Abstract
Triple negative breast cancer (TNBC) is an aggressive breast cancer subtype with few therapy options besides chemotherapy. Although platinum-based drugs have shown initial activity in BRCA1-mutated TNBCs, chemoresistance remains a challenge. Here we show that RAD6B (UBE2B), a principal mediator of translesion synthesis (TLS), is overexpressed in BRCA1 wild-type and mutant TNBCs, and RAD6B overexpression correlates with poor survival. Pretreatment with a RAD6-selective inhibitor, SMI#9, enhanced cisplatin chemosensitivity of BRCA1 wild-type and mutant TNBCs. SMI#9 attenuated cisplatin-induced PCNA monoubiquitination (TLS marker), FANCD2 (Fanconi anemia (FA) activation marker), and TLS polymerase POL η. SMI#9-induced decreases in γH2AX levels were associated with concomitant inhibition of H2AX monoubiquitination, suggesting a key role for RAD6 in modulating cisplatin-induced γH2AX via H2AX monoubiquitination. Concordantly, SMI#9 inhibited γH2AX, POL η and FANCD2 foci formation. RAD51 foci formation was unaffected by SMI#9, however, its recruitment to double-strand breaks was inhibited. Using the DR-GFP-based assay, we showed that RAD6B silencing or SMI#9 treatment impairs homologous recombination (HR) in HR-proficient cells. DNA fiber assays confirmed that restart of cisplatin-stalled replicating forks is inhibited by SMI#9 in both BRCA1 wild-type and mutant TNBC cells. Consistent with the in vitro data, SMI#9 and cisplatin combination treatment inhibited BRCA1 wild-type and mutant TNBC growth as compared to controls. These RAD6B activities are unaffected by BRCA1 status of TNBCs suggesting that the RAD6B function in TLS/FA crosstalk could occur in HR-dependent and independent modes. Collectively, these data implicate RAD6 as an important therapeutic target for TNBCs irrespective of their BRCA1 status.
Keywords: Triple negative breast cancer, BRCA1, FANCD2, POL η, H2AX, Cisplatin, Ubiquitination, Small molecule inhibitor, Homologous recombination, Replication restart
1. Introduction
Triple negative breast cancers (TNBCs) lack estrogen receptor, progesterone receptor and Her2/neu expressions, and represent a heterogeneous subset with basal-like and mesenchymal subtypes being the most prevalent [1]. TNBCs are treated with chemotherapy and despite initial response, patients frequently relapse within 5 years [2–4]. Preclinical and phase II studies suggest potential benefit for BRCA1/2 mutated TNBCs treated with cisplatin [5–7]. Platinum drug cytotoxicity results from intra- and inter-strand crosslinks, and although intra-strand DNA crosslinks represent ~95% of lesions, inter-strand crosslinks (ICLs) are more deleterious as they block DNA replication and transcription. DNA damage response activation, particularly those preventing replication fork collapse and promoting replication rescue, is critical for cell survival and eventual development of cisplatin resistance [8].
Repair of platinum-induced ICLs requires the concerted activities of nucleotide excision repair (NER), translesion synthesis (TLS), Fanconi anemia (FA) and homologous recombination (HR) repair pathways. While NER is essential for ICL removal in quiescent cells [9], ICL repair in replicating cells requires orchestration of TLS and HR pathways. FA pathway proteins regulating transactions at replicating forks are also critical for ICL repair as FA pathway mutations render hypersensitivity to ICL-inducing agents [10–12]. Assembly of FA core proteins FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FANCM at the ICL site induces FANCD2 and FANCI monoubiquitination and recruitment to the damage site [13]. RAD6, an E2 ubiquitin conjugating enzyme, is a major component of the TLS pathway. RAD6 catalytic activity is essential for TLS function as yeast with inactivating mutations display hypersensitivity to alkylating and DNA crosslinking agents, indicating the importance of RAD6 function in DNA damage tolerance [14,15]. The two closely related human homologs of yeast RAD6, UBE2A (or RAD6A) and UBE2B (or RAD6B) are localized on chromosomes Xq24–25 and 5q23–31, respectively, and share 95% amino acid identity [16]. We have shown previously that RAD6B rather than RAD6A is overexpressed in breast cancers and that constitutive overexpression of RAD6B in normal breast cells induces transformation and chemoresistance whereas silencing of RAD6B in breast cancer cells compromises TLS activity and renders them chemosensitive [17,18]. RAD6 complexes with its cognate E3 ubiquitin ligase RAD18 to monoubiquitinate proliferating cell nuclear antigen (PCNA) at lysine 164 [19]. Monoubiquitinated PCNA facilitates recruitment of low fidelity TLS polymerases POL η, POL κ, POL ι or REV1 resulting in either error-free or mutagenic repair [20,21]. TLS rescues cells from replication fork-collapse, conferring tolerance to cisplatin [22–25]. RAD6/RAD18 complex is also implicated in FA network activation as RAD6 or RAD18 silencing attenuate FANCD2 monoubiquitination [26] and increase sensitivity to ICL-inducing agents [27,28]. However, the exact sequence of events linking FA pathway and TLS are not well understood.
The co-involvement of FA and TLS pathways in platinum-induced DNA damage response was demonstrated by our findings that RAD6B silencing or inhibition with a small molecule inhibitor, SMI#9, developed in our laboratory [29] attenuates PCNA and FANCD2 ubiquitinations and sensitizes cells to platinum compounds [30]. Here we show that RAD6B plays an integral role in cisplatin-induced replication stress tolerance in TNBC cells by coordinating TLS, FA and HR activities. SMI#9 inhibits restart of cisplatin-stalled replicating forks by attenuating PCNA, FANCD2 and H2AX monoubiquitinations, inhibiting POL η and γH2AX induction and foci formation, and thwarting FANCD2, POL η and RAD51 recruitments to double-strand breaks (DSBs). Pretreatment with SMI#9 sensitizes both BRCA1 wild-type and mutant TNBC cells to cisplatin in vitro and in vivo. TCGA analyses show that RAD6B copy number gain and overexpression rather than RAD6A is associated with disease progression and poor overall survival of TNBC patients. In HR-competent cells, inhibition or silencing of RAD6B reduces HR repair efficiency. The activities of RAD6B are unaffected by the BRCA1 status of TNBC cells suggesting that RAD6B role in cisplatin tolerance involves TLS/FA crosstalk in HR-dependent and independent modes. These data offer a new strategy for treating BRCA1 wild type and mutant TNBCs and for mitigating the toxic effects of cisplatin therapy.
2. Methods
2.1. Cell lines and human breast cancer tissues
SUM1315, and MDA-MB-468, MDA-MB-231 and HCC1937 TNBC cells were purchased from Asterand (Detroit, MI) and ATCC, respectively, and maintained in Dulbecco’s Minimal Essential Medium/F12 (DMEM/F12) supplemented with 5% fetal bovine serum. MCF10A cells were obtained from the Karmanos Cancer Institute Cell Core Facility and maintained as described previously [17]. To minimize drifting, several aliquots of the authenticated cells were frozen and used between 4 and 8 passages. HeLa cells stably transfected with pDR-GFP (HeLa-DR-13–9) were kindly provided by Dr. Jeffrey Parvin at The Ohio State University, and were maintained as described previously [31]. De-identified archived formalin-fixed paraffin-embedded TNBCs from women with wild-type or mutant BRCA1 were acquired after approval by the Wayne State University Human Investigation Committee.
2.2. Cell survival analysis
The sensitivities of TNBC and HeLa cells to cisplatin, SMI#9, or SMI#9 + cisplatin were measured by MTT assays. For drug combination experiments, cells were pretreated overnight with 0.1–10 μM SMI#9 or vehicle prior to the addition of equivalent concentrations of cisplatin. Cell viability was assessed at 72–96 h and results presented from three independent experiments. For clonogenic assays, SUM1315 cells were treated overnight with vehicle, 3 μM SMI#9, 1 μM cisplatin, or SMI#9 + cisplatin, trypsinized and reseeded in drug-free media at 100 cells/well in quadruplicates in 24-well plates. Colonies containing > 50 cells were enumerated to determine colony forming efficiency.
2.3. DNA damage response analysis
SUM1315, MDA-MB-468, or HCC1937 cells were treated for 4 h with respective IC50 (1 μM, 3 μM, or 15 μM) cisplatin doses, washed and allowed to recover in drug-free media for 0–24 h prior to lysis for immunoblot analysis. To assess the effect of RAD6 inhibition on cisplatin-induced response, cells were pretreated overnight with SMI#9 (3 μM for MDA-MB-468 and SUM1315 or 2 μM for HCC1937 cells) prior to cisplatin treatment.
2.4. Western blot and immunoprecipitation
Protein-matched aliquots of whole cell lysates were subjected to SDS-PAGE and immunoblot analysis of RAD6 (17), PCNA (Dako, CA), FANCD2, KU86, KU70 (Santa Cruz Biotechnology Inc., TX), POL η (Abcam, MA), γH2AX (BioLegend, CA), RAD18 (Imgenex Corp., CA), RAD51 (Calbiochem, MA), and β-actin (Sigma-Aldrich Chemicals, MO). Since the peptide we used for generating RAD6B antibody is 91% conserved in human RAD6A, the RAD6 proteins detected by our antibody will not distinguish RAD6A and RAD6B proteins, and hence is referred as RAD6 rather than RAD6A or RAD6B [17]. For PCNA or H2AX ubiquitination analysis, lysates were immunoprecipitated with anti-PCNA, rabbit anti-H2AX (Abcam) or the corresponding normal IgG, and the captured immune complexes subjected to immunoblotting with anti-ubiquitin antibody (Santa Cruz, TX). Stripped membranes were reprobed with PCNA or mouse anti-H2AX antibody (Santa Cruz) to verify PCNA or H2AX pull-down, respectively. H2AX-depleted supernatants were immunoblotted with anti-γH2AX antibody. The relative levels of monoubiquitinated-PCNA, γH2AX and monoubiquitinated-γH2AX were quantified by Image J.
