Abstract
BACKGROUND:
Mirvetuximab soravtansine (IMGN853) is an antibody-drug conjugate that selectively targets folate receptor α (FRα). In this phase 1 dose-escalation study, the authors investigated IMGN853 in patients with FRα-positive solid tumors.
METHODS:
Patients received IMGN853 on day 1 of a 21-day cycle (once every 3 weeks dosing), with cycles repeated until patients experienced dose-limiting toxicity or progression. Dose escalation commenced in single-patient cohorts for the first 4 planned dose levels and then followed a standard 3 + 3 scheme. The primary objectives were to determine the maximum tolerated dose and the recommended phase 2 dose. Secondary objectives were to determine safety and tolerability, to characterize the pharmacokinetic profile, and to describe preliminary clinical activity.
RESULTS:
In total, 44 patients received treatment at doses escalating from 0.15 to 7.0 mg/kg. No meaningful drug accumulation was observed with the dosing regimen of once every 3 weeks. The most common treatment-related adverse events were fatigue, blurred vision, and diarrhea, the majority of which were grade 1 or 2. The dose-limiting toxicities observed were grade 3 hypophosphatemia (5.0 mg/kg) and grade 3 punctate keratitis (7.0 mg/kg). Two patients, both of whom were individuals with epithelial ovarian cancer, achieved confirmed tumor responses according to Response Evaluation Criteria in Solid Tumors 1.1, and each was a partial response.
CONCLUSIONS:
IMGN853 demonstrated a manageable safety profile and encouraging preliminary clinical activity, particularly in patients with ovarian cancer. The results establish a recommended phase 2 dosing of6.0 mg/kg (based on adjusted ideal body weight) once every 3 weeks.
Keywords: antibody-drug conjugate, clinical trial, folate receptor, phase 1, targeted therapy
INTRODUCTION
The use of chemotherapeutic drugs has long provided a foundation for systemic cancer therapy; however, their effectiveness is commonly hampered because of dose-limiting toxicities (DLTs) that arise as a consequence of adverse effects on normal tissues. In addition, standard-of-care treatments for a large number of human malignancies involve multidrug combinations, and these typically require further dose reductions to maintain an acceptable tolerability profile for patients.1 In an effort to overcome these limitations, various tumor-selective drug-delivery strategies have been developed that are designed to deliver cytocidal amounts of therapeutic agents directly to tumors.2–4 In this regard, significant translational progress has been achieved in the field of antibody-drug conjugate (ADC) technology. ADCs provide targeted delivery of cytotoxic agents through linkage to monoclonal antibodies directed against tumor-associated antigens, thereby affording a means to minimize toxicities of highly potent drugs in normal tissues while maintaining or improving their antitumor efficacy.5,6
Folate receptor α (FRα) is a member of a family of cell-surface glycoproteins that facilitate the transport and accumulation of folate, through endocytosis, into cells.7 In contrast to its highly restricted expression pattern in normal tissues,7,8 FRα is aberrantly expressed in a variety of epithelial tumors.9 Indeed, high receptor expression is characteristic of several common human malignancies, including ovarian cancer, endometrial cancer, and nonsmall cell lung cancer (NSCLC).10 Therefore, FRα has emerged as an attractive candidate for molecularly targeted strategies designed to exploit this differential distribution pattern to maximize both antitumor efficacy and tolerability. Consequently, several experimental folate receptor-targeting agents are now under clinical evaluation.9–11 The 2 primary approaches explored to date have involved either targeted cytotoxic drug delivery through small-molecule folate-cytotoxic agent conjugates, such as vintafolide,12,13 or the use of humanized anti-FRα monoclonal antibodies, exemplified by farletuzumab,14 intended to selectively induce tumor cell death. Unfortunately, neither approach has demonstrated meaningful efficacy in pivotal ovarian cancer trials to date.15,16 Of particular note, the ability of FRα to internalize large-molecule ligands underscores the potential utility of this receptor as a target for ADC-based therapeutic interventions, which couple the targeting and pharmacokinetic (PK) features of an antibody with the cancer-killing impact of a cytotoxic agent.
