K2Pt(CN)4 becomes soluble in dichloromethane upon addition of two equivalents of 18-crown-6. Crystals of [K(18-crown-6)]2 [Pt(CN)4] are obtained upon layering the dichloromethane solution with diethylether. No Pt⋯Pt interactions are observed in the crystal.
Keywords: tetracyanoplatinate, crown ether, platinum, potassium, crystal structure
Abstract
In the title compound, di-μ-cyanato-1:2κ2
N:C;2:3κ2
C:N-dicyanato-2κ2
C-bis(1,4,7,10,13,16-hexaoxacyclooctadecane)-1κ6
O;3κ6
O-1,3-dipotassium(I)-2-platinum(II), [K2Pt(CN)4(C12H24O6)2] or [K(18-crown-6)]2·[Pt(CN)4], two trans-orientated cyano groups of the square-planar [Pt(CN)4]2− dianion (Pt site symmetry 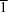 ) bind to one potassium ion each, which are additionally coordinated by the six O atoms of 18-crown-6. No Pt⋯Pt interactions occur in the crystal, but very weak Pt⋯H contacts (2.79 Å) are observed.
) bind to one potassium ion each, which are additionally coordinated by the six O atoms of 18-crown-6. No Pt⋯Pt interactions occur in the crystal, but very weak Pt⋯H contacts (2.79 Å) are observed.
Chemical context
Polycyanometallates are an important class of inorganic compounds with intriguing properties. As a result of their anionic nature and high nucleophilicity, they have been widely used as metallo-ligands in coordination chemistry. Depending on the geometry of the polycyanometallate, various topologies can be realized (Alexandrov et al., 2015 ▸). While photomagnetic effects have been predominantly realized with hexa- and octacyanometallates (Ohkoshi et al., 2012 ▸), studies on tetracyanoplatinates and their derivatives have focused on the high electrical conductivities of mixed-valent Krogmann’s salts K2[Pt(CN)4]Br0.32·2.6H2O (Krogmann, 1969 ▸), vapochromic sensor materials (e.g. Zn[Pt(CN)4] for ammonia (Varju et al., 2019 ▸) and spin-crossover compounds such as [Fe(pyrazine)][Pt(CN)4]·2H2O (Niel et al., 2001 ▸). However, alkali salts of polycyanometallates are in generally water-soluble but suffer from insolubility in organic solvents. A general way to increase the solubility of metals salts in organic solvents is the utilization of crown ethers. For example, even potassium permanganate KMnO4 becomes benzene-soluble by coordination of 18-crown-6 to the potassium cation (Doheny & Ganem, 1980 ▸). During our attempts to explore the coordination chemistry of the tetracyanoplatinate dianion [Pt(CN)4]2− in organic solvents, we realized that commercially available K2[Pt(CN)4] is insoluble in dichloromethane but dissolves rapidly upon addition of 18-crown-6. The product [K(18-crown-6)]2 [Pt(CN)4], which was already isolated many years ago by a rather complicated procedure (Almeida & Pidcock, 1981 ▸), could now be obtained in crystalline form. In contrast to other tetracyanoplatinate(II) salts with large organic cations [e.g. PPh4
