Abstract
Background:
KDIGO (Kidney Disease: Improving Global Outcomes) defines acute kidney injury (AKI) solely by serum creatinine (SCr) and urine output variation. Severe AKI is a syndrome covering various clinical situations.
Objective:
To describe severe AKI heterogeneity by department of hospitalization.
Design:
This is a prospective observational single-center study.
Setting:
Adult patients hospitalized in a French tertiary hospital from August 2016 to December 2017.
Patients:
All adults with severe AKI, defined by dialysis for AKI or an increase in SCr above 354 μmol/L.
Measurements:
Patient characteristics, clinical and laboratory presentation, AKI cause, medical indication for renal replacement therapy (RRT), planned palliative care, and vital status 30 days after severe AKI.
Methods:
A global description of patient characteristics, care, and prognosis and comparison by department of hospitalization: intensive care unit (ICU), nephrology, and others.
Results:
The study included 480 patients (73% men, median age: 72 years, range: 64-83), with medical histories including cardiovascular disease, diabetes, cancer, and chronic kidney disease. Principal causes were sepsis (104; 22%), hypovolemia (98; 20%), obstructive AKI (84; 18%), acute tubular necrosis (ATN; 74; 15%), and cardiorenal syndrome (51; 11%). Severe AKI was diagnosed in the ICU for 188 (39%) patients, the nephrology department for 130 (27%), and in other wards for 162 (34%). Patient characteristics differed by department for age, comorbidity, cause, and RRT use and indications. Palliative care was planned for 72 (15%) patients, most frequently in other wards.
Limitations:
We studied a subgroup of stage 3 KDIGO AKI patients in a single center without cardiac surgery.
Conclusion:
Patients hospitalized for severe AKI have frequent and various comorbidities, different clinical presentations, care, hospitalization in various departments, and different prognosis. The heterogeneity of this severe AKI implies the need for personalized care, which requires prognostic tools that include information besides SCr and diuresis.
Keywords: AKI, clinical epidemiology, care, prognosis, renal replacement therapy, dialysis
Abrégé
Contexte:
Le KDIGO définit l’insuffisance rénale aigüe (IRA) uniquement par une variation de la créatinine sérique (SCr) et de la diurèse. L’IRA grave est un syndrome couvrant diverses situations cliniques.
Objectif:
Décrire l’hétérogénéité de l’IRA grave selon l’unité d’hospitalisation.
Type d’étude:
Étude observationnelle prospective menée dans un seul centre.
Sujets:
Des adultes hospitalisés entre août 2016 et décembre 2017 dans un centre de soins tertiaires en France.
Participants:
Tous les adultes atteints d’IRA grave, définie par un traitement de dialyse ou un taux de SCr au-delà de 354 µmol/l.
Mesures:
Les caractéristiques du patient, le tableau clinique et de laboratoire, l’étiologie de l’IRA, l’indication médicale pour une thérapie de remplacement rénal (TRR), le plan de soins palliatifs et le statut vital 30 jours après l’épisode d’IRA grave.
Méthodologie:
Une description globale des caractéristiques des patients, des soins et du pronostic, ainsi qu’une comparaison selon l’unité d’hospitalisation: unité de soins intensifs (USI), néphrologie et autres.
Résultats:
L’étude portait sur 480 patients (73 % d’hommes) âgés de 64 à 83 ans (âge médian: 72 ans) avec des antécédents incluant maladies cardiovasculaires, diabète, cancer ou insuffisance rénale chronique. Les principales causes de l’IRA grave étaient une septicémie (104, 22 %), une hypovolémie (98, 20 %), une IRA obstructive (84, 18 %), une nécrose tubulaire aigüe (74, 15 %) ou un syndrome cardio-rénal (51, 11 %). Le diagnostic avait été posé à l’USI pour 188 patients (39 %), en néphrologie pour 130 patients (27 %) et dans d’autres unités pour 162 patients (34 %). Les caractéristiques des patients différaient entre les unités de soins en ce qui concerne l’âge, les comorbidités, l’étiologie et les indications de TRR. Un plan de soins palliatifs existait pour 72 patients (15 %), le plus souvent dans les autres unités.
Limites:
Nous avons étudié un sous-groupe de patients atteints d’IRA de stade 3 (classification KDIGO) dans un seul centre sans chirurgie cardiaque.
Conclusion:
Les patients hospitalisés pour une IRA grave présentent des comorbidités, des tableaux cliniques, des soins et des pronostics variés et sont admis dans différentes unités d’hospitalisation. Cette hétérogénéité de l’IRA grave met en relief le besoin de soins personnalisés qui nécessitent des outils pronostics basés sur des informations autres que la SCr et la diurèse.
What was known before
Acute kidney injury (AKI) is defined according to variations of SCr and diuresis, with a 3-stage prognostic classification. Most descriptions of AKI come from intensive care units (ICUs). Severe AKI could be defined as a homogenous prognostic stage.
What this adds
The clinical presentation, care, and prognosis of patients hospitalized with severe AKI in a tertiary hospital are highly heterogeneous.
Introduction
Acute kidney injury (AKI) is a syndrome characterized by a rapid reduction in kidney function that sharply decreases clearance of excess water, electrolytes, and toxins. It is highly prevalent among hospitalized patients and is associated with poor outcomes including increased length of stay and mortality.1 In 2012, KDIGO (Kidney Disease: Improving Global Outcomes), an international nonprofit organization that develops and implements clinical practice guidelines for kidney disease, issued a clinical practice guideline that proposed a new classification of AKI based on variations in serum creatinine (SCr), urine volume, and dialysis treatment.2 This definition facilitates homogeneous description and has resulted in better knowledge of AKI epidemiology worldwide.3
However, no other clinical information, including clinical presentation, is recorded,1 even though AKI is not a single homogeneous disease but a “loose collection of syndromes characterized by an abrupt decrease in glomerular filtration rate (GFR) with multiple potential causes.”4 The KDIGO definition and classification thus appear too simple for several of its patterns of clinical presentation. Recent articles have debated the definition and classification of AKI, offering conflicting opinions.5 Barasch et al5 argue that focus on creatinine-based staging has de-emphasized the traditional clinical approach of determining the cause of AKI and prevents progress in the personalized medicine needed to treat these various syndromes. Kellum and Lameire6 counter that, in the absence of current alternatives to SCr, its significant advantages in clinical and research environments include the facilitation of efforts to address variations in the delivery of AKI care.
