Abstract
This open‐label disease‐drug–drug interaction study assessed whether blockade of the interleukin (IL)‐17A pathway by secukinumab and subsequent downregulation of inflammatory cytokines like IL‐6 or high‐sensitivity C‐reactive protein affects the pharmacokinetics (PKs) of a sensitive probe substrate of the cytochrome P450 3A4 isoform (CYP3A4). The PKs of midazolam, metabolized by CYP3A4, was evaluated before and after 7 and 35 days of treatment initiation of subcutaneous secukinumab at a dose of 300 mg weekly in 24 patients with moderate‐to‐severe psoriasis. Although demonstrating the expected decrease in downstream inflammatory cytokines, secukinumab had no clinically relevant effects on the PKs of midazolam, provided substantial clinical benefit, and was generally well tolerated. In summary, blockade of IL‐17A signaling in patients with moderate‐to‐severe psoriasis does not significantly affect CYP3A4 enzyme activities and, therefore, the use of secukinumab is unlikely to influence the PKs of CYP3A4 substrates.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Secukinumab is a fully human monoclonal antibody that targets interleukin (IL)‐17A. It is approved for the treatment of psoriasis, psoriatic arthritis (PsA), and ankylosing spondylitis. It is not known whether the pharmacokinetics (PKs) of drugs metabolized by cytochrome P450 3A4 isoform (CYP4503A4) are influenced by IL‐17A or other cytokines in patients with moderate‐to‐severe psoriasis or in other patient populations.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This disease‐drug−drug interaction study investigated whether secukinumab treatment, which neutralizes the signaling of IL‐17A, affects CYP3A4 enzyme activity in patients with moderate‐severe psoriasis.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Secukinumab has no effect on CYP3A4 activity in the investigated patient population.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ These results show that secukinumab can be used in the treatment of psoriasis without significant PK interactions with drugs metabolized by CYP3A4. Due to only slightly increased cytokine levels, such as IL‐17A and IL‐6, in patients with psoriasis and patients with PsA, reduction of cytokine levels to levels observed in healthy subjects will have no impact on any of the CYP450 activities.
Secukinumab (Cosentyx; Novartis, Basel, Switzerland) is a recombinant high‐affinity fully human monoclonal antihuman interleukin‐17A (IL‐17A) antibody of the immunoglobulin 1/kappa isotype, approved for the treatment of moderate‐to‐severe psoriasis, ankylosing spondylitis (AS), and psoriatic arthritis (PsA).
Secukinumab binds to human IL‐17A and neutralizes the bioactivity of this cytokine. IL‐17A is the cornerstone cytokine of a subset of inflammatory T cells, the Th17 cells that, in several animal models, are pivotal for several autoimmune and inflammatory processes. IL‐17A is also produced by memory effector CD4+, and CD8+ T lymphocytes. IL‐17A is recognized as one of the principal pro‐inflammatory cytokines in autoimmune diseases, such as psoriasis, AS, and PsA. Its neutralization can treat the underlying structural features of immune‐mediated disease, such as inflammation and tissue destruction, as well as providing symptom relief.1, 2, 3
Therapeutic proteins (TPs), like secukinumab, typically do not undergo metabolism or involve transporters in their clearance pathway; therefore, the potential is limited for small molecule drugs to affect the pharmacokinetics (PK) of TPs through metabolism or transport pathways.
Inflammation is associated with elevation of pro‐inflammatory cytokines, including IL‐1β, IL‐17A, IL‐6, C‐reactive protein (CRP), and tumor necrosis factor (TNF‐α).4, 5 Hepatocytes have receptors for those cytokines. Elevated cytokine levels can downregulate the expression level of cytochrome P450 (CYP) isoenzymes, including CYP3A4.4, 6, 7, 8, 9 As an example, increased Il‐6 and TNF‐α downregulate CYP‐metabolizing enzymes and transporters, such as CYP3A and P‐glycoprotein, in patients with rheumatoid arthritis.6, 8 In clinical pharmacology studies with secukinumab, it was observed that IL‐6 and CRP levels decreased after the start of treatment in parallel to therapeutic effect. By reducing inflammation through blocking of IL‐17A and consequently reducing IL‐6 and CRP levels in patients with psoriasis, secukinumab might increase/restore the expression level of CYP3A4 and thereby decrease the exposure to concomitant medication that are metabolized by CYP3A4. Further, although no direct evidence exists from in vitro assays or literature, a direct effect of neutralizing free IL‐17A on CYP3A4 activity and expression cannot be excluded.10 Therefore, neutralization of pro‐inflammatory cytokines—being the scope of many marketed TPs—might restore activity and expression of CYP isozymes, including CYP3A4, CYP2C19, CYP1A2, CYP2B6, CYP2C9, CYP2D6, and CYP2E1 as the major drug‐metabolizing enymes.4
The primary objective of this disease‐drug–drug interaction (DDDI) study was to investigate the effect of secukinumab on the PKs of the sensitive CYP3A4 substrate midazolam in patients with moderate‐to‐severe psoriasis. Another objective of the study was to explore the pharmacodynamic (PD) effects of secukinumab on cytokine concentrations (IL‐6 and other cytokines) and their impact on PKs of midazolam in these patients. Assuming that CYP3A4 levels could be decreased in patients with psoriasis due to high levels of IL‐6 or other proinflammatory cytokines, secukinumab may restore CYP3A4 activity back to levels in healthy subjects by reversing the downregulating effect of cytokines. Consequently, a decrease in midazolam plasma exposure would be expected after initiation of secukinumab treatment.8 The study was conducted to support guidance on dosing recommendations for concomitant use of secukinumab and substrates of CYP3A4. We decided to focus in this study on CYP3A4 activity only, because it is the most prominent drug‐metabolizing enzyme and the majority of published evidence points to CYP3A as the major culprit of disease drug interactions via pro‐inflammatory cytokines.
