Abstract
Pancreatic Ductal Adenocarcinoma (PDAC) is a particularly insidious and aggressive disease that causes significant mortality worldwide. The direct correlation between PDAC incidence, disease progression, and mortality highlights the critical need to understand the mechanisms by which PDAC cells rapidly progress to drive metastatic disease in order to identify actionable vulnerabilities. One such proposed vulnerability is epithelial mesenchymal plasticity (EMP), a process whereby neoplastic epithelial cells delaminate from their neighbours, either collectively or individually, allowing for their subsequent invasion into host tissue. This disruption of tissue homeostasis, particularly in PDAC, further promotes cellular transformation by inducing inflammatory interactions with the stromal compartment, which in turn contributes to intratumoural heterogeneity. This review describes the role of EMP in PDAC, and the preclinical target discovery that has been conducted to identify the molecular regulators and effectors of this EMP program. While inhibition of individual targets may provide therapeutic insights, a single ‘master-key’ remains elusive, making their collective interactions of greater importance in controlling the behaviours’ of heterogeneous tumour cell populations. Much work has been undertaken to understand key transcriptional programs that drive EMP in certain contexts, however, a collaborative appreciation for the subtle, context-dependent programs governing EMP regulation is needed in order to design therapeutic strategies to curb PDAC mortality.
Keywords: pancreatic cancer, epithelial mesenchymal plasticity, target discovery, review
1. Pancreatic Cancer, Tumour Heterogeneity, and Carcinoma Vulnerabilities
Pancreatic cancer (PC) is the fourth most common cause of cancer-related deaths in Western societies, with 57,000 new cases annually, resulting in nearly 46,000 deaths in North America alone [1]. The most common type of PC is Pancreatic Ductal Adenocarcinoma (PDAC), which arises in the ductal epithelium of the exocrine tissue responsible for secreting pancreatic digestive juices. Late detection combined with early metastatic spread have limited gains in overall survival relative to other cancers such that PDAC mortality has the potential to surpass that of both colorectal and breast cancers by 2030 [2]. PDAC research therefore aims to define better diagnostic markers and novel therapeutic avenues, however is significantly complicated by the clinical heterogeneity present both within and between patient tumours. This emphasises the need for more integrative approaches aimed at developing a better understanding of targetable processes in PDAC tumourigenesis.
Cancer is a genetic disease caused by the accumulation of somatic mutations, resulting in a functional imbalance between tumour suppressive and oncogenic signals [3]. While transformed cells retain characteristics of the host to efficiently avoid being detected as foreign by the immune system, many aberrant phenotypes caused by genetic mutations and dysregulated signaling potentially render these cells susceptible to selective therapeutic interventions. Extensive examinations of the molecular traits of PDAC aimed at identifying such vulnerabilities have been conducted to date. Indeed, genomic and transcriptional profiling of patient tumours as part of large-scale studies by the The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) have allowed for insights into the scale of inter-tumour heterogeneity in a breadth of patient cohorts [4,5,6].
These studies have identified four major genetic aberrations common to pancreatic tumours [7,8,9]. 90% of tumours carry gain-of-function mutations in KRAS2, activating proliferative and cell survival pathways, whilst 95% contain either partial or complete inactivating mutations in CDKN2A, contributing to loss of cell cycle regulation, furthering proliferation. TP53, responsible for responding to DNA damage and inducing apoptosis, is altered in 60% of cases. SMAD4 inactivation is also common in pancreatic cancer development, and is found in 50% of patient cancers, disrupting the tumour suppressive signals of TGFβ, aiding proliferation [10]. As well as these four common driver mutations, genomic sequencing of tumours has identified an additional panel of consistently mutated genes [6]. These genetic mutations implicate pathways often dysregulated in cancer, including KRAS, TGFβ, WNT, NOTCH, ROBO/SLT, G1/S, SWI-SNF, and chromatin/DNA/RNA modification and repair.
Transcriptional profiling of PDAC tumours has allowed researchers to define discrete regulatory mechanisms within these networks that are associated with particular prognostic indices in different molecular subtypes of PDAC, which include squamous, pancreatic progenitor, immunogenic and aberrantly differentiated endocrine/ exocrine tumours [6]. Such classification schemes may provide clinical value by aiding in patient treatment regimen selection and planning [11], however, to date they have provided limited clinical value due to lack of targetable phenomena. It is important to note that while these studies have aimed to characterise changes within carcinoma cells, the excessive presence of desmoplastic stroma may confound these results. Indeed, microdissection of the tumour from its associated stroma has allowed the retrospective re-evaluation of large-scale transcriptional profiling efforts, highlighting the overwhelming contribution of stromal contamination to many such studies. Deconvolution based on laser capture microdissection and RNASeq profiling of 60 matched tumour/stroma pairs suggested that ICGC and TCGA samples contained stromal fractions of 46% and 55%, respectively, highlighting difficulties in deriving definitive conclusions from whole tumour analyses [12].
Such studies are invaluable as a means of understanding the intertumoural heterogeneity that exists between patients, and they form a strong set of public data that have been analysed to better appreciate the diversity of tumour presentation [13]. An increasing focus on single cell analytic technologies has yielded exciting opportunities to understand the contributions that individual cells make towards intratumoural heterogeneity, tumour progression, and patient outcomes [14,15]. These studies highlight the need for efforts aimed at distinguishing the heterogeneous nature of a tumour’s biology from that of the surrounding host tissue in which it propagates, so as to be better able to exploit cancer specific vulnerabilities [16].
As such, it is not surprising that the interactions between neoplastic epithelial cells and host myofibroblast and stellate populations, which can promote stromal inflammation, are increasingly being recognised. This desmoplastic reaction, which accounts for up to 90% of PDAC tumour volume, has pro-tumourigenic properties by leading to increased tissue stiffness and hypoxia as well as by providing physical barriers to both immune surveillance and chemotherapeutic penetrance [17,18,19]. The fibrillar collagen, hyaluronic acid and fibronectin rich extracellular matrix (ECM) deposited by stromal cells contains many soluble cytokines and growth factors secreted by both cancer and stromal compartments and contributes to both tumour initiation and progression [20,21,22,23]. Resident cells are forced to interact within this dynamic tumour microenvironment and are subject to stimuli that influence cell phenotypes in both stromal and carcinoma components. Such stimuli may propagate the invasion and dissemination of carcinoma cells by inducing epithelial mesenchymal plasticity (EMP), and thus this process is considered an important vulnerability that, when effectively targeted, may curb tumour progression [24,25].
2. EMP and PDAC Progression
EMP is often separated into two distinct but related processes—the forward process of epithelial-mesenchymal transition (EMT), and the reverse process of mesenchymal-epithelial transition (MET) [26]. These programs serve to describe the plasticity within epithelial cells that enables them to dedifferentiate into a more motile mesenchymal state, thereby allowing them to more effectively migrate. EMP is thought to play a significant role in several stages of tumour formation [27] and progression [28]. Initially, this plasticity allows tumour cells to detach and migrate from their site of origin (invasion), gaining access to lymphatic and blood vessels (intravasation), and then penetrating distant sites (extravasation), to form metastases.
A litany of reviews regarding different facets of EMP in PDAC, have been written, including those focused on molecular mechanisms of EMP regulation and metastasis [29,30,31,32,33,34,35,36], the role of epigenetic regulation [37], therapy development and resistance [38,39,40,41,42], microRNA regulation [43,44], and cancer stem cell generation [45,46,47,48,49]. This review thus focuses on some of the ongoing controversy surrounding in vivo evidence of EMP and the limitations of current approaches, highlighting the need to integrate a greater diversity of published EMP molecular regulators.
Development of PDAC frequently progresses undetected, remaining asymptomatic until it becomes an advanced stage of disease. Non-invasive precursor lesions formed either by epithelial proliferations or mucinous cysts in the pancreatic ducts, termed pancreatic intraepithelial neoplasia (PanINs), or intraductal papillary mucinous neoplasms (IPMNs), respectively, mark the onset of a histologically definable neoplasm in PDAC [50]. Such neoplasms, namely PanINs, progress through stages of dysplasia within the ductal epithelium, giving rise to the most common form of PDAC, pancreatic ductal adenocarcinoma (PDAC). The full breadth of factors that contribute to the invasive and metastatic behaviour of PDAC are vast. In this form of PDAC, there is very little latency between primary tumour formation and local and distant metastasis, implying that PDAC carcinoma cells may be readily equipped to invade and disseminate from a very early stage of development [51,52].
Invasive regions of human carcinomas are typically characterised by the presence of tumour-derived, fibroblast-like cells expressing mesenchymal markers such as vimentin, fibronectin and N-cadherin, with decreased expression of epithelial adhesion molecule E-Cadherin and increased nuclear beta-catenin relative to surrounding cells [53,54,55,56,57]. Decreased expression of E-cadherin has been shown to correlate with invasive and undifferentiated PDAC [58]. Furthermore, PDAC patients with tumour cells that express decreased E-cadherin and higher amounts of vimentin, s100A4, fibronectin and SNAI1 are more likely to have distant metastases, lymph node invasion and lower overall survival [54,59,60,61,62]. The EMP inducing transcription factor (TF) TWIST1 has been shown to be upregulated in PDAC compared to match normal tissues [63], and SNAI1 mRNA levels in PDAC fine needle aspirates are significantly correlated with lymph node and perineural invasion as well as with poorer survival [64]. A mediator of transforming growth factor beta (TGFβ) signaling, SMAD3, was also shown to accumulate in the nucleus of PDAC samples, and was correlated with higher grade tumours and lymph node metastasis, indicating a role for TGFβ in driving EMP in vivo [65]. Solitary infiltrating cancer cells displaying low E-cadherin and increased vimentin expression have proven to be significant prognostic indicators in resected clinical specimens from PDAC patients [66]. Tumour budding cells in PDAC have been observed with increased levels of ZEB1 and ZEB2, and reduced levels of E-cadherin and β-catenin, indicative of EMP mediated local invasion. ZEB2 overexpression in tumour-stroma associated cells also correlated with pathological assessment of tumour size, and lymph node metastasis [67]. Such striking pathology provides some of the clearest evidence for the role of EMP in PDAC progression.
While this clinical evidence strongly supports a role for EMP in mediating cancer invasion, the inability to accurately follow carcinoma epithelial dedifferentiation in vivo has led to some debate surrounding the extent of its role in tumour progression [68,69]. Such debate has necessitated the use of genetically engineered mouse models (GEMMs) to trace the role of EMP in cancer progression, specifically the pancreatic epithelium conditional Kras/P53 mutant (PKCY) mice Lineage labelling of epithelial cells in this spontaneous PDAC model has allowed researchers to track these cells as they adopt mesenchymal properties and migrate away from the primary tumour into the circulation, seeding liver metastases [70]. In one study, EMP was detected in 42% of labelled PDAC epithelial cells, as assessed by the expression of EMP markers Zeb1 or Fsp1 and/or lack of E-cadherin. These cells were mostly observed in regions of inflammation, supporting the idea that EMP is driven by inflammatory interactions within the tissue microenvironment. Interestingly, some labelled epithelial cells that had undergone EMP displayed evidence of delamination and fibroblast morphology prior to tumour formation, and were otherwise indistinguishable from host stromal cells [70]. This is supportive of the very early, integral role that EMP may play in PanIN formation prior to tumour development.
Further studies in this same PDAC mouse model have shown that suppression of EMP via the knock-out of Twist1 or Snai1 TFs does not reduce metastasis, despite the decreased expression of EMP markers and increased cell proliferation as evidence for EMP ablation [71]. Equivalent numbers of lineage labelled epithelial cells were found in circulation and in metastases regardless of Twist/ Snai1 knockout, suggesting that other mechanisms are involved in PDAC cellular invasion. PDAC cells do not possess a strong epithelial phenotype however, and may thus be insensitive to the loss of Snail TFs, which are potent repressors of epithelial programs but are less efficient in inducing mesenchymal properties. This possibly explains why Snail is dispensable for EMP and metastatic progression in this model [71,72], and points towards alternative mechanisms of EMP induction that may be driving factors in this PDAC system.
Indeed, there is evidence that the Zeb1 TF is largely responsible for driving EMP in this GEMM model of PDAC development [73]. Zeb1 ablation in PDAC cells was not found to affect Twist1 expression, however it was associated with decreased Zeb2, Slug and a slight reduction in Snai1 expression. Zeb1 depleted tumours were better differentiated, indicating less local invasion, and showed significantly reduced metastasis when compared to control PDAC mice [73]. This is in direct contrast to depletion of Twist1 or Snai1, which did not affect metastasis in this model system, highlighting the importance of recognising the context and tissue specific drivers of EMP.
Subsequent investigations aimed at overcoming the limitations of identifying single EMP regulatory TFs has shown that lineage labelled cancer cells are able to metastasize without expression of αSma or Fsp1, both of which are thought to be robust markers of EMP activation in this model [74]. Indeed, larger metastatic nodules were found containing exclusively cells that had never expressed αSma or Fsp1, while micrometastatic clusters of 3–5 cells were shown to have undergone EMP. Such evidence, combined with the fact that Zeb1 depletion in previous studies resulted in only a 50% reduction in metastasis underscores the pitfalls of seeking to identify individual master regulators and markers of such a complex process. Adding to this complexity, the emerging importance of hybrid EMP phenotypes, in which the expression of both epithelial and mesenchymal markers may occur at levels that are insufficient to drive the reporter constructs used in such lineage tracing models, adds a further technical challenge [75,76,77].