+ (see Nast & Moerler, 1969 ▸) and NBu4
+ (see Mason & Gray, 1968 ▸)], which are prepared by metathesis reactions in water, this new procedure makes the access to tetracyanoplatinate salts with solubility in organic solvents even more facile.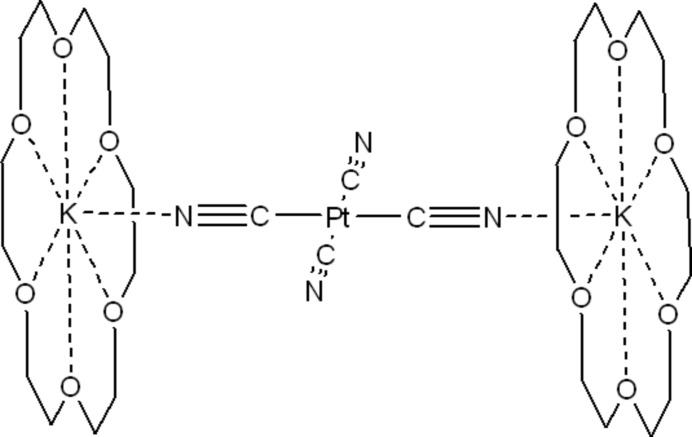
Structural Commentary
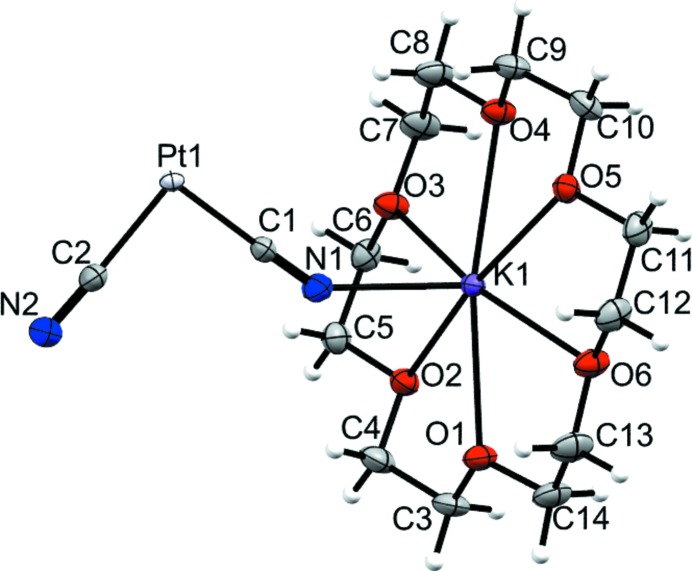
[K(18-crown-6)]2 [Pt(CN)4] (Fig. 1 ▸) crystallizes in the monoclinic space group P21/n. The tetracyanoplatinate moiety displays a square-planar molecular geometry with the platinum atom lying on a crystallographic inversion centre. Two trans-orientated cyano groups coordinate via their terminal nitrogen atoms to the potassium ions in a rather bent fashion [K1—N1—C1 = 146.76 (17)°] while the Pt—C—N bonds are almost linear [Pt1—C2—N2 = 178.81 (18)°]. The Pt—C and C—N bond lengths do not differ significantly between the terminal or bridging cyano ligands [Pt1—C2 = 1.996 (2) Å versus Pt1—C1 = 1.991 (2) Å and C2—N2 = 1.155 (3) Å versus C1—N1 = 1.154 (3) Å]. The six oxygen atoms of the crown ether coordinate to the potassium ion in a hexagonal-planar fashion. Additionally, one apical position is occupied by a nitrogen atom of a cyano group, although the K—N distance is relatively long [2.732 (2) Å]. The potassium ion is located 0.295 Å above the the O6 centroid [K—O distances = 2.769 (1)–2.837 (1) Å].
Figure 1.
The asymmetric unit of the title compound with displacement ellipsoids shown at the 50% probability level.
Supramolecular features
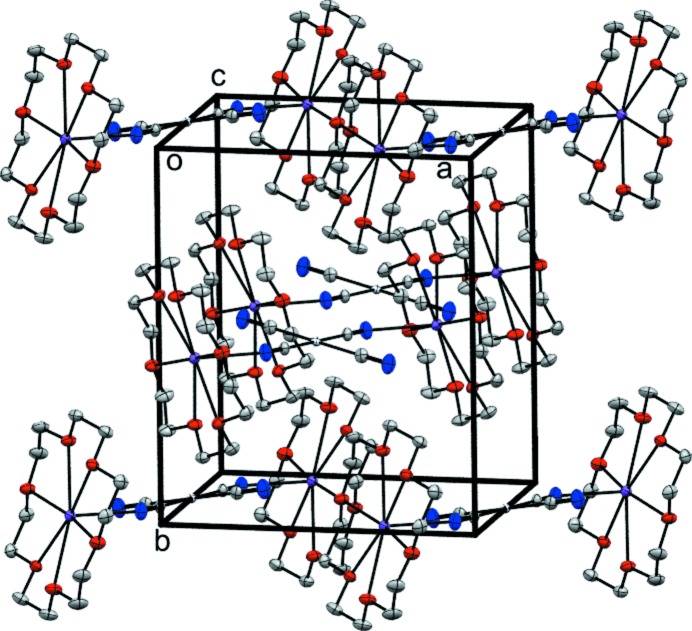
A common feature of tetracyanoplatinate salts is the formation of columnar stacks of the planar tetracyanoplatinate anions with Pt⋯Pt distances in the range of 3.0–3.8 Å, see, for example, Washecheck et al. (1976 ▸), Holzapfel et al. (1981 ▸), Mühle et al. (2004 ▸) and Neuhausen et al. (2011 ▸). However, in the crystal structure of the title compound (Fig. 2 ▸), no platinophilic interactions are observed. This is in accordance with findings of Stojanovic et al. (2011 ▸) who stated that large organic cations can suppress the formation of Pt⋯Pt contacts. Intermolecular interactions are not very pronounced in this crystal structure. However, the two uncoordinated cyano groups each point towards one neighbouring hydrogen atom in a slightly bent fashion (C—N⋯H = 152°; Table 1 ▸) although the N⋯H distance is relatively long (2.55 Å). Moreover, two hydrogen atoms from two different crown ether molecules form weak contacts to the platinum atom in a linear fashion (H⋯Pt⋯H = 180°), which results in a distorted axially elongated pseudo-octahedral PtC4H2 coordination environment for the platinum atom. The Pt⋯H distances are slightly smaller than the sum of the van der Waals radii (2.79 Å).