2.5. DNA replication restart assay
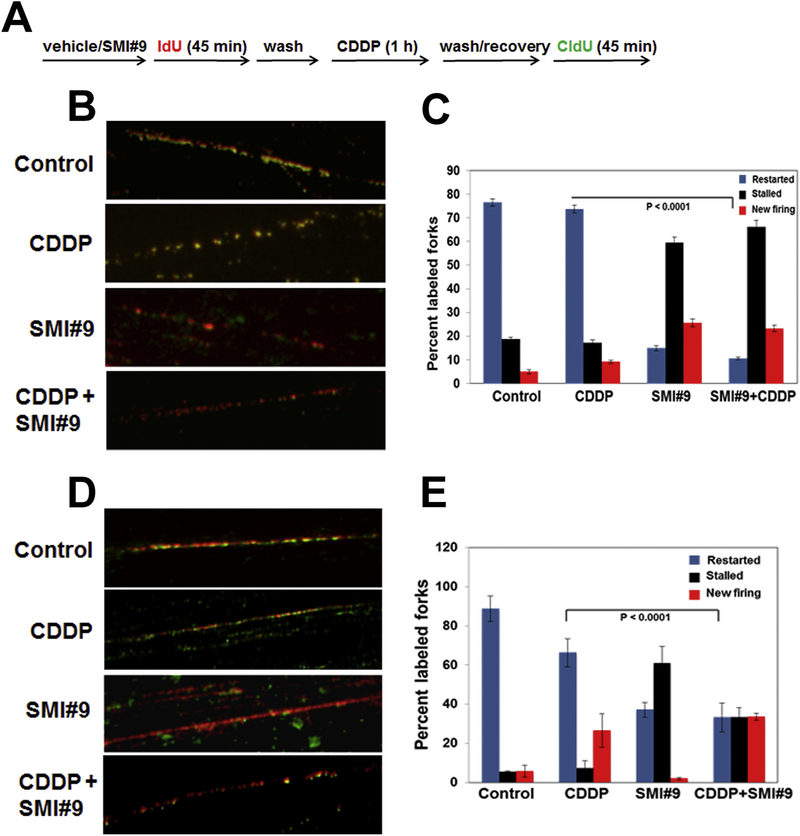
Exponentially growing MDA-MB-468 and SUM1315 cells were pulsed with 200 μM 5-iododeoxyuridine (IdU; Sigma-Aldrich Chemicals, MO) for 45 min, washed with phosphate buffered saline (PBS) and treated with vehicle or cisplatin (3 μM for MDA-MB-468 or 1 μM for SUM1315 cells) for 1 h. Cells were washed with PBS, allowed to recover for 1 h in drug-free media prior to pulse-labeling for 45 min with 200 μM 5-chlorodeoxyuridine (CldU; Sigma-Aldrich Chemicals). To determine whether BRCA1 status will influence RAD6 role in replication fork-restart following cisplatin-induced damage, MDA-MB-468 and SUM1315 cells were pretreated overnight with 1 μM SMI#9 or vehicle prior to IdU labeling and cisplatin treatment. DNA fiber spreads were prepared as previously described [30]. DNA fibers were fixed in methanol/acetic acid (3:1, v/v), denatured with 1.5 N HCl, and immunostained with mouse anti-IdU (Pierce, IL) and rat anti-CldU (Pierce) antibodies, and the corresponding Texas Red- or FITC-conjugated secondary antibodies. Images of IdU and CldU labeled DNA fibers were taken from random fields of untangled DNA fibers on an Olympus BX40 microscope equipped with a Sony high resolution/sensitivity CCD video camera and SlideBook software, and analyzed by Image J software. Approximately 35–75 individual fibers were analyzed for each experiment and the average of three independent experiments presented.
2.6. Immunohistochemical and immunofluorescence staining
RAD6 expression in breast tissues and TNBC lines was analyzed by immunohistochemical and immunofluorescence staining. Dual-immunofluorescence staining of γH2AX/FANCD2, γH2AX/POL η, or γH2AX/RAD51 was performed on SUM1315, MDA-MB-468 or HeLa cells treated for 4 h with 1, 3 or 0.4 μM cisplatin, respectively, with or without pretreatment with 1 μM SMI#9. Cells were rinsed and allowed to recover in drug-free media for 24 h prior to fixing with cold methanol/acetone and immunostaining. Images were collected on an Olympus BX40 microscope equipped with SlideBook software. Data were acquired from at least 100 cells in five-ten fields and two independent experiments.
2.7. Homologous recombination (HR) assay
HeLa-DR-13–9 cells were transfected with I-SceI-expressing plasmid pCBASce (or empty vector) kindly provided by Dr. Tej Pandita (Houston Methodist Hospital, Houston) [32]. Cells were treated with 0.4 μM cisplatin with or without overnight pretreatment with 1 μM SMI#9 or vehicle, or transiently transfected using Metafectene (Biontex, Germany) with 0.25 μg pLKO.1-puro vector encoding RAD6B shRNA#4 targeting nucleotides 451–471 of human RAD6B (UBE2B) mRNA (Accession# NM_003337) [33] or the empty vector (MISSION® shRNA, Sigma-Aldrich). Cells were transfected 24–48 h later with 0.7 μg I-SceI and 40 ng of pRL-TK Renilla luciferase vector. RAD6B knockdown was verified by immunoblotting, and Renilla luciferase reporter activity was measured to normalize transfection efficiencies. HR frequency expressed as the percent of GFP+ cells was determined three days later by flow cytometry (Amnis® ImageStream-X Mark II equipped with IDEAS software) or fluorescence microscopy.
2.8. In vivo assays
MDA-MB-468 (1 × 106) or SUM1315 (5 × 106) cells were orthotopically implanted bilaterally into the inguinal mammary gland fatpads of female athymic nude mice. When the tumors reached ~ 200 mm3, mice were randomly assigned (N = 6) to: vehicle, cisplatin (4 mg/kg body weight, once/week intraperitoneally), SMI#9 (2.5 mg/kg body weight, twice/week intratumorally), or SMI#9 + cisplatin, and mice were euthanized when the tumors reached ~750 mm3. The in vivo studies were conducted in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and institutional animal care and use committee (IACUC) guidelines of Wayne State University.
2.9. RAD6A and RAD6B copy number and TNBC patient survival
The association of RAD6A and RAD6B gene expressions with TNBC patient survival data (N = 594) was analyzed using the PROGgeneV2 database [34]. RAD6A and RAD6B copy number analysis in normal breast (111 cases), invasive lobular carcinoma (71 cases), lymph node-negative (288 cases) and lymph node-positive (340) invasive breast carcinomas, and blood DNA (702 samples) was performed with Oncomine V4.5 database.
3. Statistical analysis
Statistical analysis was performed with GraphPad Prism. Statistical comparisons were analyzed by two-tailed Student’s t-test and one way-ANOVA, and P ≤ .05 was considered significant.
4. Results
4.1. RAD6B is associated with breast cancer aggressiveness
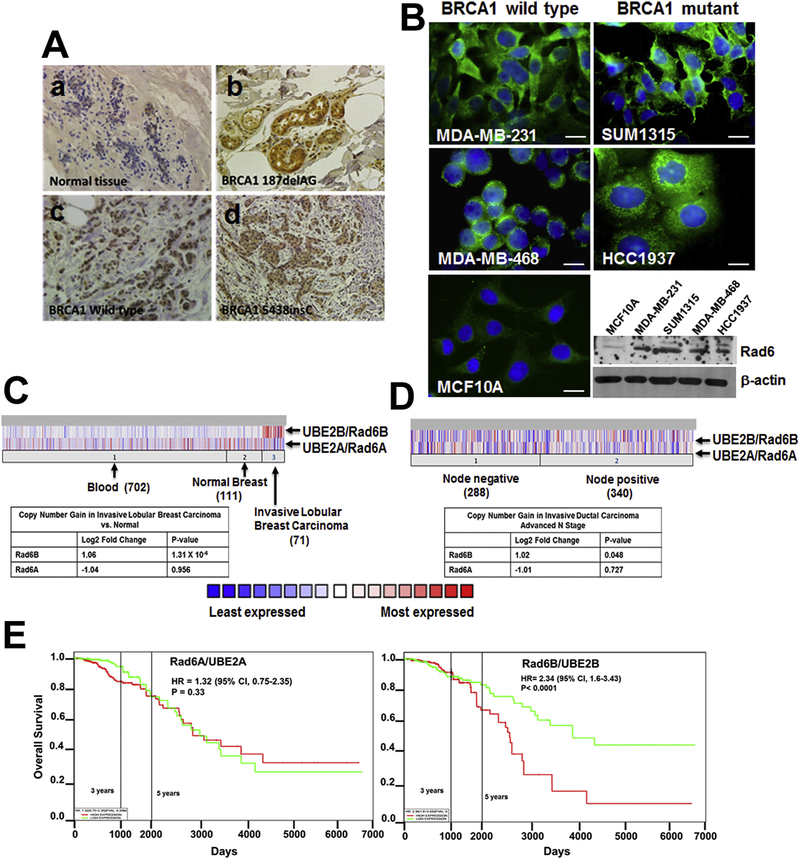
RAD6 is expressed weakly in normal ducts and overexpressed in invasive breast cancers [17]. Immunohistochemical analysis of RAD6 in BRCA1 wild-type (n = 8) and BRCA1 mutant (n = 8: three with 5438insC, two with 187delAG, one with R27P exon 4, one with 2576 delC/P1637L, and one with Q1747X mutation) TNBCs showed intense anti-RAD6 immunoreactivity in the cytoplasmic and/or nuclear compartments of all tissues tested (Fig. 1A, c and d). Interestingly, 4/4 BRCA1 mutated tumors that contained normal-looking ducts also displayed intense RAD6 staining in the cytoplasm and nuclei (Fig. 1Ab) whereas ducts in normal breast tissues showed weak staining (Fig. 1Aa). Immunofluorescence analysis of RAD6 in TNBC cell lines similarly showed strong RAD6 staining in the cytoplasmic/nuclear compartments of both BRCA1 wild-type (MDA-MB-231 and MDA-MB-468) and BRCA1 mutant (SUM1315 and HCC1937) TNBC cells whereas RAD6 staining was weak in BRCA1 wild-type nontransformed MCF10A cells (Fig. 1B). Western blot analysis corroborated 3–4.5-fold higher RAD6 levels in TNBC cells as compared to MCF10A cells (Fig. 1B), and show that RAD6 expression is not influenced by BRCA1 status.