More recent attempts to understand EMP in individual PDAC cells has shown the activation of EMP transcriptional programs within certain subsets of tumour cell populations [14]. This study highlighted a clear role for cytokines from the stromal compartment in inducing EMP in certain PDAC cell lines, and indicated that EMP activation could be observed in discrete tumour gland subunits with prognostic utility. These models have provided considerable insights into the diverse mechanisms of PDAC development, and highlight that there are context-dependent EMP programs involved in both local invasion and metastatic dissemination that require further examination [72,78].
3. In Vitro EMP Models and Exogenous Stimuli
While GEMMS, in particular the PKCY model of spontaneous PDAC formation, are currently the gold standard for studies of the biology of EMP in tumourigenesis, in vitro studies form the basis for the majority of our current molecular understanding of intracellular events which occur in EMP. Many publicly available and in-house generated cell lines are used to study PDAC, but only a very limited number of these undergo well-characterised, stimulus-driven transitions that mimic the pathophysiological induction of EMP. This is perhaps consistent with the limited number of EMP events witnessed in in vivo models, highlighting the difficulties of studying such a dynamic process.
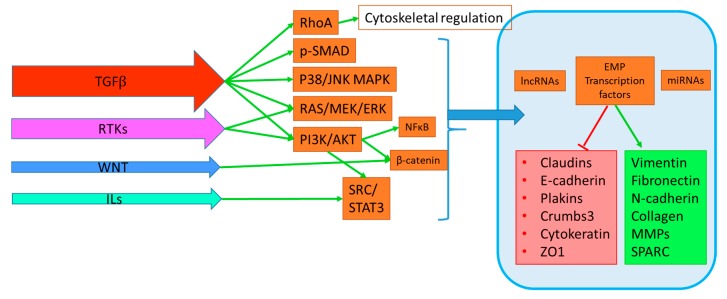
EMP is modulated by TGFβ, receptor tyrosine kinases (RTK) ligands, WNT ligands, interleukins, hypoxia via HIF1α signaling, as well as HIPPO, NOTCH signaling. Their mechanisms and specific impact on downstream EMP targets have been comprehensively reviewed elsewhere, however our understanding of their subtleties is on-going [79,80]. TGFβ acts as a tumour suppressor in normal tissue and early stage disease by regulating cell proliferation and inducing apoptosis through canonical signaling pathways, however this activity is lost as cellular transformation progresses [81,82,83,84,85]. Indeed, TGFβ is a potent activator of EMP in PDAC cells when its tumour suppressive signals are disrupted through SMAD4 mutations, found in 50% of PDAC tumours [81,86]. Similarly, activating KRAS mutations found almost ubiquitously in PDAC cooperate with TGFβ signaling to hyperactivate downstream RAS/RAF MAPK pathways to induce EMP [87]. While TGFB activates the greatest number of EMP signaling pathways, and may thus be considered a major driver in PDAC, the activation of additional pathways shown in Figure 1 by RTK, WNT and interleukin ligands may provide additional layers of crosstalk. Activation of SMAD, MAPK, PI3K, STAT, and NFκB pathways are commonly demonstrated in PDAC EMP research, however the relative extent to which each pathway governs EMP is unclear, as many studies evaluate these pathways independently [29,88,89,90,91,92,93,94].
Figure 1.
Simplified overview of cooperating signaling pathways in EMP.
These complex pathways ultimately serve to influence transcriptional programs that co-operate directly and indirectly to control the plasticity that exists between epithelial and mesenchymal phenotypes of carcinoma cells (Figure 1). Of note is the increasing recognition for the role of long non-coding RNAs (LncRNA) and micro-RNAs (miRNA) in EMP regulation. Among the cells that do undergo EMP-like transitions, there is a degree of selectivity for the ligands that are able to activate these EMP programs, and this is reflected in the limited number of commercial cell lines that are commonly manipulated within the field. This is consistent with the level of heterogeneity reported in PDAC, and suggests discrete differences in steady state signaling, which may predispose a given cell’s response or resistance to exogenous stimuli.
EMP is induced by stimuli shown within arrows on the left in order of potency. These signals activate signal transduction pathways that cooperate directly and indirectly to translocate signals to the nucleus (braced) to regulate EMP transcription factors, long non-coding RNAs (LncRNA), and micro RNAs (miRNAs).These factors then modulate EMP by discrete regulation of epithelial (Red Box) and mesenchymal (Green box) cellular properties, which in turn influence migration and invasion. Transforming growth factor (TGFB) activates the greatest number of these pathways, including direct cytoskeletal regulation by RhoA, aswell as canonical SMAD and non-canonical p38/JNK, MEK/ERK MAPK pathways and PI3K/AKT. Receptor tyrosine kinase (RTK) signaling is induced by binding of growth factor (GF) ligands such as EGF, IGF, FGF, HGF or VEGF and activates RAS/MEK/ERK, PI3K/AKT/NFκB and downstream SRC pathways. WNT signaling also modulates EMP by downstream stabilisation of B-catenin and subsequent nuclear translocation for EMP program activation by TCF/LEF transcription factors. Interleukins (ILs) can also induce EMP programs via STAT3 signaling. Additional mediators of EMP include Hypoxia, Hedgehog, Notch and Integrin signaling (not shown), and highlight the context dependent activation of EMP from micro-environmental cues.
While most studies rely upon knockdown and over-expression approaches to demonstrate the function of proteins in the context of cell migration, proliferation and EMP transitions, relatively few studies have investigated these targets in the context of the physiological induction of EMP in response to exogenous stimuli. Among PDAC cell lines, L3.6pl cells have been shown to respond to VEGF treatment [95], while the inflammatory cytokines TNF-α and IL-1β drive EMP in PaTu 8988T and AsPC-1 cells via Hedgehog signaling [96]. Collagen 1 also stimulated L3.6pl and BxPC-3 cells to become more invasive through interaction with DDR1 [97], and BMP2 was able to elicit a similar response in BxPC-3 cells [98]. PANC-1 cells are a well characterised model of inducible EMP, first shown by Ellenrieder et al to undergo a bidirectional change in response to TGFβ alongside CAPAN-1, COLO-357, IMIM-PC1 [99], HPAF-II, and CAPAN-2 cells [100]. PANC-1 cells have since been repeatedly modelled with regard to their EMP response, which has been shown to be inducible in response to TGFβ, TNF-α, HGF, or hypoxia through differing mechanisms [101,102,103,104]. SNAI1 appears to be a major driver in this model, being heavily regulated at the transcript and protein level, despite modest changes in E-cadherin and Vimentin proteins [105]. EMP is thus invariably the result of exogenous stimuli that activate discrete but conserved cellular pathways through novel intermediates that are an ongoing focus of basic cancer cell biology research.
4. Pre-Clinical Discovery of EMP Targets
As a result of the complexities of discerning cancer biology from native processes in vivo, the use of cell lines derived from primary tumours are a valuable means of modelling the molecular and phenotypic properties of cancers. Extensive investigation has been performed using gene silencing and overexpression approaches to evaluate the role that particular molecules have in regulating or effecting the EMP phenotypes of PDAC cells, however a concise summary of novel targets in the PDAC EMP field has to date been lacking. Thus, this review provides an exhaustive overview of such research as a platform for their integration, and progressive evaluation. The function of these candidate molecules can be broadly separated into secreted/soluble products (Table 1), receptors (Table 2), other membrane associated proteins (Table 3), cytoskeletal adaptors (Table 4), kinases (Table 5), intracellular mediators (Table 6), transcription factors (Table 7) and post transcriptional controllers (Table 8). The candidates shown were selected by searching Pubmed for the terms ‘pancreatic’ and ‘epithelial’, and articles investigating a novel candidate’s impact on EMP phenotypes were manually curated. These effectors have been characterised to varying extents for their influence on invasion, migration, xenograft tumour growth, prognostic associations, and impact on known EMP signaling pathways. The proposed mechanisms of candidates and assays used to assess such effects are shown within tables and may be used to gauge where further support may be warranted to confirm and extend such findings. Due to the inherent variation in models used, the statistical power granted by IHC for varying sized patient cohorts with accompanying clinical information, and the level of EMP as a primary context, it is difficult to draw direct conclusions regarding pivotal significance within the field and clinical importance from such singular studies. Candidate expression in primary patient material that correlated with lymph-node metastasis are shown in bold within tables, and provide the best surrogate for their role in EMP mediated invasion, and include membrane bound proteins IGFBP2, ITGB4, CEACAM6 [106,107,108]. The use of IHC to capture dynamic EMP processes may be limited however, as shown in the case of LIN28B, where its expression is both induced by TGFβ and high in PDAC tissue, despite its role to suppress the pro-EMP non-coding RNA LET7a [109,110]. Such studies highlight both the utility and limitations of the links between in vitro assays and clinical material, and emphasise the need for both wider cohorts of patient material for validation and the development of GEMM models to strengthen findings in a standardized manner.
Table 1.
Soluble and secreted factors that influence EMP. This table describes novel candidates that may be secreted within the ECM and act either directly through ligand-receptor interactions, or through mechanisms that remain to be demonstrated. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation (Direct or Indirect Observation) | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Human Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| BxPC-3 | DKK3 | Negative, Direct | Over-expression | DKK3 is overexpressed in tumour and is antagonist of WNT ligand activity, preventing nuclear translocation of β-catenin and EMP under hypoxia | Transwell assays, chemo-resistance, IHC in 75 matched PDAC v normal samples, xenograft growth | Not performed | Hypoxia | [111] |
| ASPC-1, PANC-1 | IGFBP2 | Positive, Direct | siRNA/Over-expression | IGFBP2 activated NF-κB through PI3K/AKT/IKK, inhibited by PTEN | WB, Transwell assays, orthotopic growth, IHC in 80 patient PDAC and lymph node samples | Survival and lymph node metastasis | - | [106] |
| PANC-1 | LTB4 | Positive, Direct | siRNA | LTB4 induced EMT through receptor BLT2 and ERK1/2 activation | WB, Transwell assays | Not performed | LTB4 | [112] |
| Patu8988, PANC-1 | DMKN | Positive, Indirect | shRNA | Knockdown reduced p-STAT3 and EMT increased ERK1/2, AKT | Proliferation, Transwell assays, Xenograft, IHC in 44 patient PDAC tumours | Correlated with T stage | - | [113] |
| PANC-1 | LGALS1 | Positive, Direct | shRNA/Over-expression | LGALS1 IHC expression correlated with MMP9 and Vimentin in PDAC. PSC LGALS1 promoted cancer cell EMT and activation of NF-κB | Xenograft, Proliferation, Invasion, IHC in 66 PDAC tumours | Not performed | [114] | |
| BxPC-3, CFPAC | SEMA3C | Positive, Indirect | shRNA/Over-expression | SEMA3C knockdown suppressed EMT and tumourigenesis, and activation of ERK1/2 signaling | Proliferation, migration, Scratch wound, Xenograft, IHC in 118 PDAC tumours | Stage, survival, recurrence | [115] | |
| Capan-1 | FUT3 | Positive, Direct | shRNA/Over-expression | FUT3 knockdown impeded proliferation, migration, tumour growth and TGFβ induced EMT | Proliferation, Scratch wound, Transwell assays, Xenograft | Not performed | TGFβ | [116] |
| PANC-1, MIAPaCa-2, Capan-2 | MIF/ NR3C2 | Positive, Indirect | siRNA/Over-expression | MIF induces miR-301b, targeting NR3C2, inducing EMT and chemo sensitivity | PDAC transcriptome by array, IHC of 173 PDAC, Proliferation, Colony formation, Transwell assays, chemo-resistance | NR3C2 inversely prognostic by RNA and IHC | - | [117] |
| PANC-1, BxPC-3 | WNT5A | Positive, Direct | siRNA, Over-expression | Wnt5a expression induced EMT and invasion and was elevated in PDAC by IHC | Scratch wound, Transwell assays, WB, orthotopic growth, IHC of 134 PDAC v normal | No | - | [118] |
| PANC-1 | LCN2 | Negative, Indirect | Over-expression | LCN2 expression correlated with better survival and lower EMT state | IHC of 60 PDAC tumours, Transwell assays | Protective by IHC | - | [119] |
| MIAPaca-2, BxPC-3, SUIT-2 | NOV | Positive, Indirect | shRNA/Over-expression | NOV expression high in PDAC by IHC, and induced EMT phenotypes in vitro/in vivo | Colony formation, soft agar, Proliferation, Transwell assays, in vivo metastasis | Not performed | - | [120] |
| PANC-1, BxPC-3 | CCL18 | Positive, Direct | CCL18 expressed in mesenchymal and cancer cells, and induced EMT | WB, Transwell assays, IHC of 62 PDAC tumours, serum ELISA from PDAC patients | Survival | - | [121] | |
| PANC-1, BxPC-3 | TUFT1 | Positive, Indirect | siRNA/Over-expression | TUFT1 expression correlated with T stage and lymph node metastasis by IHC, RNA expression correlated with HIF1a, SNAI1 and VIM | WB, Proliferation, scratch wound, Transwell assays, Xenograft, IHC of 63 PDAC tumours | Yes in TCGA by RNA | [122] | |
| SW1990, ASPC-1 | OLR1 | Positive, Direct | siRNA/Over-expression | OLR1 overexpressed in tumours and correlates with metastasis and poor survival, overexpression induced EMT | Transwell assays, scratch wound, Proliferation/apoptosis, IHC of 98 PDAC tumours | Yes survival by IHC and TCGA | - | [123] |
| MIAPaCa-2, PANC-1, ASPC-1, BxPC-3 | LOXL2 | Positive, Indirect | siRNA/Over-expression | LOXL2 IHC expression correlated with recurrence, depth of invasion and poor survival, and enhanced EMT in vitro | Transwell assays, IHC of 80 PDAC tumours | Yes by IHC | - | [124] |
| PANC-1, PK9 | TFF1 | Negative, Direct | siRNA | TFF only expressed in PanIN and intraductal neoplasia, not normal or invasive PDAC, knockdown activated EMT, loss of TFF in GEMM drove PanIN, PDAC and CAF infiltration | Transwell Invasion, Scratch wound, KC GEMM, IHC on small number of samples | Not performed | - | [125] |
Table 2.