RESULTS
Subject disposition, demographics, and baseline characteristics
A total of 25 patients were enrolled in the study, of which 1 patient withdrew consent before secukinumab was administered.
There were 13 male (54.2%) and 11 female (45.8%) patients treated with secukinumab. The mean age of the patients was 44.3 years (range 18–68 years), mean weight 100.3 kg (range 69.2–159.3 kg), and mean body mass index 33.94 (range 25.3–52.0). Patients were predominantly white (87.5%). Eight of 24 patients (33.3%) had received an anti‐TNF treatment (etanercept or adalimumab), 2 of 24 patients (8.3%) had received an anti‐IL‐12/23 treatment (ustekinumab), 13 of 24 patients (54.2%) had received other systemic treatments (e.g., methotrexate, methylprednisolone, triamcinolone acetonide, efalizumab, and apremilast). Eight of 24 patients (33.3%) had no previous systemic treatment.
Midazolam PK
As compared with midazolam alone before secukinumab administration on day 1, the peak plasma concentration (Cmax) and the area under the plasma concentration‐vs.‐time curve (AUC) for midazolam remained unchanged at 1 week (day 8) and at 5 weeks (day 36) after start of secukinumab treatment (Table 1 and Figure 1). All other PK parameters, including time of maximum plasma concentration (Tmax), terminal half‐life (t1/2), total clearance (CL/F), and apparent volume of distribution based on the terminal phase (Vz/F) were also similar at the three profiling days. Due to irregularities in some of the individual PK profiles, calculation of PK parameters, like Cmax, AUC 0–12h hours (AUC0–12 h), and/or AUC to infinity (AUCinf) was not feasible in these patients. Therefore, the number of patients with reportable PK parameters is less than the total number of patients included in the PK analysis set.
Table 1.
Summary statistics of midazolam plasma PK parameter values
| Profile day | Statistic | Cmax (ng/mL) | AUC0–12 h (hour·ng/mL) | AUCinf (hour·ng/mL) | t1/2 (h) | Tmax a (h) | Vz/F (L) | CL/F (L/h) |
|---|---|---|---|---|---|---|---|---|
| Baseline | N | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| Mean (SD) | 26.2 (9.31) | 77.0 (30.6) | 88.1 (35.4) | 4.72 (1.55) | 0.500 (0.250−1.97) | 444 (243) | 64.9 (22.9) | |
| CV% mean | 35.5 | 39.7 | 40.2 | 32.8 | 60.6 | 54.8 | 35.4 | |
| Day 8 | n | 22 | 22 | 21 | 21 | 22 | 21 | 21 |
| Mean (SD) | 29.3 (12.4) | 74.2 (31.2) | 87.1 (39.7) | 4.82 (1.50) | 0.492 (0.217−3.00) | 440 (159) | 65.2 (19.6) | |
| CV% mean | 42.3 | 42.0 | 45.6 | 31.2 | 109.6 | 36.2 | 30.1 | |
| Day 36 | n | 21 | 21 | 20 | 21 | 21 | 20 | 20 |
| Mean (SD) | 25.5 (11.4) | 70.5 (23.2) | 84.0 (27.8) | 4.82 (1.43) | 0.567 (0.233−2.07) | 436 (158) | 66.9 (25.6) | |
| CV% mean | 44.8 | 33.0 | 33.1 | 29.6 | 70.5 | 36.2 | 38.2 |
AUC0−12 h, 0–12‐hour area under the concentration‐time curve; AUCinf, area under the concentration‐time curve to infinity; CL/F, total clearance; Cmax, peak plasma concentration; CV%, percentage of coefficient of variation; PK, pharmacokinetic; t1/2, terminal half‐life; Tmax, time of maximum plasma concentration; Vz/F, volume of distribution based on the terminal phase.
Median and range is given for Tmax.
Figure 1.
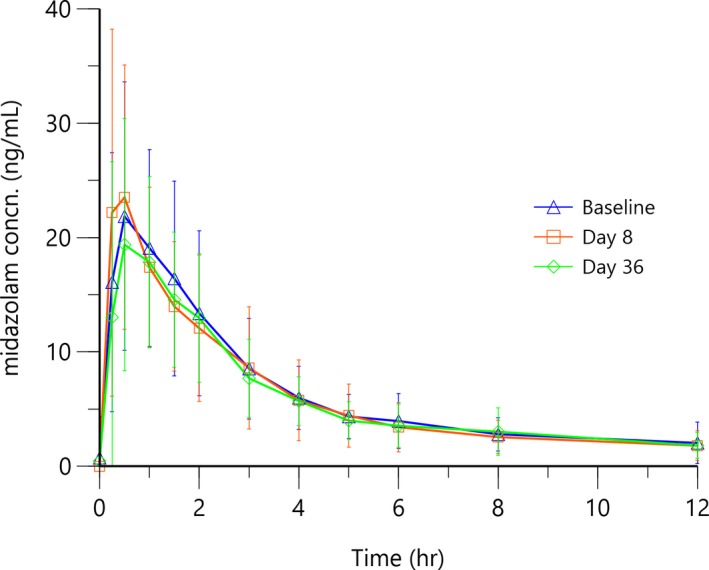
Arithmetic mean (SD) midazolam plasma pharmacokinetic concentration (concn)‐time profiles after administration of midazolam 5 mg at baseline (before secukinumab administration) and at day 8, and at day 36 during secukinumab treatment.