Fig. 1. RAD6B is overexpressed in BRCA1 wild-type and mutant TNBCs and overexpression correlates with poor overall survival.
(A) Immunohistochemical, (B) immunofluorescence and western blot analysis of RAD6. Original magnification for panels a, c, d in A is ×20, and for panel b in A is ×40. Scale bars for MDA-MB-231, SUM1315, MDA-MB-468 and MCF10A cell, 10 μm; scale bar in HCC1937 panel, 20 μm. (C,D) RAD6A and RAD6B copy number, and (E) association with TNBC patient survival (N = 594).
Our previous studies identified RAD6B, rather than RAD6A, to be overexpressed in breast cancer cell lines and tumor tissues. Constitutive RAD6B overexpression in MCF10A cells induces hyperplastic growth, cisplatin resistance and genomic instability, whereas RAD6B silencing inhibits breast cancer development [17,18,35,36]. To determine whether RAD6B overexpression in clinical breast cancers reflect copy number alterations, we analyzed RAD6A and RAD6B copy numbers using Oncomine. Invasive lobular breast carcinomas showed a 1.06 log2-fold increase in RAD6B (P = 1.31 × 10−4) copy number as compared to normal breast or blood DNA whereas RAD6A showed no association (Fig. 1C). Similar analysis showed 1.02 log2-fold increase in RAD6B copy number in lymph node-positive invasive ductal breast carcinomas as compared to lymph node-negative carcinomas (P = .048), whereas RAD6A showed no association (Fig. 1D). RAD6A and RAD6B associations with TNBC patient survival were analyzed using the PROGgeneV2 database. High RAD6B expression (N = 297) was negatively associated with overall survival (hazard ratio = 2.34 (5% CI, 1.6–3.43), P < .0001; Fig. 1E) while RAD6A expression showed no association (hazard ratio = 1.32 (95% CI, 0.75–2.35), P = .33; Fig. 1E). These data support a role for RAD6B in breast cancer aggressiveness.
4.2. RAD6 inhibition sensitizes TNBC cells to cisplatin in vitro and in vivo
Clinical data suggest that BRCA1 mutation confers cisplatin sensitivity presumably because of impaired HR pathway [37–40]. We performed MTT assays to compare the responses of BRCA1 wild-type and mutant TNBC cells treated with cisplatin. We did not observe a direct association between cisplatin sensitivity and BRCA1 status: BRCA1 mutant HCC1937 (IC50 20 μM) > BRCA1 wild-type MDA-MB-231 (IC50 12 μM; Ref. [30]) > MDA-MB-468 (IC50 3.8 μM) > BRCA1 mutant SUM1315 (IC50 1 μM) cells (Fig. 2A–C; Supplementary Fig. S1), and are consistent with those of Lehmann et al. [1].
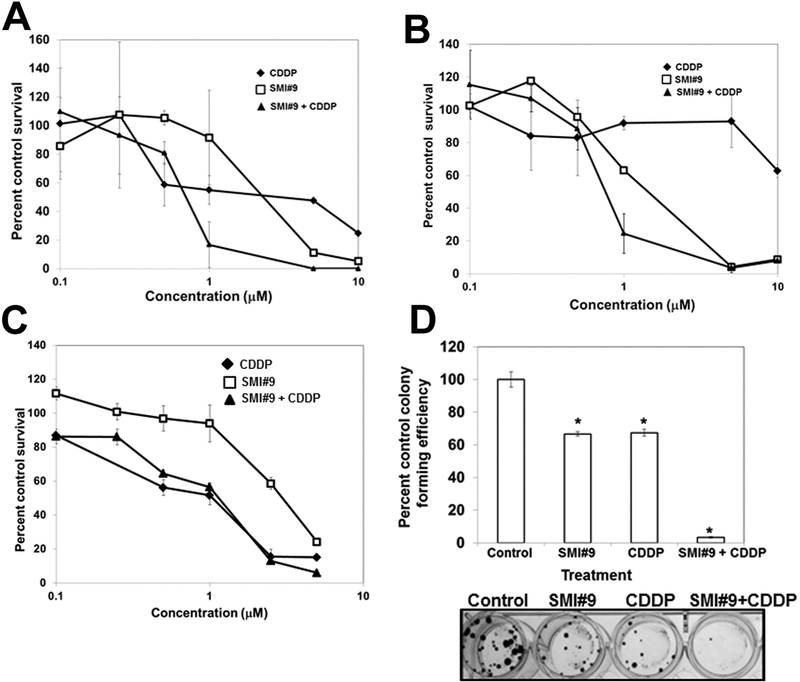
Fig. 2. RAD6 inhibition enhances cisplatin (CDDP) sensitivities of BRCA1 wild-type and mutant TNBC cells in vitro.
MTT assays of MDA-MB-468 (A), HCC1937 (B), SUM1315 (C) cells. Data are mean ± S.D. of three independent experiments. (D) Clonogenic assay. SUM1315 cells were treated overnight with SMI#9 (3 μM), CDDP (1 μM), or with a combination of SMI#9 and CDDP. Cells were trypsinized and reseeded in quadruplicates at 100 cells per well. Results are mean ± S.D. percent colony formation efficiency from two independent experiments. *Indicates P < .01.
To evaluate the impact of RAD6 inhibition on cisplatin sensitivities, TNBC cells were pretreated overnight with 0–10 μM SMI#9 prior to treatment with equivalent doses of cisplatin. Isobologram analysis revealed synergistic increases in cisplatin sensitivities in SMI#9 pretreated MDA-MB-468 and HCC1937 cells but not in SUM1315 cells (Fig. 2A–C; Supplementary Fig. S2 A–C). To determine whether this lack of synergy resulted from cytostatic cell survival, we performed colony forming assays. Treatment with 3 μM SMI#9 or 1 μM cisplatin alone significantly reduced SUM1315 clonogenic potential by ~40% (P < .05), which was inhibited by > 90% in cells treated with SMI#9 + cisplatin combinations as compared to control (P < .001; Fig. 2D). The metabolic state (measured by MTT assays) and clonogenic potentials are not necessarily parallel events, which could contribute to the discrepancies between the MTT and clonogenic assay results. At lower doses, SMI#9 could induce presenescence with retention of metabolic activity while not actively dividing during the shorter MTT assay period as compared to clonogenic assays that measures cell survival and reproductive capacity over two to three weeks. These data show that RAD6 inhibition enhances cisplatin sensitivities of both BRCA1 wild-type and mutant TNBC cells and reveal an important role for RAD6 in repair of cisplatin-induced DNA damage in TNBCs.