Receptors. This table describes known receptors that may be activated to transduce signals required for EMP modulation. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| L3.6pl | VEGFR1 activation | Positive, Direct | RTK VEGFR-1 activation induced SNAI1/2, TWIST | E-cadherin/b-catenin localization/WB | Not performed | VEGF | [95] | |
| PANC-1, MiaPaCa-2 | HTR1B, HTR1D | Positive, Indirect | siRNA | 5-HT receptor knockdown reduced uPAR and Src/FAK signaling and EMT | Scratch wound, Transwell, Colony formation | Not performed | - | [126] |
| PANC-1 HPAC |
IGF1R | Positive, Indirect | siRNA | IGF1R overexpressed in PDAC by IHC, silencing inhibits AKT/PI3K, MAPK, JAK/STAT signaling pathways | Transwell assays, soft agar, Proliferation, apoptosis, IHC of TMA | Not performed | - | [127] |
| L3.6pl, BxPC-3 | DDR1 | Positive, Direct | siRNA/ Over-expression | DDR1 expression correlates with CHD2 expression by IHC, DDR1-b signals through SHC1 adapter to PYK2 to induce CDH2 | Invasion, IHC of PDAC TMA | Not performed | COL1A | [97] |
| PANC-1 | SMO | Positive, Indirect | siRNA | Hedgehog activated in tumourspheres, SMO knockdown inhibited CSC/EMT features properties | Proliferation, sphere formation, Transwell assays, Xenograft | Not performed | - | [128] |
| PANC-1, BxPC-3 | EPHA4 | Positive, Direct | siRNA | EPHA4 knockdown suppressed EMT, MMP2 activity | Gelatin zymography, Transwell assays, scratch wound, WB | Not performed | - | [129] |
| CFPAC-1, AsPC-1 | ITGB4 | Positive, Direct | siRNA/Over-expression | ITGB4 IHC expression correlated with T stage, knockdown inhibited EMP | Transwell assays, WB, IHC of 134 PDAC tumours | Survival lymph node metastasis by IHC | TGFβ | [107] |
| PANC-1, MiaPaCa2, Capan2 | F2R | Positive, Indirect | shRNA | F2R (PAR1) expression associated with mesenchymal gene signature | Xenograft, Scratch wound | Not performed | - | [130] |
Table 3.
Membrane associated proteins. This table describes membrane bound proteins that may interact with other cells and the extracellular environment to sense cues that modulate EMP in a context dependent fashion. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| PANC-1 | CDCP1 | Positive, Indirect | siRNA | CDCP1 expression high in PDAC, induced by BMP4/ERK signaling, and knockdown inhibited EMT phenotypes | Scratch wound, Transwell, spheroid formation, chemo-resistance, IHC on 42 PDAC tumours | Not performed | - | [131] |
| Colo-357, Capan-1 | MUC16 | Positive, Indirect | siRNA, CRISPR/Cas9 | MUC16 knockdown decreased FAK mediated AKT/ERK/MAPK activation, and EMT | Proliferation, migration, Colony formation, Xenograft | Not performed | - | [132] |
| MiaPaCa2 | ANXA1 | Positive, Indirect | CRISPR | ANXA1 KO downregulated miR196a, effected cell motility and liver metastases in vivo | Scratch wound, Transwell migration, Invasion, Xenograft | Not performed | [133,134] | |
| CFPAC-1, PANC-1 | CEACAM6 | Positive, Direct | shRNA, Over-expression | CEACAM6, regulated by miR-29a/b/c, required for EMT | Transwell assays, Xenograft, WB, IHC in 99 PDAC tumours | Lymph node metastasis | - | [108] |
| SUIT-2, CAPAN-2 | TM4SF1 | Negative, Indirect | siRNA | TM4SF1 IHC expression protective, knockdown induced migration and decreased E-cadherin | Transwell assays, IHC in 74 PDAC tumours | Yes inversely prognostic by IHC | TGFβ | [135] |
| PANC-1, SW1990 | DPP4 | Positive, Indirect | siRNA/ Over-expression | DPP4 (CD26) knockdown suppressed EMT, in vivo growth | Proliferation, Transwell assays, Xenograft, WB | Not performed | - | [136] |
| PANC-1, AsPC-1 | SLC39A4 | Positive, Indirect | siRNA/ overexpression | SLC39A4 (ZIP4) IHC expression correlated with ZEB1 and EMT, increasing FAK and paxillin phosphorylation | Xenograft, Scratch wound, Transwell migration, Invasion, IHC of 72 paired PDAC v normal | Not performed | - | [137] |
Table 4.
Cytoskeletal adaptors. This table describes intracellular adapter proteins that may participate in and be required protein complex localization and transduction of signals that modulate EMP. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| PANC-1, CFPAC-1 | WASF3 | Positive, Indirect | siRNA, | WASF3 (WAVE3) knockdown suppressed PDK2, downregulating PBK/AKT pathway and EMT | Proliferation, migration, Invasion, Scratch wound, IHC of 87 paired PDAC v normal | Lymph node metastasis | - | [138] |
| PANC-1, AsPC-1, MiaPaCa-2 | NES | Positive, Direct | shRNA/Over-expression | NES (Nestin) required for EMT and induced by TGFβ in positive feedback loop promoting p-smad2 | Xenograft, Transwell assays, IHC of GEMM | Not performed | TGFβ | [139,140] |
| HPAF-II, PANC-04.03 PANC-1 |
DNM2 | Positive, Indirect | siRNA, Over-expression | Upregulated by IHC in PDAC, DNM2/VAV1 interaction required for RAC-1 induced lamellipodia formation | Transwell assays, lamellipodia formation, xenograft, IHC of 85 PDAC tumours | Not performed | EGF (HPAF-II) | [141,142] |
| SUIT-2 | RAB5A | Positive, Indirect | siRNA | RAB5 IHC expression correlated with invasion and CDH1, aids TGFβR endocytosis, stimulates FA turnover, prognostic in PDAC, breast, ovarian | Morphology, Proliferation, Transwell assays, IHC of 111 PDAC tumours | Survival IHC | - | [143] |
Table 5.
Kinases and Phosphatases. This table describes proteins with activity that may directly participate in signal transduction by phospho-regulation of intracellular substrates. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| PANC-1, MIAPaCa-2 | EEF2K, | Positive, Direct | siRNA/Over-expression | EEF2K promotes EMT through TG2/β1 integrin/SRC/uPAR/MMP2 signaling | Scratch wound, Transwell assays, WB | Not performed | - | [144] |
| Patu8988, PANC-1, BxPC-3, Capan-1 | CDK14 | Positive, Direct | siRNA | Suppression of CDK14 reduced PI3K/AKT activation and EMT | Proliferation, Colony formation, Transwell assays | Not performed | - | [145] |
| HDPE | PRAG1 | Positive, Indirect | siRNA/Over-expression | Phosphorylation of PRAG1 found in malignant cells, Over-expression induced JAK1/STAT3 mediated EMT | Transwell assays, phospho-WB | Not performed | - | [146] |
| BxPC-3, PANC-1 | AURKA | Positive, Direct | shRNA | AURKA IHC expression high in PDAC, phosphorylates and stabilizes TWIST1 in positive feedback loop, promoting EMT | Sphere formation, migration, Proliferation, Xenograft, IHC on small PDAC cohort | Not performed | - | [63] |
| PANC-1, ASPC-1 | MAP3K3 | Positive, Direct | CRISPR | MAP3K3 (MEKK3) KO reduced EMT, CSC and migration, and YAP/TAZ transcriptional activity on AXL, DKK1, FosL1, CTGF | Transwell migration Invasion, Proliferation, Xenograft, ChIP | Not performed | - | [147] |
| PANC-1, COLO357 | RAC1 | Negative, Direct | siRNA/Over-expression | RAC1b inhibits canonical and non-canonical TGFβ signaling, effecting MKK6-p38 and MEK-ERK-MAPK EMT activation | Migration, qPCR | Not performed | TGFβ | [90,148] |
| HPAF-II, CAPAN-2 | PTPN11 | Positive, Direct | shRNA/Over-expression | PTPN11 (SHP2) activity enhances the effect of EGF on TGFβ induced EMT, resulting in more complete EMT | Cell scatter, scratch wound, WB | Not performed | TGFβ/EGF | [100] |
Table 6.
Enzymes and Co-factors. This table describes intracellular proteins that may directly or indirectly participate in pathways required for EMP modulation by other enzymatic control of substrate proteins. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| PANC-1 | USP22 | Positive, Direct | shRNA/Over-expression | USP22 expression correlated with Ezrin and FAK phosphorylation and EMT | Scratch wound, Transwell assays, WB | Not performed | - | [149] |
| 779E, 1334 PDCL | EIF5A | Positive, Indirect | shRNA/Over-expression | Mutant KRAS induces EIF5A, stimulating PEAK1 mediated ECM signaling. PEAK1 binds YAP/TAZ driving stem TFs | Sphere formation, IP, WB | Not performed | - | [150] |
| AsPC-1, PANC-1 | EIF4E | Negative, Indirect | siRNA | Knockdown of MNK effector, EIF4E, induced ZEB1 through repression of miR-200c, miR-141, MNK inhibitors induce MET | Collagen 3D, qPCR | Not performed | - | [94] |
| BxPC-3 | RGCC | Positive, Direct | siRNA | RGCC regulated by HIF1α and required for hypoxia induced EMT | qPCR, WB | Not performed | hypoxia | [151] |
| PANC-1 MIA PaCa-2 |
SET | Positive, Direct | shRNA/ Over-expression | SET over-expression activated Rac1/JNK/c-Jun pathway and decreased PP2A activity, N-cadherin and EMT TFs up | Transwell assays, Colony formation, Xenograft tumour growth and liver metastases | Not performed | - | [152] |
| MiaPaCa2, SW1990, PANC-1, CFPAC1 | GPX1 | Negative, Direct | shRNA/Over-expression | GPX1 IHC expression lower in PDAC, silencing induced EMT and gemcitabine resistance through ROS activated PI3K/Akt/GSK3B/SNAIL, Over-expression sensitized in vivo | Transwell migration, chemo-resistance, Xenografts, IHC of 281 PDAC tumours, and 42 paired PDAC v normal | Yes inversely prognostic by IHC | [153] | |
| BxPC-3, PANC-1, MiaPaCa2, PSN1 | HDAC1 | Positive, Indirect | siRNA | HDAC IHC expression and activity correlated with EMT phenotype | IHC, Transwell Invasion, IHC of 103 PDAC tumours | Survival by IHC | - | [154] |
| PANC-1 BxPC-3 |
Class I HDAC | Positive, Indirect | 4SC-202 small inhibitor | HDACi (inhibition) blocked TGFβ induced EMT in PANC-1, requiring BRD4 and MYC for effect of HDACi | Migration, sphere formation, Xenograft | Not performed | TGFβ (PANC-1) | [155] |
| CFPAC-1, L3.7-2 | PAFAH1B2 | Positive, Direct | siRNA/Over-expression | PAFAH1B2 IHC expression higher in PDAC, HIF1a expression regulated PAFAH1B2 via direct promoter binding | Transwell migration, Invasion, orthotopic Xenograft/ liver metastases, HIF1a/PAFAH1B2 co-localization in PDAC, IHC of 124 PDAC tumours and 70 normal | Survival by IHC and TCGA | hypoxia | [156] |
| PANC-1, MIAPaCa-2 | KDM4B | Positive, Direct | siRNA | KDM4B IHC expression correlated with ZEB1 in PDAC, knockdown inhibited TGFβ induced EMT in PANC-1 by regulating ZEB1 methylation | CHIP, scratch wound, Transwell assays, IHC of 49 PDAC tumours | Not performed | TGFβ | [157] |
| HPAC, BxPC-3, Colo357 PANC-1, MiaPaCa-2 | SMURF2 | Negative, Direct | SMURF negative regulator of TGFβ induced EMT, suppressed by miR-15b | Scratch wound, Transwell assays, WB | Not performed | TGFβ | [158] | |
| CAPAN-1 PANC-1 |
CUL4B | Positive, Direct | miRNA | CUL4B IHC expression higher in PDAC, regulated by miR -300, required for Wnt/β-catenin induced EMP | qPCR, Transwell assays, Xenograft, IHC of 110 PDAC v normal | Not performed | - | [159] |
| PANC-1 | KMT5C | Positive, Direct | siRNA | KMT5C (SUV420H2) expression higher in PanIN and PDAC, methylates H4K20me3,suppresses epithelial drivers FOXA1, OVOL2, OVOL2 | Transwell assays, chemo-resistance, sphere formation, | Not performed | - | [160] |
| PANC-1 | NOX4 | Positive, Direct | siRNA | NOX4 IHC expression elevated in PDAC, aids ROS generation and TGFβ induced EMT | Transwell assays, WB | Not performed | TGFβ | [161] |
| BxPC-3 | PAWR | Negative, Indirect | siRNA, Over-expression | PAWR (PAR4) suppressed in cisplatin resistant EMT cells, required PI3K/AKT signaling | Transwell assays, Proliferation, WB, Xenograft | Not performed | - | [162] |
| BxPC-3 | PPM1H | Negative, Indirect | siRNA | PPM1H expression decreased by TGFβ/BMP2 treatment, knockdown induced EMT | Proliferation, Transwell assays, WB, apoptosis | Not performed | TGFβ, BMP2 | [98] |
| PANC-1 | HMGN5 | Positive, Indirect | shRNA | HMNG5 silencing reduced Wnt expression | Xenograft, Transwell migration Invasion, WB, | Not performed | - | [163] |
| PANC-1 | GOLM1 | Positive, Direct | siRNA/ overexpression | GOLM1 (GP73) overexpression induced EMT and correlated with human metastasis and Xenograft growth | Xenograft, Transwell migration Invasion, Scratch wound, WB | Not performed | - | [164] |
Table 7.
