Abstract
Small-cell lung cancer (SCLC) represents 15% of all lung cancers and it is clinically the most aggressive type, being characterized by a tendency for early metastasis, with two-thirds of the patients diagnosed with an extensive stage (ES) disease and a five-year overall survival (OS) as low as 5%. There are still no effective targeted therapies in SCLC despite improved understanding of the molecular steps leading to SCLC development and progression these last years. After four decades, the only modest improvement in OS of patients suffering from ES-SCLC has recently been shown in a trial combining atezolizumab, an anti-PD-L1 immune checkpoint inhibitor, with carboplatin and etoposide, chemotherapy agents. This highlights the need to pursue research efforts in this field. Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase that is overexpressed and activated in several cancers, including SCLC, and contributing to cancer progression and metastasis through its important role in cell proliferation, survival, adhesion, spreading, migration, and invasion. FAK also plays a role in tumor immune evasion, epithelial-mesenchymal transition, DNA damage repair, radioresistance, and regulation of cancer stem cells. FAK is of particular interest in SCLC, being known for its aggressiveness. The inhibition of FAK in SCLC cell lines demonstrated significative decrease in cell proliferation, invasion, and migration, and induced cell cycle arrest and apoptosis. In this review, we will focus on the role of FAK in cancer cells and their microenvironment, and its potential as a therapeutic target in SCLC.
Keywords: focal adhesion kinase, small-cell lung cancer, targeted therapy
1. Introduction
Lung cancer, which arises from lung epithelial cells, is histologically divided into two main types: small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), which represent 15% and 85% of the cases, respectively [1]. As opposed to SCLC, oncogenic drivers with sensitivity to targeted therapies have been discovered in NSCLC. Tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) rearrangements, or other oncogenic abnormalities have brought remarkable improvements in the outcome of oncogenic-driven NSCLC patients [2]. Immunotherapy with anti-programmed death-(ligand) 1 (PD-(L)1) immune checkpoint inhibitors (ICIs) has also significantly improved the survival of NSCLC patients without oncogenic drivers [3,4,5,6,7,8,9]. Clinically, SCLC is the most aggressive type of lung cancer, being characterized by a high growth rate, a fast doubling time, and a tendency for early metastasis, with two-thirds of the patients diagnosed with an extensive stage (ES) disease [10,11]. While a good initial response to chemotherapy and/or radiation therapy is observed in most patients, they typically recur or progress rapidly after the primary treatment, with a median overall survival (OS) of 24–38 months in limited stage (LS) [12,13] and 7–10 months in ES [14], and a five-year OS as low as 5% [1].
Despite improvements in the understanding of the molecular steps that lead to SCLC development and progression these last years, there are still no effective targeted therapies in SCLC. Rovalpituzumab tesirine (Rova-T) is an antibody-drug conjugate (pyrrolobenzodiazepine (PBD)-dimer cytotoxic) that is directed against Delta-like 3 (DLL3), an inhibitory NOTCH ligand, which has been shown to be overexpressed on the surface of SCLC cells [15]. Despite encouraging preclinical and early clinical results, targeted therapy with Rova-T underperformed in the phase II TRINITY trial, including pretreated SCLC patients with high levels of DLL3 on tumor cell surface [15,16]. After four decades, the only modest improvement in the OS of patients suffering from ES-SCLC has recently been shown in a trial combining atezolizumab, an anti-PD-L1 ICI, with carboplatin and etoposide, chemotherapy agents [17]. In this trial, the OS was 10.3 months in the chemotherapy alone arm, while it was 12.3 months in the chemotherapy plus immunotherapy arm. Based on this positive trial, atezolizumab that is associated to carboplatin an etoposide recently became the new standard of care in the first-line treatment of ES-SCLC [17]. At relapse or progression after a first-line treatment, a rechallenge with platinum and etoposide is proposed to tumors that are considered to be sensitive to platinum (relapse or progression within 60 or 90 days of completion of chemotherapy) [18], while a second-line chemotherapy with topotecan is proposed to tumors platinum-refractory (relapse or progression before three to six months). However, the response rates are poor and OS ranges from 1.2 months to 7.6 months based on systematic reviews of real-world data 15 [19]. These disappointing results highlight the need for novel therapies.
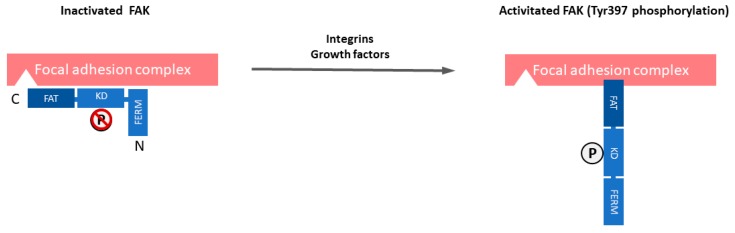
Focal adhesion kinase (FAK) is a 125 kDa non-receptor protein tyrosine kinase that is known to be overexpressed and activated in several cancers, including SCLC [20,21,22,23,24,25,26,27,28]. Unlike receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR), non-RTKs, such as FAK, are cytoplasmic enzymes that lack transmembrane and extracellular domains [29]. FAK localizes to focal adhesions and it is triggered off by extracellular signals, such as integrin-mediated adhesion and some growth factors [30]. Therefore, FAK plays a central role in the interaction between cells, including cancer cells and their microenvironment. The FAK structure includes an NH2-terminal Protein4.1-ezrin-radixin-moesin (FERM) domain, a central kinase domain, two proline-rich motifs, and a COOH-terminal focal adhesion targeting (FAT) domain. FAK is maintained in an inactive state by the binding of the FERM domain to the kinase domain, which blocks access to the catalytic site and sequesters the activation loop, as well as the key autophosphorylation site tyrosine 397 (Tyr397) (Figure 1). The engagement of integrins with the extracellular matrix (ECM) or growth factors leads to signals that displace the FERM domain, resulting in rapid autophosphorylation of Tyr397, which is a critical event in signal transduction by FAK [30,31]. Tyr397 phosphorylation provides a binding site that recruits and activates Src through the SH2 domains of Src family kinases. The FAK-Src complex therefore maintains Src and FAK in their activated states, creating a functional kinase complex [32].
Figure 1.
The domain organization and activation of focal adhesion kinase (FAK). FAK is composed of a central kinase domain (KD), an amino-terminal side that is flanked by a protein band 4.1-ezrin-radixin-moesin (FERM) homology domain, and a carboxy-terminal focal adhesion targeting (FAT) domain flanked by proline-rich regions (PRRs). FAK localizes to focal adhesions and is triggered off by extracellular signals such as integrin-mediated adhesion and some growth factors. FAK is maintained in an inactive state by the binding of the FERM domain to the kinase domain, which blocks access to the catalytic site and sequesters the activation loop, as well as the key autophosphorylation site tyrosine 397 (Tyr397). Engagement of integrins with the extracellular matrix (ECM) or growth factors leads to signals that displace the FERM domain, resulting in rapid autophosphorylation of Tyr397, which is a critical event in signal transduction by FAK.
Based on FAK overexpression and/or increased activity in cancer and its known function in multiple biological processes that play a role in the development and progression of cancers, such as crosstalk between cell and his microenvironment, cell growth, survival, adhesion, spreading, migration, invasion, angiogenesis, DNA damage repair, radioresistance, and regulation of cancer stem cells, it has been suggested that increased the expression and/or activity of FAK may have a critical role in cancer development and progression [33]. Therefore, FAK is a potential target for anti-cancer therapy, especially in SCLC, being known to be a highly invasive cancer. Small-molecule inhibitors targeting the FAK kinase domain and preventing FAK activation (Tyr397 autophosphorylation) have been developed. Phase I trials with GSK2256098 [34,35,36], VS-6062 [37], defactinib (VS-6063) [38,39,40], or BI853520 [41,42,43] have shown an acceptable safety profile and favorable pharmacokinetics. Most frequent treatment-related adverse events included digestive disorders (nausea, diarrhea, vomiting), headaches, reversible proteinuria, and unconjugated hyperbilirubinemia [34,35,36,37,38,39,40,41,42]. With GSK2256098, the best response of stable disease was observed in 37% of glioblastoma (three patients, median PFS 5, seven weeks) [36] and in 45% of advanced solid cancers (28 patients) [35]. With VS-6062, 34% of patients (31 patients) with advanced solid tumors exhibited stable disease at six weeks, including one case of SCLC for ≥6 cycles cycles [37]. VS-6063 led to the stabilization of advanced solid tumors in 43% of Caucasian patients (six cases) after six weeks of treatment [38] and in 33% of Asian patients (three cases) during more than 24 weeks (median PFS of 63 days) [40]. Recently, the combination of the FAK inhibitor GSK2256098 and the MEK inhibitor trametinib in recurrent advanced pancreatic ductal adenocarcinoma did not provide significant clinical activity in a phase II trial (PFS of 1.6 month and OS of 3.6 months) [44]. In malignant pleural mesothelioma, defactinib in maintenance after first-line chemotherapy in a phase II trial did not provide any benefit either (PFS of 4.1 months with defactinib vs 4.0 months with placebo, and OS of 12.7 months with defactinib vs. 13.6 months with placebo) [45]. Preoperative administration of defactinib in the ongoing phase II clinical trial NCT02004028 appears promising, with therapeutic activity (13% objective partial response, 67% stable disease, 17% tumor progression) and beneficial modification of the tumoral microenvironment [46]. Several clinical trials with defactinib associated with immunotherapy (NCT02758587, NCT03727880, NCT02943317), RAK/MEK inhibitor (NCT03875820), or chemotherapy (NCT02546531) are ongoing, with some of them being open to SCLC inclusion (Table 1) [34,35,36,37,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. Other small-molecules targeting the protein-protein interactions between FAK and other proteins, such as VEGFR-3, called scaffolding inhibitors, have been developed and shown to induce antitumoral effects in preclinical studies. Further research is needed to find predictive biomarkers of response to FAK TKI alone or, probably more promising, in association with another drug.
Table 1.
FAK inhibitors with anti-tumor activity in preclinical studies and clinical trials.
| Name | Type | Specificity | Cancers Targeted | Study Phase | References |
|---|---|---|---|---|---|
| TAE-226 Novartis | Kinase inhibitor ATP competitive | FAK, IGF-IR, c-Met, Pyk2 | Glioma, ovarian | Preclinical | [47,62] |
| PF-573,228 Pfizer | Kinase inhibitor ATP competitive | FAK | Prostate, breast | Preclinical | [48] |
| GSK2256098 GlaxoSmithKline | Kinase inhibitor ATP competitive Reversible | FAK, UGT1A1 | Solid tumors (ovarian, pancreatic, meningioma, glioblastoma, malignant pleural mesothelioma) | Clinical: phase I & II | [34,35,36,44,49] NCT00996671, NCT02523014 |
| NVP-TAC544 | Kinase inhibitor ATP competitive | FAK | N/A | Preclinical | [50] |
| VS-4718 (PND-1186) Verastem | Kinase inhibitor ATP competitive Reversible | FAK, Pyk2 | Solid tumors (pancreas, breast, ovarian), acute myeloid leukemia, B-cell acute lymphoblastic leukemia | Clinical: phase I | [51] |
| VS-6062 (PF-562271 and PF271) Verastem | Kinase inhibitor ATP competitive Reversible | FAK, CDK2/CyclinE, CDK3/CyclinE, CDK1/CyclinB, Pyk2 | Prostate, pancreatic, head and neck | Clinical: phase I | [37,52] |
| VS-6063 (Defactinib) Verastem | Kinase inhibitor ATP competitive | FAK, Pyk2 | NSCLC, pancreatic cancer, ovarian, malignant pleural mesothelioma, hematologic | Clinical: phase I/Ib & II | [38,39,40,45,53] NCT02758587 NCT02004028 NCT03875820 NCT03727880, NCT02943317, NCT02913716, NCT02465060, NCT02546531 |
| 1H-Pyrrolo(2,3-b) Merk Serono | Kinase inhibitor non-ATP competitive | Hinge region of FAK | N/A | Preclinical | [54] |
| C4 CureFAKtor Pharmaceuticals | Scaffold inhibitor | FAK /VEGFR-3 | Neuroblastoma, pancreatic, breast | Preclinical | [55,56,57] |
| Compound R2 (Roslins) CureFAKtor Pharmaceuticals | Scaffold inhibitor | FAK, p53 | Colon, reast | Preclinical | [58] |
| Y11 CureFAKtor Pharmaceuticals | Scaffold inhibitor | FAK Y397 site | Colon, breast | Preclinical | [59] |
| BI853520 | ATP competitive inhibitor | FAK | Malignant pleural mesothelioma, non-hematologic malignancies | Preclinical, clinical | [42,43,60] |
Abbreviations: CDK: Cyclin-dependent kinases 1, 2, 3; FAK: focal adhesion kinase; IGF-IR: insulin-like growth factor 1 (IGF-1) receptor; N/A: data not available; Pyk2: proline-rich tyrosine kinase 2; UGT1A1: UDP-glucuronosyltransferase 1-1; VEGFR-3: vascular endothelial growth factor receptor 3.
In this review, we will focus on the role of FAK in tumor development and progression and its potential as a therapeutic target in SCLC.
2. FAK Overexpression and/or Activation in Human Cancers, Its Frequency and Mechanisms
Increased FAK expression or activity has been observed by various methods (Western blot, IHC, Northern blot, quantitative real-time polymeric chain reaction, immunohistochemistry (IHC)) in many human cancers, including lung, head and neck, oral cavity, thyroid, breast, ovarian, prostate, colon, liver, stomach, pancreas, kidney, skin, and bone cancers [63,64,65,66]. Increased FAK expression or activity has also been reported in various tumor-derived cancer cell lines [64].
IHC in 85 human SCLC tissues revealed that total FAK was localized to the cytoplasm of 78/85 (92%) SCLCs, and that its expression was low in 11 (13%), moderate in 17 (20%), and high in 50 (59%) SCLCs [24]. In a more recent study, multiplex immunofluorescence staining in 105 SCLC and 95 non-NSCLC patients, as well as 37 healthy donors, revealed that FAK and phospho-FAK (Y397) expression was significantly higher in lung cancer than in normal lung, and significantly higher in SCLC when compared to NSCLC tissues (p < 0.01). Moreover, the ratio between phospho-FAK and FAK staining scores was significantly higher in SCLC than in NSCLC tissues (p < 0.01) [67]. In the SCLC cell lines, FAK and phospho-FAK (Y397) expression has also been shown to be increased [28,68].
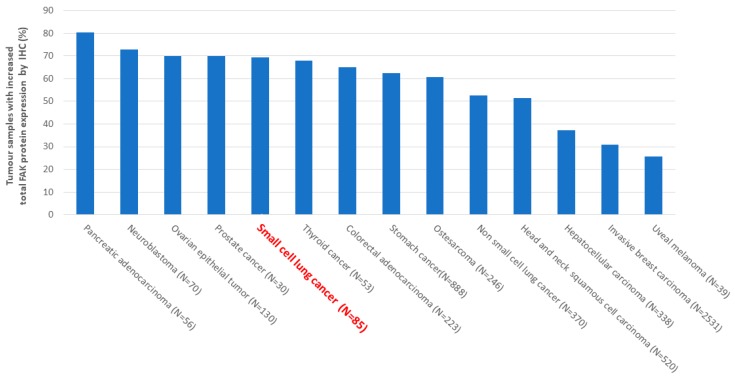
We performed a Pubmed search of studies evaluating FAK protein expression in human cancers by IHC to determine the percentage of cancer samples with increased FAK protein expression. The used methods are described in the legend of Figure 2 and Figure A1. Based on this Pubmed search, we found an overexpression of FAK at the protein level, as evaluated by IHC, in 80% of pancreatic adenocarcinoma, 72% of neuroblastoma, 70% of ovarian epithelial tumors, and many other cancers, including 52% of NSCLC and 69% of SCLC (Figure 2) [20,21,24,26,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109].
Figure 2.