Back‐transformed estimates (Table 2) in an analysis of variance of a potential secukinumab effect on midazolam PK revealed that day 8/baseline mean effect ratios for midazolam Cmax, AUC0–12 h, and AUCinf were 1.09 (90% CI, 0.94–1.28), 0.97 (90% CI, 0.87–1.09), and 0.99 (90% CI, 0.88–1.12), respectively. Day 36/baseline mean effect ratios for midazolam Cmax, AUC0–12 h, and AUCinf were 0.94 (90% CI, 0.80–1.10), 0.93 (90% CI, 0.83–1.05), and 0.97 (90% CI, 0.85–1.09), respectively. In all cases, except for the geometric Cmax ratio, day 8 vs. baseline, the geometric mean ratios and 90% CIs were very close to 1 and between the bioequivalence boundaries of 80% and 125%, respectively.
Table 2.
Geometric mean ratio (midazolam and secukinumab/midazolam) and 90% CIs for PK parameters (PK analysis set)
| Parameter | Visit | n a | Adjusted geometric mean | Comparison (day/baseline) | Geometric mean ratio | 90% CI for ratio |
|---|---|---|---|---|---|---|
| AUC0–12 h (hour·ng/mL) | Baseline | 22 | 71.84 | |||
| Day 8 | 22 | 69.90 | Day 8 vs. baseline | 0.97 | 0.87−1.09 | |
| Day 36 | 21 | 67.11 | Day 36 vs. baseline | 0.93 | 0.83−1.05 | |
| AUCinf (hour·ng/mL) | Baseline | 22 | 82.17 | |||
| Day 8 | 21 | 81.70 | Day 8 vs. baseline | 0.99 | 0.88−1.12 | |
| Day 36 | 20 | 79.35 | Day 36 vs. baseline | 0.97 | 0.85−1.09 | |
| Cmax (ng/mL) | Baseline | 22 | 24.81 | |||
| Day 8 | 22 | 27.13 | Day 8 vs. baseline | 1.09 | 0.94−1.28 | |
| Day 36 | 21 | 23.26 | Day 36 vs. baseline | 0.94 | 0.80−1.10 |
AUC0–12 h, 0–12‐hour area under the concentration‐time curve; AUCinf, area under the concentration‐time curve to infinity; CI, confidence interval; Cmax, peak plasma concentration; PK, pharmacokinetic.
n = number of patients with nonmissing values. The log transformed primary PK parameters of Cmax, AUC0–12 h, and AUCinf were analyzed using a mixed effect model with day as fixed and patients as random effect. Midazolam 5 mg was administered on baseline (reference), day 8 (test), and day 36 (test).
Secukinumab PK
The arithmetic mean of secukinumab trough concentrations is shown in Figure 2. The trough PK concentrations for secukinumab increased with time during the first 5 weeks. This PK behavior is consistent with the 5 weekly doses during induction in the first month of the study. Maximum concentration levels were reached at week 5 and then gradually decreased until week 24 with steady‐state trough concentrations thereafter (not shown here).11
Figure 2.
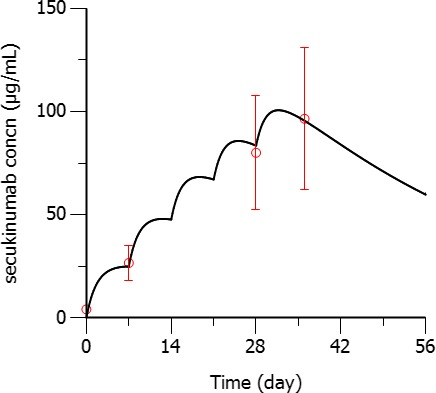
Arithmetic mean (SD) secukinumab serum concentrations (concn; circles) at week 1 (7 days post‐first dose, pre‐second dose), week 4 (pre‐fifth dose administration), and week 5 (7 days post‐fifth dose administration) compared with predicted pharmacokinetics in patients with psoriasis (black solid line). [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
Assessment of psoriasis area and severity index
At the screening visit, the mean (SD) Psoriasis Area and Severity Index (PASI) score was 18.20 (4.17), and at baseline it was 19.52 (4.03). All patients had a PASI score of >12. At week 5, the mean (SD) PASI score was 4.29 (3.09), whereas at week 24 the mean (SD) PASI score was 2.07 (2.61). As shown in Figure 3, a rapid decrease of PASI score was observed after treatment commenced. The outlying values are due to one patient who had persistently high PASI scores compared with all other patients whose values were consistently lower. At week 5, 60.9% of patients (n = 14) reached a PASI 75 response, whereas 21.7% of patients (n = 5) reached PASI 90 response. At week 24, 73.7% of patients (n = 14) reached a PASI 75 response, whereas 63.2% of patients (n = 12) reached a PASI 90 response.
Figure 3.
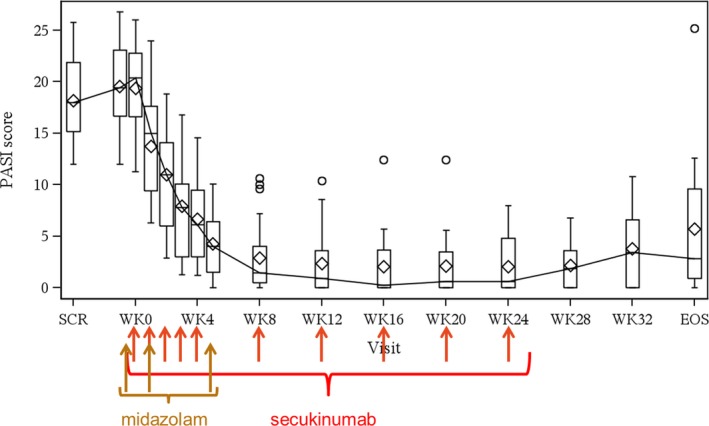
Boxplot of Psoriasis Area and Severity Index (PASI) score during secukinumab treatment. Medians are presented with a line and connected over time. Means are presented with diamonds. EOS, end of study; SCR, screening; WK, week. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
PD biomarker assessments
Arithmetic mean (SE) plots of serum levels of high‐sensitivity C‐reactive protein (hsCRP), IL‐6, TNF‐α, and beta‐defensin‐2 (BD‐2) from screening until week 24 by visit are depicted in Figure 4. The hsCRP, IL‐6, TNF‐α, and BD‐2 were all decreased following secukinumab treatment, with maximum reductions observed at week 1 (hsCRP), week 12 (BD‐2), and week 24 (IL‐6 and TNF‐α). There was no observed consistent change in IL‐1β, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), interferon gamma (IFN‐γ), IL‐2, IL‐4, IL‐5, IL‐8, and IL‐10 (results not shown here).