Mirvetuximab soravtansine (IMGN853) is an ADC comprised of a humanized anti-FRα monoclonal antibody (M9346A) linked to a cytotoxic effector molecule, the maytansinoid DM4.11,17 IMGN853 binds with high affinity and specificity to FRα, which upon antigen binding, promotes ADC internalization and intracellular release of DM4.18 Through its ability to inhibit tubulin polymerization and disrupt microtubule assembly, DM4 serves as a potent antimitotic agent to induce cell cycle arrest and apoptosis.19 Preclinically, IMGN853 has exhibited robust antitumor activity against FRα-expressing tumors, including models of ovarian cancer and NSCLC.20
Here, we present the dose-escalation phase of the first-in-human clinical evaluation of IMGN853 monotherapy in patients with advanced, FRα-positive solid tumors who were refractory to standard therapies. The primary objectives of this phase 1 study were to determine the maximum tolerated dose (MTD) and the recommended phase 2 dose (RPD2) of IMGN853 administered intravenously once every 3 weeks. Secondary objectives included an evaluation of the safety, tolerability, and plasma pharmacokinetics of IMGN853 as well as any preliminary evidence of clinical activity.
MATERIALS AND METHODS
Patient Selection and Eligibility Criteria
Adults with pathologically confirmed, advanced solid tumors who were refractory to conventional therapy, or for whom standard treatments were either not available or not an option, were eligible for inclusion. Enrollment without prior documentation of tumor FRα expression was limited to patients with the following tumor histologic subtypes, characterized by a high incidence of FRα-positivity: epithelial ovarian cancer (EOC) (serous or endometrioid), primary peritoneal cancer, fallopian tube cancer, serous or endometrioid endometrial cancer, NSCLC (adenocarcinoma or bronchoalveolar carcinoma), and renal cell cancer. Patients had measurable or nonmeasureable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.21 Patients were also required to be age 18 years or older; to have an Eastern Cooperative Oncology Group performance status 0 or 1; and to have adequate hematologic, renal, and hepatic function. Key exclusion criteria included grade >1 neuropathy, known hypersensitivity to monoclonal antibody or maytansinoid therapy, any active or chronic corneal disorder, a history of other solid tumor malignancy with a disease-free interval <3 years (except for adequately treated basal cell or squamous cell skin cancer and in situ breast or prostate cancer), concomitant administration of folate-containing vitamins, or prior allogenic or autologous bone marrow transplantation. All patients provided written informed consent in accordance with federal, local, and institutional guidelines.
Study Design and Treatment Administration
The primary objectives of this open-label, phase 1, dose-escalation study were to determine the MTD and the RP2D of single-agent IMGN853 in patients with solid tumors. Secondary objectives were to evaluate safety and tolerability, characterize the PK profile, and describe any preliminary clinical activity. IMGN853 was administered intravenously once every 3 weeks (ie, on day 1 of a 21-day cycle). Escalated doses for this schedule were 0.15, 0.5, 1.0, 2.0, 3.3, 5.0, and 7.0 mg/kg, with 1 patient enrolled at each of the first 4 dose levels. Beyond this, a standard 3 + 3 design was used. Initially, administered doses were calculated on total body weight (TBW); however, early analyses revealed dose-dependent and exposure-dependent correlations with ocular adverse events (AEs). In an effort to minimize the total milligram dose administered across the wide weight range of patients (range, 48.2–135.8 kg) and to decrease the incidence of ocular toxicity, dosing was modified to use the adjusted ideal body weight (AIBW).
Study treatment continued until patients developed progressive disease, had unacceptable toxicity, withdrew consent, or died. Patients were followed for 28 days after the last dose of study drug or until they recovered from any treatment-related toxicity, whichever came first. The trial was conducted in accordance with US Food and Drug Administration regulations, the International Conference on Harmonization Guidelines for Good Clinical Practice, and the Declaration of Helsinki. The study was compliant with all relevant Institutional Review Board and Independent Ethics Committee requirements and is registered at clinicaltrials.gov ().
Assessments
Baseline assessments included medical history and physical examination, Eastern Cooperative Oncology Group performance status, blood chemistry and hematology, serum pregnancy test, and electrocardiogram. During screening, radiologic imaging studies of the chest, abdomen, and pelvis for tumor evaluation were obtained. For patients who had measureable disease, overall tumor response was defined according RECIST 1.1 and was assessed using computerized tomography scans approximately every second cycle from the date of first dose to the 28-day follow-up visit. Patients with ovarian and endometrial cancers had concomitant cancer antigen 125 (CA 125) measurements taken approximately at the time of radiologic assessment. In accordance with Gynecologic Cancer Intergroup guidelines, a CA 125 response was defined as a reduction ≥50% in the CA 125 level from pretreatment sampling that was confirmed and maintained for ≥28 days.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 and were monitored continuously throughout the study from the time of the first study dose until 28 days after treatment cessation. Serious AEs (SAEs) were defined as any AE that was fatal or life-threatening, required prolonged existing hospitalization, resulted in persistent or significant disability/incapacity, or required medical or surgical intervention. A DLT was defined as grade 4 neutropenia for ≥7 days; grade ≥3 neutropenia with fever; grade 3 thrombocytopenia accompanied by bleeding; grade 4 thrombocytopenia; or any grade ≥3 nonhematologic toxicity considered related to IMGN853 (other than nausea, vomiting, or diarrhea in the absence of appropriate prophylaxis; AEs related to underlying disease; or grade 3 fatigue).