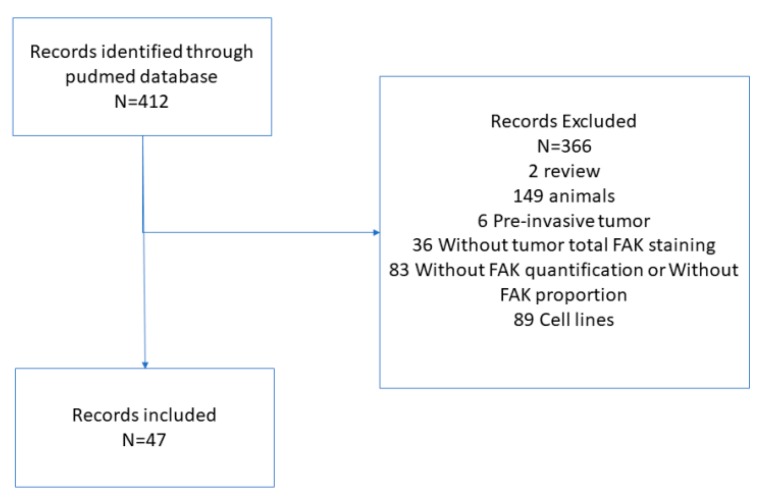
Frequency of focal adhesion kinase (FAK) overexpression at protein level in human solid cancers. A Pubmed search of studies evaluating FAK protein expression in human cancers by immunohistochemistry (IHC) was performed to determine the percentage of cancer samples with increased FAK protein expression. The following keywords were used in the search strategy: FAK [All Fields] AND (“neoplasms” [MeSH Terms] OR “neoplasms” [All Fields] OR “cancer” [All Fields]) AND (“immunohistochemistry” [MeSH Terms] OR “immunohistochemistry” [All Fields]). The results were limited to English language studies. Manual searches of reference articles from applicable studies were performed to identify articles that may have been missed by the computer-assisted search. Abstracts were excluded for cell lines, pre-invasive tumors, if insufficient data to evaluate the methodological quality, absence of tumor total FAK staining, absence of FAK quantification or proportion, absence of proportion of samples overexpressing FAK. Non-eligible trials included ecological studies, case reports, reviews, editorials, and animal trials. This work was conducted in accordance with the PRISMA guidelines (Figure A1). N = number of cancers analysed.
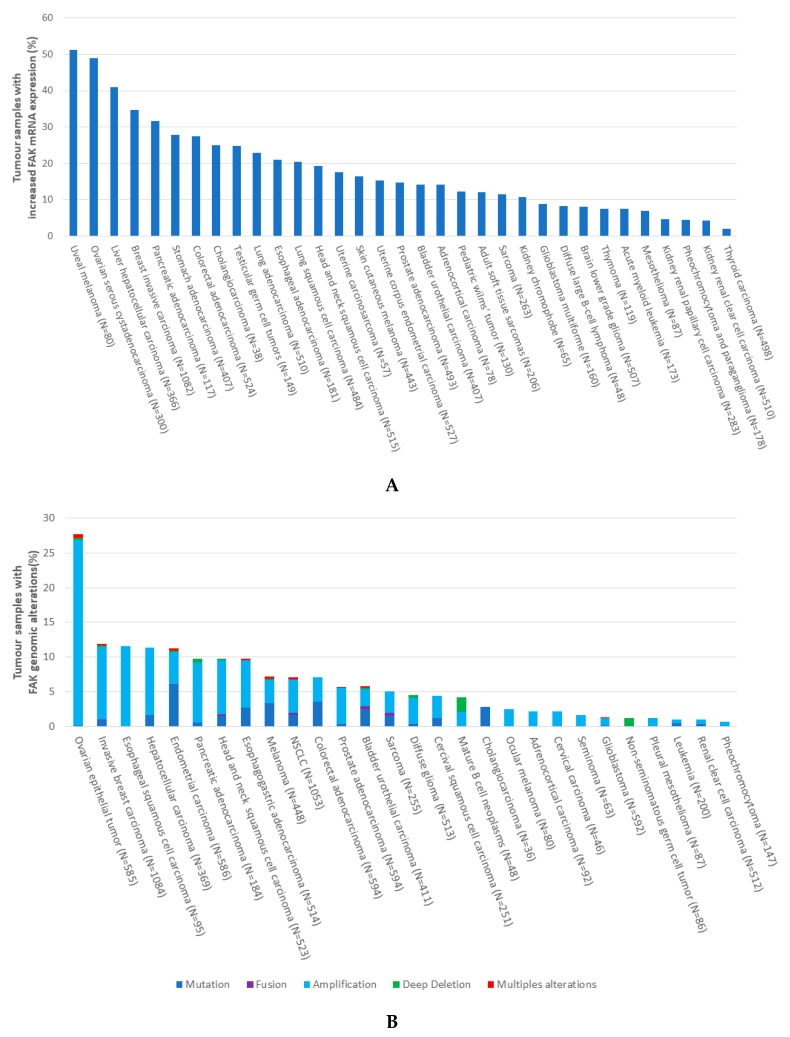
In The Cancer Genome Atlas (TCGA) database [110], we found increased FAK expression at the mRNA level in several human malignancies, including 51% of uveal melanoma, 49% of ovarian serous cystadenocarcinoma, 41% of liver hepatocellular carcinoma, 34% of breast invasive carcinoma, 23% of lung adenocarcinoma, and 20% of lung squamous cell carcinoma, while not being reported in SCLC (Figure 3A).
Figure 3.
(A) Frequency of increased focal adhesion kinase (FAK) expression at mRNA levels in human cancers. The Cancer Genome Atlas (TCGA) was queried using cbioportal.org to determine the percentage of tumor samples with increased levels of FAK mRNA expression. Search criteria included mRNA expression data (Z-scores for all genes) and tumor datasets with mRNA data. N = number of cancers analysed in the TCGA. (B) Frequency of focal adhesion kinase (FAK) genomic alterations in human cancers. The Cancer Genome Atlas (TCGA) was queried using cbioportal.org to determine the percentage of samples with FAK genomic alterations (mutations, fusions, amplifications, deep deletions, multiples alterations) in different cancers. Search criteria included PTK2 (FAK). N = number of cancers analysed in the TCGA.
Despite recent progress, the underlying mechanisms of FAK overexpression and activation in cancer, especially in SCLC, remain unclear. The control mechanisms include gene alterations, transcriptional regulation, post-translational modifications, and interaction with proteases, phosphatases, etc. Among gene alterations, FAK gene amplification within chromosome 8q24.3 and isochromosome formation has been described in many cancers [90,111].
Based on the TCGA database [110], the FAK copy number gain is found in 26% of ovarian epithelial tumors, 11.5% of oesophageal squamous cell, 10.4% of invasive breast, 9.7% of hepatocellular carcinoma, and less frequently in other tumors, such as 4.8% of NSCLC (Figure 3B), while there are no data related to SCLC. In SCLC, specifically, the genomic profiling of SCLC tumor samples while using genomic comparative hybridization revealed 70 regions of significant copy number gain and 55 regions of significant copy number loss, among which an enrichment of 11 genes associated with the focal adhesion pathway, including amplified FAK, was found [28]. The FAK gene copy number gain was confirmed by fluorescent in situ hybridization (FISH) in 80% of the SCLC tissues. FAK amplification was also correlated to increased FAK mRNA expression. At the protein level, as evaluated by IHC, FAK was expressed in the cytoplasm of 78/85 (92%) SCLC tissues [24]. In the TCGA database, point mutations with a single-base substitution in FAK gene, resulting in amino acid substitutions in FAK protein, are found in 6.1% of endometrial carcinoma, 3.5% of colorectal adenocarcinoma, 3.3% of melanoma, 2.7% of cholangiocarcinoma, and less frequently in other tumors, including NSCLC (Figure 3B), while no data are available in SCLC. Somatic mutations (A1004S point mutations) and splicing variants of FAK have been reported in 7.7% of human NSCLC (Figure 3B) [112] and they have been shown to exhibit increased autophosphorylation and increased sensitivity to FAK kinase inhibitors as compared with wild-type FAK in patient-derived xenograft models [112].
However, FAK gene copy number gains and mutations have not always been correlated with increased FAK expression or activity [28]. Therefore, epigenetic mechanisms may also play a role in increasing FAK expression or activity. Analysis of human FAK gene promoter has identified putative binding sites for transcription factors. NFκB [113], Argonaute 2 (Ago2) [114], and Nanog [59] are known to activate FAK transcription, while TP53 is a well described repressor of the FAK promotor [115]. Though not explored in SCLC, this last mechanism might be of particular interest in SCLC where TP53 is universally inactivated [116]. According to TCGA, concomitant TP53 mutation and FAK amplification/mutation co-occurred in 2% of all cancers. However, these data do not include SCLC samples. The lack of material that is dedicated to research is unfortunately a major obstacle in the study of SCLC.
Finally, FAK activation is induced by the engagement of integrins with the ECM or the binding of extracellular growth factors to their receptors. SCLC is well-known to release growth factors, such as bombesin, gastrin-related peptide (GRP), HGF, VEGF, TGF-β, HGF, and FGF, which have been shown to activate focal adhesion pathways in several cancers [117,118,119,120,121,122,123,124,125].
Similarly, it has been demonstrated that bombesin, gastrin, and bradykinin phosphorylated FAK in SCLC cell lines in vitro [126], which suggests autocrine and paracrine regulation.
3. FAK Role in Proliferation, Cell Cycle, and Survival
FAK activation during cell adhesion protects cells from anoikis, a form of apoptosis that is induced by cell detachment from ECM, favouring cancer growth and metastasis [127]. FAK is implicated in several pathways that contribute to cell survival. Phosphorylated FAK at Tyr397 can bind PIK3R2, which leads to the activation of AKT that inhibits apoptosis by regulating various molecules. Among other mechanisms, there is the suppression of apoptosis by FAK through c-JUN kinase activation downstream of CAS [33] and the inhibition of RIP interaction with the death receptor complex [128].
FAK also induces cell proliferation through the stimulation of cell cycle progression. One of the mechanisms is the formation of FAK/Src complex that allows for Src to phosphorylate FAK at Tyr925 and mediate its interaction with Grb2, which leads to the activation of the RAS-MAPK signaling pathway [40]. Another mechanism involves the FAK-induced increased expression of cyclin D1 and decreased expression of cycline-dependent kinase (Cdk) inhibitor p21 [129,130,131,132]. Other cell cycle regulators, such as cyclin E, Cdk inhibitor p27, and Skp2, also mediate FAK regulation of cell cycle progression [133,134,135,136]. Moreover, FAK is important for tumor cell-induced remodeling of the tumor matrix, which produces a rigid microenvironment and facilitates cell proliferation [137].
Specifically, in SCLC cell lines, it has been shown that the inhibition of FAK activity with PF-573,228, a FAK TKI, decreased proliferation, DNA synthesis, induced cell-cycle arrest in G2-M phases, and increased apoptosis in the NCI-H82, NCI-H146, NCI-H196, and NCI-H446 SCLC cell lines [138]. Treatment with increasing concentrations of PF-228 (0.1 to 10 µM) dose-dependently decreased the FAK phosphorylation (Tyr397) in these four cell lines, without modifying total FAK expression, and the inhibition of FAK activity with 1 to 10 µM PF-228 significantly decreased their proliferation, also dose-dependently (p < 0.001 for all tested concentrations beside 1 µM in NCI-H196), as assessed by a WST-1 assay. Cell cycle analysis showed that PF-228 inhibited progression through cell cycle by significantly reducing the S phase and inducing cell cycle arrest in the G2/M phases in the four cell lines after 24h-treatment, dose-dependently (p < 0.001). PF-228 at concentrations of 1 to 5 µM also significantly induced apoptosis in the four cell lines, as demonstrated by a dose-dependent increase of PARP p85 expression by WB after 48h-treatment. This was confirmed by flow cytometry in NCI-H82 and NCI-H446 cell lines, with a significant increase of BrdU-positive and activated Caspase 3-positive cells after 48h-treatment (p < 0.001 for all tested concentrations, except 1 µM in NCI-H446 in the Caspase-3 assay). Genetic inhibition of FAK through stable transduction with FAK shRNA and/or FAK-related non-kinase (FRNK), a splice variant lacking the N-terminal and kinase domains of FAK, revealed that the FAK-targeting (FAT) domain (attached to focal adhesion complex, where it inhibits pro-proliferative proteins) was necessary to inhibit proliferation, cell cycle progression, and survival [138]. Indeed, FAK shRNA transduction did not affect these functions, while the restoration of FAT domain by FRNK transduction inhibited proliferation, DNA synthesis, and induced apoptosis in the evaluated SCLC cell lines. Additionally, while FAK shRNA transduction increased the active Rac1 level, FRNK re-expression in cells that were previously transduced with FAK shRNA decreased it. Therefore, this study not only suggested that FAK is important in SCLC biology, but also that targeting its kinase domain might have a therapeutic potential, while targeting its FAT domain might have Rac1-mediated pro-tumoral activity and thus should be avoided.
4. FAK Role in Adhesion, Migration, and Invasion
FAK induces morphological changes in cells, including the formation of podosomes or invadopodia, contributing to cell migration [68,139,140]. Moreover, cancer cells overexpressing FAK are able to invade tissues [141]. FAK overexpression contributes to the metastatic phenotype of cancer cells by promoting cell migration and invasion.
Cell migration is a complex process that consists of several coordinated events, including protrusion of the leading edge, adhesion of the leading edge to the substrate [142], translocation of the cell body, and release of the trailing edge [143]. Thus, a strict regulation of tension at specific times and in specific areas of the cell is required for cell migration [144,145], where FAK plays an important role by sensing the mechanical forces that are generated in or exerted on cells [146], and modulating cell responses to environmental stimuli. Once activated by integrins, G protein-coupled receptors ligands, or growth factors, FAK is autophosphorylated at Tyr397 and activates proteins, such as Src, p130CAS, paxillin, and PIK3R2 [147], to regulate adhesion turnover at the cell front, a process that is central to migration [147,148,149,150,151]. FAK is indeed required for the organization of the leading edge in migrating cells [152]. The formation of a complex between FAK and Src, leading to the phosphorylation of the adaptor molecule CAS by the FAK/Src complex [153,154,155,156,157], is one of the best characterized downstream signaling pathways that mediate FAK-stimulated cell migration. A second mechanism involves FAK interaction with PIK3 and an adaptor molecule, Grb7 [158,159]. A third mechanism involves the modulation of the assembly and disassembly of actin cytoskeleton through the effect of FAK on the Rho family GTPases. Among the Rho family GTPases, FAK/Src signaling has, in particular, been implicated in regulating the activities of Rac1 and RhoA.
Besides its role in cell migration, FAK promotes invasion in normal and cancer cells by various mechanisms. In one of them, FAK promotes the formation of the Src-CAS-Crk-Dock180 complex, which activates Rac1 and JNK, and leads to increased expression or activity of metalloproteinases 2 (MMP2) and 9 (MMP9) [68]. MMPs are concentrated and activated at actin-rich cell/ECM contacts, termed podosomes or invadopodia, which are distinct from focal adhesion. In another mechanism, FAK cooperates with Src to disrupt the E-cadherin-based intercellular adherens junctions [160], contributing to EMT and, therefore, to the invasive phenotype of metastatic carcinomas through increased cell migration and remodelling of the ECM microenvironment [161,162,163]. In SCLC cell lines, the pharmacologic inhibition of FAK with PF-573,228 decreased cell adhesion [28], as well as migration and invasion [138]. In NCI-H69, NCI-H146, and NCI-H209 SCLC cell lines, PF-573,228 induced a dose-dependent decrease of cell adhesion on laminin, with the effect becoming statistically significant at the concentration of 10 µM (NCI-H69: p = 3 × 10−4, NCI-H146, and NCI-H209: p = 1 × 10−4 as compared to DMSO) [28]. Moreover, a wound healing assay combined with time-lapse microscopy showed that PF-573,228 decreased the migration velocity of two SCLC cell lines with an adherent component, from 5 to 2.5 µm/min. in NCI-H196 (p = 0.0561) and from 9 to 4 µm/min. in NCI-H446 (p = 0.0916)) [68]. Modified Boyden chambers showed that PF-573,228, at a concentration of 3 µM, also inhibited invasion, with the number of invasive cells being able to migrate to the lower side of the insert separating the two Boyden chambers, decreasing from 150 to 50 per field (20× magnification) for NCI-H196 and from 45 to five per field for NCI-H446 [68].
5. FAK in Epithelial to Mesenchymal Transition
Through epithelial-to-mesenchymal transition (EMT), cancer cells acquire a more motile phenotype, promoting invasion, metastasis, but also conferring resistance to chemotherapies and targeted therapies. Epithelial cancers undergoing EMT acquire transient mesenchymal features, like Vimentin and N-cadherin, which are associated with the loss of epithelial markers E-cadherin and β-catenin [164]. EMT is correlated with poor outcomes in SCLC [165], such as in many other cancers. Identified mechanisms inducing EMT in SCLC include inactive Notch signaling [166], activated MET receptor signaling through hepatocyte growth factor [165], and activated TGFβ/Akt signaling [167].
