Abstract
There is a need for biomarkers to improve the clinical benefit from systemic treatment of colorectal cancer. We designed a prospective, clinical study where patients receiving regorafenib as last-line treatment had sequential blood samples drawn. Effect and toxicity was monitored. The primary clinical endpoint was progression free survival (PFS). Cell-free circulating tumor (ct) DNA was measured as either the fraction with Neuropeptide Y (NPY) methylated DNA or KRAS/NRAS/BRAF mutated ctDNA. One hundred patients were included from three Danish centers. Among 95 patients who received regorafenib for at least two weeks, the median PFS was 2.1 months (95% confidence interval (CI) 1.8–3.3) and the median overall survival (OS) was 5.2 months (95% CI 4.3–6.5). Grade 3–4 toxicities were reported 51 times, most frequently hypertension, hand-food syndrome, and skin rash. In the biomarker population of 91 patients, 49 could be monitored using mutated DNA and 90 using methylated DNA. There was a strong correlation between mutated and methylated DNA. The median survival for patients with a level of methylated ctDNA above the median was 4.3 months compared to 7.6 months with ctDNA below the median, p < 0.001. The median time from increasing methylated ctDNA to disease progression was 1.64 months (range 0.46–8.38 months). In conclusion, NPY methylated ctDNA was a universal liquid biopsy marker in colorectal cancer patients treated with regorafenib. High baseline levels correlated with short survival and changes during treatment may predict early effect and later progression. We suggest plasma NPY methylation analysis as an easy and universally applicable method for longitudinal monitoring of ctDNA in metastatic colorectal cancer patients.
Keywords: ctDNA, NPY methylation, biomarker, colorectal cancer, regorafenib
1. Introduction
Last line treatment with regorafenib for patients with metastatic colorectal cancer has proved limited survival benefit in randomized trials and a severe toxicity profile [1]. Therefore, biomarkers are essential in order to optimize the patient selection before treatment. Furthermore, biomarkers are needed for early detection of resistance, in order to help stop an inefficient treatment as early as possible.
Cell-free circulating tumor specific DNA (ctDNA) in plasma is a potential surrogate for the entire tumor genome and may be used as a “liquid biopsy” [2]. Serial blood tests with analysis of ctDNA is a promising method for both initial selection of patients to receive treatment and for monitoring treatment effect during therapy [3,4]. The fraction of the total DNA in plasma that is tumor specific can be defined as the fraction with DNA sequence mutations only present in tumor tissue. Most commonly, ctDNA is detected by next generation sequencing either directly in plasma or in tumor tissue followed by PCR analysis for quantification in plasma of specific mutations. One of the major drawbacks of this method is the pronounced heterogeneity of mutations between different colorectal tumors.
Epigenetic changes, i.e., aberrant methylation of DNA, affect gene expression and are important in the carcinogenesis [5]. Aberrant methylation may be a more robust target for detecting and quantifying ctDNA [6,7,8], and preliminary results support this use [9]. Data from clinical settings are lacking.
The neurotransmitter Neuropeptide Y (NPY) is involved in cell motion and cell proliferation and can reduce the invasive potential of colon cancer cells in vitro [10]. The gene is frequently hypermethylated in certain carcinomas and gene promoter hypermethylation is correlated with inactivation of gene expression [11]. Roperch et al. proposed a panel of tumor-specific hypermethylated genes including NPY and confirmed their power to discriminate healthy individuals from patients with risk of colorectal cancer [12]. The same panel was investigated by Garrigou et al. analyzing hypermethylation in different stages of colorectal cancer to identify universal blood markers in the follow up setting [13].
The standard systemic treatments for stage IV colorectal cancer include the cytotoxic agents 5-flourouracil, irinotecan and oxaliplatin. The anti-EGFR antibodies cetuximab or panitumumab should be added in the case of KRAS, NRAS, or BRAF (RAS/RAF) wild-type tumors. Bevacizumab may be supplemented to chemotherapy, but there is no marker for optimally select patients for this agent. After exposure, intolerance or contraindications to these drugs, the patient care should be focused on supportive and palliative care or experimental treatments.
Regorafenib is an oral multikinase inhibitor which targets e.g., VEGFR1, VEGFR2, VEGFR3, TIE2, PDGF, FGFR, RET and cKIT [14]. In the pivotal phase III trial, the median survival was prolonged for 1.4 months, but with more than half of the patients experiencing grade 3+ toxicity [1].
The effect is below the bar defined by American Society of Clinical Oncology (ASCO) for clinically meaningful outcomes [15] and turns into an unfavorable score in European Society for Medical Oncology’s (ESMO’s) approach to stratify drugs based on the magnitude of clinical benefit [16]. In Denmark, the drug has not been approved by the authorities for standard use.
Based on a proven anti-cancer effect, we wanted to investigate the outcomes from regorafenib treatment in a Danish colorectal cancer patient cohort. Because of the expected limited efficacy, we also wanted to evaluate ctDNA as a marker which may help to identify patients with expected no or minimal effect of regorafinib. We hypothesized that tumor specific methylation of NPY in plasma DNA correlated with ctDNA measured with DNA nucleotide mutation. Furthermore, we hypothesized that NPY methylation changes during regorafenib treatment reflected the clinical course and could predict progression earlier than imaging.