Pharmacokinetics
Blood samples for PK analysis were collected after the first and third doses. Collection times were as follows: predose; end of infusion; and 2, 4, 24, 48, and 96 hours or 120, 168, 336, and 504 hours postdose. For the third dose on day 15, an additional sample was also taken 336 hours postdose. Plasma samples were analyzed for concentrations of IMGN853 using a validated enzyme-linked immunosorbent assay method. The assay was validated in the range from 75 to 2500 ng/mL, with a lower limit of quantification of 75 ng/mL, using a 20-μL sample volume. PK parameters were calculated by noncompartmental methods using Phoenix WinNonlinVersion 6.4 (Certara, Princeton, NJ).
Statistical Analysis
The period of patient accrual spanned from July 2012 to March 2015, and the data analysis extended to April 2016. Descriptive statistics were used to summarize demographic and baseline characteristics, and additional analyses were performed using SAS statistical software (version 9.4; SAS Inc., Cary, NC). For the safety evaluations, baseline was defined as the last available assessment before day 1, cycle 1; and any AE with an onset date that was the same as the start of study treatment or later was reported as treatment-emergent. The safety population included all patients who received at least 1 dose of IMGN853. Tumor response was based on all patients who had received at least 1 dose of study drug and from whom at least 1 tumor assessment evaluation was attained.
RESULTS
Patient Characteristics
Forty-four patients were enrolled and received treatment using the once every 3 weeks dosing schedule. Patient demographics and baseline characteristics are summarized in Table 1. The median age was 58 years (range, 37–86 years), and the majority of patients were women (89%) who were diagnosed with either recurrent ovarian (52%) or endometrial (25%) cancer. Renal cell carcinoma and NSCLC (11% and 9%, respectively) accounted for non-gynecologic malignancies that were included in the trial. All individuals were heavily pretreated, having received a median of 5 prior systemic therapies (range, 1–14 prior systemic therapies), and greater than 84% had previous platinum and/or taxane exposure.
TABLE 1.
Patient Demographics and Baseline Characteristics (N = 44)
| Characteristic | No. of Patients (%) |
|---|---|
| Age: Median [range], y | 58 [37–86] |
| Sex | |
| Men | 5 (11.4) |
| Women | 39 (88.6) |
| Race | |
| White | 41 (93.2) |
| Black or African American | 2 (4.5) |
| Asian | 1 (2.3) |
| Tumor type | |
| Ovarian cancer | 23 (52.3) |
| Endometrial | 11 (25) |
| NSCLC adenocarcinoma | 4 (9.1) |
| Renal cell cancer | 5 (11.4) |
| Cervical cancer | 1 (2.3) |
| ECOG PS | |
| 0 | 22 (50) |
| 1 | 22 (50) |
| Prior compound exposure | |
| Platinum | 38 (86.4) |
| Taxane | 37 (84.1) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status. NSCLC, nonsmall cell lung cancer.
Dose Escalation, MTD, and RP2D
Dose escalation is summarized in Table 2. DLTs were assessed only during the first cycle of treatment. IMGN853 dosing was initially escalated from 0.15 to 7.0 mg/kg (based on TBW). The first 4 treatment groups (0.15, 0.5, 1.0, and 2.0 mg/kg) were single-patient cohorts, with the exception of the initial 0.15 mg/kg level (n = 2), in which the first patient enrolled progressed within cycle 1 and was replaced. No DLTs were observed at any of these dose levels. Similarly, no DLTs were observed in the first 3 patients who received with IMGN853 at either 3.3 or 5 mg/kg, and escalation proceeded to 7.0 mg/kg. At that dose level, 1 of 5 patients experienced a DLT of grade 3 punctate keratitis. Accordingly, the dose was reduced to 5.0 mg/kg, which was received by a total of 11 patients. In this cohort, dose-limiting grade 3 hypophosphatemia was observed, along with additional ocular AEs that arose during the second cycle. These factors contributed to a further reduction back to 3.3 mg/kg dosing. No further DLTs were observed in this treatment population (n = 6).