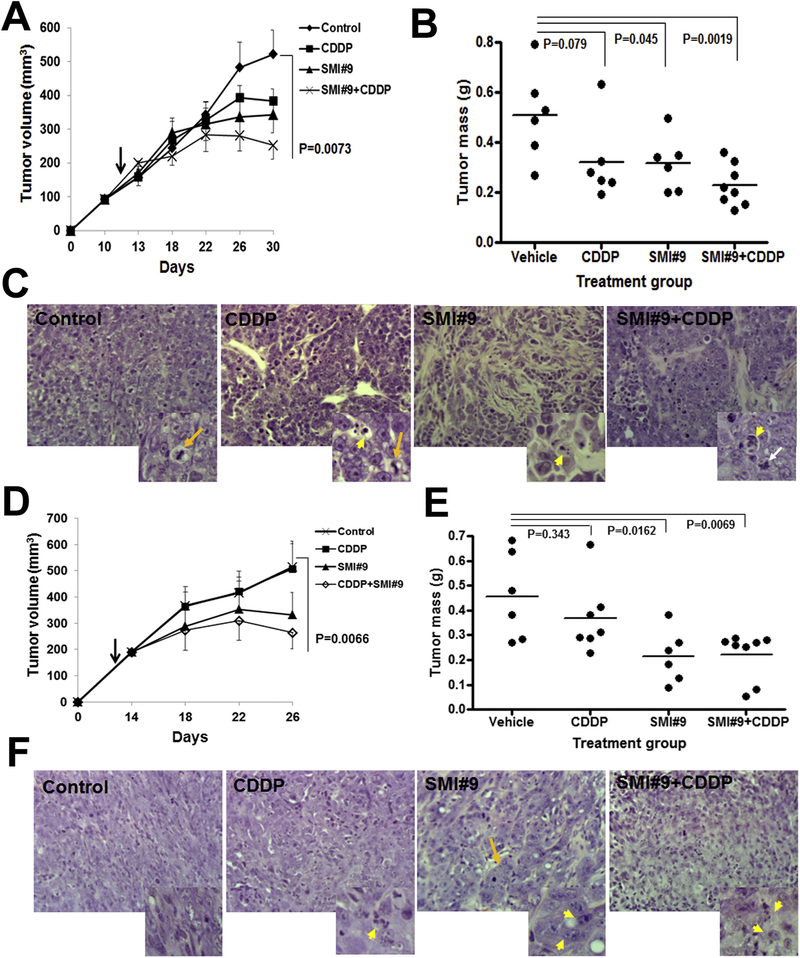
We next investigated the in vivo effects of SMI#9 administered alone or in combination with cisplatin on BRCA1 wild-type and mutant TNBC growth. Mice bearing orthotopic bilateral implants of TNBC cells were randomized and treatments with vehicle, cisplatin, SMI#9, or SMI#9 + cisplatin initiated when the lesions were ~200 mm3. Compared to controls, MDA-MB-468 (P = .0073) and SUM1315 (P = .0066) tumor volumes and excised tumor mass were significantly inhibited by SMI#9 + cisplatin treatment (Fig. 3A, B, D, E). MDA-MB-468 (P = .045) and SUM1315 (P = .0162) tumor mass in SMI#9 treated mice were significantly decreased compared to control but were not different from SMI#9 + cisplatin groups (Fig. 3B, E). H&E stained tumors showed elevated mitosis in control MDA-MB-468 tumors (Fig. 3C and inset, long arrow). Mitotic (long arrow) and rare apoptotic (short arrow) cells were observed in cisplatin-exposed tumors (Fig. 3C and inset). SMI#9 and SMI#9 + cisplatin treated tumors were sparsely populated, lacked mitotic cells and contained apoptotic cells (short arrow) and cells with nuclear morphology characteristic of mitotic catastrophe (white arrow; Fig. 3C insets). Consistent with the mesenchymal TNBC subtype, control SUM1315 tumors were robustly populated with EMT (epithelial-mesenchymal transition)-phenotype cells (Fig. 3F). While cisplatin treatment induced apoptosis (Fig. 3F, short arrow in inset), SUM1315 tumors treated with SMI#9 or SMI#9 + cisplatin (Fig. 3F and insets) showed loss of EMT typified by a glandular or differentiated phenotype (Fig. 3F), and abundant apoptotic cells (Fig. 3F, short arrows in insets). SMI#9-induced EMT loss is consistent with our previous data that showed reversal of EMT in Rad6B-silenced breast cancer cells [36]. Since SMI#9 shows variable in vitro sensitivities in TNBC cells, our data suggest that SMI#9 may differentially influence cell metabolism and consequently affect MTT assay results. However, the results of metabolism-independent assays such as colony forming (Fig. 2D) and in vivo assays (Fig. 3) provide a better picture of SMI#9 drug responses in TNBC cells.
Fig. 3. SMI#9 inhibits BRCA1 wild-type and mutant TNBC growth in vivo.
(A-C) MDA-MB-468, (D-F) SUM1315 tumors. (A,D) tumor volumes, (B,E) vertical scatter plots of excised tumor mass at time of sacrifice. (C,F) H&E analysis. (C) Long yellow arrows, mitotic cells; long white arrow, cells undergoing mitotic catastrophe. (F) Long yellow arrow in SMI#9 group indicates loss of EMT. Short yellow arrows in C and F indicate apoptosis. Original magnification ×40; data analyzed by one-way ANOVA and 2-tailed Student’s t-test.
4.3. SMI#9-induced cisplatin sensitization is associated with concomitant attenuation of TLS and FA pathway activation in BRCA1 wild-type and mutant TNBC cells
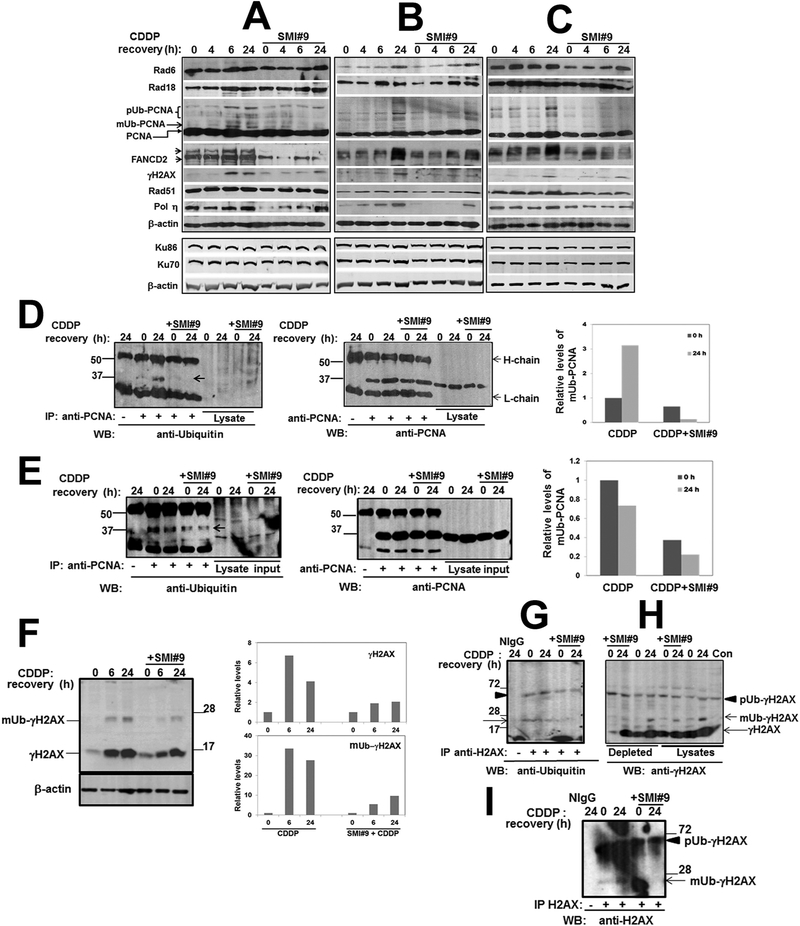
Our data from Figs. 2 and 3 implicate RAD6 in cisplatin response modulation in both BRCA1 wild-type and mutant TNBC cells. To elucidate the underlying mechanisms by which RAD6 ubiquitin conjugating activity coordinates cisplatin-induced damage response, TNBC cells were pretreated overnight with vehicle or SMI#9 prior to cisplatin treatment and recovery in drug-free media for 0–24 h. The steady-state levels of TLS (RAD6, RAD18, PCNA, POL η), FA (FANCD2), HR (RAD51), and NHEJ (KU70/KU86) pathway proteins, and γH2AX were analyzed by immunoblotting. Rad6 and Rad18 proteins showed similar patterns of regulation by cisplatin and SMI#9 (Fig. 4A–C). Densitometric quantitation showed that Rad6 protein levels in SMI#9 pretreated cells either remained unaltered (MDA-MB-468; Fig. 4A) or decreased slightly (SUM1315, HCC1937; Fig. 4B and C and Supplementary Fig. S3). Although nascent PCNA steady-state levels were unaffected, a band corresponding to monoubiquitinated-PCNA (surrogate marker of RAD6-mediated TLS activation), was detectable at 4–6 h post-cisplatin treatment which was attenuated by SMI#9 in both BRCA1 wild-type and mutant TNBC cells (Fig. 4A–C). PCNA monoubiquitination and SMI#9 inhibition were confirmed by analyzing PCNA immunoprecipitates with anti-ubiquitin antibody (Fig. 4D and E). SMI#9 pretreatment caused a 2-fold decrease in or loss of cisplatin-induced monoubiquitinated-PCNA in SUM1315 (Fig. 4D) and MDA-MB-468 (Fig. 4E) cells, respectively. The efficacy of PCNA-pulldown relative to monoubiquitinated-PCNA was confirmed by reprobing the stripped membranes with anti-PCNA antibody (middle panels in Fig. 4D and E). High molecular weight PCNA forms, potentially representing polyubiquitinated-PCNA, were also induced by cisplatin; however, SMI#9 effects were marginal or inconsistent (Fig. 4A–C). FANCD2 analysis revealed bands corresponding to unmodified (lower band) and monoubiquitinated (upper band) FANCD2 (12) which were clearly seen in MDA-MB-468 cells (Fig. 4A). Monoubiquitinated-FANCD2 was enhanced by cisplatin, and SMI#9 pretreatment downregulated FANCD2 steady-state levels in all TNBC cells suggesting a role for RAD6 in FANCD2 stability (Fig. 4A–C, and Supplementary Fig. S3). Cisplatin-induced γH2AX was also attenuated by SMI#9, linking RAD6 in cisplatin-induced DSB repair (Fig. 4A–C).
Fig. 4. SMI#9 attenuates cisplatin-induced increases in PCNA, FANCD2 and H2AX ubiquitination, and γH2AX and POL η levels.
(A-C) Western blot analysis of the indicated proteins in MDA-MB-468 (A), SUM1315 (B) and HCC1937 (C) whole cell lysates. SUM1315, MDA-MB-468 or HCC1937 cells were treated with 1, 3 or 15 μM cisplatin (CDDP) for 4 h, washed and allowed to recover for 0–24 h prior to lysis. For experiments involving Rad6 inhibition, cells were pretreated overnight with 3 μM (MDA-MB-468, SUM1315) or 2 μM (HCC1937) SMI#9 prior to CDDP treatment. (D,E) Analysis of PCNA immunoprecipitates in SUM1315 (D) and MDA-MB-468 (E) lysates. Arrow indicates monoubiquitinated-PCNA. Middle and far right panels in D and E represent PCNA pulldown and monoubiquitinated-PCNA quantification, respectively. (F) Western blot analysis of γH2AX and quantitation. (G) H2AX ubiquitination analysis. (H) Immunoblot analysis of γH2AX in H2AX-immunodepleted supernatants. (I) Stripped blot from (G) reprobed with anti-H2AX antibody to authenticate ubiquitinated-H2AX.