2. Results
2.1. Patient Characteristics
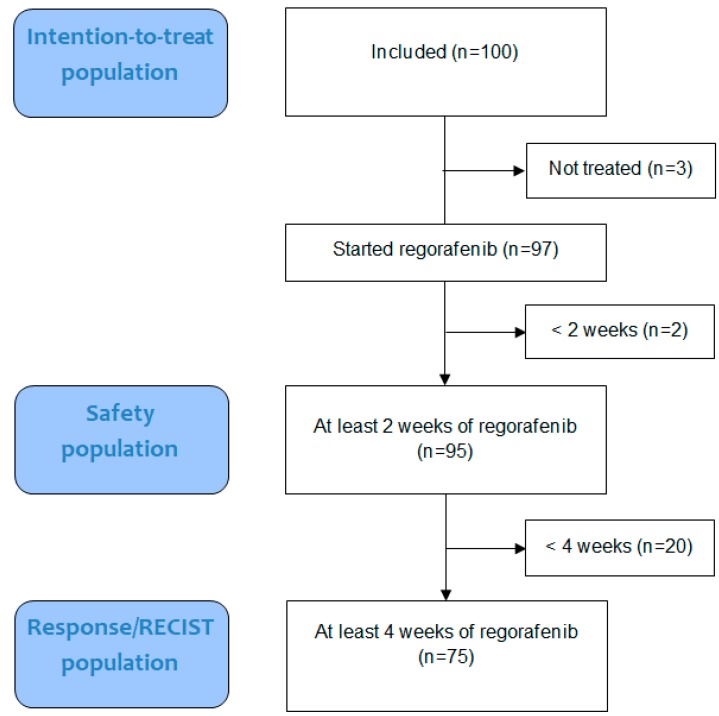
From October 2013 to May 2016, 100 patients were included. The patient flow is shown in Figure 1. Most patients were in performance status 1 (n = 54) and 43 in performance status 0. Performance status was not specified as 0 or 1 in three cases. Patient characteristics are shown in Table 1.
Figure 1.
Patient Flow with an illustration of the intention-to-treat, safety, and Response evaluation criteria in solid tumors (RECIST) population.
Table 1.
Patient characteristics for the intention-to-treat population of 100 patients. NR = not reported.
| Characteristic | Categories | Data |
|---|---|---|
| Age | Median (min–max), years | 65 (28–77) |
| Time since first chemotherapy for metastatic colorectal cancer | Median (min–max), years | 1.8 (0.4–10.9) |
| n | ||
| Sex | Female | 47 |
| Male | 53 | |
| Localization of primary tumor | Right colon | 30 |
| Left colon | 31 | |
| Rectum | 35 | |
| NR | 4 | |
| No of metastatic sites | 1–2 | 54 |
| 2+ | 46 | |
| Performance status | 0 | 43 |
| 1 | 54 | |
| NR | 3 | |
| Treatment, adjuvant | Fluoropyrimidine monotherapy | 36 |
| Fluoropyrimidine and oxaliplatin | 30 | |
| Treatment, metastatic | Fluoropyrimidine | 96 |
| Oxaliplatin | 87 | |
| Irinotecan | 98 | |
| Bevacizumab | 90 | |
| EGFR inhibitor | 33 | |
| Other | 5 | |
| NR | 2 | |
| Mutation in tumor tissue | KRAS | 51 |
| NRAS | 4 | |
| BRAF | 6 | |
| Wild-type or NR | 39 |
2.2. Treatment
Three patients were included but never received treatment. The 97 patients starting regorafenib received it for a median of 63 days (range 3 to 493 days).
2.3. Toxicity
Ninety-five patients were treated with regorafenib for at least two weeks and were considered evaluable for toxicity. Grade 3–4 toxicities were reported 51 times, most frequently hypertension, hand-food syndrome, and skin rash. Details of toxicity are given in Table 2.
Table 2.
Toxicity analysis of 95 patients evaluable for toxicity. The most common toxicities are listed according to frequency of minor toxicity, grade 1–2, and major toxicity, grade 3–4.
| Adverse Event | Grade 1–2 N (%) |
Grade 3–4 N (%) |
|---|---|---|
| Hypertension | 31 (33%) | 8 (8%) |
| Hand-foot-skin reaction | 48 (51%) | 6 (6%) |
| Rash | 20 (21%) | 6 (6%) |
| Fatigue | 77 (81%) | 4 (4%) |
| Diarrhea | 35 (37%) | 3 (3%) |
| Anorexia | 60 (63%) | 2 (2%) |
| Oral mucositis | 43 (45%) | 2 (2%) |
| Nausea | 35 (37%) | 1 (1%) |
| Voice changes | 66 (69%) | 0 (0%) |
| Conjunctivitis | 19 (20%) | 0 (0%) |
| Bleeding | 16 (17%) | 0 (0%) |
| Other toxicites | 65 (68%) | 19 (20%) |
2.4. Efficacy of Regorafenib
Patients were evaluable for response if they received regorafenib for at least four weeks and had measurable disease on the baseline scan. Seventy-five patients fulfilled these criteria, 60% had stable disease and 40% had progression as best response. There were no complete or partial responses.