Transcription Factors and Cofactors. This table describes transcription factors and cofactors that influence gene expression required for actions of EMP in their respective systems. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| PaTu 8988T, AsPC-1 | GLI1 | Positive, Direct | siRNA | GLI1 component of HH signaling, induced EMT by TNF-α/IL-1β, mediated through NF-κB pathway | Transwell assays, WB | Not performed | TNF-α/IL-1β | [96,165,166,167,168] |
| Colo-357, L3.7 | FOXM1 | Positive, Indirect | siRNA/Over-expression | FOXM1c activates uPAR promoter directly, inducing EMT | Scratch wound, Transwell migration, IHC of PDAC TMA v normal | Elevated in metastatic PDAC | - | [169] |
| BxPC-3, ASPC-1, PANC-1 | TAZ | Negative, Direct | shRNA, Over-expression | TAZ required for EMT through TEA/ATTS TFs, activation correlates with suppression of NF2 | Colony formation, Xenograft, Transwell assays, IHC of 57 PDAC v normal | Correlated with PDAC differentiation | - | [170] |
| PANC-1, CAPAN-1 | YAP | Positive, Direct | shRNA/Over-expression | YAP expression associated with activation of AKT cascade and EMT | Transwell assays, chemo-resistance, WB | Not performed | - | [171] |
| PANC-1, BxPC-3 | HSF1 | Positive, Indirect | siRNA | p-HSF1 IHC elevated in PDAC, promotes invasion and is downregulated by p-AMPK | Transwell assays, scratch, WB, GEMM | Not performed | - | [172] |
| HPAC, MiaPaCa2 | FOXC1 | Positive, Indirect | siRNA/Over-expression | IGFR1 positively regulates FOXC1, activating PI3K/Akt/ERK, promoting migration, and EMT, and tumour growth | Xenograft, Transwell migration Invasion, soft agar | Not performed | IGF | [173] |
| PANC-1, SW1990 | BHLHA15, Direct | Negative, Direct | Over-expression | BHLHA15 (MIST1) Over-expression suppressed tumour growth & metastases. Caused MET by suppressing SNAIL indirectly | Transwell migration, Invasion, Xenograft and liver met | Not performed | - | [174] |
| PANC-1 | KLF8, Indirect | Positive, Direct | siRNA, Over-expression | KLF8 IHC elevated in PDAC, directly induces FHL2 transcription via promoter binding | WB, Invasion | Not performed | - | [175] |
| GEMM | P73, Direct | Negative, Direct | GEMM | P73 deficiency led to stromal deposition and EMT in PDAC tumours, decreased BGN secretion, required for tumour suppressive functions of TGFβ | GEMM, Transwell assays | Not performed | - | [176] |
| GEMM | PRRX1 | Positive, Direct | Overexpression | PRRX1 a/b have discrete functions in MET/EMT, knockdown suppresses tumour growth and EMT | GEMM tumour model, Xenograft | Not performed | - | [177] |
| Capan-2 | TRIM28 | Positive, Indirect | Overexpression | TRIM28 Overexpression drove EMT and Invasion, correlated with T stage | Transwell assays, WB, Xenograft, IHC of 91 PDAC | Lymph node metastasis and survival | - | [178] |
| PANC-1 | ETS1 | Positive, Direct | shRNA | ETS1 knockdown epithelialized PANC-1 cells | Scratch wound, adhesion, qPCR for EMT markers | Not performed | - | [179] |
| HDPE, COLO-357 | NFE2L2 | Positive, Direct | siRNA/Over-expression | NFE2L2 activation enhanced TGFβ induced EMT in both premalignant and malignant cells | Scratch wound, Transwell assays, WB, qPCR | Not performed | TGFβ | [180] |
| PANC-1, HPAF-II | PDX1 | Positive, Indirect | shRNA, GEMM | PDX1 has dual roles in premalignant and transformed cells. PDX1 expression is reduced in tumours and EMT | Colony formation, GEMMs, IHC of 183 PDAC | Inversely prognostic for survival | TGFβ (PANC-1), HGF (HPAF-II) | [181] |
| PANC-1 | BCL9L | Positive, Direct | siRNA/Over-expression | BCL9L knockdown prevented EMT and inhibited in vivo growth | Proliferation, Transwell assays, Xenograft | Not performed | TGFβ | [182] |
| GEMM | ETV1 | Positive, Direct | Overexpression | ETV1 induces SPARC, required for tumour growth and metastasis in vivo, EMT in vitro | Xenograft, Invasion | Not performed | - | [183] |
| ASPC-1, SW1990 | EPAS1 | Positive, Direct | siRNA | EPAS1 (HIF2α) IHC expression high in PDAC, and knockdown inhibited EMT | CHIP, Transwell assays, IHC of 70 PDAC | Lymph node metastasis, differentiation | - | [184] |
| PANC-1 BxPC-3 |
SIX1 | Positive, Indirect | siRNA/shRNA | SIX1 IHC expression elevated in PDAC, knockdown reduced migration and tumour size | Migration, EMT markers, PANC-1 Xenograft, CD44-/CD24+, IHC of 139 PDAC | No | - | [185] |
| Cfpac-1 | GRHL2 | Negative, Direct | siRNA | GRHL2 IHC expression elevated in normal duct and liver metastases, drives epithelial phenotype. | Proliferation, EMT markers, Colony and sphere formation, drug resistance, IHC of 155 PDAC | No | - | [186] |
| PaTu8988S | GATA6 | Negative, Direct | shRNA/Over-expression | GATA6 IHC expression low in PDAC, Silencing induced EMT | chemo-resistance, IF, Invasion, Xenograft, IHC of 58 PDAC | Inversely prognostic for survival | - | [187] |
Table 8.
Post transcriptional effectors. This table describes factors that may post transcriptionally modulate EMP by controlling stability of mRNA and hence expression of effector proteins. Candidates that exhibit clinical correlation with lymph node metastasis are shown in bold.
| Cell Line | Target | EMT Regulation | KD/KO/Over-expression | Pathway/Mechanism | Functional Assay | Prognostic Association | EMT Activator | Reference |
|---|---|---|---|---|---|---|---|---|
| Miapaca-2, PANC-1, Patu-8988 | HNRNPA2B1 | Positive, Direct | shRNA/Over-expression | Knockdown epithelialized cells, Over-expression drove EMT through ERK/SNAI1 pathway | Cell viability, Transwell assays, PANC-1 Xenograft, EMT markers | Not performed | - | [188] |
| SW1990, BxPC-3 | YTHDF2 | Negative, Direct | shRNA | Knockdown reduced p-AKT, p-GSK-3b, promoted EMT, YAP knockdown reversed effect | Proliferation, Colony formation, Invasion, adhesion | Not performed | - | [189] |
| Panc-1, Patu8988 | Lnc TUG1 | Positive, Direct | shRNA | Lnc TUG1 sponges miR-382, preventing repression of ezh2 | Colony formation, Transwell assays, WB | Not performed | - | [190] |
| Gemcitabine resistant BxPC-3 | DYNC2H1-4 | Positive, Direct | siRNA | Lnc DYNC2H1-4 sponges miR-145, upregulating ZEB1, MMP3 and other CSC markers | Transwell assays, CSC markers, Xenograft | Not performed | - | [191] |
| ASPC-1, BxPC-3, PANC-1 | miR-23 | Positive, Direct | miRNAs | miR -23 promotes EMT by regulating ESRP1, miR-23 required for TGFβ induced EMT | WB, Transwell assays, Xenograft, qPCR of 52 paired PDAC tumour v normal | Survival by RNA | TGFβ | [192] |
| SW1990, PANC-1, BxPC-3, CAPAN-1 | NORAD | Positive, Direct | shRNA/Over-expression | Lnc NORAD acts as ceRNA of miR-125a-3p, enhancing RHOa and EMT | Scratch wound, Transwell assays, Xenograft | Not performed | Hypoxia | [193] |
| Panc-1 | Lnc H19 | Positive, Direct | siRNA | H19 antagonised LET-7, inducing HMGA-2 mediated EMT | Transwell assays, scratch wound, WB | Not performed | - | [194] |
| ASPC-1, BxPC-3 | LncRNA-ROR | Positive, Direct | shRNA/Over-expression | LncRNA-ROR expression induces ZEB1 and EMT | Scratch wound, Transwell assays, Xenograft | Not performed | - | [195] |
| PANC-1, BxPC-3, COLO357 | miR-100, miR -125b | Positive, Indirect | siRNA/CRISPR/Over-expression | TGFβ induced lnc-miR100HG, which codes for tumourigenic miR 100, miR125b and LET-7a. LIN28B also induced by TGFβ, suppresses LET-7a activity | miR Over-expression, Xenograft, Scratch wound, sphere formation, RNAseq, RIPseq | Survival by RNA | TGFβ | [109] |
| BxPC-3, PANC-1, CFPAC-1, SW1990 | miR-361-3p | Positive, Direct | Over-expression | miR-361-3p downregulates DUSP2, preventing inactivation of ERK1/2 | Orthotopic metastasis, Transwell assays | Survival by RNA | - | [196] |
| Sw1990 | miR-1271 | Negative, Direct | miR Mimics, Inhibitors | miR -1271 inhibited EMT and migration | Proliferation, Transwell migration invasion, xenograft | Not performed | - | [197] |
| Panc-1 | LSM1 | Positive, Indirect | Over-expression | Lsm1 (CaSm) induction induced EMT and proliferation, effecting apoptotic and metastasis gene expression | Proliferation, anoikis, Transwell assays, chemo-resistance, xenograft | Not performed | - | [198] |
| KPCY | MTDH | Positive, Indirect | siRNA | MTDH expression promoted CSC and metastasis, high cytoplasmic expression by IHC | Spheroid formation, orthotopic and metastatic xenograft models, IHC of 134 PDAC | Survival | - | [199] |
| ASPC-1, HS766t, BxPC-3 | LIN28B | Positive, Direct | shRNA | LIN28B IHC expression high in PDAC, suppression inhibited proliferation and EMT | Colony formation, Proliferation, migration, IHC of 185 PDAC tumours | Survival, stage, metastasis | - | [110] |
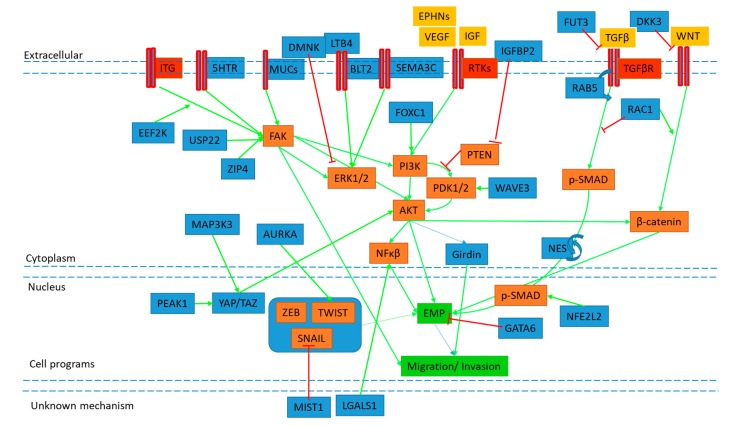
Figure 2 illustrates the proposed activity of some of these novel candidates, and how they may positively or negatively regulate discrete EMP signaling pathways. Of note are several candidates that converge to positively regulate EMP migratory phenotypes through FAK/Src and FAK/PI3K signaling, including the 5HT receptor and mucins, as well as EEF2K, USP22, and ZIP4. Their complete mechanisms of action and prevalence in PDAC tissue remain to be elucidated, however their inhibition may curb carcinoma invasion by blocking FAK activation and subsequent EMP modulation. Similarly, candidates participating in stability of EMP signaling and TF activity provide targets to modulate the EMP process specific for carcinoma cells. AURKA kinase has been shown to participate in a positive feedback loop with stabilization and activity of TWIST1, while PEAK1 and NES have been implicated in stabilization YAP/TAZ and SMAD TF activity. The discovery of discrete EMP regulation and development of combinatorial inhibitors may provide the opportunity for more personalized therapeutic approaches to curb metastatic disease.
Figure 2.
Simplified overview of the proposed mechanisms of novel candidates.