Monoubiquitinated-PCNA and FANCD2 interact with the TLS polymerase POL η and facilitate its recruitment to the damage sites [27,41]. POL η also participates in HR repair during strand invasion and D-loop formation via RAD51 interaction, and loss of POL η decreases HR [42]. To investigate the impact of RAD6 activity loss on TLS-HR link, we analyzed the levels/regulation of POL η and RAD51. SMI#9 pretreatment markedly delayed cisplatin-mediated POL η induction but had little effect on RAD51 steady-state levels (Fig. 4A–C). KU70 and KU86 proteins were unaffected by SMI#9, suggesting noninvolvement of RAD6 on NHEJ core proteins during cisplatin-induced damage response (Fig. 4A–C).
Induction of H2AX phosphorylation depends upon H2AX monoubiquitination [43]. To determine whether the reduced γH2AX levels resulted from SMI#9-induced inhibition of H2AX ubiquitination, SUM1315 cells were treated with SMI#9 and cisplatin as described above. γH2AX and monoubiquitinated-γH2AX steady-state levels increased by 6 h of cisplatin post-treatment, and SMI#9 pretreatment caused commensurate decreases in both γH2AX and monoubiquitinated-γH2AX levels (Fig. 4F). To determine SMI#9 effect on H2AX ubiquitination, cell lysates were immunoprecipitated with rabbit anti-H2AX antibody and probed with mouse anti-ubiquitin antibody. Ubiquitin-reactive H2AX bands corresponding to the monoubiquitinated (Fig. 4G, arrow) and polyubiquitinated (Fig. 4G, arrowhead) forms were detected, and SMI#9 decreased monoubiquitinated but not polyubiquitinated H2AX. Reprobing the stripped membranes with mouse anti-H2AX antibody authenticated the ubiquitinated-H2AX bands and confirmed the loss of monoubiquitinated-H2AX in SMI#9 treated cells (Fig. 4I, arrow). The H2AX antibody only showed mild immunoreactivities to phosphorylated H2AX as the bulk of the nonubiquitinated- (Fig. 4H, long arrow), monoubiquitinated- (Fig. 4H, short arrow), and polyubiquitinated- (Fig. 4H, arrowhead) γH2AX remained in the immunodepleted supernatants (Fig. 4H). Monoubiquitinated-γH2AX, but not polyubiquitinated-γH2AX, was induced by cisplatin (Fig. 4H). γH2AX and monoubiquitinated-γH2AX levels in the H2AX-depleted supernatants were lower in SMI#9-pretreated cells as compared to cisplatin whereas polyubiquitinated-γH2AX was unaffected (Fig. 4H). These data suggest that RAD6 may play an important role in maintaining cisplatin-induced γH2AX via regulation of H2AX monoubiquitination.
4.4. SMI#9-mediated cisplatin sensitization of TNBC cells involves blockade of replication-restart resulting from impaired recruitment of repair proteins to damage sites
Our data thus far show that SMI#9-induced chemosensitization is associated with delayed POL η induction and attenuation of FANCD2 and γH2AX activations. As TLS, FA and HR are required for proper repair of ICLs and stalled replication fork-restart, we compared the replication fork-restart capabilities of RAD6-inhibited BRCA1 wild-type and mutant TNBC cells. MDA-MB-468 and SUM1315 cells were labeled as described in Fig. 5A. The DNA fibers from control and cisplatin treated MDA-MB-468 cells show contiguous clusters of replicons defined by merging of IdU (red) and CldU (green) tracks indicating adjacent replicons completing replication within the total pulse time (Fig. 5B, C and Supplementary Fig. S4). SUM1315 cells showed efficient DNA synthesis in control cells which was decreased by cisplatin, potentially reflecting HR defects due to BRCA1 deficiency (Fig. 5D and E). However, consistent with SMI#9-induced cisplatin sensitization (Figs. 2 and 3), the DNA fibers from SMI#9 treated TNBC cells show single tracks of red indicating sites where replication was terminated or stalled and failed to incorporate the CldU nucleotide from the second pulse (Fig. 5B–E, and Supplementary Fig. S4). These data show that restart of cisplatin-stalled replication forks is significantly blocked by SMI#9 in both BRCA1 wild-type and mutant TNBC cells (P < .0001; Fig. 5C and E).
Fig. 5. Restart of stalled-replication forks is impeded by RAD6 inhibition.
(A) Labeling scheme. (B-E) Representative images of MDA-MB-468 (B,C) and SUM1315 (D,E) DNA fibers immunostained with anti-IdU (red) and anti-CldU (green) antibodies. (C,E) Quantification of stalled, restarted and aberrant newly fired forks. ~35–75 individual fibers were analyzed for each experiment and the average of three independent experiments presented. Data were analyzed by two-tailed Student’s t-test. Details of treatment conditions are provided under Methods.
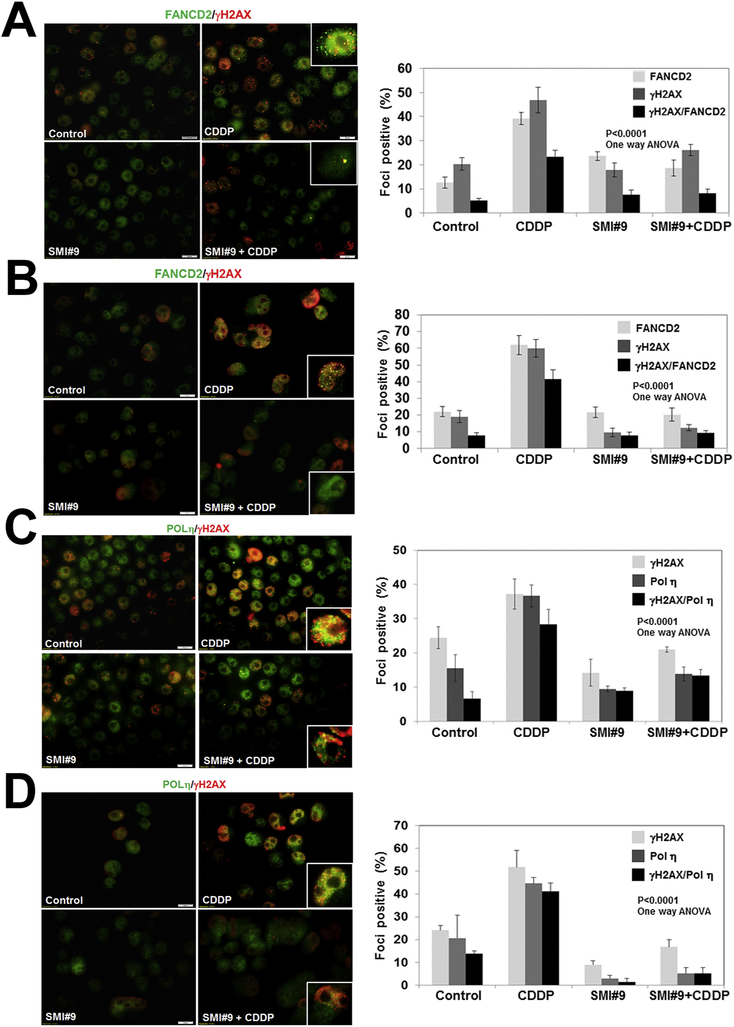
To verify whether the failure to restart cisplatin-stalled replication forks in SMI#9 treated cells results from defective TLS, FA and/or HR pathway activation(s), we analyzed foci development and recruitment of FANCD2, POL η and RAD51 to γH2AX. Whereas FANCD2 staining in the nuclei is diffuse in control and SMI#9 treated cells, cisplatin treatment induced robust FANCD2 foci formation in both MDA-MB-468 (Fig. 6A) and SUM1315 (Fig. 6B) cells. SMI#9 pretreatment abolished cisplatin-induced FANCD2 foci formation (Fig. 6A and B). FANCD2/γH2AX colocalized foci are abundantly present in cisplatin treated TNBC cells but were significantly decreased in SMI#9 treated MDA-MB-468 (P < .0001; Fig. 6A) and SUM1315 (P < .0001; Fig. 6B) cells. Pol η was detectable in the nuclei of all cells; however, the majority of cells in control and SMI#9 treated conditions displayed diffuse/weaker staining as compared to cisplatin treated cells that showed strong and granular (indicative of foci) Pol η immunoreactivity with a substantial proportion showing Pol η/γH2AX colocalization (Fig. 6C and D). Pretreatment with SMI#9 attenuated the cisplatin-induced effects on Pol η and γH2AX foci formation (Fig. 6C and D; P < .0001, one-way ANOVA). RAD51 is a key component of HR repair and RAD51 foci formation is an indicator of HR repair. In control and SMI#9-treated MDA-MB-468 cells, RAD51 is sequestered into diffuse pools which upon cisplatin treatment become mobilized into discrete foci that colocalize with γH2AX (Fig. 6E). SMI#9 pretreatment did not affect cisplatin-induced RAD51 foci formation; however, RAD51 association with γH2AX was diminished due to SMI#9 inhibition of γH2AX foci development (Fig. 6E). In BRCA1 mutant SUM1315 cells, cisplatin treatment failed to induce RAD51 foci formation confirming BRCA1 requirement for HR (Fig. 6F). Since SMI#9 treatment impedes γH2AX, POL η, and FANCD2 foci formation and interactions (including RAD51) with γH2AX, our findings suggest that regulation of H2AX ubiquitination by RAD6 may play a central role in managing TLS/FA crosstalk in HR-proficient and HR-compromised TNBC cells during repair of cisplatin-induced ICLs.