Moreover, most descriptive data for AKI come from severely ill patients treated in intensive care units (ICUs). Few data from non-ICU patients are available.4,7 A French study that recently reported a difference in AKI prognosis according to whether it is coded in the French discharge database as the principal or an associated diagnosis indicates that AKI covers a wide range of clinical situations and types of care that differ according to the department in which the patient is hospitalized.8
Acute kidney injury occurs across a wide range of diseases treated by a variety of specialists: Most episodes of AKI are managed by nonnephrologists.9 In 2009, the UK National Confidential Enquiry into Patient Outcome and Death reported that this heterogeneity of patient management led to “good care” for only 50% of AKI patients, and only 31% were referred to nephrologists.10 Descriptive data about AKI nonetheless come mainly from administrative data or from ICU patient cohorts; specific information is missing about its cause, its clinical features, and the care provided. Outcomes for severe AKI are reported mainly from ICUs and not for hospitals as a whole. Moreover, few studies describe the indications for dialysis according to the unit of hospitalization (ICU or nephrology). More precise descriptive information is needed to improve individualized patient-centered care for this inpatient population with severe AKI.
In this context, the single-center prospective study we report here aimed to describe the incidence and patterns of severe AKI diagnosed in a tertiary university hospital, from its clinical presentation to its global care, according to the department of hospitalization: ICU, nephrology, and other wards.
Methods
Study Population
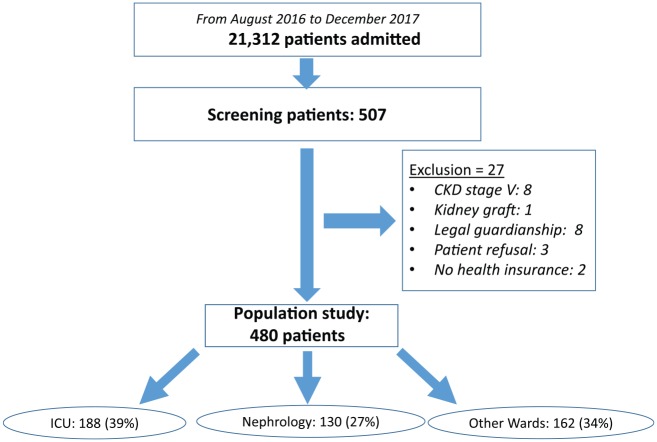
All adults (≥18 years) presenting severe AKI at or after admission to our university hospital between August 2016 and December 2017 were eligible to participate. They were prospectively identified by physicians in the ICU and the nephrology department and by the biochemistry laboratory for the hospital overall. Inclusion criteria were severe AKI, defined according to 2 of the 3 criterion definitions of KDIGO stage 3 AKI: an increase in SCr above 354 µmol/L or renal replacement therapy (RRT) indication, but not the 3-fold increase in SCr increase within 7 days with SCr below 354 µmol/L. Exclusion criteria were chronic kidney disease (CKD) KDIGO stage 5, patients living with a kidney transplant, planned dialysis for bilateral surgical nephrectomy, patients under guardianship, patients who refused, and patients with no health insurance. Exhaustiveness of inclusions in the hospital during the study period was verified regularly by the biochemistry department, which sent us information about all patients with SCr above 354 µmol/L. The patients requiring RRT for AKI were also screened weekly by the investigators throughout the study. Figure 1 presents a flowchart of the study population.
Figure 1.
Flowchart of the study.
Note. CKD = chronic kidney disease; ICU = intensive care unit.
Patient characteristics
The following information was recorded at inclusion by the hospital staff physician responsible for the patient care and during the hospitalization by the study data team: sociodemographic information (age, sex, height, weight, and body mass index [BMI]) and prespecified chronic illnesses (coronary heart disease, treated hypertension, heart failure, cardiac arrhythmia, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease or asthma, noninvasive ventilation except for supplemental oxygen, diabetes mellitus, chronic liver dysfunction, solid organ malignancies, and hemopathy). The comorbidities were defined according to the “dictionary used with the REIN registry.”11 A Charlson comorbidity score was calculated at inclusion, and the Simplified Acute Physiology score (SAPS II)12 within 24 hours after admission to the “inclusion ward.” The presence of CKD according to the KDIGO definition and care by a nephrologist at least 3 months before inclusion was also recorded. In the absence of information about the kidney function before hospitalization, the GFR was estimated at a value of 75 mL/min/1.73 m2, as recommended.1 At inclusion, we also recorded laboratory values for SCr (enzymatic method), urea, lactates, hemoglobin, potassium, phosphates, bicarbonates, albumin, and for urine sample values, including the protein/creatinine ratio.