EMP and cell migration (GREEN boxes) is induced through cell surface proteins (ITG, 5HTR, MUC, BLT2, SEMA3C, RTK, TGFβR) (RED) to activate signaling pathways (ORANGE boxes, blue arrows). These pathways are influenced by novel mediators (BLUE boxes) through activation (GREEN arrows) or inhibition (RED T) of known signaling members, however complete mechanisms of action remain to be elucidated. For full details, evidence of proposed mechanism and references of novel mediators, see tables below. Note signaling pathways shown have had intermediates removed for ease of visualisation.
5. Conclusions
Overall, investigation of the fundamental biology of EMP aims to combat local and metastatic invasion by providing a better understanding of the processes that allow cancer cells to dissociate from their epithelial adhesions to spread. EMP is a prominent driver of PDAC progression, thus highlighting the importance of our understanding of the subtleties of its regulation. The ability of EMP programs to direct cancer cells towards a drug resistant and migratory lineage capable of seeding local and distant recurrence presents a significant barrier to current treatment regimens. Therefore, the identification of new candidate molecules regulating these processes are crucial to inform targeted therapies and provide insights into the vulnerabilities of heterogeneous populations of tumour cells present in PDAC.
It is clear from this ever-growing list of EMP effectors in PDAC cells alone, that much work remains to delineate their collective interactions within and beyond our current understanding on EMP signaling pathways. While candidates have been shown to play roles in aspects of EMP signaling and associated phenotypes, significant support is required for their mechanisms of action to make concrete conclusions about their directive actions in cancer. Our understanding of receptor mediated canonical signaling through PI3K/AKT, MAPK, NFκB and other well studied cell cycle pathways has required decades to tease apart, and the subtleties of EMP programs provides a similar challenge. Open source integrative tools such as Reactome [200], WikiPathways [201], String [202], and Cytoscape [203] provide platforms for researchers to combine such analyses to build upon our current understanding and fill knowledge gaps in the field of cancer biology. In this way, progress may be made to better understand and discover properties that may be modulated in concert to control EMP in cancer.
In vitro and xenograft tumour modelling and manipulation of target molecules often demonstrates a role in cancer cell migration and tumour formation, however stronger evidence for their physiological role in regulating EMP, metastasis and therapy resistance may require GEMMs. The use of in vivo manipulation of PDAC GEMM models using targeted CRISPR approaches may be such a route towards a system that better recapitulates the spontaneity and heterogeneity of human tumours [204].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R., Ozenberger B.A., Ellrott K., Shmulevich I., Sander C., Stuart J.M. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waddell N., Pajic M., Patch A.M., Chang D.K., Kassahn K.S., Bailey P., Johns A.L., Miller D., Nones K., Quek K., et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.-M., Gingras M.-C., Miller D.K., Christ A.N., Bruxner T.J.C., Quinn M.C., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 7.Maitra A., Hruban R.H. Pancreatic cancer. Annu. Rev. Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones S., Zhang X., Parsons D.W., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Kamiyama H., Jimeno A., et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin J., Hruban H.R.R., Aguirre A.J., Moffitt R.A., Yeh J.J., Chip S.A., Robertson G., Cherniack A.D., Gupta M., Getz G., et al. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32:185–203.e113. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruban R.H., Maitra A., Schulick R., Laheru D., Herman J., Kern S.E., Goggins M. Emerging molecular biology of pancreatic cancer. Gastrointest. Cancer Res. GCR. 2008;2:S10–S15. [PMC free article] [PubMed] [Google Scholar]
- 11.Collisson E.A., Sadanandam A., Olson P., Gibb W.J., Truitt M., Gu S., Cooc J., Weinkle J., Kim G.E., Jakkula L., et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer C., Holmstrom S.R., He J., Laise P., Su T., Ahmed A., Hibshoosh H., Chabot J.A., Oberstein P.E., Sepulveda A.R., et al. Experimental microdissection enables functional harmonisation of pancreatic cancer subtypes. Gut. 2019;68:1034–1043. doi: 10.1136/gutjnl-2018-317706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L., Zhao H., Yan H. Gene expression profiling of 1200 pancreatic ductal adenocarcinoma reveals novel subtypes. BMC Cancer. 2018;18:603. doi: 10.1186/s12885-018-4546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligorio M., Sil S., Malagon-Lopez J., Nieman L.T., Misale S., Di Pilato M., Ebright R.Y., Karabacak M.N., Kulkarni A.S., Liu A., et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell. 2019;178:160–175.e27. doi: 10.1016/j.cell.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng J., Sun B.-F., Chen C.-Y., Zhou J.-Y., Chen Y.-S., Chen H., Liu L., Huang D., Jiang J., Cui G.-S., et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29:725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffitt R.A., Marayati R., Flate E.L., Volmar K.E., Loeza S.G.H., Hoadley K.A., Rashid N.U., Williams L.A., Eaton S.C., Chung A.H., et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spivak-Kroizman T.R., Hostetter G., Posner R., Aziz M., Hu C., Demeure M.J., Von Hoff D., Hingorani S.R., Palculict T.B., Izzo J., et al. Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer. Cancer Res. 2013;73:3235–3247. doi: 10.1158/0008-5472.CAN-11-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir J.A., Mann J., White S.A. The role of pancreatic stellate cells in pancreatic cancer. Surg. Oncol. 2015;24:232–238. doi: 10.1016/j.suronc.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Feig C., Gopinathan A., Neesse A., Chan D.S., Cook N., Tuveson D.A. The Pancreas Cancer Microenvironment. AACR; Philadelphia, PA, USA: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neesse A., Michl P., Frese K.K., Feig C., Cook N., Jacobetz M.A., Lolkema M.P., Buchholz M., Olive K.P., Gress T.M. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 21.Korc M. Pancreatic cancer–associated stroma production. Am. J. Surg. 2007;194:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z., Pothula S.P., Wilson J.S., Apte M.V. Pancreatic cancer and its stroma: A conspiracy theory. World J. Gastroenterol. WJG. 2014;20:11216–11229. doi: 10.3748/wjg.v20.i32.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahadevan D., Von Hoff D.D. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Therap. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 25.Thomas D., Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer. 2019;18:14. doi: 10.1186/s12943-018-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trelstad R.L., Hay E.D., Revel J.D. Cell contact during early morphogenesis in the chick embryo. Dev. Biol. 1967;16:78–106. doi: 10.1016/0012-1606(67)90018-8. [DOI] [PubMed] [Google Scholar]
- 27.Huber M.A., Kraut N., Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Tsai J.H., Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannetti E., van der Borden C.L., Frampton A.E., Ali A., Firuzi O., Peters G.J. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin. Cancer Biol. 2017;44:43–59. doi: 10.1016/j.semcancer.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Du Y.X., Liu Z.W., You L., Wu W.M., Zhao Y.P. Advances in understanding the molecular mechanism of pancreatic cancer metastasis. Hepatobiliary Pancreat. Dis. Int. 2016;15:361–370. doi: 10.1016/S1499-3872(15)60033-9. [DOI] [PubMed] [Google Scholar]
- 31.Beuran M., Negoi I., Paun S., Ion A.D., Bleotu C., Negoi R.I., Hostiuc S. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2015;15:217–225. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Satoh K., Hamada S., Shimosegawa T. Involvement of epithelial to mesenchymal transition in the development of pancreatic ductal adenocarcinoma. J. Gastroenterol. 2015;50:140–146. doi: 10.1007/s00535-014-0997-0. [DOI] [PubMed] [Google Scholar]
- 33.Hamada S., Satoh K., Masamune A., Shimosegawa T. Regulators of epithelial mesenchymal transition in pancreatic cancer. Front. Physiol. 2012;3:254. doi: 10.3389/fphys.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey J.M., Leach S.D. Signaling pathways mediating epithelial-mesenchymal crosstalk in pancreatic cancer: Hedgehog, Notch and TGFBeta. In: Grippo P.J., Munshi H.G., editors. Pancreatic Cancer and Tumor Microenvironment. Transworld Research Network; Trivandrum, India: 2012. [PubMed] [Google Scholar]
- 35.Dangi-Garimella S., Krantz S.B., Shields M.A., Grippo P.J., Munshi H.G. Epithelial-mesenchymal transition and pancreatic cancer progression. In: Grippo P.J., Munshi H.G., editors. Pancreatic Cancer and Tumor Microenvironment. Transworld Research Network; Trivandrum, India: 2012. [PubMed] [Google Scholar]
- 36.Chaffer C.L., San Juan B.P., Lim E., Weinberg R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 37.Trager M.M., Dhayat S.A. Epigenetics of epithelial-to-mesenchymal transition in pancreatic carcinoma. Int. J. Cancer. 2017;141:24–32. doi: 10.1002/ijc.30626. [DOI] [PubMed] [Google Scholar]
- 38.Elaskalani O., Razak N.B., Falasca M., Metharom P. Epithelial-mesenchymal transition as a therapeutic target for overcoming chemoresistance in pancreatic cancer. World J. Gastrointest. Oncol. 2017;9:37–41. doi: 10.4251/wjgo.v9.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaianigo N., Melisi D., Carbone C. EMT and treatment resistance in pancreatic cancer. Cancers. 2017;9:122. doi: 10.3390/cancers9090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishiwata T. Cancer stem cells and epithelial-mesenchymal transition: Novel therapeutic targets for cancer. Pathol. Int. 2016;66:601–608. doi: 10.1111/pin.12447. [DOI] [PubMed] [Google Scholar]
- 41.Kyuno D., Yamaguchi H., Ito T., Kono T., Kimura Y., Imamura M., Konno T., Hirata K., Sawada N., Kojima T. Targeting tight junctions during epithelial to mesenchymal transition in human pancreatic cancer. World J. Gastroenterol. WJG. 2014;20:10813–10824. doi: 10.3748/wjg.v20.i31.10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibue T. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawa Z., Haque I., Ghosh A., Banerjee S., Harris L., Banerjee S.K. The miRacle in pancreatic cancer by miRNAs: Tiny angels or devils in disease progression. Int. J. Mol. Sci. 2016;17:809. doi: 10.3390/ijms17060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brabletz S., Brabletz T. The ZEB/miR-200 feedback loop—A motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhim A.D. Epithelial to mesenchymal transition and the generation of stem-like cells in pancreatic cancer. Pancreatology. 2013;13:114–117. doi: 10.1016/j.pan.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan H.X., Xu J.W., Wu D., Zhang T.P., Hu S.Y. Pancreatic cancer stem cells: New insight into a stubborn disease. Cancer Lett. 2015;357:429–437. doi: 10.1016/j.canlet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Vaz A.P., Ponnusamy M.P., Seshacharyulu P., Batra S.K. A concise review on the current understanding of pancreatic cancer stem cells. J. Cancer Stem Cell Res. 2014;2:e1004. doi: 10.14343/JCSCR.2014.2e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellanos J.A., Merchant N.B., Nagathihalli N.S. Emerging targets in pancreatic cancer: Epithelial-mesenchymal transition and cancer stem cells. Onco Targets Ther. 2013;6:1261–1267. doi: 10.2147/ott.s34670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karamitopoulou E. Tumor budding cells, cancer stem cells and epithelial-mesenchymal transition-type cells in pancreatic cancer. Front. Oncol. 2012;2:209. doi: 10.3389/fonc.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuai G., Yang F., Yan J., Chen Y., Ma Q., Zhou C., Zhu C., Gu F., Liu Q. Deciphering relationship between microhomology and in-frame mutation occurrence in human CRISPR-based gene knockout. Mol. Ther. Nucleic Acids. 2016;5:e323. doi: 10.1038/mtna.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen D.X., Bos P.D., Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 52.Nieto J., Grossbard M.L., Kozuch P. Metastatic pancreatic cancer 2008: Is the glass less empty? Oncologist. 2008;13:562–576. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- 53.Schmalhofer O., Brabletz S., Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 54.Nakajima S., Doi R., Toyoda E., Tsuji S., Wada M., Koizumi M., Tulachan S.S., Ito D., Kami K., Mori T., et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004;10:4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 55.Peinado H., Portillo F., Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 56.Hotz B., Arndt M., Dullat S., Bhargava S., Buhr H.J., Hotz H.G. Epithelial to mesenchymal transition: Expression of the regulators snail, slug, and twist in pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 57.Brabletz T., Jung A., Reu S., Porzner M., Hlubek F., Kunz-Schughart L.A., Knuechel R., Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joo Y.E., Rew J.S., Park C.S., Kim S.J. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2002;2:129–137. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 59.Yin T., Wang C., Liu T., Zhao G., Zha Y., Yang M. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J. Surg. Res. 2007;141:196–203. doi: 10.1016/j.jss.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Oida Y., Yamazaki H., Tobita K., Mukai M., Ohtani Y., Miyazaki N., Abe Y., Imaizumi T., Makuuchi H., Ueyama Y., et al. Increased S100A4 expression combined with decreased E-cadherin expression predicts a poor outcome of patients with pancreatic cancer. Oncol. Rep. 2006;16:457–463. doi: 10.3892/or.16.3.457. [DOI] [PubMed] [Google Scholar]