Figure 2.
Packing in the unit cell of the title compound.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3A⋯N1i | 0.99 | 2.54 | 3.510 (3) | 165 |
| C9—H9B⋯N2ii | 0.99 | 2.55 | 3.459 (3) | 152 |
Symmetry codes: (i) 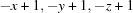 ; (ii)
; (ii) 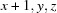 .
.
Database survey
A database survey (CSD version 5.40, update of November 2018; Groom et al., 2016 ▸) gave 348 hits for the [Pt(CN)4] moiety and 1562 hits for the [K(18-crown-6)] moiety. While the tetracyanoplatinate moiety binds to many elements from the periodic table, only a few tetracyanoplatinate salts with metal–crown ether counter-cations are known. For example, complexes of Ba2+ [Pt(CN)4]2− with 18-crown-6 (Olmstead et al., (2005 ▸), dibenzo-18-crown-6 (Olmstead et al., 2016)) and diaza-18-crown-6 (Olmstead et al., 2009 ▸). In the first two examples, the Ba2+ cation exhibits a coordination number of 10 whereas only ninefold coordination is observed in the last case. In general, these high coordination numbers result from bridging cyanide ligands and oxygen-containing donor solvents that bind to the Ba2+ cations. In [Tl(18-crown-6)]2[Pt(CN)4] (Liu et al., 2006 ▸), only a sevenfold coordination is observed for the thallium cation. Interestingly, Tl+ does not bind to a terminal cyanide group but forms a weak metallophilic contact to Pt2+ (Tl⋯Pt distance = 3.185 Å).
The combination of [K(18-crown-6)] cations with other polycyanometallates is relatively rare. Crystal structures of [K3(18-crown-6)3(H2O)4][Cr(CN)6]·3H2O (Zhou et al., 2003 ▸), [K(18-crown-6)]2[K(18-crown-6)(H2O)2][Ru(CN)6]·CH2Cl2 (Vostrikova & Peresypkina, 2011 ▸) and [K(18-crown-6)]2[K(18-crown-6)(C3H7OH)][Os(CN)6]·2C3H7OH·H2O (Vostrikova & Peresypkina, 2011 ▸) have been reported in the literature.
Synthesis and crystallization
Potassium tetracyanoplatinate (37.7 mg, 0.1 mmol) was suspended in 3 ml of CH2Cl2. Then, 52.8 mg (0.2 mmol) of 18-crown-6 were added and the mixture was stirred for several minutes until the solid had completely dissolved. A small part of the solution was placed in a narrow glass tube and layered with diethyl ether. Colourless blocks of the title compound formed overnight. IR(ATR) (cm−1): 2898–2815 [m, v(CH)], 2126 [s, n(CN)], 1451 [w, d(CH2)], 1099 [vs, n(CO)]. 1H NMR (400 MHz in CD2Cl2): 3.62 (s, crown ether) ppm. 13C(1H) NMR (101 MHz in CD2Cl2): 122.4 (CN, 1 J Pt—C = 1018 Hz), 70.1 (crown) ppm.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The H atoms were placed geometrically with a constrained C—H distance of 0.99 Å and refined as riding atoms with U iso(H) = 1.2U eq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [K2Pt(CN)4(C12H24O6)2] |
| M r | 905.99 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 100 |