Figure 4.
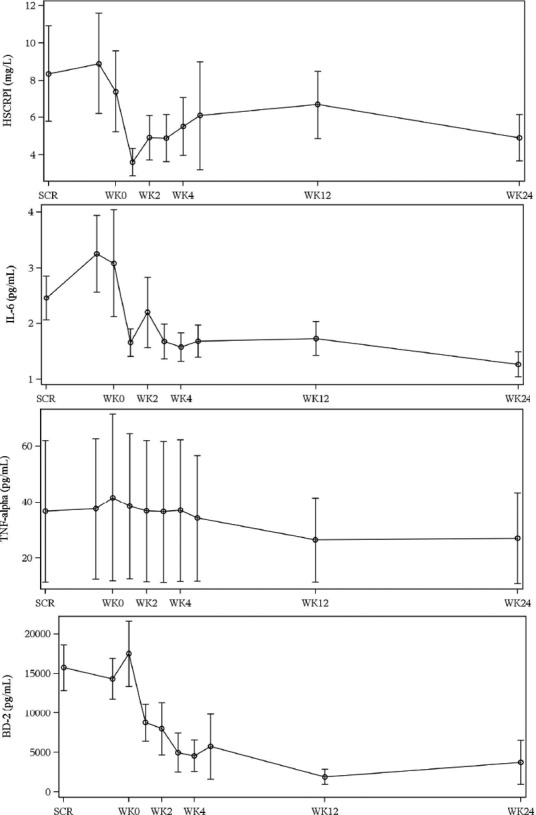
Arithmetic mean (SE) plots of high‐sensitivity C‐reactive protein (hsCRP; mg/L), interleukin (IL)‐6 (pg/mL), tumor necrosis factor (TNF)‐alpha (pg/mL), and beta‐defensin‐2 (BD‐2, pg/mL) from screening (SCR) until week (WK) 24. Very high value of hsCRP observed in one patient (67.8 mg/L on day 39/week 5) is concomitant to the adverse event of “Pharyngitis streptococcal” (days 38−42). Another patient had relatively high values of hsCRP (9.2, 5, and 31.3 mg/L at week 2, week 3, and week 4, respectively) when he presented a concomitant adverse event of “upper respiratory tract infection” (days 14−24). The high SE for TNF‐α was due to one patient who had abnormal values compared with other patients whose values are very consistent.
Mean free IL‐17A median concentrations as measured at three independent timepoints before secukinumab treatment were low and stable at screening (0.93 pg/mL), baseline (0.88 pg/mL), and at week 0 (0.64 pg/mL). See clinical study design in Figure 5 for definition of timepoints. Accumulation of total IL‐17A median concentrations was observed after initiation of secukinumab treatment and a plateau was reached, as published before,11 with this therapeutic regimen. The increase in concentrations after the start of treatment is due to slowing of IL‐17A clearance after binding to secukinumab, and is in line with expectations.
Safety
No deaths or serious adverse events (AEs) were reported. After baseline, of all patients exposed to study medication (secukinumab 300 mg + midazolam 5 mg), 16 patients (66.7%) experienced at least one treatment‐emergent AE. Overall, 48 AEs were reported in 16 patients after the start of the study medications (midazolam and secukinumab). No trends were noted in the types of AEs reported. Most frequently reported AEs were dehydration (n = 2, 8.3%) and headache (n = 2, 8.3%). For the rest, all other AEs occurred only in one patient each. The most commonly affected primary system organ classes were infections and infestations (n = 6; 25.0%), musculoskeletal and connective tissue disorders (n = 3; 12.5%), and nervous system disorders (n = 3; 12.5%). The rest of the system organ classes had AEs occurring in <2 patients.
Immunogenicity data were collected at baseline, day 59, and at the end of the study visit. Secukinumab serum concentrations were below the level of drug tolerance (53.8 μg/mL) for a polyclonal positive control anti‐secukinumab antibody in all samples.12 No anti‐secukinumab antibodies were found in serum for any of the patients.
DISCUSSION
Midazolam Cmax and AUC values at baseline were similar to those reported in healthy subjects. The administration of 5 mg midazolam to patients with psoriasis resulted in very similar plasma concentrations of midazolam compared with those reported after administration to healthy persons. After an oral administration of 15 mg to healthy subjects,13 mean AUCinf and Cmax were 277 ng·hour/mL and 69.5 ng/mL, respectively, which was approximately threefold higher than the AUCinf of 88.1 ng·hour/mL and Cmax of 26.2 ng/mL after the oral dose of 5 mg in this study. This strongly suggests that the level of CYP3A4 activity in the psoriasis disease state is close to the level in healthy subjects.
In this study, a potential CYP modulation was specifically investigated for the impact of the anti‐IL‐17A antibody secukinumab on the PK of CYP3A4 substrate midazolam. Five weekly subcutaneous administrations of 300 mg secukinumab led to expected serum secukinumab concentrations. At the highest mean secukinumab concentration of 96.5 μg/mL, as measured at week 5 (i.e., 1 week after the fifth secukinumab dose at week 4), the third dose of midazolam was administered.