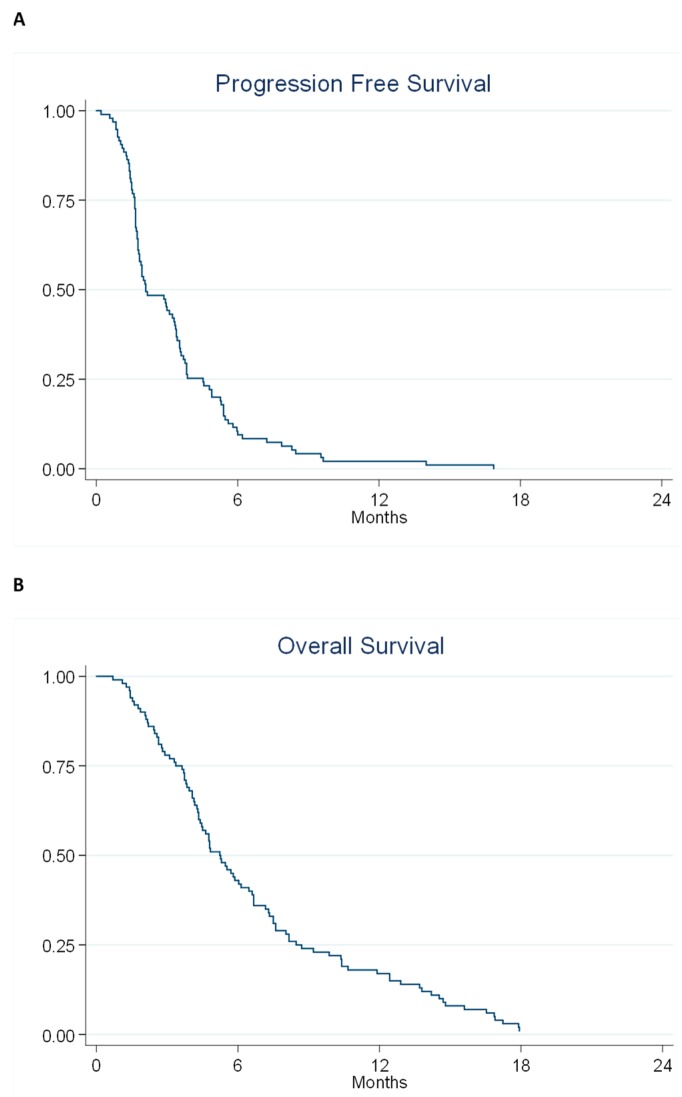
All patients were evaluable for PFS and OS and all but two patients died during followed up. The median PFS was 2.1 months (95% confidence interval (CI) 1.8–3.3) and the median OS was 5.2 months (95% CI 4.3–6.5). The PFS and OS curves are shown in Figure 2.
Figure 2.
Kaplan–Meier Plots describing progression free survival (A) and overall survival (B) for the entire cohort.
Forty-six patients were alive without progression at two months. Thus, the fraction of PFS at two months was 46% (95% CI 36.1–55.9%) of the intention-to-treat population. Efficacy data is summarized in Table 3.
Table 3.
Clinical Outcomes. Response rates are given for 75 patients evaluable for response. PFS = progression free survival at 2 months is the fraction of all patients alive without progression at the radiological evaluation after two cycles. Median PFS and OS = overall survival is given for the intention-to-treat population and reported using the Kaplan–Meier method.
| Endpoint | Categories | N | Data | 95% CI |
|---|---|---|---|---|
| Response rate | Stable disease | 45 | 60% | 48–71% |
| Progressive disease | 30 | 40% | 29–52% | |
| PFS | At 2 months | 46 | 46% | 36–56% |
| Median | 100 | 2.1 months | 1.8–3.4 | |
| OS | Median | 100 | 5.2 months | 4.3–6.5 |
2.5. Tumor Specific DNA
Plasma samples were available from 91 patients (anytime) and 82 at baseline. In 49 of 61 patients with a known tumor RAS/RAF mutation the same mutation was detected in at least one plasma sample. In 90 of 91 patients, methylated NPY was detected at least in one sample. The case where NPY methylation failed was a patient with a single blood sample who stopped regorafenib after three days, i.e., not eligible for toxicity or response evaluation.
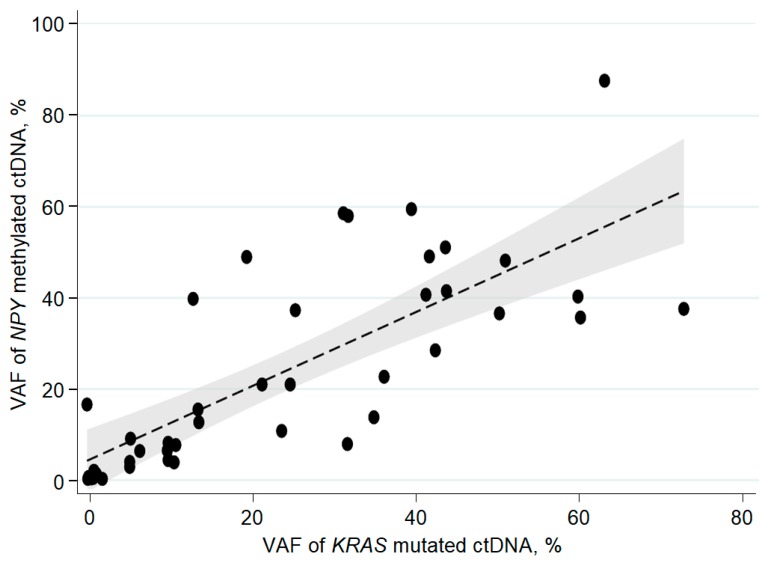
There was a strong correlation between paired observations of fraction of RAS/RAF mutation and fraction of NPY methylated ctDNA; Spearman’s rho was 0.82 (p < 0.001) for paired observations at baseline (Figure 3). Correspondingly, Spearman’s rho was 0.83 and 0.87 for the two next sampling time points during therapy (p < 0.001).