TABLE 2.
Summary of IMGN853 Dose Escalation
| IMGN853 Dose, mg/kg | No. of Patients | DLTs and Treatment-Related SAEs |
|---|---|---|
| 0.15a | 2b | None |
| 0.5a | 1 | None |
| 1.0a | 1 | None |
| 2.0a | 1 | None |
| 3.3a | 9 | None |
| 5.0a | 11 | DLT, grade 3 hypophosphatemia; SAE, grade 3 corneal opacity |
| 7.0a | 5 | DLT, grade 3 punctate keratitis |
| 5.0 | 7 | SAE, grade 3 pulmonary edema |
| 6.0 | 7 | None |
Abbreviations: DLT, dose-limiting toxicity; IMGN853, mirvetuximab soravtansine; SAE, severe adverse event.
These patients were dosed based on total body weight, and others were dosed according to adjusted ideal body weight.
The original patient enrolled in this cohort progressed during cycle 1 and was replaced. Patients in this initial cohort were monitored for a safety assessment period of 28 days.
A modification in dosing based on AIBW instead of TBW was implemented to decrease the range of variance in interpatient drug exposures.22 This was designed to improve tolerability, particularly with respect to the incidence of visual disturbances (primarily blurred vision; see below) reported at the higher dose levels. Subsequently enrolled patients received IMGN853 at 5.0 and 6.0 mg/kg AIBW (n = 7 per group). No DLTs were observed, and the 6.0 mg/kg (AIBW) dose was declared to be the RP2D for the once every 3 weeks dosing regimen. No further escalation occurred beyond this level in an effort to decrease the likelihood of ocular AEs events; accordingly, the MTD was not reached on this schedule.
Safety
All 44 patients were included in the safety analyses. The most common treatment-emergent AEs (TEAEs) that occurred in ≥10% of patients (all grades) are presented in Supporting Table 1 (see online supporting information). The most frequently reported TEAEs included diarrhea (34%), fatigue (32%), and nausea and blurred vision (25% each); the majority of these were generally mild (grade 1 or 2). In total, 33 patients (75%) had a TEAE that was deemed related to study drug. The majority of these were either grade 1 or 2. Treatment-related TEAEs that occurred in >10% of patients are listed in Table 3. The major adverse reactions observed across the study population were fatigue (25%), blurred vision (23%), and diarrhea and peripheral neuropathy (21% each). Six patients overall (14%) reported a grade 3 TEAE, and no individual event occurred in more than 1 patient. One patient experienced a grade 4 TEAE (decreased lymphocyte count), and no fatalities caused by related TEAEs were reported during the study.
TABLE 3.
Treatment-Related, Treatment-Emergent Adverse Events Reported in ≥10% of Patients
| IMGN853 Dose, mg/kg: No. of Patients (%) | ||||||
|---|---|---|---|---|---|---|
| Adverse Event | Total, n = 44 | 0.15–2.0, n = 5a | 3.3, n = 9 | 5.0, n = 18b | 6.0, n = 7 | 7.0, n = 5 |
| Fatigue | 11 (25) | 0 (0) | 2 (22.2) | 3 (16.7) | 3 (42.9) | 3 (60) |
| Vision blurred | 10 (22.7) | 0 (0) | 0 (0) | 3 (16.7) | 2 (28.6) | 5 (100) |
| Diarrhea | 9 (20.5) | 0 (0) | 1 (11.1) | 6 (33.3) | 1 (14.3) | 1 (20) |
| Peripheral neuropathyc | 9 (20.5) | 0 (0) | 2 (22.2) | 4 (22.2) | 1 (14.3) | 2 (40) |
| ALT increased | 7 (15.9) | 0 (0) | 0 (0) | 4 (22.2) | 1 (14.3) | 2 (40) |
| Keratopathyd | 7 (15.9) | 0 (0) | 0 (0) | 3 (16.7) | 2 (28.6) | 2 (40) |
| AST increased | 6 (13.6) | 0 (0) | 1 (11.1) | 3 (16.7) | 0 (0) | 2 (40) |
| Headache | 5 (11.4) | 0 (0) | 1 (11.1) | 3 (16.7) | 0 (0) | 1 (20) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; IMGN853, mirvetuximab soravtansine.
The include patients who received IMGN853 at doses of 0.15, 0.5, 1.0, and 2.0 mg/kg.
These include the initial 11 patients who were dosed based on total body weight and the additional 7 who were dosed according to adjusted ideal body weight.
These events include neuropathy peripheral, peripheral sensory neuropathy, peripheral motor neuropathy, and hypoesthesia.