Midazolam exposure did not change compared with baseline, following treatment with secukinumab and disease condition improvement, neither at the first week nor at 5 weeks after the start of treatment with secukinumab. All 90% CIs, except for Cmax day 8/baseline ratio, were within the range of 0.80–1.25, demonstrating that secukinumab treatment had no effect on the PKs of midazolam in patients with moderate‐to‐severe psoriasis.
PASI and Investigator's Global Assessment modified 2011 scores (the latter not shown here) consistently decreased following secukinumab treatment. This was accompanied by decreases in hsCRP, BD‐2, IL‐6, and TNF‐α below baseline values following secukinumab treatment, whereas no consistent changes in IL‐1β levels were noted upon treatment. Free IL‐17A levels in patients with psoriasis before treatment were low (i.e., <1 pg/mL) and in agreement with concentrations as observed in other clinical studies and literature.14 Obviously, the only slightly increased baseline IL‐17A levels in patients with psoriasis compared with healthy subjects and the free IL‐17A suppression after start of secukinumab treatment do not have any impact on CYP3A4 activity. The increase in total IL‐17A clearly demonstrates target engagement and is in line with the slower clearance of bound IL‐17A than of free IL‐17A.
Data from this study were gathered to support dosing recommendations for concomitant use of secukinumab and substrates of CYP3A4. As described in the literature,4 altered (decreased) CYP enzyme activities and CYP‐mediated PK interactions have been observed when endogenous cytokine concentrations were increased because of an inflammatory process in the patients who were studied. As observed in patients with clearly elevated IL‐6 or TNF‐α levels, increased cytokine concentrations may inhibit CYP enzyme activities.6, 8 However, in patients with psoriasis and atopic dermatitis, the situation seems to be different. DDDI studies, such as with the anti‐IL‐4/IL‐13 antibody dupilumab in patients with moderate‐to‐severe atopic dermatitis and with the anti‐IL‐23p19 monoclonal antibodies risankizumab and tildrakizumab in patients with moderate‐to‐severe psoriasis, showed no significant effect on the PK of CYP3A4, CYP2C19, CYP2C9, CYP1A2, or CYP2D6 substrates.15, 16, 17
Patients with psoriasis have increased levels of inflammation as assessed by inflammatory biomarkers. The typical biomarker used to characterize systemic inflammation level is hsCRP. It is indeed a good reflection of the effect of inflammatory cytokines on the hepatocytes, because inflammatory cytokines are known to induce the synthesis of CRP and to reduce the CYP synthesis.5 In Rocha‐Pereira et al.,18 hsCRP was 11.6 ± 0.7 mg/L in patients with active psoriasis and 3.1 ± 0.2 mg/L in controls. The average baseline hsCRP observed in this study was 8.56 mg/L, which was clearly in the expected range for this plaque psoriasis population. In two secukinumab phase III psoriasis trials, baseline CRP ranged from 3.2−5.2 mg/L. In the subgroup of patients with an Health Assessment Questionnaire without Disability Index > 0.5, baseline CRP ranged from 4.3−9.9 mg/L.19 Elevated levels of IL‐17A, BD‐2, and other pro‐inflammatory cytokines (IL‐6, IL‐1β, and TNF‐α) might be responsible for a direct effect on hepatocytes, which would reduce the CYP3A4 activity.4, 6, 8, 9 Average TNF‐α (36.4 ± 118 pg/mL) was moderately elevated at baseline and decreased by up to −16.8% following secukinumab treatment. IL‐6 concentrations decreased up to −38.4% from baseline following secukinumab treatment and were stable until week 24. Mean CRP decreased up to −35.3% from baseline. IL‐1β did not show any change following treatment. The average values of IL‐6 concentrations were in the range of 2.5–3.3 pg/mL, which is in the low range as compared with published data in review papers4, 8 or higher when compared with observed IL‐6 levels in the DDDI trial with tildrakizumab in patients with moderate‐to‐severe psoriasis.17
So far, clinically meaningful changes in CYP activity (up to twofold) due to treatment with TPs targeting soluble cytokines have not been clinically demonstrated in PsA and AS, but might be possible based on the magnitude of change in cytokine concentrations. Normalization of CYP protein concentrations could cause circulating drug concentrations of sensitive CYP substrates to fall by more than twofold, resulting in a lack of efficacy due to drug concentrations outside the therapeutic window.
Based on results in our study, in the above‐mentioned studies, and in other recently published cocktail DDDI studies in patients with moderate‐to‐severe psoriasis16, 17 in cases of minimal systemic inflammation with only moderate increases in hsCRP, IL‐6, and TNF‐α, there will be insufficient change in CYP activity to result in any clinically significant change in plasma exposure of comedications metabolized by CYP enzymes. Therefore, dedicated DDDI studies in this patient population may not be needed anymore. In inflammatory disease states, which can be regarded as moderate to severe, such as influenza, HIV infection, rheumatoid arthritis, and Crohn's disease, a greater than twofold change in plasma exposure may occur with victim drugs that are sensitive substrates of CYP3A, CYP2C9, or CYP1A2.8 Therefore, caution should prevail when dosing sensitive CYP substrates, such as cyclosporine (CYP3A), theophylline (CYP1A2), and warfarin (CYP2C9) in patients under treatment for influenza, HIV infection, and rheumatoid arthritis, and patients with active Crohn's disease.
This study demonstrated that the therapeutic dosing regimen of secukinumab did not change plasma exposure of concomitantly administered midazolam. More specifically, no “normalization” of CYP3A4 activity to a level similar to that in healthy persons with otherwise decreasing exposure to drugs metabolized by CYP3A4 was observed. This observation has clinical relevance for patients with psoriasis who take drugs metabolized by CYP3A4 and that have a narrow therapeutic range. For those patients treated with secukinumab and concomitant CYP3A4 substrates, monitoring of these comedications with potential dose adjustments is not needed.