Figure 3.
Correlation between baseline cell-free circulating tumor specific DNA (ctDNA) in the RAS mutation cohort measured by mutation or methylation, Spearman’s rho = 0.82, p < 0.001, n = 42. VAF = Variant allele frequency, dashed line is the linear regression line with 95% confidence interval in grey.
The fraction of RAS/RAF mutated ctDNA and the fraction of NPY methylated ctDNA were independent from sex, age, and PS at baseline. Table 4 depicts the biomarker population and correlations with basic characteristics.
Table 4.
Patient characteristics of the biomarker population. Correlation between patient characteristics and the mean level of circulating tumor DNA (ctDNA) at baseline determined either by RAS/RAF mutation (Mut ctDNA) or NPY methylation (Meth ctDNA) in the biomarker population, n = number, CI = confidence interval. In 82 patients a baseline plasma sample was available and the correlations are based on these patients.
| Characteristic | Categories | Clinical Trial, n | Mut ctDNA | Meth ctDNA | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean % (95%CI) | p-Value | n | Mean % (95%CI) | p-Value | |||
| Age | Median (min–max), years 65 (28–77) | 100 | 46 | 25 (19–32) | 0.19 | 82 | 25 (20–30) | 0.24 |
| Sex | Female | 47 | 21 | 25 (14–36) | 40 | 21 (14–27) | ||
| Male | 53 | 25 | 25 (18–33) | 0.77 | 41 | 30 (22–37) | 0.11 | |
| Localization of primary tumor | Right colon | 30 | 18 | 26 (16–37) | 24 | 30 (19–44) | ||
| Left colon | 31 | 14 | 27 (12–41) | 24 | 21 (10–31) | |||
| Rectum | 35 | 13 | 23 (12–35) | 0.98 | 31 | 23 (17–30) | 0.32 | |
| Performance status | 0 | 43 | 22 | 24 (15–33) | 34 | 21 (15–30) | ||
| 1 | 54 | 24 | 27 (17–36) | 0.96 | 46 | 27 (19–34) | 0.58 | |
There was a negative correlation between the level of baseline mutated ctDNA and the primary endpoint PFS at two months, p = 0.004 (n = 46), but not for the fraction of methylated ctDNA, p = 0.26 (n = 82).
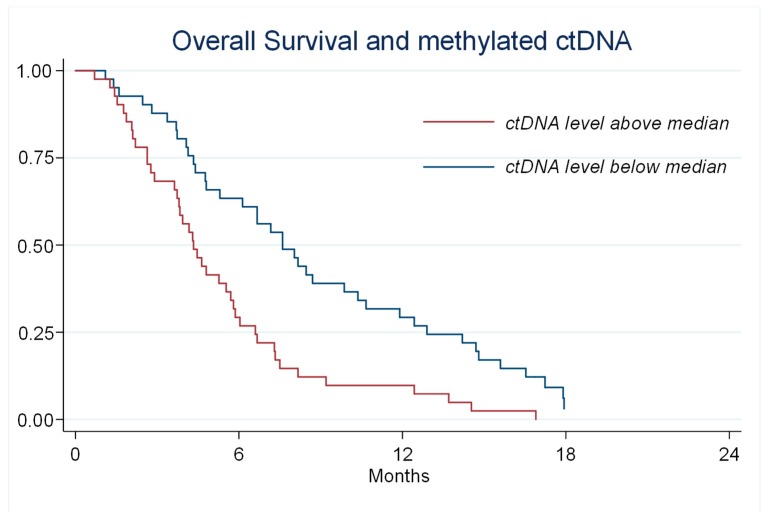
The fraction of ctDNA at baseline correlated with OS both for mutated ctDNA, p < 0.001 (n = 46) and for methylated ctDNA. The median OS for patients with a level of methylated ctDNA above the median was 4.3 months compared to 7.6 months with ctDNA below the median, p < 0.001 (Figure 4). In a multivariate model including age, sex, location of primary tumor, performance status, and level of methylated ctDNA, the latter was the only significant predictor of survival (p = 0.001).
Figure 4.
Kaplan–Meier Plots describing overall survival for patients with a level of methylated circulating tumor (ct) DNA above or below the median. Median survival was 7.6 vs. 4.3 months, p < 0.001.
2.6. Dynamics of ctDNA
The initial effect of regorafenib on ctDNA was evaluated by comparing 74 patients who had a baseline sample and a follow-up sample. The fraction of NPY methylated DNA fell initially in 68 of 74 patients (92%).