These events include corneal epithelial microcysts, corneal opacity, corneal erosion, corneal pigmentation, and punctate keratitis.
Treatment-related SAEs were reported in 4 individuals (9%). These included DLTs of grade 3 hypophosphatemia and punctate keratitis and 1 episode each of grade 3 corneal opacity and pulmonary edema (Table 2). The corneal abnormalities, along with the blurred-vision TEAEs reported above, were reversible.
Pharmacokinetics
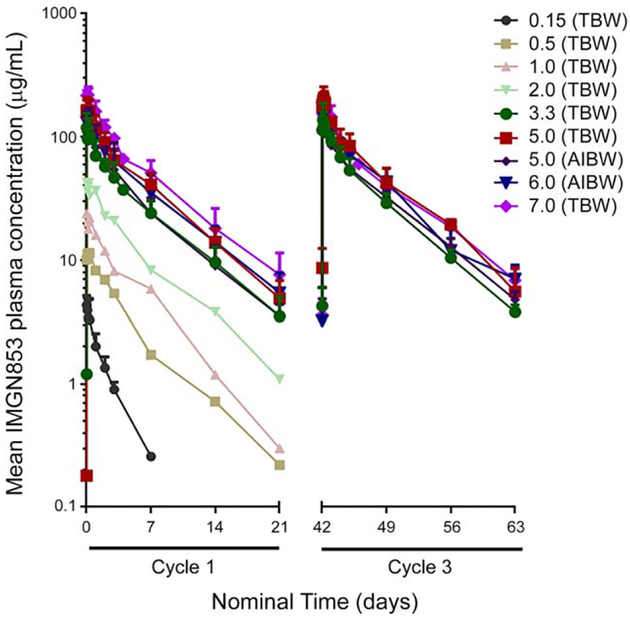
PK evaluations were based on all 44 patients who received treatment with IMGN853. The mean plasma concentration-time profiles of IMGN853 for cycles 1 and 3 are presented in Figure 1, and PK parameters are reported in Supporting Table 2 (see online supporting information). After the first administration of IMGN853, mean exposure (maximum plasma concentration [Cmax] and area under the concentration-time curve from zero to infinity [AUC0−∞]) increased with dose in a generally proportional manner from 1.0 to 7.0 mg/kg. For cycle 1, the mean half-life (t1/2) values for IMGN853 ranged from approximately 79 hours to 121 hours across doses, with no meaningful dose-dependence noted in total clearance (CL) or volume of distribution (Vss) for cohorts that received ≥1.0 mg/kg. In the 2 lowest dose cohorts examined (ie, 0.15 and 0.5 mg/kg), there appeared to be a trend toward higher CL; however, because of low patient numbers in these groups, definitive conclusions were not possible. After multiple doses, there appeared to be a trend toward time-dependent changes in CL (decreasing) and Vss (decreasing), leading to a slight increase in t1/2 by cycle 3. However, the exposure metrics at cycle 3 indicated that there was no meaningful accumulation after multiple doses of IMGN853.
Figure 1.
Mean concentration-time profiles are illustrated in cycles 1 and 3 after infusion of mirvetuximab soravtansine on dosing schedule of once every 3 weeks. All doses listed are in mg/kg and were calculated based on total body weight (TBW) or adjusted ideal body weight (AIBW), as indicated.
Clinical Activity
Forty-three patients were evaluable for best response to therapy by RECIST and/or CA 125 response. Confirmed tumor responses were observed in 2 patients, both comprising partial responses (PRs), for an objective response rate (ORR) of 5%. Each of these involved patients with EOC, including 1 who remained on treatment for 33 weeks. Stable disease (SD) was observed in an additional 22 patients, including 4 who had SD that lasted ≥4 months. Five patients (4 with EOC and 1 with endometrial cancer) experienced a confirmed CA 125 response, resulting in an overall clinical benefit rate (ORR + SD ≥4 months + CA 125 response) of 23% (Table 4).
TABLE 4.
Patients With Clinical Benefit
| IMGN853 Dose, mg/kg | Diagnosis | Clinical Benefita | Duration on Study, wk |
|---|---|---|---|
| 3.3 | EOC | CA 125 response | 15 |
| EOC | PR | 33 | |
| NSCLC | SD ≥4 mo | 21 | |
| 5.0 | EOC | CA 125 response | 19 |
| EOC | SD ≥4 mo | 29 | |
| Endometrial | CA 125 response | 16 | |
| Endometrial | SD ≥4 mo | 28 | |
| 6.0 | EOC | CA 125 response | 9 |
| 7.0 | EOC | PR | 23 |
| EOC | CA 125 response, SD ≥4 mo | 23 |
Abbreviations: CA 125, cancer antigen 125; EOC, epithelial ovarian cancer; IMGN853, mirvetuximab soravtansine; NSCLC, nonsmall cell lung cancer; PR, partial response; SD, stable disease.