Fig. 6. SMI#9 treatment impedes cisplatin-induced recruitment and colocalization of DNA damage response proteins in TNBC cells.
Dual immunofluorescence staining of FANCD2/γH2AX, POL η/γH2AX, or RAD51/γH2AX in MDA-MB-468 (A,C,E) or SUM1315 (B,D,F) cells treated with 1 μM (SUM1315) or 3 μM (MDA-MB-468) CDDP with or without pretreatment with 1 μM SMI#9. Scale bars, 20 μm. Graphs show foci positive cells scored from at least 100 cells in five-ten fields from two independent experiments by Image J. Cells containing > 5 colocalized foci were counted for protein colocalization. Results were analyzed by 2-tailed Student’s t-test. Details of treatment conditions are provided under Methods.
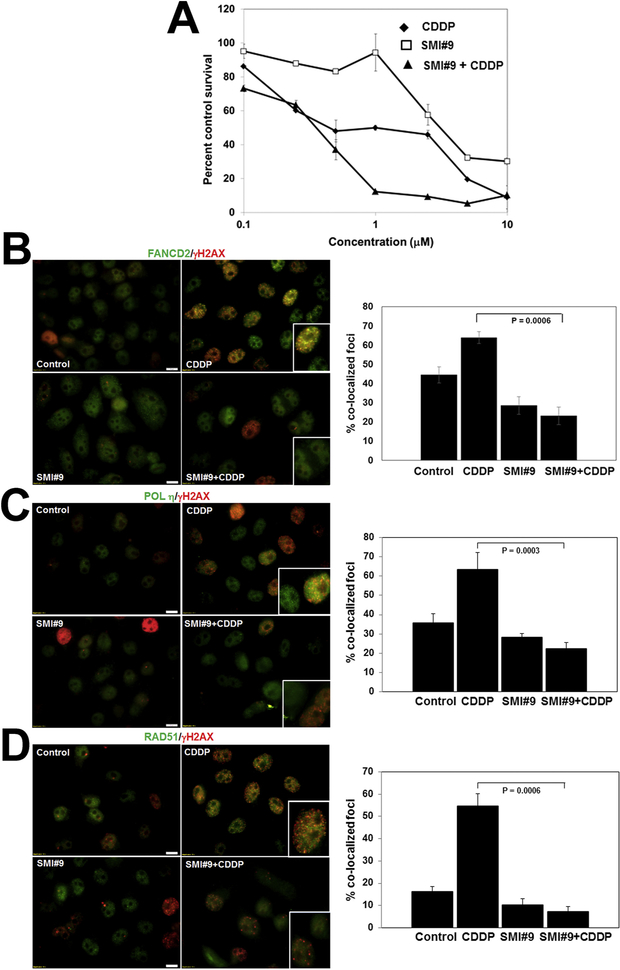
To determine the general relevance of RAD6 in cisplatin-induced ICL repair, we assessed the impact of SMI#9 pretreatment on cisplatin sensitivity of HR-proficient HeLa cells. As with TNBC cells, SMI#9 pretreatment increased cisplatin sensitivity of HeLa cells (Fig. 7A and Supplementary Fig. S5). SMI#9 effects on FANCD2, POL η and RAD51 foci formation and colocalization with γH2AX foci were evaluated as described above. Cisplatin induced robust foci formation and colocalization of FANCD2 (Fig. 7B) and POL η (Fig. 7C) with γH2AX, which was inhibited by SMI#9 (Fig. 7B, C). Although SMI#9 had no effect upon cisplatin-induced RAD51 foci formation, RAD51 interaction with γH2AX was impaired by SMI#9 (Fig. 7D). These data establish a general requirement for RAD6 ubiquitin conjugating activity in coordinating TLS/FA/HR crosstalk in HR-proficient cells during cisplatin-induced DNA damage response.
Fig. 7. RAD6B plays a general role in repair of cisplatin-induced DNA damage.
(A) SMI#9 effect on cisplatin (CDDP) sensitivity of HeLa cells by MTT assay. Data are mean ± S.D. of three independent experiments. (B-F) Representative images of FANCD2/γH2AX (B), POL η/γH2AX (C), or RAD51/γH2AX (D) dual immunofluorescence staining. Scale bars, 10 μm. Foci positive cells were scored from ~100 cells in five-ten fields, and cells containing > 5 colocalized foci were enumerated for protein colocalization. Data were analyzed by 2-tailed Student’s t-test.
4.5. RAD6B silencing/inhibition mitigates HR repair
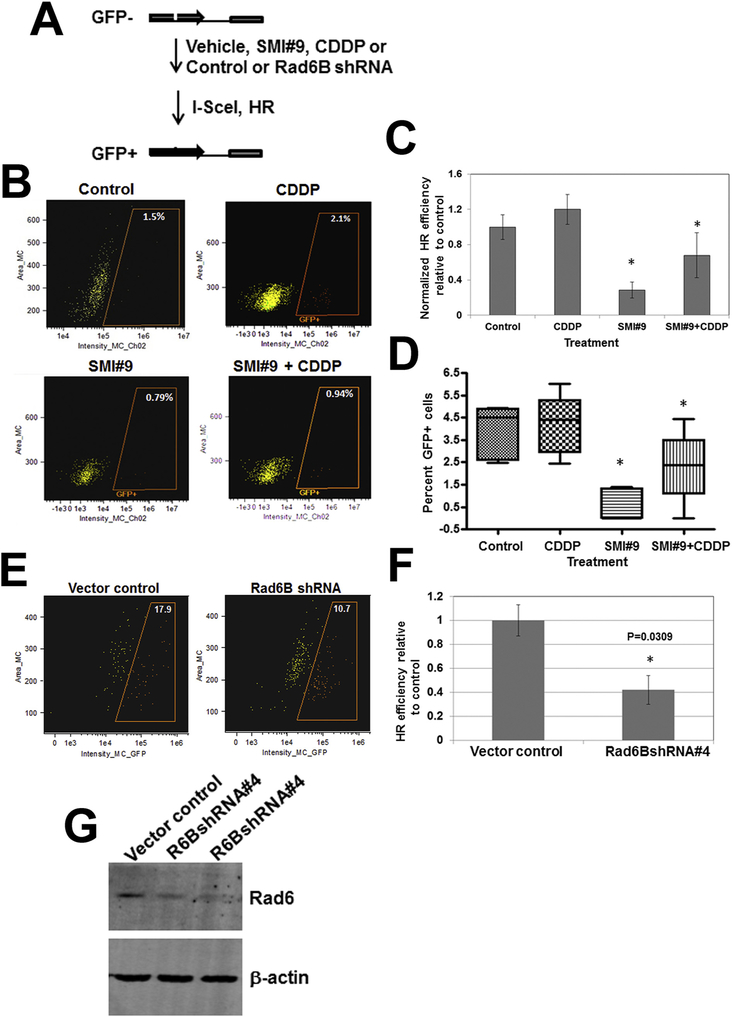
To confirm whether RAD6B plays a direct role in HR repair, HeLa-DR-GFP cells were cotransfected with I-SceI-expressing plasmid pCBASce (or vector control) and pRL-TK with or without prior treatment with SMI#9 as described in Fig. 8A. In experiments involving cisplatin, cells were treated with cisplatin for 4 h, washed and then allowed to recover in cisplatin-free media prior to I-SceI transfection. GFP+ cells were quantified by flow cytometry or fluorescence microscopy. Flow cytometry analysis showed a slight but nonsignificant increase in GFP+ cells (HR efficiency) in cisplatin-treated group as compared to control, which was significantly reduced by SMI#9 pretreatment (P < .05; Fig. 8B, C). Visual scoring of GFP+ cells similarly showed SMI#9 inhibition of HR efficiency (P < .05; Fig. 8D and Supplementary Fig. S6). RAD6B relevance in HR was further verified by RAD6B knockdown. HR efficiency determined by flow cytometry and visual scoring of GFP+ cells showed 40–50% decrease in RAD6BshRNA#4 transfected cells as compared to control vector transfected cells (Fig. 8E, F and Supplementary Fig. S7). Concordant with these data, immunoblot analysis confirmed > 50% RAD6B suppression in RAD6BshRNA#4 transfected cells as compared to control vector (Fig. 8G). The HR efficiency decreases were not due to transfection efficiency variations as confirmed by coexpressed luciferase reporter activities. Taken together, the data from SMI#9 and RAD6B gene silencing experiments suggest that inhibition or loss of RAD6B decreases HR efficiency, establishing RAD6B requirement in HR repair of cisplatin-induced ICLs.
Fig. 8.
Rad6B silencing or inhibition decreases homologous recombination (HR) efficiency. (A) HR assay scheme with DR-GFP HeLa cells. Flow cytometry (B,C) and fluorescence microscopy (D) analysis of GFP positive cells in SMI#9 (1 μM) and CDDP (0.4 μM) treated cells. (E) Flow cytometry analysis of GFP positive cells in vector control and Rad6BshRNA transfected cells. Results were analyzed by one-way ANOVA and Student’s t-test. *Indicates P < .05. (F) Western blot analysis of RAD6.