| a, b, c (Å) | 11.7341 (10), 13.7280 (12), 11.8876 (10) |
| β (°) | 94.999 (3) |
| V (Å3) | 1907.6 (3) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 3.96 |
| Crystal size (mm) | 0.44 × 0.44 × 0.12 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2016 ▸) |
| T min, T max | 0.306, 0.564 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 57788, 5839, 4658 |
| R int | 0.047 |
| (sin θ/λ)max (Å−1) | 0.716 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.019, 0.051, 1.05 |
| No. of reflections | 5839 |
| No. of parameters | 215 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.25, −1.54 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019015238/hb4322sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019015238/hb4322Isup2.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [K2Pt(CN)4(C12H24O6)2] | F(000) = 912 |
| Mr = 905.99 | Dx = 1.577 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.7341 (10) Å | Cell parameters from 9630 reflections |
| b = 13.7280 (12) Å | θ = 2.3–30.6° |
| c = 11.8876 (10) Å | µ = 3.96 mm−1 |
| β = 94.999 (3)° | T = 100 K |
| V = 1907.6 (3) Å3 | Block, colourless |
| Z = 2 | 0.44 × 0.44 × 0.12 mm |
Data collection
| Bruker APEXII CCD diffractometer | 4658 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.047 |
| Absorption correction: multi-scan (SADABS; Bruker, 2016) | θmax = 30.6°, θmin = 2.3° |
| Tmin = 0.306, Tmax = 0.564 | h = −16→16 |
| 57788 measured reflections | k = −19→19 |
| 5839 independent reflections | l = −17→16 |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.019 | w = 1/[σ2(Fo2) + (0.0186P)2 + 1.9161P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.051 | (Δ/σ)max = 0.001 |
| S = 1.05 | Δρmax = 1.25 e Å−3 |
| 5839 reflections | Δρmin = −1.54 e Å−3 |
| 215 parameters | Extinction correction: SHELXL2018 (Sheldrick, 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0120 (4) |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Pt1 | 0.500000 | 0.500000 | 0.000000 | 0.01307 (4) | |
| K1 | 0.81107 (3) | 0.50085 (2) | 0.38736 (3) | 0.01684 (7) | |
| O5 | 0.93998 (11) | 0.65091 (10) | 0.29546 (12) | 0.0220 (3) | |
| O2 | 0.71541 (11) | 0.34705 (10) | 0.50409 (12) | 0.0232 (3) | |
| O4 | 0.97580 (12) | 0.45517 (10) | 0.23439 (12) | 0.0256 (3) | |
| O3 | 0.82893 (12) | 0.31294 (10) | 0.30722 (12) | 0.0254 (3) | |
| O1 | 0.68912 (13) | 0.54392 (11) | 0.57027 (12) | 0.0258 (3) | |
| O6 | 0.84409 (13) | 0.68057 (10) | 0.50316 (12) | 0.0273 (3) | |
| C2 | 0.34516 (17) | 0.47354 (16) | 0.05042 (17) | 0.0234 (4) | |
| N2 | 0.25437 (16) | 0.45768 (17) | 0.07632 (17) | 0.0358 (4) | |
| N1 | 0.61178 (17) | 0.50228 (13) | 0.25023 (17) | 0.0284 (4) | |
| C9 | 0.99412 (18) | 0.53486 (16) | 0.15999 (18) | 0.0268 (4) | |
| H9A | 0.922679 | 0.549960 | 0.112661 | 0.032* | |
| H9B | 1.054051 | 0.517845 | 0.109679 | 0.032* | |