METHODS
Study population and design
This was an open‐label, multicenter, confirmatory study investigating a potential DDDI of secukinumab in male and female patients with moderate‐to‐severe plaque psoriasis. A total of 25 male and female patients aged at least 18 years at screening with (i) a PASI score of 12 or greater, (ii) Investigator's Global Assessment modified 2011 score of 3 or greater (based on a scale of 0–4), and (iii) body surface area affected by plaque‐type psoriasis of 10% or greater were eligible for inclusion in this study. Main exclusion criteria included other forms of psoriasis or alternative inflammatory disease, underlying disease that poses an unacceptable risk for immunomodulatory treatment, and evidence of ongoing infection or malignancy of any organ system. Any medications with known history of interactions with CYP 3A4/5 or P‐glycoprotein were prohibited from 2 months prior to the first dosing of the study treatment until the end of the study. Ongoing use of some psoriasis treatments required a washout of up to 12 weeks prior to first dosing of the study treatment. All subjects were informed about the nature and purpose of the study, participation/termination conditions, and risks and benefits of treatment. Patients provided written informed consent before any protocol‐specific procedure.
Secukinumab 150 mg/mL (Novartis, Basel, Switzerland) was supplied as open‐labeled bulk medication. It was provided in prefilled syringes with an extractable volume of 1 mL (2 syringes required for 300 mg dose). Midazolam HCl oral syrup (e.g., 2 mg/mL cherry‐flavored midazolam syrup) was sourced by the study site. The study design is shown in Figure 5. Midazolam was administered orally in the morning to allow for PK sampling over a 12‐hour period. All patients were fasted for at least 10 hours prior to administration of midazolam and remained fasted for at least 4 hours thereafter. On day 8, secukinumab was administered between 1 hour before and 1 hour after midazolam.
Figure 5.
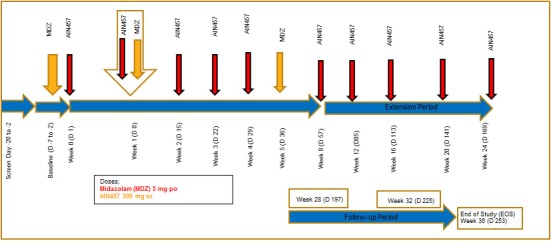
Scheme of clinical study design.
During the baseline visit, a full midazolam PK profile after a single oral dose of 5 mg was assessed. On day 1 (week 0), day 8 (week 1), day 15 (week 2), day 22 (week 3), and day 29 (week 4) patients received 300 mg s.c. secukinumab (two injections of 150 mg s.c.). On day 8 (week 1) and on day 36 (week 5), patients received additional single doses of 5 mg oral midazolam (total of three single doses).
At screening, baseline, day 1, weeks 1, 2, 3, 4, 5, 12, and 24, serum samples were collected and analyzed for a panel of inflammatory cytokines (IL‐6, TNF‐α, IL‐1β, IL‐2, IL‐4, IL‐5, IL‐8, IL‐10, GM‐CSF, and IFN‐γ), BD‐2, free IL‐17A (collected at screening, baseline, and predose day 1 visits only), total IL‐17A (starting at day 8, following initiation of secukinumab treatment), and for hsCRP. BD‐2 is a responsive biomarker of IL‐17A‐driven skin pathology in psoriasis and elevated BD‐2 levels in patients with psoriasis that decreases shortly after treatment with secukinumab.14
The dosing regimen of secukinumab was selected to reflect clinical practice. To evaluate the overall safety and tolerability of 24‐week administration of secukinumab, there was an extension period during which patients received secukinumab 300 mg s.c. monthly from weeks 8−24. Following the extended treatment period, there was a follow‐up period of 12 weeks (after the last dose administered at week 24) with visits at weeks 28, 32, and 36.
This trial was conducted in accordance with the principles of the Declaration of Helsinki and the guidelines of the International Conference on Harmonization requirements for Good Clinical Practice, and with the approval of the Schulman Associates IRB (Cincinnati, OH).
Safety and tolerability
Safety assessments were performed during the treatment and follow‐up periods. Safety assessments included physical examinations, electrocardiograms, vital signs, standard clinical laboratory evaluations (hematology, blood chemistry, and urinalysis), and monitoring of AEs and serious adverse events. For each patient, the total duration of the trial participation was ~ 40 weeks from screening to end‐of‐study visit at week 36, see Figure 1.
Study objectives
The primary objective of this trial was to investigate the effect of secukinumab on the PK of midazolam (sensitive probe substrate for CYP3A4, recommended by health authorities as an index substrate for the study of CYP3A interactions20) in patients with moderate‐to‐severe plaque psoriasis. The secondary objectives were to evaluate the overall safety and tolerability of 24‐week administration of secukinumab and of the coadministration of midazolam and secukinumab in patients with moderate‐to‐severe plaque psoriasis. An exploratory objective was to assess PK/PD relationships of secukinumab in these patients.
Sample collections
Blood samples (2 mL) for measurement of plasma concentrations of midazolam were collected into precooled (ice bath) lithium heparinized tubes before each dose and 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, and 12 hours after each dose. Within 30 minutes of collection, plasma was prepared by centrifugation of blood at ~ 2,000g at 4°C for 10 minutes.
Blood samples were collected for the measurement of serum concentrations of free IL‐17A (at screening, baseline, and day 1 pre‐secukinumab dose), total IL‐17A (on days 8, 15, 22, 29, 36, 85, and 169), other cytokines in an inflammatory panel as well as BD‐2 and hsCRP (at screening, baseline, and day 1 pre‐secukinumab dose, on days 8, 15, 22, 29, 36, 85, and 169). Blood samples for serum secukinumab measurements were collected before secukinumab administrations at baseline, day 8, day 29, and on day 36 (i.e., 1 week after dosing on day 29). Blood samples were collected into serum‐separating tubes. The blood sample was allowed to clot over a minimum of 30 minutes at room temperature prior to harvest serum. The serum was obtained by centrifugation at ~ 1,000–2,000g for 10 minutes. Patients who terminated study participation early had their last blood samples collected at the termination visits. Blood samples collected before secukinumab administration at baseline, on day 59, and day 169 were also evaluated for the presence of antidrug antibodies (ADAs) in serum.