While FAK-mediated EMT has not yet been explored in SCLC, its important role has been demonstrated in other cancers and non-malignant cells [168,169,170,171]. Impaired FAK functions lead to a defective mesenchymal phenotype during EMT. Hence, upon TGF β-induced EMT, hepatocyte cell lines transduced with FRNK, a genomic method for inhibiting FAK, underwent an incomplete mesenchymal transition, exhibiting a lack of mesenchymal markers MMP9 and fibronectin and a persistence of membrane-bound E-cadherin [168]. Mammary tumor cells with deficient FAK scaffolding function due to Pro 878/881 mutation also displayed incomplete mesenchymal phenotype with increased E-cadherin and decreased N-cadherin, Vimentin, and fibronectin in a mice model [169]. It was associated with decreased metastasis potential and decreased expression of EMT-inducing gene Snail 1 [169]. A similar reduction of Snail 1 in embryonic FAK-null cells has been associated with the inability to display mesenchymal cell characteristics, while the reexpression of FAK restored mesenchymal phenotype and Snail 1 level through PI3K/Akt signaling [170]. In ovarian cancer, FAK controls EMT by upregulating transcription factor KLF8 via the PI3K/Akt pathway [171]. It has been shown that transcription factors Snail 1 and KLF8 repress E-cadherin expression, promoting EMT in various normal and malignant cells [172,173,174]. The inhibition of FAK by a genetic or a pharmacologic method decreased the EMT features and aggressiveness in colorectal carcinoma cell lines [175,176] and triple negative breast cancer cell lines in vitro [177], but not in NSCLC cell lines in vitro [178].
6. FAK-Mediated Angiogenesis and Vascular Permeability
The role of angiogenesis and vascular permeability is fundamental to the progression of cancer from localized to advanced-stage disease [179,180,181]. The tumors induce local generation (vasculogenesis) and subsequent growth (angiogenesis) of new vasculature that facilitates the supply of oxygen and nutrients to cancer cells [180]. Moreover, it has been shown that SCLC cells in tumors or in the blood harbours markers of vascular mimicry, including the expression of vascular endothelial cadherin (VE-Cadherin). Therefore, vascular mimicry could supply nutrient and oxygen required for the expansion of SCLC cells [182]. Furthermore, several molecules have been shown to promote angiogenesis and/or vascular permeability, for instance vascular endothelial growth factor (VEGF), hypoxia inducible factor (HIF), fibroblast growth factor (FGF), transforming growth factor beta (TGF-β), hepatocyte growth factor (HGF), tumor necrosis factor-α, angiogenin, ephrins, and angiopoietins [123,179,181,183,184]. SCLC produces many of these pro-angiogenic factors, including VEGF, TGF-β, HGF, and FGF [117,118,119,120,121,122]. Moreover, SCLC displays a higher vascularisation when compared to other tumours. Both high tumor vascularisation and high VEGF expression are associated with a poor outcome in SCLC [122,185]. High VEGF expression has also been correlated to an increased risk of extensive disease [185]. This stressed out the strong connexion between angiogenesis, vascular permeability, and the development of metastases in SCLC, which is a highly metastatic disease with a high prevalence of circulating tumour cells (CTCs) (Figure 4A) [1,11,186,187,188]. Several clinical trials have demonstrated that antiangiogenic agents, such as bevacizumab, pazopanib, and sunitinib, increased the progression-free survival PFS in SCLC, despite that they failed to show a significant benefit in terms of OS [189,190,191,192]. These results are probably related to the absence of relevant biomarkers to select patients that might benefit from antiangiogenic agents.
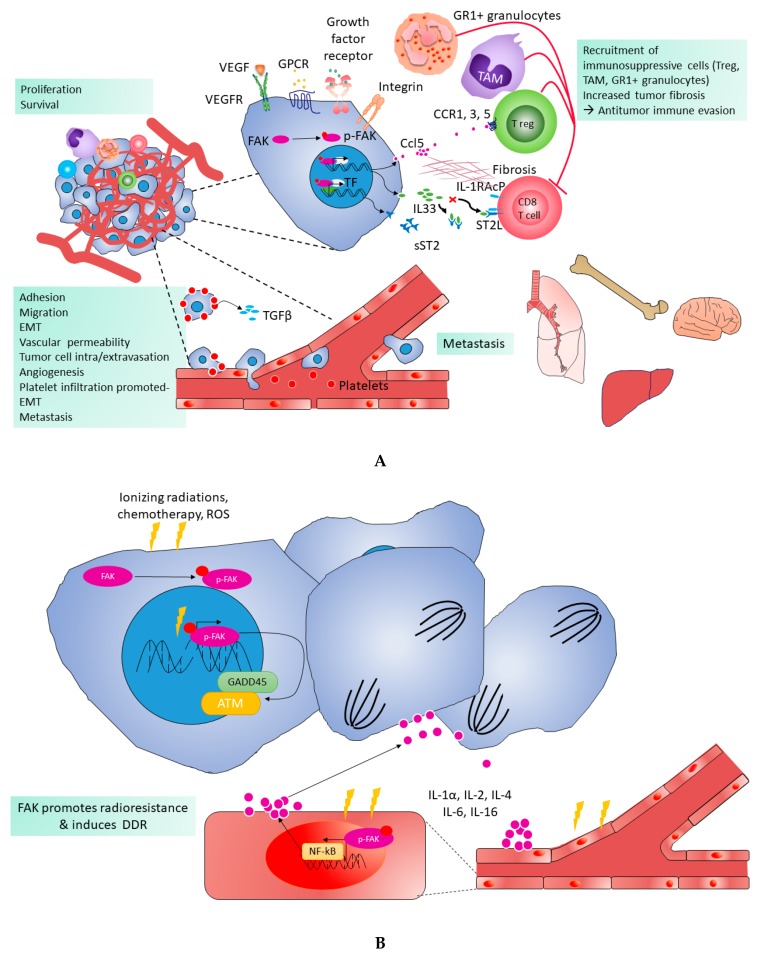
Figure 4.
Pro-tumoral functions of FAK. (A). FAK is triggered off by integrins, G protein-coupled receptors (GPCR), growth factor receptors, and vascular endothelial growth factor receptor (VEGFR). Activated FAK promotes cell proliferation and survival. FAK also contributes to tumor progression and metastasis via cell adhesion, migration, and promotion of epithelial to mesenchymal transition (EMT). Transient contact between platelets and tumor cells induces TGFβ production by the platelets, which promotes EMT-like transformation and invasive behaviour. In endothelial cell (EC), FAK drives angiogenesis, increases vascular permeability, and regulates platelet extravasation; this facilitates intravasation or extravasation of tumor cells, leading to metastasis. Additionally, FAK induces a tumor protective fibrotic and immunosuppressive tumor microenvironment that promotes antitumor immune evasion. Indeed, FAK induces cytokines (short soluble (sST2), IL33, Ccl5), which lead to the recruitment of immunosuppressive cells, such as regulatory T cells (Treg), tumor-associated macrophages (TAM), and GR1+ granulocytes, as well as to increased tumor fibrosis. Pro-tumoral functions of FAK. (B). Ionizing radiations, chemotherapy, and reactive oxygen species (ROS) increase DNA damage and activate FAK in tumor cells. Activated FAK favors the expression of DNA damage repair (DDR) genes such as Growth Arrest and DNA Damage-inducible 45 (GADD45), Ataxia Telangiectasia Mutated (ATM) genes, and Ataxia Telangiectasia and Rad3-related (ATR) genes which play an important role in resistance to drug and radiation. Additionally, in endothelial cells (EC), ionizing radiations activate FAK and NF-kB, which induces the production of various cytokines (IL-1α, IL-2, IL-4 IL-6, IL-16) activating the proliferation of tumor cells. Abbreviations used in the figure and not described in the legend: IL-1RAcP: interleukin-1 receptor accessory protein, ST2L: longer membrane bound form.
Interestingly, FAK has a crucial role in angiogenesis and vascular permeability, as demonstrated by the vascular defects in FAK double knockout mice, resulting from the inability of FAK-deficient endothelial cells to organise themselves into vascular networks [193]. Additionally, the overexpression of FAK in vascular endothelial cells promotes angiogenesis [194]. Additionally, VEGF-induced vascular permeability is mediated by FAK signaling (Figure 4A), with the inhibition of FAK activity in endothelial cells suppressing VEGF-stimulated vascular permeability [195]. It has been shown that FAK trigger off by VEGF is abrogated by FAK inhibitors, which decrease vascular permeability and tumor vasculature, preventing tumor growth, metastasis, and immunosuppressive tumor infiltration by cells, such as tumor macrophages and T regulatory cells (Figure 4A) [109,195,196,197,198,199]. Additionally, it has been shown that the withdrawal of antiangiogenic therapy results in accelerated tumor growth and that FAK activation mediates this tumor rebound, which increases angiogenesis and platelet infiltration (Figure 4A) [200]. Interestingly, FAK inhibition prevents tumor rebound after the cessation of antiangiogenic therapy through the inhibition of tumor angiogenesis, platelet-induced tumor cell proliferation, and vascular leakage (Figure 4A) [200,201,202,203]. Of note, there is no data regarding the role of FAK in angiogenesis and vascular permeability, specifically in SCLC.
7. FAK and DNA Damage Repair
Exposure to endogenous and exogenous carcinogens (reactive oxygen species, UV light, tobacco smoking, ionizing radiation, platinum chemotherapy…) generates DNA damage in both normal and cancer cells [204]. Signaling pathways that are activated by cells to sense and repair DNA damage, preventing genomic instability, are known as DNA damage repair (DDR) [205,206]. DNA-damaging chemotherapy and radiotherapy are used alone or in combination in the treatment of ES- and LS-SCLC, respectively. SCLC tumors are initially responsive to the treatment, but the development of early resistance limits outcomes. Objective response rates of 80–90% are achieved in LS-SCLC treated by concurrent radiochemotherapy [12,13] and of 60–70% in ES-SCLC treated by platinum-based chemotherapy [207,208], but the median OS is only 25–30 months in LS-SCLC and 12 months in ES-SCLC [12,17,209]. Understanding the underlying mechanisms of acquired or intrinsic radioresistance and/or chemoresistance is important in the improvement of SCLC survival.
It has been shown that DDR genes and proteins are more highly expressed and activated in SCLC as compared to NSCLC and that blocking these DDR pathways has antitumoral activity in both preclinical [210] and clinical [211] studies, including many different types of cancer. In SCLC specifically, the association of the PARP inhibitor olaparib and the anti-PD-L1 ICI durvalumab in a phase II trial did not meet efficacy criteria, but revealed that responses were only observed in tumors with an inflamed phenotype on tissue biopsies at baseline, which suggests that the tumor microenvironment inflammation phenotype is a potential predictive biomarker [212]. Another phase II trial with the PARP inhibitor veliparib combined or not to the chemotherapy agent temozolomide in recurrent SCLC showed improved overall response rate without improvement of PFS and OS in the combination arm, but patients with SLNF11 (inhibitor of DNA replication)-positive tumors treated with the association had a significantly improved PFS and OS, which suggests that SLNF11 is a predictive biomarker [211].
Interestingly, FAK promotes DDR by promoting the transcription of genes favoring DDR, such as growth arrest and DNA damage-inducible 45 (GADD45), ataxia telangiectasia mutated (ATM), and ataxia telangiectasia and Rad3-related (ATR) (Figure 4B) [213,214]. Furthermore, FAK inhibition promotes the hyperactivation of downstream targets of ATM/ATR, such as checkpoint kinase 2 (CHK2) [215]. In in vitro and in vivo preclinical models of NSCLC harbouring KRAS mutations, ionizing radiation leads to FAK activation (Tyr397 phosphorylation), which persists for several hours, while the inhibition of FAK activity leads to an inherent loss of DNA repair capacity and radiosensitizing effects that promote the therapeutic effect of ionizing radiation [213,214,216]. Similarly, FAK has also been shown to regulate human ductal carcinoma in situ (DCIS) cancer stem cells (CSC) activity and response to radiotherapy [217]. While CSC harbor the ability to avoid or efficiently repair DNA damage from radiotherapy and chemotherapy, which play a role in disease recurrence, inhibition of FAK activity potentiated the effect of irradiation in DCIS CSC [217]. Finally, it has been shown that FAK regulates tumor resistance to DNA-damaging therapies through NF-kB activation and subsequent cytokine production. Interestingly, FAK inhibition sensitizes tumour cells to chemotherapy by suppressing NF-kB activation and subsequent cytokine production (IL-1α, IL-2, IL-4, IL-6, IL-16…) (Figure 4B) [217]. Even though no data are available regarding the role of FAK in DDR, specifically in SCLC, we hypothesize that FAK TKI might also be used in SCLC to improve the efficiency of chemotherapy and/or radiotherapy by impairing DDR and/or increasing DNA damage based on these findings in other cancers.
8. FAK and Radioresistance
Radiotherapy that is associated with chemotherapy remains the cornerstone of LS-SCLC treatment, despite the frequent emergence of resistance and cancer recurrence. Understanding the underlying mechanisms of acquired or intrinsic radioresistance is important in the improvement of SCLC survival. Several mechanisms have been involved in tumor radioresistance. Among those, adhesion molecules have a key role against radio-induced apoptosis, in a phenomenon called “cell adhesion-mediated resistance” [218,219,220,221,222]. In SCLC, the spontaneous transformation of cell lines in culture, since several months into more adherent and radioresistant sublines highlights this mechanism [223,224]. FAK, as a key player in the focal adhesion pathway, mediates this anti-apoptotic action against ionizing radiation. Hence, the irradiation of a promyelocytic leukemia cell line overexpressing FAK induced less DNA fragmentation and cell death than in the control cells [225]. Accordingly, a proteomic analysis showed that FAK expression was strongly correlated with radioresistance in a large panel of head and neck (HN) squamous cell carcinoma (SCC) cell lines [226]. Moreover, ionizing radiation upregulated the in vitro expression and activation of FAK in breast cancer, glioblastoma, and lung cancer cell lines, leading to acquired radioresistance [227,228]. The inhibition of FAK using genetic (FAK shRNA transduction) or pharmacological (FAK TKI) methods radiosensitized KRAS-mutated NSCLC significantly decreased radiation survival in vitro and in vivo [215]. Similar results have been reported in HNSCC [226,229,230] and in pancreatic carcinoma [218].
Several FAK downstream signaling pathways have been involved in FAK-mediated survival after ionizing radiation. In a promyelocytic leukemia cell line overexpressing FAK, the Phosphoinositide 3-kinase (PI-3K)-Akt survival pathway is constitutively activated. Moreover, FAK prevents radiation-induced cell death by downregulating the mediator of apoptosis Caspase 8 and by upregulating inhibitor-of-apoptosis proteins, like c-IAP and XIAP [225]. Concomitant activation of NF-κB has also been reported [225]. FAK inhibition radiosensitized HNSCC cells lines in vitro through MAPK and Akt signaling dephosphorylation [230]. In spontaneous radioresistant SCLC cell lines, constitutive activation of Akt and MAPK pathways and increased level of active NF-κB are similarly observed [224]. FAK interaction with JNK1 also has an important role for radioresistance in pancreatic carcinoma cell lines [218] and in HNSCC cell lines [218].
Even though not explored in vivo yet, FAK inhibition might be a useful approach for improving radiotherapy efficacy in SCLC. Nevertheless, cautions are mandatory, since the effects of FAK inhibition on radiosensitivity depend on the tumor type. While FAK pharmacological inhibition combined with radiation radiosensitized HNSCC, it did not show any additional effect in vitro on ionizing radiation lethality in non-Kras mutated NSCLC, colorectal carcinoma, and pancreatic carcinoma cell lines [229].