Clinical benefit was defined as a confirmed objective response (complete or partial response) according to Response Evaluation Criteria in Solid Tumors 1.1; a CA 125 response according to Gynecologic Cancer Intergroup criteria, or SD ≥4 months.
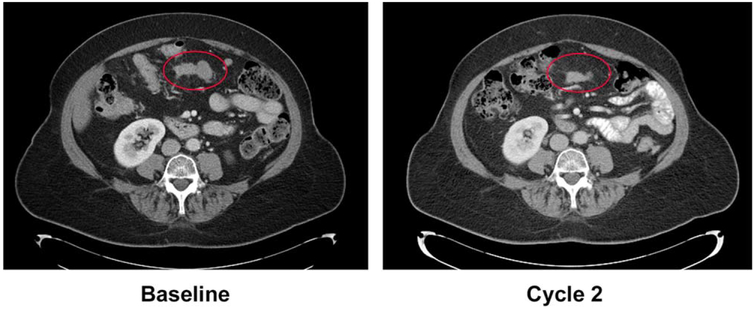
An example of a radiologic response is provided in Figure 2. This case involved a heavily pretreated woman aged 65 years who was diagnosed with platinum-resistant, transitional cell ovarian cancer in 2008 and who had received 3 previous lines of chemotherapy before receiving treatment with IMGN853 at 7.0 mg/kg (TBW). The patient achieved a PR after 2 cycles, which was subsequently confirmed at cycle 4.
Figure 2.
The activity of mirvetuximab soravtansine in a patient who had platinum-resistant ovarian cancer reveals a partial response.
DISCUSSION
Here, we report dose-escalation findings from the first-in-human phase 1 study of IMGN853, an FRα-Targeting ADC, administered to heavily pretreated patients with advanced solid tumors. Systemic exposure to IMGN853, assessed by Cmax and AUC0−∞, increased in a generally dose-proportional manner across the 1.0 to 7.0 mg/kg range. It is noteworthy that the terminal half-life of IMGN853 determined at doses ≥1.0 mg/kg demonstrated no dose dependence with respect to clearance or volume of distribution parameters. In addition, no meaningful drug accumulation was observed with this dosing regimen.
IMGN853 was well tolerated, with escalation proceeding to doses of 5.0 and 7.0 mg/kg (TBW) before DLTs were observed. After the modification to AIBW-based calculations, patients received doses of 5.0 and 6.0 mg/kg. No DLTs or SAEs were reported in the 6.0-mg/kg AIBW cohort; and, based on a collective evaluation of safety, activity, and PK data, this dose was declared the RP2D. It is worth noting that, after the escalation stage of the current study, this RP2D has been used in several expansion cohorts, in which its tolerability has been confirmed.
The most frequent treatment-related AEs observed with IMGN853 included mild (primarily grade 1 or 2) fatigue and diarrhea, which were readily managed without requiring discontinuation of therapy. We note that this safety profile was similar to that reported during the phase 1 evaluation of farletuzumab, but without the high incidence of hypersensitivity and infusion-related reactions.23,24 Peripheral neuropathy, typically associated with cytotoxic chemotherapy, was experienced by 9 patients (21%), 5 of whom had a prior history of neuropathy after previous platinum/taxane therapy. All episodes were either grade 1 or 2 and were likely a consequence of the maytansinoid payload in IMGN853.25
Ocular TEAEs, which primarily included reversible blurred vision and/or keratopathy, were considered AEs of interest in the current study. Although these have been reported for a diverse range of ADCs targeting different antigens and using different cytotoxic payloads, the molecular mechanisms underlying such events remain poorly defined.26 Grade 3 punctate keratitis and corneal opacity were reported as a DLT and an SAE in individuals who received 7.0 and 5.0 mg/kg, respectively, once every 3 weeks. It is important to note that IMGN853 dosing in these particular patients was calculated based on TBW; PK analysis subsequently suggested an association between the AEs and initial exposure to higher plasma levels of IMGN853 when TBW dosing was used. In an effort to decrease variability in exposure levels, AIBW dosing was subsequently used for the duration of the study. After this change, the visual and corneal abnormalities observed were generally mild (grade ≤2) and were similar to those reported for other DM4-conjugated antibodies.27 These effects appeared to be independent of the target antigen, consistent with the profile observed for other ADCs, because FRα does not exhibit significant expression in the eye. In addition to the inherent potential toxicity of the payload, it has been proposed that such events may also be a function of the linker used for drug attachment, because prolonged retention in the circulation conferred by stable linkers may be sufficient to enhance overall exposure in normal (including ocular) tissues.28