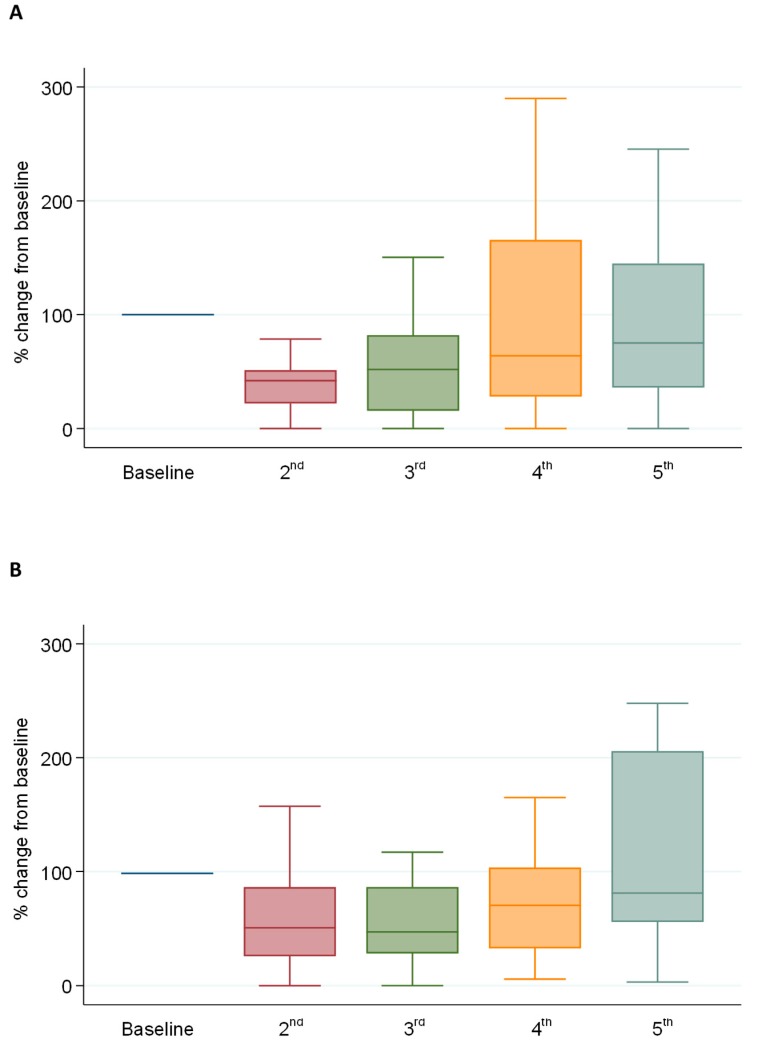
The longitudinal changes during treatment were described by normalizing the fraction of methylated ctDNA to 100 at baseline for patients with at least five serial plasma samples (n = 38). The relative change during treatment is shown in Figure 5A. There is an immediate decline initially followed by an increase. The mean normalized fraction of methylated ctDNA was 100%, 48%, 71%, 185%, and 220% for the first five samples, respectively, with a significant decline in Sample 2 and 3 compared to the baseline (p < 0.001 and p = 0.04). For comparison, the same is shown in Figure 5B for RAS/RAF mutated ctDNA (n = 23) with a similar pattern. The means were 100%, 60%, 69%, 128%, and 198% for Sample 1–5. The decrease was significant for Sample 2 and 3 compared to the baseline (p < 0.001 and p = 0.04).
Figure 5.
(A) methylated circulating tumor (ct) ctDNA and (B) mutated ctDNA. Baseline and five serial plasma samples were available from 40 and 23 patients, respectively. The boxplots of medians show the longitudinal changes of normalized values of the fraction of ctDNA.
Collection of longitudinal samples made it possible to evaluate the time span for individual patients from the increase in NPY methylated ctDNA during treatment to time of radiologic progression. Fifty-three patients evaluable according to response evaluation criteria in solid tumors (RECIST) received more than one full cycle of regorafenib and had at least three plasma samples analyzed. Patients were followed in the protocol until progression, and 48 of these 53 patients (91%) showed an increase in the fraction of NPY ctDNA before progression according to RECIST. The median time from increasing ctDNA to RECIST progression was 1.64 months (range 0.46–8.38 months).
3. Discussion
In this trial of last-line regorafenib in metastatic colorectal cancer patients, we showed that monitoring of the disease during treatment was possible using ctDNA. Most commonly, the detection of tumor specific mutations in the DNA is used to define tumor specificity. Early reports have pointed towards NPY methylation as a marker of tumor specificity. We show for the first time in last-line treatment with regorafenib that measuring NPY methylated ctDNA is superior to measuring RAS/RAF mutated ctDNA, as it can be measured in almost all patients irrespective of mutational status. Furthermore, the dynamics of NPY methylated ctDNA during treatment reflect the dynamics of RAS/RAF ctDNA. A smaller study has reached the same conclusion in a mixed population of patients with metastatic colorectal cancer [9]. Future studies should focus on evaluating NPY ctDNA and treatment effect in larger, prospective trials.
The main clinical endpoint in the present trial was the fraction of PFS at two months (46%). The median PFS was 2.1 months and median OS was 5.2 months. In the CORRECT [1] and the CONCUR [17] trials, the median PFS was 1.9 and 3.2 and median OS 6.4 and 8.8 months, respectively. In conclusion, our data does not support a clinically relevant benefit or value [15,16] of regorafenib in unselected Danish colorectal cancer patients after standard systemic treatment for metastatic disease.
Given the low clinical effect in an unselected cohort, the question is whether defined subgroups may benefit. We used ctDNA to group patients and monitor the disease during treatment. By dividing the patients according to the median level of NPY methylated ctDNA, it was possible to identify a subgroup of patients with a low median OS of 4.3 months (Figure 4). These patients would probably benefit more from palliative care than specific antineoplastic treatment with the risk of toxicity [18].