Bioanalytical methods
Plasma midazolam concentrations were determined by a validated liquid chromatography tandem mass spectrometry assay in MRM positive mode using electrospray ionization as the ionization technique. The lower limit of quantification (LLOQ) for midazolam was 0.05 ng/mL with a calibration range up to 50 ng/mL. The precision of the midazolam assay, as determined from the analysis of quality control plasma samples, ranged from 2.7−6.7%.
Total secukinumab (i.e., free secukinumab plus secukinumab bound to IL‐17A; secukinumab–IL‐17A complex) serum concentrations were quantified using a validated enzyme‐linked immunosorbent assay with an LLOQ of 80 ng/mL. The method was based on a purified, nonneutralizing, anti‐idiotype anti‐secukinumab antibody coated on microtiter plates. Serum samples (calibration samples, quality controls, or unknown samples) and biotin‐labeled secukinumab were simultaneously incubated and competed for binding on the anti‐idiotypic anti‐secukinumab antibody. Unbound material was removed by washing. Bound biotinylated secukinumab was detected by incubating horseradish peroxidase conjugated to streptavidin with o‐phenylenediamine dihydrochloride as enzymatic substrate. The intra day accuracy and intra day precision were within the ranges 76.4−118% and 1.4−17.8%, respectively. The interaay accuracy and interday precision were within the ranges 89.6−99.0% and 7.2−15.6%, respectively. Secukinumab has been proven to be stable at −20°C for at least 20 months.
The ADA assessment followed a three‐tiered approach: screening, confirmation, and titer determination. At the timepoints of ADA assessment, all secukinumab concentrations were below the drug tolerance level, and therefore drug levels did not interfere with ADA determination.12
Serum samples were quantified for IL‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐8, IL‐10, GM‐CSF, IFN‐γ, and TNF‐α using a validated multiplexed immunoassay where analyte‐specific antibodies were precoated onto color‐coded microparticles (Luminex Performance Assay, R&D Systems). LLOQ ranged between 0.200 (IL‐5) and 45.2 (IL‐4) pg/mL. BD‐2 was measured in serum using a validated enzyme‐linked immunosorbent assay (Alpha Diagnostics) with an LLOQ of 32.5 pg/mL.
IL‐17A at baseline (i.e., in the absence of secukinumab) was quantified via a method based on a sandwich immunoassay using a biotinylated monoclonal anti‐human IL‐17A capture antibody coated onto streptavidin paramagnetic microparticles. IL‐17A in calibrators, quality control samples, or study samples binds to the immobilized antibody, and any unbound material was removed by washing. An AlexaFluor647‐labeled polyclonal antihuman IL‐17A antibody was added and unbound material was again removed by washing. Bound detection antibody was eluted and detected by a single molecule counting platform (Erenna system). The LLOQ was 0.096 pg/mL.
Total IL‐17A was quantified via a sandwich ligand binding assay. First, a saturating concentration of secukinumab was added to each calibrator, quality control sample, or study sample to form IL‐17A/secukinumab complexes. Then, all samples were added to an MSD Streptavidin Multi‐Array 96‐Well plate coated with a biotinylated antihuman IL‐17 antibody (in‐house), and IL‐17A/secukinumab complexes were captured. Because IL‐17A is a homodimer, detection of the bound complex was performed via a Fab fragment of the capturing antibody, which was labeled with Ruthenium(II) tris‐bipyridine 4‐methylsulfonate N‐hydroxysuccinimide ester, the ordinary MSD sulfoTAG label. Detection was based on electrochemiluminescence, and the LLOQ of that assay was 20 pg/mL.
PK analysis
PK parameters for midazolam were calculated from plasma concentration‐vs.‐time data, using noncompartmental methods with WinNonlin Phoenix version 6.4 (Certara, Mountain View, CA). The derived PK parameters included the maximum observed serum concentration (Cmax), time to reach Cmax (Tmax), area under the serum concentration from time zero to time of last measured concentration (AUClast), AUCinf, t1/2, CL/F, and Vz/F. The linear trapezoidal rule was used for AUC calculation. Regression analysis of the terminal serum elimination phase for the determination of t1/2 included at least three data points after Cmax. If the adjusted R 2 value of the regression analysis of the terminal phase was <0.75, no values were reported for t1/2, AUCinf, Vz/F, and CL/F.
Statistical analysis
Descriptive statistics were used to summarize serum midazolam concentrations at each scheduled sampling timepoint and to summarize the derived PK parameters of midazolam, including arithmetic mean and SD or range. The effect of secukinumab on the PK of midazolam in patients with moderate‐to‐severe plaque psoriasis was analyzed by a fixed effect model. Log transformed PK parameters, Cmax, AUC0–12 h, and AUCinf of midazolam were analyzed using a fixed effect model with day as fixed and patient as random effect. The difference in adjusted means along with 90% CIs was calculated for day 8 (week 1 (test)) and day 36 (week 5 (test)) vs. baseline (reference). The results were back transformed to the original scale to obtain adjusted geometric mean ratios (day 8 vs. baseline; day 36 vs. baseline) and the corresponding 90% CIs.
All PD biomarkers, including inflammatory panel (IL‐6, TNF‐α, IL‐1β, IL‐2, IL‐4, IL‐5, IL‐8, IL‐10, baseline and total IL‐17A, GM‐CSF, and IFN‐γ), BD‐2, and hsCRP data were listed by patient and visit/time. Summary statistics were provided by visit/time.