Acute kidney injury cause was defined according to standard nephrology definitions13 and the clinical context, as suggested by Kellum and Prowle.4 If a kidney biopsy was done for AKI, we used the results to specify the precise cause. The different causes considered were prerenal kidney injury with a clinical context of real hypovolemia and rapid reversibility of AKI after fluid administration; obstructive kidney failure with documented urinary tract obstruction; and renal kidney injury including acute tubular necrosis (ATN), glomerulopathy, vascular or acute interstitial nephritis, as assessed by a nephrologist based on medical history, clinical presentation, kidney imaging, and urine analysis. Acute tubular necrosis was diagnosed in cases of sustained renal ischemia, drug direct tubular injury, rhabdomyolysis, cast nephropathy, or persistent AKI more than 72 hours after hemodynamic correction. Acute kidney injury in the context of type I cardiorenal syndrome was defined by acute heart failure complicated by AKI,14 and AKI with liver-renal syndrome by AKI with cirrhosis or liver failure.15 AKI associated with sepsis was defined according to the 2016 definition16 and AKI with multiorgan failure by the failure of at least 3 organs. Finally, hospital-acquired AKI was defined by its occurrence ≥48 hours after hospital admission, surgery-associated AKI by its occurrence within 72 hours after surgery, and community-acquired AKI as that not acquired in a hospital. Two investigators (C.A. and O.M.) reached a consensus about each probable cause of AKI. As Kellum et al point out, this diagnosis is sometimes complex; therefore, we first describe causes according to clinical syndrome, as in sepsis or multiorgan failure, and then as a cause as hypovolemic, renal, or obstructive.
Trajectory and care
We recorded patient trajectory during hospitalization with information about different departments of hospitalization for patients. The “inclusion ward” was defined as the ward where the patient presented severe AKI requiring inclusion. In this context, we defined 3 groups of inclusion wards; the ICU and the nephrology ward (NW) both had RRT available, whereas the third group was “all other wards, without RRT care.” The “ICU department” contains 2 units, 1 surgical and 1 medical, with different patient profiles. Other departments recorded included the ward, if any, before the “inclusion ward” and the last ward of stay before discharge, transfer to another facility, or inpatient death.
The reasons for patient admission were separated into 9 main groups according to the principal reason: sepsis, cardiovascular, neurologic, digestive disease, respiratory distress, hypovolemia or hemorrhage, AKI, or other diagnoses. More than 1 condition could be selected in each case. During hospitalization, dates of hospital admission, inclusion, discharge or death, and need for RRT were recorded.
At inclusion, that is, at the diagnosis of AKI, physicians reported any palliative care plan. When RRT was indicated, the attending physician specified the reason from the following items selected in advance: oligoanuria, hyperkalemia, acute pulmonary edema, metabolic acidosis, hypercalcemia volume overload with diuretic resistance, toxin clearance, or refractory shock. Several causes could be selected. Similarly, when dialysis was not considered to be indicated, physicians were asked to select the reason from a list of situations including the absence of clinical and laboratory indications and patient refusal or preference for palliative care. The following specific treatments were also recorded during hospitalization: need for mechanical ventilation and intravenous infusion of inotropes or vasopressors.
Statistical Analysis
We reported the incidence of hospitalization for severe AKI in adults over the study period according to the overall number of stays in this tertiary university hospital for adults hospitalized in medical, surgical, and obstetric wards. The population characteristics, clinical presentation at inclusion, trajectory, and care are reported for the overall population and by inclusion ward. Quantitative values with Gaussian distribution are expressed as means with their standard deviations (SDs) and compared with the analysis of variance (ANOVA) test; those with non-Gaussian distribution are expressed as medians and their interquartile ranges (IQRs) and compared by inclusion ward with the Kruskal-Wallis nonparametric test. Qualitative values are expressed by numbers (with percentages) for qualitative values and compared by inclusion ward with the chi-square test. Significance was defined as P < .05 with a bilateral test. Statistical analyses were performed with SAS software version 9.3 (SAS Institute Inc). The statistical comparison of the variables according to group is a global comparison between the ICU (surgical and medical combined), the NW, and other wards (OW).
Ethics
This study was approved by the Ethics Committee (REB) and the French Data Protection Authority (Commission Nationale Informatique & Libertés; CNIL number: 1963867v0). All patients or their substitute decision-makers received clear information that they could object to the collection of their health records, and none expressed any opposition.
Results
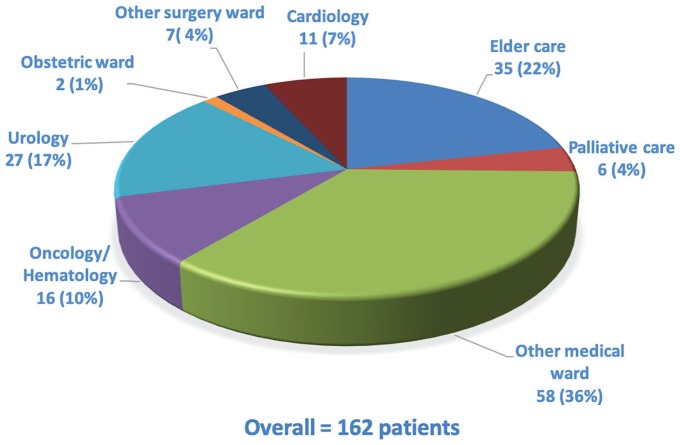
During the study period, 480 (mean age: 72 years, IQR: [64, 83], 73% male) patients were included among the 507 who were eligible (Figure 1). Overall, 188 (39%) were included from the ICU: 91 (19%) from the surgical ICU and 97 (20%) from the medical ICU. The NW was the inclusion ward for 130 (27%) and the OW for 162 (34%). Figure 2 lists the OW, together with the number of patients included in each; they included elder care, oncology/hematology, palliative care, urology, obstetrics, cardiology, and other medical and surgical wards. Of the overall hospital patient admissions (N = 21 312) during the study period, 2.3% were of patients diagnosed with severe AKI at admission or during their stay. The prespecified chronic illnesses and sociodemographic information are presented for the overall population and according to the inclusion ward in Table 1. Patients included in the ICU were younger (mean age: 66 years) than those from the NW (73) or OW (83) (P < .001). Comorbidities were numerous, especially cardiovascular and neoplastic. Nearly half the patients (n = 234; 49%) were known to have had CKD before this hospitalization; only 82 (35%) had previous nephrology care. A history of CKD was most frequent in patients included in the NW.