9. Regulation of Cancer Stem Cells
CSC hypothesis has been developed over the last 150 years [231] and progressively replaced the clonal evolution theory in carcinogenesis [232]. This model postulates that the tumor arises from a subpopulation of pluripotent cells that are capable of extensive self-renewal and resistance to ionizing radiation and chemotherapies. Altogether, these aggressive subtypes of malignant cells are presumed to be responsible for recurrence after treatment [233]. The existence of CSCs in SCLC has been demonstrated in cell lines and primary tumors [234,235,236,237,238], participating in therapy resistance and the rapid recurrence of SCLC [237,239,240]. CSCs have been identified in SCLC based on the analysis of cell surface markers and functional properties, such as the capacity to exclude Hoechst dye, to form tumorspheres, and to initiate tumor after xenotransplantation in mice, mirroring their tumorigenicity. In SCLC, common markers that are used to study CSCs are CD133, ALDH1, pluripotency-related gene Nanog, Oct3/4, and Sox 2, among others (reviewed in [241]). Some of these markers have been correlated with poor prognosis [242,243,244]
While not explored yet in SCLC, the critical role of FAK in CSCs maintenance has been described in several cancers. It has been demonstrated that the CSC marker Nanog upregulates FAK, which, in turn, phosphorylates Nanog in CRC cell lines [59]. Upregulation and activation of FAK has also been observed in the presence of Oct 3/4-surexpressing glioblastoma primary cell cultures [245]. CD133, another CSC marker, enhanced cells migration through Src-FAK signaling activation [246]. Furthermore, a strong influence of ECM in sustaining CSCs through FAK signaling has been demonstrated in pancreatic ductal adenocarcinoma, colorectal cancer, and breast cancer [247,248,249]. Additional proof of FAK implication in CSCs is that several drugs that are effective against CSCs act through FAK inhibition [250,251,252,253]. Several studies have demonstrated that FAK inhibition preferentially eliminates CSCs pool in vivo and in vitro in various cancers [217,247,254,255,256,257,258,259]. In pancreatic ductal adenocarcinoma, FAK inhibition with a TKI or shRNA impacted tumor-initiating potential, self-renewal, and metastasis, and improved the response to chemotherapy via CSCs regulation in vitro and in vivo [247]. FAK TKI more efficiently decreased proliferation and survival of the CSCs subpopulation in malignant mesothelioma [254,255], and its administration after chemotherapy improved disease-free survival in a mouse model [255]. In breast cancer, similar effects of FAK inhibition were obtained on the CSCs pool in vivo and in vitro [217,256,257] and on the duration of response after chemotherapy [257]. FAK knockout mice prevented the induction and growth of skin SCC, which suggested the decreased capacity of CSCs generation and maintenance [258]. Finally, colorectal CSCs were preferentially targeted by FAK TKI in vitro in human cell lines as compared to non-CSCs [259]. FAK kinase dependent and independent-functions have both been implicated in CSCs maintenance and regulation in breast cancer [260]. Interestingly, FAK inhibition suppressed β-catenin activation, which confirmed a crosstalk between FAK and Wnt/β-catenin pathway [217,257]. We hypothesize that combination of FAK TKI with conventional treatment might be a pertinent strategy to explore in order to improve outcome given the poor response and rapid recurrence of SCLC after chemotherapy.
10. FAK in Tumor Immune Escape
ICIs induced remarkable improvements in tumor response and OS in many types of solid tumors, including NSCLC, both in pretreated and treatment-naive advanced-stage disease [3,4,6,9,261,262]. The most robust objective response rates to ICIs have been shown in tumors with high PD-L1 expression, even though PD-L1 remains an imperfect biomarker [263]. As opposed to NSCLC, there is a lack of correlation between PD-L1 expression and the response to ICIs in SCLC [264] and the efficacy of ICIs in terms of response rates and OS is limited in SCLC patients [17]. The IMpower133 trial, comparing carboplatin plus etoposide with or without atezolizumab, a PD-L1 inhibitor, in the first-line treatment of patients with ES-SCLC, showed only a two-month improvement in OS in the atezolizumab arm. [6]. Nevertheless, it was the first time since several decades that an improved survival was obtained in ES-SCLC. Based on this study, chemotherapy combined with atezolizumab recently became the new standard of care in the first-line treatment of ES-SCLC.
SCLC displays high capacities to escape immune surveillance through several processes. Among those, it has been demonstrated that SCLC cell lines have the capacity to induce regulatory T cell (Tregs) in vitro [265]. This is an important mechanism, as Tregs infiltration in SCLC biopsies has been correlated with the poor survival of patients [265]. Interestingly, a study recently demonstrated a role for FAK in controlling Treg levels in cutaneous and pancreatic tumors [17,266]. In skin SCC, FAK drove the recruitment and expansion of Tregs within the tumor, subsequently impairing the antitumor response of CD8+ cytotoxic T lymphocytes [266]. The xenograft of FAK-deficient SCC in mice failed to durably develop and exhibit a CD8+ T cells-dependent tumor regression within 21 days, as opposed to FAK-wild type tumor cells [266]. The pharmacological inhibition of FAK in a skin SCC mouse model decreased the levels of Tregs and increased those of CD8+, which confirmed the key role of FAK in immune escape [266]. Similar results were observed in pancreatic ductal adenocarcinoma and colorectal cancer, where association of FAK inhibitors with immunotherapy markedly improved survival of the mice [17,267]. Mechanistically, FAK controls Tregs infiltration in skin SCC through the transcription of chemokines and cytokines via its nuclear interaction with transcription factors and regulators [199,266]. Among those increased genes, Ccl1, Ccl5, and TGFβ2 have been involved in Tregs conversion and recruitment in various cancers [109,268,269,270,271,272].
Additionally, the immunosuppressive role of myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) promoting tumor development by impairing antitumor immunity has been described in various cancers [273]. In SCLC, the peripheric MDSC count has been correlated with poor prognosis [274] and tumor progression induced by TAM has been demonstrated in vitro [275]. Interestingly, FAK TKI also decreased the tumor-infiltrating immunosuppressive cells in pancreatic [17,276] and breast cancers [277]. In SCC, FAK TKI promoted tumor control by reducing tumor-infiltrating regulatory T cells and increasing the T CD8+ T cells [266]. Furthermore, it has been shown that FAK promotes the expression of interleukin-33 (IL-33), soluble secreted form of the IL-33 receptor, called soluble ST2 (sST2), and chemokine CCL5 (CCL5) in SCC cells. Therefore, IL-33 and ST2 mediate FAK kinase-dependent antitumor immune evasion [199].
Even though the role of FAK in immune tumor escape has not been proven yet in SCLC, these studies raise the hope of improving the outcome of patients through the association of FAK TKI with immunotherapy or conventional chemotherapy. In advanced pancreatic cancer, mesothelioma, and NSCLC, a clinical trial evaluating the association of FAK (VS6063) and PD-1 (pembrolizumab) inhibitors is ongoing (NCT02758587).
11. Prognostic and Predictive Value of FAK Alterations
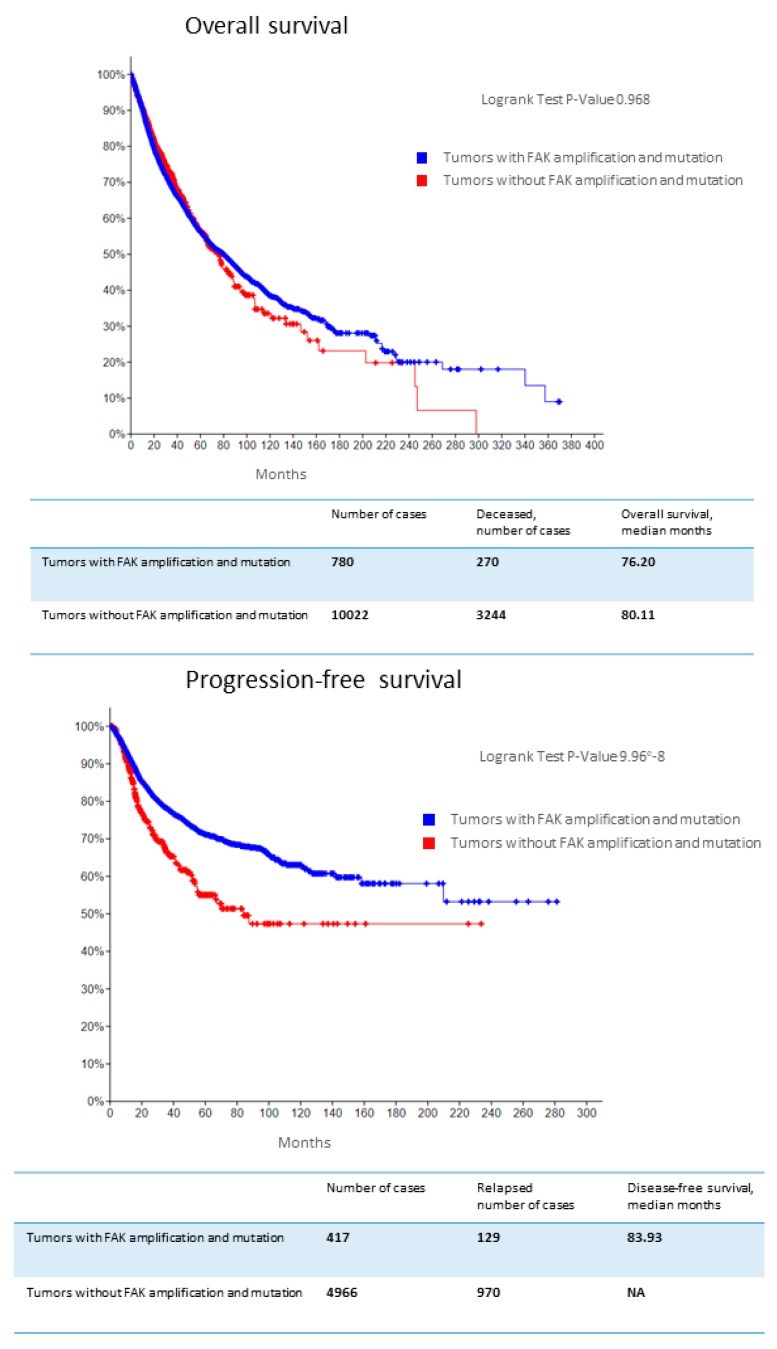
FAK genetic alterations that were reported in the Cancer Cohort of TCGA project were correlated with PFS (Figure 5), and FAK overexpression at mRNA and protein levels were correlated with poor OS in several cancers [200,278]. FAK protein overexpression was associated with worse OS in gastric cancer (HR = 2.646, 95% CI:1.743–4.017, p = 0.000), hepatocellular cancer (HR = 1.788, 95% CI: 1.228–2.602, p = 0.002), ovarian cancer (HR = 1.815, 95% CI: 1.193–2.762, p = 0.005), endometrial cancer (HR = 4.149, 95% CI: 2.832–6.079, p = 0.000), gliomas (HR = 2.650, 95% CI: 1.205–5.829, p = 0.015), and squamous cell head and neck and digestive cancers (HR = 1.696, 95% CI: 1.030–2.793, p = 0.038) [200].
Figure 5.
Association of focal adhesion kinase (FAK) amplification with survival. Kaplan-Meier overall survival and progression-free survival analysis of patients with versus without FAK amplification in their tumors (many different cancers included) in The Cancer Genome Atlas (TCGA) database [110].
In SCLC, no correlation was found between total FAK expression evaluated by IHC on 85 SCLC tissues and SCLC disease stage, response to therapy, PFS, or OS [24]. Similarly, total FAK and phospho-FAK (Y397) expression evaluated by multiplex immunofluorescence in tissues from 105 SCLC and 95 NSCLC patients did not correlate with PFS or OS [67]. However, a predictive value of response to FAK TKIs cannot be ruled out, even in the absence of a prognostic value. Several clinical trials have evaluated FAK TKI in patients suffering from various advanced-stage cancers, showing antitumor activity (up to 33% objective response rates) and safety [35,36,38,40], while they did not use biomarkers, such as FAK or phospho-FAK expression to identify patients that are likely to respond to FAK TKI. It would be interesting for future clinical trials evaluating FAK TKI to prospectively test total FAK and activated FAK expression as potential predictive biomarkers of response to FAK TKI.
12. Conclusions and Therapeutic Perspectives
In this review, we have presented a brief overview on the role of FAK in cancer development and progression, through its functions in cell growth, survival, adhesion, spreading, migration, invasion, angiogenesis, DNA damage repair, radioresistance, and regulation of CSC. This constitutes the biological rationale to consider FAK as a potential therapeutic target in SCLC. The association of FAK inhibitors with standard therapies of SCLC—platinum-based chemotherapy, radiochemotherapy, or immunotherapy—might have synergistic effects and improve the outcomes of SCLC patients. We hope that the development of specific FAK inhibitors will have clinical translational significance in SCLC by targeting, among others, antitumor immunity, angiogenesis, EMT, regulation of CSC, DDR, and therapy resistance, including radioresistance, which are crucial in SCLC biology.
Acknowledgments
F. Aboubakar Nana was supported by Télévie (Fonds National de la Recherche Scientifique (FNRS)) (7.4624.15), Fonds Spécial de Recherche (FSR) (Communauté Française de Belgique), and Fondation Willy and Marcy De Vooght, Belgium. M. Vanderputten was supported by Fund for Research Training in Industry and Agriculture (FRIA) (FNRS) (1.E.108.19F), Belgium. Ocak was supported by grants from Fondation Mont-Godinne (FMG-2011-BR-02, FMG-2013-BR-02, FMG-2014-BR-01, FMG-2015-BR-02, FMG-2016-BR-02, FMG-2017-BR-04, and FMG-2018-BR-01), Télévie (FNRS) (7.4588.10F and 7.4624.15), FRIA (FNRS) (1.E.108.19F), FSR, and Secteurs des Sciences de la Santé, Université catholique de Louvain (UCLouvain), Belgium.
Appendix A
Figure A1.
PRISMA guidelines for methodological review of literature related to frequency of focal adhesion kinase (FAK) overexpression at protein level in human solid cancers.