Clinical benefit from IMGN853 monotherapy was observed across a range of solid tumor types, most notably within the subset of patients with EOC. Ovarian cancer represents an attractive indication for the application of folate receptor-targeting therapeutics,29,30 in part because of the high constitutive expression of FRα (approximately 80% of nonmucinous tumors) in this malignancy31—a feature that also has been proposed as a negative prognostic factor with respect to patient response to chemotherapeutics.32 RECIST-defined objective responses (PRs) were reported in 2 patients with EOC, with individuals remaining on study for periods ranging between 23 and 33 weeks. Clinical benefit was observed in additional patients with EOC, including 4 who had confirmed CA 125 responses and 2 with SD that lasted >4 months. On the basis of these findings, an expansion cohort evaluating the safety and efficacy of IMGN853 in a population of patients with FRα-positive, platinum-resistant ovarian cancer was opened as part of the current trial.33
Overall, the results of this study support the continued exploration of IMGN853 as a novel, FRα-Targeting therapeutic based on its favorable tolerability and encouraging signs of clinical efficacy, particularly in patients with ovarian cancer. The RP2D is 6 mg/kg AIBW administered once every 3 weeks. The trial is continuing, including an expansion cohort focused on the prophylactic use of corticosteroid eye drops for the management of ocular disorders. Moreover, additional trials of IMGN853 in advanced ovarian cancer have recently been initiated, including a pivotal phase 3 monotherapy trial in patients with platinum-resistant disease (FORWARD I; clinicaltrials.gov identifier ) as well as a phase 1b study in which it is being evaluated in combination with both standard-of-care chemotherapeutics and targeted agents (FORWARD II; clinicaltrials.gov identifier ).
Supplementary Material
FUNDING SUPPORT
This work was funded by ImmunoGen, Inc.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Kathleen N. Moore reports honoraria for advisory board service from AstraZeneca, Clovis, Immunogen, and Genentech/Roche; service on the Tesaro steering committee; and service on the Advaxis steering committee/advisory board outside the submitted work. Hossein Borghaei reports personal fees from and service on the advisory boards of Genentech, Lilly, Bristol-Myers-Squibb, Celgene, and Clovis; personal fees from AstraZeneca, Pfizer, Merck, EMD-Serono, and Trovagene; and clinical trial funding from Merck/Celgene and Millennium outside the submitted work. David M. O’Malley reports personal fees from Clovis, Janssen, AstraZeneca, Genentech/Roche, Amgen, and Eisai outside the submitted work. Shelly M. Seward reports service on the AstraZeneca Speakers’ Bureau. Ursula A. Matulonis reports service on the advisory boards of AstraZeneca, Clovis, Eli Lilly, Genentech, and Immunogen outside the submitted work. Kelli L. Running reports employment at ImmunoGen. Xiaoyan Zhang reports employment at ImmunoGen. Jose F. Ponte reports employment at ImmunoGen and has a patent pending (US. Pub. no. 2015 of 0132323; International Pub. no. WO2015 of 054400). Rodrigo Ruiz-Soto reports employment at ImmunoGen. Woondong Jeong, Todd M. Bauer, Raymond P. Perez, and Michael J. Birrer made no disclosures.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Frei E 3rd, Elias A, Wheeler C, Richardson P, Hryniuk W. The relationship between high-dose treatment and combination chemo-therapy: the concept of summation dose intensity. Clin Cancer Res. 1998;4:2027–2037. [PubMed] [Google Scholar]
- 2.Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res. 2008;41:98–107. [DOI] [PubMed] [Google Scholar]
- 3.Yewale C, Baradia D, Vhora I, Misra A. Proteins: emerging carrier for delivery of cancer therapeutics. Expert Opin Drug Deliv. 2013;10: 1429–1448. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee B, Karmakar SD, Hossain CM, Bhattacharya S. Peptides, proteins and peptide/protein-polymer conjugates as drug delivery system. Protein Pept Lett. 2014;21:1121–1128. [DOI] [PubMed] [Google Scholar]
- 5.Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angewandte Chemie. 2014;53: 3796–3827. [DOI] [PubMed] [Google Scholar]
- 6.Chudasama V, Maruani A, Caddick S. Recent advances in the construction of antibody-drug conjugates. Nat Chem. 2016;8:114–119. [DOI] [PubMed] [Google Scholar]