Figure 2.
Distribution of the group of other wards.
Table 1.
Sociodemographic Data, Medical History, and Kidney Information.
| General (N = 480) | ICU (N = 188; 39%) | Surgical ICU (N = 91; 19%) | Medical ICU (N = 97; 20%) | Nephrology ward (NW) (N = 130; 27%) | Other wards (OW) (N = 162; 34%) | P (general) | |
|---|---|---|---|---|---|---|---|
| Sociodemographic data | |||||||
| Men, n (%) | 351 (73.1) | 145 (77.1) | 70 (76.9) | 75 (77.3) | 88 (67.7) | 118 (72.8) | .32 |
| Age (years), median [IQR] | 72 [64, 83] | 68 [60, 76] | 70 [64, 78] | 66 [57, 74] | 73 [63, 82] | 83 [71, 89] | <.0001 |
| Past medical history, n (%) | |||||||
| Cardiovascular disease | 440 (91.7) | 174 (93) | 82 (90.1) | 92 (94.9) | 115 (88.5) | 151 (93) | .28 |
| Coronary heart disease | 116 (24.3) | 46 (24.5) | 21 (23.1) | 25 (25.8) | 29 (22.3) | 41 (25.8) | .88 |
| Heart failure | 128 (26.7) | 42 (22.3) | 15 (16.5) | 27 (27.8) | 37 (28.5) | 49 (30.3) | .10 |
| Peripheral vascular disease | 86 (17.9) | 35 (18.6) | 19 (20.9) | 16 (16.5) | 23 (17.7) | 28 (17.3) | .87 |
| Cardiac arrhythmia | 108 (22.6) | 30 (16.0) | 15 (16.5) | 15 (15.5) | 29 (22.3) | 49 (30.8) | .012 |
| Cerebrovascular disease | 61 (12.8) | 17 (9.0) | 7 (7.7) | 10 (10.3) | 11 (8.5) | 33 (20.8) | .003 |
| Treated hypertension | 320 (67.1) | 127 (67.6) | 58 (63.7) | 69 (71.1) | 93 (71.5) | 100 (62.9) | .31 |
| Diabetes mellitus | 176 (36.7) | 72 (38.3) | 33 (36.3) | 39 (40.2) | 49 (37.7) | 55 (34.0) | .77 |
| Chronic liver dysfunction | 29 (6) | 17 (9.0) | 9 (9.9) | 8 (8.3) | 7 (5.4) | 5 (3.1) | .11 |
| Solid organ malignancies | 130 (27.1) | 43 (22.9) | 21 (23.1) | 22 (22.7) | 27 (20.8) | 60 (37.0) | .006 |
| Hemopathy | 40 (8.4) | 13 (7.0) | 6 (6.7) | 7 (7.4) | 11 (8.5) | 16 (9.9) | .85 |
| Asthma or COPD | 66 (13.8) | 31 (16.6) | 17 (18.9) | 14 (14.4) | 16 (12.4) | 19 (11.7) | .42 |
| NIV or supplemental oxygen | 37 (7.7) | 20 (10.6) | 13 (14.3) | 7 (7.2) | 8 (6.2) | 9 (5.6) | .069 |
| Renal information (previous 6 months) | |||||||
| CKD, n (%) | 235 (48.9) | 77 (41.0) | 33 (36.3) | 44 (45.4) | 83 (63.9) | 74 (45.7) | <.001 |
| GFR (mL/min/1.73 m2), median [IQR] | 56 [37, 81] | 71 [46, 90] | 73 [47, 90] | 68 [46, 91] | 54 [30, 71] | 46 [32, 75] | <.0001 |
Note. “P” for the global comparison of values between ICU, NW, and OW. ICU = intensive care unit; IQR = interquartile range; COPD = chronic obstructive pulmonary disease; NIV = noninvasive ventilation; CKD = chronic kidney disease; GFR = glomerular filtration rate.
Clinical Characteristics and Laboratory Values
Other characteristics including reason for hospitalization, community- vs hospital-acquired AKI, surgery-associated AKI, clinical data, and AKI cause are reported in Table 2. The principal reasons for admission were gastrointestinal disease (24.8%), sepsis (21.9%), hypovolemia (19.5%), and AKI (19.5%). Acute kidney injury was hospital acquired for 28% of the study population and surgery associated for 20.3%, including 78% of the cases regarded as emergency surgery. At inclusion, the median Charlson index was 7 (IQR: [4, 9]) and was highest in the OW inclusion group. The median BMI was 26.6 (IQR: [23.3, 30.9]) with significant differences according to inclusion ward, and the values were lower for patients included in OW than in the ICU and NW. The median SCr (423 μmol/L, IQR: [367, 557]) also differed between these groups and was lower in the ICU, as were bicarbonate levels.
Table 2.