| C1 | 0.56960 (16) | 0.50133 (12) | 0.15887 (17) | 0.0192 (3) | |
| C5 | 0.68672 (17) | 0.26882 (14) | 0.42770 (18) | 0.0265 (4) | |
| H5A | 0.628172 | 0.290733 | 0.368065 | 0.032* | |
| H5B | 0.654414 | 0.214014 | 0.468750 | 0.032* | |
| C10 | 1.03093 (17) | 0.62118 (15) | 0.23170 (19) | 0.0276 (4) | |
| H10A | 1.098625 | 0.603787 | 0.283356 | 0.033* | |
| H10B | 1.052471 | 0.675460 | 0.182981 | 0.033* | |
| C4 | 0.61633 (18) | 0.38299 (16) | 0.5518 (2) | 0.0309 (4) | |
| H4A | 0.579757 | 0.329734 | 0.591811 | 0.037* | |
| H4B | 0.560472 | 0.407414 | 0.491136 | 0.037* | |
| C6 | 0.79142 (17) | 0.23559 (14) | 0.37517 (17) | 0.0250 (4) | |
| H6A | 0.852386 | 0.218411 | 0.434705 | 0.030* | |
| H6B | 0.773614 | 0.177181 | 0.328031 | 0.030* | |
| C8 | 0.9510 (2) | 0.36800 (15) | 0.1721 (2) | 0.0335 (5) | |
| H8A | 1.016517 | 0.350456 | 0.129004 | 0.040* | |
| H8B | 0.882998 | 0.377767 | 0.117934 | 0.040* | |
| C14 | 0.7268 (2) | 0.62271 (15) | 0.64242 (18) | 0.0308 (4) | |
| H14A | 0.795873 | 0.603522 | 0.691114 | 0.037* | |
| H14B | 0.666168 | 0.640928 | 0.691447 | 0.037* | |
| C11 | 0.97505 (19) | 0.72802 (15) | 0.37123 (18) | 0.0295 (4) | |
| H11A | 1.002946 | 0.783790 | 0.328515 | 0.035* | |
| H11B | 1.038243 | 0.705558 | 0.425670 | 0.035* | |
| C12 | 0.8756 (2) | 0.75891 (14) | 0.43299 (19) | 0.0311 (4) | |
| H12A | 0.896408 | 0.816886 | 0.479879 | 0.037* | |
| H12B | 0.810247 | 0.776359 | 0.378360 | 0.037* | |
| C7 | 0.9286 (2) | 0.28768 (15) | 0.2533 (2) | 0.0329 (5) | |
| H7A | 0.916538 | 0.225258 | 0.212284 | 0.039* | |
| H7B | 0.994834 | 0.280173 | 0.310186 | 0.039* | |
| C3 | 0.65005 (18) | 0.46399 (16) | 0.63312 (18) | 0.0280 (4) | |
| H3A | 0.583590 | 0.484034 | 0.673536 | 0.034* | |
| H3B | 0.711603 | 0.441769 | 0.689539 | 0.034* | |
| C13 | 0.7534 (2) | 0.70708 (15) | 0.5689 (2) | 0.0342 (5) | |
| H13A | 0.684720 | 0.724656 | 0.518624 | 0.041* | |
| H13B | 0.775999 | 0.764336 | 0.616299 | 0.041* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Pt1 | 0.01451 (5) | 0.01192 (5) | 0.01299 (6) | 0.00149 (3) | 0.00243 (3) | −0.00065 (3) |
| K1 | 0.01690 (16) | 0.01710 (16) | 0.01701 (17) | −0.00121 (12) | 0.00425 (13) | −0.00143 (13) |
| O5 | 0.0216 (6) | 0.0192 (6) | 0.0254 (7) | −0.0033 (5) | 0.0037 (5) | −0.0028 (5) |
| O2 | 0.0223 (6) | 0.0220 (6) | 0.0260 (7) | −0.0027 (5) | 0.0065 (5) | −0.0029 (5) |
| O4 | 0.0296 (7) | 0.0201 (7) | 0.0283 (7) | 0.0010 (5) | 0.0104 (6) | −0.0033 (6) |
| O3 | 0.0289 (7) | 0.0185 (6) | 0.0301 (8) | 0.0011 (5) | 0.0105 (6) | −0.0019 (5) |
| O1 | 0.0319 (7) | 0.0256 (7) | 0.0213 (7) | −0.0017 (6) | 0.0103 (6) | −0.0027 (6) |
| O6 | 0.0382 (8) | 0.0194 (6) | 0.0255 (7) | −0.0025 (6) | 0.0099 (6) | −0.0032 (5) |
| C2 | 0.0251 (9) | 0.0275 (9) | 0.0176 (8) | 0.0004 (7) | 0.0018 (7) | −0.0031 (7) |