In the biomarker study associated with the CORRECT trial, the relative effect, but not the absolute effect, of regorafenib was maintained across subgroups of patients divided by high or low concentration of total circulating DNA [19]. Furthermore, ctDNA, defined as level of KRAS mutation, had a prognostic effect. Our data supports their findings in the minority with detectable RAS/RAF mutations. In addition, our data adds further information that NPY methylated ctDNA is detectable in practically every colorectal cancer patient, correlates with RAS/RAF mutated ctDNA, and has the same prognostic information. This calls for trials evaluating deselection of patients with high ctDNA for last-line treatment.
Another interesting observation was the initial decrease in ctDNA in almost all patients. It can be speculated that regorafenib has a specific antineoplastic effect in the vast majority of colorectal cancer patients, but redundancy in cancer [20] leads to early resistance. If this is true, then personalized, targeted medicine, in the sense that pretreatment marker analysis can predict clinical effect [21], is destined to fail. What decides clinical benefit may instead be mechanisms of resistance [20]. We suggest performing studies in which predictions of clinical benefits of a drug is based not on a snapshot of molecular biology but on biological systems incorporating the temporal effects. These systems are under development using 3D tumor cell cultures combined with sensitivity analysis [22].
Traditionally, the duration of benefit from a drug is reflected by PFS. We showed that progression according to RECIST was preceded by an increase in ctDNA with a median of 1.6 months. Thus, ineffective treatment may be stopped earlier if based on increasing NPY ctDNA. We suggest that future trials should test early termination based on increasing ctDNA compared to RECIST progression with the perspective of sparing the patients from ineffective treatments and unnecessary toxicity.
The limitations of our study are primarily caused by the relatively small patient number. It is not possible to give narrow estimates of efficacy of regorafenib in just 100 patients, and subgrouping based on markers is even more uncertain. Furthermore, there is no untreated control group. Subsequently, our conclusions and suggestions based on the results are made with caution.
4. Materials and Methods
4.1. Patients
Patients with histologically confirmed metastatic adenocarcinoma of the colon or rectum were included from three Danish cancer centres. Refractory disease was required and it was defined as progressive disease within six months of the last administration of each drug or regimen including fluoropyrimidine, oxaliplatin, irinotecan, and bevacizumab. In cases with RAS/RAF wild-type tumor, progression of treatment with cetuximab or panitumumab was also mandatory. If there were contraindications to any of the drugs, resistance to that specific drug was not required. Contraindications could be grade 2 or higher neurotoxicity after previous adjuvant oxaliplatin, cardiotoxicity to fluoropyrimidine, or hypersensitivity reactions despite pretreatment. Imaging performed within one month of inclusion should be evaluable, i.e., not necessarily with measurable disease, according to RECIST version 1.1 [23]. Other inclusion criteria were performance status 0–1, adequate organ function based on laboratory tests, age of at least 18 years, and informed consent. Patients were excluded in the case of uncontrolled hypertension or proteinuria, symptomatic medical conditions requiring prompt interventions, or pregnancy including chance of pregnancy and breast feeding.
4.2. Ethics and Approvals
The trial was approved by the Regional Ethics Committee on Health Research, Region of Southern Denmark No. S-20130057, 2013 and reported to the Danish Data Protection Agency.
4.3. Trial Design and Treatment
In this single-arm, prospective phase II biomarker trial, eligible patients received an initial dose of 120 or 160 mg regorafenib once daily according to institutional practice. The starting dose of 120 mg was allowed to escalate to 160 mg if tolerable. One cycle was defined as 21 days with regorafenib followed by seven days without regorafenib. Treatment continued until progression or unacceptable toxicity including patient request for stopping. Procedures for dose modifications and pausing of regorafenib were specified in the protocol according to observed toxicity.
Reporting of the study followed the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) [24] whenever relevant.
4.4. Evaluations
Hematology, biochemistry, and organ function tests (Stivarga, Summary of Product Characteristics, European Medicines Agency, 2013) were performed every second week for two months and then monthly if stable. Imaging of the chest and abdomen and disease evaluation according to RECIST 1.1 was performed every second cycle of treatment.
4.5. Blood Samples and Tumor Tissue
Before treatment, after two weeks of treatment, and then before every new cycle, 9 mL of blood was drawn in EDTA tubes for marker analyses. After treatment ended and until progression, blood was drawn before every evaluation scan. The samples were centrifuged at 2000 g for 10 minutes and stored at −80 degrees Celsius within four hours. Beside plasma samples, serum, buffy coat, and tumor biopsies were collected and stored in a biobank.
4.5.1. DNA Purification
DNA was purified from 4 mL plasma after thawing and centrifugation for 10 min at 10,000 g using QIAsymphony Circulating DNA kit (Qiagen, Venlo, The Netherlands). An internal control, 20 µL CPP1, was added [25] and the DNA was eluted in 60 µL before the addition of 340 µL water. The QIAsymphony robot (Qiagen) was used for purification.
4.5.2. Quantification of Total Plasma DNA
Quantitative PCR analysis of B2M and CPP1 was performed on a Quantstudio 12k Flex real-time PCR system (Life Technologies, Waltham, MA, USA) according to [25]. DNA from lymphocytes and water were included as external controls in the qPCR.