Reason for Admission, Clinical Data, Laboratory Findings, and Causes of AKI.
| General population (N = 480) | ICU (N = 188; 39%) | Surgical ICU (N = 91; 19%) | Medical ICU (N = 97; 20%) | Nephrology ward (NW) (N = 130; 27%) | Other wards (OW) (N = 162; 34%) | P | |
|---|---|---|---|---|---|---|---|
| Reason for admission, n (%) | |||||||
| Gastrointestinal | 120 (25) | 62 (33.0) | 35 (38.5) | 27 (27.8) | 26 (20.0) | 32 (19.8) | .004 |
| Infectious | 105 (21.9) | 65 (34.6) | 41 (45.1) | 24 (24.7) | 3 (2.3) | 37 (22.8) | <.001 |
| AKI (alone) | 99 (19.5) | 11 (5.9) | 4 (4.4) | 7 (7.2) | 60 (45.8) | 28 (17.3) | .2 |
| Hypovolemia/hemorrhage | 93 (19.4) | 39 (20.7) | 17 (18.7) | 22 (22.7) | 30 (23.1) | 24 (14.8) | .26 |
| Cardiovascular | 70 (14.6) | 40 (21.3) | 15 (16.5) | 25 (25.8) | 16 (12.3) | 14 (8.6) | .001 |
| Respiratory distress | 63 (13.1) | 40 (21.3) | 18 (19.8) | 22 (22.7) | 5 (3.9) | 18 (11.1) | <.0001 |
| Neurological | 37 (7.7) | 9 (4.8) | 5 (5.5) | 4 (4.1) | 10 (7.7) | 18 (11.1) | .17 |
| Polytrauma | 13 (2.7) | 7 (3.7) | 6 (6.6) | 1 (1.0) | 1 (0.8) | 5 (3.1) | .042 |
| Other | 27 (16.3) | 0 | 0 | 0 | 0 | 27 (16.7) | <.0001 |
| Hospital-acquired AKI, n (%) | 135 (28.1) | 68 (36.2) | 42 (46.2) | 26 (26.8) | 27 (20.8) | 40 (24.7) | .0003 |
| Surgery-associated AKI, n (%) | 98 (20.4) | 60 (31.9) | 47 (51.7) | 13 (13.4) | 12 (9.2) | 26 (16.1) | <.0001 |
| Clinical data, median [IQR] | |||||||
| BMI (kg/m2) | 26.6 [23.3, 30.9] | 28.1 [24.7, 32.4] | 29.1 [24.3, 32.8] | 27.8 [24.7, 32.3] | 26.5 [23.0, 30.4] | 24.9 [22.5, 28.4] | <.001 |
| Charlson index | 7 [4, 9] | 6 [3, 8] | 5 [3, 8] | 6 [3, 8] | 7 [4, 9] | 8 [6, 10] | <.001 |
| Laboratory data at inclusion, median [IQR] | |||||||
| Creatinine (μmol/L) | 423 [367, 557] | 379 [257, 482] | 381 [239, 468] | 376 [295, 500] | 535 [415, 732] | 428 [382, 505] | <.0001 |
| Urea (mmol/L) | 29.6 [21, 38] | 26.9 [17, 36] | 22.9 [14.9, 31.7] | 29.9 [21, 38.2] | 30.6 [23, 37.2] | 30.9 [22.6, 38.8] | <.0001 |
| Potassium (mmol/L) | 4.9 [4.2, 5.6] | 4.8 [4.2, 5.5] | 4.9 [4.3, 5.6] | 4.8 [4.0, 5.5] | 4.8 [4.1, 5.4] | 5 [4.2, 5.6] | .096 |
| Bicarbonates (mmol/L) | 18.6 [14.5, 21.8] | 16.7 [13.5, 21.0] | 16.1 [13.4, 20.7] | 16.7 [13.8, 21.0] | 19.4 [15.8, 22.6] | 19.5 [16.1, 21.8] | .0007 |
| Cause of AKI, n (%) | <.001 | ||||||
| Hypovolemia | 98 (20.4) | 24 (12.8) | 11 (12.1) | 13 (13.4) | 29 (22.3) | 45 (27.8) | |
| Renal | 112 (23.5) | 27 (14.4) | 12 (13.2) | 15 (15.5) | 61 (46.9) | 24 (14.8) | |
| Acute tubular necrosis | 74 (15.4) | 21 (11.1) | 10 (11) | 11 (11.3) | 32 (24.6) | 21 (13) | |
| Acute interstitial nephritis | 13 (2.7) | 2 (1) | 1 (1.1) | 1 (1) | 9 (7) | 2 (1.2) | |
| Glomerulonephritis | 19 (4) | 2 (1) | 1 (1.1) | 1 (1) | 16 (12.3) | 1 (0.6) | |
| Vascular nephropathy | 7 (1.5) | 3 (3.1) | 0 | 3 (3.1) | 4 (3.1) | 0 | |
| Obstructive | 85 (17.5) | 14 (7.5) | 8 (8.8) | 6 (6.2) | 19 (14.6) | 52 (32.1) | |
| Sepsis | 104 (21.7) | 76 (40.4) | 45 (49.6) | 31 (32.0) | 8 (6.2) | 20 (12.4) | |
| Multiorgan failure | 25 (5.2) | 20 (10.6) | 8 (8.8) | 12 (12.4) | 0 | 5 (3.1) | |
| Cardiorenal syndrome | 51 (10.6) | 22 (11.7) | 5 (5.5) | 17 (17.5) | 13 (10) | 16 (9.9) | |
| Hepatorenal syndrome | 5 (1) | 5 (2.7) | 2 (2.2) | 3 (3.1) | 0 | 0 | |
Note. “P” for the global comparison of values between ICU, NW, and OW. AKI = acute kidney injury; ICU = intensive care unit; BMI = body mass index; IQR = interquartile range.
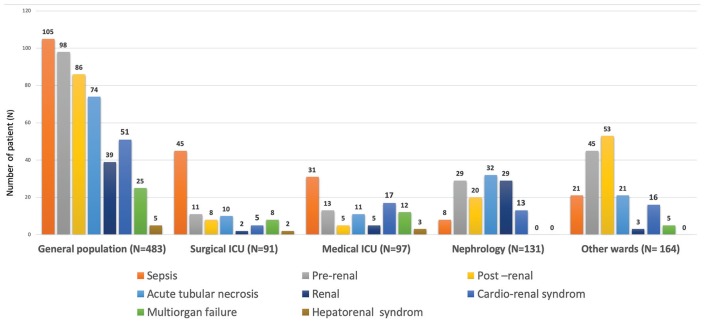
AKI Causes
The main causes of AKI were sepsis (22%), hypovolemia AKI (20%), obstructive AKI (18%), ATN (15%), and cardiorenal syndrome (11%) (Figure 3). These causes differed significantly between wards: septic AKI, multiorgan failure, and cardiorenal and hepatorenal syndromes were most frequent in the ICU, whereas hypovolemia and obstructive AKI were predominant in the OW inclusion group. Finally, renal AKI, which includes ATN, acute glomerular injury, acute vascular nephritis, and acute interstitial nephritis, was predominant in the NW.