| N2 | 0.0260 (9) | 0.0547 (13) | 0.0273 (9) | −0.0040 (9) | 0.0062 (7) | −0.0055 (9) |
| N1 | 0.0228 (8) | 0.0430 (11) | 0.0193 (8) | 0.0027 (7) | 0.0013 (7) | −0.0011 (7) |
| C9 | 0.0278 (10) | 0.0273 (9) | 0.0272 (10) | 0.0016 (8) | 0.0138 (8) | 0.0007 (8) |
| C1 | 0.0156 (8) | 0.0228 (9) | 0.0193 (8) | 0.0009 (6) | 0.0030 (6) | −0.0009 (6) |
| C5 | 0.0282 (10) | 0.0226 (9) | 0.0291 (10) | −0.0070 (7) | 0.0052 (8) | −0.0030 (8) |
| C10 | 0.0202 (9) | 0.0268 (9) | 0.0373 (11) | −0.0026 (7) | 0.0101 (8) | 0.0013 (8) |
| C4 | 0.0261 (10) | 0.0301 (10) | 0.0390 (12) | −0.0041 (8) | 0.0167 (9) | −0.0026 (9) |
| C6 | 0.0300 (10) | 0.0180 (8) | 0.0268 (10) | −0.0017 (7) | 0.0020 (8) | −0.0013 (7) |
| C8 | 0.0426 (12) | 0.0252 (10) | 0.0355 (12) | −0.0025 (9) | 0.0194 (10) | −0.0103 (9) |
| C14 | 0.0422 (12) | 0.0276 (10) | 0.0243 (10) | 0.0007 (9) | 0.0131 (9) | −0.0070 (8) |
| C11 | 0.0360 (11) | 0.0226 (9) | 0.0301 (10) | −0.0121 (8) | 0.0037 (8) | −0.0037 (8) |
| C12 | 0.0475 (13) | 0.0174 (9) | 0.0292 (10) | −0.0060 (8) | 0.0075 (9) | −0.0046 (8) |
| C7 | 0.0376 (11) | 0.0207 (9) | 0.0427 (13) | 0.0024 (8) | 0.0169 (10) | −0.0080 (9) |
| C3 | 0.0280 (10) | 0.0310 (10) | 0.0273 (10) | 0.0011 (8) | 0.0162 (8) | 0.0006 (8) |
| C13 | 0.0496 (13) | 0.0227 (10) | 0.0322 (11) | 0.0032 (9) | 0.0147 (10) | −0.0070 (8) |
Geometric parameters (Å, º)
| Pt1—C2i | 1.996 (2) | C9—C10 | 1.501 (3) |
| Pt1—C2 | 1.996 (2) | C5—H5A | 0.9900 |
| Pt1—C1i | 1.991 (2) | C5—H5B | 0.9900 |
| Pt1—C1 | 1.991 (2) | C5—C6 | 1.497 (3) |
| K1—K1ii | 4.9761 (9) | C10—H10A | 0.9900 |
| K1—O5 | 2.8308 (14) | C10—H10B | 0.9900 |
| K1—O2 | 2.8133 (14) | C4—H4A | 0.9900 |
| K1—O4 | 2.8369 (14) | C4—H4B | 0.9900 |
| K1—O3 | 2.7642 (14) | C4—C3 | 1.503 (3) |
| K1—O1 | 2.7691 (14) | C6—H6A | 0.9900 |
| K1—O6 | 2.8354 (14) | C6—H6B | 0.9900 |
| K1—N1 | 2.732 (2) | C8—H8A | 0.9900 |
| K1—C4 | 3.527 (2) | C8—H8B | 0.9900 |
| O5—C10 | 1.422 (2) | C8—C7 | 1.503 (3) |
| O5—C11 | 1.427 (2) | C14—H14A | 0.9900 |
| O2—C5 | 1.428 (2) | C14—H14B | 0.9900 |
| O2—C4 | 1.425 (2) | C14—C13 | 1.500 (3) |
| O4—C9 | 1.435 (3) | C11—H11A | 0.9900 |
| O4—C8 | 1.424 (2) | C11—H11B | 0.9900 |
| O3—C6 | 1.427 (2) | C11—C12 | 1.493 (3) |
| O3—C7 | 1.425 (2) | C12—H12A | 0.9900 |
| O1—C14 | 1.426 (2) | C12—H12B | 0.9900 |
| O1—C3 | 1.426 (3) | C7—H7A | 0.9900 |
| O6—C12 | 1.429 (2) | C7—H7B | 0.9900 |
| O6—C13 | 1.421 (3) | C3—H3A | 0.9900 |
| C2—N2 | 1.155 (3) | C3—H3B | 0.9900 |
| N1—C1 | 1.154 (3) | C13—H13A | 0.9900 |
| C9—H9A | 0.9900 | C13—H13B | 0.9900 |
| C9—H9B | 0.9900 | ||
| C2i—Pt1—C2 | 180.0 | O2—C5—H5B | 109.7 |
| C1—Pt1—C2 | 91.47 (8) | O2—C5—C6 | 109.75 (16) |
| C1i—Pt1—C2i | 91.47 (8) | H5A—C5—H5B | 108.2 |
| C1—Pt1—C2i | 88.53 (8) | C6—C5—H5A | 109.7 |
| C1i—Pt1—C2 | 88.53 (8) | C6—C5—H5B | 109.7 |