- 61.Javle M.M., Gibbs J.F., Iwata K.K., Pak Y., Rutledge P., Yu J., Black J.D., Tan D., Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann. Surg. Oncol. 2007;14:3527–3533. doi: 10.1245/s10434-007-9540-3. [DOI] [PubMed] [Google Scholar]
- 62.Yamada S., Fuchs B.C., Fujii T., Shimoyama Y., Sugimoto H., Nomoto S., Takeda S., Tanabe K.K., Kodera Y., Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946–954. doi: 10.1016/j.surg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Nikhil K., Viccaro K., Chang L., Jacobsen M., Sandusky G., Shah K. The Aurora-A-Twist1 axis promotes highly aggressive phenotypes in pancreatic carcinoma. J. Cell Sci. 2017;130:1078–1093. doi: 10.1242/jcs.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z., Zhao L., Xiao Y., Gao Y., Zhao C. Snail transcript levels in diagnosis of pancreatic carcinoma with fine-needle aspirate. Br. J. Biomed. Sci. 2015;72:107–110. doi: 10.1080/09674845.2015.11666805. [DOI] [PubMed] [Google Scholar]
- 65.Yamazaki K., Masugi Y., Effendi K., Tsujikawa H., Hiraoka N., Kitago M., Shinoda M., Itano O., Tanabe M., Kitagawa Y., et al. Upregulated SMAD3 promotes epithelial-mesenchymal transition and predicts poor prognosis in pancreatic ductal adenocarcinoma. Lab. Investing. J. Tech. Methods Pathol. 2014;94:683–691. doi: 10.1038/labinvest.2014.53. [DOI] [PubMed] [Google Scholar]
- 66.Masugi Y., Yamazaki K., Hibi T., Aiura K., Kitagawa Y., Sakamoto M. Solitary cell infiltration is a novel indicator of poor prognosis and epithelial-mesenchymal transition in pancreatic cancer. Hum. Pathol. 2010;41:1061–1068. doi: 10.1016/j.humpath.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Galvan J.A., Zlobec I., Wartenberg M., Lugli A., Gloor B., Perren A., Karamitopoulou E. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br. J. Cancer. 2015;112:1944–1950. doi: 10.1038/bjc.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarin D., Thompson E.W., Newgreen D.F. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- 69.Ledford H. Cancer theory faces doubts. Nature. 2011;472:273. doi: 10.1038/472273a. [DOI] [PubMed] [Google Scholar]
- 70.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A., Bailey J.M., McAllister F., Reichert M., Beatty G.L., Rustgi A.K., Vonderheide R.H., et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H., Wu C.C., LeBleu V.S., Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aiello N.M., Brabletz T., Kang Y., Nieto M.A., Weinberg R.A., Stanger B.Z. Upholding a role for EMT in pancreatic cancer metastasis. Nature. 2017;547:E7–E8. doi: 10.1038/nature22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krebs A.M., Mitschke J., Lasierra Losada M., Schmalhofer O., Boerries M., Busch H., Boettcher M., Mougiakakos D., Reichardt W., Bronsert P., et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017;19:518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y., LeBleu V.S., Carstens J.L., Sugimoto H., Zheng X., Malasi S., Saur D., Kalluri R. Dual reporter genetic mouse models of pancreatic cancer identify an epithelial-to-mesenchymal transition-independent metastasis program. EMBO Mol. Med. 2018;10:e9085. doi: 10.15252/emmm.201809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson E.W., Nagaraj S.H. Transition states that allow cancer to spread. Nature. 2018;556:442–444. doi: 10.1038/d41586-018-04403-x. [DOI] [PubMed] [Google Scholar]
- 76.Pastushenko I., Brisebarre A., Sifrim A., Fioramonti M., Revenco T., Boumahdi S., Van Keymeulen A., Brown D., Moers V., Lemaire S., et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 77.Pastushenko I., Blanpain C. EMT Transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Brabletz S., Brabletz T., Stemmler M.P. Road to perdition: Zeb1-dependent and -independent ways to metastasis. Cell Cycle. 2017;16:1729–1730. doi: 10.1080/15384101.2017.1360648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonzalez D.M., Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.David C.J., Huang Y.-H., Chen M., Su J., Zou Y., Bardeesy N., Iacobuzio-Donahue C.A., Massagué J. TGF-β Tumor Suppression through a Lethal EMT. Cell. 2016;164:1015–1030. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glazer E.S., Welsh E., Pimiento J.M., Teer J.K., Malafa M.P. TGFβ1 overexpression is associated with improved survival and low tumor cell proliferation in patients with early-stage pancreatic ductal adenocarcinoma. Oncotarget. 2017;8:999. doi: 10.18632/oncotarget.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowland-Goldsmith M.A., Maruyama H., Kusama T., Ralli S., Korc M. Soluble type II transforming growth factor-beta (TGF-beta) receptor inhibits TGF-beta signaling in COLO-357 pancreatic cancer cells in vitro and attenuates tumor formation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001;7:2931–2940. [PubMed] [Google Scholar]
- 84.Wagner M., Kleeff J., Friess H., Buchler M.W., Korc M. Enhanced expression of the type II transforming growth factor-beta receptor is associated with decreased survival in human pancreatic cancer. Pancreas. 1999;19:370–376. doi: 10.1097/00006676-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Chuvin N., Vincent D.F., Pommier R.M., Alcaraz L.B., Gout J., Caligaris C., Yacoub K., Cardot V., Roger E., Kaniewski B., et al. Acinar-to-Ductal Metaplasia Induced by Transforming Growth Factor Beta Facilitates KRAS(G12D)-driven Pancreatic Tumorigenesis. Cell. Mol. Gastroenterol. Hepatol. 2017;4:263–282. doi: 10.1016/j.jcmgh.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subramanian G., Schwarz R.E., Higgins L., McEnroe G., Chakravarty S., Dugar S., Reiss M. Targeting endogenous transforming growth factor beta receptor signaling in SMAD4-deficient human pancreatic carcinoma cells inhibits their invasive phenotype1. Cancer Res. 2004;64:5200–5211. doi: 10.1158/0008-5472.CAN-04-0018. [DOI] [PubMed] [Google Scholar]
- 87.Janda E., Lehmann K., Killisch I., Jechlinger M., Herzig M., Downward J., Beug H., Grunert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: Dissection of Ras signaling pathways. J. Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Ismaeel Q., Neal C.P., Al-Mahmoodi H., Almutairi Z., Al-Shamarti I., Straatman K., Jaunbocus N., Irvine A., Issa E., Moreman C., et al. ZEB1 and IL-6/11-STAT3 signaling cooperate to define invasive potential of pancreatic cancer cells via differential regulation of the expression of S100 proteins. Br. J. Cancer. 2019;121:65–75. doi: 10.1038/s41416-019-0483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ram Makena M., Gatla H., Verlekar D., Sukhavasi S., Pandey M.K., Pramanik K.C. Wnt/beta-Catenin signaling: The culprit in pancreatic carcinogenesis and therapeutic resistance. Int. J. Mol. Sci. 2019;20:4242. doi: 10.3390/ijms20174242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Witte D., Otterbein H., Forster M., Giehl K., Zeiser R., Lehnert H., Ungefroren H. Negative regulation of TGF-beta1-induced MKK6-p38 and MEK-ERK signaling and epithelial-mesenchymal transition by Rac1b. Sci. Rep. 2017;7:17313. doi: 10.1038/s41598-017-15170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang G., Yu Y., Sun C., Liu T., Liang T., Zhan L., Lin X., Feng X.H. STAT3 selectively interacts with Smad3 to antagonize TGF-beta signaling. Oncogene. 2016;35:4388–4398. doi: 10.1038/onc.2015.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheng W., Chen C., Dong M., Wang G., Zhou J., Song H., Li Y., Zhang J., Ding S. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017;8:e3147. doi: 10.1038/cddis.2017.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witte D., Bartscht T., Kaufmann R., Pries R., Settmacher U., Lehnert H., Ungefroren H. TGF-beta1-induced cell migration in pancreatic carcinoma cells is RAC1 and NOX4-dependent and requires RAC1 and NOX4-dependent activation of p38 MAPK. Oncol. Rep. 2017;38:3693–3701. doi: 10.3892/or.2017.6027. [DOI] [PubMed] [Google Scholar]
- 94.Kumar K., Chow C.R., Ebine K., Arslan A.D., Kwok B., Bentrem D.J., Eckerdt F.D., Platanias L.C., Munshi H.G. Differential regulation of ZEB1 and EMT by MAPK-interacting protein Kinases (MNK) and eIF4E in pancreatic cancer. Mol. Cancer Res. MCR. 2016;14:216–227. doi: 10.1158/1541-7786.MCR-15-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang A.D., Camp E.R., Fan F., Shen L., Gray M.J., Liu W., Somcio R., Bauer T.W., Wu Y., Hicklin D.J., et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y., Jin G., Li Q., Wang Z., Hu W., Li P., Li S., Wu H., Kong X., Gao J., et al. Hedgehog Signaling non-canonical activated by pro-inflammatory cytokines in pancreatic ductal adenocarcinoma. J. Cancer. 2016;7:2067–2076. doi: 10.7150/jca.15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang H., Svoboda R.A., Lazenby A.J., Saowapa J., Chaika N., Ding K., Wheelock M.J., Johnson K.R. Up-regulation of N-cadherin by collagen i-activated discoidin domain receptor 1 in pancreatic cancer requires the adaptor molecule Shc1. J. Biol. Chem. 2016;291:23208–23223. doi: 10.1074/jbc.M116.740605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu H., Qin H., Li D.M., Liu J., Zhao Q. Effect of PPM1H on malignant phenotype of human pancreatic cancer cells. Oncol. Rep. 2016;36:2926–2934. doi: 10.3892/or.2016.5065. [DOI] [PubMed] [Google Scholar]
- 99.Ellenrieder V., Hendler S.F., Boeck W., Activation S.-R.K., Seufferlein T., Menke A., Ruhland C., Adler G., Gress T.M. Transforming growth factor β 1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61:4222–4228. [PubMed] [Google Scholar]
- 100.Buonato J.M., Lan I.S., Lazzara M.J. EGF augments TGFBeta-induced epithelial-mesenchymal transition by promoting SHP2 binding to GAB1. J. Cell Sci. 2015;128:3898–3909. doi: 10.1242/jcs.169599. [DOI] [PubMed] [Google Scholar]
- 101.Maier H.J., Schmidt-Strassburger U., Huber M.A., Wiedemann E.M., Beug H., Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295:214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Oyanagi J., Kojima N., Sato H., Higashi S., Kikuchi K., Sakai K., Matsumoto K., Miyazaki K. Inhibition of transforming growth factor-beta signaling potentiates tumor cell invasion into collagen matrix induced by fibroblast-derived hepatocyte growth factor. Exp. Cell Res. 2014;326:267–279. doi: 10.1016/j.yexcr.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 103.Wajant H., Pfizenmaier K., Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 104.Xu J., Lamouille S., Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu M., Quek L.E., Sultani G., Turner N. Epithelial-mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 2016;4:19. doi: 10.1186/s40170-016-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao S., Sun Y., Zhang X., Hu L., Liu Y., Chua C.Y., Phillips L.M., Ren H., Fleming J.B., Wang H., et al. IGFBP2 Activates the NF-kappaB Pathway to Drive Epithelial-Mesenchymal Transition and Invasive Character in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2016;76:6543–6554. doi: 10.1158/0008-5472.CAN-16-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Masugi Y., Yamazaki K., Emoto K., Effendi K., Tsujikawa H., Kitago M., Itano O., Kitagawa Y., Sakamoto M. Upregulation of integrin beta4 promotes epithelial-mesenchymal transition and is a novel prognostic marker in pancreatic ductal adenocarcinoma. Lab. Investig. J. Tech. Methods Pathol. 2015;95:308–319. doi: 10.1038/labinvest.2014.166. [DOI] [PubMed] [Google Scholar]
- 108.Chen J., Li Q., An Y., Lv N., Xue X., Wei J., Jiang K., Wu J., Gao W., Qian Z., et al. CEACAM6 induces epithelial-mesenchymal transition and mediates invasion and metastasis in pancreatic cancer. Int. J. Oncol. 2013;43:877–885. doi: 10.3892/ijo.2013.2015. [DOI] [PubMed] [Google Scholar]