Funding
The APC was funded by Fondation Mont-Godinne (FMG-2018-BR-01).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Govindan R., Page N., Morgensztern D., Read W., Tierney R., Vlahiotis A., Spitznagel E.L., Piccirillo J. Changing Epidemiology of Small-Cell Lung Cancer in the United States Over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database. J. Clin. Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Recondo G., Facchinetti F., Olaussen K.A., Besse B., Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: First-generation or next-generation TKI? Nat. Rev. Clin. Oncol. 2018;15:694–708. doi: 10.1038/s41571-018-0081-4. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J., Reckamp K.L., Baas P., Crino L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet Lond. Engl. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gümüş M., Mazières J., Hermes B., Çay Şenler F., Csőszi T., Fülöp A., et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 10.Wang J.C., Sone S., Feng L., Yang Z.G., Takashima S., Maruyama Y., Hasegawa M., Kawakami S., Honda T., Yamanda T. Rapidly growing small peripheral lung cancers detected by screening CT: Correlation between radiological appearance and pathological features. Br. J. Radiol. 2000;73:930–937. doi: 10.1259/bjr.73.873.11064644. [DOI] [PubMed] [Google Scholar]
- 11.Thomas A., Pattanayak P., Szabo E., Pinsky P. Characteristics and Outcomes of Small Cell Lung Cancer Detected by CT Screening. Chest. 2018;154:1284–1290. doi: 10.1016/j.chest.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turrisi A.T., Kim K., Blum R., Sause W.T., Livingston R.B., Komaki R., Wagner H., Aisner S., Johnson D.H. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 13.Kubota K., Hida T., Ishikura S., Mizusawa J., Nishio M., Kawahara M., Yokoyama A., Imamura F., Takeda K., Negoro S., et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): A randomised phase 3 study. Lancet Oncol. 2014;15:106–113. doi: 10.1016/S1470-2045(13)70511-4. [DOI] [PubMed] [Google Scholar]
- 14.Pujol J.L., Daures J.P., Riviere A., Quoix E., Westeel V., Quantin X., Breton J.L., Lemarie E., Poudenx M., Milleron B., et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: A French Federation of Cancer Institutes multicenter phase III randomized study. J. Natl. Cancer Inst. 2001;93:300–308. doi: 10.1093/jnci/93.4.300. [DOI] [PubMed] [Google Scholar]
- 15.Saunders L.R., Bankovich A.J., Anderson W.C., Aujay M.A., Bheddah S., Black K., Desai R., Escarpe P.A., Hampl J., Laysang A., et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015;7:302ra136. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudin C.M., Pietanza M.C., Bauer T.M., Ready N., Morgensztern D., Glisson B.S., Byers L.A., Johnson M.L., Burris H.A., Robert F., et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet. Oncol. 2017;18:42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn L., Mansfield A.S., Szczęsna A., Havel L., Krzakowski M., Hochmair M.J., Huemer F., Losonczy G., Johnson M.L., Nishio M., et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 18.Stinchcombe T.E. Current Treatments for Surgically Resectable, Limited-Stage, and Extensive-Stage Small Cell Lung Cancer. Oncologist. 2017;22:1510–1517. doi: 10.1634/theoncologist.2017-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Povsic M., Enstone A., Wyn R., Kornalska K., Penrod J.R., Yuan Y. Real-world effectiveness and tolerability of small-cell lung cancer (SCLC) treatments: A systematic literature review (SLR) PLoS ONE. 2019;14:e0219622. doi: 10.1371/journal.pone.0219622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carelli S., Zadra G., Vaira V., Falleni M., Bottiglieri L., Nosotti M., Di Giulio A.M., Gorio A., Bosari S. Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer. 2006;53:263–271. doi: 10.1016/j.lungcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Dy G.K., Ylagan L., Pokharel S., Miller A., Brese E., Bshara W., Morrison C., Cance W.G., Golubovskaya V.M. The Prognostic Significance of Focal Adhesion Kinase Expression in Stage I Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2014;9:1278–1284. doi: 10.1097/JTO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C., Yang R., Yue D., Zhang Z. Expression of FAK and PTEN in Bronchioloalveolar Carcinoma and Lung Adenocarcinoma. Lung. 2009;187:104–109. doi: 10.1007/s00408-008-9130-6. [DOI] [PubMed] [Google Scholar]
- 23.Imaizumi M., Nishimura M., Takeuchi S., Murase M., Hamaguchi M. Role of tyrosine specific phosphorylation of cellular proteins, especially EGF receptor and p125FAK in human lung cancer cells. Lung Cancer. 1997;17:69–84. doi: 10.1016/S0169-5002(97)00650-8. [DOI] [PubMed] [Google Scholar]
- 24.Ocak S., Chen H., Callison C., Gonzalez A.L., Massion P.P. Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer. 2012;118:1293–1301. doi: 10.1002/cncr.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu N.Y., Chen C.Y., Hsu C.P., Lin T.Y., Chou M.C., Chiou S.H., Chow K.C. Prognostic significance of expression of nm23-H1 and focal adhesion kinase in non-small cell lung cancer. Oncol. Rep. 2007;18:81–85. doi: 10.3892/or.18.1.81. [DOI] [PubMed] [Google Scholar]
- 26.Ji H.F., Pang D., Fu S.B., Jin Y., Yao L., Qi J.P., Bai J. Overexpression of focal adhesion kinase correlates with increased lymph node metastasis and poor prognosis in non-small-cell lung cancer. J. Cancer Res. Clin. Oncol. 2013;139:429–435. doi: 10.1007/s00432-012-1342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens L.V., Xu L., Craven R.J., Dent G.A., Weiner T.M., Kornberg L., Liu E.T., Cance W.G. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 28.Ocak S., Yamashita H., Udyavar A.R., Miller A.N., Gonzalez A.L., Zou Y., Jiang A., Yi Y., Shyr Y., Estrada L., et al. DNA copy number aberrations in small-cell lung cancer reveal activation of the focal adhesion pathway. Oncogene. 2010;29:6331–6342. doi: 10.1038/onc.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blume-Jensen P., Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 30.Schaller M.D., Borgman C.A., Parsons J.T. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol. Cell. Biol. 1993;13:785–791. doi: 10.1128/MCB.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson A., Parsons T. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 32.Kleinschmidt E.G., Schlaepfer D.D. Focal adhesion kinase signaling in unexpected places. Curr. Opin. Cell Biol. 2017;45:24–30. doi: 10.1016/j.ceb.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida E.A., Ilic D., Han Q., Hauck C.R., Jin F., Kawakatsu H., Schlaepfer D.D., Damsky C.H. Matrix survival signaling: From fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J. Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak G., Soria J.C., Blagden S.P., Plummer R., Fleming R.A., Nebot N., Zhang J., Mazumdar J., Rogan D., Gazzah A., et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br. J. Cancer. 2019;120:975–981. doi: 10.1038/s41416-019-0452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soria J.C., Gan H.K., Blagden S.P., Plummer R., Arkenau H.T., Ranson M., Evans T.R., Zalcman G., Bahleda R., Hollebecque A., et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 2016;27:2268–2274. doi: 10.1093/annonc/mdw427. [DOI] [PubMed] [Google Scholar]
- 36.Brown N.F., Williams M., Arkenau H.T., Fleming R.A., Tolson J., Yan L., Zhang J., Swartz L., Singh R., Auger K.R., et al. A study of the focal adhesion kinase inhibitor GSK2256098 in patients with recurrent glioblastoma with evaluation of tumor penetration of [11C] GSK2256098. Neuro Oncol. 2018;20:1634–1642. doi: 10.1093/neuonc/noy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Infante J.R., Camidge D.R., Mileshkin L.R., Chen E.X., Hicks R.J., Rischin D., Fingert H., Pierce K.J., Xu H., Roberts W.G., et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J. Clin. Oncol. 2012;30:1527–1533. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 38.Jones S.F., Siu L.L., Bendell J.C., Cleary J.M., Razak A.R., Infante J.R., Pandya S.S., Bedard P.L., Pierce K.J., Houk B., et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Investig. New Drugs. 2015;33:1100–1107. doi: 10.1007/s10637-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 39.Patel M.R., Infante J.R., Moore K.N., Keegan M., Poli A., Padval M., Jones S.F., Horobin J., Burris H.A. Phase 1/1b study of the FAK inhibitor defactinib (VS-6063) in combination with weekly paclitaxel for advanced ovarian cancer. J. Clin. Oncol. 2014;32:5521. doi: 10.1200/jco.2014.32.15_suppl.5521. [DOI] [Google Scholar]
- 40.Shimizu T., Fukuoka K., Takeda M., Iwasa T., Yoshida T., Horobin J., Keegan M., Vaickus L., Chavan A., Padval M., et al. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother. Pharm. 2016;77:997–1003. doi: 10.1007/s00280-016-3010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doi T., Yang J.C., Shitara K., Naito Y., Cheng A.L., Sarashina A., Pronk L.C., Takeuchi Y., Lin C.C. Phase I Study of the Focal Adhesion Kinase Inhibitor BI 853520 in Japanese and Taiwanese Patients with Advanced or Metastatic Solid Tumors. Target Oncol. 2019;14:57–65. doi: 10.1007/s11523-019-00620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Jonge M.J.A., Steeghs N., Lolkema M.P., Hotte S.J., Hirte H.W., van der Biessen D.A.J., Abdul Razak A.R., De Vos F., Verheijen R.B., Schnell D., et al. Phase I Study of BI 853520, an Inhibitor of Focal Adhesion Kinase, in Patients with Advanced or Metastatic Nonhematologic Malignancies. Target Oncol. 2019;14:43–55. doi: 10.1007/s11523-018-00617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verheijen R.B., van der Biessen D.A.J., Hotte S.J., Siu L.L., Spreafico A., de Jonge M.J.A., Pronk L.C., De Vos F., Schnell D., Hirte H.W., et al. Randomized, Open-Label, Crossover Studies Evaluating the Effect of Food and Liquid Formulation on the Pharmacokinetics of the Novel Focal Adhesion Kinase (FAK) Inhibitor BI 853520. Target Oncol. 2019;14:67–74. doi: 10.1007/s11523-018-00618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aung K.L., McWhirter E., Welch S., Wang L., Lovell S., Stayner L.-A., Ali S., Malpage A., Makepeace B., Ramachandran M., et al. A phase II trial of GSK2256098 and trametinib in patients with advanced pancreatic ductal adenocarcinoma (PDAC) (MOBILITY-002 Trial, NCT02428270) J. Clin. Oncol. 2018;36:409. doi: 10.1200/JCO.2018.36.4_suppl.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fennell D.A., Baas P., Taylor P., Nowak A.K., Gilligan D., Nakano T., Pachter J.A., Weaver D.T., Scherpereel A., Pavlakis N., et al. Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND—A Double-Blind, Randomized, Phase II Study. J. Clin. Oncol. 2019;37:790–798. doi: 10.1200/JCO.2018.79.0543. [DOI] [PubMed] [Google Scholar]
- 46.Bueno R., Gill R.R., Lizotte P.H., Sprott K., Jackman D.M., Barlow J., Sharma S., Yeap B.Y., Chirieac L.R., Lebenthal A., et al. Effect of FAK inhibitor defactinib on tumor immune changes and tumor reductions in a phase II window of opportunity study in malignant pleural mesothelioma (MPM) J. Clin. Oncol. 2017;35:8555. doi: 10.1200/JCO.2017.35.15_suppl.8555. [DOI] [Google Scholar]
- 47.Liu T.-J., LaFortune T., Honda T., Ohmori O., Hatakeyama S., Meyer T., Jackson D., de Groot J., Yung W.K.A. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol. Cancer Ther. 2007;6:1357–1367. doi: 10.1158/1535-7163.MCT-06-0476. [DOI] [PubMed] [Google Scholar]
- 48.Slack-Davis J.K., Martin K.H., Tilghman R.W., Iwanicki M., Ung E.J., Autry C., Luzzio M.J., Cooper B., Kath J.C., Roberts W.G., et al. Cellular Characterization of a Novel Focal Adhesion Kinase Inhibitor. J. Biol. Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 49.Auger K.R., Smitheman K.N., Korenchuk S., McHugh C., Kruger R., Van Aller G.S., Smallwood A., Gontarek R.R., Faitg T., Johnson N. 387 The Focal Adhesion Kinase Inhibitor GSK2256098: A Potent and Selective Inhibitor for the Treatment of Cancer. Eur. J. Cancer. 2012;48:118. doi: 10.1016/S0959-8049(12)72185-8. [DOI] [Google Scholar]
- 50.Weis S.M., Lim S.T., Lutu-Fuga K.M., Barnes L.A., Chen X.L., Gothert J.R., Shen T.L., Guan J.L., Schlaepfer D.D., Cheresh D.A. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J. Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh C., Tanjoni I., Uryu S., Tomar A., Nam J.O., Luo H., Phillips A., Patel N., Kwok C., McMahon G., et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol. 2010;9:778–790. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts W.G., Ung E., Whalen P., Cooper B., Hulford C., Autry C., Richter D., Emerson E., Lin J., Kath J., et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 53.Kang Y., Hu W., Ivan C., Dalton H.J., Miyake T., Pecot C.V., Zand B., Liu T., Huang J., Jennings N.B., et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J. Natl. Cancer Inst. 2013;105:1485–1495. doi: 10.1093/jnci/djt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinrich T., Seenisamy J., Emmanuvel L., Kulkarni S.S., Bomke J., Rohdich F., Greiner H., Esdar C., Krier M., Grädler U., et al. Fragment-Based Discovery of New Highly Substituted 1H-Pyrrolo[2,3-b]- and 3H-Imidazolo[4,5-b]-Pyridines as Focal Adhesion Kinase Inhibitors. J. Med. Chem. 2013;56:1160–1170. doi: 10.1021/jm3016014. [DOI] [PubMed] [Google Scholar]
- 55.Kurenova E., Liao J., He D.H., Hunt D., Yemma M., Bshara W., Seshadri M., Cance W.G. The FAK scaffold inhibitor C4 disrupts FAK-VEGFR-3 signaling and inhibits pancreatic cancer growth. Oncotarget. 2013;4:1632–1646. doi: 10.18632/oncotarget.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurenova E.V., Hunt D.L., He D., Magis A.T., Ostrov D.A., Cance W.G. Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. J. Med. Chem. 2009;52:4716–4724. doi: 10.1021/jm900159g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart J.E., Ma X., Megison M., Nabers H., Cance W.G., Kurenova E.V., Beierle E.A. Inhibition of FAK and VEGFR-3 binding decreases tumorigenicity in neuroblastoma. Mol. Carcinog. 2015;54:9–23. doi: 10.1002/mc.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golubovskaya V.M., Ho B., Zheng M., Magis A., Ostrov D., Morrison C., Cance W.G. Disruption of focal adhesion kinase and p53 interaction with small molecule compound R2 reactivated p53 and blocked tumor growth. BMC Cancer. 2013;13:342. doi: 10.1186/1471-2407-13-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho B., Olson G., Figel S., Gelman I., Cance W.G., Golubovskaya V.M. Nanog increases focal adhesion kinase (FAK) promoter activity and expression and directly binds to FAK protein to be phosphorylated. J. Biol. Chem. 2012;287:18656–18673. doi: 10.1074/jbc.M111.322883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiede S., Meyer-Schaller N., Kalathur R.K.R., Ivanek R., Fagiani E., Schmassmann P., Stillhard P., Hafliger S., Kraut N., Schweifer N., et al. The FAK inhibitor BI 853520 exerts anti-tumor effects in breast cancer. Oncogenesis. 2018;7:73. doi: 10.1038/s41389-018-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laszlo V., Valko Z., Ozsvar J., Kovacs I., Garay T., Hoda M.A., Klikovits T., Stockhammer P., Aigner C., Groger M., et al. The FAK inhibitor BI 853520 inhibits spheroid formation and orthotopic tumor growth in malignant pleural mesothelioma. J. Mol. Med. 2019;97:231–242. doi: 10.1007/s00109-018-1725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Q., Hjelmeland A.B., Keir S.T., Song L., Wickman S., Jackson D., Ohmori O., Bigner D.D., Friedman H.S., Rich J.N. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol. Carcinog. 2007;46:488–496. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]
- 63.Golubovskaya V.M. Focal adhesion kinase as a cancer therapy target. Anti-Cancer Agents Med. Chem. 2010;10:735–741. doi: 10.2174/187152010794728648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabarra-Niecko V., Schaller M.D., Dunty J.M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/A:1023725029589. [DOI] [PubMed] [Google Scholar]
- 65.Van Nimwegen M.J., van de Water B. Focal adhesion kinase: A potential target in cancer therapy. Biochem. Pharm. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Golubovskaya V.M., Cance W.G. Focal adhesion kinase and p53 signaling in cancer cells. Int. Rev. Cytol. 2007;263:103–153. doi: 10.1016/s0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 67.Aboubakar Nana F., Hoton D., Ambroise J., Lecocq M., Vanderputten M., Sibille Y., Vanaudenaerde B., Pilette C., Bouzin C., Ocak S. Increased Expression and Activation of FAK in Small-Cell Lung Cancer Compared to Non-Small-Cell Lung Cancer. Cancers. 2019;11:1526. doi: 10.3390/cancers11101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aboubakar Nana F., Lecocq M., Ladjemi M.Z., Detry B., Dupasquier S., Feron O., Massion P.P., Sibille Y., Pilette C., Ocak S. Therapeutic Potential of Focal Adhesion Kinase Inhibition in Small Cell Lung Cancer. Mol. Cancer. 2019;18:17–27. doi: 10.1158/1535-7163.MCT-18-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cance W.G., Harris J.E., Iacocca M.V., Roche E., Yang X., Chang J., Simkins S., Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: Correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- 70.Ayaki M., Komatsu K., Mukai M., Murata K., Kameyama M., Ishiguro S., Miyoshi J., Tatsuta M., Nakamura H. Reduced Expression of Focal Adhesion Kinase in Liver Metastases Compared with Matched Primary Human Colorectal Adenocarcinomas. Clin. Cancer Res. 2001;7:3106–3112. [PubMed] [Google Scholar]