| C1i—Pt1—C1 | 180.0 | O5—C10—C9 | 109.71 (16) |
| O5—K1—K1ii | 74.34 (3) | O5—C10—H10A | 109.7 |
| O5—K1—O4 | 59.78 (4) | O5—C10—H10B | 109.7 |
| O5—K1—O6 | 59.94 (4) | C9—C10—H10A | 109.7 |
| O5—K1—C4 | 160.31 (5) | C9—C10—H10B | 109.7 |
| O2—K1—K1ii | 96.09 (3) | H10A—C10—H10B | 108.2 |
| O2—K1—O5 | 170.43 (4) | K1—C4—H4A | 159.0 |
| O2—K1—O4 | 118.28 (4) | K1—C4—H4B | 82.0 |
| O2—K1—O6 | 117.20 (4) | O2—C4—K1 | 49.29 (9) |
| O2—K1—C4 | 22.59 (4) | O2—C4—H4A | 109.8 |
| O4—K1—K1ii | 73.68 (3) | O2—C4—H4B | 109.8 |
| O4—K1—C4 | 139.85 (5) | O2—C4—C3 | 109.49 (17) |
| O3—K1—K1ii | 95.19 (3) | H4A—C4—H4B | 108.2 |
| O3—K1—O5 | 119.15 (4) | C3—C4—K1 | 82.54 (11) |
| O3—K1—O2 | 61.00 (4) | C3—C4—H4A | 109.8 |
| O3—K1—O4 | 59.77 (4) | C3—C4—H4B | 109.8 |
| O3—K1—O1 | 121.99 (4) | O3—C6—C5 | 108.32 (16) |
| O3—K1—O6 | 165.50 (5) | O3—C6—H6A | 110.0 |
| O3—K1—C4 | 80.51 (5) | O3—C6—H6B | 110.0 |
| O1—K1—K1ii | 94.33 (3) | C5—C6—H6A | 110.0 |
| O1—K1—O5 | 118.53 (4) | C5—C6—H6B | 110.0 |
| O1—K1—O2 | 61.13 (4) | H6A—C6—H6B | 108.4 |
| O1—K1—O4 | 167.99 (5) | O4—C8—H8A | 109.9 |
| O1—K1—O6 | 59.40 (4) | O4—C8—H8B | 109.9 |
| O1—K1—C4 | 42.15 (5) | O4—C8—C7 | 108.77 (18) |
| O6—K1—K1ii | 70.44 (3) | H8A—C8—H8B | 108.3 |
| O6—K1—O4 | 115.57 (4) | C7—C8—H8A | 109.9 |
| O6—K1—C4 | 101.40 (5) | C7—C8—H8B | 109.9 |
| N1—K1—K1ii | 175.95 (5) | O1—C14—H14A | 110.2 |
| N1—K1—O5 | 102.88 (5) | O1—C14—H14B | 110.2 |
| N1—K1—O2 | 86.68 (5) | O1—C14—C13 | 107.71 (17) |
| N1—K1—O4 | 102.40 (5) | H14A—C14—H14B | 108.5 |
| N1—K1—O3 | 83.55 (5) | C13—C14—H14A | 110.2 |
| N1—K1—O1 | 89.59 (5) | C13—C14—H14B | 110.2 |
| N1—K1—O6 | 110.92 (5) | O5—C11—H11A | 109.9 |
| N1—K1—C4 | 76.81 (6) | O5—C11—H11B | 109.9 |
| C4—K1—K1ii | 106.82 (4) | O5—C11—C12 | 109.03 (17) |
| C10—O5—K1 | 116.61 (11) | H11A—C11—H11B | 108.3 |
| C10—O5—C11 | 111.15 (15) | C12—C11—H11A | 109.9 |
| C11—O5—K1 | 115.51 (11) | C12—C11—H11B | 109.9 |
| C5—O2—K1 | 109.45 (11) | O6—C12—C11 | 109.03 (17) |
| C4—O2—K1 | 108.12 (11) | O6—C12—H12A | 109.9 |
| C4—O2—C5 | 110.99 (15) | O6—C12—H12B | 109.9 |
| C9—O4—K1 | 111.94 (11) | C11—C12—H12A | 109.9 |
| C8—O4—K1 | 113.54 (11) | C11—C12—H12B | 109.9 |
| C8—O4—C9 | 110.80 (17) | H12A—C12—H12B | 108.3 |
| C6—O3—K1 | 117.58 (11) | O3—C7—C8 | 107.85 (17) |
| C7—O3—K1 | 118.16 (11) | O3—C7—H7A | 110.1 |
| C7—O3—C6 | 112.30 (15) | O3—C7—H7B | 110.1 |
| C14—O1—K1 | 118.68 (12) | C8—C7—H7A | 110.1 |
| C3—O1—K1 | 117.32 (11) | C8—C7—H7B | 110.1 |
| C3—O1—C14 | 111.44 (16) | H7A—C7—H7B | 108.4 |
| C12—O6—K1 | 113.75 (11) | O1—C3—C4 | 108.13 (17) |
| C13—O6—K1 | 114.36 (12) | O1—C3—H3A | 110.1 |
| C13—O6—C12 | 111.85 (16) | O1—C3—H3B | 110.1 |
| N2—C2—Pt1 | 177.97 (18) | C4—C3—H3A | 110.1 |
| C1—N1—K1 | 146.76 (17) | C4—C3—H3B | 110.1 |
| O4—C9—H9A | 110.2 | H3A—C3—H3B | 108.4 |