Figure 3.
Cause of acute kidney injury with differences between inclusion groups.
Note. ICU = intensive care unit.
Trajectory and Care
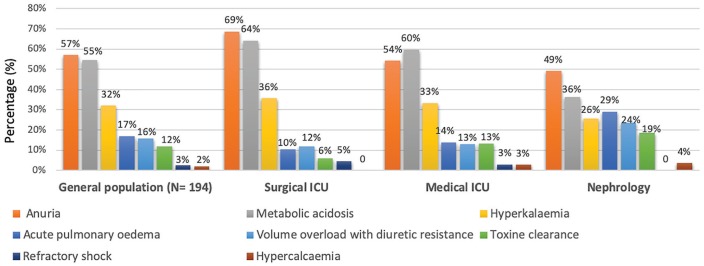
Table 3 summarizes the data about care plans, indications for RRT, and specific treatment during hospitalization. At inclusion, treatment limitations (such as “do not resuscitate” orders) were in effect for 72 patients (15%), with the largest proportion of them being included in OW. During hospitalization, 194 patients (40%) required RRT, with more of them being included in the ICU (73%) than in the NW (43%); none of the OW inclusions received RRT. The most common indications for RRT reported by physicians were anuria (57%), metabolic acidosis (54%), hyperkalemia (32%), and diuretic-resistant volume overload (15%). Acute pulmonary edema was most frequent in patients included in the NW, whereas metabolic acidosis and anuria were the principal indications in the ICU (Figure 4). In OW, none of them were treated by RRT because of treatment limitations in 58 (36%) and because of the causes of AKI that can be treated by specific treatment other than RRT such as intravenous (IV) infusion for hypovolemia (45; 28%), urine derivation for obstructive (52; 32%), and AKI or cardiac treatment for cardiorenal syndrome (16; 16%). Finally, 143 patients (30%) needed vasopressors and 115 (24%) mechanical ventilation, mostly in the ICU group. Slightly more than half of the patients (n = 245; 51%) were admitted via the emergency department to their inclusion ward. A total of 141 (29%) of these patients died during this hospitalization. The rates of death in the hospital and at 30 days differed according to department of inclusion and were highest among those included from the ICU.
Table 3.
Trajectory and Care.
| General population (N = 480) | ICU (N = 188; 39%) | Surgical ICU (N = 91; 19%) | Medical ICU (N = 97; 20%) | Nephrology ward (NW) (N = 130; 27%) | Other wards (OW) (N = 162; 34%) | P | |
|---|---|---|---|---|---|---|---|
| Palliative care, n (%) | 72 (14.9) | 10 (5.3) | 3 (3.3) | 7 (7.2) | 4 (3.1) | 58 (35.4) | <.0001 |
| RRT delivery | 194 (40.4) | 139 (73.9) | 67 (73.6) | 72 (74.2) | 55 (42.3) | 0 | <.0001 |
| Reason for RRT, n (%) | |||||||
| Anuria | 112 (23.3) | 85 (45.2) | 46 (68.7) | 39 (40.2) | 27 (20.8) | — | .07 |
| Metabolic acidosis | 106 (22.1) | 86 (45.7) | 43 (64.2) | 43 (44.3) | 20 (15.4) | — | .005 |
| Hyperkalemia | 62 (12.9) | 48 (25.5) | 24 (35.8) | 24 (24.7) | 14 (10.8) | — | .45 |
| Acute pulmonary edema | 33 (6.9) | 17 (9.0) | 7 (10.5) | 10 (10.3) | 16 (12.3) | — | .02 |
| Volume overload with diuretic resistance | 30 (6.3) | 17 (9.0) | 8 (8.8) | 9 (9.3) | 13 (10.0) | — | .16 |
| Toxic clearance | 23 (4.8) | 13 (6.9) | 4 (4.4) | 9 (9.3) | 10 (7.7) | — | .10 |
| Refractory shock | 5 (1.0) | 5 (2.7) | 3 (3.3) | 2 (2.1) | 0 | — | .32 |
| Hypercalcemia | 4 (0.8) | 2 (1.1) | 0 | 2 (2.9) | 2 (3.6) | — | .32 |
| Specific treatment, n (%) | |||||||
| Vasopressor use | 143 (29.8) | 67 (35.6) | 71 (78.0) | 67 (69.1) | 1 (0.8) | 4 (2.4) | <.0001 |
| Mechanical ventilation | 115 (23.9) | 54 (47) | 61 (67.0) | 54 (55.7) | 0 | 0 | <.0001 |
| Trajectory, n (%) | |||||||
| Ward before inclusion | <.0001 | ||||||
| Medicine | 67 (13.9) | 24 (12.8) | 8 (8.8) | 16 (16.5) | 31 (23.9) | 12 (7.4) | |
| Surgery | 23 (4.8) | 17 (9.0) | 12 (13.2) | 5 (5.2) | 6 (4.6) | 0 | |
| Emergency | 245 (51.0) | 62 (32.9) | 33 (36.3) | 29 (29.9) | 58 (44.6) | 125 (77.2) | |
| Inhospital death, n (%) | 141 (29.4) | 80 (42.6) | 34 (37.4) | 46 (47.4) | 12 (9.3) | 49 (30.3) | <.0001 |
| 30-day mortality, n (%) | 141 (29.4) | 70 (37.2) | 30 (33.0) | 40 (41.2) | 12 (9.2) | 59 (36.4) | <.0001 |
Note. “P” for global comparison between ICU, NW, and OW. ICU = intensive care unit; RRT = renal replacement therapy.