4.5.3. Concentration
In samples where both RAS/RAF mutation and methylation analysis were performed, DNA was concentrated to 25 µL using Amicon Ultra-0.5 Centrifugal Filter Unit (Merck Millipore, Burlington, MA, USA), and 12 µL was used for mutation analysis and 12 µL for bisulfite conversion. If only methylation analysis was performed, half of the DNA was concentrated to 20 µL. In samples with high total DNA, the amount of DNA for conversion was reduced accordingly.
4.5.4. RAS/RAF Mutation Analysis
The specific RAS/RAF mutation known from the tumor tissue analysis was analyzed in duplicates with five µL samples in 20 µL reactions using digital droplet (dd) PCR supermix for probes (no dUTP) and Bio-Rad PrimePCR assays. Controls included gBlock mixed with wild-type donor DNA as positive control and wild-type donor DNA and water as negative controls for each mutation assay. Droplets were generated with QX200 Automated Droplet Generator (Bio-Rad, Hercules, CA, USA) and PCR run on a Veriti thermal cycler (Applied Biosystems, Waltham, MA, USA). The QX100 Droplet Digital Reader (Bio-Rad) was used for reading of the samples.
4.5.5. Methylation Analysis
DNA underwent bisulphite conversion in a 50 µL reaction using EZ DNA Methylation Lightning Kit (Zymo Research, Irvine, CA, USA) before eluting to 12 µL. NPY/Albumin analysis was done according to Garrigou [13] with ddPCR as described above. Data analysis was completed with QuantaSoft ver. 1.7.4 (Bio-Rad).
All analyses were performed by personal blinded to the study end points.
4.6. Statistics
The clinical primary endpoint was the fraction of progression free survival (PFS) at two months defined as patients alive without progression at the radiological evaluation after two cycles divided with the number of all patients who entered the trial on an intention-to-treat principle. It was anticipated that 50% of the patients would progress at two months [1]. We considered it of clinical interest to detect a 15% point change in PFS at two months. With a power of 90% and a 5% risk of a type-one error, 56 of 97 included patients should be without progression two months after inclusion to indicate an effect of 15% point [26]. Hence, we aimed at including 100 patients to allow for a clinical test and a biomarker evaluation.
Two central experimental plasma biomarkers were evaluated in this study. The first was ctDNA measured as the fraction of mutated ctDNA with specific RAS/RAF sequence mutation relative to total DNA in plasma. The second was ctDNA measured as the fraction of ctDNA with methylated NPY gene relative to total DNA in plasma. Spearman’s rank-order correlation described the correlation between these two biomarkers.
Baseline characteristics such as age, sex, performance status, histopathology, date of diagnosis, and type of previous treatments were described using descriptive statistics. Toxicity was evaluated at baseline and at every cycle using common terminology criteria for adverse events version 4 (National Cancer Institute 2009), and the worst grade was noted. Treatment was described by dates of first and last administration, dose, and dose modifications. The effect of regorafenib was measured according to RECIST 1.1 [23] with respect to response and progression. PFS was defined as the time from inclusion in the trial to the first date of progression or death from any cause. Overall survival (OS) was defined as the time from inclusion to death independent from cause. The Kaplan–Meier method was used for PFS and OS in this setting and comparisons were done with the log rank test. Patients who received at least two weeks of regorafenib were evaluable for toxicity and those receiving at least one cycle were evaluable for response. All patients were evaluable for PFS and OS according to the intention-to-treat principle. Level of significance was 5% and calculations were done using the statistical software STATA version 14.0 (StataCorp, College Station, TX, USA).
5. Conclusions
In conclusion, NPY methylation and RAS/RAF mutation analysis to determine ctDNA are interchangeable, but NPY is superior as it can quantify ctDNA in nearly all the patients. High baseline levels correlate with short survival, and monitoring plasma tumor DNA using NPY methylation during treatment may predict early effect and later progression. We suggest plasma NPY methylation analysis as an easy and universally applicable method for longitudinal monitoring of ctDNA in metastatic colorectal cancer patients.
Author Contributions
Conceptualization, L.H.J., R.O., L.N.P., A.J. and T.F.H.; methodology, L.H.J., R.F.A., J.L., A.J. and T.F.H.; formal analysis, L.H.J., R.F.A. and L.N.; clinical investigators, L.H.J., R.O., L.N.P., A.K.B., C.E.B.T., B.M.H. and T.F.H.; data curation, L.N.; writing—original draft preparation, L.H.J.; writing—review and editing, R.O., L.N.P., A.K.B., R.F.A., J.L., L.N., C.E.B.T., B.M.H., A.J. and T.F.H.; supervision, A.J. and T.F.H.; funding acquisition, A.J. All authors have approved the final manuscript.
Funding
This research was partly funded by public funds from the Healthcare Region of Southern Denmark.