| O4—C9—H9B | 110.2 | O6—C13—C14 | 109.05 (17) |
| O4—C9—C10 | 107.64 (17) | O6—C13—H13A | 109.9 |
| H9A—C9—H9B | 108.5 | O6—C13—H13B | 109.9 |
| C10—C9—H9A | 110.2 | C14—C13—H13A | 109.9 |
| C10—C9—H9B | 110.2 | C14—C13—H13B | 109.9 |
| N1—C1—Pt1 | 178.81 (18) | H13A—C13—H13B | 108.3 |
| O2—C5—H5A | 109.7 |
Symmetry codes: (i) −x+1, −y+1, −z; (ii) −x+2, −y+1, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3A···N1iii | 0.99 | 2.54 | 3.510 (3) | 165 |
| C9—H9B···N2iv | 0.99 | 2.55 | 3.459 (3) | 152 |
Symmetry codes: (iii) −x+1, −y+1, −z+1; (iv) x+1, y, z.
Funding Statement
This work was funded by Freie Universität Berlin grant . Deutsche Forschungsgemeinschaft grant .
References
- Alexandrov, E. V., Virovets, A. V., Blatov, V. A. & Peresypkina, E. V. (2015). Chem. Rev. 115, 12286–12319. [DOI] [PubMed]
- Almeida, J. F. & Pidcock, A. (1981). J. Organomet. Chem. 208, 273–278.
- Bruker (2016). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Doheny, A. J. & Ganem, B. (1980). J. Chem. Educ. 57, 308.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Holzapfel, W., Yersin, H. & Gliemann, G. (1981). Z. Kristallogr. 157, 47–67.
- Krogmann, K. (1969). Angew. Chem. Int. Ed. Engl. 8, 35–42.
- Liu, F.-H., Chen, W.-Z. & Wang, D.-Q. (2006). Chin J. Struct. Chem. 25, 677–680.
- Mason, W. R. III & Gray, H. B. (1968). J. Am. Chem. Soc. 90, 5721–5729.
- Mühle, C., Nuss, J., Dinnebier, R. E. & Jansen, M. (2004). Z. Anorg. Allg. Chem. 630, 1462–1468.
- Nast, R. & Moerler, H.-D. (1969). Chem. Ber. 102, 2050–2056.
- Neuhausen, C., Pattison, P. & Schiltz, M. (2011). CrystEngComm, 13, 430–432.
- Niel, V., Martinez-Agudo, J. M., Muñoz, M. C., Gaspar, A. B. & Real, J. A. (2001). Inorg. Chem. 40, 3838–3839. [DOI] [PubMed]
- Ohkoshi, S.-I. & Tokoro, H. (2012). Acc. Chem. Res. 45, 1749–1758. [DOI] [PubMed]
- Olmstead, M. M., Beavers, C. M. & Paw, U. L. (2009). Acta Cryst. E65, m408–m409. [DOI] [PMC free article] [PubMed]
- Olmstead, M. M., Lee, M. A. & Stork, J. R. (2005). Acta Cryst. E61, m1048–m1050.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Stojanovic, M., Robinson, N. J., Ngo, T. & Sykora, R. E. (2011). J. Chem. Crystallogr. 41, 1425–1432.
- Varju, D. R., Wollschlaeger, S. A. & Leznoff, D. B. (2019). Chem. Eur. J. 25, 9017–9025. [DOI] [PubMed]
- Vostrikova, K. E. & Peresypkina, E. V. (2011). Eur. J. Inorg. Chem. pp. 811–815.
- Washecheck, D. M., Peterson, S. W., Reis, A. H. Jr & Williams, J. M. (1976). Inorg. Chem. 15, 74–78.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zhou, B.-C., Kou, H.-Z., He, Y., Wang, R.-J., Li, Y.-D. & Wang, H.-G. (2003). Chin. J. Chem. 21, 352–355.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019015238/hb4322sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019015238/hb4322Isup2.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report