- 71.Rovin J.D., Frierson H.F., Ledinh W., Parsons J.T., Adams R.B. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–132. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 72.Schneider G.B., Kurago Z., Zaharias R., Gruman L.M., Schaller M.D., Hendrix M.J. Elevated focal adhesion kinase expression facilitates oral tumor cell invasion. Cancer. 2002;95:2508–2515. doi: 10.1002/cncr.10992. [DOI] [PubMed] [Google Scholar]
- 73.Aronsohn M.S., Brown H.M., Hauptman G., Kornberg L.J. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in squamous cell carcinoma of the larynx. Laryngoscope. 2003;113:1944–1948. doi: 10.1097/00005537-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 74.Lark A.L., Livasy C.A., Calvo B., Caskey L., Moore D.T., Yang X., Cance W.G. Overexpression of Focal Adhesion Kinase in Primary Colorectal Carcinomas and Colorectal Liver Metastases. Clin. Cancer Res. 2003;9:215–222. [PubMed] [Google Scholar]
- 75.Oktay M.H., Oktay K., Hamele-Bena D., Buyuk A., Koss L.G. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum. Pathol. 2003;34:240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- 76.Theocharis S.E., Kouraklis G.P., Kakisis J.D., Kanelli H.G., Apostolakou F.E., Karatzas G.M., Koutselinis A.S. Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur. J. Surg. Oncol. 2003;29:571–574. doi: 10.1016/S0748-7983(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 77.Itoh S., Maeda T., Shimada M., Aishima S., Shirabe K., Tanaka S., Maehara Y. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004;10:2812–2817. doi: 10.1158/1078-0432.CCR-1046-03. [DOI] [PubMed] [Google Scholar]
- 78.Kim S.J., Park J.W., Yoon J.S., Mok J.O., Kim Y.J., Park H.K., Kim C.H., Byun D.W., Lee Y.J., Jin S.Y., et al. Increased expression of focal adhesion kinase in thyroid cancer: Immunohistochemical study. J. Korean Med. Sci. 2004;19:710–715. doi: 10.3346/jkms.2004.19.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lightfoot H.M., Lark A., Livasy C.A., Moore D.T., Cowan D., Dressler L., Craven R.J., Cance W.G. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res. Treat. 2004;88:109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 80.Lark A.L., Livasy C.A., Dressler L., Moore D.T., Millikan R.C., Geradts J., Iacocca M., Cowan D., Little D., Craven R.J., et al. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod. Pathol. 2005;18:1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- 81.Canel M., Secades P., Rodrigo J.-P., Cabanillas R., Herrero A., Suarez C., Chiara M.-D. Overexpression of Focal Adhesion Kinase in Head and Neck Squamous Cell Carcinoma Is Independent of ak Gene Copy Number. Clin. Cancer Res. 2006;12:3272–3279. doi: 10.1158/1078-0432.CCR-05-1583. [DOI] [PubMed] [Google Scholar]
- 82.Rodrigo J.P., Dominguez F., Suárez V., Canel M., Secades P., Chiara M.D. Focal Adhesion Kinase and E-Cadherin as Markers for Nodal Metastasis in Laryngeal Cancer. Arch. Otolaryngol. Head Neck Surg. 2007;133:145–150. doi: 10.1001/archotol.133.2.145. [DOI] [PubMed] [Google Scholar]
- 83.Beierle E.A., Massoll N.A., Hartwich J., Kurenova E.V., Golubovskaya V.M., Cance W.G., McGrady P., London W.B. Focal adhesion kinase expression in human neuroblastoma: Immunohistochemical and real-time PCR analyses. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:3299–3305. doi: 10.1158/1078-0432.CCR-07-1511. [DOI] [PubMed] [Google Scholar]
- 84.Murata T., Naomoto Y., Yamatsuji T., Okawa T., Shirakawa Y., Gunduz M., Nobuhisa T., Takaoka M., Sirmali M., Nakajima M., et al. Localization of FAK is related with colorectal carcinogenesis. Int. J. Oncol. 2008;32:791–796. [PubMed] [Google Scholar]
- 85.Cai L., Han J., Zhuo X., Xiong Y., Dong J., Li X. Overexpression and significance of focal adhesion kinase in hepatocellular carcinoma and its relationship with HBV infection. Med. Oncol. 2009;26:409–414. doi: 10.1007/s12032-008-9137-0. [DOI] [PubMed] [Google Scholar]
- 86.Golubovskaya V.M., Conway-Dorsey K., Edmiston S.N., Tse C.K., Lark A.A., Livasy C.A., Moore D., Millikan R.C., Cance W.G. FAK overexpression and p53 mutations are highly correlated in human breast cancer. Int. J. Cancer. J. Int. Du Cancer. 2009;125:1735–1738. doi: 10.1002/ijc.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lau G.M., Lau G.M., Yu G.L., Gelman I.H., Gutowski A., Hangauer D., Fang J.W. Expression of Src and FAK in hepatocellular carcinoma and the effect of Src inhibitors on hepatocellular carcinoma in vitro. Dig. Dis. Sci. 2009;54:1465–1474. doi: 10.1007/s10620-008-0519-0. [DOI] [PubMed] [Google Scholar]
- 88.Theocharis S.E., Klijanienko J.T., Padoy E., Athanassiou S., Sastre-Garau X.X. Focal adhesion kinase (FAK) immunocytochemical expression in breast ductal invasive carcinoma (DIC): Correlation with clinicopathological parameters and tumor proliferative capacity. Med. Sci. Monit. Int. Med J. Exp. Clin. Res. 2009;15:221–226. [PubMed] [Google Scholar]
- 89.Lai I.R., Chu P.Y., Lin H.S., Liou J.Y., Jan Y.J., Lee J.C., Shen T.L. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am. J. Pathol. 2010;177:1629–1637. doi: 10.2353/ajpath.2010.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park J.H., Lee B.L., Yoon J., Kim J., Kim M.A., Yang H.K., Kim W.H. Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum. Pathol. 2010;41:1664–1673. doi: 10.1016/j.humpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Yuan Z., Zheng Q., Fan J., Ai K.X., Chen J., Huang X.Y. Expression and prognostic significance of focal adhesion kinase in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2010;136:1489–1496. doi: 10.1007/s00432-010-0806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yom C.K., Noh D.Y., Kim W.H., Kim H.S. Clinical significance of high focal adhesion kinase gene copy number and overexpression in invasive breast cancer. Breast Cancer Res. Treat. 2011;128:647–655. doi: 10.1007/s10549-010-1150-2. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y., Xia X., Wang S., Wu X., Zhang J., Zhou Y., Tan Y., He S., Qiang F., Li A., et al. High FAK combined with low JWA expression: Clinical prognostic and predictive role for adjuvant fluorouracil-leucovorin-oxaliplatin treatment in resectable gastric cancer patients. J. Gastroenterol. 2013;48:1034–1044. doi: 10.1007/s00535-012-0724-7. [DOI] [PubMed] [Google Scholar]
- 94.De Vicente J.C., Rosado P., Lequerica-Fernandez P., Allonca E., Villallain L., Hernandez-Vallejo G. Focal adhesion kinase overexpression: Correlation with lymph node metastasis and shorter survival in oral squamous cell carcinoma. Head Neck. 2013;35:826–830. doi: 10.1002/hed.23038. [DOI] [PubMed] [Google Scholar]
- 95.Faingold D., Filho V.B., Fernandes B., Jagan L., de Barros A.M., Orellana M.E., Antecka E., Burnier M.N. Expression of focal adhesion kinase in uveal melanoma and the effects of Hsp90 inhibition by 17-AAG. Pathol. Res. Pract. 2014;210:739–745. doi: 10.1016/j.prp.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 96.Golubovskaya V.M., Ylagan L., Miller A., Hughes M., Wilson J., Wang D., Brese E., Bshara W., Edge S., Morrison C., et al. High focal adhesion kinase expression in breast carcinoma is associated with lymphovascular invasion and triple-negative phenotype. BMC Cancer. 2014;14:769. doi: 10.1186/1471-2407-14-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stone R.L., Baggerly K.A., Armaiz-Pena G.N., Kang Y., Sanguino A.M., Thanapprapasr D., Dalton H.J., Bottsford-Miller J., Zand B., Akbani R., et al. Focal adhesion kinase: An alternative focus for anti-angiogenesis therapy in ovarian cancer. Cancer Biol. Ther. 2014;15:919–929. doi: 10.4161/cbt.28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li M., Hong L.I., Liao M., Guo G. Expression and clinical significance of focal adhesion kinase and adrenomedullin in epithelial ovarian cancer. Oncol. Lett. 2015;10:1003–1007. doi: 10.3892/ol.2015.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ren K., Lu X., Yao N., Chen Y., Yang A., Chen H., Zhang J., Wu S., Shi X., Wang C., et al. Focal adhesion kinase overexpression and its impact on human osteosarcoma. Oncotarget. 2015;6:31085–31103. doi: 10.18632/oncotarget.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomez Del Pulgar T., Cebrian A., Fernandez-Acenero M.J., Borrero-Palacios A., Del Puerto-Nevado L., Martinez-Useros J., Marin-Arango J.P., Carames C., Vega-Bravo R., Rodriguez-Remirez M., et al. Focal adhesion kinase: Predictor of tumour response and risk factor for recurrence after neoadjuvant chemoradiation in rectal cancer. J. Cell. Mol. Med. 2016;20:1729–1736. doi: 10.1111/jcmm.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Omura G., Ando M., Saito Y., Kobayashi K., Yoshida M., Ebihara Y., Kanaya K., Fujimoto C., Sakamoto T., Kondo K., et al. Association of the upregulated expression of focal adhesion kinase with poor prognosis and tumor dissemination in hypopharyngeal cancer. Head Neck. 2016;38:1164–1169. doi: 10.1002/hed.24176. [DOI] [PubMed] [Google Scholar]
- 102.Almstedt K., Sicking I., Battista M.J., Huangfu S., Heimes A.S., Weyer-Elberich V., Hasenburg A., Schmidt M. Prognostic Significance of Focal Adhesion Kinase in Node-Negative Breast Cancer. Breast Care. 2017;12:329–333. doi: 10.1159/000477895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thanapprapasr K., Nartthanarung A., Thanapprapasr D., Jinawath A. pFAK-Y397 overexpression as both a prognostic and a predictive biomarker for patients with metastatic osteosarcoma. PLoS ONE. 2017;12:e0182989. doi: 10.1371/journal.pone.0182989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu H.J., Zhou B. Focal adhesion kinase promotes progression and predicts poor clinical outcomes in patients with osteosarcoma. Oncol. Lett. 2018;15:6225–6232. doi: 10.3892/ol.2018.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Munguia-Calzada P., Fernandez-Vega I., Martinez-Camblor P., Diaz-Coto S., Garcia-Pedrero J.M., Vivanco B., Osuna C.G., Vazquez-Lopez F., Rodrigo J.P., Santos-Juanes J. Correlation of focal adhesion kinase expression with nodal metastasis in patients with head and neck cutaneous squamous cell carcinoma. Head Neck. 2018;41:1291–1296. doi: 10.1002/hed.25556. [DOI] [PubMed] [Google Scholar]
- 106.Schmitz K.J., Grabellus F., Callies R., Otterbach F., Wohlschlaeger J., Levkau B., Kimmig R., Schmid K.W., Baba H.A. High expression of focal adhesion kinase (p125FAK) in node-negative breast cancer is related to overexpression of HER-2/neu and activated Akt kinase but does not predict outcome. Breast Cancer Res. BCR. 2005;7:194–203. doi: 10.1186/bcr977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fujii T., Koshikawa K., Nomoto S., Okochi O., Kaneko T., Inoue S., Yatabe Y., Takeda S., Nakao A. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J. Hepatol. 2004;41:104–111. doi: 10.1016/j.jhep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 108.Sun C.K., Ng K.T., Sun B.S., Ho J.W.Y., Lee T.K., Ng I., Poon R.T.P., Lo C.M., Liu C.L., Man K., et al. The significance of proline-rich tyrosine kinase2 (Pyk2) on hepatocellular carcinoma progression and recurrence. Br. J. Cancer. 2007;97:50. doi: 10.1038/sj.bjc.6603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang H., Hegde S., Knolhoff B.L., Zhu Y., Herndon J.M., Meyer M.A., Nywening T.M., Hawkins W.G., Shapiro I.M., Weaver D.T., et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016;22:851. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.In The Cancer Genome Atlas (TCGA) Database. [(accessed on 20 May 2019)]; Available online: http://www.cbioportal.org/
- 111.Agochiya M., Brunton V.G., Owens D.W., Parkinson E.K., Paraskeva C., Keith W.N., Frame M.C. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646–5653. doi: 10.1038/sj.onc.1202957. [DOI] [PubMed] [Google Scholar]
- 112.Damstrup L., Rygaard K., Spang-Thomsen M., Skovgaard Poulsen H. Expression of transforming growth factor beta (TGF beta) receptors and expression of TGF beta 1, TGF beta 2 and TGF beta 3 in human small cell lung cancer cell lines. Br. J. Cancer. 1993;67:1015–1021. doi: 10.1038/bjc.1993.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dowell J.E., Amirkhan R.H., Lai W.S., Frawley W.H., Minna J.D. Survival in small cell lung cancer is independent of tumor expression of VEGF and COX-2. Anticancer Res. 2004;24:2367–2373. [PubMed] [Google Scholar]
- 114.Ma P.C., Tretiakova M.S., Nallasura V., Jagadeeswaran R., Husain A.N., Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: Implications for tumour invasion. Br. J. Cancer. 2007;97:368–377. doi: 10.1038/sj.bjc.6603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L., Yu H., Badzio A., Boyle T.A., Schildhaus H.-U., Lu X., Dziadziuszko R., Jassem J., Varella-Garcia M., Heasley L.E., et al. Fibroblast Growth Factor Receptor 1 and Related Ligands in Small-Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2015;10:1083–1090. doi: 10.1097/JTO.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thomas A., Lee J.H., Abdullaev Z., Park K.S., Pineda M., Saidkhodjaeva L., Miettinen M., Wang Y., Pack S.D., Giaccone G. Characterization of fibroblast growth factor receptor 1 in small-cell lung cancer. J. Thorac. Oncol. 2014;9:567–571. doi: 10.1097/JTO.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lucchi M., Mussi A., Fontanini G., Faviana P., Ribechini A., Angeletti C.A. Small cell lung carcinoma (SCLC): The angiogenic phenomenon. Eur. J. Cardio Thorac. Surg. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2002;21:1105–1110. doi: 10.1016/S1010-7940(02)00112-4. [DOI] [PubMed] [Google Scholar]
- 118.Beviglia L., Kramer R.H. HGF induces FAK activation and integrin-mediated adhesion in MTLn3 breast carcinoma cells. Int. J. Cancer. J. Int. Du Cancer. 1999;83:640–649. doi: 10.1002/(SICI)1097-0215(19991126)83:5<640::AID-IJC13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 119.Rodríguez-Fernández J.L., Rozengurt E. Bombesin, Vasopressin, Lysophosphatidic Acid, and Sphingosylphosphorylcholine Induce Focal Adhesion Kinase Activation in Intact Swiss 3T3 Cells. J. Biol. Chem. 1998;273:19321–19328. doi: 10.1074/jbc.273.30.19321. [DOI] [PubMed] [Google Scholar]
- 120.Leyton J., Garcia-Marin L.J., Tapia J.A., Jensen R.T., Moody T.W. Bombesin and gastrin releasing peptide increase tyrosine phosphorylation of focal adhesion kinase and paxillin in non-small cell lung cancer cells. Cancer Lett. 2001;162:87–95. doi: 10.1016/S0304-3835(00)00639-X. [DOI] [PubMed] [Google Scholar]
- 121.Zhou B., Wang G.-Z., Zhou G.-B., Wen Z.-S., Zhou Y.-C., Huang Y.-C., Chen Y. Somatic Mutations and Splicing Variants of Focal Adhesion Kinase in Non–Small Cell Lung Cancer. JNCI J. Natl. Cancer Inst. 2017;110:195–204. doi: 10.1093/jnci/djx157. [DOI] [PubMed] [Google Scholar]
- 122.Sulzmaier F.J., Jean C., Schlaepfer D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeng X.Q., Li N., Ma L.L., Tseng Y.J., Zhao N.Q., Chen S.Y. Prognostic Value of Focal Adhesion Kinase (FAK) in Human Solid Carcinomas: A Meta-Analysis. PLoS ONE. 2016;11:e0162666. doi: 10.1371/journal.pone.0162666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Golubovskaya V., Kaur A., Cance W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: Nuclear factor kappa B and p53 binding sites. Biochim. Biophys. Acta. 2004;1678:111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 125.Cheng N., Li Y., Han Z.G. Argonaute2 promotes tumor metastasis by way of up-regulating focal adhesion kinase expression in hepatocellular carcinoma. Hepatology. 2013;57:1906–1918. doi: 10.1002/hep.26202. [DOI] [PubMed] [Google Scholar]
- 126.Golubovskaya V.M. FAK and Nanog cross talk with p53 in cancer stem cells. Anticancer Agents Med. Chem. 2013;13:576–580. doi: 10.2174/1871520611313040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.George J., Lim J.S., Jang S.J., Cun Y., Ozretic L., Kong G., Leenders F., Lu X., Fernandez-Cuesta L., Bosco G., et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tallett A., Chilvers E.R., MacKinnon A.C., Haslett C., Sethi T. Neuropeptides stimulate tyrosine phosphorylation and tyrosine kinase activity in small cell lung cancer cell lines. Peptides. 1996;17:665–673. doi: 10.1016/0196-9781(96)00055-1. [DOI] [PubMed] [Google Scholar]