Conflicts of Interest
The authors declare no other conflict of interest. Neither the funders nor other parties had any role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Grothey A., van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., Humblet Y., Bouché O., Mineur L., Barone C., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 2.Heitzer E., Ulz P., Geigl J.B. Circulating tumor DNA as a liquid biopsy for cancer. Clin. Chem. 2015;61:112–123. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- 3.Jung K., Fleischhacker M., Rabien A. Cell-free DNA in the blood as a solid tumor biomarker—A critical appraisal of the literature. Clin. Chim. Acta. 2010;411:1611–1624. doi: 10.1016/j.cca.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Esposito A., Bardelli A., Criscitiello C., Colombo N., Gelao L., Fumagalli L., Minchella I., Locatelli M., Goldhirsch A., Curigliano G. Monitoring tumor-derived cell-free DNA in patients with solid tumors: Clinical perspectives and research opportunities. Cancer Treat. Rev. 2014;40:648–655. doi: 10.1016/j.ctrv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Kanwal R., Gupta K., Gupta S. Cancer epigenetics: An introduction. In: Clifton N.J., editor. Methods in Molecular Biology. Vol. 1238. Humana Press; New York, NY, USA: 2015. pp. 3–25. [DOI] [PubMed] [Google Scholar]
- 6.Li B., Gan A., Chen X., Wang X., He W., Zhang X., Huang R., Zhou S., Song X., Xu A. Diagnostic performance of DNA hypermethylation markers in peripheral blood for the detection of colorectal cancer: A meta-analysis and systematic review. PLoS ONE. 2016;11:e0155095. doi: 10.1371/journal.pone.0155095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warton K., Mahon K.L., Samimi G. Methylated circulating tumor DNA in blood: Power in cancer prognosis and response. Endocr. Relat. Cancer. 2016;23:R157–R171. doi: 10.1530/ERC-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen R.F. Tumor-specific methylations in circulating cell-free DNA as clinically applicable markers with potential to substitute mutational analyses. Expert Rev. Mol. Diagn. 2018;18:1011–1019. doi: 10.1080/14737159.2018.1545576. [DOI] [PubMed] [Google Scholar]
- 9.Boeckx N., de Beeck K.O., Beyens M., Deschoolmeester V., Hermans C., de Clercq P., Garrigou S., Normand C., Monsaert E., Papadimitriou K., et al. Mutation and methylation analysis of circulating tumor DNA can be used for follow-up of metastatic colorectal cancer patients. Clin. Colorectal Cancer. 2018;17:e369–e379. doi: 10.1016/j.clcc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara M., Murata J., Ayukawa K., Saiki I. Differential effect of intestinal neuropeptides on invasion and migration of colon carcinoma cells in vitro. Cancer Lett. 1997;119:125–130. doi: 10.1016/S0304-3835(97)81762-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Fang L., Zang Y., Xu Z. Identification of core genes and key pathways via integrated analysis of gene expression and DNA methylation profiles in bladder cancer. Med. Sci. Monit. 2018;24:3024–3033. doi: 10.12659/MSM.909514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roperch J.-P., Incitti R., Forbin S., Bard F., Mansour H., Mesli F., Baumgaertner I., Brunetti F., Sobhani I. Aberrant methylation of NPY, PENK, and WIF1 as a promising marker for blood-based diagnosis of colorectal cancer. BMC Cancer. 2013;13:566. doi: 10.1186/1471-2407-13-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrigou S., Perkins G., Garlan F., Normand C., Didelot A., le Corre D., Peyvandi S., Mulot C., Niarra R., Aucouturier P., et al. A study of hypermethylated circulating tumor DNA as a universal colorectal cancer biomarker. Clin. Chem. 2016;62:1129–1139. doi: 10.1373/clinchem.2015.253609. [DOI] [PubMed] [Google Scholar]
- 14.Strumberg D., Schultheis B. Regorafenib for cancer. Expert Opin. Investig. Drugs. 2012;21:879–889. doi: 10.1517/13543784.2012.684752. [DOI] [PubMed] [Google Scholar]
- 15.Schnipper L.E., Davidson N.E., Wollins D.S., Tyne C., Blayney D.W., Blum D., Dicker A.P., Ganz P.A., Hoverman J.R., Langdon R., et al. American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J. Clin. Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherny N.I., Sullivan R., Dafni U., Kerst J.M., Sobrero A., Zielinski C., de Vries E.G.E., Piccart M.J. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann. Oncol. 2015;26:1547–1573. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Qin S., Xu R., Yau T.C.C., Ma B., Pan H., Xu J., Bai Y., Chi Y., Wang L., et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 18.Baszanger I. One more chemo or one too many? Defining the limits of treatment and innovation in medical oncology. Soc. Sci. Med. 2012;75:864–872. doi: 10.1016/j.socscimed.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Tabernero J., Lenz H.-J., Siena S., Sobrero A., Falcone A., Ychou M., Humblet Y., Bouché O., Mineur L., Barone C., et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937–948. doi: 10.1016/S1470-2045(15)00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavi O. Redundancy: A critical obstacle to improving cancer therapy. Cancer Res. 2015;75:808–812. doi: 10.1158/0008-5472.CAN-14-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.le Tourneau C., Delord J.-P., Gonçalves A., Gavoille C., Dubot C., Isambert N., Campone M., Trédan O., Massiani M.-A., Mauborgne C., et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 22.Jeppesen M., Hagel G., Glenthoj A., Vainer B., Ibsen P., Harling H., Thastrup O., Jørgensen L.N., Thastrup J. Short-term spheroid culture of primary colorectal cancer cells as an in vitro model for personalizing cancer medicine. PLoS ONE. 2017;12:e0183074. doi: 10.1371/journal.pone.0183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Altman D.G., McShane L.M., Sauerbrei W., Taube S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallisgaard N., Spindler K.-L.G., Andersen R.F., Brandslund I., Jakobsen A. Controls to validate plasma samples for cell free DNA quantification. Clin. Chim. Acta. 2015;446:141–146. doi: 10.1016/j.cca.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Simon R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]