Immunogenicity assessment
Subjects were classified as being treatment‐emergent ADA‐positive if ADAs were detected in the sample evaluated after exposure to secukinumab accompanied by a negative sample at baseline.
Funding
This study was funded by Novartis Pharma AG.
Conflict of Interest
G.B., A.H., J.M., C.C., R.S., R.W., and B.B‐D are full‐time employees and shareholders of Novartis Pharma AG. I.K. was a Novartis employee until study completion, and is now working for Rubius Therapeutics. D.M.P. is an employee of Virginia Clinical Research. All authors declared no competing interests for this work.
Author Contributions
G.B. wrote the manuscript. G.B., R.S., R.W., and B.B.‐D. designed the research. D.M.P. performed the research. G.B., A.H., I.K., J.M., C.C., R.W., and B.B.‐D. analyzed the data.
ACKNOWLEDGMENTS
We thank the patients and clinical site staff for their contributions to this study.
References
- 1. Blauvelt, A. T‐helper 17 cells in psoriatic plaques and additional genetic links between IL‐23 and psoriasis. J. Invest. Dermatol. 128, 1064–1067 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynde, C.W. , Poulin, Y. , Vender, R. , Bourcier, M. & Khalil, S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J. Am. Acad. Dermatol. 71, 141–150 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Krueger, J.G. et al IL‐17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 130, 145–154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang, J. , Wang, C. & Ahn, H.Y. Biological products for the treatment of psoriasis: therapeutic targets, pharmacodynamics and disease‐drug–drug interaction implications. AAPS J. 16, 938–947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eklund, C.M. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 48, 111–136 (2009). [DOI] [PubMed] [Google Scholar]
- 6. Schmitt, C. , Kuhn, B. , Zhang, X. , Kivitz, A.J. & Grange, S. Disease‐drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin. Pharmacol. Ther. 89, 735–740 (2011). [DOI] [PubMed] [Google Scholar]
- 7. Gorski, J.C. , Hall, S.D. , Becker, P. , Affrime, M.B. , Cutler, D.L. & Haehner‐Daniels, B. In vivo effects of interleukin‐10 on human cytochrome P450 activity. Clin. Pharmacol. Ther. 67, 32–43 (2000). [DOI] [PubMed] [Google Scholar]
- 8. Coutant, D.E. & Hall, S.D. Disease‐drug interactions in inflammatory states via effects on CYP‐mediated drug clearance. J. Clin. Pharmacol. 58, 849–863 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Lee, J.I. , Zhang, L. , Men, A.Y. , Kenna, L.A. & Huang, S.M. CYP‐mediated therapeutic protein‐drug interactions: clinical findings, proposed mechanisms and regulatory implications. Clin. Pharmacokinet. 49, 295–310 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Al‐Harbi, N.O. et al Psoriatic inflammation causes hepatic inflammation with concomitant dysregulation in hepatic metabolism via IL‐17A/IL‐17 receptor signaling in a murine model. Immunobiology 222, 128–136 (2017). [DOI] [PubMed] [Google Scholar]
- 11. Bruin, G. , Loesche, Ch , Nyirady, J. & Sander, O. Population pharmacokinetic modeling of secukinumab in patients with moderate to severe psoriasis. J. Clin. Pharm. 57, 876–885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reich, K. et al Secukinumab, a fully human anti‐interleukin‐17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate‐to‐severe plaque psoriasis. Br. J. Dermatol. 176, 752–758 (2017). [DOI] [PubMed] [Google Scholar]
- 13. Backman, J.T. , Kivisto, K.T. , Olkkola, K.T. & Neuvonen, P.J. The area under the plasma concentration‐time curve for oral midazolam is 400‐fold larger during treatment with itraconazole than with rifampicin. Eur. J. Clin. Pharmacol. 54, 53–58 (1998). [DOI] [PubMed] [Google Scholar]
- 14. Kolbinger, K. et al Beta‐defensin‐2 is a responsive biomarker of IL‐17A‐driven skin pathology in psoriasis. J. Allergy Clin. Immunol. 139, 923–932 (2017). [DOI] [PubMed] [Google Scholar]
- 15. Davis, J.D. et al Evaluation of potential disease‐mediated drug–drug interaction in patients with moderate‐to‐severe atopic dermatitis receiving dupilumab. Clin. Pharmacol. Ther. 104, 1146–1154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khatri, A. , Cheng, L. , Camez, A. , Ignatenko, S. , Pang, Y. & Othman, A.A. Lack of effect of 12‐week treatment with risankizumab on the pharmacokinetics of cytochrome P450 probe substrates in patients with moderate to severe chronic plaque psoriasis. Clin. Pharmacokinet. 58, 805–814 (2019). [DOI] [PubMed] [Google Scholar]
- 17. Khalilieh, S. et al Effect of tildrakizumab (MK‐3222), a high affinity, selective anti‐IL23p19 monoclonal antibody, on cytochrome P450 metabolism in subjects with moderate to severe psoriasis. Br. J. Clin. Pharmacol. 84, 2292–2302 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocha‐Pereira, P. , Santos‐Silva, A. , Rebelo, I. , Figueiredo, A. , Quintanilha, A. & Teixeira, F. The inflammatory response in mild and in severe psoriasis. Br. J. Dermatol. 150, 917–928 (2004). [DOI] [PubMed] [Google Scholar]
- 19. Gottlieb, A.B. et al Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J. Drugs Dermatol. 14, 821–833 (2015). [PubMed] [Google Scholar]
- 20. Clinical Drug Interaction Studies – Study Design, Data Analysis, and Clinical Implications, Guidance for Industry, CDER, FDA. October 2017. <https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-study-design-data-analysis-and-clinical-implications-guidance> (2017).