5. Discussion
This is the first study demonstrating the integrating role of RAD6B ubiquitin conjugating activity in modulating cisplatin-induced H2AX phosphorylation and TLS/FA crosstalk in BRCA1 wild type and BRCA1 mutant TNBC cells. In this study, using a small molecule inhibitor targeting the RAD6 catalytic activity [29], we provide direct evidence of RAD6B activity in TLS/FA/HR crosstalk and the importance of targeting RAD6B for BRCA1 wild-type and mutant TNBC therapy. Although RAD6A and RAD6B homologs have similar functions, we have shown previously that RAD6B is the major player in breast cancer development/progression [17,18] and our present data show that silencing/inhibiting RAD6B diminishes HR repair in HR-proficient cells. However, the RAD6B activities are independent of BRCA1 as SMI#9 treatment sensitizes both BRCA1 wild type and mutant TNBCs to cisplatin. The clinical relevance of RAD6B (and not RAD6A) is further supported by TCGA data that show a direct association between RAD6B copy number and advanced breast cancer, and RAD6B overexpression and poor survival of TNBC patients.
RAD6-mediated PCNA monoubiquitination, a DNA damage-induced event, is imperative for FANCD2 ubiquitination and recruitment of POL η to the damage sites [26–28]. Consistent with these reports, our data show that RAD6B silencing [30] or inhibition attenuates cisplatin-induced PCNA and FANCD2 ubiquitinations and delays POL η accumulation. Interestingly, SMI#9 treatment also attenuates cisplatin-induced γH2AX levels, γH2AX foci formation and γH2AX recruitment to replication-stalled sites [30]. To determine whether the inhibition of stalled-replication fork-restart results from disruption of protein recruitments, we examined FANCD2, POL η and RAD51 foci formation, and their localization to γH2AX foci. Consistent with FA and TLS functions in platinum-induced ICL repair, FANCD2 and POL η foci formation and their interactions with γH2AX foci are inhibited by SMI#9 primarily because of the SMI#9 inhibitory effects on γH2AX. These activities of RAD6 are unaffected by the BRCA1 status of TNBC cells implicating a critical upstream role for RAD6. Concordantly, irrespective of the BRCA1 status, SMI#9 and SMI#9 + cisplatin treatments cause accumulation of stalled forks and inhibit TNBC growth establishing its in vivo relevance.
RAD51 is a key component of the HR repair pathway and RAD51 foci formation on the resected DNA strand is indicative of HR repair [44]. Consistent with BRCA1 requirement for RAD51 foci development [45], RAD51 foci formation is impaired in BRCA1 mutant TNBC cells whereas FANCD2, POL η and γH2AX activations by cisplatin are unaffected. Cisplatin-induced RAD51 foci formation in BRCA1 wild-type TNBC and HeLa cells is unaffected by SMI#9, yet HR repair is reduced in SMI#9 treated and RAD6B-depleted cells. SMI#9-induced HR repair defect correlates with the failure to activate POL η and γH2AX foci formation, and recruitment of RAD51 and POL η to DSBs. Since SMI#9 inhibits and sensitizes both HR-proficient and HR-compromised TNBC cells, our data reveal a BRCA1-independent role of RAD6B ubiquitinating activity in repair of cisplatin-induced lesions.
The E2 ubiquitin conjugating enzyme Ubc13 in partnership with RNF8 and RNF168 E3 ligases has been linked to H2AX ubiquitination [46,47]. Screening of E2 enzymes important for ionizing radiation-induced ubiquitination and recruitment to DSBs identified RAD6A and RAD6B working with RNF168 [48]. Since RAD6B loss by gene silencing or inactivation by SMI#9 impedes cisplatin-induced γH2AX levels and foci formation [30], we analyzed SMI#9 effects on H2AX ubiquitination and phosphorylation. Immunoprecipitation and ubiquitination analysis showed that SMI#9 selectively inhibits H2AX monoubiquitination with minimal effect on H2AX polyubiquitination. Cisplatin induced the nonubiquitinated and monoubiquitinated γH2AX forms but not polyubiquitinated γH2AX, and strikingly SMI#9 decreased only the cisplatin-responsive γH2AX forms. Interestingly, the H2AX immunoprecipitates predominantly contained unphosphorylated H2AX derivatives as the bulk of phosphorylated H2AX forms were retained in the immunodepleted supernatants. Although the reasons for this are not yet clear, it is possible that the phosphorylated epitope in γH2AX hindered its pulldown by the H2AX antibody. Besides RNF168, RAD18 and RNF20/RNF40 are known partners of RAD6A and RAD6B, and complexes of RAD6 with RNF20/RNF40 have been implicated in DNA damage response [49,50]. Repair of DSBs in the heterochromatin requires local disassembly of HP1α to facilitate RAD51 nucleoprotein filament formation for HR repair [51,52], and a role for RAD6 in autophagy-mediated HP1α degradation and HR repair has been reported [53]. These data support our results demonstrating a direct role for RAD6B activity in HR repair. SMI#9 treatment induced no changes in the NHEJ core Ku proteins. However, since γH2AX foci represent DSBs undergoing both NHEJ and HR, and γH2AX foci formation is disrupted by SMI#9 with augmented cisplatin sensitization of both BRCA1 wild-type and mutant TNBC cells, our results establish a ubiquitous regulatory role for RAD6B in DSB repair in TNBC cells.
6. Conclusion
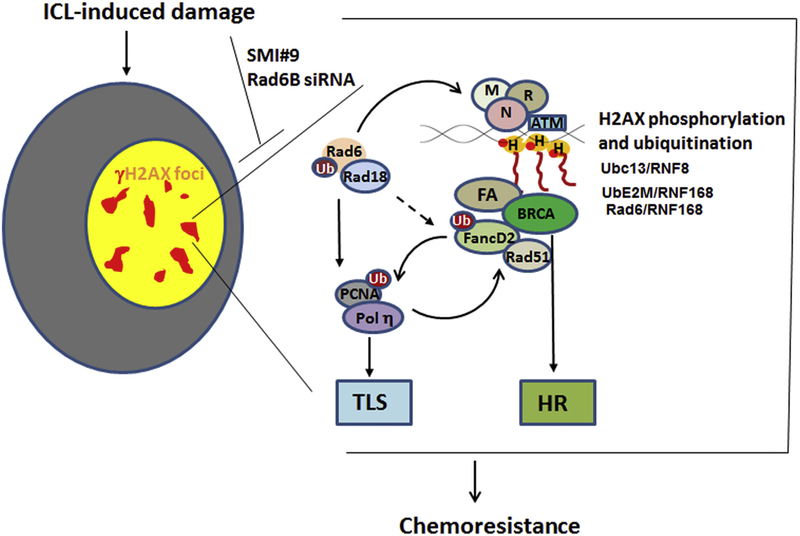
Our results reveal a key role for RAD6B ubiquitin conjugating activity in coordinating cisplatin-induced H2AX phosphorylation and TLS/FA activities via HR-dependent and independent modes (Fig. 9). Our data implicate the general importance of RAD6B in cisplatin resistance and the benefits of targeting it for TNBC treatment.
Fig. 9. Model for RAD6-dependent activity in platinum-induced ICL repair.
RAD6 participates in cisplatin-induced DNA crosslink repair by activating TLS and FA networks and coordinating crosstalk with HR-mediated repair. Inhibition of RAD6B E2 ubiquitin conjugating activity with SMI#9 inhibits cisplatin-induced H2AX monoubiquitination resulting in decreased H2AX phosphorylation that is required for proper DSB signaling via HR and non-HR modes. γH2AX, POL η and FANCD2 foci formation, and recruitments of POL η, FANCD2 and RAD51 to DSBs are impaired by SMI#9, implicating the significance of RAD6 in ICL repair in BRCA1 wild type and mutant TNBC cells.
Supplementary Material
Acknowledgements
We thank Drs. Jeffrey Parvin and Tej Pandita for providing HeLa-DR-13-9 cells and pCBASce vectors, respectively. Financial information: This work was supported by National Cancer Institute (grant number R21CA178117 to MPS), Molecular Therapeutics Program of Karmanos Cancer Institute (to MPS) and Bridge grant from Wayne State University (to MPS). The histology core facility is supported by NCI Comprehensive Cancer Center Center grant P30 CA022453 to the Karmanos Cancer Institute at Wayne State University. BH was supported by Initiative for Maximizing Student Diversity (IMSD) award from National Institutes of Health (GM058905) and Ruth L. Kirschstein National Research Service Award T32-CA009531 training grant from NIH.
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
Transparency document
The Transparency document associated with this article can be found, in online version.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbadis.2019.165561.