Figure 4.
Indications for renal replacement therapy and differences between inclusion groups for 194 patients (40.4%).
Note. ICU = intensive care unit.
Discussion
Our prospective study, conducted over an 18-month period in a tertiary teaching hospital, included 480 patients with severe AKI, that is, 2.3% of overall hospital admissions. Our study showed that this population was relatively old and had frequent comorbidities, which were cardiovascular (92%), diabetes mellitus (37%), cancer (35%), and CKD (50%) before hospitalization. These patients were managed in several different wards, not only in the ICU and NW, and these wards varied according to patients’ clinical presentation and laboratory results. The causes of severe AKI varied, with sepsis being predominant in the ICU and renal AKI in the NW. Indications for RRT for AKI also differed between the ICU and the NW, and no patients initially diagnosed in OW had RRT. Finally, the prognosis of our study population with severe AKI and a high rate of comorbidities was poor, with a high inhospital mortality of almost one third. It nonetheless differed according to inclusion ward and was associated with different patterns and care. In this population comprising a subgroup of patients with KDIGO stage 3, the high inhospital mortality rate was probably due not only to AKI but also to the causes of AKI and the high rate of comorbidities. This study underlines that severe AKI is not limited to the ICU and that its clinical presentation and care are extremely heterogeneous.
The demographics of the study population were consistent with previous epidemiologic studies, in particular with 2 French cohort studies based on the national discharge database.8,17 We observed that most patients in our cohort had major comorbidities, including but not limited to diabetes, cardiovascular disease, and cancer. The median Charlson index score was 7. Moreover, the median patient age was 72 years. Patients included in the ICU were significantly younger than those included in the NW and OW, as in a nationwide French study.17 A novelty for our cohort is information about CKD history, which was frequent in this population: Around 50% of the patients had previously met the definition for CKD, but only 17% of them were managed by nephrologists. This CKD history rate is higher than that in French administrative database studies or in other studies of AKI, which report rates from 10% to 35%.18-23 The high frequency of CKD history may be explained in part by the quality of information collected, due to the study’s prospective design and its use of the KDIGO definition of CKD, rather than estimated GFR or administrative codes. It may also be explained by the age profile of our cohort.
Gastrointestinal diseases, sepsis, hypovolemia, AKI, and cardiovascular disorders were the leading reasons for hospitalization. These reasons can vary in populations by hospital type, especially in relation to both solid organ transplantation and cardiac surgery, the latter being strongly associated with AKI occurrence. Reports indicate that AKI complicates 18% of hospitalizations for cardiac surgery, with 2% to 6% requiring RRT.24 Otherwise, we observed that 28% of AKI cases were hospital acquired and 72% community acquired. The same ratio was observed in a UK study in 2 tertiary centers (6% of ICU stays and 25% AKI stage 3): 27% hospital acquired vs 73% community acquired.25
In our study, the incidence of severe adult AKI was 2.3% of overall hospitalizations. This finding is consistent with the UK data from a multicentre randomized trial performed in 5 UK hospitals that showed an incidence of 2.5% of severe AKI.26
Sepsis was the leading cause of AKI in our study population, followed by prerenal, postrenal, and cardiorenal AKI. In a Scottish population study of all hospitalized patients (including 8.5% patients in ICU and 35% in the failure stage of the RIFLE classification), sepsis and hypovolemia were the leading causes of AKI.27 In comparison, the study of all hospitals in Madrid (and with 27% of patients in the ICU) by Liano and Pascual22 found the 3 leading causes of AKI to be ATN (45%), hypovolemic AKI (21%), and obstructive AKI (10%). Nonetheless, the definition of AKI was different then, and the cases of ATN probably include some cases of septic AKI, which has only become understood more recently.4 The main indications for RRT in our study were oligoanuria, metabolic acidosis, hyperkalemia, and volume overload—causes similar to those from a Canadian study of a cohort of elderly ICU patients.28 RRT in our hospital is performed by a continuous venovenous hemodiafiltration technique in the ICU (medical and surgical) and by intermittent hemodialysis in the NW. Because the study design was observational, we described the frequency of RRT indication according to the ward of inclusion. The frequency of RRT differed according to not only inclusion ward but also the causes of RRT, patient comorbidities, and AKI causes. We described the population and practices according to the ward and observed different rates of RRT according to the ward but also for indication or no indication of RRT showing heterogeneity of this population with severe AKI.
For the overall population, we report that hospital mortality and 30-day mortality were similar, around 29%. Hospital mortality for RRT patients was 40.2%. A multicentre, stepped-wedge cluster randomized trial performed in 5 UK hospitals included patients with AKI aged ≥18 years and observed an overall 30-day mortality rate of 24.5%.26 In the French cohort studies based on the national discharge database for patients with AKI requiring RRT, the inhospital mortality of 47% was similar to ours.17
The strength of this study is its prospective design and the quality of information collected for medical history. We used the entire KDIGO definition of CKD, because it is frequently underestimated by focusing only on GFR in studies based on medical administrative databases. Thus, this prospective analysis of all adults with severe AKI in a university hospital allows us to show the frequent comorbidities and heterogeneity of severe AKI in clinical presentation, severity, and care in different hospital departments. Its results emphasize that the care of patients with severe AKI depends on the presence of treatment limitations and that the indications for RRT differ between intensivists and nephrologists.