- 129.Frisch S.M., Vuori K., Ruoslahti E., Chan-Hui P.Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kurenova E., Xu L.H., Yang X., Baldwin A.S., Craven R.J., Hanks S.K., Liu Z.G., Cance W.G. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol. Cell. Biol. 2004;24:4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schlaepfer D.D., Hanks S.K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 132.Oktay M., Wary K.K., Dans M., Birge R.B., Giancotti F.G. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol. 1999;145:1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao J., Bian Z.C., Yee K., Chen B.P., Chien S., Guan J.L. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol. Cell. 2003;11:1503–1515. doi: 10.1016/S1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 134.Zhao J., Pestell R., Guan J.L. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol. Biol. Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao J.H., Reiske H., Guan J.L. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bond M., Sala-Newby G.B., Newby A.C. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. J. Biol. Chem. 2004;279:37304–37310. doi: 10.1074/jbc.M404307200. [DOI] [PubMed] [Google Scholar]
- 137.Bryant P., Zheng Q., Pumiglia K. Focal adhesion kinase controls cellular levels of p27/Kip1 and p21/Cip1 through Skp2-dependent and -independent mechanisms. Mol. Cell. Biol. 2006;26:4201–4213. doi: 10.1128/MCB.01612-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carrano A.C., Eytan E., Hershko A., Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 139.Ding Q., Grammer J.R., Nelson M.A., Guan J.L., Stewart J.E., Gladson C.L. p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J. Biol. Chem. 2005;280:6802–6815. doi: 10.1074/jbc.M409180200. [DOI] [PubMed] [Google Scholar]
- 140.Walker J.L., Fournier A.K., Assoian R.K. Regulation of growth factor signaling and cell cycle progression by cell adhesion and adhesion-dependent changes in cellular tension. Cytokine Growth Factor Rev. 2005;16:395–405. doi: 10.1016/j.cytogfr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 141.Haskell H., Natarajan M., Hecker T.P., Ding Q., Stewart J., Grammer J.R., Gladson C.L. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003;9:2157–2165. [PubMed] [Google Scholar]
- 142.Hauck C.R., Hsia D.A., Ilic D., Schlaepfer D.D. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 2002;277:12487–12490. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- 143.Hsia D.A., Mitra S.K., Hauck C.R., Streblow D.N., Nelson J.A., Ilic D., Huang S., Li E., Nemerow G.R., Leng J., et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003;160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kornberg L.J. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 1998;20:745–752. doi: 10.1002/(SICI)1097-0347(199812)20:8<745::AID-HED14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 145.Burridge K., Turner C.E., Romer L.H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: A role in cytoskeletal assembly. J. Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., Parsons J.T., Horwitz A.R. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 147.Sheetz M.P., Felsenfeld D.P., Galbraith C.G. Cell migration: Regulation of force on extracellular-matrix-integrin complexes. Trends Cell. Biol. 1998;8:51–54. doi: 10.1016/S0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 148.Pelham R.J., Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Katsumi A., Orr A.W., Tzima E., Schwartz M.A. Integrins in mechanotransduction. J. Biol. Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 150.Hanks S.K., Ryzhova L., Shin N.Y., Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front. Biosci. A J. Virtual Libr. 2003;8:982–996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 151.Mitra S.K., Hanson D.A., Schlaepfer D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 152.Parsons J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 153.Siesser P.M., Hanks S.K. The signaling and biological implications of FAK overexpression in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 154.Webb D.J., Donais K., Whitmore L.A., Thomas S.M., Turner C.E., Parsons J.T., Horwitz A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 155.Tilghman R.W., Slack-Davis J.K., Sergina N., Martin K.H., Iwanicki M., Hershey E.D., Beggs H.E., Reichardt L.F., Parsons J.T. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J. Cell Sci. 2005;118:2613–2623. doi: 10.1242/jcs.02380. [DOI] [PubMed] [Google Scholar]
- 156.Klemke R.L., Leng J., Molander R., Brooks P.C., Vuori K., Cheresh D.A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cary L.A., Chang J.F., Guan J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 158.Cary L.A., Han D.C., Polte T.R., Hanks S.K., Guan J.L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Owen J.D., Ruest P.J., Fry D.W., Hanks S.K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. doi: 10.1128/MCB.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sieg D.J., Hauck C.R., Schlaepfer D.D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 161.Han D.C., Guan J.L. Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem. 1999;274:24425–24430. doi: 10.1074/jbc.274.34.24425. [DOI] [PubMed] [Google Scholar]
- 162.Han D.C., Shen T.L., Guan J.L. Role of Grb7 targeting to focal contacts and its phosphorylation by focal adhesion kinase in regulation of cell migration. J. Biol. Chem. 2000;275:28911–28917. doi: 10.1074/jbc.M001997200. [DOI] [PubMed] [Google Scholar]
- 163.Irby R.B., Yeatman T.J. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 164.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell. Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 165.Avizienyte E., Frame M.C. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 166.McLean G.W., Carragher N.O., Avizienyte E., Evans J., Brunton V.G., Frame M.C. The role of focal-adhesion kinase in cancer—A new therapeutic opportunity. Nat. Rev. Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 167.Lu W., Kang Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell. 2019;49:361–374. doi: 10.1016/j.devcel.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Canadas I., Taus A., Gonzalez I., Villanueva X., Gimeno J., Pijuan L., Domine M., Sanchez-Font A., Vollmer I., Menendez S., et al. High circulating hepatocyte growth factor levels associate with epithelial to mesenchymal transition and poor outcome in small cell lung cancer patients. Oncotarget. 2014;5:5246–5256. doi: 10.18632/oncotarget.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ito T., Kudoh S., Ichimura T., Fujino K., Hassan W.A., Udaka N. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: Significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum. Cell. 2017;30:1–10. doi: 10.1007/s13577-016-0149-3. [DOI] [PubMed] [Google Scholar]
- 170.Zhao L., Li J., Liu Y., Zhou W., Shan Y., Fan X., Zhou X., Shan B., Song Y., Zhan Q. Flotillin1 promotes EMT of human small cell lung cancer via TGF-beta signaling pathway. Cancer Biol. Med. 2018;15:400–414. doi: 10.20892/j.issn.2095-3941.2018.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cicchini C., Laudadio I., Citarella F., Corazzari M., Steindler C., Conigliaro A., Fantoni A., Amicone L., Tripodi M. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp. Cell Res. 2008;314:143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 172.Fan H., Zhao X., Sun S., Luo M., Guan J.L. Function of focal adhesion kinase scaffolding to mediate endophilin A2 phosphorylation promotes epithelial-mesenchymal transition and mammary cancer stem cell activities in vivo. J. Biol. Chem. 2013;288:3322–3333. doi: 10.1074/jbc.M112.420497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li X.Y., Zhou X., Rowe R.G., Hu Y., Schlaepfer D.D., Ilic D., Dressler G., Park A., Guan J.L., Weiss S.J. Snail1 controls epithelial-mesenchymal lineage commitment in focal adhesion kinase-null embryonic cells. J. Cell Biol. 2011;195:729–738. doi: 10.1083/jcb.201105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang X., Urvalek A.M., Liu J., Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J. Biol. Chem. 2008;283:13934–13942. doi: 10.1074/jbc.M709300200. [DOI] [PubMed] [Google Scholar]
- 175.Wang X., Zheng M., Liu G., Xia W., McKeown-Longo P.J., Hung M.C., Zhao J. Kruppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67:7184–7193. doi: 10.1158/0008-5472.CAN-06-4729. [DOI] [PubMed] [Google Scholar]
- 176.Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., De Herreros A.G. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 177.Cano A., Pérez-Moreno M.A., Rodrigo I., Locascio A., Blanco M.J., del Barrio M.G., Portillo F., Nieto M.A. The transcription factor Snail controls epithelial—Mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 178.Liu S.Q., Xu C.Y., Wu W.H., Fu Z.H., He S.W., Qin M.B., Huang J.A. Sphingosine kinase 1 promotes the metastasis of colorectal cancer by inducing the epithelialmesenchymal transition mediated by the FAK/AKT/MMPs axis. Int. J. Oncol. 2019;54:41–52. doi: 10.3892/ijo.2018.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu C.Y., Liu S.Q., Qin M.B., Zhuge C.F., Qin L., Qin N., Lai M.Y., Huang J.A. SphK1 modulates cell migration and EMT-related marker expression by regulating the expression of p-FAK in colorectal cancer cells. Int. J. Mol. Med. 2017;39:1277–1284. doi: 10.3892/ijmm.2017.2921. [DOI] [PubMed] [Google Scholar]
- 180.Taliaferro-Smith L., Oberlick E., Liu T., McGlothen T., Alcaide T., Tobin R., Donnelly S., Commander R., Kline E., Nagaraju G.P., et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget. 2015;6:4757. doi: 10.18632/oncotarget.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wilson C., Nicholes K., Bustos D., Lin E., Song Q., Stephan J.P., Kirkpatrick D.S., Settleman J. Overcoming EMT-associated resistance to anti-cancer drugs via Src/FAK pathway inhibition. Oncotarget. 2014;5:7328–7341. doi: 10.18632/oncotarget.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Azzi S., Hebda J., Gavard J. Vascular Permeability and Drug Delivery in Cancers. Front. Oncol. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kuczynski E.A., Vermeulen P.B., Pezzella F., Kerbel R.S., Reynolds A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 184.Reymond N., d’Água B.B., Ridley A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer. 2013;13:858. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 185.Williamson S.C., Metcalf R.L., Trapani F., Mohan S., Antonello J., Abbott B., Leong H.S., Chester C.P.E., Simms N., Polanski R., et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 187.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 188.Salven P., Ruotsalainen T., Mattson K., Joensuu H. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int. J. Cancer. 1998;79:144–146. doi: 10.1002/(SICI)1097-0215(19980417)79:2<144::AID-IJC8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 189.Hodgkinson C.L., Morrow C.J., Li Y., Metcalf R.L., Rothwell D.G., Trapani F., Polanski R., Burt D.J., Simpson K.L., Morris K., et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 190.Gaspar L.E., McNamara E.J., Gay E.G., Putnam J.B., Crawford J., Herbst R.S., Bonner J.A. Small-Cell Lung Cancer: Prognostic Factors and Changing Treatment Over 15 Years. Clin. Lung Cancer. 2012;13:115–122. doi: 10.1016/j.cllc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 191.Hamilton G., Moser D., Hochmair M. Metastasis: Circulating Tumor Cells in Small Cell Lung Cancer. Trends Cancer. 2016;2:159–160. doi: 10.1016/j.trecan.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 192.Sun J.-M., Lee K.H., Kim B.-S., Kim H.-G., Min Y.J., Yi S.Y., Yun H.J., Jung S.-H., Lee S.-H., Ahn J.S., et al. Pazopanib maintenance after first-line etoposide and platinum chemotherapy in patients with extensive disease small-cell lung cancer: A multicentre, randomised, placebo-controlled Phase II study (KCSG-LU12-07) Br. J. Cancer. 2018;118:648. doi: 10.1038/bjc.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marcello T., Boni L., Ambrosio F., Camerini A., Baldini E., Cinieri S., Brighenti M., Zanelli F., Defraia E., Chiari R., et al. Italian, Multicenter, Phase III, Randomized Study of Cisplatin Plus Etoposide With or Without Bevacizumab as First-Line Treatment in Extensive-Disease Small-Cell Lung Cancer: The GOIRC-AIFA FARM6PMFJM Trial. J. Clin. Oncol. 2017;35:1281–1287. doi: 10.1200/jco.2016.69.4844. [DOI] [PubMed] [Google Scholar]
- 194.Spigel D.R., Townley P.M., Waterhouse D.M., Fang L., Adiguzel I., Huang J.E., Karlin D.A., Faoro L., Scappaticci F.A., Socinski M.A. Randomized Phase II Study of Bevacizumab in Combination with Chemotherapy in Previously Untreated Extensive-Stage Small-Cell Lung Cancer: Results From the SALUTE Trial. J. Clin. Oncol. 2011;29:2215–2222. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 195.Ready N.E., Pang H.H., Gu L., Otterson G.A., Thomas S.P., Miller A.A., Baggstrom M., Masters G.A., Graziano S.L., Crawford J., et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance) J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:1660–1665. doi: 10.1200/JCO.2014.57.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ilic D., Kovacic B., McDonagh S., Jin F., Baumbusch C., Gardner David G., Damsky Caroline H. Focal Adhesion Kinase Is Required for Blood Vessel Morphogenesis. Circ. Res. 2003;92:300–307. doi: 10.1161/01.RES.0000055016.36679.23. [DOI] [PubMed] [Google Scholar]
- 197.Ueda H., Nagy T., Shen T.-L., Peng X., Guan J.-L., Stokol T., Zhou H., Vassalli J.-D., Alcaraz A. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc. Res. 2004;64:421–430. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 198.Chen X.L., Nam J.-O., Jean C., Lawson C., Walsh C.T., Goka E., Lim S.-T., Tomar A., Tancioni I., Uryu S., et al. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jean C., Chen X.L., Nam J.-O., Tancioni I., Uryu S., Lawson C., Ward K.K., Walsh C.T., Miller N.L.G., Ghassemian M., et al. Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J. Cell Biol. 2014;204:247–263. doi: 10.1083/jcb.201307067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stokes J.B., Adair S.J., Slack-Davis J.K., Walters D.M., Tilghman R.W., Hershey E.D., Lowrey B., Thomas K.S., Bouton A.H., Hwang R.F., et al. Inhibition of Focal Adhesion Kinase by PF-562,271 Inhibits the Growth and Metastasis of Pancreatic Cancer Concomitant with Altering the Tumor Microenvironment. Mol. Cancer Ther. 2011;10:2135–2145. doi: 10.1158/1535-7163.MCT-11-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ward K.K., Tancioni I., Lawson C., Miller N.L.G., Jean C., Chen X.L., Uryu S., Kim J., Tarin D., Stupack D.G., et al. Inhibition of focal adhesion kinase (FAK) activity prevents anchorage-independent ovarian carcinoma cell growth and tumor progression. Clin. Exp. Metastasis. 2013;30:579–594. doi: 10.1007/s10585-012-9562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Serrels B., McGivern N., Canel M., Byron A., Johnson S.C., McSorley H.J., Quinn N., Taggart D., Von Kreigsheim A., Anderton S.M., et al. IL-33 and ST2 mediate FAK-dependent antitumor immune evasion through transcriptional networks. Sci. Signal. 2017;10:aan8355. doi: 10.1126/scisignal.aan8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Haemmerle M., Bottsford-Miller J., Pradeep S., Taylor M.L., Choi H.J., Hansen J.M., Dalton H.J., Stone R.L., Cho M.S., Nick A.M., et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J. Clin. Investig. 2016;126:1885–1896. doi: 10.1172/JCI85086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Borsig L. The role of platelet activation in tumor metastasis. Expert Rev. Anticancer Ther. 2008;8:1247–1255. doi: 10.1586/14737140.8.8.1247. [DOI] [PubMed] [Google Scholar]