- 109.Ottaviani S., Stebbing J., Frampton A.E., Zagorac S., Krell J., de Giorgio A., Trabulo S.M., Nguyen V.T.M., Magnani L., Feng H., et al. TGF-β induces miR-100 and miR-125b but blocks let-7a through LIN28B controlling PDAC progression. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y., Li J., Guo S., Ouyang Y., Yin L., Liu S., Zhao Z., Yang J., Huang W., Qin H., et al. Lin28B facilitates the progression and metastasis of pancreatic ductal adenocarcinoma. Oncotarget. 2017;8:60414–60428. doi: 10.18632/oncotarget.19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo Q., Qin W. DKK3 blocked translocation of beta-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J. Cell. Mol. Med. 2015;19:2832–2841. doi: 10.1111/jcmm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim Y.R., Park M.K., Kang G.J., Kim H.J., Kim E.J., Byun H.J., Lee M.Y., Lee C.H. Leukotriene B4 induces EMT and vimentin expression in PANC-1 pancreatic cancer cells: Involvement of BLT2 via ERK2 activation. Prostaglandins Leukotrienes Essential Fatty Acids. 2016;115:67–76. doi: 10.1016/j.plefa.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 113.Huang C., Xiang Y., Chen S., Yu H., Wen Z., Ye T., Sun H., Kong H., Li D., Yu D., et al. DMKN contributes to the epithelial-mesenchymal transition through increased activation of STAT3 in pancreatic cancer. Cancer Sci. 2017;108:2130–2141. doi: 10.1111/cas.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang D., Zhang J., Yuan Z., Zhang H., Chong Y., Huang Y., Wang J., Xiong Q., Wang S., Wu Q., et al. PSC-derived Galectin-1 inducing epithelial-mesenchymal transition of pancreatic ductal adenocarcinoma cells by activating the NF-kappaB pathway. Oncotarget. 2017;8:86488–86502. doi: 10.18632/oncotarget.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu X., Zhao Z., Guo S., Li J., Liu S., You Y., Ni B., Wang H., Bie P. Increased semaphorin 3c expression promotes tumor growth and metastasis in pancreatic ductal adenocarcinoma by activating the ERK1/2 signaling pathway. Cancer Lett. 2017;397:12–22. doi: 10.1016/j.canlet.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Zhan L., Chen L., Chen Z. Knockdown of FUT3 disrupts the proliferation, migration, tumorigenesis and TGF-β induced EMT in pancreatic cancer cells. Oncol. Lett. 2018;16:924–930. doi: 10.3892/ol.2018.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang S., He P., Wang J., Schetter A., Tang W., Funamizu N., Yanaga K., Uwagawa T., Satoskar A.R., Gaedcke J., et al. A Novel MIF signaling pathway drives the malignant character of pancreatic cancer by targeting NR3C2. Cancer Res. 2016;76:3838–3850. doi: 10.1158/0008-5472.CAN-15-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bo H., Zhang S., Gao L., Chen Y., Zhang J., Chang X., Zhu M. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer. 2013;13:496. doi: 10.1186/1471-2407-13-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu B., Jin D.Y., Lou W.H., Wang D.S. Lipocalin-2 is associated with a good prognosis and reversing epithelial-to-mesenchymal transition in pancreatic cancer. World J. Surg. 2013;37:1892–1900. doi: 10.1007/s00268-013-2009-6. [DOI] [PubMed] [Google Scholar]
- 120.Cui L., Xie R., Dang S., Zhang Q., Mao S., Chen J., Qu J., Zhang J. NOV promoted the growth and migration of pancreatic cancer cells. Tumor Biol. 2014;35:3195–3201. doi: 10.1007/s13277-013-1418-3. [DOI] [PubMed] [Google Scholar]
- 121.Meng F., Li W., Li C., Gao Z., Guo K., Song S. CCL18 promotes epithelial-mesenchymal transition, invasion and migration of pancreatic cancer cells in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2015;46:1109–1120. doi: 10.3892/ijo.2014.2794. [DOI] [PubMed] [Google Scholar]
- 122.Zhou B., Zhan H., Tin L., Liu S., Xu J., Dong Y., Li X., Wu L., Guo W. TUFT1 regulates metastasis of pancreatic cancer through HIF1-Snail pathway induced epithelial-mesenchymal transition. Cancer Lett. 2016;382:11–20. doi: 10.1016/j.canlet.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 123.Zhang J., Zhang L., Li C., Yang C., Li L., Song S., Wu H., Liu F., Wang L., Gu J. LOX-1 is a poor prognostic indicator and induces epithelial-mesenchymal transition and metastasis in pancreatic cancer patients. Cell. Oncol. (Dordr.) 2017;41:73–84. doi: 10.1007/s13402-017-0360-6. [DOI] [PubMed] [Google Scholar]
- 124.Park J.S., Lee J.H., Lee Y.S., Kim J.K., Dong S.M., Yoon D.S. Emerging role of LOXL2 in the promotion of pancreas cancer metastasis. Oncotarget. 2016;7:42539–42552. doi: 10.18632/oncotarget.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamaguchi J., Yokoyama Y., Kokuryo T., Ebata T., Enomoto A., Nagino M. Trefoil factor 1 inhibits epithelial-mesenchymal transition of pancreatic intraepithelial neoplasm. J. Clin. Investig. 2018;128:3619–3629. doi: 10.1172/JCI97755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gurbuz N., Ashour A.A., Alpay S.N., Ozpolat B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PLoS ONE. 2014;9:e110067. doi: 10.1371/journal.pone.0105245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Subramani R., Lopez-Valdez R., Arumugam A., Nandy S., Boopalan T., Lakshmanaswamy R. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS ONE. 2014;9:e97016. doi: 10.1371/journal.pone.0097016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang F., Ma L., Zhang Z., Liu X., Gao H., Zhuang Y., Yang P., Kornmann M., Tian X., Yang Y. Hedgehog signaling regulates epithelial-mesenchymal transition in pancreatic cancer stem-like cells. J. Cancer. 2016;7:408–417. doi: 10.7150/jca.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu C., Huang H., Wang C., Kong Y., Zhang H. Involvement of ephrin receptor A4 in pancreatic cancer cell motility and invasion. Oncol. Lett. 2014;7:2165–2169. doi: 10.3892/ol.2014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tekin C., Shi K., Daalhuisen J.B., Ten Brink M.S., Bijlsma M.F., Spek C.A. PAR1 signaling on tumor cells limits tumor growth by maintaining a mesenchymal phenotype in pancreatic cancer. Oncotarget. 2018;9:32010–32023. doi: 10.18632/oncotarget.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miura S., Hamada S., Masamune A., Satoh K., Shimosegawa T. CUB-domain containing protein 1 represses the epithelial phenotype of pancreatic cancer cells. Exp. Cell Res. 2014;321:209–218. doi: 10.1016/j.yexcr.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 132.Muniyan S., Haridas D., Chugh S., Rachagani S., Lakshmanan I., Gupta S., Seshacharyulu P., Smith L.M., Ponnusamy M.P., Batra S.K. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer. 2016;7:110–124. doi: 10.18632/genesandcancer.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Belvedere R., Bizzarro V., Forte G., Dal Piaz F., Parente L., Petrella A. Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of Formyl Peptide Receptor pathway. Sci. Rep. 2016;6:29660. doi: 10.1038/srep29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Belvedere R., Saggese P., Pessolano E., Memoli D., Bizzarro V., Rizzo F., Parente L., Weisz A., Petrella A. miR-196a Is able to restore the aggressive phenotype of annexin a1 knock-out in pancreatic cancer cells by CRISPR/Cas9 genome editing. Int. J. Mol. Sci. 2018;19:1967. doi: 10.3390/ijms19071967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zheng B., Ohuchida K., Cui L., Zhao M., Shindo K., Fujiwara K., Manabe T., Torata N., Moriyama T., Miyasaka Y., et al. TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma is involved in migration and invasion of cancer cells. Int. J. Oncol. 2015;47:490–498. doi: 10.3892/ijo.2015.3022. [DOI] [PubMed] [Google Scholar]
- 136.Ye C., Tian X., Yue G., Yan L., Guan X., Wang S., Hao C. Suppression of CD26 inhibits growth and metastasis of pancreatic cancer. Tumour Biol. 2016;37:15677–15686. doi: 10.1007/s13277-016-5315-4. [DOI] [PubMed] [Google Scholar]
- 137.Liu M., Yang J., Zhang Y., Zhou Z., Cui X., Zhang L., Fung K.M., Zheng W., Allard F.D., Yee E.U., et al. ZIP4 promotes pancreatic cancer progression by repressing ZO-1 and claudin-1 through a ZEB1-dependent transcriptional mechanism. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:3186–3196. doi: 10.1158/1078-0432.CCR-18-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang S. WAVE3 promotes proliferation, migration and invasion via the AKT pathway in pancreatic cancer. Int. J. Oncol. 2018;53:672–684. doi: 10.3892/ijo.2018.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Su H.T., Weng C.C., Hsiao P.J., Chen L.H., Kuo T.L., Chen Y.W., Kuo K.K., Cheng K.H. Stem cell marker nestin is critical for TGF-beta1-mediated tumor progression in pancreatic cancer. Mol. Cancer Res. MCR. 2013;11:768–779. doi: 10.1158/1541-7786.MCR-12-0511. [DOI] [PubMed] [Google Scholar]
- 140.Hagio M., Matsuda Y., Suzuki T., Ishiwata T. Nestin regulates epithelial-mesenchymal transition marker expression in pancreatic ductal adenocarcinoma cell lines. Mol. Clin. Oncol. 2013;1:83–87. doi: 10.3892/mco.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Razidlo G.L., Wang Y., Chen J., Krueger E.W., Billadeau D.D., McNiven M.A. Dynamin 2 potentiates invasive migration of pancreatic tumor cells through stabilization of the Rac1 GEF Vav1. Dev. Cell. 2013;24:573–585. doi: 10.1016/j.devcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eppinga R.D., Krueger E.W., Weller S.G., Zhang L., Cao H., McNiven M.A. Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene. 2012;31:1228–1241. doi: 10.1038/onc.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Igarashi T., Araki K., Yokobori T., Altan B., Yamanaka T., Ishii N., Tsukagoshi M., Watanabe A., Kubo N., Handa T., et al. Association of RAB5 overexpression in pancreatic cancer with cancer progression and poor prognosis via E-cadherin suppression. Oncotarget. 2017;8:12290–12300. doi: 10.18632/oncotarget.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ashour A.A., Gurbuz N., Alpay S.N., Abdel-Aziz A.A., Mansour A.M., Huo L., Ozpolat B. Elongation factor-2 kinase regulates TG2/beta1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion. J. Cell. Mol. Med. 2014;18:2235–2251. doi: 10.1111/jcmm.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng L., Zhou Z., He Z. Knockdown of PFTK1 inhibits tumor cell proliferation, invasion and epithelial-to-mesenchymal transition in pancreatic cancer. Int. J. Clin. Exp. Pathol. 2015;8:14005–14012. [PMC free article] [PubMed] [Google Scholar]
- 146.Tactacan C.M., Phua Y.W., Liu L., Zhang L., Humphrey E.S., Cowley M., Pinese M., Biankin A.V., Daly R.J. The pseudokinase SgK223 promotes invasion of pancreatic ductal epithelial cells through JAK1/Stat3 signaling. Mol. Cancer. 2015;14:139. doi: 10.1186/s12943-015-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Santoro R., Zanotto M., Carbone C., Piro G., Tortora G., Melisi D. MEKK3 sustains EMT and stemness in pancreatic cancer by regulating YAP and TAZ transcriptional activity. Anticancer Res. 2018;38:1937–1946. doi: 10.21873/anticanres.12431. [DOI] [PubMed] [Google Scholar]
- 148.Ungefroren H., Sebens S., Giehl K., Helm O., Groth S., Fandrich F., Rocken C., Sipos B., Lehnert H., Gieseler F. Rac1b negatively regulates TGF-beta1-induced cell motility in pancreatic ductal epithelial cells by suppressing Smad signaling. Oncotarget. 2014;5:277–290. doi: 10.18632/oncotarget.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ning Z., Wang A., Liang J., Xie Y., Liu J., Yan Q., Wang Z. USP22 promotes epithelial-mesenchymal transition via the FAK pathway in pancreatic cancer cells. Oncol. Rep. 2014;32:1451–1458. doi: 10.3892/or.2014.3354. [DOI] [PubMed] [Google Scholar]
- 150.Strnadel J., Choi S., Fujimura K., Wang H., Zhang W., Wyse M., Wright T., Gross E., Peinado C., Park H.W., et al. eIF5A-PEAK1 signaling regulates YAP1/TAZ protein expression and pancreatic cancer cell growth. Cancer Res. 2017;77:1997–2007. doi: 10.1158/0008-5472.CAN-16-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu L., Zhao Q. Hypoxia-inducible factor 1alpha participates in hypoxia-induced epithelial-mesenchymal transition via response gene to complement 32. Exp. Ther. Med. 2017;14:1825–1831. doi: 10.3892/etm.2017.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mody H.R., Hung S.W., Naidu K., Lee H., Gilbert C.A., Hoang T.T., Pathak R.K., Manoharan R., Muruganandan S., Govindarajan R. SET contributes to the epithelial-mesenchymal transition of pancreatic cancer. Oncotarget. 2017;8:67966–67979. doi: 10.18632/oncotarget.19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meng Q., Shi S., Liang C., Liang D., Hua J., Zhang B., Xu J., Yu X. Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3beta/Snail signaling. Oncogene. 2018;37:5843. doi: 10.1038/s41388-018-0392-z. [DOI] [PubMed] [Google Scholar]
- 154.Shinke G., Yamada D., Eguchi H., Iwagami Y., Asaoka T., Noda T., Wada H., Kawamoto K., Gotoh K., Kobayashi S., et al. Role of histone deacetylase 1 in distant metastasis of pancreatic ductal cancer. Cancer Sci. 2018;109:2520–2531. doi: 10.1111/cas.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mishra V.K. Histone deacetylase class-I inhibition promotes epithelial gene expression in pancreatic cancer cells in a BRD4- and MYC-dependent manner. Nucleic Acids Res. 2017;45:6334–6349. doi: 10.1093/nar/gkx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ma C., Guo Y., Zhang Y., Duo A., Jia Y., Liu C., Li B. PAFAH1B2 is a HIF1a target gene and promotes metastasis in pancreatic cancer. Biochem. Biophys. Res. Commun. 2018;501:654–660. doi: 10.1016/j.bbrc.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 157.Li S., Wu L., Wang Q., Li Y., Wang X. KDM4B promotes epithelial-mesenchymal transition through up-regulation of ZEB1 in pancreatic cancer. Acta Biochim. Biophys. Sin. 2015;47:997–1004. doi: 10.1093/abbs/gmv107. [DOI] [PubMed] [Google Scholar]
