1):59–65. doi: 10.1016/0168-1656(94)00143-z. [DOI] [PubMed] [Google Scholar]
- Lim H.K., Jung K.H., Park D.H., Chung S.I. Production characteristics of interferon-Α using an L-arabinose promoter system in a high-cell-density culture. Appl. Microbiol. Biotechnol. 2000;53(2):201–208. doi: 10.1007/s002530050009. [DOI] [PubMed] [Google Scholar]
- Maier R.M. Introduction to Environmental Microbiology. second ed. Academic Press; Cape Town, South Africa: 2009. Bacterial growth; pp. 37–53. [Google Scholar]
- Maldonado L.M., Hernandez V.E., Rivero E.M., Barba de la Rosa A.P., Flores J.L., Acevedo L.G. Optimization of culture conditions for a synthetic gene expression in Escherichia coli using response surface methodology: the case of human interferon beta. Biomol. Eng. 2007;24(2):217–222. doi: 10.1016/j.bioeng.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Mergulhao F.J., Summers D.K., Monteiro G.A. Recombinant protein secretion in Escherichia coli. Biotechnol. Adv. 2005;23(3):177–202. doi: 10.1016/j.biotechadv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Morowvat M.H., Babaeipour V., Memari H.R., Vahidi H. Optimization of fermentation conditions for recombinant human interferon beta production by Escherichia coli using the response surface methodology. Jundishapur J. Microbiol. 2015;8(4):1–9. doi: 10.5812/jjm.8(4)2015.16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong H., Yamazaki M., Ishizaki M., Endo I., Harada N., Aramaki M. Clinical evaluation of the immunochromatographic system using silver amplification for the rapid detection of Mycoplasma pneumoniae. Sci. Rep. 2018;8(1):1–7. doi: 10.1038/s41598-018-19734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournejati R., Karbalaei-Heidari H.R., Budisa N. Secretion of recombinant archeal lipase mediated by SVP2 signal peptide in Escherichia coli and its optimization by response surface methodology. Protein Expr. Purif. 2014;101:84–90. doi: 10.1016/j.pep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Salihu A., Alam M.Z., Abdul K.M.I., Salleh M.H. Optimization of lipase production by Candida cylindracea in palm oil mill effluent based medium using statistical experimental design. J. Mol. Catal. B Enzym. 2011;69(1):66–73. [Google Scholar]
- Setiawan D., Fadhlillah M., Pertiwi W., Idar I., Gaffar S., Subroto T. Optimization of the expression of recombinant universal infleunza vaccine candidate in Escherichia coli using response surface methodology. IOSR J. Pharm. 2018;8(2):53–60. [Google Scholar]
- Shojaosadati S.A., Varedi K.S.M., Babaeipour V., Farnoud A.M. Recent advances in high cell density cultivation for production of recombinant protein. Iran. J. Biotechnol. 2008;6(2):63–84. [Google Scholar]
- Stevenson K., Mcvey A.F., Clark I.B.N., Swain P.S., Pilizota T. Nature Publishing Group; 2016. General Calibration of Microbial Growth in Microplate Readers; pp. 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toihir A.O.S., Rasolofo V., Andrianarisoa S.H., Ranjalahy G.M., Ramarokoto H. Validation of an immunochromatographic assay kit for the identification of the Mycobacterium tuberculosis complex. Mem. Inst. Oswaldo Cruz. 2011;106(6):777–780. doi: 10.1590/s0074-02762011000600022. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H., Ouchi K., Ohsaki M., Yamanaka T., Kuniya Y., Takeuchi Y. Immunochromatography test for rapid diagnosis of Adenovirus respiratory tract infections: comparison with virus isolation in tissue culture. J. Clin. Microbiol. 1999;37(6):2007–2009. doi: 10.1128/jcm.37.6.2007-2009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C., Gregory M.E., Turner M.K. A study of the effect of specific growthrate and acetate on recombinant protein production of Escherichia coli JM107. Biotechnol. Lett. 1994;16(9):891–896. [Google Scholar]
- Yates G.T., Smotzer T. On the lag phase And initial decline of microbial growth curves. J. Theor. Biol. 2007;244(3):511–517. doi: 10.1016/j.jtbi.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Zhang J., Greasham R. Chemically defined media for commercial fermentations. Appl. Microbiol. Biotechnol. 1999;51(4):407–421. [Google Scholar]
- Zhang H., Zheng Y., Liu Q., Tao X., Zheng W., Ma X., Wei D. Development of a fed-batch process for the production of anticancer drug tatm-survivin (T34A) in Escherichia coli. Biochem. Eng. J. 2009;43(2):163–168. [Google Scholar]
- Zhang C., Daidi F., Shang L.A., Xiaoxuan M., Wenjiao X., Pengfei G. Optimization of fermentation process for human-like collagen production of recombinant Escherichia coli using response surface methodology. Chin. J. Chem. Eng. 2010;18(1):137–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.