- 205.Labelle M., Begum S., Hynes R. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cho M.S., Bottsford-Miller J., Vasquez H.G., Stone R., Zand B., Kroll M.H., Sood A.K., Afshar-Kharghan V. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869–4872. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Roos W.P., Thomas A.D., Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer. 2015;16:20. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 208.Hoeijmakers J.H.J. DNA Damage, Aging, and Cancer. N. Engl. J. Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 209.Tubbs A., Nussenzweig A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell. 2017;168:644–656. doi: 10.1016/j.cell.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sabari J.K., Lok B.H., Laird J.H., Poirier J.T., Rudin C.M. Unravelling the biology of SCLC: Implications for therapy. Nat. Rev. Clin. Oncol. 2017;14:549–561. doi: 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Behera M., Ragin C., Kim S., Pillai R.N., Chen Z., Steuer C.E., Saba N.F., Belani C.P., Khuri F.R., Ramalingam S.S., et al. Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer. 2015;122:50–60. doi: 10.1002/cncr.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Faivre-Finn C., Snee M., Ashcroft L., Appel W., Barlesi F., Bhatnagar A., Bezjak A., Cardenal F., Fournel P., Harden S., et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Byers L.A., Wang J., Nilsson M.B., Fujimoto J., Saintigny P., Yordy J., Giri U., Peyton M., Fan Y.H., Diao L., et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pietanza M.C., Waqar S.N., Krug L.M., Dowlati A., Hann C.L., Chiappori A., Owonikoko T.K., Woo K.M., Cardnell R.J., Fujimoto J., et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J. Clin. Oncol. 2018;36:2386–2394. doi: 10.1200/JCO.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thomas A., Vilimas R., Trindade C., Erwin-Cohen R., Roper N., Xi L., Krishnasamy V., Levy E., Mammen A., Nichols S., et al. Durvalumab in Combination with Olaparib in Patients with Relapsed SCLC: Results from a Phase II Study. J. Thorac. Oncol. 2019;14:1447–1457. doi: 10.1016/j.jtho.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Constanzo J.D., Tang K.-j., Rindhe S., Melegari M., Liu H., Tang X., Rodriguez-Canales J., Wistuba I., Scaglioni P.P. PIAS1-FAK Interaction Promotes the Survival and Progression of Non-Small Cell Lung Cancer. Neoplasia. 2016;18:282–293. doi: 10.1016/j.neo.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tang K.-J., Constanzo J.D., Venkateswaran N., Melegari M., Ilcheva M., Morales J.C., Skoulidis F., Heymach J.V., Boothman D.A., Scaglioni P.P. Focal Adhesion Kinase Regulates the DNA Damage Response and Its Inhibition Radiosensitizes Mutant KRAS Lung Cancer. Clin. Cancer Res. 2016;22:5851–5863. doi: 10.1158/1078-0432.CCR-15-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Beinke C., Van Beuningen D., Cordes N. Ionizing radiation modules of the expression and tyrosine phosphorylation of the focal adhesion-associated proteins focal adhesion kinase (FAK) and its substrates p130cas and paxillin in A549 human lung carcinoma cells in vitro. Int. J. Radiat. Biol. 2003;79:721–731. doi: 10.1080/09553000310001610231. [DOI] [PubMed] [Google Scholar]
- 219.Williams K.E., Bundred N.J., Landberg G., Clarke R.B., Farnie G. Focal adhesion kinase and Wnt signaling regulate human ductal carcinoma in situ stem cell activity and response to radiotherapy. Stem Cells. 2015;33:327–341. doi: 10.1002/stem.1843. [DOI] [PubMed] [Google Scholar]
- 220.Tavora B., Reynolds L.E., Batista S., Demircioglu F., Fernandez I., Lechertier T., Lees D.M., Wong P.P., Alexopoulou A., Elia G., et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature. 2014;514:112–116. doi: 10.1038/nature13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cordes N., Frick S., Brunner T.B., Pilarsky C., Grutzmann R., Sipos B., Kloppel G., McKenna W.G., Bernhard E.J. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene. 2007;26:6851–6862. doi: 10.1038/sj.onc.1210498. [DOI] [PubMed] [Google Scholar]
- 222.Eke I., Sandfort V., Mischkus A., Baumann M., Cordes N. Antiproliferative effects of EGFR tyrosine kinase inhibition and radiation-induced genotoxic injury are attenuated by adhesion to fibronectin. Radiother. Oncol. 2006;80:178–184. doi: 10.1016/j.radonc.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 223.Estrugo D., Fischer A., Hess F., Scherthan H., Belka C., Cordes N. Ligand bound beta1 integrins inhibit procaspase-8 for mediating cell adhesion-mediated drug and radiation resistance in human leukemia cells. PLoS ONE. 2007;2:e269. doi: 10.1371/journal.pone.0000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fuks Z., Vlodavsky I., Andreeff M., McLoughlin M., Haimovitz-Friedman A. Effects of extracellular matrix on the response of endothelial cells to radiation in vitro. Eur. J. Cancer. 1992;28:725–731. doi: 10.1016/0959-8049(92)90104-A. [DOI] [PubMed] [Google Scholar]
- 225.Li L., Dong X., Peng F., Shen L. Integrin beta1 regulates the invasion and radioresistance of laryngeal cancer cells by targeting CD147. Cancer Cell Int. 2018;18:80. doi: 10.1186/s12935-018-0578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brodin O., Arnberg H., Bergh J., Nilsson S. Increased radioresistance of an in vitro transformed human small cell lung cancer cell line. Lung Cancer. 1995;12:183–198. doi: 10.1016/0169-5002(95)00444-6. [DOI] [PubMed] [Google Scholar]
- 227.Kraus A.C., Ferber I., Bachmann S.O., Specht H., Wimmel A., Gross M.W., Schlegel J., Suske G., Schuermann M. In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene. 2002;21:8683–8695. doi: 10.1038/sj.onc.1205939. [DOI] [PubMed] [Google Scholar]
- 228.Kasahara T., Koguchi E., Funakoshi M., Aizu-Yokota E., Sonoda Y. Antiapoptotic action of focal adhesion kinase (FAK) against ionizing radiation. Antioxid. Redox Signal. 2002;4:491–499. doi: 10.1089/15230860260196290. [DOI] [PubMed] [Google Scholar]
- 229.Skinner H.D., Giri U., Yang L., Woo S.H., Story M.D., Pickering C.R., Byers L.A., Williams M.D., El-Naggar A., Wang J., et al. Proteomic Profiling Identifies PTK2/FAK as a Driver of Radioresistance in HPV-negative Head and Neck Cancer. Clin. Cancer Res. 2016;22:4643–4650. doi: 10.1158/1078-0432.CCR-15-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nguemgo Kouam P., Buhler H., Hero T., Adamietz I.A. The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition. Radiat. Oncol. 2019;14:25. doi: 10.1186/s13014-019-1230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hehlgans S., Lange I., Eke I., Cordes N. 3D cell cultures of human head and neck squamous cell carcinoma cells are radiosensitized by the focal adhesion kinase inhibitor TAE226. Radiother. Oncol. 2009;92:371–378. doi: 10.1016/j.radonc.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 232.Hehlgans S., Eke I., Cordes N. Targeting FAK radiosensitizes 3-dimensional grown human HNSCC cells through reduced Akt1 and MEK1/2 signaling. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:669–676. doi: 10.1016/j.ijrobp.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 233.Eke I., Deuse Y., Hehlgans S., Gurtner K., Krause M., Baumann M., Shevchenko A., Sandfort V., Cordes N. beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Investig. 2012;122:1529–1540. doi: 10.1172/JCI61350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wicha M.S., Liu S., Dontu G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 235.Afify S.M., Seno M. Conversion of Stem Cells to Cancer Stem Cells: Undercurrent of Cancer Initiation. Cancers. 2019;11:345. doi: 10.3390/cancers11030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Suresh R., Ali S., Ahmad A., Philip P.A., Sarkar F.H. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv. Exp. Med. Biol. 2016;890:57–74. doi: 10.1007/978-3-319-24932-2_4. [DOI] [PubMed] [Google Scholar]
- 237.Salcido C.D., Larochelle A., Taylor B.J., Dunbar C.E., Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br. J. Cancer. 2010;102:1636–1644. doi: 10.1038/sj.bjc.6605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang P., Gao Q., Suo Z., Munthe E., Solberg S., Ma L., Wang M., Westerdaal N.A., Kvalheim G., Gaudernack G. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS ONE. 2013;8:e57020. doi: 10.1371/journal.pone.0057020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 239.Zhang Z., Zhou Y., Qian H., Shao G., Lu X., Chen Q., Sun X., Chen D., Yin R., Zhu H., et al. Stemness and inducing differentiation of small cell lung cancer NCI-H446 cells. Cell Death Dis. 2013;4:e633. doi: 10.1038/cddis.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sarvi S., Mackinnon A.C., Avlonitis N., Bradley M., Rintoul R.C., Rassl D.M., Wang W., Forbes S.J., Gregory C.D., Sethi T. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res. 2014;74:1554–1565. doi: 10.1158/0008-5472.CAN-13-1541. [DOI] [PubMed] [Google Scholar]
- 241.Qiu X., Wang Z., Li Y., Miao Y., Ren Y., Luan Y. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett. 2012;323:161–170. doi: 10.1016/j.canlet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 242.Eramo A., Lotti F., Sette G., Pilozzi E., Biffoni M., Di Virgilio A., Conticello C., Ruco L., Peschle C., De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 243.Gutova M., Najbauer J., Gevorgyan A., Metz M.Z., Weng Y., Shih C.C., Aboody K.S. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS ONE. 2007;2:e243. doi: 10.1371/journal.pone.0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Codony-Servat J., Verlicchi A., Rosell R. Cancer stem cells in small cell lung cancer. Transl. Lung Cancer Res. 2016;5:16–25. doi: 10.3978/j.issn.2218-6751.2016.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Morise M., Hishida T., Takahashi A., Yoshida J., Ohe Y., Nagai K., Ishii G. Clinicopathological significance of cancer stem-like cell markers in high-grade neuroendocrine carcinoma of the lung. J. Cancer Res. Clin. Oncol. 2015;141:2121–2130. doi: 10.1007/s00432-015-1985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sodja E., Rijavec M., Koren A., Sadikov A., Korosec P., Cufer T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiol. Oncol. 2016;50:188–196. doi: 10.1515/raon-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yang F., Gao Y., Geng J., Qu D., Han Q., Qi J., Chen G. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int. J. Clin. Exp. Pathol. 2013;6:2846–2854. [PMC free article] [PubMed] [Google Scholar]
- 248.Kobayashi K., Takahashi H., Inoue A., Harada H., Toshimori S., Kobayashi Y., Goto K., Sugimoto K., Yano H., Ohnishi T., et al. Oct-3/4 promotes migration and invasion of glioblastoma cells. J. Cell Biochem. 2012;113:508–517. doi: 10.1002/jcb.23374. [DOI] [PubMed] [Google Scholar]
- 249.Liu C., Li Y., Xing Y., Cao B., Yang F., Yang T., Ai Z., Wei Y., Jiang J. The Interaction between Cancer Stem Cell Marker CD133 and Src Protein Promotes Focal Adhesion Kinase (FAK) Phosphorylation and Cell Migration. J. Biol. Chem. 2016;291:15540–15550. doi: 10.1074/jbc.M115.712976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Begum A., Ewachiw T., Jung C., Huang A., Norberg K.J., Marchionni L., McMillan R., Penchev V., Rajeshkumar N.V., Maitra A., et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS ONE. 2017;12:e0180181. doi: 10.1371/journal.pone.0180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ou J., Deng J., Wei X., Xie G., Zhou R., Yu L., Liang H. Fibronectin extra domain A (EDA) sustains CD133(+)/CD44(+) subpopulation of colorectal cancer cells. Stem Cell Res. 2013;11:820–833. doi: 10.1016/j.scr.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guan J.L. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 2010;62:268–276. doi: 10.1002/iub.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jang H.-J., Bak Y., Pham T.-H., Kwon S.-B., Kim B.-Y., Hong J., Yoon D.-Y. STK899704 inhibits stemness of cancer stem cells and migration via the FAK-MEK-ERK pathway in HT29 cells. BMB Rep. 2018;51:596–601. doi: 10.5483/BMBRep.2018.51.11.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ginestier C., Liu S., Diebel M.E., Korkaya H., Luo M., Brown M., Wicinski J., Cabaud O., Charafe-Jauffret E., Birnbaum D., et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sun J., Luo Q., Liu L., Yang X., Zhu S., Song G. Salinomycin attenuates liver cancer stem cell motility by enhancing cell stiffness and increasing F-actin formation via the FAK-ERK1/2 signalling pathway. Toxicology. 2017;384:1–10. doi: 10.1016/j.tox.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 256.Thakur R., Trivedi R., Rastogi N., Singh M., Mishra D.P. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci. Rep. 2015;5:10194. doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Blum W., Pecze L., Felley-Bosco E., Wu L., de Perrot M., Schwaller B. Stem Cell Factor-Based Identification and Functional Properties of In Vitro-Selected Subpopulations of Malignant Mesothelioma Cells. Stem Cell Rep. 2017;8:1005–1017. doi: 10.1016/j.stemcr.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shapiro I.M., Kolev V.N., Vidal C.M., Kadariya Y., Ring J.E., Wright Q., Weaver D.T., Menges C., Padval M., McClatchey A.I., et al. Merlin deficiency predicts FAK inhibitor sensitivity: A synthetic lethal relationship. Sci. Transl. Med. 2014;6:237–268. doi: 10.1126/scitranslmed.3008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Luo M., Fan H., Nagy T., Wei H., Wang C., Liu S., Wicha M.S., Guan J.L. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69:466–474. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kolev V.N., Tam W.F., Wright Q.G., McDermott S.P., Vidal C.M., Shapiro I.M., Xu Q., Wicha M.S., Pachter J.A., Weaver D.T. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget. 2017;8:51733–51747. doi: 10.18632/oncotarget.18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Schober M., Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. USA. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vishnubalaji R., Manikandan M., Fahad M., Hamam R., Alfayez M., Kassem M., Aldahmash A., Alajez N.M. Molecular profiling of ALDH1(+) colorectal cancer stem cells reveals preferential activation of MAPK, FAK, and oxidative stress pro-survival signalling pathways. Oncotarget. 2018;9:13551–13564. doi: 10.18632/oncotarget.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Luo M., Zhao X., Chen S., Liu S., Wicha M.S., Guan J.L. Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res. 2013;73:5591–5602. doi: 10.1158/0008-5472.CAN-13-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Reck M., Mok T.S.K., Nishio M., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodríguez-Abreu D., Moro-Sibilot D., et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 265.Gandini S., Massi D., Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 266.Ott P.A., Elez E., Hiret S., Kim D.-W., Morosky A., Saraf S., Piperdi B., Mehnert J.M. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J. Clin. Oncol. 2017;35:3823–3829. doi: 10.1200/JCO.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 267.Wang W., Hodkinson P., McLaren F., MacKinnon A., Wallace W., Howie S., Sethi T. Small cell lung cancer tumour cells induce regulatory T lymphocytes, and patient survival correlates negatively with FOXP3+ cells in tumour infiltrate. Int. J. Cancer. 2012;131:928–937. doi: 10.1002/ijc.27613. [DOI] [PubMed] [Google Scholar]
- 268.Serrels A., Lund T., Serrels B., Byron A., McPherson R.C., von Kriegsheim A., Gomez-Cuadrado L., Canel M., Muir M., Ring J.E., et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163:160–173. doi: 10.1016/j.cell.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ring J., Li Y., Shapiro I., Wang Y., Weaver D., Pachter J. FAK/PYK2 inhibitors defactinib and VS-4718 enhance immune checkpoint inhibitor efficacy. J. Immunother. Cancer. 2015:3. doi: 10.1186/2051-1426-3-s2-p354. [DOI] [Google Scholar]
- 270.Hoelzinger D.B., Smith S.E., Mirza N., Dominguez A.L., Manrique S.Z., Lustgarten J. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J. Immunol. 2010;184:6833–6842. doi: 10.4049/jimmunol.0904084. [DOI] [PubMed] [Google Scholar]
- 271.Kuehnemuth B., Piseddu I., Wiedemann G.M., Lauseker M., Kuhn C., Hofmann S., Schmoeckel E., Endres S., Mayr D., Jeschke U., et al. CCL1 is a major regulatory T cell attracting factor in human breast cancer. BMC Cancer. 2018;18:1278. doi: 10.1186/s12885-018-5117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang X., Lang M., Zhao T., Feng X., Zheng C., Huang C., Hao J., Dong J., Luo L., Li X., et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3(+)Treg cells in pancreatic ductal adenocarcinoma. Oncogene. 2017;36:3048–3058. doi: 10.1038/onc.2016.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Tan M.C., Goedegebuure P.S., Belt B.A., Flaherty B., Sankpal N., Gillanders W.E., Eberlein T.J., Hsieh C.S., Linehan D.C. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Aggarwal S., Sharma S.C., Das S. Dynamics of regulatory T cells (Tregs ) in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2017;116:1103–1113. doi: 10.1002/jso.24782. [DOI] [PubMed] [Google Scholar]
- 275.Ghiringhelli F., Puig P.E., Roux S., Parcellier A., Schmitt E., Solary E., Kroemer G., Martin F., Chauffert B., Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tian T., Gu X., Zhang B., Liu Y., Yuan C., Shao L., Guo Y., Fan K. Increased circulating CD14(+)HLA-DR-/low myeloid-derived suppressor cells are associated with poor prognosis in patients with small-cell lung cancer. Cancer Biomark. 2015;15:425–432. doi: 10.3233/CBM-150473. [DOI] [PubMed] [Google Scholar]
- 278.Iriki T., Ohnishi K., Fujiwara Y., Horlad H., Saito Y., Pan C., Ikeda K., Mori T., Suzuki M., Ichiyasu H., et al. The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer. 2017;106:22–32. doi: 10.1016/j.lungcan.2017.01.003. [DOI] [PubMed] [Google Scholar]