- 158.Zhang W.L., Zhang J.H., Wu X.Z., Yan T., Lv W. miR-15b promotes epithelial-mesenchymal transition by inhibiting SMURF2 in pancreatic cancer. Int. J. Oncol. 2015;47:1043–1053. doi: 10.3892/ijo.2015.3076. [DOI] [PubMed] [Google Scholar]
- 159.Zhang J.Q., Chen S., Gu J.N., Zhu Y., Zhan Q., Cheng D.F., Chen H., Deng X.X., Shen B.Y., Peng C.H. MicroRNA-300 promotes apoptosis and inhibits proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/beta-catenin signaling pathway by targeting CUL4B in pancreatic cancer cells. J. Cell. Biochem. 2017;119:1027–1040. doi: 10.1002/jcb.26270. [DOI] [PubMed] [Google Scholar]
- 160.Viotti M., Wilson C., McCleland M., Koeppen H., Haley B., Jhunjhunwala S., Klijn C., Modrusan Z., Arnott D., Classon M., et al. SUV420H2 is an epigenetic regulator of epithelial/mesenchymal states in pancreatic cancer. J. Cell Biol. 2017;217:763–777. doi: 10.1083/jcb.201705031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hiraga R., Kato M., Miyagawa S., Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431–4438. [PubMed] [Google Scholar]
- 162.Tan J., You Y., Xu T., Yu P., Wu D., Deng H., Zhang Y., Bie P. Par-4 downregulation confers cisplatin resistance in pancreatic cancer cells via PI3K/Akt pathway-dependent EMT. Toxicol. Lett. 2014;224:7–15. doi: 10.1016/j.toxlet.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 163.Zhao J., Wang Y., Wu X. HMGN5 promotes proliferation and invasion via the activation of Wnt/β-catenin signaling pathway in pancreatic ductal adenocarcinoma. Oncol. Lett. 2018;16:4013–4019. doi: 10.3892/ol.2018.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Song Y.X., Xu Z.C., Li H.L., Yang P.L., Du J.K., Xu J. Overexpression of GP73 promotes cell invasion, migration and metastasis by inducing epithelial-mesenchymal transition in pancreatic cancer. Pancreatology. 2018;18:812–821. doi: 10.1016/j.pan.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 165.Xu X., Su B., Xie C., Wei S., Zhou Y., Liu H., Dai W., Cheng P., Wang F., Xu X., et al. Sonic hedgehog-Gli1 signaling pathway regulates the epithelial mesenchymal transition (EMT) by mediating a new target gene, S100A4, in pancreatic cancer cells. PLoS ONE. 2014;9:e96441. doi: 10.1371/journal.pone.0096441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Inaguma S., Kasai K., Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene. 2011;30:714–723. doi: 10.1038/onc.2010.459. [DOI] [PubMed] [Google Scholar]
- 167.Nagai S., Nakamura M., Yanai K., Wada J., Akiyoshi T., Nakashima H., Ohuchida K., Sato N., Tanaka M., Katano M. Gli1 contributes to the invasiveness of pancreatic cancer through matrix metalloproteinase-9 activation. Cancer Sci. 2008;99:1377–1384. doi: 10.1111/j.1349-7006.2008.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Inaguma S., Kasai K., Hashimoto M., Ikeda H. GLI1 modulates EMT in pancreatic cancer—Letter. Cancer Res. 2012;72:3702–3703. doi: 10.1158/0008-5472.CAN-12-0379. [DOI] [PubMed] [Google Scholar]
- 169.Huang C., Xie D., Cui J., Li Q., Gao Y., Xie K. FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014;20:1477–1488. doi: 10.1158/1078-0432.CCR-13-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xie D., Cui J., Xia T., Jia Z., Wang L., Wei W., Zhu A., Gao Y., Xie K., Quan M. Hippo transducer TAZ promotes epithelial mesenchymal transition and supports pancreatic cancer progression. Oncotarget. 2015;6:35949–35963. doi: 10.18632/oncotarget.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yuan Y., Li D., Li H., Wang L., Tian G., Dong Y. YAP overexpression promotes the epithelial-mesenchymal transition and chemoresistance in pancreatic cancer cells. Mol. Med. Rep. 2016;13:237–242. doi: 10.3892/mmr.2015.4550. [DOI] [PubMed] [Google Scholar]
- 172.Chen K., Qian W., Li J., Jiang Z., Cheng L., Yan B., Cao J., Sun L., Zhou C., Lei M., et al. Loss of AMPK activation promotes the invasion and metastasis of pancreatic cancer through an HSF1-dependent pathway. Mol. Oncol. 2017;11:1475–1492. doi: 10.1002/1878-0261.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Subramani R., Camacho F.A., Levin C.I., Flores K., Clift A., Galvez A., Terres M., Rivera S., Kolli S.N., Dodderer J., et al. FOXC1 plays a crucial role in the growth of pancreatic cancer. Oncogenesis. 2018;7:52. doi: 10.1038/s41389-018-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li X., Chen H., Liu Z., Ye Z., Gou S., Wang C. Overexpression of MIST1 reverses the epithelial-mesenchymal transition and reduces the tumorigenicity of pancreatic cancer cells via the Snail/E-cadherin pathway. Cancer Lett. 2018;431:96–104. doi: 10.1016/j.canlet.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 175.Yi X., Zai H., Long X., Wang X., Li W., Li Y. Kruppel-like factor 8 induces epithelial-to-mesenchymal transition and promotes invasion of pancreatic cancer cells through transcriptional activation of four and a half LIM-only protein 2. Oncol. Lett. 2017;14:4883–4889. doi: 10.3892/ol.2017.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thakur A.K., Nigri J., Lac S., Leca J., Bressy C., Berthezene P., Bartholin L., Chan P., Calvo E., Iovanna J.L., et al. TAp73 loss favors Smad-independent TGF-β signaling that drives EMT in pancreatic ductal adenocarcinoma. Cell Death Differ. 2016;23:1358–1370. doi: 10.1038/cdd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takano S., Reichert M., Bakir B., Das K.K., Nishida T., Miyazaki M., Heeg S., Collins M.A., Marchand B., Hicks P.D., et al. Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 2016;30:233–247. doi: 10.1101/gad.263327.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu C., Zhan L., Jiang J., Pan Y., Zhang H., Li X., Pen F., Wang M., Qin R., Sun C. KAP-1 is overexpressed and correlates with increased metastatic ability and tumorigenicity in pancreatic cancer. Med. Oncol. (Northwood Lond. Engl.) 2014;31:25. doi: 10.1007/s12032-014-0025-5. [DOI] [PubMed] [Google Scholar]
- 179.Li C., Wang Z., Chen Y., Zhou M., Zhang H., Chen R., Shi F., Wang C., Rui Z. Transcriptional silencing of ETS-1 abrogates epithelial-mesenchymal transition resulting in reduced motility of pancreatic cancer cells. Oncol. Rep. 2015;33:559–565. doi: 10.3892/or.2014.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arfmann-Knubel S., Struck B., Genrich G., Helm O., Sipos B., Sebens S., Schafer H. The Crosstalk between Nrf2 and TGF-beta1 in the epithelial-mesenchymal transition of pancreatic duct epithelial cells. PLoS ONE. 2015;10:e0132978. doi: 10.1371/journal.pone.0132978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Roy N., Takeuchi K.K., Ruggeri J.M., Bailey P., Chang D., Li J., Leonhardt L., Puri S., Hoffman M.T., Gao S., et al. PDX1 dynamically regulates pancreatic ductal adenocarcinoma initiation and maintenance. Genes Dev. 2016;30:2669–2683. doi: 10.1101/gad.291021.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sannino G., Armbruster N., Bodenhofer M., Haerle U., Behrens D., Buchholz M., Rothbauer U., Sipos B., Schmees C. Role of BCL9L in transforming growth factor-beta (TGF-beta)-induced epithelial-to-mesenchymal-transition (EMT) and metastasis of pancreatic cancer. Oncotarget. 2016;7:73725–73738. doi: 10.18632/oncotarget.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heeg S., Das K.K., Reichert M., Bakir B., Takano S., Caspers J., Aiello N.M., Wu K., Neesse A., Maitra A., et al. ETS-Transcription Factor ETV1 Regulates Stromal Expansion and Metastasis in Pancreatic Cancer. Gastroenterology. 2016;151:540–553.e14. doi: 10.1053/j.gastro.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang J., Zhang X., Zhang Y., Zhu D., Zhang L., Li Y., Zhu Y., Li D., Zhou J. HIF-2alpha promotes epithelial-mesenchymal transition through regulating Twist2 binding to the promoter of E-cadherin in pancreatic cancer. J. Exp. Clin. Cancer Res. CR. 2016;35:26. doi: 10.1186/s13046-016-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lerbs T., Bisht S., Scholch S., Pecqueux M., Kristiansen G., Schneider M., Hofmann B.T., Welsch T., Reissfelder C., Rahbari N.N., et al. Inhibition of Six1 affects tumour invasion and the expression of cancer stem cell markers in pancreatic cancer. BMC Cancer. 2017;17:249. doi: 10.1186/s12885-017-3225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nishino H., Takano S., Yoshitomi H., Suzuki K., Kagawa S., Shimazaki R., Shimizu H., Furukawa K., Miyazaki M., Ohtsuka M. Grainyhead-like 2 (GRHL2) regulates epithelial plasticity in pancreatic cancer progression. Cancer Med. 2017;6:2686–2696. doi: 10.1002/cam4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martinelli P., Carrillo-de Santa Pau E., Cox T., Sainz B., Jr., Dusetti N., Greenhalf W., Rinaldi L., Costello E., Ghaneh P., Malats N., et al. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut. 2017;66:1665–1676. doi: 10.1136/gutjnl-2015-311256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dai S., Zhang J., Huang S., Lou B., Fang B., Ye T., Huang X., Chen B., Zhou M. HNRNPA2B1 regulates the epithelial-mesenchymal transition in pancreatic cancer cells through the ERK/snail signaling pathway. Cancer Cell. Int. 2017;17:12. doi: 10.1186/s12935-016-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen J., Sun Y., Xu X., Wang D., He J., Zhou H., Lu Y., Zeng J., Du F., Gong A., et al. YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16:2259–2271. doi: 10.1080/15384101.2017.1380125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao L., Sun H., Kong H., Chen Z., Chen B., Zhou M. The Lncrna-TUG1/EZH2 Axis promotes pancreatic cancer cell proliferation, migration and EMT phenotype formation through sponging Mir-382. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;42:2145–2158. doi: 10.1159/000479990. [DOI] [PubMed] [Google Scholar]
- 191.Gao Y., Zhang Z., Li K., Gong L., Yang Q., Huang X., Hong C., Ding M., Yang H. Linc-DYNC2H1-4 promotes EMT and CSC phenotypes by acting as a sponge of miR-145 in pancreatic cancer cells. Cell Death Dis. 2017;8:e2924. doi: 10.1038/cddis.2017.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu G., Li Z., Jiang P., Zhang X., Xu Y., Chen K., Li X. MicroRNA-23a promotes pancreatic cancer metastasis by targeting epithelial splicing regulator protein 1. Oncotarget. 2017;8:82854–82871. doi: 10.18632/oncotarget.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li H., Wang X., Wen C., Huo Z., Wang W., Zhan Q., Cheng D., Chen H., Deng X., Peng C., et al. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol. Cancer. 2017;16:169. doi: 10.1186/s12943-017-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ma C., Nong K., Zhu H., Wang W., Huang X., Yuan Z., Ai K. H19 promotes pancreatic cancer metastasis by derepressing let-7’s suppression on its target HMGA2-mediated EMT. Tumor Biol. 2014;35:9163–9169. doi: 10.1007/s13277-014-2185-5. [DOI] [PubMed] [Google Scholar]
- 195.Zhan H.X., Wang Y., Li C., Xu J.W., Zhou B., Zhu J.K., Han H.F., Wang L., Wang Y.S., Hu S.Y. LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer Lett. 2016;374:261–271. doi: 10.1016/j.canlet.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 196.Hu J., Li L., Chen H., Zhang G., Liu H., Kong R., Chen H., Wang Y., Li Y., Tian F., et al. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:807. doi: 10.1038/s41419-018-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li N., Wang C., Zhang P., You S. Emodin inhibits pancreatic cancer EMT and invasion by up-regulating microRNA-1271. Mol. Med. Rep. 2018;18:3366–3374. doi: 10.3892/mmr.2018.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Little E.C., Camp E.R., Wang C., Watson P.M., Watson D.K., Cole D.J. The CaSm (LSm1) oncogene promotes transformation, chemoresistance and metastasis of pancreatic cancer cells. Oncogenesis. 2016;5:e182. doi: 10.1038/oncsis.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Suzuki K., Takano S., Yoshitomi H., Nishino H., Kagawa S., Shimizu H., Furukawa K., Miyazaki M., Ohtsuka M. Metadherin promotes metastasis by supporting putative cancer stem cell properties and epithelial plasticity in pancreatic cancer. Oncotarget. 2017;8:66098–66111. doi: 10.18632/oncotarget.19802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fabregat A., Jupe S., Matthews L., Sidiropoulos K., Gillespie M., Garapati P., Haw R., Jassal B., Korninger F., May B., et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kelder T., Pico A.R., Hanspers K., van Iersel M.P., Evelo C., Conklin B.R. Mining biological pathways using WikiPathways web services. PLoS ONE. 2009;4:e6447. doi: 10.1371/journal.pone.0006447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maresch R., Mueller S., Veltkamp C., Ollinger R., Friedrich M., Heid I., Steiger K., Weber J., Engleitner T., Barenboim M., et al. Multiplexed pancreatic genome engineering and cancer induction by transfection-based CRISPR/Cas9 delivery in mice. Nat. Commun. 2016;7:10770. doi: 10.1038/ncomms10770. [DOI] [PMC free article] [PubMed] [Google Scholar]