References
- [1].Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. , Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies, J. Clin. Invest 121 (2011) 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Malorni L, Shetty PB, De Angelis C, Hilsenbeck S, Rimawi MF, Elledge R, et al. , Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up, Breast Cancer Res. Treat 136 (2012) 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guarneri V, Dieci MV, Conte P, Relapsed triple-negative breast cancer: challenges and treatment strategies, Drugs 73 (2013) 1257–1265. [DOI] [PubMed] [Google Scholar]
- [4].Billar JA, Dueck AC, Stucky CC, Gray RJ, Wasif N, Northfelt DW, et al. , Triple-negative breast cancers: unique clinical presentations and outcomes, Ann. Surg. Oncol 17 (Suppl. 3) (2010) 384–390. [DOI] [PubMed] [Google Scholar]
- [5].Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP, The role of BRCA1 in the cellular response to chemotherapy, J. Natl. Cancer Inst 96 (2004) 1659–1668. [DOI] [PubMed] [Google Scholar]
- [6].Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. , TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer, J. Clin. Oncol 33 (2015) 1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tovey H, Bliss J, Tutt A, Morden J, Jarman K, Martin S, et al. , Managing nonproportionality of hazards (PH) within TNT: a randomised phase III trial of carboplatin compared to docetaxel for patients with metastatic or recurrent locally advanced triple negative (TN) or brca1/2 breast cancer (BC), Trials 16 (2015). [Google Scholar]
- [8].Aguilera A, Gomez-Gonzalez B, Genome instability: a mechanistic view of its causes and consequences, Nat Rev Genet 9 (2008) 204–217. [DOI] [PubMed] [Google Scholar]
- [9].Reed E, Platinum-DNA adduct, nucleotide excision repair and platinum based anticancer chemotherapy, Cancer Treat. Rev 24 (1998) 331–344. [DOI] [PubMed] [Google Scholar]
- [10].Moldovan GL, D’Andrea AD, How the fanconi anemia pathway guards the genome, Annu. Rev. Genet 43 (2009) 223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alpi AF, Patel KJ, Monoubiquitylation in the Fanconi anemia DNA damage response pathway, DNA Repair (Amst) 8 (2009) 430–435. [DOI] [PubMed] [Google Scholar]
- [12].Thompson LH, Hinz JM, Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: mechanistic insights, Mutat. Res 668 (2009) 54–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yao CJ, Du W, Zhang Q, Zhang F, Zeng F, Chen FP, Fanconi anemia pathway—the way of DNA interstrand cross-link repair, Pharmazie 68 (2013) 5–11. [PubMed] [Google Scholar]
- [14].Sung P, Prakash S, Prakash L, Mutation of cysteine-88 in the Saccharomyces cerevisiaeRAD6 protein abolishes its ubiquitin-conjugating activity and its various biological functions, Proc. Natl. Acad. Sci. U. S. A 87 (1990) 2695–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ulrich HD, The RAD6 pathway: control of DNA damage bypass and mutagenesis by ubiquitin and SUMO, Chembiochem 6 (2005) 1735–1743. [DOI] [PubMed] [Google Scholar]
- [16].Koken MHM, Reynolds P, Jaspers-Dekker I, et al. , Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6, Proc. Natl. Acad. Sci. U. S. A 88 (1991) 8865–8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shekhar MP, Lyakhovich A, Visscher DW, Heng H, Kondrat N, Rad6 overexpression induces multinucleation, centrosome amplification, abnormal mitosis, aneuploidy, and transformation, Cancer Res 62 (2002) 2115–2124. [PubMed] [Google Scholar]
- [18].Lyakhovich A, Shekhar MP, RAD6B overexpression confers chemoresistance: RAD6 expression during cell cycle and its redistribution to chromatin during DNA damage-induced response, Oncogene 23 (2004) 3097–3106. [DOI] [PubMed] [Google Scholar]
- [19].Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S, RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO, Nature 419 (2002) 135–141. [DOI] [PubMed] [Google Scholar]
- [20].Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, et al. , Translesion synthesis: Y-family polymerases and the polymerase switch, DNA Repair (Amst) 6 (2007) 891–899. [DOI] [PubMed] [Google Scholar]
- [21].Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, et al. , Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis, Science 310 (2005) 1821–1824. [DOI] [PubMed] [Google Scholar]
- [22].Mamenta EL, Poma EE, Kaufmann WK, Delmastro DA, Grady HL, Chaney SG, Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines, Cancer Res 54 (1994) 3500–3505. [PubMed] [Google Scholar]
- [23].Zhao Y, Biertumpfel C, Gregory MT, Hua YJ, Hanaoka F, Yang W, Structural basis of human DNA polymerase eta-mediated chemoresistance to cisplatin, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 7269–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen YW, Cleaver JE, Hanaoka F, Chang CF, Chou KM. A novel role of DNA polymerase eta in modulating cellular sensitivity to chemotherapeutic agents, Mol. Cancer Res 4 (2006) 257–265. [DOI] [PubMed] [Google Scholar]
- [25].Xie K, Doles J, Hemann MT, Walker GC, Error-prone translesion synthesis mediates acquired chemoresistance, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 20792–20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang J, Zhao D, Wang H, Lin CJ, Fei P, FANCD2 monoubiquitination provides a link between the HHR6 and FA-BRCA pathways, Cell Cycle 7 (2008) 407–413. [DOI] [PubMed] [Google Scholar]
- [27].Geng L, Huntoon CJ, Karnitz LM, RAD18-mediated ubiquitination of PCNA activates the Fanconi anemia DNA repair network, J. Cell Biol 191 (2010) 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fu D, Dudimah FD, Zhang J, Pickering A, Paneerselvam J, Palrasu M, et al. , Recruitment of DNA polymerase eta by FANCD2 in the early response to DNA damage, Cell Cycle 12 (2013) 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sanders MA, Brahemi G, Nangia-Makker P, Balan V, Morelli M, Kothayer H, et al. , Novel inhibitors of Rad6 ubiquitin conjugating enzyme: design, synthesis, identification, and functional characterization. Mol Cancer Ther 12 (2013) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sanders MA, Haynes B, Nangia-Makker P, Polin LA, Shekhar MP, Pharmacological targeting of RAD6 enzyme-mediated translesion synthesis overcomes resistance to platinum-based drugs, J. Biol. Chem 292 (2017) 10347–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ransburgh D, Chiba N, Ishioka C, Toland AE, Parvin JD, Identification of breast tumor mutations in BRCA1 that abolish its function in homologous DNA recombination, Cancer Res 70 (2010) 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nakanishi K, Cavallo F, Brunet E, Jasin M, Homologous recombination assay for interstrand cross-link repair, Methods Mol. Biol 745 (2011) 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shekhar MP, Gerard B, Pauley RJ, Williams BO, Tait L, Rad6B is a positive regulator of beta-catenin stabilization, Cancer Res 68 (2008) 1741–1750. [DOI] [PubMed] [Google Scholar]
- [34].Goswami CP, Nakshatri H, PROGgene: gene expression based survival analysis web application for multiple cancers, J Clin Bioinforma 3 (2013) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shekhar MP, Tait L, Gerard B, Essential role of T-cell factor/beta-catenin in regulation of Rad6B: a potential mechanism for Rad6B overexpression in breast cancer cells, Mol. Cancer Res 4 (2006) 729–745. [DOI] [PubMed] [Google Scholar]
- [36].Gerard B, Tait L, Nangia-Makker P, Shekhar MP, Rad6B acts downstream of Wnt signaling to stabilize beta-catenin: implications for a novel Wnt/beta-catenin target, J. Mol. Signal 6 (2011) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann Oncol 2012;23 Suppl 6:vi40–5. [DOI] [PubMed] [Google Scholar]
- [38].Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. , Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy, J. Clin. Oncol 28 (2010) 375–379. [DOI] [PubMed] [Google Scholar]
- [39].Byrski T, Dent R, Blecharz P, Foszczynska-Kloda M, Gronwald J, Huzarski T, et al. , Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer, Breast Cancer Res 14 (2012) R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tassone P, Di Martino MT, Ventura M, Pietragalla A, Cucinotto I, Calimeri T, et al. , Loss of BRCA1 function increases the antitumor activity of cisplatin against human breast cancer xenografts in vivo, Cancer Biol Ther 8 (2009) 648–653. [DOI] [PubMed] [Google Scholar]
- [41].Zhang W, Qin Z, Zhang X, Xiao W, Roles of sequential ubiquitination of PCNA in DNA-damage tolerance, FEBS Lett 585 (2011) 2786–2794. [DOI] [PubMed] [Google Scholar]
- [42].McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC, Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination, Mol. Cell 20 (2005) 783–792. [DOI] [PubMed] [Google Scholar]
- [43].Pan MR, Peng G, Hung WC, Lin SY, Monoubiquitination of H2AX protein regulates DNA damage response signaling, J. Biol. Chem 286 (2011) 28599–28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Baumann P, Benson FE, West SC, Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro, Cell 87 (1996) 757–766. [DOI] [PubMed] [Google Scholar]
- [45].Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK, The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin, J. Biol. Chem 275 (2000) 23899–23903. [DOI] [PubMed] [Google Scholar]
- [46].Zhao GY, Sonoda E, Barber LJ, Oka H, Murakawa Y, Yamada K, et al. , A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination, Mol. Cell 25 (2007) 663–675. [DOI] [PubMed] [Google Scholar]
- [47].Doil C, Mailand N, Bekker-Jensen S, Menard P, Larsen DH, Pepperkok R, et al. , RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins, Cell 136 (2009) 435–446. [DOI] [PubMed] [Google Scholar]
- [48].Liu C, Wang D, Wu J, Keller J, Ma T, Yu X, RNF168 forms a functional complex with RAD6 during the DNA damage response, J. Cell Sci 126 (2013) 2042–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Moyal L, Lerenthal Y, Gana-Weisz M, Mass G, So S, Wang SY, et al. requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks, Mol. Cell 41 (2011) 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nakamura K, Kato A, Kobayashi J, Yanagihara H, Sakamoto S, Oliveira DV, et al. , Regulation of homologous recombination by RNF20-dependent H2B ubiquitination, Mol. Cell 41 (2011) 515–528. [DOI] [PubMed] [Google Scholar]
- [51].Dinant C, Luijsterburg MS, The emerging role of HP1 in the DNA damage response, Mol. Cell. Biol 29 (2009) 6335–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH, Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair, Cell 144 (2011) 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen S, Wang C, Sun L, Wang DL, Chen L, Huang Z, et al. , RAD6 promotes homologous recombination repair by activating the autophagy-mediated degradation of heterochromatin protein HP1, Mol. Cell. Biol 35 (2015) 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.