Limitations
Our study has several limitations that must be considered in interpreting our data. First, we studied a subgroup of patients with KDIGO stage 3 AKI with a restricted definition that does not include the urine output criteria or the tripling of baseline SCr with a maximum SCr below 354 µmol/L. The single-center design in a tertiary hospital with no cardiac surgery ward or solid organ transplantation limits the generalizability of this result without limiting the extent of heterogeneity in the population.
This study indicates the substantial heterogeneity of severe AKI and reinforces the fact that AKI is not a single disease and is not managed only in ICUs. This probably implies the need for an individualized patient-centered approach and the wide implementation of the KDIGO AKI classification to discuss the planning of care.
Conclusion
This study reports 3 hospitalization patterns in patients with severe AKI. Only 39% of these patients are hospitalized in the ICU, 27% are in the NW, and 34% in OW. Clinical presentation, AKI cause, and thus its care, including reasons for RRT, differ according to the department of hospitalization with broad heterogeneity for the same AKI stage. The heterogeneity of severe AKI and of its treatment in this population of a subgroup of patients with stage 3 KDIGO AKI implies the need for more individualized and patient-centered care and for improvement of prognostic tools to include more information than simply SCr and diuresis.
Acknowledgments
The authors thank the University Hospital of Nîmes for its structural, human, and financial support through the award obtained by our team during the internal call for tenders “Thématiques émergentes.” The authors also thank Jo Ann Cahn for reviewing the English.
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the Ethics Committee and the French Data Protection Authority (Commission Nationale Informatique & Libertés; CNIL number: 1963867v0).
Consent for Publication: All authors consent to the publication of this study.
Availability of Data and Materials: The data and materials are not available for this study.
Author Contributions: O.M. conceived and designed the study. C.A., L.M., S.D.B., R.T., Z.M., J.-Y.L., and O.M. analyzed and interpreted the data. C.A., L.M., S.D.B., J.-Y.L., D.-P.D.B., and O.M. drafted and revised the article. C.A., L.M., P.R., S.C., S.D.B., R.T., Z.M., J.-Y.L., and O.M. provided intellectual content of critical importance to the work described. C.A., L.M., P.R., S.C., S.D.B., R.T., Z.M., D.-P.D.B., J.-Y.L., and O.M. approved the final version to be published.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The current study is part of the thematic portfolio for clinical research studies which were at least partly supported through the University Hospital of Nîmes call for internal bids “Thématiques émergentes.”
ORCID iD: Olivier Moranne  https://orcid.org/0000-0002-3127-1415
https://orcid.org/0000-0002-3127-1415
References
- 1. Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kellum JA, Lameire N, Aspelin P, et al. Work group membership. Kidney Int. 2012;2:1. [Google Scholar]
- 3. Mehta RL, Burdmann EA, Cerda J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017-2025. [DOI] [PubMed] [Google Scholar]
- 4. Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14:217-230. [DOI] [PubMed] [Google Scholar]
- 5. Barasch J, Zager R, Bonventre JV. Acute kidney injury: a problem of definition. Lancet. 2017;389:779-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kellum JA, Lameire N. The definition of acute kidney injury. Lancet. 2018;391:202-203. [DOI] [PubMed] [Google Scholar]
- 7. Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol. 2012;7:533-540. [DOI] [PubMed] [Google Scholar]
- 8. Riffaut N, Moranne O, Hertig A, Hannedouche T, Couchoud C. Outcomes of acute kidney injury depend on initial clinical features: a national French cohort study. Nephrol Dial Transplant. 2018;33:2218-2227. [DOI] [PubMed] [Google Scholar]
- 9. Sykes L, Sinha S, Hegarty J, et al. Reducing acute kidney injury incidence and progression in a large teaching hospital. BMJ Open Qual. 2018;7:e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NCEPOD—Acute Kidney Injury: Adding Insult to Injury Report (2009). www.ncepod.org.uk/2009report1/Downloads/AKI_report.pdf
- 11. Couchoud C, Stengel B, Landais P, et al. The Renal Epidemiology and Information Network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006;21:411-418. [DOI] [PubMed] [Google Scholar]
- 12. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957-2963. [DOI] [PubMed] [Google Scholar]
- 13. Lameire N, Biesen WV, Vanholder R. Acute renal failure. Lancet. 2005;365:417-430. [DOI] [PubMed] [Google Scholar]
- 14. Ronco C, Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10:251-280. [DOI] [PubMed] [Google Scholar]
- 15. Ginès P, Guevara M, Arroyo V, et al. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [DOI] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garnier F, Couchoud C, Landais P, Moranne O. Increased incidence of acute kidney injury requiring dialysis in metropolitan France. PLoS ONE. 2019;14:e0211541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlson N, Hommel K, Olesen JB, et al. Trends in one-year outcomes of dialysis-requiring acute kidney injury in Denmark 2005-2012: a population-based nationwide study. PLoS ONE. 2016;11(7):e0159944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolhe NV, Muirhead AW, Wilkes SR, et al. The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: retrospective analysis of hospital episode statistics. Int J Clin Pract. 2016;70:330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nisula S, Kaukonen K-M, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428. [DOI] [PubMed] [Google Scholar]
- 21. Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol. 2014;9:1007-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811-818. [DOI] [PubMed] [Google Scholar]
- 23. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813-818. [DOI] [PubMed] [Google Scholar]
- 24. Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10:500-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol. 2014;9:1007-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Selby NM, Casula A, Lamming L, et al. An organizational-level program of intervention for AKI: A pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol. 2019;30:505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292-1298. [DOI] [PubMed] [Google Scholar]
- 28. Bagshaw SM, Adhikari NKJ, Burns KEA, et al. Selection and receipt of kidney replacement in critically ill older patients with AKI. Clin J Am Soc Nephrol. 2019;14:496-505. [DOI] [PMC free article] [PubMed] [Google Scholar]