- 7.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. [DOI] [PubMed] [Google Scholar]
- 8.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. [DOI] [PubMed] [Google Scholar]
- 9.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17:89–95. [DOI] [PubMed] [Google Scholar]
- 10.Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26:2034–2043. [DOI] [PubMed] [Google Scholar]
- 11.Lutz RJ. Targeting the folate receptor for the treatment of ovarian cancer. Transl Cancer Res. 2015;4:118–126. [Google Scholar]
- 12.Dosio F, Milla P, Cattel L. EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Curr Opin Investig Drugs. 2010;11:1424–1433. [PubMed] [Google Scholar]
- 13.Ambrosio AJ, Suzin D, Palmer EL, Penson RT. Vintafolide (EC145) for the treatment of folate-receptor-alpha positive platinum-resistant ovarian cancer. Expert Rev Clin Pharmacol. 2014;7:443–450. [DOI] [PubMed] [Google Scholar]
- 14.Spannuth WA, Sood AK, Coleman RL. Farletuzumab in epithelial ovarian carcinoma. Expert Opin Biol Ther. 2010;10:431–437. [DOI] [PubMed] [Google Scholar]
- 15.Vergote I, Armstrong D, Scambia G, et al. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J Clin Oncol. 2016;34:2271–2278. [DOI] [PubMed] [Google Scholar]
- 16.Endocye Inc. Merck and Endocyte Announce Independent DSMB Recommends Vintafolide PROCEED Phase 3 Trial Be Stopped for Futility Following Interim Analysis. Endocyte Inc. Available at: http://investor.endocyte.com/releasedetail.cfm?ReleaseID=844838. Accessed October 25, 2016.
- 17.Lambert JM. Drug-conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol. 2013;76:248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson HK, Widdison WC, Mayo MF, et al. Tumor delivery and in vivo processing of disulfide-linked and thioether-linked antibody-maytansinoid conjugates. Bioconjug Chem. 2010;21:84–92. [DOI] [PubMed] [Google Scholar]
- 19.Hong EE, Erickson H, Lutz RJ, et al. Design of coltuximab ravtansine, a CD19-targeting antibody-drug conjugate (ADC) for the treatment of B-Cell malignancies: structure-activity relationships and preclinical evaluation. Mol Pharm. 2015;12:1703–1716. [DOI] [PubMed] [Google Scholar]
- 20.Ab O, Whiteman KR, Bartle LM, et al. IMGN853, a folate receptor-alpha (FRalpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRalpha-expressing tumors. Mol Cancer Ther. 2015;14:1605–1613. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 22.Moore KN, Ponte J, Lorusso PM, et al. Relationship of pharmacokinetics (PK), toxicity, and initial evidence of clinical activity with IMGN853, a folate receptor alpha (FRa) targeting antibody drug conjugate in patients (Pts) with epithelial ovarian cancer (EOC) and other FRa-positive solid tumors [abstract]. J Clin Oncol. 2014;32: 15S Abstract 5571. [Google Scholar]
- 23.Konner JA, Bell-McGuinn KM, Sabbatini P, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16: 5288–5295. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki Y, Miwa K, Yamashita K, et al. A phase I study of farletuzumab, a humanized anti-folate receptor alpha monoclonal antibody, in patients with solid tumors. Invest New Drugs. 2015;33:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Roca CA, Boni V, Moreno V, et al. A phase I study of SAR566658, an anti CA6-antibody drug conjugate (ADC), in patients (Pts) with CA6-positive advanced solid tumors (STs) () [abstract]. J Clin Oncol. 2016;34:15S Abstract 2511. [Google Scholar]
- 26.Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parslow AC, Parakh S, Lee F, Gan HK, Scott AM. Antibody-drug conjugates for cancer therapy. Biomedicines. 2016;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polakis P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. 2016;68:3–19. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti C, Palaia I, Giorgini M, et al. Targeted drug delivery via folate receptors in recurrent ovarian cancer: a review. Onco Targets Ther. 2014;7:1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015;34:41–52. [DOI] [PubMed] [Google Scholar]
- 31.Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toffoli G, Russo A, Gallo A, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer. 1998;79:121–126. [DOI] [PubMed] [Google Scholar]
- 33.Moore KN, Martin LP, O’Malley DM, et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol. 2017;35:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.